Abstract
Background
Natural disasters expose entire communities to stress and trauma, leading to increased risk for psychiatric symptoms. Yet, the majority of exposed individuals are resilient, highlighting the importance of identifying underlying factors that contribute to outcomes.
Methods
The current study was part of a larger prospective study of children in Long Island, NY (N = 260). At age 9, children viewed unpleasant and pleasant images while the late positive potential (LPP), an event-related potential (ERP) component that reflects sustained attention towards salient information, was measured. Following the ERP assessment, Hurricane Sandy, the second costliest hurricane in U.S. history, hit the region. Eight weeks after the hurricane, mothers reported on exposure to hurricane-related stress and children’s internalizing and externalizing symptoms. Symptoms were re-assessed 8 months after the hurricane.
Results
The LPP predicted both internalizing and externalizing symptoms after accounting for pre-hurricane symptomatology, and interacted with stress to predict externalizing symptoms. Among children exposed to higher levels of hurricane-related stress, enhanced neural reactivity to unpleasant images predicted greater externalizing symptoms 8 weeks after the disaster, while greater neural reactivity to pleasant images predicted lower externalizing symptoms. Moreover, interactions between the LPP and stress continued to predict externalizing symptoms 8 months after the hurricane.
Conclusions
Results indicate that heightened neural reactivity and attention towards unpleasant information, as measured by the LPP, predisposes children to psychiatric symptoms when exposed to higher levels of stress related to natural disasters, while greater reactivity to and processing of pleasant information may be a protective factor.
Keywords: stress, vulnerability, internalizing, externalizing, natural disaster, event-related potentials
Exposure to stress in childhood and adolescence prospectively predicts increases in both internalizing and externalizing symptoms across development (for a review, 1). At the same time, most children experience some level of stress and remain resilient; thus, identifying vulnerability factors that moderate the association between stress and the development of psychopathology is of paramount public health importance (2; 3).
Natural disasters are one stressor linked to increased risk of both internalizing (e.g., post-traumatic stress, depression, anxiety) and externalizing symptoms (e.g., oppositional and aggressive behavior) that may persist for months or even years after the disaster (4–11). Individuals who experience a greater number of disaster-related stressors tend to exhibit higher levels of symptoms (10; 12; 13). Nonetheless, only a minority of those exposed to natural disasters will develop serious psychological symptoms (14).
Natural disasters are circumscribed in time and affect entire communities; thus, they are less likely than other stressors to be confounded with pre-existing child and family characteristics. Examining children exposed to disasters can provide insights into neural mechanisms underlying risk and resilience (15; 16). However, as disasters typically occur with little warning, it is often impossible to determine whether factors that moderate children’s responses reflect vulnerabilities that predispose to symptoms or consequences of stress exposure.
Most studies have focused on psychosocial factors that influence reactivity to stress in youth experiencing natural disasters. For example, child temperament and symptoms of psychopathology prior to the disaster predict symptoms following the disaster (13; 15; 17; 18). In addition, social influences contribute to responses to disasters: greater social support is a protective factor, whereas negative family dynamics predict poorer outcomes (11; 18; 19).
A few prospective functional magnetic resonance imaging (fMRI) studies have evaluated neural processes that predispose to developing symptoms in response to trauma, including war and terrorism (20; 21) and general life stress (22), but not natural disasters. The little existing evidence suggests that amygdala hyper-reactivity to threat may contribute to risk (20–22). For example, in a small fMRI study of adolescents, greater amygdala reactivity to threatening images prospectively predicted increased post-traumatic stress symptoms after the Boston Marathon bombing (20). In addition, a large fMRI study of adults indicated that greater amygdala reactivity to threat interacted with stressful life events 1–4 years later to predict increased internalizing symptoms (22).
The late positive potential (LPP) is an event-related potential (ERP) component that provides a neural measure of emotional processing that can be reliably assessed across development (23). The LPP is a relative positivity in the ERP that begins as early as 200 ms after stimulus onset and is increased for emotional compared to neutral stimuli; this emotional modulation of the LPP is pronounced throughout the duration of picture presentation and reflects sustained attention towards motivationally salient information (24–26).
Abnormalities in the LPP are associated with internalizing disorders, such as anxiety and depression (27–33), as well as externalizing symptoms in both youth and adults (34; 35). Cross-sectional research suggests the LPP may measure emotional processing styles that contribute to vulnerability for psychopathology. For example, offspring of parents with fear disorders (i.e., panic disorder, social anxiety disorder, and specific phobia) and children with fearful temperament showed enhanced LPPs to unpleasant stimuli (36; 37). In addition, blunted LPPs to emotional faces have been observed in youth at risk for depression based both on parental history of depression and temperament style (37–39). Importantly, there is evidence that individual differences in processing and coping with emotions contribute to children’s responses to stress (40), suggesting that the magnitude of the LPP may moderate effects of stress on the development of symptoms. However, no research has investigated whether neural processing of emotion, as measured by the LPP, prospectively predicts changes in symptoms in children, either alone or in conjunction with stress.
The current study examined neural reactivity to emotional images as a vulnerability for psychiatric symptoms following a natural disaster. Hurricane Sandy struck the Long Island, NY region on October 29, 2012, destroying 100,000 homes, and is estimated to be the second costliest hurricane in United States history, after Hurricane Katrina (41). As part of a pre-existing study, a large sample of 9-year-old children completed an emotional processing task in which ERPs were recorded as participants viewed pleasant, neutral, and unpleasant images. In addition, mothers reported on child internalizing and externalizing symptoms. Approximately 8 weeks after Hurricane Sandy, participants’ mothers completed a questionnaire assessing exposure to hurricane-related stressors and children’s symptoms. To assess long-term impact, mothers completed questionnaires again approximately 8 months after the hurricane. We evaluated neural reactivity to emotional images (i.e., LPP) as a prospective predictor of symptoms after the hurricane, both as a main effect and interaction with hurricane-related stress. We hypothesized that an enhanced LPP to unpleasant images would predict a greater increase in symptoms, and consistent with vulnerability-stress models (3), that these effects would be most apparent among children who experienced higher levels of stress. Additional exploratory analyses evaluated whether the LPP to pleasant images also predicted symptoms.
Method and Materials
Participants
Participants were 9–12 year olds from a larger prospective community sample of children, initially recruited between 3–6 years old. All children living with at least one English-speaking biological parent and free of significant medical or developmental disabilities were eligible (42). A total of 323 children completed the emotional interrupt EEG task and pre-hurricane symptom measures, were in the area at the time of Hurricane Sandy, and completed the 8-week post-hurricane questionnaire. Of these participants, 63 were excluded for excessively noisy EEG data, fewer than 15 correct, artifact-free trials per condition and/or poor accuracy (<65%) on the emotional interrupt task. The excluded sample had higher mother-reported internalizing, t(321) = 2.70, p = .01; mean difference = 2.47, and externalizing symptoms, t(321) = 3.13, p < .01; mean difference = 2.93, than the included sample prior to the hurricane, and the groups continued to differ on symptoms at the initial post-hurricane assessment (ps < .05) but did not significantly differ on hurricane-related stress (p = .16). The final sample included 260 children with a mean age of 9.16 (SD = .34) at the EEG assessment and 10.41 (SD = .78) at the 8-week post-hurricane assessment. Participants were 45.8% female; 89.6% Caucasian, 6.9% African American, 3.1% Asian, and 0.4% Native American; 8.1% identified as Hispanic/Latino.
Procedure
Study protocols were approved by the Institutional Review Board at Stony Brook University. Informed consent was obtained from all parents and verbal assent from children. Participants and one parent visited the laboratory as close as possible to the child’s 9th birthday to complete the EEG assessment and measures of child symptoms. Following the hurricane, mothers were asked to complete an online questionnaire to assess hurricane-related stressors and child’s symptoms. On average, these questionnaires were completed 8.52 weeks (SD = 1.55) following the hurricane and 53.58 weeks (SD = 31.69) after the EEG assessment. Seven months after the hurricane, mothers were again asked to report on current child symptoms. On average, this questionnaire was completed 7.69 months (SD = 0.81) after the hurricane (58 mothers did not complete the follow-up questionnaire, leaving data for 202 children).
Measures
Emotional interrupt task
Prior to the hurricane, participants completed a version of the emotional interrupt paradigm (43; 44) previously used to measure the LPP in youth (37; 45). Sixty developmentally appropriate pictures from the International Affective Picture System (IAPS; 46) were presented: 20 pleasant images (e.g., children playing, cute animals), 20 neutral images (e.g., people in neutral situations, household objects), and 20 unpleasant or threatening images (e.g., sad/angry people, aggressive animals, weapons). See Supplemental Information for image numbers. Each image was randomly presented once in each of two blocks for a total of 120 trials. Each trial began with an 800 ms fixation, then an image for 1000 ms followed by a target for 150 ms and the same picture for an additional 400 ms. The target was an arrow pointed to the left or right, and participants pressed the left or right mouse button to indicate the direction of the arrow. Intertrial interval varied randomly between 1500 and 2000 ms.
Electroencephalogram (EEG) data acquisition/analysis
EEG was recorded using a 34-channel Biosemi system (32 channel cap plus Iz and FCz; 10/20 system). Ground electrode was formed by the Common Mode Sense and Driven Right Leg electrodes. Two electrodes were placed on the left/right mastoids, and electrooculogram (EOG) was recorded from facial electrodes one cm above and below the left eye, one cm to the left of the left eye and one cm to the right of the right eye. Data were digitized at 24-bit resolution with a LSB value of 31.25nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with −3dB cutoff points at 208 Hz.
Off-line analysis was performed using Brain Vision Analyzer software (Brain Products). Data were converted to an averaged mastoid reference, band-pass filtered from 0.1 to 30 Hz, segmented for each trial 200 ms before picture onset to 1000 ms after onset. Data were corrected for eye blinks (47), and artifacts were removed using semiautomated procedures to identify: voltage step of more than 50 μV between sample points, voltage difference of 300 μV within a trial, and voltage difference of less than .50 μV within 100 ms intervals. Visual inspection was used to remove additional artifacts. Data were baseline corrected to 200 ms prior to stimulus onset.
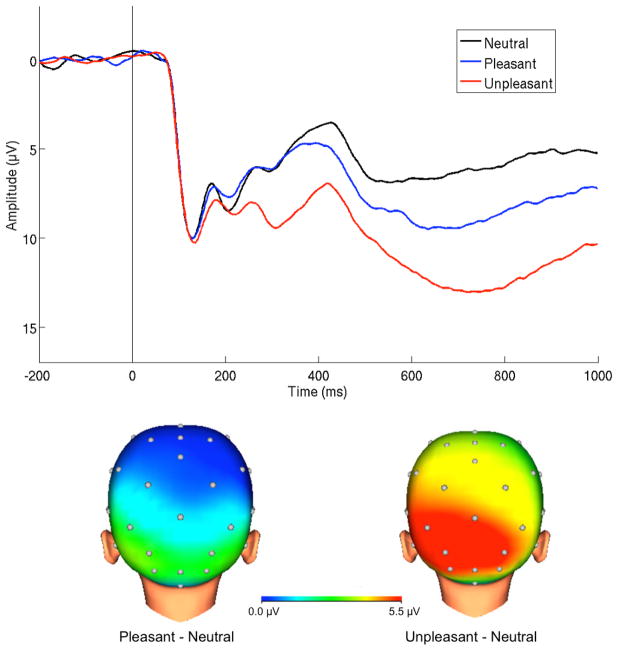
ERPs were averaged across unpleasant, pleasant and neutral trials. To ensure that children were attending to the images, only correct trials were included in averages. The LPP was scored as the mean amplitude where the differences between emotional and neutral images were maximal (Figure 1): 400–1000 ms after feedback at a pooling of occipital and parietal sites (O1, O2, Oz, PO3, PO4, P3, P4, Pz; 29). The emotional (pleasant or unpleasant) minus neutral difference score was computed and used for analyses to isolate neural activity modulated by emotional content.
Figure 1.
ERPs (negative up) at occipital-parietal sites and scalp distributions depicting reactivity to emotional compared to neutral images 400–1000 ms after onset in the overall sample.
Hurricane-related stress
Mothers completed a 13-item questionnaire assessing experiences that are common during hurricane-related disasters with potential impacts on children. The items were drawn from questionnaires administered in studies of Hurricane Ike (48) and Hurricane Katrina (49). Reports of hurricane-related experiences have previously been demonstrated to be stable across time (50). Eight items (life disrupted by hurricane, children fearing for safety, difficulty finding food or warmth, difficulty finding gasoline, children complaining more than usual, damage to home or possessions, family’s safety threatened, and financial hardship) were rated on a 5-point scale (1 = not at all affected; 5 = extremely affected). Two items were rated on duration (time school was closed, time without power), and 3 items were rated as present/absent (evacuating the home, using FEMA services, and injury or robbery of family or friends). To create an overall sum of exposure severity (ranging from 0–13), non-dichotomous items were rescored such that 1 = present and 0 = absent. For items rated on a 5-point scale, scores of 3+ indicating moderate-extreme difficulty were scored as 1. Very commonly endorsed items (i.e., life disrupted, difficulty finding gasoline) required a higher threshold (scores of 4+ and 5+, respectively) to be scored as present in order to ensure sufficient variability. For duration items, scores indicating one week or longer were scored as 1. The final scale demonstrated good internal consistency (Cronbach’s alpha = .72).
Child psychiatric diagnoses and symptoms
For rates of pre-hurricane clinical disorders, one parent and the child were interviewed by advanced clinical psychology doctoral students or Master’s level clinicians using the Schedule of Affective Disorders and Schizophrenia for School-Age Children (K-SADS; 51) at the age 9 assessment (see 52 for more information).
To measure change in symptoms, mothers completed the Child Behavior Checklist (CBCL), a 113-item parent-report measure of behavioral and emotional problems in children (53). To measure the broad range of possible reactions in children following a disaster, analyses focused on the higher order externalizing and internalizing scales. Mothers completed the CBCL on 3 occasions: age 9 assessment to control for pre-hurricane symptoms, approximately 8 weeks after the hurricane, and approximately 8 months following the hurricane.
Data analysis
Hierarchical regression analyses were computed to examine predictors of symptoms following Hurricane Sandy. Variables were centered to evaluate interactions. Pre-hurricane internalizing and externalizing symptoms and child sex was entered into Step 1, followed by main effects of hurricane-related stress, unpleasant and pleasant LPP in Step 2, and interactions between stress and LPP in Step 3. To account for non-normality and heteroscedasticity in CBCL symptoms, we computed heteroscedasticity-consistent standard errors and p values (HC3; 54) for all regression analyses.
Results
Clinical Characteristics
At the assessment prior to the hurricane, 15.8% (n = 41) of the sample met current criteria for an internalizing or externalizing disorder. Specifically, 0.8% had a mood disorder, 8.8% anxiety disorder (0.8% separation anxiety disorder, 2.3% social anxiety disorder, 3.8% specific phobia, 3.1% generalized anxiety disorder), 6.5% attention deficit hyperactivity disorder, 1.5% oppositional defiant disorder, and 0.4% conduct disorder.
Descriptive statistics and correlations between measures are presented in Table 1. Both externalizing, t(259) = 7.09, p < .001, and internalizing, t(259) = 5.92, p < .001, symptoms decreased from pre- to post-hurricane, possibly due to repeated administration of the questionnaire (55), and indicating that the hurricane did not increase symptoms overall. Rates of borderline or clinical levels of symptoms on externalizing and internalizing CBCL scales at the 8-week post-hurricane assessment were 4.2% and 4.6%, respectively. Males exhibited higher post-hurricane externalizing symptoms than females, but sex was not related to pre-hurricane externalizing or internalizing symptoms at any assessment. Externalizing and internalizing symptoms were moderately to strongly correlated at each assessment and across time. Associations between hurricane-related stress and internalizing and externalizing symptoms 8 weeks following the hurricane were modest but significant.
Table 1.
Descriptive Statistics and Bivariate Correlations between Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 Sex | - | |||||||||
| 2 Pre-hurricane CBCL-EXT | −.06 | - | ||||||||
| 3 Pre-hurricane CBCL-INT | .01 | .48*** | - | |||||||
| 4 Hurricane Stress | −.07 | .10 | .00 | - | ||||||
| 5 Unpleasant LPP | −.01 | .00 | −.08 | .06 | - | |||||
| 6 Pleasant LPP | .01 | −.01 | −.07 | .01 | .49*** | - | ||||
| 7 Post-hurricane CBCL-EXT | −.15* | .56*** | .25*** | .19** | .09 | −.10 | - | |||
| 8 Post-hurricane CBCL-INT | −.03 | .27*** | .42*** | .18** | .09 | −.05 | .66*** | - | ||
| 9 8-Month CBCL-EXT | −.17* | .63*** | .36*** | .04 | .02 | −.14* | .82*** | .46*** | - | |
| 10 8-Month CBCL-INT | −.10 | .39*** | .60*** | .03 | .02 | −.09 | .55*** | .63*** | .65*** | - |
|
| ||||||||||
| M(SD) | 3.97(4.59) | 3.45(4.24) | 2.38(2.30) | 5.53(6.53) | 2.34(6.37) | 2.20(3.81) | 1.95(3.20) | 2.55(4.31) | 2.05(3.44) | |
p < .001;
p < .01;
p • .05;
CBCL-EXT = Child Behavior Checklist Externalizing Scale; CBCL-INT = Child Behavior Checklist Internalizing Scale; LPP = late positive potential
Behavioral Measures
Accuracy was lower for both unpleasant (M = 84.8%, SD = 8.98) and pleasant (M = 84.9%, SD = 9.12) compared to neutral trials (M = 89.0%, SD = 7.53), t(259) = 8.75, p < .001 and t(259) = 8.30, p < .001, respectively. Reaction time (RT) was longer for both unpleasant (M = 610.31 ms, SD = 154.05) and pleasant (M = 609.91, SD = 151.40) compared to neutral trials (M = 581.17, SD = 139.57), t(259) = 10.04, p < .001 and t(259) = 10.34, p < .001, respectively. Hierarchical multiple regression analyses were computed to evaluate whether emotional interference on behavioral responses (e.g., unpleasant RT minus neutral RT) predicted symptoms following the hurricane. None of the main effects of RT or accuracy or interactions with stress were significant in predicting symptoms (ps > .12).
LPP Predicting Post-Hurricane Symptoms
Results of multiple regression analyses to evaluate effects of the LPP on symptoms 8 weeks following the hurricane are presented in Table 2. For externalizing symptoms, the main effects of stress, unpleasant LPP and pleasant LPP were significant but were qualified by significant stress X unpleasant LPP, t(259) = 2.71, p < .01, and stress X pleasant LPP, t(259) = −2.85, p < .01, interactions. An enhanced unpleasant LPP predicted greater externalizing symptoms with high (+1 SD) stress (simple slope = .21, SE = .06, t = 3.73, p < .001) and average stress (simple slope = .11, SE = .03, t = 3.44, p < .001), but not low (−1 SD) stress (p = 0.63). An enhanced pleasant LPP predicted lower externalizing symptoms with high (simple slope = −.23, SE = .07, t = −3.48, p < .001) and average stress (simple slope = −.12, SE = .04, t = −2.67, p < .001, but not low stress (p = .90; Figures 2 and 3).
Table 2.
Hierarchical Multiple Regression Analyses Prospectively Predicting Symptoms Following Hurricane Sandy (N = 260)
| Post-hurricane CBCL-EXT | Post-hurricane CBCL-INT | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Predictor | •R2 | β | b(HC-SE) | •R2 | β | b(HC-SE) |
| Step 1 | .33 | .18 | ||||
| Sex | .12 | .89(.38)* | .03 | .20(.37) | ||
| Pre-hurricane CBCL-INT | −.01 | −.01(.06) | .38 | .29(.13)* | ||
| Pre-hurricane CBCL-EXT | .56 | .46(.09)*** | .09 | .06(.06) | ||
| Step 2 | .04 | .05 | ||||
| Hurricane stress | .12 | .20(.09)* | .16 | .23(.10)* | ||
| Unpleasant LPP | .16 | .10(.03)** | .16 | .08(.04)* | ||
| Pleasant LPP | −.17 | −.10(.04)* | −.10 | −.05(.04) | ||
| Step 3 | .03 | .03 | ||||
| Stress X Unpleasant LPP | .15 | .04(.015)** | .17 | .04(.025)‡ | ||
| Stress X Pleasant LPP | −.19 | −.05(.02)** | −.12 | −.03(.02) | ||
| Total Model | .40 | .25 | ||||
p < .001;
p < .01;
p • .05;
p < .10;
CBCL-EXT = Child Behavior Checklist Externalizing Scale; CBCL-INT = Child Behavior Checklist Internalizing Scale; LPP = late positive potential; HC-SE = heteroscedasticity-consistent standard error
Figure 2.
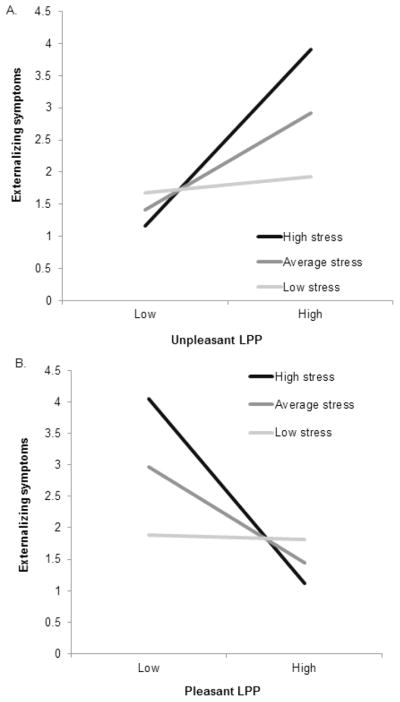
Interactions between stress and unpleasant LPP predicting externalizing symptoms following the hurricane (A), and stress and pleasant LPP predicting externalizing symptoms following the hurricane (B).
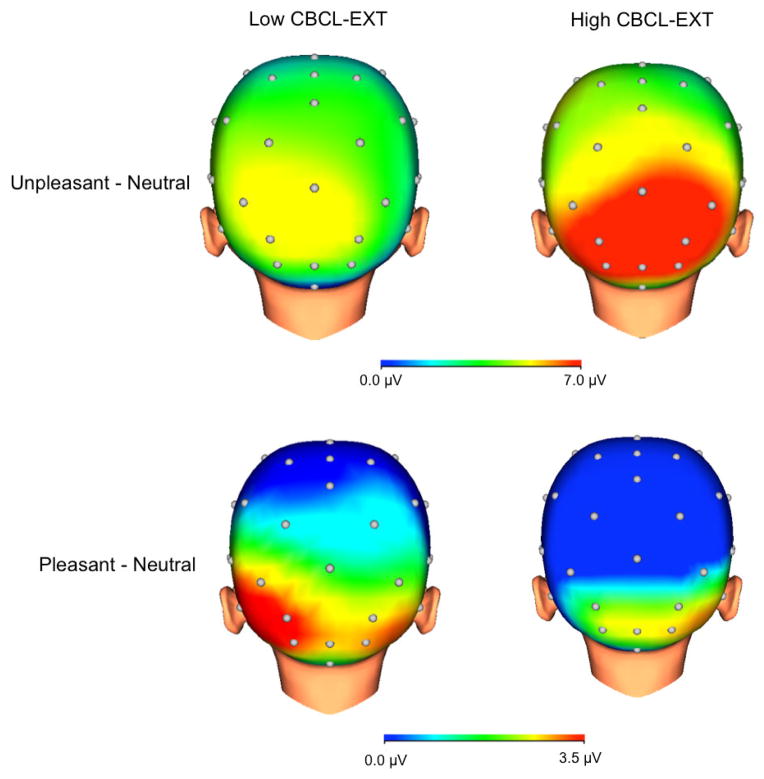
Figure 3.
Scalp distributions depicting reactivity to unpleasant (top) and pleasant (bottom) compared to neutral images among children with high (above median) exposure to stress. Children on the left exhibited low (below median) externalizing symptoms (CBCL-EXT) after the hurricane controlling for symptoms prior to the hurricane (n = 37), whereas children on the right exhibited high (above median) CBCL-EXT symptoms (n = 38).
For internalizing symptoms, more hurricane-related stress, t(259) = −2.37, p = .02, and larger LPPs to unpleasant stimuli, t(259) = 2.12, p = .04, predicted greater symptoms. The stress X unpleasant LPP interaction approached significance for internalizing symptoms (p < .10). See Supplement for analyses of CBCL subscales and scatter plots.
In order to evaluate whether effects were driven by children with clinical disorders prior to the hurricane, we computed these models excluding 41 children with clinical diagnoses prior to the hurricane. Both the stress X unpleasant LPP, b = .04, t(218) = 2.39, p = .02, and stress X pleasant LPP, b = −.04, t(218) = −2.15, p = .03, interaction effects on externalizing symptoms remained significant.
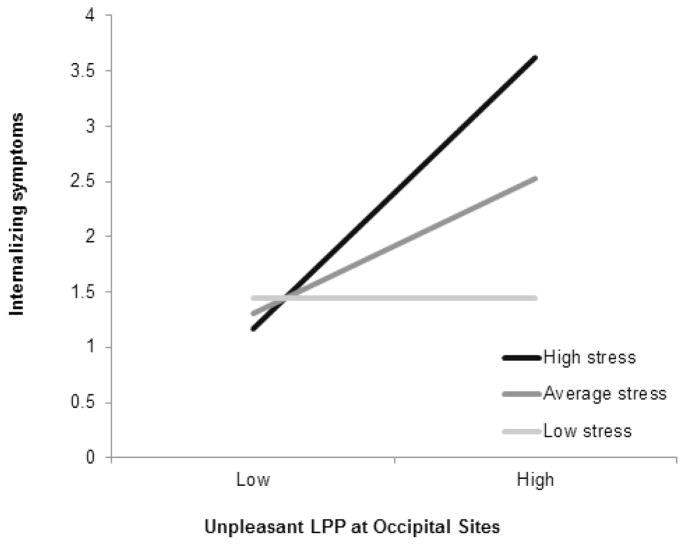
Given evidence of developmental changes in the scalp distribution of the LPP (23), we also evaluated the LPP prediction models separately for the LPP at parietal (P3, P4, and Pz) and occipital (O1, O2, and Oz) sites. The stress X unpleasant LPP and stress X pleasant LPP interactions were significant for externalizing symptoms at both parietal and occipital sites (see Supplement). For internalizing symptoms, the stress X unpleasant LPP interaction reached significance at occipital, b = .04, t(259) = 2.03, p = .04, but not parietal sites. An enhanced unpleasant LPP at occipital sites predicted greater internalizing symptoms at high (simple slope = .17, SE = .06, t = 2.71, p < .01) and average (simple slope = .08, SE = .03, t = 2.61, p < .01) but not low levels of stress (p = .98; Figure 4).
Figure 4.
Interaction between stress and unpleasant LPP scored at occipital sites (O1, O2, Oz) predicting externalizing symptoms following the hurricane.
We also examined models controlling for income and time between the age 9 and hurricane assessment with no substantive change in results, and additional exploratory analyses examined interactions with sex and the LPP to neutral images (see Supplement).
LPP Predicting Symptoms 8 Months Following the Hurricane
Models evaluating long-term impact on symptoms 8 months following the hurricane are presented in Table 3. The main effects of stress reported 8 weeks after the hurricane were no longer significant in predicting symptoms (ps > .80). Both the stress X unpleasant LPP, t(201) = 2.15, p = .03, and stress X pleasant LPP, t(201) = −1.98, p < .05, interactions remained significant predictors of externalizing symptoms. Similar to the earlier assessment, an enhanced unpleasant LPP predicted greater externalizing symptoms with high (simple slope = .21, SE = .07, t = 3.11, p < .01) and average stress (simple slope = .11, SE = .05, t = 2.44, p = .02), but not low stress (p = 0.86). An enhanced pleasant LPP continued to predict lower externalizing symptoms at high (simple slope = −.23, SE = .07, t = −3.16, p < .01) and average stress (simple slope = −.14, SE = .05, t = −2.62, p < .01), but not low stress (p = .50). None of the LPP effects reached significance for internalizing symptoms, though the main effect of unpleasant LPP approached significance (p = .08).
Table 3.
Hierarchical Multiple Regression Analyses Prospectively Predicting Symptoms 8 Months after the Hurricane (N = 202)
| 8-Month Follow-up CBCL-EXT | 8-Month Follow-up CBCL-INT | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Predictor | •R2 | β | b(HC-SE) | •R2 | β | b(HC-SE) |
| Step 1 | .41 | .38 | ||||
| Sex | .10 | .84(.49) ‡ | .07 | .50(.40) | ||
| Pre-hurricane CBCL-INT | .08 | .09(.10) | .55 | .48(.09)*** | ||
| Pre-hurricane CBCL- | .57 | .53(.10)*** | .11 | .08(.06) | ||
| EXT | ||||||
| Step 2 | .03 | .01 | ||||
| Hurricane Stress | −.01 | −.02(.09) | .01 | .02(.09) | ||
| Unpleasant LPP | .14 | .10(.05)* | .13 | .07(.04)‡ | ||
| Pleasant LPP | −.19 | −.13(.05)* | −.12 | −.06(.05) | ||
| Step 3 | .01 | .01 | ||||
| Stress X Unpleasant LPP | .13 | .04(.02)* | .10 | .02(.02) | ||
| Stress X Pleasant LPP | −.14 | −.04(.02)* | −.10 | −.02(.02) | ||
| Total Model | .45 | .40 | ||||
p < .001;
p < .01;
p • .05;
p < .10;
CBCL-EXT = Child Behavior Checklist Externalizing Scale; CBCL-INT = Child Behavior Checklist Internalizing Scale; LPP = late positive potential; HC-SE = heteroscedasticity-consistent standard error
Discussion
The current study evaluated neural reactivity to pleasant and unpleasant images as a prospective predictor of children’s symptoms after Hurricane Sandy. A larger LPP to unpleasant images predicted greater internalizing and externalizing symptoms 8 weeks after the hurricane, whereas an enhanced LPP to pleasant images predicted lower externalizing symptoms. Moreover, the LPP interacted with hurricane-related stress to predict changes in externalizing symptoms. For children exposed to higher levels of stress, enhanced reactivity to unpleasant images predicted greater symptoms, and enhanced reactivity to pleasant images predicted lower externalizing symptoms. Interactions between the LPP and stress were most apparent for externalizing, rather than internalizing symptoms, with the interaction between stress and unpleasant LPP only reaching significance for internalizing symptoms when scored at occipital sites. Both unpleasant and pleasant LPPs continued to predict long-term effects on externalizing symptoms 8 months after the hurricane, though effects were no longer apparent for internalizing symptoms. Importantly, the effects of the LPP in predicting symptoms were apparent at both high and average stress exposure, indicating that neural processing of emotional information shapes responses to even moderate levels of stress.
The LPP reflects sustained attention towards salient information, and activation of motivational systems in the brain (24; 25; 56). It has previously been demonstrated that attending to more emotional or arousing aspects of a stimulus enhances the LPP, while employing reappraisal or other emotional regulation strategies reduces the magnitude of the LPP (25; 57–63). Thus, children with an enhanced LPP to unpleasant images likely attend more to negative or threatening aspects of a situation or have difficulties regulating negative emotional responses, processes that predispose to greater symptoms of psychopathology following stressors.
Consistent with interpretations of the LPP as reflecting attention towards emotional information, the LPP has been linked to activation in the visual cortex, as well as increased bidirectional coupling between occipitoparietal and frontal cortex in response to emotional stimuli (64–66). In combined fMRI-ERP studies, the LPP has also been correlated with activation in subcortical regions, including amygdala and insula (67; 68). The current results suggest that activation in this extensive brain network when processing emotional information contributes to risk for psychopathology, findings that may be consistent with previous evidence that heightened amygdala activation to threat is associated with greater risk of symptoms following stress (20–22).
In addition to reactivity to unpleasant information, our study is unique in examining the effects of processing pleasant stimuli on subsequent symptoms. The results suggest that neural reactivity to pleasant information may contribute to resilience in response to stress. Children with enhanced LPPs to pleasant images may demonstrate greater attentional allocation towards positive aspects of the environment, which is likely to be protective under conditions of higher stress. It is important to note, however, that blunted LPPs to emotional images appear to be a risk factor for depressive disorders in youth (37–39), possibly indicating tendencies for emotional disengagement. Thus, an alternative explanation is that withdrawal from positive stimuli increases risk for symptoms. Importantly, both the pleasant and unpleasant LPP demonstrated opposite and unique effects on externalizing symptoms, suggesting that they may measure distinct aspects of emotional processing that contribute to risk and resilience.
Interestingly, concurrent associations between the LPP and symptoms were not significant, but the effect of the LPP to emotional images in predicting symptoms following the hurricane was comparable in magnitude to the effects of hurricane-related stress. In addition, at the 8-month follow-up assessment, mother-reported stress exposure immediately following the hurricane was no longer a significant independent predictor of symptoms, though the LPP continued to predict externalizing symptoms. Taken together, these findings indicate that rather than being a correlate of psychiatric symptoms, individual differences in neural reactivity to emotion reflect a vulnerability contributing to both short- and longer-term outcomes following stress exposure.
A few limitations of the study should be noted. First, analyses focused on maternal report of stress exposure and symptoms, which are limited with regard to measuring child experiences, though parent reports may be more valid for externalizing symptoms (69). Second, the effects of the LPP on symptoms were small in magnitude; however, both the pleasant and unpleasant LPP accounted for unique variance in predicting externalizing symptoms, and outcomes of disasters likely depend on the combination of a range of risk and resilience factors (14). Third, as the current study focused on broad measures of internalizing and externalizing symptoms, additional work is needed to examine whether similar patterns are observed in predicting PTSD symptoms specifically. Lastly, most of the children exhibited subclinical levels of symptoms after the hurricane, and as the post-hurricane assessment relied on questionnaire measures rather than clinical interviews, we were unable to evaluate change in clinical diagnoses. Though subthreshold symptoms are a strong predictor of full-threshold disorders (70; 71), additional work is needed to evaluate whether our results generalize to prediction of clinical disorders.
In conclusion, this is the first study to demonstrate that a neural measure predicts children’s adjustment following exposure to a natural disaster, and that these effects persist for at least 8 months. These findings highlight the importance of considering individual differences in neural reactivity to both pleasant and unpleasant emotional information in identifying children at risk of developing symptoms after exposure to disasters, though future work should examine whether similar patterns are observed with other measures of emotional reactivity and optimize methods for screening and assessing individuals. The current findings also provide a specific neural marker to target for early intervention or prevention, with future research needed to develop interventions that impact the LPP.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health Grants RO1 MH069942 to Daniel N. Klein, and F31 MH09530701 to Autumn Kujawa.
Footnotes
Financial Disclosures
Dr. Gabrielle Carlson reports receiving research funding from GlaxoSmithKline, Otsuka/Bristol-Myers Squibb, Pfizer, and Merck/Schering Plough. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant KE, McMahon SD, Carter JS, Carleton RA, Adam EK, Chen E. The influence of stressors on the development of psychopathology. In: Lewis M, Rudolph KD, editors. Handb Dev Psychopathol. 3. New York: Springer; 2014. pp. 205–224. [Google Scholar]
- 2.Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY. Stressors and child and adolescent psychopathology: Measurement issues and prospective effects. J Clin Child Adolesc Psychol. 2004;33:412–425. doi: 10.1207/s15374424jccp3302_23. [DOI] [PubMed] [Google Scholar]
- 3.Ingram RE, Luxton DD. Vulnerability-stress models. In: Hankin BL, Abela JRZ, editors. Dev Psychopathol A vulnerability-stress Perspect. Thousand Oaks, CA US: Sage Publications, Inc; 2005. pp. 32–46. [Google Scholar]
- 4.Becker-Blease KA, Turner HA, Finkelhor D. Disasters, victimization, and children’s mental health. Child Dev. 2010;81:1040–52. doi: 10.1111/j.1467-8624.2010.01453.x. [DOI] [PubMed] [Google Scholar]
- 5.Marsee MA. Reactive aggression and posttraumatic stress in adolescents affected by Hurricane Katrina. J Clin Child Adolesc Psychol. 2008;37:519–529. doi: 10.1080/15374410802148152. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin KA, Fairbank JA, Gruber MJ, Jones RT, Lakoma MD, Pfefferbaum B, et al. Serious emotional disturbance among youths exposed to Hurricane Katrina 2 years postdisaster. J Am Acad Child Adolesc Psychiatry. 2009;48:1069–1078. doi: 10.1097/CHI.0b013e3181b76697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.La Greca AM, Silverman WK, Wasserstein SB. Children’s predisaster functioning as a predictor of posttraumatic stress following Hurricane Andrew. J Consult Clin Psychol. 1998;66:883–892. doi: 10.1037//0022-006x.66.6.883. [DOI] [PubMed] [Google Scholar]
- 8.Lonigan CJ, Shannon MP, Taylor CM, Finch A, Sallee FR. Children exposed to disaster: II. Risk factors for the development of post-traumatic symptomatology. J Am Acad Child Adolesc Psychiatry. 1994;33:94–105. doi: 10.1097/00004583-199401000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Scheeringa MS, Zeanah CH. Reconsideration of harm’s way: Onsets and comorbidity patterns of disorders in preschool children and their caregivers following Hurricane Katrina. J Clin Child Adolesc Psychol. 2008;37:508–518. doi: 10.1080/15374410802148178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Greca AM, Silverman WK, Vernberg EM, Prinstein MJ. Symptoms of posttraumatic stress in children after Hurricane Andrew: a prospective study. J Consult Clin Psychol. 1996;64:712–723. doi: 10.1037//0022-006x.64.4.712. [DOI] [PubMed] [Google Scholar]
- 11.La Greca AM, Lai BS, Joormann J, Auslander BB, Short MA. Children’s risk and resilience following a natural disaster: Genetic vulnerability, posttraumatic stress, and depression. J Affect Disord. 2013;151:860–867. doi: 10.1016/j.jad.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002;65:207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- 13.Weems CF, Pina AA, Costa NM, Watts SE, Taylor LK, Cannon MF. Predisaster trait anxiety and negative affect predict posttraumatic stress in youths after hurricane Katrina. J Consult Clin Psychol. 2007;75:154–159. doi: 10.1037/0022-006X.75.1.154. [DOI] [PubMed] [Google Scholar]
- 14.Bonanno GA, Brewin CR, Kaniasty K, LaGreca AM. Weighing the costs of disaster: Consequences, risks, and resilience in individuals, families, and communities. Psychol Sci Public Interes. 2010;11:1–49. doi: 10.1177/1529100610387086. [DOI] [PubMed] [Google Scholar]
- 15.Weems C. Biological correlates of child and adolescent responses to disaster exposure: A bio-ecological model. Curr Psychiatry Rep. 2015;17:1–7. doi: 10.1007/s11920-015-0588-7. [DOI] [PubMed] [Google Scholar]
- 16.Carrion VG, Weems CF, Bradley T. Natural disasters and the neurodevelopmental response to trauma in childhood: A brief overview and call to action. Future Neurol. 2010;5:667–674. [Google Scholar]
- 17.Weems CF, Scott BG, Banks DM, Graham RA. Is TV traumatic for all youths? The role of preexisting posttraumatic-stress symptoms in the link between disaster coverage and stress. Psychol Sci. 2012;23:1293–1297. doi: 10.1177/0956797612446952. [DOI] [PubMed] [Google Scholar]
- 18.Pine DS, Cohen JA. Trauma in children and adolescents: Risk and treatment of psychiatric sequelae. Biol Psychiatry. 2002;51:519–531. doi: 10.1016/s0006-3223(01)01352-x. [DOI] [PubMed] [Google Scholar]
- 19.Pfefferbaum B, Noffsinger MA, Wind LH, Allen JR. Children’s coping in the context of disasters and terrorism. J Loss Trauma. 2013;19:78–97. doi: 10.1080/15325024.2013.791797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, Sheridan Ma. Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depress Anxiety. 2014;31:834–842. doi: 10.1002/da.22284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Admon R, Lubin G, Stern O, Rosenberg K, Sela L, Ben-ami H, Hendler T. Human vulnerability to stress depends on amygdala’s predisposition and hippocampal plasticity. Proc Natl Acad Sci. 2009;106:14120–14125. doi: 10.1073/pnas.0903183106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kujawa A, Klein DN, Proudfit GH. Two-year stability of the late positive potential across middle childhood and adolescence. Biol Psychol. 2013;94:290–296. doi: 10.1016/j.biopsycho.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 25.Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman E, editors. Handb event-related potential components. New York: Oxford University Press; 2011. pp. 441–472. [Google Scholar]
- 26.Schupp HT, Flaisch T, Stockburger J, Junghöfer M, Jungho M, Junghöfer M. Emotion and attention: Event-related brain potential studies. Prog Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- 27.Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: An electrophysiological study. Biol Psychol. 2008;78:93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Mühlberger A, Wieser MJ, Herrmann MJ, Weyers P, Tröger C, Pauli P. Early cortical processing of natural and artificial emotional faces differs between lower and higher socially anxious persons. J Neural Transm. 2009;116:735–746. doi: 10.1007/s00702-008-0108-6. [DOI] [PubMed] [Google Scholar]
- 29.Kujawa A, MacNamara A, Fitzgerald KD, Monk CS, Phan KL. Enhanced neural reactivity to threatening faces in anxious youth: Evidence from event-related potentials. J Abnorm Child Psychol. 2015 doi: 10.1007/s10802-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacNamara A, Hajcak G. Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depress Anxiety. 2010;27:234–243. doi: 10.1002/da.20679. [DOI] [PubMed] [Google Scholar]
- 31.Foti D, Olvet DM, Klein DN, Hajcak G. Reduced electrocortical response to threatening faces in major depressive disorder. Depress Anxiety. 2010;27:813–820. doi: 10.1002/da.20712. [DOI] [PubMed] [Google Scholar]
- 32.Kayser J, Bruder GE, Tenke CE, Stewart JE, Quitkin FM. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int J Psychophysiol. 2000;36:211–36. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 33.MacNamara A, Post D, Kennedy AE, Rabinak CA, Phan KL. Electrocortical processing of social signals of threat in combat-related post-traumatic stress disorder. Biol Psychol. 2013;94:441–449. doi: 10.1016/j.biopsycho.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Cheng Y, Hung A-Y, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev Psychopathol. 2012;24:623–636. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- 35.Sadeh N, Verona E. Visual complexity attenuates emotional processing in psychopathy: Implications for fear-potentiated startle deficits. Cogn Affect Behav Neurosci. 2012;12:346–360. doi: 10.3758/s13415-011-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solomon B, DeCicco JM, Dennis TA. Emotional picture processing in children: an ERP study. Dev Cogn Neurosci. 2012;2:110–119. doi: 10.1016/j.dcn.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson BD, Perlman G, Hajcak G, Klein DN, Kotov R. Familial risk for distress and fear disorders and emotional reactivity in adolescence: An event-related potential investigation. Psychol Med. 2015;45:2545–2556. doi: 10.1017/S0033291715000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. J Child Psychol Psychiatry. 2012;53:207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Speed BC, Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Personality and emotional processing: A relationship between extraversion and the late positive potential in adolescence. Psychophysiology. 2015;52:1039–1047. doi: 10.1111/psyp.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant KE, Compas BE, Thurm AE, McMahon SD, Gipson PY, Campbell AJ, et al. Stressors and child and adolescent psychopathology: Evidence of moderating and mediating effects. Clin Psychol Rev. 2006;26:257–283. doi: 10.1016/j.cpr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Neria Y, Shultz JM. Mental health effects of Hurricane Sandy: characteristics, potential aftermath, and response. J Am Med Assoc. 2012;308:2571–2. doi: 10.1001/jama.2012.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. J Abnorm Psychol. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. J Cogn Neurosci. 2011;23:2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell DGV, Richell Ra, Leonard A, Blair RJR. Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. J Abnorm Psychol. 2006;115:559–566. doi: 10.1037/0021-843X.115.3.559. [DOI] [PubMed] [Google Scholar]
- 45.Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Dev Cogn Neurosci. 2012;2:458–467. doi: 10.1016/j.dcn.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International Affective Picture System (IAPS): Affective ratings of pictures and instructional manual. [Google Scholar]
- 47.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 48.Norris FH, Sherrieb K, Galea S. Prevalence and consequences of disaster-related illness and injury from Hurricane Ike. Rehabil Psychol. 2010;55:221–230. doi: 10.1037/a0020195. [DOI] [PubMed] [Google Scholar]
- 49.Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, et al. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64:1427–1434. doi: 10.1001/archpsyc.64.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weems CF, Russell JD, Banks DM, Graham RA, Neill EL, Scott BG. Memories of traumatic events in childhood fade after experiencing similar less stressful events: Results from two natural experiments. J Exp Psychol Gen. 2014:143. doi: 10.1037/xge0000016. [DOI] [PubMed] [Google Scholar]
- 51.Axelson D, Birmaher B, Zelazny J, Kaufman J, Gill MK. The Schedule for Affective Disorders and Schizophrenia--Present and Lifetime Version (K-SADS-PL) 2009 Working Draft. n.d. [Google Scholar]
- 52.Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. J Abnorm Psychol. 2014;123:287–297. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR, and TRF profiles. Burlington, VT: 1991. [Google Scholar]
- 54.Hayes AF, Cai L. Using heteroskedasticity-consistent standard error estimators in OLS regression: An introduction and software implementation. Behav Res Methods. 2007;39:709–722. doi: 10.3758/bf03192961. [DOI] [PubMed] [Google Scholar]
- 55.Jorm A, Duncan-Jones P, Scott R. An analysis of the re-test artefact in longitudinal studies of psychiatric symptoms and personality. Psychol Med. 1989 doi: 10.1017/s0033291700012514. [DOI] [PubMed] [Google Scholar]
- 56.Schupp HT, Cuthbert B, Bradley M, Hillman C, Hamm A, Lang P. Brain processes in emotional perception: Motivated attention. Cogn Emot. 2004;18:593–611. [Google Scholar]
- 57.Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci. 2006;6:291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- 58.Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8:132–137. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- 59.Moser JS, Hajcak G, Bukay E, Simons RF. Intentional modulation of emotional responding to unpleasant pictures: An ERP study. Psychophysiology. 2006;43:292–296. doi: 10.1111/j.1469-8986.2006.00402.x. [DOI] [PubMed] [Google Scholar]
- 60.Dennis TA, Hajcak G. The late positive potential: A neurophysiological marker for emotion regulation in children. J Child Psychol Psychiatry. 2009;50:1373–83. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dunning JP, Hajcak G. See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46:28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- 62.Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- 63.MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9:531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- 64.Moratti S, Saugar C, Strange BA. Prefrontal-occipitoparietal coupling underlies late latency human neuronal responses to emotion. J Neurosci. 2011;31:17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: Correlation of functional MRI and event-related potentials. Cereb Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- 66.Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture-processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. J Neurosci. 2012;32:14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sabatinelli D, Keil A, Frank DW, Lang PJ. Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol. 2013;92:513–519. doi: 10.1016/j.biopsycho.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen PS, Rubo-Stipec M, Canino G, Bird HR, Dulcan MK, Schwab-Stone ME, Lahey BB. Parent and child contributions to diagnosis of mental disorder: Are both informants always necessary? J Am Acad Child Adolesc Psychiatry. 1999;38:1569–1579. doi: 10.1097/00004583-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 70.Shankman SA, Lewinsohn PM, Klein DN, Small JW, Seeley JR, Altman SE. Subthreshold conditions as precursors for full syndrome disorders: A 15-year longitudinal study of multiple diagnostic classes. J Child Psychol Psychiatry. 2009;50:1485–1494. doi: 10.1111/j.1469-7610.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Copeland WE, Wolke D, Shanahan L, Costello EJ. Adult functional outcomes of common childhood psychiatric problems: A prospective, longitudinal study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.