Abstract
Background & Aims
Rectal indomethacin, a non-steroidal anti-inflammatory drug, is given to prevent pancreatitis in high-risk patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), based on findings from clinical trials. European Society for Gastrointestinal Endoscopy guidelines recently recommended prophylactic rectal indomethacin for all patients undergoing ERCP, including those at average risk for pancreatitis. We performed a randomized controlled trail to investigate the efficacy of this approach.
Methods
We performed a prospective, double-blind, placebo-controlled trial of 449 consecutive patients undergoing ERCP at Dartmouth Hitchcock Medical Center, from March 2013 through December 2014. Approximately 70% of the cohort were at average-risk for PEP. Subjects were randomly assigned to groups given either a single 100 mg dose of rectal indomethacin (n=223) or a placebo suppository (n=226) during the procedure. The primary outcome was the development of post-ERCP pancreatitis (PEP), defined by new upper-abdominal pain, a level of lipase greater than 3-fold the upper limit of normal, and hospitalization following ERCP for 2 consecutive nights.
Results
There were no differences between the groups in baseline clinical or procedural characteristics. Sixteen patients in the indomethacin group (7.2%) and 11 in the placebo group (4.9%) developed PEP (P=.33). Complications and the severity of PEP were similar between groups. Per a priori protocol guidelines, the study was stopped due to futility.
Conclusions
In a randomized controlled study of consecutive patients undergoing ERCP, rectal indomethacin did not prevent post-ERCP pancreatitis. ClincialTrials.gov no: NCT01774604
Keywords: pancreas, NSAID, inflammation, ESGE recommendation
Introduction
Acute pancreatitis (AP) is the most common gastrointestinal indication for admission to the hospital in the United States.1 Post-endoscopic retrograde cholangiopancreatography (ERCP) pancreatitis (PEP) is the most prevalent iatrogenic cause, leading to substantial morbidity, occasional mortality, and a significant economic impact to the United States healthcare system.2,3 Due to the clinical and economic burden of PEP, extensive research efforts have been devoted to its prevention.4-5 Among the most promising interventions to prevent PEP is the use of periprocedural rectal non-steroidal anti-inflammatory drugs (NSAIDs).6-7
Rectal NSAIDs are thought to regulate pro-inflammatory mediators in AP by inhibiting phospholipase A2 activity, including arachidonic acid products and platelet-activating factors.6,8 One NSAID in particular, rectal indomethacin, has been used extensively since 2012 following the publication of a randomized, placebo-controlled trial in patients undergoing ERCP considered to be at high risk for PEP.8 The trial found that a single 100 mg dose of rectal indomethacin significantly reduced the risk of PEP from 16.9% in those receiving placebo to 9.2% in those receiving indomethacin. As a result of this study and others, the European Society for Gastrointestinal Endoscopy (ESGE) in 2014 recommended routine rectal administration of 100 mg indomethacin or diclofenac during ERCP in all patients without contraindication.9 However, despite these recommendations, the use of rectal NSAIDs in patients not considered to be at high-risk for PEP (the “average-risk” patient) is unproven.
In order to determine the benefit of rectal indomethacin in preventing PEP in all patients, we conducted a prospective, randomized, double-blind, placebo-controlled trial in consecutive patients undergoing ERCP.
Methods
Study Design
We enrolled patients at a single, tertiary care, academic medical center in the United States after approval from the Committee for the Protection of Human Subjects (IRB) at Dartmouth-Hitchcock Medical Center (CPHS#23749). An independent data and safety monitoring board provided regulatory oversight by reviewing blinded subject data, analyzing complications, and performing scheduled in-term analysis. The study was designed under the auspices of the CONSORT guidelines.10
Patients
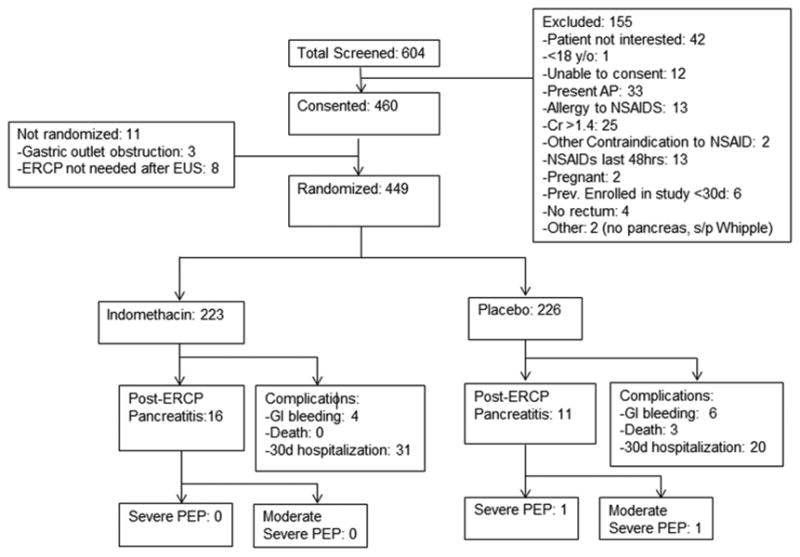
The inclusion criteria were defined as consecutive patients undergoing ERCP (+/- endoscopic ultrasound [EUS]) at Dartmouth-Hitchcock Medical Center in Lebanon, NH. All patients were adults greater than 18 years-old who were able to provide written, informed consent. Consent was obtained by the therapeutic endoscopist or interventional fellow at the time of informed consent for the procedure. Exclusion criteria included all patients with active acute pancreatitis, those in whom ERCP was performed for diagnosis and/or treatment of acute pancreatitis, contraindication to NSAID therapy (serum creatinine >1.4 mg/dL or active peptic ulcer disease), previously documented allergy to NSAIDs, pregnant or nursing mothers, inability to provide written informed consent, those who had been previously randomized within the last 30 days, those <18 years of age, or those without a rectum (i.e. status post total proctocolectomy). Eligible patients who provided written informed consent and met inclusion criteria were randomized after the major papilla was reached and attempts at cannulation initiated (Figure 1). Randomization was performed in a block format prior to study initiation by the Dartmouth Investigational Pharmacy with the investigators blinded to treatment allocation. Pre-made envelopes with allocation and study number ensured randomization concealment until interventions were assigned.
Figure 1. Enrollment and Outcomes.
Intervention
All procedure-related maneuvers and interventions were managed by two experienced therapeutic endoscopists. Following attempted cannulation, two 50 mg indomethacin suppositories (Cardinal Health, Dublin, OH) or two inert placebo suppositories (Letco Medical, Decatur, AL) were administered by the nurse in the procedure room if the patient had met all inclusion criteria and signed written, informed consent. The suppository was given per rectum during the ERCP. The endoscopist and patient were blinded to the study allocation.
The number of cannulation attempts, the use and type of pancreatic duct stents, the use of wire-guided cannulation, the amount of periprocedural intravenous fluid, and the participation of an advanced endoscopy fellow were factors all at the discretion of the treating endoscopist and were not specifically outlined in the study protocol.
Outcomes
The primary study outcome was whether 100 mg of rectal indomethacin compared to placebo would lower the rate of PEP in all patients undergoing ERCP. The secondary outcome was to assess the severity of PEP in those receiving indomethacin versus placebo. PEP was defined if the following three conditions were met – new onset upper abdominal pain, an elevated lipase greater than three times the upper limit of normal 24 hours after the onset of pain, and hospitalization for at least two nights. Severity of pancreatitis was defined per the Revised Atlanta Classification.11
Following ERCP, patients were observed in the recovery area per institutional guidelines for at least ninety minutes. If there was new pain requiring admission, the patient was admitted to the hospitalist medicine service. Subsequent care was left to the discretion of the inpatient service team and supporting gastrointestinal consult service, both of whom were unaware of study-group assignments.
All patients were contacted by telephone five days following the ERCP to determine whether they developed PEP. All patients were then again contacted thirty days after the procedure to assess for delayed complications and/or PEP. ERCP procedural elements, patient demographics, and follow-up data were recorded on standardized data-collection forms by the primary investigator who was unaware of the patient/study-group assignments. All co-authors had access to the study data, reviewed, and approved the final manuscript.
Adverse Events
All complications of the procedure, adverse events that were potentially attributed to the study drugs, and any death from any cause within 30 days of enrollment were reported to the local institutional review board (IRB) as well as the data and safety monitoring board. The IRB requested not to report PEP as it is an expected event following ERCP.
Statistical Analysis
We estimated that 1,398 patients (699 per study group) would provide a power of 80% to detect a 50% reduction in the rate of PEP from 5% in the placebo group to 2.5% in the indomethacin group using the two-tailed Fisher's Exact test with a two-sided significance of 0.05.12 The prevalence of PEP was based on internal estimates of the past rate of PEP in patients undergoing ERCP at our medical center.
Continuous variables were evaluated using the student's t-test and categorical variables using the Fisher's Exact test. Exploratory subgroup analysis by calculating relative risks was performed for pre-specified factors which conveyed a higher risk for PEP - pancreatic stent placement, suspected sphincter of Oddi dysfunction, history of PEP, difficult cannulation, wire cannulation of the pancreatic duct, pancreatography, pancreatic acinarization, therapeutic biliary sphincterotomy, therapeutic pancreatic sphincterotomy, balloon dilation of the biliary sphincter, and trainee involvement in the ERCP.13
Scheduled interim analysis was to be performed after 350 patients were enrolled (25% of predicted enrollment) to ensure safety and assess the primary outcome. A priori stopping guidelines were instituted including a p-value of <0.020 showing benefit of indomethacin versus placebo, or evidence of futility to reach a statistical different outcome between the study groups.
Results
Patients
From March 2013 to December 2014, all patients undergoing ERCP at our institution were screened for eligibility and offered inclusion into the study if eligible (See Figure 1). 449 patients were eventually enrolled into the study. In May 2014, the data and safety monitoring committee evaluated the first 350 patients and due to indomethacin's lack of efficacy, recommended continued enrollment with 75 more patients randomized. In December 2014, the board recommended termination of the study due to futility of the anticipated primary end point after reviewing these additional 75 patients. Due to the lag time between enrollment and 30 day study follow-up requirements, an additional 24 patients were enrolled and are included in the study analysis. Thus, a total of 223 patients received indomethacin and 226 received placebo. Follow-up of all patients for primary and secondary end-points was completed.
Baseline characteristics were similar in both groups (See Table 1). Only 14 patients (2.8%) had suspected sphincter of Oddi dysfunction and 37 (7.4%) had planned interventions to their pancreatic duct due to a pancreatic duct stricture, disruption, or stone. Table 2 demonstrates the procedural characteristics. There was no difference in the rates of sphincter manipulation, biliary and/or pancreatic duct stenting, or previous biliary and/or pancreatic sphincterotomy.
Table 1. Baseline Patient Characteristics.
| Characteristic | Indomethacin (n=223) | Placebo (n=226) | P value |
|---|---|---|---|
|
| |||
| Age – yr | 64.9 | 64.3 | 0.68 |
| Female Sex – no. (%) | 118 (52.9) | 118 (52.2) | 0.92 |
| Indication – no. (%) | |||
| Acute Cholangitis | 12 (5.4) | 13 (5.8) | 1.00 |
| Choledocholithiasis | 59 (26.4) | 52 (23.0) | 0.44 |
| Malignant Biliary Obstruction* | 53 (23.8) | 50 (22.1) | 0.74 |
| Biliary Stent Change | 25 (11.2) | 25 (11.1) | 1.00 |
| Biliary Leak | 11 (4.9) | 12 (5.3) | 1.00 |
| Elevated Liver Tests/Jaundice | 13 (5.8) | 9 (4.0) | 0.39 |
| Pancreatic Stricture | 3 (1.3) | 5 (2.2) | 0.72 |
| Suspected Sphincter of Oddi Dysfunction | 6 (2.7) | 8 (3.5) | 0.79 |
| Pancreatic Leak/Disruption | 11 (4.9) | 12 (5.3) | 1.00 |
| Pancreatic Duct Stone | 3 (1.3) | 2 (0.9) | 0.68 |
| Recurrent Acute Pancreatitis | 5 (2.2) | 2 (0.9) | 0.28 |
| Ampullectomy | 6 (2.7) | 5 (2.2) | 0.77 |
| Other† | 16 (7.2) | 31 (13.7) | 0.03 |
| History of post-ERCP pancreatitis – no. (%) | 9 (4.0) | 9 (4.0) | 1.00 |
| Previous Sphincterotomy – no. (%) | 72 (31.8) | 71 (31.4) | 0.61 |
| Previous ERCP – no. (%) | 81 (36.3) | 79 (35.0) | 0.77 |
Malignant biliary obstruction includes pancreatic head malignancy, cholangiocarcinoma and indeterminate biliary stricture
Most common indications for “other” included primary sclerosing cholangitis, papillary stenosis, and choledochal cyst evaluation
Table 2. Procedural Characteristics.
| Characteristic | Indomethacin (n=223) | Placebo (n=226) | P value |
|---|---|---|---|
|
| |||
| Sphincter Manipulation | |||
| Difficult Cannulation (>8 attempts) – no. (%) | 46 (20.6) | 42 (18.4) | 0.64 |
| Precut Biliary Sphincterotomy | 11 (4.9) | 16 (7.0) | 0.43 |
| Therapeutic Biliary Sphincterotomy – no. (%) | 106 (46.9) | 114 (50.9) | 0.57 |
| Therapeutic Pancreatic Sphincterotomy – no. (%) | 12 (5.4) | 5 (2.2) | 0.08 |
| Minor Duct Sphincterotomy – no. (%) | 5 (2.2) | 4 (1.8) | 0.75 |
| Balloon Dilation of Biliary Sphincter – no. (%) | 21 (9.4) | 20 (8.8) | 0.87 |
| Pancreatic Duct Manipulation | |||
| Wire Cannulation of Pancreatic Duct – no. (%) | 90 (40.3) | 89 (31.4) | 0.85 |
| Pancreatography – no. (%) | 50 (22.4) | 49 (21.7) | 0.97 |
| Pancreatic Acinarization – no. (%) | 5 (2.2) | 4 (1.8) | 0.75 |
| Stent Placement | |||
| Biliary Stent Placement – no. (%) | 89 (39.9) | 84 (37.2) | 0.56 |
| Pancreatic Stent Placement – no. (%) | 36 (16.1) | 35 (15.5) | 0.90 |
| Other Interventions | |||
| Trainee Involvement in ERCP – no. (%) | 152 (68.2) | 168 (74.3) | 0.18 |
| Concomittant EUS/FNA – no. (%) | 41 (18.4) | 40 (17.7) | 0.90 |
| Periprocedural Fluid Volume - ml | 705 | 703 | 0.95 |
Study Outcomes
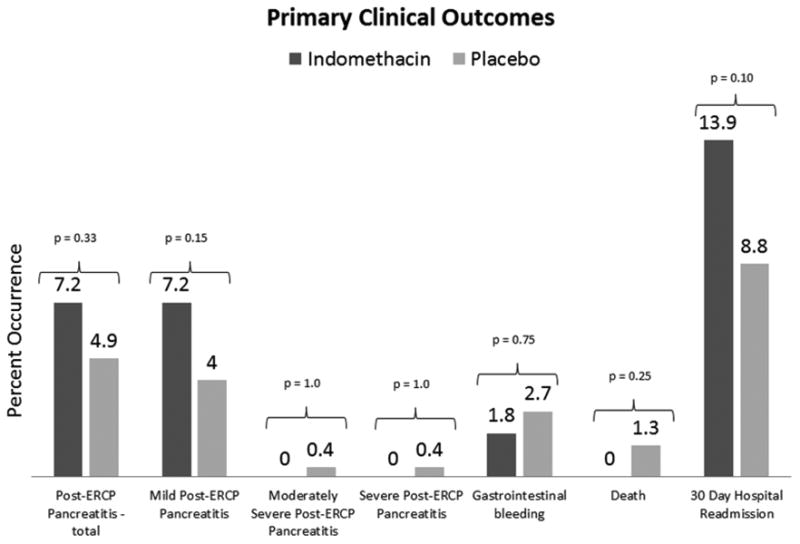
The primary outcome of PEP occurred in 27 of 449 patients (6.0%) - 16 of 223 (7.2%) occurred in the indomethacin group and 11 of 226 (4.9%) occurred in the placebo group (p=0.33) (Figure 2). All 27 patients with PEP completed 30 days of follow-up (or until time of death) to assess the severity of pancreatitis. Severe or moderately severe PEP occurred in 0 of 223 receiving indomethacin while 1 of 226 (0.4%) had severe PEP (p=1.0) and 1 of 226 had moderately severe PEP receiving placebo (0.4%) (p=1.0). The patient with severe PEP in the placebo group died secondary to untreated acute renal failure following diagnostic and palliative EUS/FNA and ERCP with biliary stenting for metastatic pancreatic cancer, opting for comfort care only. The two other deaths in the placebo group were not secondary to pancreatitis: one with known multifocal hepatocellular carcinoma died from secondary bacterial peritonitis in the setting of decompensated ascites and one from a witnessed aspiration event in the hospital two days post-procedure. No necrotizing pancreatitis occurred in either group. There was no difference in rates of gastrointestinal bleeding, death, or 30 day hospital readmission (p= 0.75, 0.25, and 0.10 respectively) (Figure 2).
Figure 2. Primary Clinical Outcomes.
Exploratory subgroup analysis
Table 3 demonstrates that there was no one-directional trend toward benefit in terms of relative risk reduction for indomethacin vs. placebo. Of note, there was an increased rate of pancreatitis in those who underwent dual therapy of rectal indomethacin and pancreatic duct stenting, although this did not meet statistical significance (8 of 16 patients in the indomethacin group and 4 of 11 in the placebo group with a relative risk reduction of -28%).
Table 3. Exploratory SubGroup Analysis of Patients with Post-ERCP Pancreatitis.
| Patients with PEP | % Relative Risk Reduction (Indomethacin vs. Placebo) | ||
|---|---|---|---|
| Indomethacin (n=16) | Placebo (n=11) | ||
|
| |||
| Pancreatic Stent Placement – no. (%) | 8 (50) | 4 (36) | -28% |
| Suspected Sphincter of Oddi Dysfunction – no. (%) | 1 (6) | 1 (9) | +33% |
| History of Post-ERCP Pancreatitis | 2 (13) | 1 (9) | -21% |
| Difficult Cannulation | 6 (38) | 5 (45) | +16% |
| Wire Cannulation of Pancreatic Duct – no. (%) | 13 (81) | 7 (64) | -21% |
| Pancreatography – no. (%) | 8 (50) | 8 (73) | +32% |
| Pancreatic Acinarization – no. (%) | 2 ( 13) | 0 (0) | NA |
| Therapeutic Biliary Sphincterotomy – no. (%) | 7 (44) | 3 (27) | -39% |
| Therapeutic Pancreatic Sphincterotomy – no. (%) | 2 (13) | 3 (27) | +52% |
| Balloon Dilation of Biliary Sphincter – no. (%) | 0 (0) | 1 (9) | NA |
| Trainee Involvement in ERCP – no. (%) | 12 (75) | 8 (73) | -3% |
In patients with biliary manipulation only, 3 of 124 (2.4%) in the placebo group and 2 of 125 (1.6%) in the indomethacin group had PEP (p=0.68). In patients with pancreatic ductal instrumentation only, 3 of 28 (10.7%) in the placebo group and 6 of 32 (18.8%) in the indomethacin group developed PEP (p=0.48). Manipulation of both ducts (including wire access only) resulted in PEP in 5 of 70 (7.1%) patients in the placebo group and 8 of 70 (11.4%) in the indomethacin group (p=0.79).
Discussion
Our findings demonstrate that giving a single 100 mg of rectal indomethacin in consecutive individuals undergoing ERCP does not prevent PEP. These results are in contrast to recent studies highlighting the benefit of rectal NSAIDS to prevent PEP in high-risk patients.8 In addition, our results counter the guidelines espoused by the European Society for Gastrointestinal Endoscopy, which recently recommended giving rectal indomethacin to prevent PEP in all patients undergoing ERCP.9
This study did not exclude patients based on indication or intervention; it was deliberately designed to mirror the unenhanced patient population that is encountered most frequently in general gastroenterology practice. It also did not pre-determine the interventions of the treating endoscopist for each patient; again this was by design in order to most directly mirror typical clinical practice. Fortunately, randomization was successful in terms of both baseline patient characteristics and procedural interventions. In addition, the study deliberately did not categorize patients into high and low-risk for PEP to maintain appropriate randomization and there was no evidence of increased PEP in high-risk patients, nor benefit of rectal indomethacin in this group. A potential study limitation, however, is that patients were enrolled at a single center.
The overall rate of PEP (6%) was consistent with our pre-study estimates, which is much lower than the mean rate of PEP in previous studies of high-risk patients in NSAID pharmacoprevention studies.8 This finding supports the characterization of this study population as unenhanced toward a high-risk group and suggests that the conclusions reached in prior studies are not generalizable to all patients undergoing ERCP.
There is a clear benefit of using rectal NSAIDs to prevent PEP in high-risk individuals as demonstrated in the study by Elmunzer et al.8 Since this manuscript was published, many institutions have begun using rectal indomethacin in all patients undergoing ERCP.14 However, our findings suggest that universal prophylaxis using rectal indomethacin for PEP is not beneficial and should not be recommended.
The ESGE guidelines are based on meta-analyses of several small studies and then larger studies in high-risk patients.15-22 Currently, there are no major American endoscopy society guidelines that specifically recommend using rectal NSAIDs to prevent PEP in all patients.23 One might argue that since rectal NSAIDs are inexpensive with little chance of causing clinically relevant side effects, the benefit of occasionally preventing PEP outweighs the minimal risk. However, guidelines that promulgate universal prophylaxis based on presumptive application to groups not fully studied are not justified and should not be endorsed. Not only can assumptions lead to unnecessary medication usage and charges, they can also lead to unsubstantiated medico-legal liability.
The role of pancreatic duct stenting in combination with rectal NSAIDs has yet to be determined.24-5 In this study, there was an increased rate of pancreatitis in those who had the dual therapy of rectal indomethacin and pancreatic duct stenting. Further investigation is needed to evaluate the role of rectal NSAID therapy to prevent PEP in the context of pancreatic duct stenting. Until definitive comparative effectiveness trials are performed, pancreatic duct stenting has been, and should remain, a critical component of reducing PEP and in our opinion should be employed whenever wire access to the pancreatic duct and/or pancreatography is performed.
There was no protective benefit found with previous sphincterotomy in this study, which mirrors similar results in other large studies evaluating risk factors for PEP. For example, in a large prospective multi-center cohort study, previous sphincterotomy had a non-significant increased risk of PEP compared to those with a native papilla (7.8 vs 6.2%, p=0.22).26 Furthermore, prior sphincterotomy was only one of nine of the 32 variables in that study which did not reach significance in univariate or multivariate analysis as protective against PEP.
In summary, prophylactic rectal indomethacin did not reduce the incidence or severity of PEP in consecutive patients undergoing ERCP. Guidelines that recommend the administration of rectal indomethacin in all patients undergoing ERCP should be reconsidered.
Acknowledgments
Grant support: National Pancreas Foundation supported the cost of the medications; Dr. Gardner is supported in part by NIH grant 1K23DK088832
Abbreviations
- endoscopic retrograde cholangiopancreatography (ERCP)
endoscopic ultrasound (EUS), fine needle aspiration (FNA), nonsteroidal anti-inflammatory drugs (NSAID), post-ERCP pancreatitis (PEP)
Footnotes
Disclosures: None
M Rockacy, SB Bensen, BE Lacy, LC Levy, S Hyder: Acquisition of data, critical revision
Author Contribution: JM Levenick, SR Gordon, and TB Gardner: Study concept, design, obtained funding, acquisition of data, analysis, interpretation, drafting and editing of manuscript, study supervision.
L Fadden, D Parr: technical and material support
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peery AF, Dellon, ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143(5):1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335(13):909–18. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 3.National Inpatient Sample – Healthcare Cost and Utilization Project. Rockville, MD: Agency for Healthcare Research and Quality; ( Http://hcupnet.ahrq.gov) [Google Scholar]
- 4.Fazel A, Quadri A, Catalano MF, et al. Does a pancreatic duct stent prevent post-ERCP pancreatitis? A prospective randomized study Gastrointest Endosc. 2003;57:291–4. doi: 10.1067/mge.2003.124. [DOI] [PubMed] [Google Scholar]
- 5.Tarnasky PR, Palesch YY, Cunningham JT, et al. Pancreatic stenting prevents pancreatitis after biliary sphincterotomy in patients with sphincter of Oddi dysfunction. Gastroenterology. 1998;115:1518–24. doi: 10.1016/s0016-5085(98)70031-9. [DOI] [PubMed] [Google Scholar]
- 6.Sotoudehmanesh R, Khatibian M, Kolahdoozan S, et al. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007;102:978–83. doi: 10.1111/j.1572-0241.2007.01165.x. [DOI] [PubMed] [Google Scholar]
- 7.Murray B, Carter R, Imrie C, et al. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003;124:1786–91. doi: 10.1016/s0016-5085(03)00384-6. [DOI] [PubMed] [Google Scholar]
- 8.Elmunzer BJ, Scheiman JM, Lehman GA, et al. A Randomized Trial of Rectal Indomethacin to Prevent Post-ERCP Pancreatitis. N Engl J Med. 2012;366:1414–22. doi: 10.1056/NEJMoa1111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline – updated June 2014. Endoscopy. 2014;46:799–815. doi: 10.1055/s-0034-1377875. [DOI] [PubMed] [Google Scholar]
- 10.http://www.consort-statement.org/
- 11.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis-2012: revision of Atlanta classification and definitions by international consensus. Gut. 2013;62:102–11. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 12.Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–1168. [PubMed] [Google Scholar]
- 13.Chen JJ, Wang XM, Liu XQ, et al. Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res. 2014 May;19(1):26. doi: 10.1186/2047-783X-19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia MJ, Santiago SL, Martin CA, et al. Factors involved in post-ERCP pancreatitis in a tertiary care hospital. DDW 2015, presentation Su1632 [Google Scholar]
- 15.Dai HF, Wang XW, Zhao K. Role of nonsteroidal anti-inflammatory drugs in the prevention of post-ERCP pancreatitis: a meta-analysis. Hepatobiliary Pancreat Dis Int. 2009;8:11–16. [PubMed] [Google Scholar]
- 16.Elmunzer B, Waljee A, Elta G, et al. A meta-analysis of rectal NSAIDS in the prevention of post-ERCP pancreatitis. Gut. 2008;57:1262. doi: 10.1136/gut.2007.140756. [DOI] [PubMed] [Google Scholar]
- 17.Zheng M-H, Xia H, Chen Y-P. Rectal administration of NSAIDs in the prevention of post-ERCP Pancreatitis: a complementary meta-analysis. Gut. 2008;57:1632. [PubMed] [Google Scholar]
- 18.Ding X, Chen M, Huang S, et al. Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis: a meta-analysis. Gastrointest Endosc. 2012;76:1152–1159. doi: 10.1016/j.gie.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Yahoobi M, Rolland S, Waschke KA, et al. Meta-analysis: rectal indomethacin for the prevention of post-ERCP pancreatitis. Aliment Pharmacol Ther. 2013;38:995–1001. doi: 10.1111/apt.12488. [DOI] [PubMed] [Google Scholar]
- 20.Sun HL, Han B, Zhai HP, et al. Rectal NSAIDs for the prevention of post-ERCP pancreatitis: A meta-analysis of randomized controlled trials. Surgeon. 2014;12:141–147. doi: 10.1016/j.surge.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Yuhara H, Ogawa M, Kawaguchi Y, et al. Pharmacologic prophylaxis of post-endoscopic retrograde cholangiopancreatography pancretitis: protease inhibitors and NSAIDs in a meta-analysis. J Gastroenterol. 2014;49:388–399. doi: 10.1007/s00535-013-0834-x. [DOI] [PubMed] [Google Scholar]
- 22.Sethi S, Sethi N, Wadhwa V, et al. A meta-analysis on the role of rectal diclofenac and indomethacin in the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Pancreas. 2014;43:190–197. doi: 10.1097/MPA.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 23.ASGE Standard of Practice Committee. Complications of ERCP. Gastrointest Endosc. 2012;75(3):476–73. doi: 10.1016/j.gie.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Elmunzer BJ, Higgins PD, Saini SD, et al. Does rectal indomethacin eliminate the need for prophylactic stent placement in patients undergoing high-risk ERCP? Post hoc efficacy and cost-benefit analyses using prospective clinical trial data. Am J Gastroenterol. 2013 Mar;108(3):410–5. doi: 10.1038/ajg.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baron TH, Abu Dayyeh BK, Zinsmeister AR. Rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. 2012 Jul 19;367(3):277–8. doi: 10.1056/NEJMc1205928. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001;54(4):425–34. doi: 10.1067/mge.2001.117550. [DOI] [PubMed] [Google Scholar]


