Abstract
Objective
To determine the expected systolic, mean and diastolic blood pressures at birth and respective rates of change during the first 72 hours of life in infants born <28 weeks EGA with a favorable short-term outcome, defined as survival to 14 days with grade II or less IVH.
Study Design
Systolic, mean and diastolic blood pressures were continuously sampled at 0.5 Hz via umbilical artery catheter from birth through 72 hours. The raw data were aligned by postnatal hour and underwent error correction. For each infant, the mean values of systolic, mean and diastolic blood pressure were calculated for each postnatal hour. The slope and intercept of best-fit line for each of the three blood pressure parameters was then calculated. Infants that received inotropic medications, died in the first 14 days of life, or had IVH grade III or IV were excluded.
Result
Using 11.9 million valid data points from 35 infants (mean EGA = 25.7±1.5 weeks, mean birth weight = 865 ± 201 grams), we found independent associations of African-American race (p<0.01) and a complete course of antenatal steroids (p<0.01) with higher blood pressures at birth and a slower rate of increase. Acute chorioamnionitis was independently associated (p=0.02) with lower blood pressures at birth and a faster rate of increase. EGA and birth weight were not independently predictive of blood pressure parameters.
Conclusion
We found that (i) the estimated mean blood pressure at birth is approximately 33 mmHg in a cohort of very preterm infants (ii) blood pressure gradually increases with postnatal age (iii) systolic blood pressure increases at a faster rate than diastolic blood pressure, (iv) race, antenatal steroid exposure, and chorioamnionitis are independent modulators of blood pressure while EGA and birth weight are not.
Keywords: Preterm infants, blood pressure, massive data, regression analysis
Introduction
Determining the target blood pressure range in very preterm infants (<28 weeks gestation) has been the subject of debate for several decades. Hypotension has been previously linked to the development of intraventricular hemorrhage (1,2) and white matter injury (3). Description of a normative set of blood pressure values in very preterm infants is confounded by varied methodology, heterogeneous patient populations used in prior studies, substantial changes in strategies for hemodynamic support and monitoring methodology, and survival rates among preterm infants since Versmold et al. published the first normative data set in 1981.(4)
Previous studies can be categorized into two groups by blood pressure measurement methodology, non-invasive oscillometric or intra-arterial manometric measurement. The results of the two largest studies, one conducted by Zubrow et al. (5) using oscillometric measurements taken every eight hours from birth until term-equivalent age and the other by Cunningham et al. (6) using intra-arterial measurements taken every minute for the first 7 days of life, form the basis for the most common clinical practice of setting a goal mean arterial blood pressure (MABP) in mmHg equal to the estimated gestational age (EGA) in weeks of the infant. Other studies have been conducted in the intervening years using similar methodologies with inconsistent results. This variance likely arises from two sources: (i) different sampling intervals ranging from three times during the entire hospital stay (7) to once per hour (8) and (ii) systematic variation in measurement by device manufacturer and patient state (9). The systematic bias introduced by non-invasive blood pressure measurement can be avoided by the use of intra-arterial measurement, although studies that employ this methodology include heterogeneous sampling periods (ranging from every 8.8 minutes (10) to once during the entire hospitalization (4)) which confound clinical applicability.
Another confounding issue across studies is underrepresentation of infants born <28 weeks completed gestation. Versmold et al. examined a cohort with a mean EGA <28 weeks (mean EGA was 26 weeks, although biased by a 92% exclusion rate intended to capture the 16 most stable infants who weighed <1,000 grams among the 207 candidates) while other studies have examined more mature cohorts with mean EGAs ranging between 29 (7) and 32.5 weeks (7,8,10–13). While Batton et al. reported a more homogeneous very preterm cohort, the data they collected from multiple centers lacked standardization of measurement methodology (mixed umbilical arterial, peripheral arterial and non-invasive) and used a sampling rate of once per hour (14). The paucity and heterogeneity of data in this very preterm population have led to considerable variance in clinical practice, e.g., different rates of volume expansion and inotrope use for resuscitation in similar cohorts of infants at different centers (15).
In this this study, we present the empirical analysis results of 11.9 million data points of intra-arterial manometric blood pressure data, prospectively collected during the first three days following birth in a cohort of very preterm infants <28 weeks EGA who had a favorable short term outcome, defined as no clinical need for inotropes or volume resuscitation, no greater than grade II IVH and survival at two weeks of life.
Material and Methods
Participants
Between 2012 and 2014 we prospectively recruited infants, after informed parental consent, who were born < 28 weeks gestation by best obstetrical estimate and admitted to the Neonatal Intensive Care Unit at St. Louis Children’s Hospital for a hemodynamic monitoring study. Infants were excluded if they were born ≥ 28 weeks EGA, if the clinical team did not elect to place an umbilical arterial catheter, if inotropic agents (dopamine or epinephrine) or volume resuscitation were used, if the infant had greater than grade II interventricular hemorrhage or if the infant died before 14 days of life. The study protocol was reviewed and approved by the Washington University School of Medicine Human Research Protection Office.
Infant clinical characteristics
Records regarding antenatal steroid or magnesium sulfate administration, diagnosis of chorioamnionitis, delivery route, and use of delayed cord clamping were obtained from the maternal medical chart. Infant clinical characteristics including birth weight, sex, race (by maternal report), Apgar scores, inotropic medication use, sedative use, patent ductus arteriosus requiring medical or surgical treatment and cranial ultrasound (CUS) reports were obtained from the infant’s medical record.
Data collection
Umbilical arterial lines (3.5 French Argyle single lumen umbilical vessel catheter, Covidien, Mansfield, MA, USA) were placed, shortly after hospital admission, in the high-lying position, located between the sixth and eighth thoracic vertebrae on chest radiograph as per standard clinical practice. Arterial blood pressure was then determined by use of a pressure transducer connected to the patient monitor (Intellivue MP70, Philips Medical, Andover, MA, USA). Systolic, mean and diastolic arterial blood pressures were digitally sampled continuously for the first 72 hours. A time-integrated sample of the beat-to-beat blood pressure was taken every two seconds yielding a sampling rate of 0.5 Hz. Periods of time where the pressure reading was known to be unreliable (e.g. while collecting specimen for arterial blood gas or during infusion of medication or fluids through the catheter) were noted in the research record by a research assistant or the bedside nurse. At our institution, umbilical arterial lines are not routinely accessed or flushed except for specimen withdrawal or for infusion. Study data were collected and managed using REDCap electronic data capture tools (16) hosted at Washington University.
Data pre-processing and analysis
Pre-processing
The captured data underwent multistep pre-processing to eliminate missing or invalid data using in-house software written in MATLAB 8.4 (The Mathworks Inc., Natick, MA, USA). The time trace was loaded in serial, non-overlapping 10 second (5 sample) blocks and inspected for (i) interrupted regions of the recording (as indicated in the research record) and (ii) periods with motion artifact, defined as sudden, non-physiologic changes in the baseline or excessive variance using the sliding-window motion artifact rejection methodology proposed by Ayaz et. Al (17). Blocks of data containing errors were rejected.
Data analysis
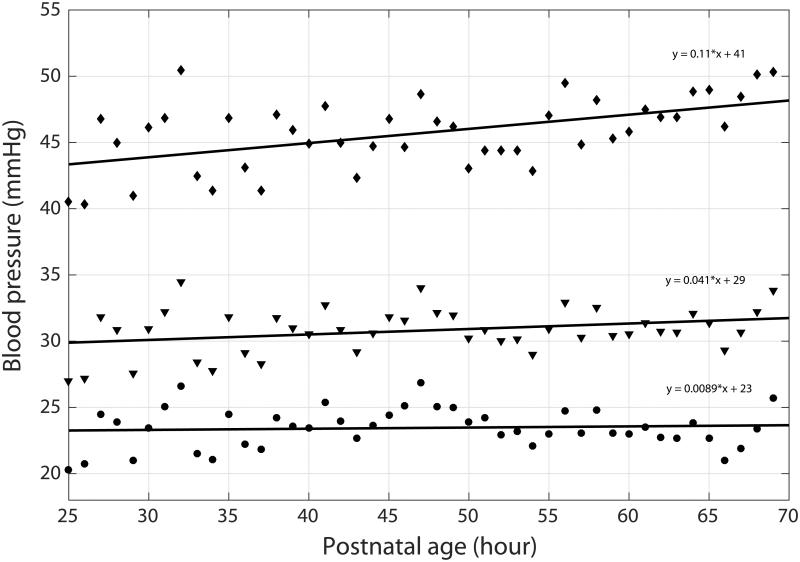
Mean values for systolic, mean and diastolic blood pressure were calculated on an infant-by-infant basis for each postnatal hour, from the start of the recording through 72 hours. Ordinary least squares regression was used to calculate the slope and intercept of the best-fit line for each of the three blood pressure parameters. An example of raw data and regression lines for a single subject are shown in Figure 1.
Figure 1.
Mean hourly blood pressure values shown for a single subject. SBP values shown as diamond, MABP values shown as triangles, DBP values shown as circles. Ordinary least squares regression lines shown for each parameter. Equations are shown for each line.
Statistical methodology
A multiple linear regression model was developed to evaluate the impact of important clinical factors (gestational age, birth weight, race, antenatal magnesium sulfate or steroid exposure, delayed umbilical cord clamping, method of ventilation, patent ductus arteriosus (PDA) requiring treatment, and acute chorioamnionitis) on the intercept and slope values for each of the three blood pressure measures. Correlation between predictors was assessed using the variance inflation factor (VIF), a measure of the degree of multicollinearity where VIF > 5 is indicative of highly correlated predictors. A population estimate was derived averaging the calculated blood pressure measures for each postnatal hour across the entire cohort.
Statistical analysis, including descriptive statistics and regression modeling, were conducted using R version 3.1.0 (R Project for Statistical Computing, Vienna, Austria) and Minitab 17.0 (Minitab Inc., State College, PA, USA).
Results
Sample characteristics and data quality
Sixty-two infants were initially recruited into the study. Seventeen infants (27%) were excluded due to inotrope use, six (10%) were excluded due to high grade IVH, and four (6%) were excluded due to death in the first 14 days, leaving thirty-five infants with a mean ± standard deviation EGA of 25.7 ± 1.5 weeks and a mean birth weight of 865.4 ± 201.1 grams. A summary of the clinical characteristics of the cohort is displayed in Table 1. The median postnatal age at recording start was 12 hours (range 5-24 hours) and a median of 66 hours of data was captured per infant. Preprocessing resulted in an average rejection of 8% of each recording, leaving a median of 63 hours of data captured per infant for a total of 11.9 million valid data points.
Table 1.
Sample descriptive statistics
| N=35 | |
|---|---|
| EGA, mean (SD), weeks | 25.7 (1.5) |
| Birthweight, mean (SD), grams | 865 (201) |
| SGA status, n (%) | 2 (6) |
| Male sex, n (%) | 23 (66) |
| Caucasian race, n (%) | 19 (54) |
| Vaginal delivery, n (%) | 9 (26) |
| Antenatal magnesium sulfate, n (%) | 17 (49) |
| Any antenatal steroids, n (%) | 27 (77) |
| Complete course of antenatal steroidsa, n (%) | 14 (40) |
| 5 minute Apgar score, median (min-max) | 6 (1-9) |
| Acute chorioamnionitis, n (%) | 11 (31) |
| Delayed cord clamping, n (%) | 3 (9) |
| IVH, n (%) | 11 (31) |
| PDA requiring treatment, n (%) | 19 (54) |
| Sedation in first 72h, n (%) | 7 (20) |
| Intubated at 72h of life, n (%) | 22 (63) |
Footnote:
Defined as receiving two doses of betamethasone over a 48-hour period.
Multiple linear regression models
Blood pressure intercept (estimation of blood pressure at birth)
The prediction models for systolic, mean and diastolic blood pressure intercepts were each statistically significant. African-American race and completion of a course of antenatal steroids were independently associated with higher intercept values of systolic, mean and diastolic blood pressure. Acute chorioamnionitis was independently associated with a lower intercept value for systolic and mean blood pressures. The impact of invasive and non-invasive ventilation was not able to be systematically assessed given the sample size and considerable heterogeneity in timing of extubation. A PDA requiring medical or surgical treatment was not associated with a change in intercept values in univariate analysis and was not included in the final model. The complete model output is shown in Table 2.
Table 2.
Multiple linear regression models for blood pressure intercepts
| Systolic | Mean | Diastolic | ||||
|---|---|---|---|---|---|---|
| Covariate | β | P | β | P | β | P |
| EGA | 0.60 | 0.59 | 0.68 | 0.42 | 0.723 | 0.40 |
| BW | −0.003 | 0.70 | −0.003 | 0.53 | −0.003 | 0.51 |
| African-American race | 4.49 | 0.02* | 4.74 | <0.01* | 4.86 | <0.01* |
| Acute chorioamnionitis | −6.18 | <0.01* | −3.45 | 0.02* | −2.11 | 0.17 |
| Antenatal MgSO4 | 1.25 | 0.57 | 1.03 | 0.54 | 0.92 | 0.59 |
| Complete course of antenatal corticosteroidsa |
4.89 | 0.02* | 4.81 | <0.01* | 4.77 | <0.01* |
| Delayed umbilical cord clamping | 5.32 | 0.16 | 4.25 | 0.13 | 3.72 | 0.20 |
Footnote:
Denotes significance at p < 0.05.
Defined as receiving two doses of betamethasone over a 48-hour period. All β coefficients are unstandardized.
Model summary: Systolic- R2=0.503, Mean- R2=0.556, Diastolic- R2=0.512.
Blood pressure slope (depicting rate of rise of blood pressure over time)
The prediction models for systolic, mean and diastolic blood pressure slopes were all statistically significant. Delayed cord clamping and completion of a course of antenatal steroids were independently associated with a decreased value for systolic blood pressure slope. Delayed cord clamping, antenatal steroids and African-American race were associated with a decreased value for mean and diastolic blood pressure slope. A PDA requiring medical or surgical treatment was not associated with a change in slope values in univariate analysis and was not included in the final model. The complete model output is shown in Table 3.
Table 3.
Multiple linear regression model for blood pressure slopes
| Systolic | Mean | Diastolic | ||||
|---|---|---|---|---|---|---|
| Covariate | β | P | β | P | β | P |
| EGA | 0.05 | 0.15 | 0.02 | 0.29 | 0.01 | 0.53 |
| BW | 1.7×10−4 | 0.41 | 2.9×10−5 | 0.85 | 4.2×10−5 | 0.78 |
| African-American race | −0.04 | 0.41 | −0.10 | 0.01* | −0.13 | <0.01* |
| Acute chorioamnionitis | 0.13 | 0.02* | 0.07 | 0.07 | 0.05 | 0.23 |
| Antenatal MgSO4 | −0.07 | 0.23 | −0.05 | 0.257 | −0.04 | 0.366 |
| Complete course of antenatal corticosteroidsa |
−0.05 | 0.40 | −0.09 | 0.04* | −0.11 | <0.01* |
| Delayed umbilical cord clamping | −0.221 | 0.04* | −0.19 | 0.01* | −0.18 | 0.02* |
Footnote:
Denotes significance at p < 0.05.
Defined as receiving two doses of betamethasone over a 48-hour period. All β coefficients are unstandardized.
Model summary: Systolic- R2=0.332, Mean- R2=0.465, Diastolic- R2=0.514.
Population estimate
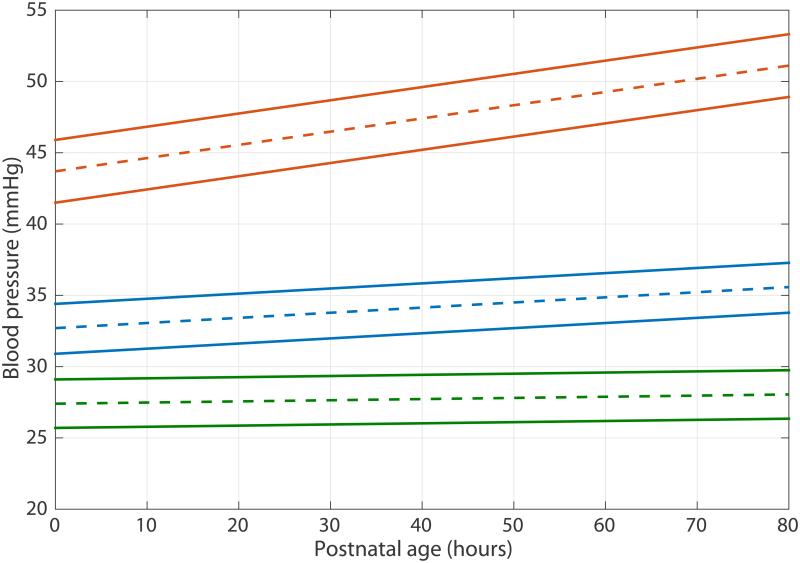
Mean intercept and slope values are presented in Table 4. A model of expected blood pressure values at each postnatal hour is shown in Figure 2.
Table 4.
Arterial blood pressure characteristics for postnatal hours 0-72
| Systolic | Mean | Diastolic | |
|---|---|---|---|
| Intercept, mean (SEM), mmHg | 43.7 (1.1) | 32.8 (0.9) | 27.4 (0.9) |
| Slope, mean (SEM), mmHg h−1 | 0.09 (0.02) | 0.04 (0.02) | 0.01 (0.02) |
Footnote: SEM- standard error of the mean.
Figure 2.
Population estimate of blood pressure values by postnatal age in hours. Dashed line represents the blood pressure estimate while solid line represents the boundaries of the 95% confidence interval. Orange- SBP, blue – MABP, green DBP.
Discussion
The high-density nature of the data collected in this study permits estimation of blood pressure measures with precision greater than available in previous studies. Our data suggest that the blood pressure of preterm infants with favorable short-term outcomes should increase steadily with increasing postnatal age. African-American race and completion of a course of antenatal steroids were independently associated with higher starting blood pressure values with a slower rate of rise, while the presence of chorioamnionitis was associated with lower starting blood pressure values and a faster rate of rise. Furthermore, the values derived from this model suggest higher normative values than previously described.
In the setting of poor cerebral autoregulation in this very preterm population, this finding may suggest a biologically programmed minimum mean arterial blood pressure of approximately 30 mmHg required for optimal brain perfusion. This speculation is supported by previous work demonstrating a higher incidence of IVH in infants who spent greater periods of time with MABP < 30 mmHg (1,2) presumably resulting from ischemia-reperfusion injury when blood pressure rises again often in response to inotropic or fluid volume support. Our data also demonstrates a relatively tight range of 95% confidence intervals (±3 mmHg) for each blood pressure measure, supporting the concept of a narrow range of normal blood pressures during this transition phase. Physiologically, this phenomenon may help maintain normal brain perfusion given the lack of autoregulatory ability.
A marked difference in the regression line slopes was noted, particularly between the systolic (0.09 mmHg h−1) and the diastolic (0.01 mmHg h−1) blood pressures. One potential explanation may lie in cardiac function: echocardiographic studies examining ventricular function in a very premature cohort have demonstrated a similar pattern, with delayed or altered diastolic function and preserved systolic function (18,19). Another potential explanation may be the diastolic runoff caused by a patent ductus arteriosus, becoming increasing prominent, by way of a widening pulse pressure, entering into the fourth day of life. Although the presence of a PDA requiring medical treatment was not associated with changes in the slope or intercept of any blood pressure parameter in this study, these data represent a time period (first 72 hours) during which hemodynamically significant PDAs are less common. Future studies empirically examining blood pressure over the course of the first week of life should be performed to better assess this effect.
Additionally, although it is mechanistically plausible that delayed cord clamping would result in the slower rate-of-rise in blood pressure associated with better hemodynamics (potentially as a result of improved vena cava blood flow and right ventricular function (20)), only 10% of the study population underwent this procedure, as it became routine clinical practice only during the final year of the study. Further evaluation of this effect should be conducted in a larger cohort.
Our findings have potentially important implications for the management of blood pressure in this population. Although Tyszczuk et al. demonstrated (21) that the premature brain can maintain adequate cerebral blood flow in the setting of wide range of mean arterial blood pressures (24-39 mmHg), considerable evidence suggests that these autoregulatory mechanisms may be disrupted in sick or very preterm infants (22,23) and that autoregulatory maintenance of adequate blood pressure is compromised. Careful control of blood pressure, avoiding hypo- and hypertension is likely of greater import in this population.
Our data should be interpreted with some caution. Although our reference curves may be used to determine whether a single blood pressure measurement is outside of the 95% confidence interval, these data cannot be used to estimate the risk of adverse outcomes. Furthermore, these data represent a cohort estimate and cannot inform decisions about the appropriate threshold at which inotropic medications should be initiated. Future studies should compare the response of hypotensive infants treated with inotropes to these curves.
An additional limitation of our study is a relative lack of data from the first 12 hours of life. The nature of obtaining informed consent from recently post-partum families, transport of some infants from remote facilities and the setup of the monitoring equipment created substantial difficulty in acquiring data in this time period. Other researchers have examined this specific period in great detail (13) and have found a proportionally higher blood pressure in the first few hours following birth, perhaps owing to epinephrine release as a part of parturition (24), which decayed to values consistent with the slope and intercept calculations from our data.
Development of a “hypotension index” by application of these blood pressure reference curves to measures of adverse outcome would provide the next step in developing an evidenced-based approach to blood pressure management in preterm infants.
Acknowledgements
The authors wish to thank Anthony Barton for his tireless efforts at patient recruitment and data collection.
Dr. Vesoulis’s work was supported by an NIH grant, KL2 TR000450-08 (NCATS).
Dr. Wallendorf’s work was supported an NIH grant, P30 HD062171 (NICHD), to the Intellectual and Developmental Disabilities Research Center at Washington University, and funding from The McDonnell Centers for Systems Neuroscience and Cellular & Molecular Neurobiology.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. J Pediatr. 1990 Oct;117(4):607–14. doi: 10.1016/s0022-3476(05)80700-0. [DOI] [PubMed] [Google Scholar]
- 2.Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Arch Dis Child. 1987 Oct;62(10):1068–9. doi: 10.1136/adc.62.10.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddle A, Maire J, Cai V, Nguyen T, Gong X, Hansen K, et al. Hemodynamic and metabolic correlates of perinatal white matter injury severity. PloS One. 2013;8(12):e82940. doi: 10.1371/journal.pone.0082940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versmold HT, Kitterman JA, Phibbs RH, Gregory GA, Tooley WH. Aortic Blood Pressure During the First 12 Hours of Life in Infants with Birth Weight 610 to 4,220 Grams. Pediatrics. 1981 May 1;67(5):607–13. [PubMed] [Google Scholar]
- 5.Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. J Perinatol Off J Calif Perinat Assoc. 1995 Dec;15(6):470–9. [PubMed] [Google Scholar]
- 6.Cunningham S, Symon AG, Elton RA, Zhu C, McIntosh N. Intra-arterial blood pressure reference ranges, death and morbidity in very low birthweight infants during the first seven days of life. Early Hum Dev. 1999 Dec;56(2-3):151–65. doi: 10.1016/s0378-3782(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 7.Georgieff MK, Mills MM, Gómez-Marín O, Sinaiko AR. Rate of change of blood pressure in premature and full term infants from birth to 4 months. Pediatr Nephrol Berl Ger. 1996 Apr;10(2):152–5. doi: 10.1007/BF00862059. [DOI] [PubMed] [Google Scholar]
- 8.Hegyi T, Carbone MT, Anwar M, Ostfeld B, Hiatt M, Koons A, et al. Blood pressure ranges in premature infants. I. The first hours of life. J Pediatr. 1994 Apr;124(4):627–33. doi: 10.1016/s0022-3476(05)83146-4. [DOI] [PubMed] [Google Scholar]
- 9.Dannevig I, Dale HC, Liestøl K, Lindemann R. Blood pressure in the neonate: three non-invasive oscillometric pressure monitors compared with invasively measured blood pressure. Acta Paediatr Oslo Nor 1992. 2005 Feb;94(2):191–6. doi: 10.1111/j.1651-2227.2005.tb01889.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Rajadurai VS, Tan KW. Blood pressure standards for very low birthweight infants during the first day of life. Arch Dis Child Fetal Neonatal Ed. 1999 Nov;81(3):F168–70. doi: 10.1136/fn.81.3.f168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pejovic B, Peco-Antic A, Marinkovic-Eric J. Blood pressure in non-critically ill preterm and full-term neonates. Pediatr Nephrol. 2006 Dec 18;22(2):249–57. doi: 10.1007/s00467-006-0311-3. [DOI] [PubMed] [Google Scholar]
- 12.Kent AL, Kecskes Z, Shadbolt B, Falk MC. Normative blood pressure data in the early neonatal period. Pediatr Nephrol Berl Ger. 2007 Sep;22(9):1335–41. doi: 10.1007/s00467-007-0480-8. [DOI] [PubMed] [Google Scholar]
- 13.Pichler G, Cheung P-Y, Binder C, O’Reilly M, Schwaberger B, Aziz K, et al. Huang L-M, editor. Time Course Study of Blood Pressure in Term and Preterm Infants Immediately after Birth. PLoS ONE. 2014 Dec 16;9(12):e114504. doi: 10.1371/journal.pone.0114504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batton B, Li L, Newman NS, Das A, Watterberg KL, Yoder BA, et al. Evolving blood pressure dynamics for extremely preterm infants. J Perinatol. 2014 Apr;34(4):301–5. doi: 10.1038/jp.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aweel I, Pursley DM, Rubin LP, Shah B, Weisberger S, Richardson DK. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. J Perinatol Off J Calif Perinat Assoc. 2001 Aug;21(5):272–8. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayaz H, Izzetoglu M, Shewokis PA, Onaral B. Sliding-window motion artifact rejection for Functional Near-Infrared Spectroscopy. Conf Proc Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf. 2010;2010:6567–70. doi: 10.1109/IEMBS.2010.5627113. [DOI] [PubMed] [Google Scholar]
- 18.Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right Ventricular Function in Preterm and Term Neonates: Reference Values for Right Ventricle Areas and Fractional Area of Change. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2015 Mar;:6. doi: 10.1016/j.echo.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose A, Khoo NS, Aziz K, Al-Rajaa N, van den Boom J, Savard W, et al. Evolution of left ventricular function in the preterm infant. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2015 Mar;28(3):302–8. doi: 10.1016/j.echo.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Sommers R, Stonestreet BS, Oh W, Laptook A, Yanowitz TD, Raker C, et al. Hemodynamic Effects of Delayed Cord Clamping in Premature Infants. PEDIATRICS. 2012 Mar 1;129(3):e667–72. doi: 10.1542/peds.2011-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyszczuk L, Meek J, Elwell C, Wyatt JS. Cerebral blood flow is independent of mean arterial blood pressure in preterm infants undergoing intensive care. Pediatrics. 1998 Aug 102;(2):337–41. doi: 10.1542/peds.102.2.337. Pt 1. [DOI] [PubMed] [Google Scholar]
- 22.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007 Apr;61(4):467–73. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 23.Alderliesten T, Lemmers PMA, Smarius JJM, van de Vosse RE, Baerts W, van Bel F. Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr. 2013 Apr;162(4):698–704. doi: 10.1016/j.jpeds.2012.09.038. e2. [DOI] [PubMed] [Google Scholar]
- 24.Eliot RJ, Klein AH, Glatz TH, Nathanielsz PW, Fisher DA. Plasma norepinephrine, epinephrine, and dopamine concentrations in maternal and fetal sheep during spontaneous parturition and in premature sheep during cortisol-induced parturition. Endocrinology. 1981 May;108(5):1678–82. doi: 10.1210/endo-108-5-1678. [DOI] [PubMed] [Google Scholar]