Abstract
Inhaled nitric oxide (iNO) is approved for use in persistent pulmonary hypertension of the newborn (PPHN) but does not lead to sustained improvement in oxygenation in a third of patients with PPHN. Inhaled NO is less effective in the management of PPHN secondary to congenital diaphragmatic hernia (CDH), extreme prematurity and bronchopulmonary dysplasia (BPD). Intravenous pulmonary vasodilators such as prostacyclin, alprostadil, sildenafil and milrinone have been successfully used in PPHN resistant to iNO. Oral pulmonary vasodilators such as endothelin-receptor antagonist bosentan and phosphodiesterase-5 inhibitors such as sildenafil and tadalafil are used both during acute and chronic phase of PPHN. In the absence of infection, glucocorticoids may also be effective in PPHN. Many of these pharmacologic agents are not approved for use in PPHN and our knowledge is based on case reports and small trials. Large multicenter randomized controlled trials with long-term follow-up are required to evaluate pharmacologic strategies in PPHN.
Keywords: hypoxia, oxygen, nitric oxide, prostacyclin, sildenafil, bosentan, iloprost, milrinone
Introduction
The fetus is in a state of physiologic pulmonary hypertension with low pulmonary blood flow, while the placenta functions as the site for gas exchange. At birth, successful adaptation to extra-uterine life requires a rapid increase in pulmonary blood flow to establish the lungs as the site of gas exchange. Persistent pulmonary hypertension of the newborn (PPHN) is a syndrome in which normal circulatory transition at birth fails to occur, and pulmonary blood flow remains low with right-to-left shunting at the patent foramen ovale (PFO) and/or patent ductus arteriosus (PDA). Pulmonary vasoconstriction, and vascular proliferation and remodeling contribute to elevated pulmonary vascular resistance (PVR) in PPHN. The incidence of PPHN has been reported as 1.9 per 1000 live births (range: 0.4–6.8) in the United States and 0.43 – 6 per 1000 live births in the United Kingdom with mortality rate ranging between 4–33%1,2.
Management of PPHN includes supportive therapies, lung recruitment strategies, and pharmacologic pulmonary vasodilation. Inhaled NO (iNO) has been the primary agent studied in large randomized clinical trials, and is currently the only FDA approved specific pulmonary vasodilator therapy for infants. The response to iNO, however, remains suboptimal. A meta-analysis of randomized trials of iNO in newborns with PPHN revealed that almost a third to half of near-term and term infants with hypoxic respiratory failure/PPHN had a suboptimal response to iNO3.
Alternative agents for iNO-resistant PPHN are under active investigation based on their potential physiologic effects and complementary action with NO. These include systemic and inhaled vasodilators such as PDE 5 inhibitors, prostaglandins, the PDE 3 inhibitor milrinone, and ET-1 receptor antagonists. These promising therapeutic strategies are used by clinicians and centers with expertise in pulmonary vasodilator therapy. In addition, novel areas of investigation include emerging agents such as recombinant human superoxide dismutase (rhSOD), L-citrulline, sGC stimulators and activators, Rho-kinase inhibitors, and proliferator-activated receptor-γ agonists.
Endothelium Derived Mediators
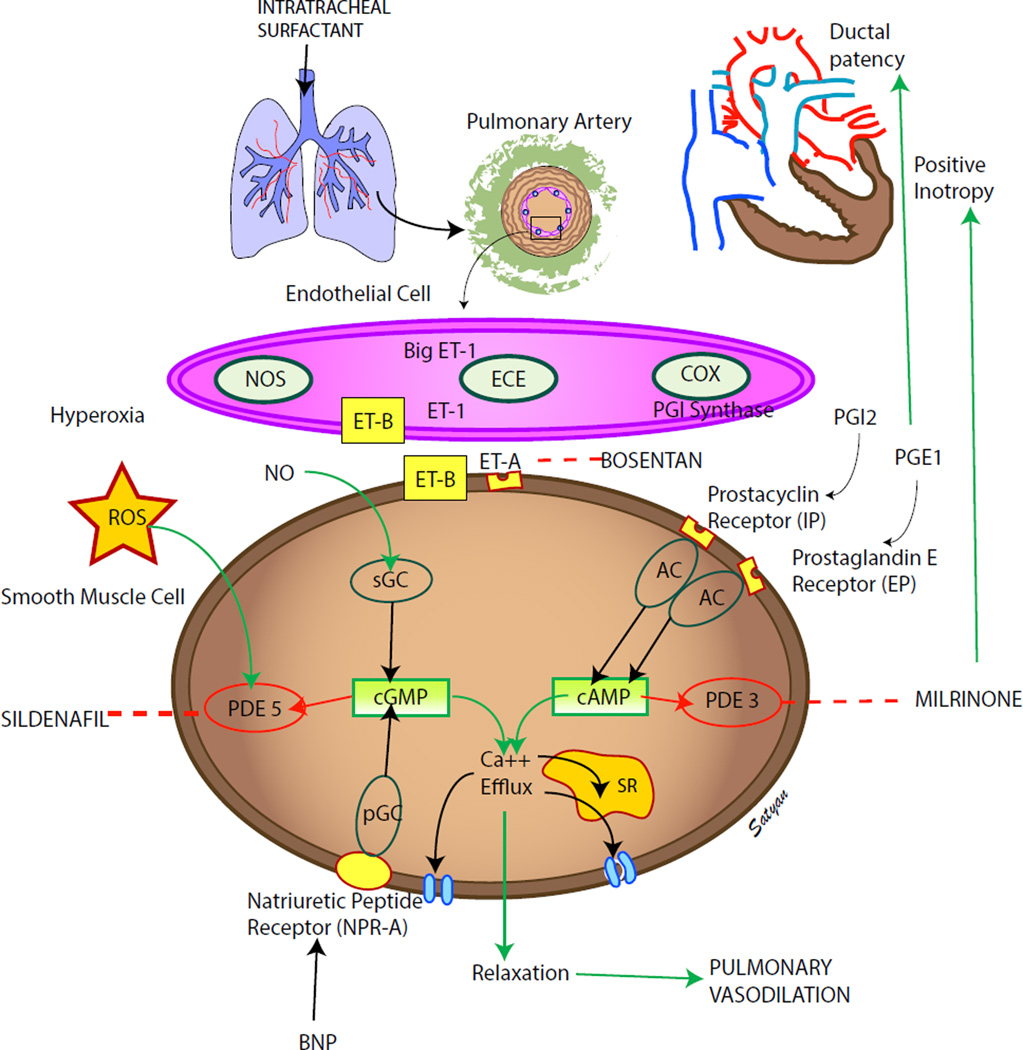
The pulmonary vascular endothelium releases vasoactive mediators that play an important role in cardiopulmonary transition at birth. In PPHN, the function of the endothelium is impaired and the balance between vasodilators and vasoconstrictors is altered. There is decreased production of vasodilators such as prostacyclin and nitric oxide (NO) and increased production of vasoconstrictors such as endothelin (Fig 1).
Figure 1. Endothelium derived mediators: vasodilators – prostacyclin (PGI2) and nitric oxide (NO) and vasoconstrictor (endothelin, ET-1).
Cyclooxygenase (COX) and prostacyclin synthase (PGIS) are involved in the production of prostacyclin. Prostacyclin acts on its receptor (IP) in the smooth muscle cell and stimulates adenylate cyclase (AC) to produce cyclic adenosine monophosphate (cAMP). Cyclic AMP is broken down by phosphodiesterase 3A (PDE 3A, the enzyme most prevalent in vasculature) in the smooth muscle cell. Milrinone inhibits PDE 3A and increases cAMP levels in arterial smooth muscle cells and cardiac myocytes resulting in pulmonary (and systemic) vasodilation and inotropy. Nitric oxide (NO) stimulates PDE 3A. Endothelin is a powerful vasoconstrictor and acts on ET-A receptors in the smooth muscle cell and increases ionic calcium concentration. A second endothelin receptor (ET-B) on the endothelial cell stimulates NO release and vasodilation. Endothelial nitric oxide synthase (eNOS) produces NO which diffuses from the endothelium to the smooth muscle cell and stimulates soluble guanylate cyclase (sGC) enzyme to produce cyclic guanosine monophosphate (cGMP). Cyclic GMP is broken down by PDE5 enzyme in the smooth muscle cell. Sildenafil inhibits PDE5 and increases cGMP levels in pulmonary arterial smooth muscle cells. Cyclic AMP and cGMP reduce cytosolic ionic calcium concentrations and induce smooth muscle cell relaxation and pulmonary vasodilation. Nitric oxide is a free radical and can avidly combine with superoxide anions to form a toxic vasoconstrictor, peroxynitrite. Hence, the bioavailability of NO in a tissue is determined by the local concentration of superoxide anions. Hyperoxic ventilation with 100% oxygen can increase the risk of formation of superoxide anions in the pulmonary arterial smooth muscle cells and limit the bioavailability of NO and stimulate PDE5 activity. Medications used in PPHN are shown in black boxes (modified from ref114 copyright Satyan Lakshminrusimha).
Many of these mediators and their derivatives or inhibitors are effective pulmonary vasodilators and are beneficial in the treatment of PPHN. These mediators are broadly classified into three categories based on their action via the cGMP, cAMP, and endothelin pathways.
Pulmonary vasodilators acting via the cGMP pathway
Pulmonary endothelial NO production increases markedly at the time of birth. The shear stress resulting from increased pulmonary blood flow and increased oxygenation induces endothelial nitric oxide synthase (eNOS) expression, contributing to NO-mediated pulmonary vasodilation after birth4.
Nitric oxide exerts its action through soluble guanylate cyclase (sGC) and the important second messenger, cGMP (Fig 1). The enzyme phosphodiesterase 5 (PDE 5) breaks down cGMP to inactive GMP. Hyperoxia and superoxide anions may inactivate eNOS, oxidize sGC and decrease cGMP production and stimulate PDE 5 to enhance breakdown of cGMP5. Natriuretic peptides (ANP, BNP and CNP) stimulate particulate guanylate cyclase (pGC) and produce cGMP. Plasma BNP levels are elevated in PPHN6–8 and may be an alternate source of cGMP. Inhaled NO and PDE 5 inhibitors are commonly used agents in the management of PPHN.
Sildenafil (Viagra®, Revatio®, Pfizer) is the prototype PDE 5 inhibitor. Considerable research in pulmonary arterial hypertension (PAH) and rebranding with a new trade name Revatio® has resulted in sildenafil being a common agent in the chronic management of PAH in adults9. Tadalafil (Adcirca®) is also a PDE 5 inhibitor approved for use in pulmonary arterial hypertension in adults. Sildenafil is the most common enteral pulmonary vasodilator used to treat infants, although this has been controversial.
(http://www.cbsnews.com/news/viagra-for-kids/). The FDA recently reignited the controversy by issuing a warning and recommending against its use in children (http://www.fda.gov/Drugs/DrugSafety/ucm317123.htm). Subsequent publications by experts10 and a clarification from FDA (http://www.fda.gov/Drugs/DrugSafety/ucm390876.htm) have acknowledged that there may be situations in which the risk-benefit profile of sildenafil may be acceptable in individual children, especially when treatment options are limited. It is important to note that sildenafil is not approved in neonates, and that the study that triggered this controversy did not enroll any neonates or infants under the age of 1 year11. Sildenafil is available in intravenous form, oral tablets and recently as a 10mg/ml suspension.
Currently, sildenafil is used for the following indications in neonates: (a) as an acute adjuvant to iNO in NO-resistant PPHN or to facilitate weaning of iNO; (b) as an acute primary treatment of PPHN where iNO is not available or is contraindicated and (c) in chronic primary treatment of pulmonary hypertension in conditions such as BPD and CDH.
There are no randomized trials evaluating sildenafil in neonates with PPHN. The available evidence from animal models and neonatal reports is summarized below.
Animal models of PPHN. Intravenous sildenafil was noted to be as effective as iNO in piglets with PPHN induced by intratracheal instillation of meconium 12. Intravenous sildenafil (2 mg/kg) administered to the same model while receiving iNO resulted in systemic hypotension demonstrating that sildenafil-induced vasodilation is not limited to the pulmonary circulation13. In a nitrofen-induced rat model of CDH, antenatal sildenafil administration improved lung structure, increased pulmonary vessel density, reduced right ventricular hypertrophy and improved postnatal NO-mediated pulmonary vasodilation 14. In a rat model of BPD induced by antenatal lipopolysaccharide (LPS) and postnatal hyperoxia exposure, intraperitoneal sildenafil improved alveolarization, and increased vascular distribution in the lung tissue by acting through the hypoxia-inducible-factor (HIF) pathway15. Exposure to hyperoxia increases PDE5 expression and activity in pulmonary vasculature and reduces cGMP impairing pulmonary vasodilation 16, 17. Hence, sildenafil may be an effective agent during management of neonates with PPHN with prolonged exposure to hyperoxic ventilation.
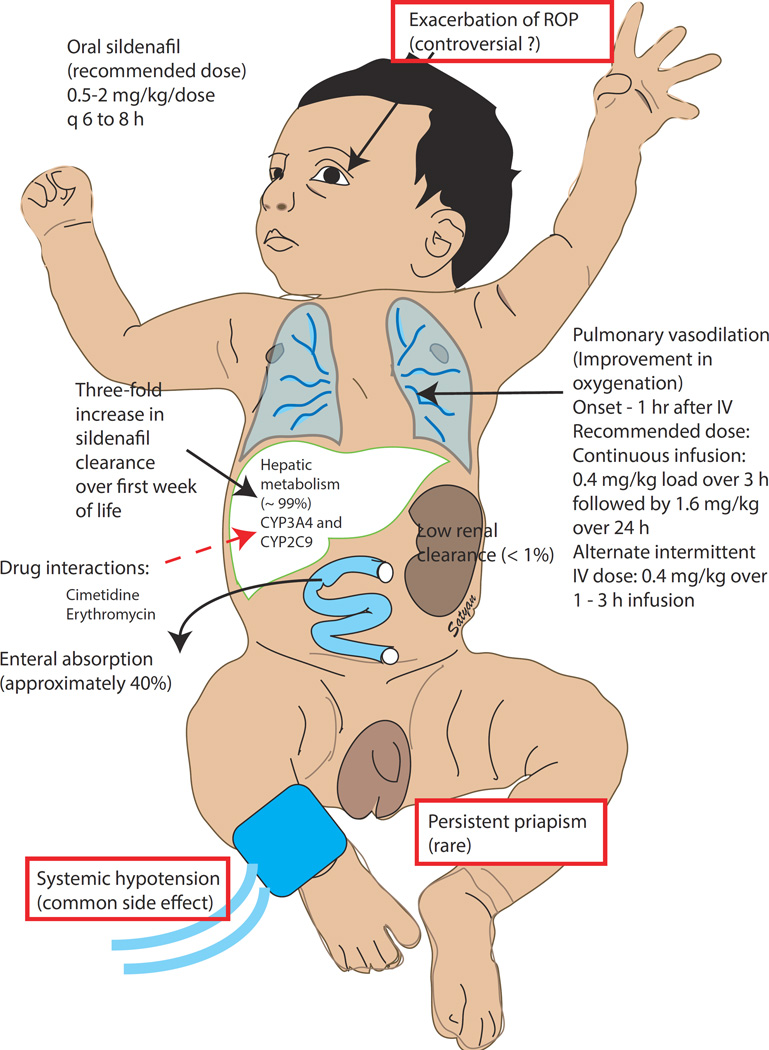
Infants with PPHN. A randomized trial of oral sildenafil in term infants with severe PPHN without access to iNO demonstrated improved survival (6/7) compared to placebo (1/6)18. A pharmacokinetic trial involving 8 different dosing regimens showed that a loading dose of sildenafil − 0.4 mg/kg over 3 hours (0.14 mg/kg/h), followed by 0.07 mg/kg/h (or approximately 1.6 mg/kg/day) continuous infusion provided the best therapeutic levels and was effective in improving oxygenation (Figure 2)19.
Figure 2. Sildenafil use in PPHN.
Sildenafil can be administered orally or by intravenous route. In adult volunteers approximately 40% of orally administered sildenafil is bioavailable. The recommended doses are shown in the figure. Sildenafil is predominantly metabolized in the liver through the CYP pathway (and its metabolism can be inhibited by cimetidine and erythromycin). The metabolism of sildenafil is low in a newly born infant due to hepatic immaturity but increases rapidly over the first week of life in term infants. The most common side-effect is systemic hypotension although priapism has been rarely reported. Deterioration of preexisting retinopathy of prematurity (ROP) has been described but not confirmed by other studies (these side effects are shown in boxes) (copyright Satyan Lakshminrusimha).
Infants with BPD. Small retrospective studies suggest that sildenafil decreases right ventricular pressures 20, 21 and potentially improves right ventricular function over time in infants with BPD-associated pulmonary hypertension. When compared to historic rates, mortality in this population may be decreased with sildenafil22. Sildenafil has not been shown to improve gas exchange in these infants however, and as a nonselective pulmonary vasodilator, it can lead to systemic hypotension and worse ventilation-perfusion mismatch21. A randomized trial to evaluate the efficacy of sildenafil in BPD-associated pulmonary hypertension is very much needed but may be difficult to conduct given the widespread off-label use and reasonable safety profile 23.
Infants with CDH. Sildenafil is used in many units for treatment of chronic pulmonary hypertension associated with CDH 24, 25. In one center, 17% of CDH patients had been discharged on sildenafil therapy and slowly weaned after discharge. A trial evaluating chronic sildenafil for severe CDH was recently terminated due to the change in clinical practice (allowing routine administration of sildenafil beyond 6 weeks of age) incompatible with the possibility of placebo enrollment (NCT00133679 – clinicaltrials.gov).
Pharmacokinetics. In adults, sildenafil is 41% bioavailable (90% confidence interval - 36–47%) after oral administration26 and has a half-life of 3.7 hours27. In neonates, the volume of distribution is 4 times higher and the clearance is slower resulting in a longer terminal half-life of 48 – 56 hours. Sildenafil clearance increases threefold in the first week of life and likely reflects maturation of the CYP mediated N-demethylation28.
Glucocorticoids. Recent evidence derived from animal models of PPHN suggests a potential role for glucocorticoids in restoring normal pulmonary vascular function. In the lamb model of PPHN induced by ductal ligation, hydrocortisone significantly improved arterial-to-alveolar ratios and attenuated oxidative stress, in part by increasing SOD activity 29, 30. Hydrocortisone increased cGMP by normalizing sGC and PDE 5 activity and by attenuating abnormalities induced by oxidative stress.
The glucocorticoids have potent anti-inflammatory properties and intravenous methylprednisolone (0.5 mg/kg/day in two divided doses) has been shown to reduce the duration of oxygen dependence in neonates with MAS 31. There is anecdotal evidence that hydrocortisone improves oxygenation in neonates with PPHN, and it is used in some centers as a rescue strategy prior to ECMO. Generally favorable results from studies have indicated that glucocorticoids may be beneficial, particularly in severe MAS in the presence of lung edema, pulmonary vasoconstriction, and inflammation. Caution must be exercised when considering hydrocortisone therapy, as it could mask the signs of infection. Hydrocortisone also stabilizes systemic blood pressure and reduces right-to-left shunting in PPHN.
Pulmonary vasodilators acting via the cAMP pathway
The arachidonic acid-prostacyclin pathway also plays an important role in pulmonary vascular transition at birth; prostaglandins activate adenylate cyclase (AC) to increase cAMP concentrations in vascular smooth muscle cells (Figure 1). Prostacyclin derivatives are the mainstay of pulmonary hypertension management in adults. In vascular smooth muscle, PDE 3A is an important enzyme that breaks down cAMP and this enzyme is inhibited by milrinone (Primacor®). Exposure to NO appears to enhance PDE 3A in animal studies32, 33 and may explain the increased efficacy of milrinone in iNO-resistant PPHN34–38.
Prostaglandins: Two classes of prostaglandin have therapeutic applications for treatment of PPHN: prostacyclin (Prostaglandin I2, PGI2) and prostaglandin E1 (PGE1). Prostacyclin (PGI2) mediates vasodilation by activating adenylate cyclase and increasing cAMP in the pulmonary arterial smooth muscle cell (figure 1). In newborns, prostacyclin partly mediates pulmonary vasodilation at birth in response to ventilation of the lungs; it does not play a significant role in vasodilation in response to oxygenation 39,40,41. In a study of lambs with PPHN induced by antenatal ductal ligation, pulmonary prostacyclin synthase and PGI2 receptor protein levels in the lung were decreased, but the adenylate cyclase levels were not altered42.
PGI2 analogs are the mainstay of pulmonary vasodilator therapy in adults and children with pulmonary arterial hypertension (PAH), and the intravenous route is the most studied. All PGI2 analogs have the limitation of an extremely short half-life. Prostacyclins are currently approved in multiple forms and are listed below:
Epoprostenol (intravenous)
Treprostinil (oral, intravenous, subcutaneous)
Iloprost (intravenous, inhaled)
Beraprost (oral)
Epoprostenol (Flolan®, Glaxo-Wellcome, Middlesex, UK) is commonly used as a continuous intravenous infusion in the adult intensive care unit setting for pulmonary arterial hypertension 43,44,45. However, the associated systemic hypotension has limited intravenous use in infants with PPHN. In addition, epoprostenol has a short half-life of 6 minutes and needs to be administered continuously. To minimize the systemic effects, the localized delivery of epoprostenol through inhalation has been achieved by aerosolizing the intravenous formulation. Inhaled epoprostenol has reduced pulmonary hypertension and improved oxygenation in animal studies and clinical trials without decreasing systemic blood pressure46–49.
The experience in infants with PPHN treated with inhaled epoprostenol thus far has been limited to case reports or small series. Inhaled epoprostenol improved oxygenation in infants with PPHN who had failed iNO therapy and also who had not received iNO. In one series, four preterm infants with PPHN received epoprostenol as an endotracheal bolus or continuous endotracheal infusion 50. Their OI improved significantly without systemic vascular compromise. Another series looked at 4 term neonates with hypoxic respiratory failure and PPHN that was refractory to I NO 51. Inhaled epoprostenol rapidly improved oxygenation in all four neonates although one neonate with alveolar capillary dysplasia subsequently deteriorated. The authors suggest that neonates with PPHN and an inadequate response to NO may have impaired cGMP-mediated pulmonary vasodilation and might benefit from PGI2, which acts through cAMP (figure 1). Inhaled epoprostenol is commonly nebulized at a dose of 50 ng/kg/min 51. The intravenous formulation Flolan® is dissolved in 20 ml of manufacturer’s diluent (a glycine buffer, pH −10). Fresh solution is added to the nebulization chamber every 4 hours 51. The effect of such alkaline pH on neonatal respiratory tract is not known.
Treprostinil (Remodulin®, United Therapeutics) is a stable prostacyclin analog with a half-life of 3 hours in adults and can be administered by oral, subcutaneous or intravenous route. There is limited data on its use in infants 52.
Beraprost sodium (BPS, Dorner® 20µg tablet, Toray Industries Inc, Tokyo, Japan) is an oral formulation developed in Japan with a half-life of 35–40 min, and has been shown to improve pulmonary hypertension in adults 53, 54 and children with congenital heart disease 55, 56. In a case series of 7 infants with PPHN refractory to alkali therapy and high frequency ventilation, beraprost reduced OI but also decreased systemic blood pressure by an average of 11 mm Hg by 6 hours57. In this study, infants received a 1µg/kg of beraprost every 6 hours. One quarter of the 20µg tablet was crushed and dissolved in 5 ml of sterile water (1µg/ml) and this suspension was administered to the infant via an orogastric tube. Further studies to evaluate the appropriate dose and minimize the risk of systemic hypotension are warranted.
Iloprost (Ventavis®, Actelion Pharmaceuticals, US) administered in intravenous or inhaled form, and alone or in combination with NO has resulted in improvements in PPHN in a number of reports 58–60. In a study of 47 infants with PPHN, inhaled iloprost appeared to be more effective than sildenafil in time to, and duration of clinical response, and the iloprost group had less need for inotropic support60. In a study of 33 infants with severe PPHN, intravenous iloprost significantly reduced the OI, although the need for inotropic support was increased 59. lloprost was administered by inhalation using a jet nebulizer at doses of 1 – 2.5 µg/kg with an interval of 2–4 hours between doses. In intubated patients, the jet nebulizer was adapted to the respiratory circuit with a T-connector. In the US, iloprost is available as 2.5 and 5 µg discs to be administered using an l-neb adaptive aerosol delivery device (AAD).
PGE1 - The prostaglandin PGE1, Alprostadil (Prostin VR Pediatric, Pharmacia and Upjohn Company) is widely used as a continuous intravenous infusion to maintain ductal arteriosus patency in newborns as a bridge to operative correction or palliation of cyanotic congenital heart lesions. In one retrospective analysis of infants with PPHN but without ductal dependent cardiac lesions, in whom PGE1 was initiated during transport, PGE1 treatment was associated with significantly shortened length of stay. The proposed mechanisms include the pulmonary vasodilator effects of PGE1 and the advantage ductal patency offers in providing a bypass or pop-off that permits improved function in the otherwise volume and pressure loaded right ventricle 61.
PGE1 is also available in inhalation form. Aerosolized alprostadil has been used to treat pulmonary hypertension in adults as well as in experimental animal models. In infants with PPHN, a small pilot phase I-Il study demonstrated that inhaled PGE1 was a safe, selective pulmonary vasodilator in hypoxemic respiratory failure 62, 63. The commonly used dose in iNO-resistant PPHN is at 150–300 ng/kg/min diluted in saline to provide 4 ml/hour as a continuous nebulization 63.
Milrinone is a phosphodiesterase 3 (PDE3) inhibitor with inotropic and lusitropic (myocardial relaxation) effects that was approved in the 1980;s for intravenous use in decompensated congestive heart failure. Vascular PDE 3 breaks down cAMP in arterial smooth muscle cells and myocardium (figures 1 and 3). By inhibiting PDE 3, milrinone also functions as a vasodilator, independent of β1-adrenergic receptor stimulation and has been called an “inodilator” because of these dual effects 64–66. Milrinone increases cAMP levels in cardiac muscle and vascular cells, improving ventricular function both directly and by reducing afterload. The common indications of milrinone in pulmonary hypertension include:
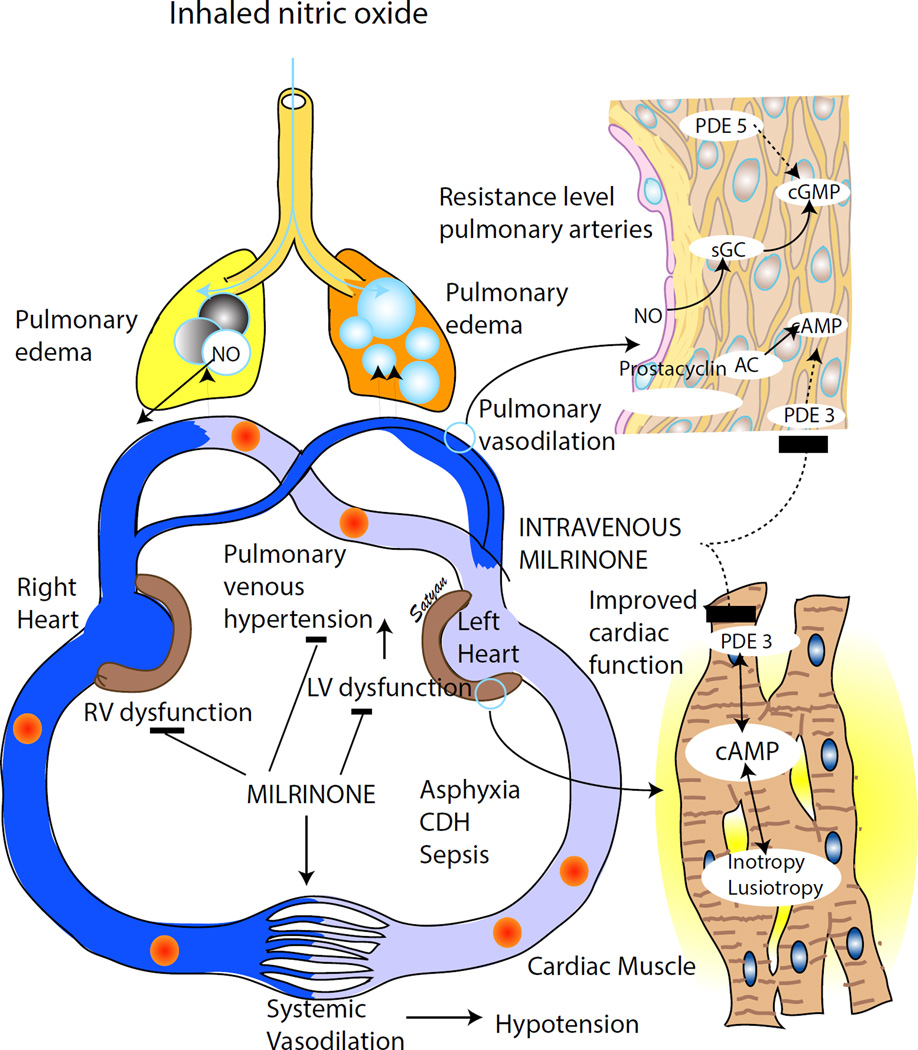
Ventricular dysfunction – especially if associated with pulmonary venous hypertension or high left atrial pressures; these infants demonstrate a left to right shunt at PFO because of left ventricular dysfunction and high left atrial pressure in spite of a right to left shunt at PDA due to high pulmonary arterial pressures (this situation is a contraindication for iNO and is common in asphyxia, CDH and sepsis associated with PPHN – figure 3).
As an adjuvant to iNO to promote pulmonary vasodilation and provide synergy
Figure 3. Milrinone in PPHN.
Pulmonary hypertension with ventricular dysfunction is common in neonates with asphyxia, congenital diaphragmatic hernia (CDH) and sepsis. Left ventricular dysfunction leads to pulmonary venous hypertension and pulmonary edema. Administration of inhaled NO in the presence of pulmonary venous hypertension can worsen pulmonary edema and deterioration of oxygenation. Milrinone improves left ventricular function through its cAMP mediated lusitropic (augment cardiac relaxation) and inotropic (enhance cardiac contraction) effect and decreases pulmonary venous hypertension. In addition, milrinone causes pulmonary and systemic vasodilation and reduces afterload on both ventricles, (copyright Satyan Lakshminrusimha).
There are no randomized trials evaluating milrinone in neonates with PPHN. The available evidence from animal models and pediatric trials and neonatal case reports is summarized below.
Animal models of PPHN: In the ovine model of PPHN induced by antenatal ductal ligation, milrinone relaxed pulmonary arterial rings in a dose dependent manner42. In the same model, an intravenous loading dose of 10 µg/kg followed by 1µg/kg/min for an hour reduced pulmonary vascular resistance from 0.503 ± 0.06 mmHg/mL/kg/min to 0.383 ± 0.03 mmHg/mL/kg/min (p<0.05) and was not associated with a significant decrease in systemic mean blood pressure (54 ± 3 to 51 ± 4mmHg)67. In lungs isolated from lambs with PPHN, protein levels of adenylate cyclase and PDE3A (the predominant PDE3 isoform in vascular tissue) were similar to control lambs without any change in PDE3A activity suggesting that the target enzymes for milrinone were unchanged by vascular remodeling in PPHN 42. In addition, exposure to iNO and oxygen both in vivo33 and at a cellular level32 markedly increases PDE 3 expression and may potentially increase the efficacy of milrinone.
Clinical studies: The benefits of milrinone in children following surgery for congenital heart disease have been well established in several studies (including the randomized, double-masked PRIMACORP study, n=238)68–71. In addition, anecdotal reports have shown that milrinone can be an effective therapeutic option in PPHN.35, 72 These retrospective case reports from two hospitals in Ontario, Canada looked at 24 critically ill late preterm/term infants (except one infant at 26 weeks postmenstrual age PMA) with HRF or PPHN unresponsive to iNO. In the first report, Bassler et al reported 4 infants with PPHN. Some of the infants were “primed” with normal saline (15ml/kg) and administered a loading dose of 50 µg/kg over 30 min followed by 0.33 µg/kg/min. None of the infants developed systemic hypotension, and all of them showed consistent improvement in oxygenation. One of the infants was born at 26 weeks PMA and developed bilateral intraventricular hemorrhage (IVH) with moderate dilation of all ventricles. A term infant also developed IVH, and a third infant (39 weeks PMA) showed a small left subependymal hemorrhage.
In a subsequent study, McNamara et al reported 9 term infants with PPHN with poor response to iNO who received intravenous milrinone. Because of the potential risk of systemic hypotension, a loading dose was avoided in these patients with PPHN. The infusion was started at 0.33 µg/kg/min and increased in increments of 0.33 according to clinical response to a maximum of 0.99 µg/kg/min. There was a significant improvement in oxygenation after commencement of milrinone, particularly in the first 24 h of infusion and there was no systemic hypotension. The same authors performed pharmacokinetic studies in 11 late preterm and term infants with PPHN resistant to iNO with a loading dose of 50 µg/kg over 60 min followed by an infusion of 0.33 to 0.99 µg/kg/min and demonstrated an improvement in oxygenation and cardiac output and a reduction in pulmonary arterial pressure by echocardiography without any IVH 73.
Preterm infants: Recently, James et al have described 7 preterm infants with PPHN resistant to iNO and treated with milrinone infusion 37. An echocardiogram was obtained one hour (median) prior to milrinone infusion. Milrinone infusion increased left ventricular output, right ventricular output and reduced right ventricular pressure. These echocardiographic changes were associated with a reduction in iNO dose and oxygen requirement over the subsequent 72 hours 37.
Infants with CDH: Patel recently reported improved right ventricular diastolic function and oxygenation in 6 neonates with CDH following milrinone infusion 74. All of them were treated with iNO or intravenous sildenafil or both. Four of these patients had undergone surgical repair prior to initiation of milrinone. Oxygenation index decreased from 10.6 ± 5.6 to 7.9 ± 6.2 by 12–24 hours and to 5.1 ± 2.6 by 48–72 hours after commencement of milrinone infusion 75. Milrinone is commonly used during the management of CDH without any randomized trials conducted to show benefit. Thirty percent of infants with CDH in the Children’s Hospital Neonatal Database (CHND)76 and 22% of late-preterm and term infants with CDH in the Pediatrix database 77 received milrinone. Milrinone appears to be an effective therapeutic option in infants with PPHN resistant to iNO and/or in the presence of cardiac dysfunction. Randomized trials evaluating its use in PPHN are warranted75.
Pharmacokinetics. The half-life, total body clearance, volume of distribution and steady state concentration of milrinone in neonates with PPHN are shown in table 1. Similar values were obtained from 48 neonatal, post-op cardiac patients in the PRIMACORP study (table 1) which also showed that the clearance in neonates is only 25% of that in children 68. With a constant-rate infusion, neonates take a much longer time to achieve steady-state concentration. Without a loading dose, neonates reached 50% of steady-state concentration by 2 hours (compared to 45 minutes in older children). However, for the same infusion rate, the steady-state concentration will be higher in neonates than older patients because of decreased renal function. These findings underscore the necessity of a loading dose for rapid achievement of a therapeutic blood concentration in neonates, but lower infusion rate to avoid higher levels secondary to poor clearance 68. Milrinone pharmacokinetics have also been evaluated in preterm infants78, 79, infants following cardiac surgery 71, 80 and more recently in neonates with iNO resistant PPHN 73 (table 1).
Table 1.
Pharmacokinetic data on milrinone in adults, children, term and preterm neonates
| Study | Adult111 | Child 112 | Neonate (post- op CHD)68 |
Neonate (PPHN)113 |
Preterm neonate79 |
|---|---|---|---|---|---|
| Gestational age/age |
Healthy adult volunteers |
Pediatric patients |
Neonates | 39.2 ±1.3 weeks 14h (10–30h) |
26 weeks (23- 28) |
| Weight (kg) | 5.9 ±4* | 3.481 ± 0.603 |
0.85 (0.52- 1.26) |
||
| Half-life (h) | 0.8 ± 0.22 | 3.7 | 4.1 ±1.1 | 10.3 | |
| Total body clearance (mL/kg/min) |
6.1 ±1.3 | 2.5 to 10.6 (increases with age) |
1.64 ±0.37 | 1.83 ±0.17 | 0.64 |
| Volume of distribution (L/kg) |
0.32 ± 0.08 | 0.7–0.9 | 0.523 ± 0.028 | 0.56 ±0.19 | 0.576 |
| Steady-state concentration (ng/mL) |
290.9 ±77.7 | 195 (78–257) at 21h after infusion |
|||
| Dose regimen | 12.5 to 75 µg/kg load followed by 0.5 µg/kg/min |
25 to 75 µg/kg load followed by 0.25 to 0.75 µg /kg/min infusion |
25 µg /kg over 60 min followed by a 0.25 µg/kg/ min (low dose) or 75 mcg/kg load and 0.75 µg/kg/min (high dose) |
50 mcg/kg load over 1h followed by 0.33 (to 0.99) µg /kg/min |
0.75 mcg/kg/min for 3h followed by 0.2 µg/kg/min |
This weight is an approximate value - based on high dose arm in PRIMACORP study
Pulmonary vasodilators acting via the Endothelin pathway
Endothelin-1 (ET-1) synthesized by vascular endothelial cells is a potent vasoconstrictor81 and acts through two receptors; ETA and ETB (figure 1). The ETA receptor plays a critical role in vasoconstriction while the ETB receptor promotes vasodilation82,83 mediated by endothelium-derived nitric oxide 84, 85. Selective blockade of the ETA receptor causes fetal pulmonary vasodilation86. ET-1 gene expression and levels are increased87 in the lungs and pulmonary arterial endothelial cells in the fetal lamb model of PPHN 87,88 while ETB protein is decreased in pulmonary artery endothelial cells isolated from lambs with PPHN induced by antenatal ductal ligation88. This model of PPHN is associated with significant remodeling of the pulmonary artery with smooth muscle hypertrophy and muscularization of small, distal pulmonary arteries 89 similar to that described in infants with PPHN and was used in preclinical studies for iNO 90.
Chronic intrauterine ETA receptor blockade following ductal ligation decreases pulmonary arterial pressure and distal muscularization of small pulmonary arteries in utero, decreases right ventricular hypertrophy, and increases the fall in PVR at delivery in newborn lambs with PPHN91. Elevated plasma ET-1 levels are observed in infants with CDH and PPHN and maybe a marker of disease severity and poor prognosis 92, 93
Bosentan (Tracleer™ tablets, Actelion Pharmaceuticals) is an ET-1 antagonist acting at both endothelin A and B receptors (ET-A and ET-B, figure 1). PPHN has been shown to be associated with increased ET-A mediated vasoconstriction and loss of ET-B mediated vasorelaxation in a newborn lamb model of PPHN 86, 91, 94. Elevated plasma levels of ET-1 has been documented in newborn infants with PPHN 92, 93.
Bosentan has proven efficacy in adults with pulmonary hypertension 95 and is commonly used in chronic therapy of primary pulmonary hypertension and thromboembolic PAH. In neonates, studies of bosentan in PPHN is limited mostly to case reports and two small randomized controlled trials. Successful use of bosentan has also been reported in pulmonary hypertension associated with congenital heart disease in newborn infants. Mohamed et al in a prospective, randomized controlled trial in newborn infants with PPHN showed both improved short-term outcomes and longer term outcomes at 6 months in infants who received bosentan as compared to placebo 96. A study by Steinhom et al with bosentan as adjuvant therapy in patients receiving inhaled nitric oxide for PPHN did not show an additive effect97. Bosentan is well absorbed following enteral administration (~98% in adults), is metabolized in the liver and is eliminated by biliary excretion. The dose of bosentan used in newborns is 1–2 mg/kg twice daily (usually prepared from 32 mg dispersible tablets).
Bosentan is available only in oral formulation and this limits its use in the acute stage of PPHN in the immediate newborn period. However it may be used as chronic therapy in infants with pulmonary hypertension associated with BPD or CDH. It may also have a role in the management of PPHN in resource-poor settings without access to inhaled nitric oxide.
Adverse effects associated with bosentan therapy include elevation of transaminases and liver failure. Hence liver function should be checked prior to onset of therapy and during treatment with bosentan. As per manufacturer recommendations bosentan should not be initiated in patients with transaminase levels more than 3 times the upper limits of normal and must be discontinued with elevation of bilirubin more than twice the upper limit of normal. Other reported side effects include angioedema, anemia, leukopenia and thrombocytopenia.
Bosentan is not currently approved for treatment of pulmonary arterial hypertension in children or for PPHN. Bosentan is currently available only through a restricted distribution program and requires physician certification and enrollment in the program. Other endothelin receptor antagonists including the selective ET-A blockers have not been carefully evaluated in PPHN.
Combination Therapy
The combination of pharmacologic agents with different mechanisms of action in treatment of PPHN may offer the benefit of an additive effect or may induce the same effect at lower doses of each agent. For example, inhaled epoprostenol may augment the response to NO in infants because its mechanism of action is different from and complementary to that of NO. When combined with NO, it also may prevent the rebound hypertension seen with NO weaning.
A number of clinical studies in adults have examined the effect of combining prostacyclins (epoprostenol, treprostinil, iloprost) with the phosphodiesterase inhibitor, sildenafil or endothelin receptor antagonist, bosentan. These have shown promising results with improvement in hemodynamic parameters and exercise tolerance 98. In a case report of a preterm infant following repair of CDH with right ventricular failure and severe PPHN, the combination of PGE1 infusion and oral sildenafil normalized right ventricular pressure and function99.
Emerging targets and therapies
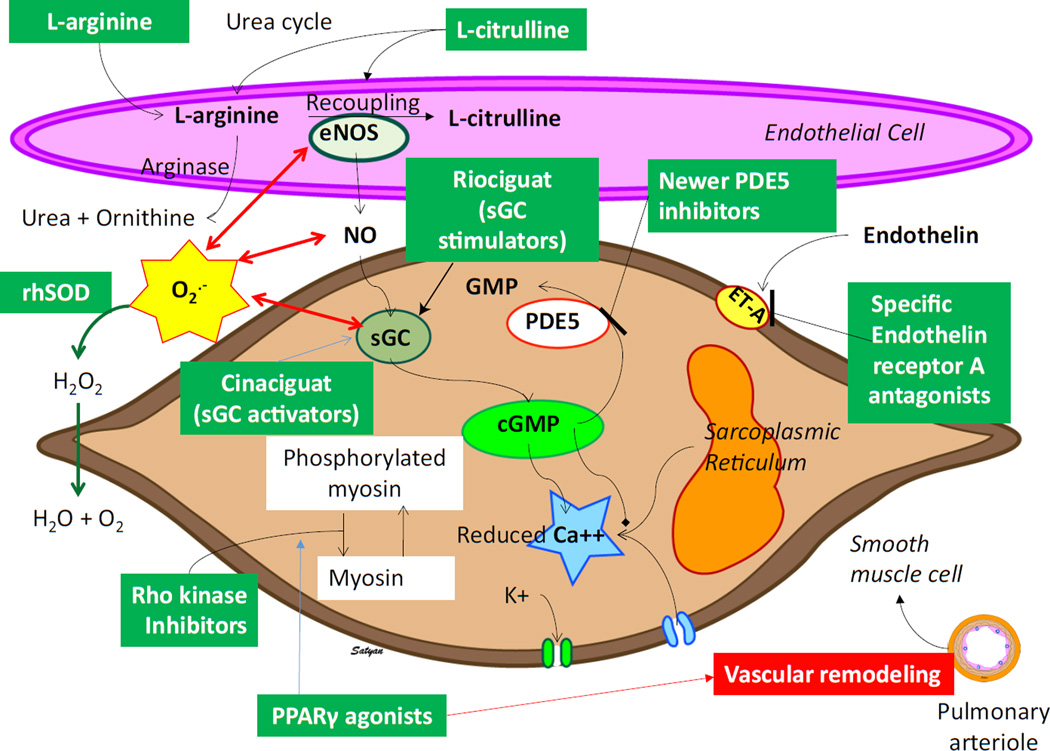
Emerging targets and therapies currently under investigation for PPHN are shown in Fig 4.
Figure 4. Emerging targets and therapies for PPHN.
L-citrulline, soluble guanylate cyclase (sGC) stimulators and activators, Rho-kinase inhibitors, PPAR γ agonists, antioxidants, newer phosphodiesterase (PDE) 5 inhibitors (e.g., tadalafil) and specific endothelin receptor – A antagonists (Sitaxsentan and Ambrisentan) are potential new therapies in PPHN that need further evaluation and clinical studies (see text for details). Copyright Satyan Lakshminrusimha
L-citrulline: With increased understanding of the pathobiology of pulmonary vascular disease in infants, and specifically the role of NO and prostaglandin signaling has revealed novel pharmacologic approaches to treat PPHN. Fike et al have demonstrated an impaired L-citrulline–L-arginine–nitric oxide pathway is involved in the pathogenesis of hypoxia-induced pulmonary hypertension100. Hypoxia reduces NO production in pulmonary arterial endothelial cells by uncoupling endothelial nitric oxide synthase, which is responsible for synthesizing NO from L-arginine. It is interesting that although L-arginine supplementation can improve NO signaling, oral citrulline is more effective than arginine in increasing serum L-arginine concentrations with fewer side effects. Rescue therapy with L-citrulline has been shown to ameliorate hypoxia-induced-pulmonary hypertension in newborn piglets101. In adult patients with idiopathic pulmonary hypertension or Eisenmenger syndrome, oral L-citrulline malate reduced pulmonary arterial pressure and improved six minute walking distance102. To date, no clinical trials of L-citrulline have been published in the neonatal population.
Soluble guanylate cyclase stimulators and activators: The target enzyme for iNO is sGC (figure 1 and 3). However, resistance to NO and tolerance may limit cGMP production in pulmonary arterial smooth muscle cell and limit vasodilation. Such circumstances have led to development of heme-dependent sGC stimulators and heme-independent sGC activators. Riociguat (Adempas®) is a stimulator and was approved by FDA in 2013 for the treatment of adults with chronic thromboembolic pulmonary hypertension (CTEPH) and some forms of pulmonary arterial hypertension (PAH). A related agent, cinaciguat is a sGC activator and has been shown to be effective as a pulmonary vasodilator in a lamb model of PPHN induced by ligation of the ductus arteriosus103, 104. The effectiveness of NO donors to increase cGMP is maximal in 21% oxygen in the absence of oxidative stress. Cinaciguat increased cGMP production in pulmonary artery smooth muscle cells from control and PPHN lambs even in the presence of oxidative stress induced by exposure to 50% oxygen or 1H-[1,2,4] oxadiazolo[4,3-a] quinoxalin-1-one (ODQ), an sGC oxidizer or hydrogen peroxide. In the presence of oxidized sGC, unlike NO donors, cinaciguat may still be effective in increasing cGMP production and vasodilation. Thus, cinaciguat may provide a novel treatment option for severe PPHN treated with prolonged hyperoxia.
Rho-kinase inhibitors: Vascular smooth muscle contraction is regulated by cytosolic Ca2+ levels [Ca2+]i. With an elevation of [Ca2+]l, formation of the calcium-calmodulin (CaM) complex increases, and myosin light-chain kinase (MLCK) is activated. MLCK phosphorylates the myosin light chain (MLC), enhancing cross-bridge cycling and inducing vascular smooth muscle contraction105. A small GTP-binding protein RhoA increases Rho-kinase (ROCK) activity leading to phosphorylation of MLC and vascular contraction. The RhoA-Rho-kinase pathway “sensitizes” the contractile proteins to [Ca2+]l, and plays an important role in hypoxic pulmonary vasoconstriction106. RhoA-Rho-kinase is an important mediator of elevated myogenic tone contributing to high PVR in fetal lambs107.
PPHN induced by partial constriction of the ductus arteriosus in fetal lambs is associated with increased ROCK activity in pulmonary arterial endothelial cells and contributes to impaired angiogenesis108. In a fetal sheep model, ROCK inhibition increased left pulmonary artery blood flow and decreased PVR107. ROCK inhibitors such as fasudil and Y27632 may have therapeutic potential in infants with PPHN
PPAR γ agonists: Both ROCK and peroxisome proliferator-activated receptor-γ (PPARγ) regulate smooth muscle cell proliferation and contribute to vascular remodeling in pulmonary hypertension. PPAR-γ is an essential regulator of pulmonary arterial smooth muscle cell proliferation and vascular tone109. PPARγ agonists produce vasodilation through inhibition of ROCK and there may be a potential role for PPARγ agonists in the management of PPHN.
Antioxidants: Oxidant stress appears to play an important role in pulmonary hypertension. The use of intratracheal recombinant human superoxide dismutase (rhSOD) induces pulmonary vasodilation, improves oxygenation and decreases oxidative stress in lambs with PPHN110.
Conclusion
Unavailability of iNO in developing countries coupled with its high cost and lack of efficacy in almost a third of patients with PPHN has triggered research evaluating newer pharmacologic approaches to PPHN. In addition, two remaining challenges where large knowledge gaps persist include management of pulmonary hypoplasia and pulmonary hypertension in CDH and BPD-associated pulmonary hypertension in the premature infant. Newer pulmonary vasodilators outlined in this chapter are currently under investigation. Further research to develop appropriate cost-effective strategies to ameliorate pulmonary vascular disease associated with conditions such as pneumonia and asphyxia and meconium aspiration, common in developing countries is warranted. Lack of equipoise among clinicians, ethical considerations (such as providing placebo to a preterm infant with BPD and pulmonary hypertension) and rapid progression of disease and clinical deterioration following failure of iNO in acute PPHN (limiting time for consent and randomization) have impaired our ability to conduct randomized control trials to evaluate pharmacological therapies outlined in this manuscript leading to significant knowledge gaps in PPHN management.
Acknowledgments
Grant support: NICHD 1R01HD072929 (SL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure:
Satyan Lakshminrusimha (SL) was previously a member of the speaker’s bureau for Ikaria (until October 2014), manufacturer of inhaled nitric oxide. He also declares that he has no competing interest in published data and Ikaria had no role in preparation of this manuscript. He was supported by NICHD HD072929 during the preparation of this manuscript.
Bobby Mathew and Corinne Leach declare that they have no relevant competing interests
Off label use:
This paper contains information about unapproved use of certain pharmacologic agents (prostaglandins, bosentan, sildenafil, milrinone, riociguat, ciniciguat and rhSOD are not approved for use in newborn period).
Suggested Reading
- 1.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 2.Bendapudi P, Rao GG, Greenough A. Diagnosis and management of persistent pulmonary hypertension of the newborn. Paediatr Respir Rev. 2015 doi: 10.1016/j.prrv.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Finer NN, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. The Cochrane database of systematic reviews. 2006:CD000399. doi: 10.1002/14651858.CD000399.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Lakshminrusimha S, Steinhom RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26:601–619. [PubMed] [Google Scholar]
- 5.Farrow KN, Lee KJ, Perez M, et al. Brief Hyperoxia Increases Mitochondrial Oxidation and Increases Pde5 Activity in Fetal Pulmonary Artery Smooth Muscle Cells. Antioxidants & redox signaling. 2012 doi: 10.1089/ars.2011.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baptista MJ, Correia-Pinto J, Rocha G, Guimaraes H, Areias JC. Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics. 2005;115:1111. doi: 10.1542/peds.2004-2780. author reply 1112. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds EW, Ellington JG, Vranicar M, Bada HS. Brain-type natriuretic peptide in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatrics. 2004;114:1297–1304. doi: 10.1542/peds.2004-0525. [DOI] [PubMed] [Google Scholar]
- 8.Soukka H, Jalonen J, Kero P, Kaapa P. Endothelin-1, atrial natriuretic peptide and pathophysiology of pulmonary hypertension in porcine meconium aspiration. Acta paediatrica (Oslo, Norway : 1992) 1998;87:424–428. doi: 10.1080/08035259850157039. [DOI] [PubMed] [Google Scholar]
- 9.Wardle AJ, Tulloh RM. Paediatric pulmonary hypertension and sildenafil: current practice and controversies. Archives of disease in childhood. Education and practice edition. 2013;98:141–147. doi: 10.1136/archdischild-2013-303981. [DOI] [PubMed] [Google Scholar]
- 10.Abman SH, Kinsella JP, Rosenzweig EB, et al. Implications of the U.S. Food and Drug Administration warning against the use of sildenafil for the treatment of pediatric pulmonary hypertension. American journal of respiratory and critical care medicine. 2013;187:572–575. doi: 10.1164/rccm.201210-1928PP. [DOI] [PubMed] [Google Scholar]
- 11.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation. 2014;129:1914–1923. doi: 10.1161/CIRCULATIONAHA.113.005698. [DOI] [PubMed] [Google Scholar]
- 12.Shekerdemian LS, Ravn HB, Penny DJ. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. American journal of respiratory and critical care medicine. 2002;165:1098–1102. doi: 10.1164/ajrccm.165.8.2107097. [DOI] [PubMed] [Google Scholar]
- 13.Shekerdemian LS, Ravn HB, Penny DJ. Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatric research. 2004;55:413–418. doi: 10.1203/01.PDR.0000112033.81970.C2. [DOI] [PubMed] [Google Scholar]
- 14.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123:2120–2131. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 15.Park HS, Park JW, Kim HJ, et al. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. American journal of respiratory cell and molecular biology. 2013;48:105–113. doi: 10.1165/rcmb.2012-0043OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrow KN, Lakshminrusimha S, Czech L, et al. Superoxide Dismutase and Inhaled Nitric Oxide Normalize Phosphodiesterase 5 Expression and Activity in Neonatal Lambs with Persistent Pulmonary Hypertension. American journal of physiology. Lung cellular and molecular physiology. 2010 doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farrow KN, Groh BS, Schumacker PT, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circulation research. 2008;102:226–233. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117:1077–1083. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 19.Steinhom RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. The Journal of pediatrics. 2009;155:841–847. e841. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Mourani PM, Sontag MK, Ivy DD, Abman SH. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. The Journal of pediatrics. 2009;154:379–384. 384, e371–e372. doi: 10.1016/j.jpeds.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyp M, Sandritter T, Poppinga N, Simon C, Truog WE. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: any benefits? Journal of perinatology: official journal of the California Perinatal Association. 2012;32:64–69. doi: 10.1038/jp.2011.131. [DOI] [PubMed] [Google Scholar]
- 22.Wardle AJ, Wardle R, Luyt K, Tulloh R. The utility of sildenafil in pulmonary hypertension: a focus on bronchopulmonary dysplasia. Arch Dis Child. 2013;98:613–617. doi: 10.1136/archdischild-2012-303333. [DOI] [PubMed] [Google Scholar]
- 23.Wardle AJ, Connolly GM, Stonier T, Tulloh R. Sildenafil in bronchopulmonary dysplasia: safe to use? Archives of disease in childhood. Fetal and neonatal edition. 2015;100:F369. doi: 10.1136/archdischild-2015-308164. [DOI] [PubMed] [Google Scholar]
- 24.Behrsin J, Cheung M, Patel N. Sildenafil Weaning After Discharge in Infants With Congenital Diaphragmatic Hernia. Pediatric cardiology. 2013 doi: 10.1007/s00246-013-0725-1. [DOI] [PubMed] [Google Scholar]
- 25.Keller RL, Hamrick SE, Kitterman JA, Fineman JR, Hawgood S. Treatment of rebound and chronic pulmonary hypertension with oral sildenafil in an infant with congenital diaphragmatic hernia. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2004;5:184–187. doi: 10.1097/01.pcc.0000113266.26638.ad. [DOI] [PubMed] [Google Scholar]
- 26.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29:297–310. doi: 10.1080/004982599238687. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee A, Dombi T, Wittke B, Lalonde R. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85:56–63. doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- 29.Perez M, Lakshminrusimha S, Wedgwood S, et al. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. American journal of physiology. Lung cellular and molecular physiology. 2012;302:1595–603. doi: 10.1152/ajplung.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez M, Wedgwood S, Lakshminrusimha S, Farrow KN, Steinhorn RH. Hydrocortisone normalizes phosphodiesterase-5 activity in pulmonary artery smooth muscle cells from lambs with persistent pulmonary hypertension of the newborn. Pulm Circ. 2014;4:71–81. doi: 10.1086/674903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi S, Saili A. The effect of steroids on the clinical course and outcome of neonates with meconium aspiration syndrome. Journal of tropical pediatrics. 2007;53:8–12. doi: 10.1093/tropej/fml018. [DOI] [PubMed] [Google Scholar]
- 32.Busch CJ, Graveline AR, Jiramongkolchai K, Liu H, Sanchez LS, Bloch KD. Phosphodiesterase 3A expression is modulated by nitric oxide in rat pulmonary artery smooth muscle cells. J Physiol Pharmacol. 2010;61:663–669. [PMC free article] [PubMed] [Google Scholar]
- 33.Chen B, Lakshminrusimha S, Czech L, et al. Regulation of Phosphodiesterase 3 in the Pulmonary Arteries During the Perinatal Period in Sheep. Pediatric research. 2009 doi: 10.1203/PDR.0b013e3181bce574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassler D, Choong K, McNamara P, Kirpalani H. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biology of the neonate. 2006;89:1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- 35.McNamara PJ, Laique F, Muang-ln S, Whyte HE. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. Journal of critical care. 2006;21:217–222. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A. Pharmacology of milrinone in neonates with persistent pulmonary hypertension of the newborn and suboptimal response to inhaled nitric oxide. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14:74–84. doi: 10.1097/PCC.0b013e31824ea2cd. [DOI] [PubMed] [Google Scholar]
- 37.James AT, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. The effect of milrinone on right and left ventricular function when used as a rescue therapy for term infants with pulmonary hypertension. Cardiology in the young. 2015:1–10. doi: 10.1017/S1047951114002698. [DOI] [PubMed] [Google Scholar]
- 38.James AT, Bee C, Corcoran JD, McNamara PJ, Franklin O, El-Khuffash AF. Treatment of premature infants with pulmonary hypertension and right ventricular dysfunction with milrinone: a case series. Journal of perinatology: official journal of the California Perinatal Association. 2015;35:268–273. doi: 10.1038/jp.2014.208. [DOI] [PubMed] [Google Scholar]
- 39.Morin FC, 3rd, Egan EA, Lundgren CE, Swartz DD. Prostacyclin does not change during an oxygen induced increase in pulmonary blood flow in the fetal lamb. Prostaglandins, leukotrienes, and essential fatty acids. 1988;32:139–144. [PubMed] [Google Scholar]
- 40.Morin FC, 3rd, Egan EA, Norfleet WT. Indomethacin does not diminish the pulmonary vascular response of the fetus to increased oxygen tension. Pediatric research. 1988;24:696–700. doi: 10.1203/00006450-198812000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Morin FC., 3rd Hyperventilation, alkalosis, prostaglandins, and pulmonary circulation of the newborn. J Appl Physiol (1985) 1986;61:2088–2094. doi: 10.1152/jappl.1986.61.6.2088. [DOI] [PubMed] [Google Scholar]
- 42.Lakshminrusimha S, Porta NF, Farrow KN, et al. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2009;10:106–112. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simonneau G, Rubin U, Galie N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Annals of internal medicine. 2008;149:521–530. doi: 10.7326/0003-4819-149-8-200810210-00004. [DOI] [PubMed] [Google Scholar]
- 44.Frantz RP, Schilz RJ, Chakinala MM, et al. Hospitalization and survival in patients using epoprostenol for injection in the PROSPECT observational study. Chest. 2015;147:484–494. doi: 10.1378/chest.14-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McLaughlin VV, Genthner DE, Panella MM, Rich S. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension.[comment] New England Journal of Medicine. 1998;338:273–277. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 46.Mikhail G, Gibbs J, Richardson M, et al. An evaluation of nebulized prostacyclin in patients with primary and secondary pulmonary hypertension. European heart journal. 1997;18:1499–1504. doi: 10.1093/oxfordjournals.eurheartj.a015478. [DOI] [PubMed] [Google Scholar]
- 47.De Wet CJ, Affleck DG, Jacobsohn E, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. The Journal of thoracic and cardiovascular surgery. 2004;127:1058–1067. doi: 10.1016/j.jtcvs.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Hill LL, Pearl RG. Combined inhaled nitric oxide and inhaled prostacyclin during experimental chronic pulmonary hypertension. J Appl Physiol (1985) 1999;86:1160–1164. doi: 10.1152/jappl.1999.86.4.1160. [DOI] [PubMed] [Google Scholar]
- 49.Zobel G, Dacar D, Rodl S, Friehs I. Inhaled nitric oxide versus inhaled prostacyclin and intravenous versus inhaled prostacyclin in acute respiratory failure with pulmonary hypertension in piglets. Pediatric research. 1995;38:198–204. doi: 10.1203/00006450-199508000-00011. [DOI] [PubMed] [Google Scholar]
- 50.De Jaegere AP, van den Anker JN. Endotracheal instillation of prostacyclin in preterm infants with persistent pulmonary hypertension. European Respiratory Journal. 1998;12:932–934. doi: 10.1183/09031936.98.12040932. [DOI] [PubMed] [Google Scholar]
- 51.Kelly LK, Porta NF, Goodman DM, Carroll CL, Steinhom RH. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. Journal of Pediatrics. 2002;141:830–832. doi: 10.1067/mpd.2002.129849. [DOI] [PubMed] [Google Scholar]
- 52.Mclntyre CM, Hanna BD, Rintoul N, Ramsey EZ. Safety of epoprostenol and treprostinil in children less than 12 months of age. Pulm Circ. 2013;3:862–869. doi: 10.1086/674762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okano Y, Yoshioka T, Shimouchi A, Satoh T, Kunieda T. Orally active prostacyclin analogue in primary pulmonary hypertension. Lancet. 1997;349:1365. doi: 10.1016/S0140-6736(97)24019-5. [DOI] [PubMed] [Google Scholar]
- 54.Ikeda D, Tsujino I, Sakaue S, et al. Pilot study of short-term effects of a novel long-acting oral beraprost in patients with pulmonary arterial hypertension. Circulation journal: official journal of the Japanese Circulation Society. 2007;71:1829–1831. doi: 10.1253/circj.71.1829. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki H, Sato S, Tanabe S, Hayasaka K. Beraprost sodium for pulmonary hypertension with congenital heart disease. Pediatrics international: official journal of the Japan Pediatric Society. 2002;44:528–529. doi: 10.1046/j.1442-200x.2002.01597.x. [DOI] [PubMed] [Google Scholar]
- 56.Limsuwan A, Pienvichit P, Khowsathit P. Beraprost therapy in children with pulmonary hypertension secondary to congenital heart disease. Pediatric cardiology. 2005;26:787–791. doi: 10.1007/s00246-005-0925-4. [DOI] [PubMed] [Google Scholar]
- 57.Nakwan N, Nakwan N, Wannaro J. Persistent pulmonary hypertension of the newborn successfully treated with beraprost sodium: a retrospective chart review. Neonatology. 2011;99:32–37. doi: 10.1159/000298137. [DOI] [PubMed] [Google Scholar]
- 58.Ehlen M, Wiebe B. Iloprost in persistent pulmonary hypertension of the newborn. Cardiology in the young. 2003;13:361–363. doi: 10.1017/s1047951103000726. [DOI] [PubMed] [Google Scholar]
- 59.Janjindamai W, Thatrimontrichai A, Maneenil G, Chanvitan P, Dissaneevate S. Effectiveness and safety of intravenous iloprost for severe persistent pulmonary hypertension of the newborn. Indian pediatrics. 2013;50:934–938. doi: 10.1007/s13312-013-0263-1. [DOI] [PubMed] [Google Scholar]
- 60.Kahveci H, Yilmaz O, Avsar UZ, et al. Oral sildenafil and inhaled iloprost in the treatment of pulmonary hypertension of the newborn. Pediatric pulmonology. 2014;49:1205–1213. doi: 10.1002/ppul.22985. [DOI] [PubMed] [Google Scholar]
- 61.Gupta N, Kamlin CO, Cheung M, Stewart M, Patel N. Prostaglandin El use during neonatal transfer: potential beneficial role in persistent pulmonary hypertension of the newborn. Archives of disease in childhood. Fetal and neonatal edition. 2013;98:F186–F188. doi: 10.1136/archdischild-2012-303294. [DOI] [PubMed] [Google Scholar]
- 62.Sood BG, Delaney-Black V, Aranda JV, Shankaran S. Aerosolized PGE1: a selective pulmonary vasodilator in neonatal hypoxemic respiratory failure results of a Phase l/ll open label clinical trial. Pediatric research. 2004;56:579–585. doi: 10.1203/01.PDR.0000139927.86617.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sood BG, Keszler M, Garg M, et al. Inhaled PGE1 in neonates with hypoxemic respiratory failure: two pilot feasibility randomized clinical trials. Trials. 2014;15:486. doi: 10.1186/1745-6215-15-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Opie LH. “Inodilators”. Lancet. 1986;1:1336. [PubMed] [Google Scholar]
- 65.Lakshminrusimha S, Steinhom RH. eNeonatal Review. Vol. 7. Baltimore, MD: Hopkins CME; 2009. Phosphodiesterase Inhibitors in the Management of Persistent Pulmonary Hypertension of the Newborn (PPHN) [Google Scholar]
- 66.Lakshminrusimha S, Steinhom RH. “Inodilators” in Nitric Oxide Resistant PPHN. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013 doi: 10.1097/PCC.0b013e318250af44. in press. [DOI] [PubMed] [Google Scholar]
- 67.Rashid N, Morin FC, 3rd, Swartz DD, et al. Effects of prostacyclin and milrinone on pulmonary hemodynamics in newborn lambs with persistent pulmonary hypertension induced by ductal ligation. Pediatric research. 2006;60:624–629. doi: 10.1203/01.pdr.0000242343.84510.81. [DOI] [PubMed] [Google Scholar]
- 68.Bailey JM, Miller BE, Lu W, Tosone SR, Kanter KR, Tarn VK. The pharmacokinetics of milrinone in pediatric patients after cardiac surgery. Anesthesiology. 1999;90:1012–1018. doi: 10.1097/00000542-199904000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Chang AC, Atz AM, Wemovsky G, Burke RP, Wessel DL. Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Critical care medicine. 1995;23:1907–1914. doi: 10.1097/00003246-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 70.Hoffman TM, Wemovsky G, Atz AM, et al. Prophylactic intravenous use of milrinone after cardiac operation in pediatrics (PRIMACORP) study. Prophylactic Intravenous Use of Milrinone After Cardiac Operation in Pediatrics. American heart journal. 2002;143:15–21. doi: 10.1067/mhj.2002.120305. [DOI] [PubMed] [Google Scholar]
- 71.Hoffman TM, Wemovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107:996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 72.Bassler D, Kreutzer K, McNamara P, Kirpalani H. Milrinone for persistent pulmonary hypertension of the newborn. The Cochrane database of systematic reviews. 2010:CD007802. doi: 10.1002/14651858.CD007802.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A. Pharmacology of Milrinone in Neonates with Persistent Pulmonary Hypertension of the Newborn and Suboptimal Response to Inhaled Nitric Oxide*. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2012 doi: 10.1097/PCC.0b013e31824ea2cd. [DOI] [PubMed] [Google Scholar]
- 74.Patel N. Use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatology. 2012;102:130–136. doi: 10.1159/000339108. [DOI] [PubMed] [Google Scholar]
- 75.Giaccone A, Kirpalani H. Judgment often impossible without randomized trials. Commentary on N. Patel: use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatology. 2012;102:130–136. doi: 10.1159/000339108. [DOI] [PubMed] [Google Scholar]; Neonatology. 2012;102:137–138. doi: 10.1159/000339112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grover TR, Murthy K, Brozanski B, et al. Short-Term Outcomes and Medical and Surgical Interventions in Infants with Congenital Diaphragmatic Hernia. American journal of perinatology. 2015 doi: 10.1055/s-0035-1548729. [DOI] [PubMed] [Google Scholar]
- 77.Malowitz JR, Hornik CP, Laughon MM, et al. Management Practice and Mortality for Infants with Congenital Diaphragmatic Hernia. American journal of perinatology. 2015 doi: 10.1055/s-0035-1544949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paradisis M, Evans N, Kluckow M, Osborn D. Randomized trial of milrinone versus placebo for prevention of low systemic blood flow in very preterm infants. The Journal of pediatrics. 2009;154:189–195. doi: 10.1016/j.jpeds.2008.07.059. [DOI] [PubMed] [Google Scholar]
- 79.Paradisis M, Jiang X, McLachlan AJ, Evans N, Kluckow M, Osborn D. Population pharmacokinetics and dosing regimen design of milrinone in preterm infants. Archives of disease in childhood. Fetal and neonatal edition. 2007;92:F204–F209. doi: 10.1136/adc.2005.092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindsay CA, Barton P, Lawless S, et al. Pharmacokinetics and pharmacodynamics of milrinone lactate in pediatric patients with septic shock. The Journal of pediatrics. 1998;132:329–334. doi: 10.1016/s0022-3476(98)70454-8. [DOI] [PubMed] [Google Scholar]
- 81.Luscher TF. Endothelin: systemic arterial and pulmonary effects of a new peptide with potent biologic properties. American Review of Respiratory Disease. 1992;146:S56–S60. doi: 10.1164/ajrccm/146.5_Pt_2.S56. [DOI] [PubMed] [Google Scholar]
- 82.Perreault T, Coceani F. Endothelin in the perinatal circulation. Can J Physiol Pharmacol. 2003;81:644–653. doi: 10.1139/y03-013. [DOI] [PubMed] [Google Scholar]
- 83.Mann J, Farrukh IS, Michael JR. Mechanisms by which endothelin 1 induces pulmonary vasoconstriction in the rabbit. Journal of Applied Physiology. 1991;71:410–416. doi: 10.1152/jappl.1991.71.2.410. [DOI] [PubMed] [Google Scholar]
- 84.Tod ML, Cassin S. Endothelin-1-induced pulmonary arterial dilation is reduced by N omega-nitro-L-arginine in fetal lambs. Journal of Applied Physiology. 1992;72:1730–1734. doi: 10.1152/jappl.1992.72.5.1730. [DOI] [PubMed] [Google Scholar]
- 85.Wong J, Reddy VM, Hendricks-Munoz K, Liddicoat JR, Gerrets R, Fineman JR. Endothelin-1 vasoactive responses in lambs with pulmonary hypertension and increased pulmonary blood flow. American Journal of Physiology. 1995;269:H1965–H1972. doi: 10.1152/ajpheart.1995.269.6.H1965. [DOI] [PubMed] [Google Scholar]
- 86.Ivy DD, Kinsella JP, Abman SH. Physiologic characterization of endothelin A and B receptor activity in the ovine fetal pulmonary circulation. The Journal of clinical investigation. 1994;93:2141–2148. doi: 10.1172/JCI117210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ivy DD, Le Cras TD, Horan MP, Abman SH. Chronic intrauterine pulmonary hypertension increases preproendothelin-1 and decreases endothelin B receptor mRNA expression in the ovine fetal lung. Chest. 1998;114:65S. doi: 10.1378/chest.114.1_supplement.65s. [DOI] [PubMed] [Google Scholar]
- 88.Gien J, Tseng N, Seedorf G, Roe G, Abman SH. Endothelin-1 impairs angiogenesis in vitro through Rho-kinase activation after chronic intrauterine pulmonary hypertension in fetal sheep. Pediatric research. 2013;73:252–262. doi: 10.1038/pr.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wild LM, Nickerson PA, Morin FC., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatric research. 1989;25:251–257. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 90.Zayek M, Cleveland D, Morin FC., 3rd Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. The Journal of pediatrics. 1993;122:743–750. doi: 10.1016/s0022-3476(06)80020-x. [DOI] [PubMed] [Google Scholar]
- 91.Ivy DD, Parker TA, Ziegler JW, et al. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. The Journal of clinical investigation. 1997;99:1179–1186. doi: 10.1172/JCI119274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Keller RL, Tacy TA, Hendricks-Munoz K, et al. Congenital diaphragmatic hernia: endothelin-1, pulmonary hypertension, and disease severity. American journal of respiratory and critical care medicine. 2010;182:555–561. doi: 10.1164/rccm.200907-1126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar P, Kazzi NJ, Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. American journal of perinatology. 1996;13:335–341. doi: 10.1055/s-2007-994352. [DOI] [PubMed] [Google Scholar]
- 94.Steinhom RH, Millard SL, Morin FC., 3rd Persistent pulmonary hypertension of the newborn. Role of nitric oxide and endothelin in pathophysiology and treatment. Clinics in perinatology. 1995;22:405–428. [PubMed] [Google Scholar]
- 95.Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study.[comment] Lancet. 2001;358:1119–1123. doi: 10.1016/S0140-6736(01)06250-X. [DOI] [PubMed] [Google Scholar]
- 96.Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. Journal of perinatology: official journal of the California Perinatal Association. 2012 doi: 10.1038/jp.2011.157. [DOI] [PubMed] [Google Scholar]
- 97.Steinhom RH, Fineman J, Kusic-Pajic A, et al. Bosentan as adjunctive therapy for persistent pulmonary hypertension of the newborn: results of the FUTURE-4 study. Circulation. 2014;130:A13503. doi: 10.1016/j.jpeds.2016.06.078. [DOI] [PubMed] [Google Scholar]
- 98.Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients receiving background endothelin receptor antagonist and phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C2 study): a randomized controlled trial. Chest. 2013;144:952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 99.Filan PM, McDougall PN, Shekerdemian LS. Combination pharmacotherapy for severe neonatal pulmonary hypertension. Journal of paediatrics and child health. 2006;42:219–220. doi: 10.1111/j.1440-1754.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 100.Fike CD, Summar M, Aschner JL. L-citrulline provides a novel strategy for treating chronic pulmonary hypertension in newborn infants. Acta paediatrica (Oslo, Norway: 1992) 2014;103:1019–1026. doi: 10.1111/apa.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fike CD, Dikalova A, Kaplowitz MR, Cunningham G, Summar M, Aschner JL. Rescue Treatment with L-Citrulline Inhibits Hypoxia-lnduced Pulmonary Hypertension in Newborn Pigs. American journal of respiratory cell and molecular biology. 2015;53:255–264. doi: 10.1165/rcmb.2014-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharif Kashani B, Tahmaseb Pour P, Malekmohammad M, et al. Oral l-citrulline malate in patients with idiopathic pulmonary arterial hypertension and Eisenmenger Syndrome: a clinical trial. Journal of cardiology. 2014;64:231–235. doi: 10.1016/j.jjcc.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Chester M, Seedorf G, Tourneux P, et al. Cinaciguat, a soluble guanylate cyclase activator, augments cGMP after oxidative stress and causes pulmonary vasodilation in neonatal pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2011;301:L755–L764. doi: 10.1152/ajplung.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chester M, Tourneux P, Seedorf G, Grover TR, Gien J, Abman SH. Cinaciguat, a soluble guanylate cyclase activator, causes potent and sustained pulmonary vasodilation in the ovine fetus. American journal of physiology. Lung cellular and molecular physiology. 2009;297:L318–L325. doi: 10.1152/ajplung.00062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nossaman BD, Nossaman VE, Murthy SN, Kadowitz PJ. Role of the RhoA/Rho-kinase pathway in the regulation of pulmonary vasoconstrictor function. Can J Physiol Pharmacol. 2010;88:1–8. doi: 10.1139/Y09-092. [DOI] [PubMed] [Google Scholar]
- 106.Fagan KA, Oka M, Bauer NR, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of Rho-kinase. American journal of physiology. Lung cellular and molecular physiology. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- 107.Parker TA, Roe G, Grover TR, Abman SH. Rho kinase activation maintains high pulmonary vascular resistance in the ovine fetal lung. American journal of physiology. Lung cellular and molecular physiology. 2006;291:L976–L982. doi: 10.1152/ajplung.00512.2005. [DOI] [PubMed] [Google Scholar]
- 108.Gien J, Seedorf GJ, Balasubramaniam V, Tseng N, Markham N, Abman SH. Chronic intrauterine pulmonary hypertension increases endothelial cell Rho kinase activity and impairs angiogenesis in vitro. American journal of physiology. Lung cellular and molecular physiology. 2008;295:680–687. doi: 10.1152/ajplung.00516.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wolf D, Tseng N, Seedorf G, Roe G, Abman SH, Gien J. Endothelin-1 decreases endothelial PPARgamma signaling and impairs angiogenesis after chronic intrauterine pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2014;306:L361–L371. doi: 10.1152/ajplung.00277.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lakshminrusimha S, Russell JA, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. American journal of respiratory and critical care medicine. 2006;174:1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young RA, Ward A. Milrinone. A preliminary review of its pharmacological properties and therapeutic use. Drugs. 1988;36:158–192. doi: 10.2165/00003495-198836020-00003. [DOI] [PubMed] [Google Scholar]
- 112.Bailey JM, Hoffman TM, Wessel DL, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. J Pharmacokinet Pharmacodyn. 2004;31:43–59. doi: 10.1023/b:jopa.0000029488.45177.48. [DOI] [PubMed] [Google Scholar]
- 113.McNamara PJ, Shivananda SP, Sahni M, Freeman D, Taddio A. Pharmacology of milrinine in neonates with persistent pulmonary hypertension of the newborn (PPHN) and suboptimal response to inhaled nitric oxide. pediatric critical care medicine. 2012 doi: 10.1097/PCC.0b013e31824ea2cd. [DOI] [PubMed] [Google Scholar]
- 114.sharma V, Berkelhamer SK, Lakshminrusimha S. Persistent pulmonary hypertension of the newborn. Maternal Health, Neonatology and Perinatology: BMC. 2015;1:1–18. doi: 10.1186/s40748-015-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]