Abstract
Purpose
Previous study has demonstrated that adventitial delivery of lentivirus mediated Vegf-A shRNA (LV-shRNA-Vegf-A, small hairpin RNA that inhibits Vegf-A gene expression) at the time of arteriovenous fistula (AVF) creation increases the lumen vessel area (LVA) of the outflow vein and reduces venous neointimal hyperplasia (VNH). However, the effect of decreasing Vegf-A and improving LVA decreases by 2-weeks as stenosis develops. The aim of the present study was to determine if a second dose of LV-shRNA-Vegf-A could improve LVA and decrease VNH.
Methods
Chronic kidney disease was created in C57BL/6 mice and 28 days later, a AVF was created by connecting right carotid artery to ipsilateral jugular vein. 5×106 plaque forming units of LV-shRNA-Vegf-A or control shRNA was administered to the adventitia of the outflow vein immediately after AVF creation and a second dose of the same treatment fourteen days later. Animals were sacrificed at 21, 28, and 42 days after AVF creation for reverse transcription polymerase chain reaction (RT-PCR) and histomorphometric analyses.
Results
By day 21, there was a 25% increase in the average LVA (day 21: P=0.11), with a decrease in cell proliferation (day 21: P=0.0079, day 28: P=0.28; day 42: P=0.5), decrease in α-smooth muscle cell actin staining (day 21: P<0.0001, day 28: P<0.05; day 42: P=0.59), and decrease in hypoxic stress (day 21: P<0.001, day 28: P=0.28; day 42: P=0.46) in LV versus control shRNA vessels, respectively.
Conclusion
A second dose of LV-shRNA-Vegf-A administration results in a moderate improvement in LVA at day 21.
Keywords: arteriovenous fistula, chronic kidney disease, murine model, restenosis, veins
Introduction
The arteriovenous fistula (AVF) is the preferred vascular access and maintaining its function is required for adequate hemodialysis. At one year, approximately 38% of the AVF will fail because of venous stenosis formation which is caused by venous neointimal hyperplasia (VNH) [1]. Histologic analysis of VNH is characterized by angiogenesis localized to the neointima and adventitia ([2]. This is accompanied with increased proliferation of cells staining positive for α-smooth muscle actin (α-SMA) in the neointima [2]. A recent study demonstrated that lentivirus mediated Vegf-A shRNA (small hairpin RNA that inhibits Vegf-A expression) transduction to the adventitia of the outflow vein immediately after AVF creation lead to an increase in lumen vessel area compared to the outflow veins transduced with control shRNA. Moreover, fibroblast to myofibroblast differentiation accompanied with a reduction in constrictive vascular remodeling was observed in outflow veins of AVFs transduced with Vegf-A shRNA [3]. However, unexpectedly, VNH started to recur two weeks after Vegf-A shRNA administration to the outflow veins of AVFs [3].
The aim of the present study is to determine whether a two doses of LV-shRNA-Vegf-A could improve lumen vessel area and decrease VNH. Experiments were performed in a murine model of chronic kidney disease (CKD) with AVF. The readouts were gene expression for Vegf-A, matrix metalloproteinase-2 and -9 (Mmp-2 and Mmp-9), proliferation, apoptosis, smooth muscle, and hypoxyprobe staining with histomorphometric analyses.
Methods
Experimental animals
Institutional Animal Care and Use Committee approval was obtained prior to conducting any experiments. The animals were housed and handled in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals revised in 2000 [4]. C57BL/6 male mice (Jackson Laboratories, Bar Harbor, ME) weighing 25–30 grams were used for the present study. The mice were maintained at room temperature of 22°C temperature with 41% relative humidity, and 12-/12-hour light/dark cycles. The animals were allowed access to water and food ad libitum.
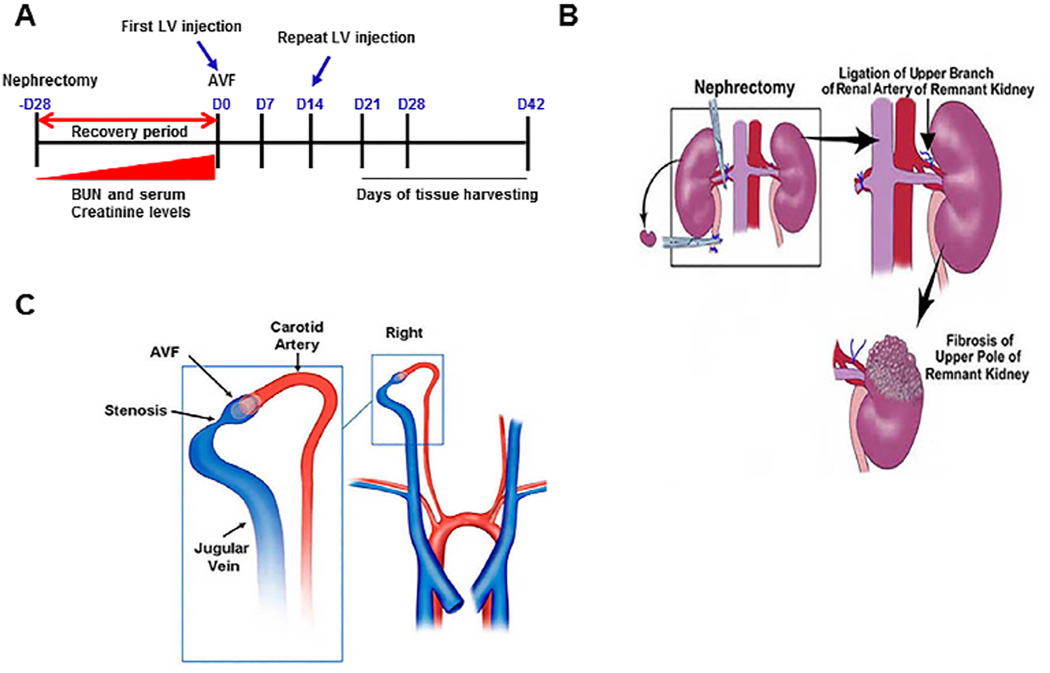
Anesthesia was obtained using a combination of ketamine hydrochloride (0.20 mg/g) and xylazine (0.02 mg/g), which was administered using intraperitoneal (i.p.) delivery and further anesthesia was maintained using pentobarbital (20–40 mg/kg, i.p.). All surgical procedures were performed by B.Y. Chronic renal insufficiency was created by performing a nephrectomy of the right kidney combined with ligation of the upper polar branch of the renal artery to the left kidney [5]. In this model, after this procedure, the average BUN and creatinine are significantly elevated twenty-eight days later [5]. Four weeks later, a carotid artery to jugular vein AVF was created (Fig. 1) [5, 6]. The shRNA for Vegf-A was purchased from Open Biosystems (Huntsville, AL) and it was prepared according to the manufacturer's protocol [7]. 5 × 106 particle forming units (PFU) of either lentivirus-shRNA-Vegf-A (LV-shRNA-Vegf-A) or scrambled-shRNA (control shRNA, non targeting empty vector) in 5-µL of PBS were administered to the adventitia of the proximal outflow vein using a 30G needle at the time of AVF creation. Fourteen days later, the same therapy was repeated using a separate surgical procedure [8]. Previously, experiments conducted using this technique have shown that venous stenosis forms in this model at the outflow vein [5–7, 9–11]. Seventy-nine male C57BL/6 mice weighing 25–30 grams underwent nephrectomy. One mouse died after nephrectomy. Six mice were excluded because of arterial thickening and inflammation and a fistula could not be placed. Animals were sacrificed at day 21 (D21), day 28 (D28), and day 42 (D42) following placement of the AVF for RT-PCR and histomorphometric analyses at each time point (Fig. 1).
Figure 1. Schematic of the study.
Adapted from reference 10.
Tissue harvesting
Prior to euthanasia, the mice were anesthetized as described previously. Next, the fistula was dissected free of any surrounding tissue. Patency of the fistula was assessed at the time of harvesting the tissue by gently obstructing the outflow vein and assessing if the vein proximally dilated which was recorded as patent. CO2 asphyxiation was used and the outflow veins harvested for RT-PCR or histologic analyses. The vessels were perfusion fixed prior to removal for histologic analysis [7].
RNA isolation and Real time polymerase chain reaction (RT-PCR) analysis
The tissue was stored in RNA stabilizing reagent (Qiagen, Gaithersburg, MD) according to the manufactures guidelines. The vessels were homogenized and the RNA was isolated using RNeasy mini kit (Qiagen) [5, 6]. The gene expression of interest was determined using RT-PCR analysis [6]. The primers used are listed in Table 1. Samples were different animals were not pooled. Typically, 1–2.5-ug of RNA can be obtained from the graft vein which can be used to perform RT-PCR for 10 genes.
Table 1.
PCR primers used in current study
| Gene | Sequence | Amplicon Length |
Cycles |
|---|---|---|---|
| Vegf-A | 5’ –atgaagtgatcaagttcatgg– 3’ (sense) 5’ –ggatcttggacaaacaaatgc– 3’ (antisense) |
360 | 35 |
| Vegfr-1 | 5’ –tttccatttgatactcttac– 3’ (sense) 5’ –tcttagttgctttaccaggg– 3’ (antisense) |
310 | 35 |
| Mmp-2 | 5’ – agatcttcttcttcaaggaccggtt– 3’ (sense) 5’ – ggctggtcagtggcttggggta– 3’ (antisense) |
225 | 35 |
| Mmp-9 | 5’ – gtttttgatgctattgctgagatcca– 3’ (sense) 5’ – cccacatttgacgtccagagaagaa3’(antisense) |
208 | 35 |
| 18S | 5’ –agctaggaataatggaatag-3’ (sense) 5’-aatcaagaacgaaagtcggag- 3’(antisense) |
150 | 19 |
LV= LV-shRNA-Vegf-A treated vessels
C= control shRNA treated vessels
Immunohistochemistry
Ki-67 staining was used to determine cellular proliferation and α-SMA staining was used to determine smooth muscle density on sections removed from the outflow vein at the different time points. The following antibodies were used: mouse monoclonal antibody for Ki-67 (DAKO, Carpentaria, CA; 1:400) or rabbit polyclonal antibody to mouse for α-SMA (Abcam, Cambridge, MA; 1:400) [7, 10]. Quantification was performed as described later [7, 10].
Hypoxyprobe staining of LV-shRNA-Vegf-A and scrambled shRNA Vegf-A transduced vessels
Hypoxyprobe™-1 was used to assess hypoxic changes after treatment in the graft vein. Each mouse was injected with 60 mg/kg Hypoxyprobe™-1 i.p. (EMD Millipore, Billerica, MA) and thirty minutes following injection, mice were sacrificed and outflow veins were dissected and fixed as specified for histological analysis. Four-micrometer thick paraffin embedded sections was stained with the anti-hypoxyprobe-1 antibody using the manufacturer’s directions [7, 10].
TUNEL staining of LV-shRNA-Vegf-A and scrambled shRNA Vegf-A transduced vessels
In order to assess apoptosis, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was used on paraffin-embedded sections from the outflow vein after LV-shRNA-Vegf-A or control shRNA vessels according to the manufacturer’s directions (DeadEnd Colorimetric tunnel assay system, G7360, Promega) [7, 10].
Picrosirius red staining of LV-shRNA-Vegf-A and scrambled shRNA Vegf-A transduced vessels
In order to assess collagen deposition, picrosirius red staining was performed on unstained sections from LV-shRNA-Vegf-A and scrambled shRNA at different time points as described elsewhere [7, 10].
Morphometry and image analysis of LV-shRNA-Vegf-A and scrambled shRNA Vegf-A transduced vessels
Morphometric analysis was performed as described previously [12, 13]. Four-micron sections stained with Hematoxylin and eosin (H and E) were digitally acquired using an Axioplan 2 Microscope (Zeiss, Oberkochen, Germany) which is equipped with a Neo-Fluor × 20/0.50 objective and Axiocam camera that allows images to capture a minimum of 1030 × 1300 pixels. The KS-400 (Zeiss) software was used to determine the lumen vessel area, neointima, and media + adventitia area, which can be determined on the H and E stained sections. These different areas were manually traced (R.J. or B.Y.). The KS-400 software has an automated program which provides the area of the lumen vessel area, neointima, and media + adventitia along with the cellular density (neointima and media + adventitia). Staining quantification was performed using the following. For nuclear staining (Ki-67 and TUNEL), the number of cells with nuclei staining brown was determined divided by total number of cells (brown + blue) and multiplied by 100 (Ki-67 index and TUNEL index). For cytoplasmic staining (α-SMA and hypoxyprobe), the number of cells with cytoplasm staining brown was divided by total number the total number of cells and multiplied by 100 (α-SMA index and hypoxyprobe index).
Statistical methods
Data are expressed as mean ± SEM. Multiple comparisons were performed using a two-way ANOVA followed by Student t-test with post hoc Bonferroni’s correction. Kaplan-Meier survival model was used to assess for differences in patency between groups using a log-rank test. Significant difference from control value was indicated by *P<0.05, **P<0.01, #P< 0.001, or ##P<0.0001. SAS version 9 (SAS Institute Inc., Cary, N.C.) was used for statistical analyses.
Results
Surgical outcomes
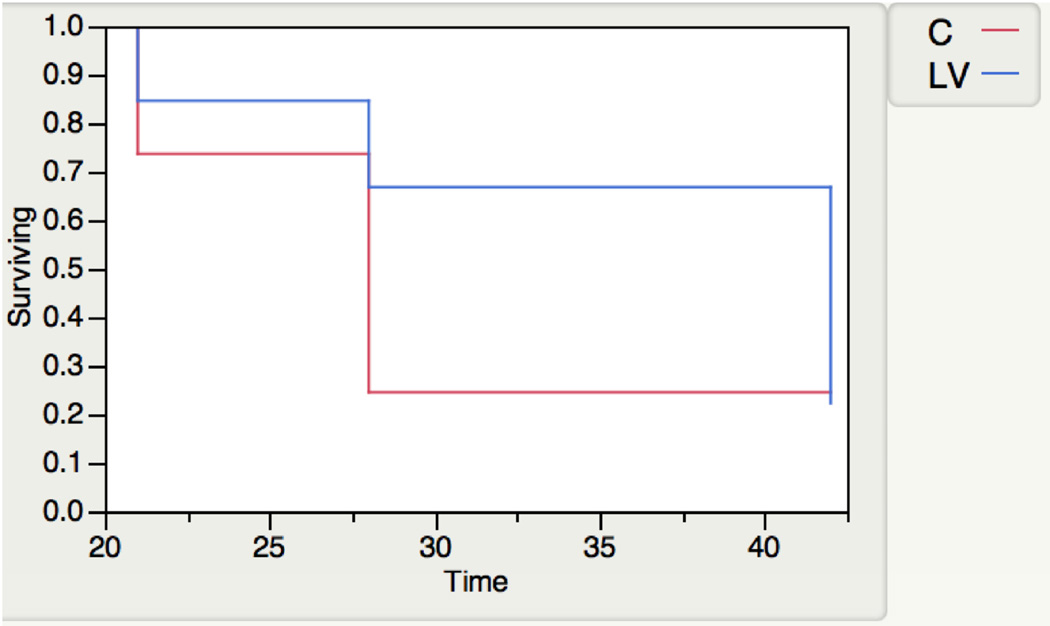
Seventy-two mice underwent placement of an AVF to connect the right carotid artery to the ipsilateral jugular vein with either 5×106 plaque forming unit of LV-shRNA-Vegf-A (LV, n=36) or scrambled-shRNA (control, C, n=36) administered to the adventitia of the outflow vein where the stenosis forms in this model followed (Fig. 1). Three mice died after fistula placement. Kaplan Meier analysis was performed to assess differences in patency between the two groups which demonstrated that there was the patency of LV-shRNA-Vegf-A transduced vessels was improved compared to controls (P=0.07, Fig. 2).
Figure 2. Kaplan-Meier estimates for patency between LV-shRNA-Vegf-A and control shRNA transduced vessels.
Second administration of LV-shRNA-Vegf-A to the outflow vein is associated with an increase in average lumen vessel area with a decrease in constrictive remodeling
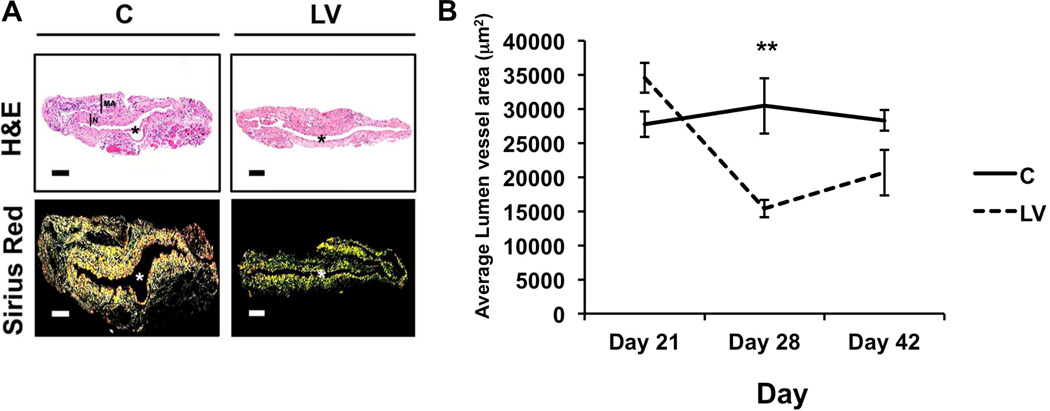
There was a 125% increase in the average lumen vessel area in the LV-shRNA-Vegf-A transduced vessels when compared to controls at day 21 (34545 ± 2208 vs. 27745 ± 1874, respectively, average increase: 125%, P=0.11) with a significant decrease in the mean lumen vessel area by day 28 (15382 ± 1259 vs. 30435 ± 4081, respectively, average reduction: 49%, P=0.0033), but not day 42 (20665 ± 3343 vs. 28304 ± 1516, respectively, average reduction: 27%, P=0.06) (Fig. 3). There was no difference in the mean wall area between the LV-shRNA-Vegf-A and control shRNA. Picrosirius staining was performed to qualitatively assess the collagen 1 and 3 content. Yellow color signifies collagen 1 or 3 staining (Fig. 3). Qualitatively, there was a decrease in the collagen 1 or 3 staining by picorsirius red at day 21 in the LV-shRNA-Vegf-A transduced vessels when compared to control shRNA. There was no difference between the two groups at day 28 or 42.
Figure 3. Hematoxylin and eosin (H and E) with Picrosirius red staining of the LV-shRNA-Vegf-A transduced vessels shows increased lumen vessel area with decreased collagen expression.
Representative sections after Hematoxylin and eosin (H and E) staining and Picrosirius red staining at the venous stenosis of the LV-shRNA-Vegf-A (LV) and scrambled-VEGF-A (C) transduced vessels at day 21 after the first administration. Pooled data for the LV-shRNA-Vegf-A and control shRNA groups each at day 21, 28, and 42 is shown in B. Semiquantitative analysis for lumen vessel area (B) shows an increase in the average lumen vessel area of LV transduced vessels when compared to C at day 21 after the first administration. Two-way ANOVA followed by Student t-test with post hoc Bonferroni’s correction was performed. Significant difference from control value was indicated by **P<0.01. Each bar shows mean ± SEM of 4–6 animals per group (A–B).
Second administration of LV-shRNA-Vegf-A to the outflow vein is associated with a decrease in cellular proliferation
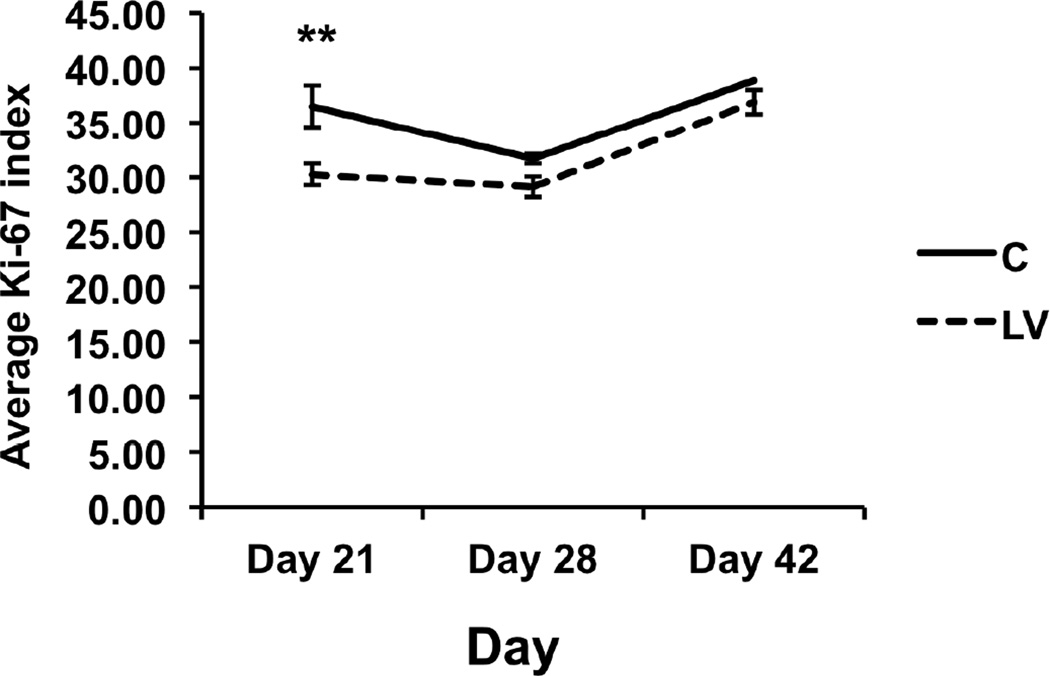
Using Ki-67 staining, by day 21, there was a significant reduction in the average Ki-67 index in the LV-shRNA-Vegf-A transduced vessels when compared to controls (30 ± 1.9 vs. 36.5 ± 1, respectively, average reduction: 17%, P<0.05). There was no difference in the average Ki-67 index between the two groups (D28: P=0.28 and D42: P =0.46) (Fig. 4).
Figure 4. Cellular proliferation index is decreased in LV-shRNA-Vegf-A transduced vessels when compared to controls at day 21.
Pooled data for the LV-shRNA-Vegf-A and control shRNA groups each at day 21, 28, and 42 is shown. This demonstrates a significant decrease in the mean Ki-67 index in the LV transduced vessels when compared to C at day 21 after first administration. Two-way ANOVA followed by Student t-test with post hoc Bonferroni’s correction was performed. Significant difference from control value was indicated by **P<0.01. Each bar shows mean ± SEM of 4 animals per group.
Second administration of LV-shRNA-Vegf-A to the outflow vein is associated with a decrease in α-SMA expression
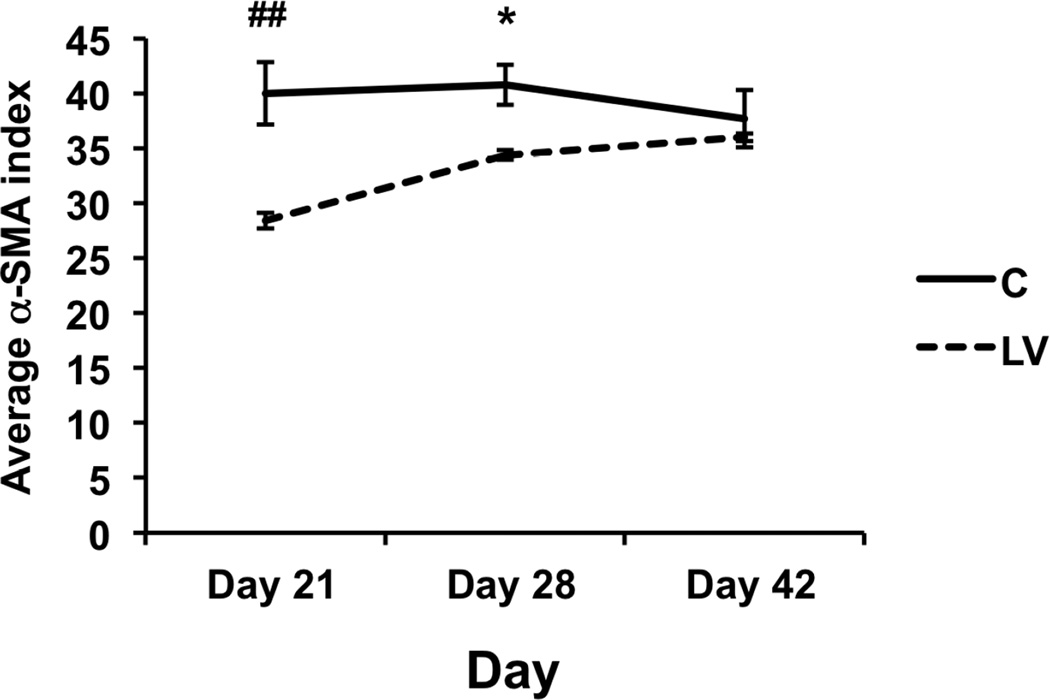
In order to assess for smooth muscle density, the outflow veins from LV-shRNA-Vegf-A and control groups were stained for α-SMA (Fig. 5). By day 21, the average α-SMA index was significantly reduced in the LV-shRNA-Vegf-A transduced vessels when compared to controls (28.4 ± 0.72 vs. 39.9 ± 2.83, respectively, P<0.0001, average reduction: 29%) and it remained lower by day 28 (34.4 ± 0.45 vs. 40.7 ± 1.82, respectively, P<0.05, average reduction: 14%). There was no difference in average α-SMA staining between the two groups at day 42 (P=0.59)
Figure 5. Smooth muscle cell index is reduced in LV-shRNA-Vegf-A transduced vessels when compared to controls at day 21.
Pooled data for the LV-shRNA-Vegf-A and control shRNA groups each at day 21, 28, and 42 is shown. This demonstrates a significant reduction in average α-SMA index in the LV transduced vessels when compared to C at day 21. Two-way ANOVA followed by Student t-test with post hoc Bonferroni’s correction was performed. Significant difference from control value was indicated by *P<0.05 or ##P<0.0001. Each bar shows mean ± SEM of 4–6 animals per group.
Second administration of LV-shRNA-Vegf-A to the outflow vein is associated with a decrease in hypoxyprobe staining
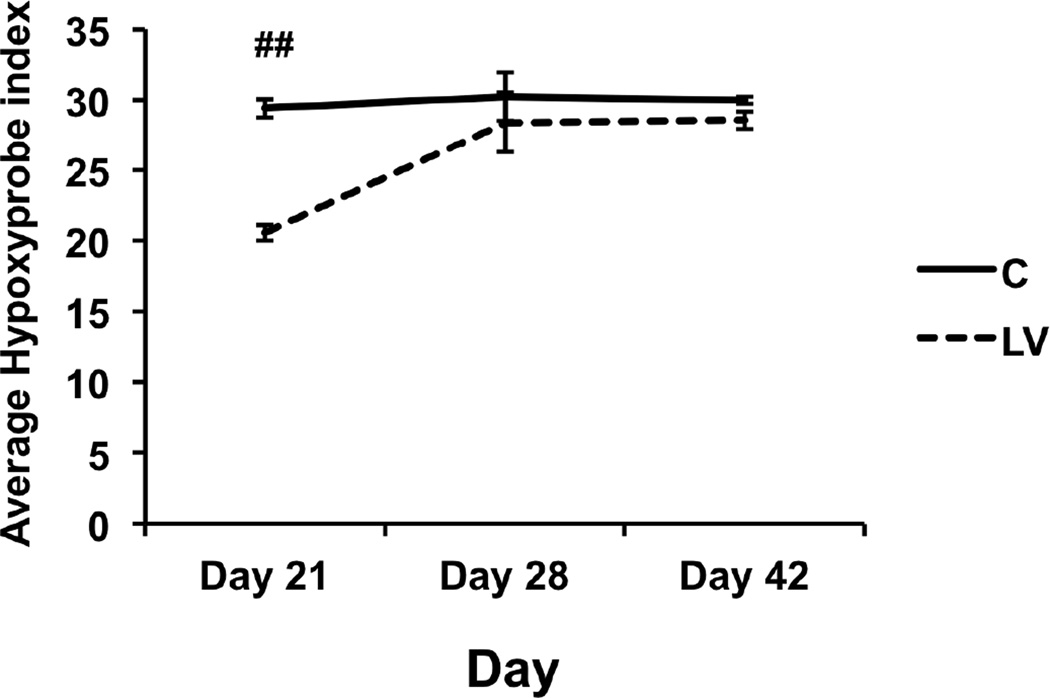
The hypoxic regions in the outflow vein after treatment with LV-shRNA-Vegf-A or control shRNA were assessed using hypoxyprobe staining (Fig. 6). By day 21, the average hypoxyprobe index was significantly reduced in the LV-shRNA-Vegf-A transduced vessels when compared to controls (20.6 ± 0.6 vs. 29.4 ± 0.6, respectively, P<0.001, average reduction: 31%). By days 28 and 42, there was no difference in the average hypoxyprobe index between the two groups (D28: P=0.28 and D42: P=0.46).
Figure 6. There is a significant decrease in the hypoxyprobe index in LV-shRNA-Vegf-A transduced vessels when compared to controls.
Pooled data for the LV-shRNA-Vegf-A and control shRNA groups each at day 21, 28, and 42 is shown. This demonstrates a significant reduction in the average hypoxyprobe index in the LV-shRNA-Vegf-A transduced vessels when compared to controls at day 21 after first administration. Two-way ANOVA followed by Student t-test with post hoc Bonferroni’s correction was performed. Significant difference from control value was indicated by ##P<0.0001. Each bar shows mean ± SEM of 4–6 animals per group.
Second administration of LV-shRNA-Vegf-A to the outflow vein does not increase apoptosis
Apoptosis was assessed using TUNEL staining performed on sections removed from the outflow vein after transduction with either LV-shRNA-Vegf-A or control shRNA at day 21, 28, and 42. This demonstrated no difference in average TUNEL index between both groups (D21: P=0.24; D28: P=0.63; and D42: P=0.96).
Second administration of LV-shRNA-Vegf-A to the outflow vein does not reduce gene expression of Vegf-A, Mmp-2, or Mmp-9
Gene expression for Vegf-A, Mmp-2, or Mmp-9 was assessed using RT-PCR at day 21, 28, and 42 days between the two groups. There was no significant difference in the average gene expression of Vegf-A, Mmp-2, or Mmp-9 between the two groups.
Discussion
Previous study has demonstrated that reducing Vegf-A gene expression using a short hairpin RNA that inhibits gene expression of Vegf-A at the time of AVF placement results in an increase in the average lumen vessel area with a reduction in constrictive remodeling. However, the authors observed an “catch-up” phenomena where the benefit of the Vegf-A reduction decreased by 14 to 28 days after delivery [7]. The goal of the present study was to determine if the beneficial effects of reducing Vegf-A could be extended by a second delivery of an shRNA. We observed that a second administration of LV-shRNA-Vegf-A two weeks after the first had a modest improvement in lumen vessel area with a reduction in smooth muscle density, proliferation and hypoxyprobe staining. Interestingly, an increase in apoptosis or reduction in Vegf-A and downstream gene products such as Mmp-2 and Mmp-9 was not observed. These observations have implications for the development of clinical therapies aimed at reducing venous stenosis formation. For example, they provide guidance for the development of a treatment with respect to which part of the vessel wall to target (adventitia, media, or endothelium), the duration of the treatment, and timing of the treatment with relationship to the placement of the AVF.
The mechanisms of hemodialysis vascular access failure are very complicated. Upstream mechanisms such as inflammation, shear stress, uremia, oxidative stress, and others have been implicated for contributing to the vascular injury, which results in venous stenosis formation [14–16]. Multiple different cytokines both proliferative, inflammatory, and angiogenic have been associated with hemodialysis vascular access failure including Vegf-A, Mmps, monocyte chemoattractant protein-1 (Mcp-1), and others [16–18]. Recently, elastin deposition within experimental fistulas has been described [19]. Local pharmaceutical delivery has been felt to be a potential strategy for treating access failure [9, 10, 20]. In fact, adventitial delivery of blood outgrowth endothelial progenitor cells has been shown to alleviate vascular injury associated with hemodialysis vascular access grafts [21]. In arterial injury, the presence of local stem cells and circulating stem cells contributing to the pathologic process is very well recognized but the role of these cells in VNH is less well recognized [22, 23]. Finally, a recent study demonstrated that adventitial delivery of human adipose derived mesenchymal stem cells to the outflow vein of murine AVF reduces VNH mediated by a reduction in gene expression of Mcp-1 [24].
Many studies have demonstrated a role for differentiation of fibroblasts to myofibroblasts and their contribution to venous stenosis formation associated with hemodialysis vascular access failure [12, 25, 26]. In the present study, we observed a reduction in smooth muscle cell staining with the anti-Vegf-A therapy is likely due to a reduction in fibroblast to myofibroblast transition. In addition, we observed a decrease in proliferation as assessed using Ki-67 staining. This consistent with other studies demonstrating the Vegf-A reduction results in a decrease in proliferation because Vegf-A is necessary for cell survival and proliferation [27, 28]. A recent experimental study determined different proliferation patterns using the pig model [29]. They observed that within the first week of vascular access placement, there was increased proliferation localized to the adventitia and they proposed that anti-proliferative therapies during this time period could potentially be a therapeutic target.
The reduction in cellular proliferation and α-SMA expression implies that there was a reduction in Vegf-A gene expression [7]. As shown previously in many different studies, Vegf-A is necessary for cellular proliferation [7]. One reason for the lack of demonstration of reduction in Vegf-A maybe due to fibrosis around the vessel and this fibrosis may have decreased the overall efficacy of the reducing Vegf-A to the vessel wall. We have previously shown that adventitial delivery of LV-shRNA-Vegf-A results in a “top down” effect by reducing mRNA expression for Vegf-A that is localized to the adventitia and media at day 3 and the whole vessel wall by day 7 after transfection. Alternatively, it is quite plausible that there was a reduction in Vegf-A, which occurred at an earlier time point, maybe a few days after delivery, which may explain the reduced effects seen in the current study. The Vegf-A dependent phase for vascular injury has been previously noted in experimental animal models of arterial injury [30]. It has been observed that there is a Vegf-A dependent phase for reducing stenosis formation [30]. Interestingly, when Avastin was used for a 4-week period of time, similar results were observed with a catch-up phenomena occurring at the later time periods.
There are several limitations to the present study. One is that a larger second dose of LV-Vegf-A would have resulted in further reduction in VNH. A second possibility is that the optimal timing could be further modified so an earlier dose may have resulted in reduction in VNH. Alternatively, targeting the endothelium may have proven to be more beneficial as has been recently described for in arterial injury caused by ADAMTS-7 [31]. Finally, the present study may have been underpowered and significance may have been achieved with increasing the number of animals in each group [30].
In conclusion, we propose that a second delivery of LV-shRNA-Vegf-A using the same dose moderately improves vascular remodeling associated with hemodialysis AVF. We postulate that this is in part due to a Vegf-A dependent and independent phase of venous stenosis formation associated with hemodialysis AVF. Finally, we hypothesize that anti-Vegf-A therapy needs to be used at the time of AVF creation to decrease vascular injury and therefore improve outcomes in patients with hemodialysis AVF.
Table 2.
Patent and occluded vessels at day 21, 28, and 42 after AVF placement
| Time | Patent | Occluded | Not sure |
|---|---|---|---|
| Day21LV | 3 | 4 | 5 |
| Day21C | 5 | 5 | 1 |
| Day28LV | 3 | 4 | 5 |
| Day28C | 2 | 6 | 3 |
| Day42LV | 4 | 8 | 0 |
| Day42C | 1 | 9 | 1 |
LV= LV-shRNA-Vegf-A treated vessels
C= control shRNA treated vessels
D21: day 21 after AVF placement
D28: day 28 after AVF placement
D42: day 42 after AVF placement
Acknowledgements
We are grateful to Dr. Nagy (University of Toronto) for providing us the probe for the in situ hybridization experiments. This work was funded by a HL098967 (SM) from the National Heart, Lung, And Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures none
References
- 1.Rooijens PPGM, Tordoir JHM, Stijnen T, Burgmans JPJ, Smet de AAEA, Yo TI. Radiocephalic Wrist Arteriovenous Fistula for Hemodialysis: Meta-analysis Indicates a High Primary Failure Rate. European Journal of Vascular and Endovascular Surgery. 2004;28:583–589. doi: 10.1016/j.ejvs.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Kelly BS, Miller MA, et al. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney international. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Yang B, Janardhanan R, Vohra P, et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney Int. 2014;85:289–306. doi: 10.1038/ki.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Committee on care and use of laboratory animals of the institute of laboratory animal resources. Washington, DC: Government print office; 1996. [Google Scholar]
- 5.Misra S, Shergill U, Yang B, Janardhanan R, Misra KD. Increased expression of HIF-1alpha, VEGF-A and its receptors, MMP-2, TIMP-1, and ADAMTS-1 at the venous stenosis of arteriovenous fistula in a mouse model with renal insufficiency. J Vasc Interv Radiol. 2010;21:1255–1261. doi: 10.1016/j.jvir.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang B, Shergill U, Fu AA, Knudsen B, Misra S. The mouse arteriovenous fistula model. J Vasc Interv Radiol. 2009;20:946–950. doi: 10.1016/j.jvir.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 7.Yang B, Janardhanan R, Vohra P, et al. Adventitial transduction of lentivirus-shRNA-VEGF-A in arteriovenous fistula reduces venous stenosis formation. Kidney international. 2014 Feb;85:289–306. doi: 10.1038/ki.2013.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turunen MP, Lehtola T, Heinonen SE, et al. Efficient regulation of VEGF expression by promoter-targeted lentiviral shRNAs based on epigenetic mechanism: a novel example of epigenetherapy. Circ Res. 2009;105:604–609. doi: 10.1161/CIRCRESAHA.109.200774. [DOI] [PubMed] [Google Scholar]
- 9.Brahmbhatt A, NievesTorres E, Yang B, et al. The role of Iex-1 in the pathogenesis of Venous Neointimal Hyperplasia associated with hemodialysis arteriovenous fistula. PLoS ONE. 2014;9:e102542. doi: 10.1371/journal.pone.0102542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janardhanan R, Yang B, Vohra P, et al. Simvastatin reduces venous stenosis formation in a murine hemodialysis vascular access model. Kidney international. 2013 Aug;:338–352. doi: 10.1038/ki.2013.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieves Torres EC, Yang B, Janardhanan R, et al. Adventitial Delivery of Lentivirus-shRNA-ADAMTS-1 Reduces Venous Stenosis Formation in Arteriovenous Fistula. PLoS ONE. 2014;9:e94510. doi: 10.1371/journal.pone.0094510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra S, Doherty MG, Woodrum D, et al. Adventitial remodeling with increased matrix metalloproteinase-2 activity in a porcine arteriovenous polytetrafluoroethylene grafts. Kidney international. 2005;68:2890–2900. doi: 10.1111/j.1523-1755.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- 13.Misra S, Fu AA, Puggioni A, et al. Increased shear stress with up regulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol. 2008;294:H2219–H2230. doi: 10.1152/ajpheart.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee T. Novel paradigms for dialysis vascular access: downstream vascular biology--is there a final common pathway? Clinical journal of the American Society of Nephrology : CJASN. 2013;8:2194–2201. doi: 10.2215/CJN.03490413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remuzzi A, Ene-Iordache B. Novel paradigms for dialysis vascular access: upstream hemodynamics and vascular remodeling in dialysis access stenosis. Clinical journal of the American Society of Nephrology : CJASN. 2013;8:2186–2193. doi: 10.2215/CJN.03450413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Misra S, Fu AA, Puggioni A, et al. Increased shear stress with upregulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol. 2008;294:H2219–H2230. doi: 10.1152/ajpheart.00650.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juncos JP, Grande JP, Kang L, et al. MCP-1 contributes to arteriovenous fistula failure. Journal of the American Society of Nephrology : JASN. 2011;22:43–48. doi: 10.1681/ASN.2010040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juncos JP, Tracz MJ, Croatt AJ, et al. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney international. 2008;74:47–51. doi: 10.1038/ki.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong CY, Rothuizen TC, de Vries MR, et al. Elastin is a key regulator of outward remodeling in arteriovenous fistulas. Eur J Vasc Endovasc Surg. 2015;49:480–486. doi: 10.1016/j.ejvs.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Kelly B, Melhem M, Zhang J, et al. Perivascular paclitaxel wraps block arteriovenous graft stenosis in a pig model. Nephrol Dial Transplant. 2006;21:2425–2431. doi: 10.1093/ndt/gfl250. [DOI] [PubMed] [Google Scholar]
- 21.Hughes D, Fu AA, Puggioni A, et al. Adventitial transplantation of blood outgrowth endothelial cells in porcine haemodialysis grafts alleviates hypoxia and decreases neointimal proliferation through a matrix metalloproteinase-9-mediated pathway--a pilot study. Nephrology Dialysis Transplantation. 2008;24:85–96. doi: 10.1093/ndt/gfn433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 23.Castier Y, Lehoux S, Hu Y, Fonteinos G, Tedgui A, Xu Q. Characterization of neointima lesions associated with arteriovenous fistulas in a mouse model. 2006;70:315–320. doi: 10.1038/sj.ki.5001569. [DOI] [PubMed] [Google Scholar]
- 24.Yang B, Brahmbhatt A, NievesTorres E, et al. Tracking and therapeutic value of human adipose derived mesenchymal stem cell transplantation in reducing venous neointimal hyperplasia associated with arteriovenous fistula. Radiology. 2015 Nov;19:150947. doi: 10.1148/radiol.2015150947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, Terry CM, Blumenthal DK, et al. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm415. [DOI] [PubMed] [Google Scholar]
- 26.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 27.Gerber HP, Hillan KJ, Ryan AM, et al. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 29.Chan JS, Campos B, Wang Y, et al. Proliferation patterns in a pig model of AV fistula stenosis: can we translate biology into novel therapies? Seminars in dialysis. 2014;27:626–632. doi: 10.1111/sdi.12240. [DOI] [PubMed] [Google Scholar]
- 30.Khurana R, Zhuang Z, Bhardwaj S, et al. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Yu F, Wang L, et al. ADAMTS-7 promotes vascular smooth muscle cells proliferation in vitro and in vivo. Science China Life sciences. 2015;58:674–681. doi: 10.1007/s11427-015-4843-2. [DOI] [PubMed] [Google Scholar]