Abstract
STUDY OBJECTIVE
To understand how adolescents and parents describe a sexually transmitted infection prevention study to a friend.
DESIGN
Adolescents and parents participating in a study about willingness to participate in a hypothetical microbicide clinical trial were interviewed separately and asked to describe the clinical trial to a friend. Qualitative responses were written down verbatim and coded using a thematic framework analysis.
SETTING
Adolescent medicine clinics in New York City.
PARTICIPANTS
The participants consisted of adolescents, 14–17 years old, and a parent (n = 301 dyads) who spoke English or Spanish. Most adolescents (72%) identified as Hispanic and 65% reported minimal sexual experience (i.e. nothing more than kissing).
INTERVENTIONS
None
MAIN OUTCOME MEASURES
Qualitative responses were content coded for: 1) overall approach, 2) opinion rendered, and 3) details mentioned using thematic framework. The relationship of demographics, sexual history and recruitment method to how adolescents/parents described the study was evaluated.
RESULTS
Adolescents differed from parents in their overall approach to describing the study (p < 0.01) with more adolescents than parents providing a “purpose with detail” (54% adolescents vs 31% parents) and less providing a “commentary” description (6% adolescents vs 28% parents). Fewer adolescents (25%) provided an opinion compared to parents (75%) (p < 0.01). A higher proportion of adolescents (70% adolescents vs 48% parents) provided a detail (p < 0.01). Adolescents provided a greater number of details than parents (p < 0.01).
CONCLUSION
Adolescents in this sample were more focused on the details of the study. Parents were focused on their impression of the study. Adolescents and parents may need to be approached differently about reproductive health studies.
Keywords: adolescent parent dyads, reproductive health, recruitment, descriptive
INTRODUCTION
Given the continuing public health problem of sexually transmitted infections (STIs),1,2 the development of new bio-medical options, such as microbicides are needed.3 With adolescents as a high-risk group for STIs,1,4 the safety, efficacy, and acceptability of microbicides will need to be evaluated in this age group.5 Understanding how adolescents and parents view and describe reproductive health studies may foster strategies to enhance adolescent enrollment.
Particularly for Phase I clinical trials (e.g., microbicide safety studies), it is highly likely that both parental consent as well as adolescent assent will be required. However, parents and adolescents may approach potential participation differently. Studies show that predictors of parental consent for their adolescent’s participation in sexual health research include parents believing that their teenager is already sexually active6 or parents perceiving a benefit for their adolescent to participate, such as the adolescent receiving sex education.7 Others show that adolescent predictors of participation focus on the role of peers, 8,9,10 altruism, 11,12,13 privacy assurance,14,15 and compensation or incentives.16
One way to understand how adolescents and parents perceive a study is by examining how they would describe it to their respective peers. Their descriptions may reflect salient aspects which, in turn, may impact study recruitment, final decision-making regarding participation, and/or retention. 17,18,19 Understanding what information about studies adolescents/parents might share with their peers in the community may provide insight regarding the use of snowball sampling (where an individual is referred into the study by a current study participant), respondent-driven sampling (or chain-referral sampling with good estimability to compensate for any non-random selection often used when accessing hard-to-reach populations), or community advisory boards (using representatives of the general public to advise representatives of an institution about research recruitment and/or design).20,21,22
Thus, we analyzed adolescent and parent responses to “how they would describe a hypothetical phase I microbicide clinical trial to a friend” after being read a consent form for such a trial. We examined adolescent and parent’s overall approach to describing the study, if they expressed opinions while describing the study, and finally, what details they chose to include. We evaluated whether demographics, sexual history or recruitment method might influence how each group (adolescent and parent) chose to describe the study.
MATERIAL AND METHODS
Recruitment and Enrollment
Participants were recruited from the adolescent medicine clinics of two large medical centers in New York City, and through snowball sampling (where an individual is referred into the study by a current study participant), to participate in a longitudinal survey study addressing willingness to participate in a hypothetical clinical trial that examined the safety of a topical microbicide in adolescents. To be included into the study, both the adolescent (14–17 years of age) and their parent/legal guardian had to agree to participate and speak either English or Spanish. The study was approved by the Institutional Review Boards of Columbia University Medical Center and Weill Cornell Medical College, and all participants provided written informed consent/assent. Only baseline data was used for the present analysis.
Procedures
At baseline, adolescents and parents were interviewed separately with a research assistant who read a structured interview aloud in the participants language of choice (English or Spanish) and participant responses were written down verbatim by the research assistant. Demographics assessed in the interview included adolescent and parent age, Hispanic ethnicity, gender of the adolescent, relationship of the parent to the adolescent (e.g., mother), and parent educational level. Adolescents’ report of their sexual experience was collapsed into those who reported nothing more than kissing versus those who reported some type of sexual contact – touching, oral, anal, or penile-vaginal sex. Parental report was divided into those who reported that their adolescent had no sexual contact beyond kissing, had sexual contact beyond kissing or the parent was not sure.
Research coordinators reviewed an informed consent document with each individual about a hypothetical study examining the safety of a topical microbicide for STIs/human immunodeficiency virus prevention in adolescents. The hypothetical study’s consent document described a randomized controlled trial in which an experimental or control gel would be randomly assigned to each participant to use once daily for a week. The gel would be applied intravaginally, or topically to the penis, and the adolescent would be asked to abstain from sexual contact during use. The study duration was approximately one month, which included three study visits, each consisting of a genital exam, blood draw, urine test, as well as answering a series of questions. Total compensation for participation in this hypothetical study would be $300 cash plus round-trip subway fare at each visit.
After listening to the hypothetical study’s informed consent, each participant was asked, “If you were to describe the study to one of your friends (or the parent of the adolescent’s friend) what would you tell them?” Responses were written down verbatim.
Analysis
All written responses were coded in NVivo (qualitative data analysis software; QSR International Pty Ltd. Version 10, 2012) by two independent coders. The coders used a thematic framework analysis approach to code for specific themes that emerged from the responses.23 Preliminary codes were generated and modified until the sub-codes captured the range of responses within each theme and consensus was reached between the independent coders. The participant responses were coded for content using three major themes. First, the responses were coded into mutually exclusive themes representing the main approach used by the participant to describe the study (overall approach). Second, regardless of overall approach, we coded whether the participant rendered an opinion of the study (opinion), and third, how many and which specific details were mentioned (details).
Within the three themes, codes were given numerical scores for quantitative analysis. Bivariate analysis using chi-square tests compared rates of overall approach, opinions, and details between adolescents and parents. In addition, we examined the frequency of details mentioned using the Wilcoxon Rank Sum test for non-parametric distributions to compare counts of details between adolescent and parent groups. For purposes of evaluating associations between demographics, sexual history and recruitment method to the adolescents’ or parents’ overall approach to describing the study, we used bivariate analysis (chi-square or ANOVA) to explore possible relationships using SAS® Version 9.4 (SAS Institute Inc., Cary, NC, © 2013).
RESULTS
Study Sample
Three hundred forty-three families were enrolled; one family withdrew and was not included in any analyses. Two families enrolled twice and only their initial data were included. When examining the demographics of those approached through clinic or snowball sampling (where an individual is referred into the study by a current study participant), there were no differences with regard to gender, Hispanic ethnicity or age of the adolescents between those who participated and those who did not. Of the 340 adolescent-parent dyads, there were 31 families with 2 siblings, and 4 families with 3 siblings per family. Given that these parents were asked to describe the hypothetical study twice, only the first adolescent-parent dyad was retained for analysis. Thus, our final analysis included 301 unique adolescent-parent dyads.
The sample represented enrollment from hospital clinical settings (91%) and the remaining (9%) from snowball sampling. Demographics of the participants are presented in Table 1 reflecting a largely Hispanic sample, with the majority of adolescents reporting minimal sexual experience. The demographic characteristics of the 301 families that were analyzed were not statistically different than the total sample of 340.
Table 1.
Baseline Characteristics of Adolescents (n = 301) and Parents (n = 301)
| Adolescent Characteristics | Mean (SD) or n (%) |
|---|---|
|
| |
| Age mean (SD) | 15.5 (1.1) |
| Hispanic | 218 (72) |
| Survey completed in Spanish | 15 (5) |
| Female | 186 (62) |
| Highest level of intimacy reported by adolescent | |
| • Nothing more than kissing | 196 (65) |
| • Touching, oral, anal, or penile-vaginal sex | 105 (35) |
|
| |
| Parent Characteristics | Mean (SD) or n (%) |
|
| |
| Age mean (SD) | 43.4 (7.7) |
| Hispanic | 213 (71) |
| Survey completed in Spanish | 133 (44) |
| Relationship to adolescent | |
| • Mother | 268 (89) |
| • Father | 20 (7) |
| • Other (grandmother, grandfather, aunt, uncle, stepmom, stepfather) | 13 (4) |
| Education Level | |
| • Did not finish high school | 95 (32) |
| • Graduated high school or some college | 142 (47) |
| • Graduated college or advanced degree | 64 (21) |
| Parent’s belief that child has had touching, oral, anal, or penile-vaginal sex | |
| • No | 138 (46) |
| • Yes | 76 (25) |
| • Not sure | 87 (29) |
Overall Approach
For both adolescents and parents, the overall approach to describing the study ranged from focusing on the 1) purpose of the study, 2) the details, 3) both purpose and details, or providing a 4) commentary on the study. There were a few adolescents (n = 8) and parents (n = 3) whose responses were too vague to code in a meaningful way and hence, not included in analysis for overall approach, as seen from a 17 year-old female: “[Quiet for a while.] You can only read it, I can’t explain it.”
A typical example describing the purpose, or aim of the study was provided by a 16 year-old female, “A study to see if the gel helps to prevent any infections like STIs.” An example of a detailed response, where only specific details related to the study were mentioned came from a 15 year-old male:
It’s something you’re volunteering to do. They’re going to give you a gel for your private parts and you can’t do nothing sexual, you have to use a gel for seven days on your private parts. You have to go to 3 doctor visits. Second visit is 30 days after the first visit, and they’re for one hour long. They’re going to talk to you about your private parts.
A purpose and detailed response, where the participant discussed the purpose of the study supported by study-specific details was described by a 14 year-old female:
It’s a research study that is seeing if a gel is safe to use in adolescents and all you have to do is apply the gel - either gel - for seven days, two times a day. And all your information is confidential and you have to go through exams - gyn exams.
Finally, an example of commentary included those responses in which an emotional response was provided and the participant did not mention any study purpose or detail, as highlighted by a 49 year-old mother:
I think she would say for her daughter to participate. Adolescents are living ‘La Vida Loca’ and need different ways of protecting themselves in addition to condoms. Parents have to learn that there other ways to protect themselves. We are in the 21st century and some parents still don’t talk to children about menstrual periods.
Table 2 represents the frequency of the overall approach responses by adolescents and parents. A higher proportion of adolescents provided a ‘purpose and detail’ response than parents (54% of adolescents vs 31% of parents), and fewer adolescents provided a commentary than parents (6% of adolescents vs 28% of parents; x2(3) = 60.26, p < 0.01).
Table 2.
Distribution of Overall Approach between Adolescents and Parents
| Primary Response | Adolescents n = 293 |
Parents n = 298 |
||
|---|---|---|---|---|
|
| ||||
| n (%) | p-value | X2 (3) | ||
|
| ||||
| Purpose | 69 (24) | 70 (24) | <0.0001 | 60.26 |
| Detail | 48 (16) | 52 (17) | ||
| Purpose with Detail | 158 (54) | 92 (31) | ||
| Commentary | 18 (6) | 84 (28) | ||
None of the factors (i.e., age, gender, Hispanic ethnicity, sexual history, parental education level, or recruitment location) were related to the adolescent overall approach. When evaluating relationships with parental overall approach, two factors (recruitment site and parental education level) were significantly associated. A higher proportion of parents (52%) recruited through snowball sampling provided a ‘purpose with detail’ than parents (29%) recruited at clinic (x2(3) = 7.84, p = 0.049). A higher proportion of parents with less than a high school education (47%) provided a ‘commentary’ than parents with a college degree (13%), and a lower proportion of parents with less than a high school education (18%) answered with a ‘purpose with detail’ than parents with a college degree (47%) (x2(6) = 31.16, p < 0.01). No other factors (i.e., adolescent age or gender, age of parent, relationship to adolescent, ethnicity of parent, nor parental belief regarding adolescent sexual experience) were associated with parental overall approach.
Opinion Rendered
Regardless of the overall approach, some adolescents and parents gave an opinion about the study. Opinion was coded from each adolescent and parent response by denoting the language used within each response into four types of mutually exclusive opinions (no opinion, positive, negative or mixed – both positive and negative; see Table 3). An example of a positive opinion was described by a 35 year-old mother:
It’s a study for young men about a cream or gel to protect them in a sexual encounter. Good to try because there are so many diseases out there and HPV and HIV are coming up. It’s not a cure but it could prevent diseases.
Table 3.
Distribution of Opinion Rendered by Adolescents and Parents
| Opinion | Adolescents n = 301 |
Parents n = 301 |
||
|---|---|---|---|---|
|
| ||||
| n (%) | p-value | X2(3) | ||
|
| ||||
| None | 225 (75) | 75 (25) | <0.0001 | 151.96 |
| Positive | 49 (16) | 119 (40) | ||
| Negative | 12 (4) | 45 (15) | ||
| Mixed | 15 (5) | 62 (20) | ||
An example of a negative opinion from a 16 year-old female was:
It’s like being a prostitute! Doctors are sticking things up your vagina for research…I’d also tell them about the risk of getting an infection from the gel or an STI if the gel doesn’t work. The hospital won’t pay if you have any problems…
An example of a mixed opinion occurs when two opinions, both positive and negative, were made in a single response, as reported by a 17 year-old female, “It’s good and bad at the same time. Good because they are trying to see if the gel works, bad because of the side effects.”
When the responses for opinion were collapsed into either providing an opinion (positive, negative or mixed) versus not (no opinion), parents had a higher proportion (75%) who offered an opinion than adolescents (25%) (x2 (1) = 149.50, p < 0.01).
Details Mentioned
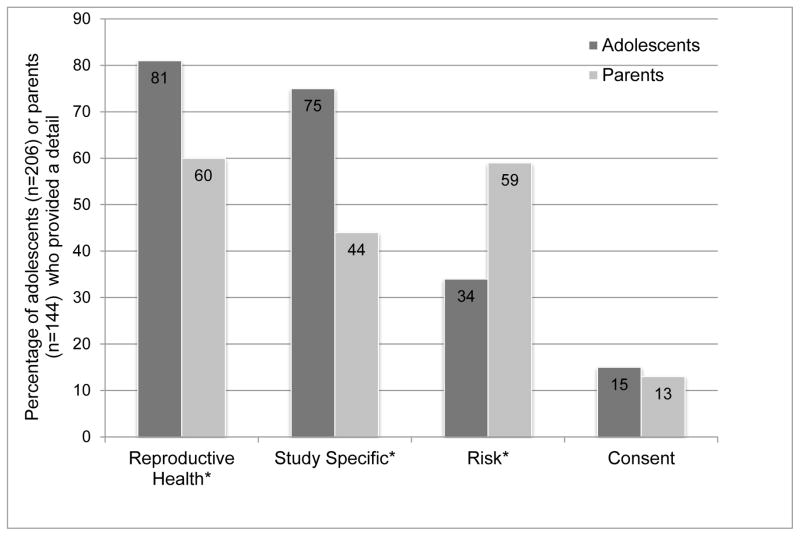
Overall, adolescents provided a detail more than parents (70% adolescents vs 48% parents; x2(1) = 25.4; p < 0.01). For purposes of examining types of details, the percentages are based only on those that provided any detail (adolescents n = 206; parents n = 144). There were 21 specific details (Table 4), which then were collapsed into four broad categories of details (reproductive health details, study-specific details, risk details, or consent details). There were too few ‘wrong’ details (n = 8) to evaluate them. This type of detail represented inaccurate information, as noted by a 14 year-old male, “Each visit is in 8 days.” Of note, one “risk” detail that was mentioned more than anticipated was “not paying for care,” which 5% of adolescents and 12% of adults mentioned.
Table 4.
Frequency of Different Types of Detail Mentioned
| Type of Detail | Adolescents n = 206 (%)a |
Parents n = 144 (%)a |
|---|---|---|
|
| ||
| Reproductive Health Detail | 166 (81) | 86 (60) |
|
| ||
| Gel use | 137 (67) | 53 (37) |
| Blood draws | 44 (21) | 21 (15) |
| Genital exams | 36 (18) | 26 (18) |
| Abstaining from sex | 33 (16) | 11 (8) |
| Other tests | 25 (12) | 10 (7) |
| Any exam | 14 (7) | 13 (9) |
| Urine test | 13 (6) | 7 (5) |
| Colposcopy or pictures | 10 (5) | 4 (3) |
| STI tests | 9 (4) | 4 (3) |
| Pregnancy test | 4 (2) | 1 (1) |
|
| ||
| Study Specific Detail | 155 (75) | 64 (44) |
|
| ||
| Number of visits | 88 (42) | 29 (20) |
| Money | 84 (41) | 20 (14) |
| Length of study | 39 (19) | 18 (13) |
| Survey questions | 22 (11) | 7 (5) |
| Pretested in adults | 22 (11) | 14 (10) |
| Number of participants | 9 (4) | 1 (1) |
| Specific age of participant | 3 (2) | 7 (5) |
|
| ||
| Risk Detail | 70 (34) | 85 (59) |
|
| ||
| Risks of study | 67 (33) | 77 (54) |
| Not paying for care | 10 (5) | 17 (12) |
|
| ||
| Consent Detail | 30 (15) | 18 (13) |
|
| ||
| Confidential or private | 16 (8) | 9 (6) |
| Voluntary | 16 (8) | 12 (8) |
|
| ||
| Wrong/Inaccurate Detailb | 4 (2) | 4 (3) |
Percentages are based only on those that provided a detail.
Wrong/inaccurate details were not included in analysis.
Figure 1 characterizes the types of details mentioned by adolescents and parents according to the above conceptual groups. Of those who provided details, adolescents provided more reproductive health details (x2(1)= 18.3, p < 0.01) and study specific details (x2(1)= 35.3, p < 0.01) and less risk details (x2(1)= 21.5, p < 0.01) than parents. Adolescents and parents were equally likely to report consent details (p < 0.58). For those participants who chose to provide a detail, adolescents provided a greater number of details than the parent group (Wilcoxon Rank, z = 4.71, p < 0.01).
Figure 1.
Type of Details Mentioned by Adolescents and Parents
*p < 0.0001
DISCUSSION
The results of this study suggest that in the context of a clinical trial in which the adolescent would be participating and the parent would need to provide consent, adolescents and parents approach the task of describing a clinical trial to a friend differently. Adolescents were more likely than parents to give the ‘purpose and detail’ of the study, which included the main point of the study as well as supporting details about the study procedures. Adolescents were also more focused than parents on the details of the study, with adolescents providing a greater number of details than parents. Specifically, adolescents focused more on reproductive health and study-specific types of details than parents. These findings suggest that adolescents may be focused on what would be expected of their friend (a potential participant) and the reasons for those activities.
On the other hand, parents were focused on their impression of the study, its value, and the risks associated with participation. This may be consistent with research which suggests that parents are focused on the overall benefit to their adolescent.24 In this study, benefits could be construed as a “preventive misperception” because there is no actual direct benefit to the adolescent. Or it could be that parents are correct in assuming that participation in a study on reproductive health has some educational benefit. Brody 25,26 suggests that risk also may be particularly important to parents for decision-making, which perhaps in this sample, suggests a higher sense of responsibility among parents.
Similarly, parents also provided an opinion (positive, negative or mixed) more often than adolescents. While we do not know if the impact of providing opinions particularly in the context of few details would encourage or discourage enrollment, understanding the role of providing opinions would be particularly important in utilizing community members for recruitment.
Even though no associations of the adolescents’ overall approach were found, parents who were recruited through snowball sampling were more likely to provide a ‘purpose and detailed’ response. This may be related to the way those that were recruited through snowball sampling first learned about the study. This warrants further investigation since parents recruited from snowball sampling described the study using a different type of response (purpose and detail) than those recruited in clinic. Furthermore, parents with less than a high school degree were more likely to provide a commentary compared to those parents with college degrees. It is unclear why this might be, but perhaps these parents were more concerned with the overall study than the specifics.
One interesting detail that was mentioned more frequently than anticipated was the “not paying for care” detail (5% of adolescents and 12% of parents). These participants found the statement concerning, and it seemed to engender a sense of potential distrust of research or of institutions. Participant’s perceptions of the “legal” or indemnification clauses in a consent form may be a preventable barrier to participation and perhaps needs greater attention during the consent process since it is used even in studies that contain no risk. Further investigation should seek to understand how to best present this information, particularly to adolescents, in an accurate and supportive manner.
The findings of this study are limited by the fact that we do not know whether individual descriptions are actually a valid proxy for saliency, nor if what was described in the context of the research study represents what adolescents and parents would actually say to a peer. We did not assess how they would respond to any questions that peers might ask, or how their description would influence potential participation. In addition, since the clinical trial being described was hypothetical, the lack of actual potential involvement may have altered their responses. Finally, our sample population primarily identified as Hispanic which is critical given the historic under-representation of Hispanics in clinical trials. However, other populations may respond differently.
Despite these limitations the findings of this study have implications for recruitment of adolescents and parents into studies of reproductive health and offers direction for future research. As might have been predicted, but rarely considered, adolescents and parents appear to be focusing on different information as they think about a study, in this case as they describe it to a friend. By respecting adolescents’ emerging capacity to make independent judgments and to ensure they have the opportunity to participate in research, an emphasis should be placed on the need to approach adolescents and parents differently about research participation.24–27
As researchers look to engage community advisory boards or use community level recruitment, understanding what adolescents and parents are likely to say to friends and helping them develop the most appropriate strategy for communication during recruitment would be beneficial. This study allows a unique glimpse into how adolescent and their parents may discuss research among their friends. Ultimately, this study shows that understanding adolescent or parent perceptions of studies may provide an opportunity to more effectively engage snowball sampling and community advisory board in the recruitment of adolescents for future reproductive health studies.
Acknowledgments
Funding: This research was supported by an R01 grant (Grant Number: 5R01HD067287-03), from the National Institutes of Health, awarded to Susan L. Rosenthal, Ph.D. and the National Center for Advancing Translational Sciences, National Institutes of Health (Grant Number: UL1 TR000040, UL1 TR000457).
We would like to acknowledge the following research coordinators and post-doctoral fellow who helped with data collection and management: Gabriela Bisono, Noe Chavez, Lauren Dapena Fraiz, Sophia Ebel, Katharine Hargreaves, and Camille Williams, and the clinic staff who helped with the recruitment of families.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among U.S. women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40:3. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Forhan SE, Gottlieb SL, Sternberg MR, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:6. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- 3.Rupp R, Stanberry LR, Rosenthal SL. New biomedical approaches for sexually transmitted infection prevention: vaccines and microbicides. Adolesc Med Clin. 2004;15:2. doi: 10.1016/j.admecli.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Eaton DK, Kann L, Kinchen S. Youth risk behavior surveillance – United States, 2011. MMWR Surveill Summ. 2012;8:4. [PubMed] [Google Scholar]
- 5.Tolley EE, Morrow KM, Owen DH. Designing a multipurpose technology for acceptability and adherence. Antiviral Res. 2013;100:S54–9. doi: 10.1016/j.antiviral.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moilanen KL. Predictors of parental consent for adolescent participation in sexual health-related research. J Empir Res Hum Res. 2015;10:2. doi: 10.1177/1556264615575510. [DOI] [PubMed] [Google Scholar]
- 7.Ott MA, Rosenberger JG, Fortenberry JD. Parental permission and perceived research benefits in adolescent STI research. J Empir Res Hum Res. 2010;5:2. doi: 10.1525/jer.2010.5.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villarruel AM, Jemmott LS, Jemmott JB, et al. Recruitment and retention of latino adolescents to a research study: lessons learned from a randomized clinical trial. J Spec Pediatr Nurs. 2006;11:4. doi: 10.1111/j.1744-6155.2006.00076.x. [DOI] [PubMed] [Google Scholar]
- 9.Robbins SCC, Rawsthorne M, Paxton K, et al. “You can help people”: adolescents’ views on engaging young people in longitudinal research. J Res Adolescence. 2011;22:1. [Google Scholar]
- 10.Giocos G, Kagee A, Swartz L. Predicting hypothetical willingness to participate (WTP) in a future phase III HIV vaccine trial among high-risk adolescents. AIDS Behav. 2008;12:6. doi: 10.1007/s10461-007-9289-5. [DOI] [PubMed] [Google Scholar]
- 11.Hoover DR, Carfioli B, Moench EA. Attitudes of adolescent/young adult women toward human papillomavirus vaccination and clinical trials. Health Care Women In. 2000;21:5. doi: 10.1080/07399330050082227. [DOI] [PubMed] [Google Scholar]
- 12.Short MB, Wiemann C, Rosenthal SL. Participation of adolescent girls in a study of sexual behaviors: balancing autonomy and parental involvement. J Pediatr Adolesc Gynecol. 2009;22:2. doi: 10.1016/j.jpag.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chávez NR, Williams CY, Ipp LS, et al. Altruistic Reasoning in Adolescent-Parent Dyads Considering Participation in a Hypothetical Sexual Health Clinical Trial for Adolescents. Research Ethics. 2015 doi: 10.1177/1747016115587963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanford PD, Monte DA, Briggs FM, et al. Recruitment and retention of adolescent participants in HIV research: findings from the REACH (reaching for excellence in adolescent care and health) project. J Adolescent Health. 2003;32:3. doi: 10.1016/s1054-139x(02)00392-0. [DOI] [PubMed] [Google Scholar]
- 15.Weimann CM, Chacko MR, Tucker JC, et al. Enhancing recruitment and retention of minority young women in community-based clinical research. J Pediatr Adolesc Gynecol. 2005;18:6. doi: 10.1016/j.jpag.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Jones FC, Broome ME. Focus groups with African American adolescents: enhancing recruitment and retention in intervention studies. J Pediatr Nurs. 2001;16:2. doi: 10.1053/jpdn.2001.23151. [DOI] [PubMed] [Google Scholar]
- 17.Alexander AB, Ott MA, Lally MA, et al. Adolescent trials network for HIV/AIDS interventions. Vaccine. 2015;10:33. [Google Scholar]
- 18.Ruhe KM, Wangmo T, Badarau DO, et al. Decision-making capacity of children and adolescents-suggestions for advancing the concept’s implementation in pediatric healthcare. Eur J Pediatr. 2015;174:6. doi: 10.1007/s00431-014-2462-8. [DOI] [PubMed] [Google Scholar]
- 19.Lipstein EA, Brinkman WB, Fiks AG, et al. An emerging field of research: challenges in pediatric decision making. Med Decis Making. 2015;35:3. doi: 10.1177/0272989X14546901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckathorn DD. Snowball versus respondent-driven sampling. Social Methodol. 2011;41:1. doi: 10.1111/j.1467-9531.2011.01244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Institute of Mental Health (NIMH) Multisite HIV/STD Prevention Trial. The role of community advisory boards (CABs) in project eban. J Acquir Immune Defic Syndr. 2008;49:1. doi: 10.1097/QAI.0b013e31818447f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadler GR, Lee HC, Lim RSH, et al. Recruitment of hard-to-reach population subgroups via adaptations of the snowball sampling strategy. Nurs Health Sci. 2010;12:3. doi: 10.1111/j.1442-2018.2010.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley EH, Curry LA, Devers JK. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Serv Res. 2007;42:4. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherer DG, Annett RD, Brody JL. Ethical issues in adolescent and parent informed consent for pediatric asthma research participation. J Asthma. 2007;44:7. doi: 10.1080/02770900701247137. [DOI] [PubMed] [Google Scholar]
- 25.Brody JL, Turner CW, Annett RD, et al. Predicting adolescent asthma research participation decisions from a structural equations model of protocol factors. J Adolesc Health. 2012;51:3. doi: 10.1016/j.jadohealth.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brody JL, Annett RD, Scherer DG, et al. Comparisons of adolescent and parent willingness to participate in minimal and above-minimal risk pediatric asthma research protocols. J Adolesc Health. 2005;37:3. doi: 10.1016/j.jadohealth.2004.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipstein EA, Brinkman WB, Fiks AG, et al. An emerging field of research: challenges in pediatric decision making. Med Decis Making. 2015;35:3. doi: 10.1177/0272989X14546901. [DOI] [PMC free article] [PubMed] [Google Scholar]