Abstract
Advances in the understanding of the neurobiology of fear extinction resulted in the development of d-cycloserine (DCS), a partial glutamatergic N-methyl-D-aspartate agonist, as an augmentation strategy for exposure treatment. We review a decade of research that has focused on the efficacy of DCS for augmenting the mechanisms (e.g., fear extinction) and outcome of exposure treatment across the anxiety disorders. Following a series of small-scale studies offering strong support for this clinical application, more recent larger-scale studies have yielded mixed results, with some showing weak or no effects. We discuss possible explanations of the mixed findings, pointing to both patient and session (i.e., learning experiences) characteristics as possible moderators of efficacy, and offer directions for future research in this area. We also review recent studies that have aimed to extend the work on DCS augmentation of exposure therapy for the anxiety disorders to DCS enhancement of learning-based interventions for addiction, anorexia nervosa, schizophrenia, and depression. Here, we attend to both DCS effects on facilitating therapeutic outcomes and additional therapeutic mechanisms beyond fear extinction (e.g., appetitive extinction, hippocampal-dependent learning).
Keywords: d-cycloserine, dcs, extinction, anxiety, addiction, depression
Introduction
One of the particular achievements of translational research has been the documentation of the importance of N-methyl-d-aspartate (NMDA) glutamatergic receptor to extinction learning, and the application of its partial agonist, d-cycloserine (DCS), as a strategy to aid the consolidation of extinction learning in animal models (1), and subsequently in human clinical applications (2). It is well accepted that NMDA receptors are involved in the acquisition, consolidation, and retrieval of extinction memory (3, 4). DCS activates NMDA receptors by binding with their glycine binding sites, enhancing the conditions for long term potentiation, and consequently, enhancing memory (2).
When DCS is administered orally to augment exposure therapy, it may be exerting its effects on multiple regions of interest at different points in the extinction learning process. Identification of DCS effects on specific brain regions is aided by animal models of exposure therapy (i.e., extinction training) investigating region-specific DCS administrations. This work has supported efficacy for DCS augmentation when infused into each of the major regions identified in fear extinction circuits –amygdala, hippocampus, and prefrontal cortex (5–10), including the infusion of DCS after extinction training (7), supporting the role of DCS in aiding the consolidation of extinction learning.
The initial translation of DCS augmentation into clinical application (11) was followed by a number of small placebo-controlled trials across the anxiety disorders that often reported large effects for the advantage of DCS over placebo for augmenting a small number of exposure sessions (12, 13). Yet, as research on DCS augmentation progressed to more diverse protocols and large multicenter trials, the effect size for its benefit began to falter (14–16; see Table 1). Sequential meta-analytic reviews help document this decline. In 2008, Norberg et al. (15), reported an effect size of d = .60 for 8 clinical trials of DCS, in 2012, Bontempo et al. (14), reported an effect size d =.46 for 9 trials, and in 2014, Rodrigues (16) reported a small to moderate effect (13 studies, d = .34, 95% Confidence Interval: .14 to .54) for the advantage of DCS vs. placebo augmentation of exposure-based treatment. In addition to some negative trials in the anxiety-related disorders (17), failures appeared in other applications, such as the application of DCS augmentation to cue exposure treatments of substance use disorders (18, 19). In the context of these faltering estimates of the DCS augmentation efficacy, however, important moderators were discovered (20, 21), enough so that guidance on judicious use of DCS for augmentation appears to be at hand. This review is concerned with the mechanism, moderators, and future directions for the application of DCS as an augmenting agent for CBT.
Table 1.
Placebo-controlled studies of DCS to augment exposure therapy in adults with anxiety spectrum disorders, addiction, schizophrenia, or anorexia nervosa.
| Publication | Sample Size Randomized | Condition | # of Total Sessions / Exposure Sessions / DCS administered | DCS Dose (mg) | Description of Findings |
|---|---|---|---|---|---|
|
Anxiety
Spectrum | |||||
| Ressler 2004 (11) | 27 | Specific phobia | 2/2/2 | 50 or 500 | DCS (50mg and 500mg combined) group displayed significantly greater improvement than the PBO group on all main outcome measures at post-treatment and 3- month follow up. No significant difference between 50mg or 500mg DCS groups. |
| Hofmann 2006 (12) | 32 | Social anxiety disorder | 5/4/4 | 50 | DCS group displayed significantly greater improvement than the PBO group on 2/3 outcome measures at post-treatment and 1-month follow up (medium-large effects). |
| Guastella 2007 (82), study 1 | 63 | Spider-fearful | 1/1/1 | 50 | No differences in improvement between DCS and PBO group on all outcomes at post- treatment or 3.5- week follow up |
| Guastella 2007 (82), study 2 | 37 | Spider-fearful | 1/1/1 | 50 or 500 | No differences in improvement between DCS and PBO group on all outcomes at post- treatment or 3.5- week follow up |
| Kushner 2007 (35) | 32 | Obsessive-compulsive disorder | 10/10/10 | 125 | No differences in improvement between DCS and PBO group on all outcomes measures at post-treatment and 3-month follow up. Some evidence of faster improvement and lower dropout. |
| Storch 2007 (86) | 24 | Obsessive-compulsive disorder | 12/12/12 | 250 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment. |
| Guastella 2008 (37) | 56 | Social anxiety disorder | 5/4/4 | 50 | DCS group displayed significantly greater improvement than the PBO group on 4/5 outcome measures at post- treatment and 1- month follow up (mostly medium effects). |
| Wilhelm 2008 (87) | 23 | Obsessive-compulsive disorder | 10/10/10 | 100 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment and 1- month follow up. Some evidence of faster improvement and improvement in depressive symptoms. Reanalysis indicated significant faster response for participants receiving DCS (88). |
| Otto 2010 (13) | 31 | Panic disorder | 5/4/3 | 50 | DCS group displayed significantly greater improvement than the PBO group on all main outcome measures at post- treatment and 1- month follow up (large effects). |
| Seigmund 2011 (39) | 44 | Panic disorder, Agoraphobia | 8/3/3 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment and 1- month follow up. Some evidence of faster improvement for more severe patients. |
| De Kleine 2012 (41) | 67 | Posttraumatic stress disorder | 10/9/9 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment and 3- month follow up; some evidence of higher post-treatment response rate. Some evidence of benefit for participants with severe pretreatment symptoms who needed longer treatment. |
| Litz 2012 (17) | 26 | Posttraumatic stress disorder | 6/4/4 | 50 | PBO group displayed significantly greater improvement than DCS group on all main outcome measures at post- treatment. |
| Nave 2012 (89) | 20 | Specific phobia | 1/1/1 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment. Some evidence of faster improvement. |
| Tart 2013 (59) | 29 | Specific phobia | 2/2/2 | 50a | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment or 1- month follow up. Evidence of exposure success as moderator. Reanalysis indicated significant greater DCS benefits for participants achieving low fear during exposure sessions (21). |
| Hofmann 2013 (38) | 169 | Social anxiety disorder | 12/5/5 | 50 | No differences between DCS and PBO in completion, response, and remission rates and post-treatment and 1, 3, 6-month follow up. Evidence of faster (24–33%) improvement. Reanalysis indicated significant greater DCS benefits for participants achieving low fear during exposure sessions (20). |
| Rothbaum 2014 (56) | 106 | Posttraumatic stress disorder | 6/5/5 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment or 3, 6, 12-month follow up. Between-session extinction learning was a treatment- specific enhancer of outcome for DCS group. |
| Andersson 2015 (62) | 128 | Obsessive-compulsive disorder | 12/5/5 | 50 | No differences between DCS and PBO in main outcome measures at post-treatment and 3- month follow up. Evidence of significant interaction of DCS effects with antidepressant medication. |
| Addiction | |||||
| Santa Ana 2009 (67) | 25 | Nicotine dependence | 2/2/2 | 50 | DCS group displayed significantly greater improvement than PBO group on 3 out of 7 main outcomes at post-treatment and 1-week follow up (large effect at follow up). |
| Kamboj 2011 (72) | 40 | Heavy drinkers | 2/2/2 | 125 | No differences in improvement between DCS and PBO group on main outcome measures at two sessions or follow up ~4 days later. |
| Watson 2011 (70) | 16 | Alcohol dependence | 3/3/2 | 250 | No differences in improvement between DCS and PBO group on main outcome measures at all 3 sessions. |
| Hofmann 2012 (71) | 20 | Heavy drinkers | 3/3/3 | 50 | No differences in DCS and PBO group on most outcomes. Some evidence of poorer outcomes in DCS group than PBO group at first of 2 post-treatment test sessions. |
| Kamboj 2012 (18) | 32 | Heavy smokers | 2/2/2 | 125 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment or 2- week follow up. |
| Price 2013 (19) | 32 | Cocaine dependence | 3/3/3 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment. Some evidence of significantly poorer session-specific outcomes and 5–12 day follow up outcomes in DCS group. |
| Yoon 2013 (68) | 47 | Cocaine and nicotine dependence | 12/12/4 | 50 | No differences in improvement between DCS and PBO group on main outcome measures at post-treatment or 6- month follow up. |
| Kiefer 2015 (73) | 76 | Alcohol dependence | 9/9/9 | 50 | No differences in improvement between DCS and PBO group on subjective outcome measures at post- treatment. PBO group displayed higher fMRI activation than DCS group in brain regions associated with negative outcomes. |
| MacKillop 2015 (74) | 37 | Alcohol use disorder | 4/2/2 | 50 | DCS group displayed greater improvements than PBO group on session-specific measures and post- treatment outcomes but not 3-week follow up. |
| Schizophreniab | |||||
| Gottlieb 2011 (96) | 21 | Schizophrenia/schizoaffective | 2/2/2 | 50 | No differences in improvement between DCS and PBO on main outcome measures at post-treatment. Significant order effectsc; DCS first displayed greater improvements than PBO first on some outcomes at post- treatment (moderate- large effects). |
| Cain 2014 (97) | 40 | Schizophrenia | 24–40/8d | 50 | DCS group displayed greater improvements than PBO on one outcome, while PBO displayed greater improvement than DCS on another. Some evidence of more improvement for subjects with more severe pretreatment symptoms. |
|
Anorexia
Nervosa | |||||
| Levinson 2015 (76) | 36 | Anorexia nervosa | 4/4/3 | 250 | DCS group displayed greater improvements than PBO on main outcome measure at post-treatment but not 1-month follow up. |
Notes. PBO = placebo
All studies administered DCS prior to CBT sessions, except for the designated study, which administered DCS immediately after session;
See supplemental information for discussion of the application of DCS to therapeutic learning in schizophrenia;
Within-participant cross-over design;
This study did not utilize exposure but utilized a "Brain Fitness" cognitive remediation program which took place 3–5x/week across 8 weeks.
A New Approach to Combination Treatment
At the outset, it is important to consider the context in which DCS augmentation first emerged into the anxiety treatment literature. Decades of effort have been applied to examine the benefit of combining pharmacotherapy and cognitive-behavior therapy for anxiety disorders, and, as meta-analytic reviews attest, the result has often been disappointing – with limited acute advantages that are often lost over follow-up intervals (22–24). Moreover, concerns have arisen that antidepressant or benzodiazepine medications, while offering anxiolytic benefit on their own, may also hinder the short-term and long-term efficacy of exposure-based treatments (25, 26). In the context of these frustrating results, augmentation of CBT with DCS represented an important innovation in the application of pharmacotherapy to combined treatment. Rather than directly targeting anxiolysis, DCS was instead used to enhance the consolidation of the therapeutic learning offered by CBT. What are the likely effects of memory enhancement of this kind? First, and most obviously, DCS may speed the onset of benefit from CBT by allowing more to be learned/retained from fewer sessions. Second, DCS may act as a rescue strategy for poor extinction learners, helping them achieve benefits that they may not be able to achieve without the help of memory enhancement.
The Nature of DCS Augmentation Benefits
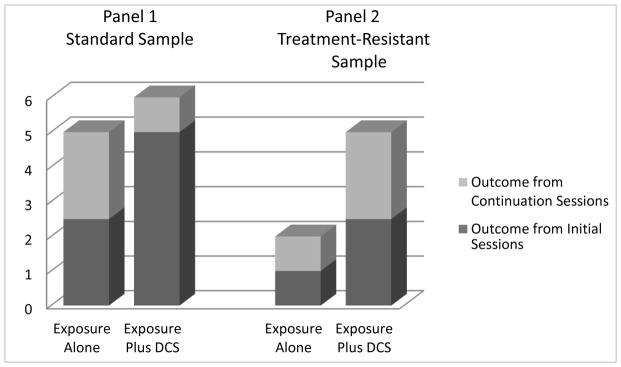
Initial studies of DCS augmentation did little to clarify which of these two potential actions might be operative. In animal and the initial human paradigms investigating DCS augmentation (6, 11, 27) the number of extinction trials/sessions were reduced, often by half or more, to ensure that extinction would be incomplete. This was done to avoid floor effects that would obscure DCS augmentation of extinction outcomes. Also, in human application, DCS is most often given in a limited number of individual, weekly doses, based on concerns about the development of tolerance that may result from more frequent or prolonged use (28–30). For example, Hofmann et al. (12) examined DCS augmentation in the context of only five total sessions of CBT. The first session was devoted to presenting the model of treatment and DCS (vs. placebo) was used on the remaining four sessions that emphasized exposure to social challenges (e.g., public speaking). For initial application to panic disorder, we used two sessions to help our patients orient to treatment and the interoceptive (internal cue) exposure used in the protocol, and then provided three sessions of DCS (or placebo) combined with 90-minute exposure sessions (13). These brief 5-session protocols stand in contrast to the 12- to 16-session protocols of treatment suggested in manualized treatment protocols (31–34). Both of these placebo-controlled trials showed strong benefit from DCS augmentation. Yet, with such a brief protocol of treatment, the outcome advantage at endpoint could have simply reflected a speeding of treatment effects rather than an overall enhancement of outcome relative to that which could be achieved with a standard protocol of CBT (see Figure 1). Additional trials of DCS enhancement appear to show that the acceleration of treatment gains may be the dominant effect of DCS enhancement, at least for standard clinical samples of patients. For example, Kushner et al. (35) was the first to show that with repeated exposure sessions, exposure alone can catch up to the early advantage provided by DCS augmentation (36). This apparent “catch up” effect has also been shown in animal models. Ren and colleagues (9) found that DCS facilitated the speed of improvement in extinction retention, but that animals undergoing saline augmentation caught up to the DCS group by the third out of five days of extinction training.
Figure 1.
Model of DCS effects showing in Panel 1 a clear advantage when few sessions of exposure are provided (dark bars), but providing little benefit over full length treatment (stacked outcomes), and in Panel 2, rescuing treatment effects in individuals who a poor responders to exposure alone. In both panels, initial exposure sessions are represented by dark bar sections, and the additional sessions characterizing standard treatment lengths are represented by the stacked gray-scale sections. Trials using limited sessions only do not allow differentiation between speeded treatment and rescued treatment outcomes. Rescued treatment outcomes (if they occur) should be more evident when treatment-resistant samples are selected.
Comparisons between trials also show the apparent loss of an advantage for DCS augmentation in the context of additional exposure sessions. Both Hofmann et al. (12) and Guastella et al. (37) showed strong efficacy for DCS augmentation for social anxiety disorder following 5 sessions of treatment, but then Hofmann et al. (38) showed no advantage in endpoint response or remission rates for DCS following 12 sessions of treatment, but faster response earlier in treatment. Likewise, Otto (13) showed a clear advantage for DCS augmentation for panic disorder following 5 sessions of treatment, but Siegmund (39) showed strong improvement overall with no group differences following 11 sessions of treatment, but a trend toward a speeding of treatment response for more severe patients receiving DCS vs. placebo augmentation. It is important to note that a faster treatment response can have far reaching effects. In addition to the more rapid reduction of the distress and disability associated with anxiety disorders, more efficient treatment also means a reduction in limited treatment resources (CBT therapist time), lower cost of treatment, less barrier to ongoing treatment (e.g., time off work, travel to sessions), and potentially less dropout (35) as treatment gains are realized more quickly (40).
In addition to these important effects on the efficiency of treatment, it remains an open question whether DCS has additional rescue effects, helping select individuals who have difficulty achieving fear reduction from exposure, despite repeated sessions. For these individuals, DCS may provide crucial enhancement of learning, providing a response when it would otherwise be unlikely (Figure 1). For individuals who are slow responders to CBT or who are otherwise unable to consolidate the safety learning from exposure, augmentation with DCS may set them on a course for fuller response by the end of a standard course of treatment. For example, in a trial of 67 outpatients with PTSD undergoing up to 10 sessions of CBT (augmented by DCS or placebo), de Kleine et al. (41) did not find an overall augmentation effect at treatment endpoint, but exploratory session-by-session analyses indicated that DCS was more beneficial for individuals who had more severe pretreatment PTSD and needed longer treatment. At present, only pilot studies inform the application of DCS augmentation to treatment-refractory samples (see Supplemental Information). For example, an open report of two cases of patients with obsessive-compulsive disorder (OCD) who were unresponsive to a program of exposure and response prevention, documents improvements after additional sessions augmented with DCS (42). Likewise, Farrell and colleagues (43) found superior outcome for DCS vs. placebo augmentation at 1-month follow-up in a small (N=17) treatment-refractory pediatric OCD sample undergoing exposure and response prevention treatment. These findings suggest that, in addition to a clear role in speeding treatment, DCS augmentation may have a role in rescuing inadequate treatment response in selected treatment-refractory samples.
Identification of the scope of such rescue-treatment applications is made difficult by the relative absence of research linking learning impairments to outcome in CBT. Cognitive and extinction-learning deficits are ubiquitous in adults with anxiety and mood disorders (44, 45), but we are aware of only three randomized treatment studies that link cognitive deficits to poorer CBT outcome (46–48). Research is needed to clarify whether DCS can rescue treatment outcome for these individuals, including research on whether cognitive deficits influence: (a) within-session learning of extinction, (b) retention of extinction across sessions, or (c) both. These distinctions are relevant because it is the retention of extinction learning that appears to be the primary domain of DCS augmentation (7).
In summary, our interpretation of the DCS clinical anxiety literature to date is that effects appear to be achieved primarily in terms of a speeding of clinical outcomes. When the number of CBT sessions provided is low, this speeding of treatment is reflected by large effects at treatment endpoint. However, when a greater number of exposure sessions are provided--providing ample opportunities for learning without the aid of memory enhancement--only subtle differences are observed for the full sample at study endpoint, despite an apparent advantage for DCS augmentation early in treatment.
Moderators of DCS Augmentation
There is also strong evidence for variable DCS augmentation efficacy based on the quality of learning within exposure sessions. Because DCS is targeted to enhancing the degree of therapeutic learning achieved from CBT, any positive effects of DCS on exposure therapy outcome should be dependent on the degree to which adequate extinction learning has occurred at the time the drug is active (2, 8). Consistent with this account, animal studies indicate that augmentation effects are achieved only with animals that demonstrate extinction at the time DCS is administered (49, 50). Likewise, there is now evidence extending this effect to the clinic. Smits et al. (21) examined whether the clinical benefit of DCS augmentation was a function of response to the exposure session. They found that DCS offered an advantage only for individuals who had achieved low fear by the end of the CBT session. In contrast, placebo augmentation offered greater benefit than DCS augmentation for patients with elevated fear at the end of the session. This effect was replicated for a large-scale trial of DCS augmentation for social anxiety disorder. Reanalysis of overall limited benefits for DCS (38) revealed that patients who reported low fear at the end of a CBT session showed a clear advantage for DCS vs. placebo augmentation, and an opposite pattern emerged for those with high fear at the end of a CBT session (20). This interaction effect was evident from one CBT session to the next, and was also evident for predicting clinical outcomes at post-treatment. These moderating effects of exposure success were indexed by fear ratings provided by patients at the conclusion of exposure, and were not better accounted for by the degree of within-session change in fear ratings. It is noteworthy that the moderating effect for end fear was further replicated for different putative extinction enhancers, yohimbine and methylene blue (51, 52), raising confidence in the generalizability of this effect, and supporting the notion that the degree of benefit offered by these augmenting agents is dependent on adequate learning from exposure. If the exposure inadequately achieves within-session extinction, then there is little therapeutic benefit available for enhancement via greater memory consolidation. Indeed, combining DCS with less-than-successful exposure sessions has been used to explain data showing impaired treatment response with DCS relative to placebo augmentation (17).
These findings are also consistent with studies showing that DCS not only augments extinction learning but can also enhance reconsolidation of fear memories in animals and humans (8, 53–55). Therefore, if fear does not decrease during exposure, fear memory reconsolidation may occur and DCS may facilitate this counter-therapeutic process. In other words, DCS appears to make “good” exposures better and “bad” exposures worse (55). Accordingly, the nature of the disorder under treatment (e.g. simple phobia vs. complex PTSD) and patient variables (e.g. personality traits, cognitive flexibility or impairment) may influence the degree of in-session extinction learning, and hence DCS augmentation effects. These considerations are apt as the field confronts current failures of DCS to successfully augment exposure therapy for PTSD, with a recent null result reported by Rothbaum et al. (56) joining an earlier unsuccessful trial by Litz and associates (17). There is some evidence that these null results may again be moderated by exposure success; Rothbaum and associates (56) found that DCS-augmentation showed a specific advantage for the stronger extinction learners within their sample. As such, successful application of DCS augmentation to PTSD may require particularly judicious use.
One strategy to achieve such judicious use is to administer DCS post- instead of pre-session, limiting the administration to exposure sessions that are characterized by low end fear (adequate extinction learning; exposure success). Support for tailored post-session administration of DCS comes from animal studies that have documented success with post-session DCS administration up to two hours following training (7, 57). This stands in contrast to the typical administration strategy for DCS augmentation for exposure therapy; DCS is typically administered orally one or more hours before an exposure session to achieve adequate concentrations of DCS centrally by the conclusion of the exposure session, consistent with expectation of peak blood levels two hours after oral administration (58). We are aware of only two studies investigating post-session administration in humans. Tart et al. (59) investigated post-session DCS administration in a sample of acrophobic adults treated with two sessions of virtual reality exposure therapy. Mataix-Cols et al. (60) investigated this strategy in 27 youth with OCD treated with 10 sessions of CBT. Both studies reported overall null results. However, reanalysis of the null result for the study by Tart et al. (59) revealed significant DCS effects when exposure success was treated as a moderator (21); thereby showing that post-session administration can work in human applications. Research by our group is now underway to further test the adequacy of judicious post-treatment application of DCS augmentation (61).
Recent research also raises the possibility that other medication use may alter DCS augmentation effects. In a post-hoc analysis of an OCD trial, Andersson and associates (62) observed an interaction between DCS and antidepressant medication; DCS was found to be superior to placebo augmentation only in individuals who were free of antidepressant medication. It is not clear whether this effect is due to direct effects of antidepressants or to other selection factors that led this subsample of patients to be on medication. Nonetheless, there is preliminary evidence for similar attenuation of DCS effects by chronic antidepressant pretreatment in an animal model (30) as well as evidence that antidepressant pretreatment can impair extinction learning (25, 26), thereby attenuating the within-session learning needed for beneficial DCS augmentation. As such, future studies will need to evaluate whether there are differences in within-session extinction learning vs. between-session retention in these medicated individuals.
Exposure Efficacy and DCS Augmentation of Addiction Treatment
Attending to the adequacy of extinction learning can also help clarify the variable results observed for the application of DCS augmentation to addiction treatment. Despite encouraging evidence from animal models of addiction (63–65), the human literature on DCS augmentation of cue exposure therapy (CET) for substance use has been marked by more negative than positive findings (66; see Table 1). Early positive DCS augmentation results for addiction treatment were reported by Santa Ana (67) for smokers randomized to DCS or placebo in combination with two sessions of CET. In a second study, Kamboj et al. (18) examined DCS vs. placebo augmentation of 2 sessions of cue exposure in smokers and reported no benefit on core outcomes. Likewise, Yoon and associates (68) reported no benefit for DCS in 29 cocaine-dependent cigarette smokers undergoing CET in a virtual reality environment. One trial reported an increase in cocaine craving with DCS vs. placebo (19). In trying to understand these mixed findings, attention has been placed on issues of sample size, dose and timing of DCS administration, and participant and design characteristics (66).
In addition, attending to the adequacy of CET sessions for reducing craving and/or the presence of other sensitizing/conditioning experiences can help clarify the variable results within the DCS addiction literature. First, there has been relatively poor control over sensitizing experiences. For example, both the Yoon et al. (68) and Kamboj et al. (18) studies of smoking cessation provide data indicating that participants were smoking around the study sessions, perhaps renewing the association between smoking cues and nicotine reward at times proximal to DCS administration. DCS has a half-life of approximately 10 hours (69); smoking during this period may lead to reconditioning and should attenuate beneficial effects of DCS augmentation of CET. Second, of the studies reporting within-session extinction results, those that provided evidence of inconsistent or inadequate within-session extinction also reported null affects for DCS (18, 70, 71). Conversely, one of two studies reporting consistent reductions in cravings across individual trials within the exposure session found the expected DCS augmentation benefits (cf., 67, 72).
To minimize opportunity for re-establishing cue-drug reward associations proximal to DCS administration, we recommend studying DCS augmentation for drug use conditions in a relapse prevention model, randomizing only participants who are successfully abstinent (NCT01399866). Likewise, a recent study of alcohol dependence examined DCS augmentation of CET primarily during the protective environment of inpatient treatment (73); the neuroimaging results supported DCS augmentation of CET for alcohol-associated cues. Finally, consistent with recommendations (70) for examining CET enhancement only in individuals shown to be reactive to the substance cue, MacKillop et al. (74) found beneficial effects of DCS augmentation on alcohol cue elicited cravings. These recent studies suggest that DCS augmentation may indeed offer benefit to CET for addictions, provided that cue relevance, adequate cue exposure, and protection from sensitizing experiences are managed.
Application to Exposure Therapy for Anorexia Nervosa
Following documentation that exposure to feared foods can aid in weight restoration among individuals with anorexia nervosa (75), Levinson and associates (76) conducted a small (N=36) trial investigating the augmenting effects of DCS on this exposure. In this randomized trial, four exposure sessions were completed over a two-week period, with DCS or placebo given prior to the first three sessions. Those individuals who received DCS achieved significantly higher weight gain than those in the placebo group. Additional research is needed to confirm these findings, and investigate whether the degree of anxiety reduction during eating is meaningfully linked to DCS augmentation effects, and whether, like the application of DCS to anxiety disorders (20–21), judicious use of DCS might be warranted.
DCS Augmentation in the Treatment of Depression
Our research team is now seeking to extend DCS augmentation to CBT that does not rely on extinction learning for its therapeutic effects. The animal literature is notable for positive DCS augmentation effects for hippocampal-dependent learning tasks (53, 77–79), but similar findings have yet to be reliably demonstrated in humans. Otto et al. (80) found no benefit for 50mg of DCS for augmenting verbal and nonverbal declarative learning in healthy participants. Yet, Onur et al. (81) found that a single 250mg dose of DCS facilitated hippocampal-dependent declarative learning, and suggested that DCS effects on hippocampal functioning may be dose dependent, requiring a 250mg dose rather than the 50mg that is sufficient for augmentation of extinction learning. Of note, retention over time was not assessed by Onur et al. (81), and it is the session-by-session promotion of therapeutic learning that has been of value for the treatment of anxiety disorders. Investigation of whether 250 mg can enhance retention of therapeutic content from CBT for depression is now underway (NCT02376257).
Failures of Human De Novo Fear Extinction Paradigms
Despite the success of DCS augmentation for the treatment of clinical fears in humans, and the success of DCS augmentation in de novo fear extinction models in animals (d = 1.19, 20 studies) (15), de novo fear conditioning models have generally failed in human work (54, 82–84). Grillon (85) has offered an important accounting of these effects, suggesting that the failure of DCS augmentation in these human paradigms may be due to the reliance on higher-order conditioning. Higher-order conditioning is slow and explicit and relies less on limbic activation and more on hippocampal structures, whereas lower-order conditioning is automatic and implicit and relies more on limbic activation and less on hippocampal structures. Grillon (85) argues that de novo fear conditioning in animals as well as clinical fears in humans reliably activate a lower-order learning mechanism as the unconditioned stimulus (UCS) is interpreted as life threatening and triggers the automatic flight or fight response and limbic activation. In contrast, de novo fear conditioning in humans activates higher-order associative processes in part because the frequently milder (e.g., self-selected) UCSs used in the laboratory are not perceived as life threatening and does not activate the automatic fight or flight response. In addition, humans enter the laboratory with expectations and conscious thought; their conditioned response is somewhat reliant on higher-order cognition as it is influenced by their awareness of the contingency between the CS and UCS. As noted above, there is only limited evidence for DCS-enhancement of hippocampal-dependent memories in humans, with initial suggestion that successful DCS augmentation may require a higher dose of DCS than typically used (81).
Grillon (85) further proposes that DCS, at least at the dose typically used, is more effective for lower-order conditioned fears and acts on lower-order learning during exposure therapy for clinical fears. If this is accurate, this would mean that de novo fear conditioning will not be influenced by DCS, unless a particularly strong UCS is used. This suggests that caution is warranted when interpreting failures of DCS augmentation of extinction when applied to nonclinical or de novo fears (82, 83), and indicates that de novo fear conditioning paradigms in humans may provide a poor model for studying the potential of DCS augmentation benefits for clinical anxiety disorders.
Summary
The last decade of research has established DCS augmentation of exposure therapy as potentially valuable strategy for accelerating treatment response in the anxiety disorders. As a memory augmentation strategy, DCS relies on effective exposure interventions for efficacy; without effective fear reduction in session, there appears to be no beneficial therapeutic memory to enhance. As such, judicious application of DCS to the most efficacious exposure sessions is becoming the new model for applying DCS in the anxiety disorders. In the same way, the failure of DCS augmentation for cue exposure for substance use disorders may be a result of inadequate extinction or subsequent sensitizing experiences. Recent applications of DCS to substance use disorders suggest that these issues may be addressed by applying DCS augmentation of cue exposure during a relapse prevention phase or in inpatient settings where sensitization experiences proximal to DCS dosing can be avoided. Ongoing research is also investigating whether DCS can be applied to CBT that does not rely on exposure interventions. Ongoing work in depression will provide initial evidence whether DCS can be applied successfully to enhancing benefits from cognitive restructuring interventions.
Supplementary Material
Acknowledgments
Dr. Otto’s effort on this manuscript was supported by NIH/NIMH R21MH102646. Ms. Kredlow’s effort on this manuscript was supported by NIH/NIMH 1F31MH103969. Dr. Smits’ effort on this grant was supported by NIH/NIMH R34MH099318. Dr. Hofmann’s effort on this manuscript was supported by NIH/NIMH R34MH086668. Dr. Evins’ work on this manuscript was supported by NIDA K24 DA030443. Dr. Pollack’s effort on this manuscript was supported by NIH/NIMH 5R34MH099309-02. The National Institutes of Health had no role in the writing of the report; or in the decision to submit the manuscript for publication. The National Institute of Health had no role other than financial support.
Footnotes
Financial Disclosures
Dr. Otto reports serving, in the last three years, as a paid consultant for MicroTransponder Inc., Concert Pharmaceuticals, and ProPhase, providing expert consensus opinion for Otsuka Pharmaceuticals, receiving royalty support for use of the SIGH-A from ProPhase, and receiving book royalties from Oxford University Press, Routledge, and Springer. Dr. Hofmann reports serving on the Advisory Board of Palo Alto Health Sciences and Otsuka America Pharmaceutical, Inc., and receiving financial compensation for his participation as an advisor, and receiving royalties from various book publishers and financial compensation for his editorial work from Springer Publications and the American Psychological Association. Dr. Tolin reports receiving research funding from Palo Alto Health Sciences, Inc. Dr. Evins has received grant support to her institution from Forum Pharmaceuticals (formerly Envivo pharmaceuticals), GSK, and Pfizer. She has provided consultation on behalf of her institution to Reckitt Benckiser and Pfizer. Dr. Pollack reports serving on the advisory board or consulting for Clintara, Concert Pharmaceuticals, Corcept Therapeutics, Edgemont Pharmaceuticals, Eli Lilly, Ironwood Pharmaceuticals, Medavante, Merck, Palo Alto Health Sciences, and Project Plus, receiving equity from Doyen Medical, Medavante, Mensante Corporation, Mindsite, and Targia Pharmaceuticals, and receiving royalties/patents for SIGH-A and SAFER interviews. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson R, Ledgerwood L, Cranney J. Facilitation of fear extinction by D-cycloserine: theoretical and clinical implications. Learn Mem. 2004;11:510–516. doi: 10.1101/lm.78204. [DOI] [PubMed] [Google Scholar]
- 2.Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 3.Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- 4.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- 8.Bolkan SS, Lattal KM. Opposing effects of D-cycloserine on fear despite a common extinction duration: interactions between brain regions and behavior. Neurobiology of learning and memory. 2014;113:25–34. doi: 10.1016/j.nlm.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren J, Li X, Zhang X, Li M, Wang Y, Ma Y. The effects of intra-hippocampal microinfusion of D-cycloserine on fear extinction, and the expression of NMDA receptor subunit NR2B and neurogenesis in the hippocampus in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:257–264. doi: 10.1016/j.pnpbp.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Chang CH, Maren S. Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learn Mem. 2011;18:221–225. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of general psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Bontempo A, Panza KE, Bloch MH. D-cycloserine augmentation of behavioral therapy for the treatment of anxiety disorders: a meta-analysis. J Clin Psychiatry. 2012;73:533–537. doi: 10.4088/JCP.11r07356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues H, Figueira I, Lopes A, Goncalves R, Mendlowicz MV, Coutinho ES, et al. Does D-cycloserine enhance exposure therapy for anxiety disorders in humans? A meta-analysis. PloS One. 2014;9:e93519. doi: 10.1371/journal.pone.0093519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, et al. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Kamboj SK, Joye A, Das RK, Gibson AJ, Morgan CJ, Curran HV. Cue exposure and response prevention with heavy smokers: a laboratory-based randomised placebo-controlled trial examining the effects of D-cycloserine on cue reactivity and attentional bias. Psychopharmacology (Berl) 2012;221:273–284. doi: 10.1007/s00213-011-2571-2. [DOI] [PubMed] [Google Scholar]
- 19.Price KL, Baker NL, McRae-Clark AL, Saladin ME, Desantis SM, Santa Ana EJ, et al. A randomized, placebo-controlled laboratory study of the effects of D-cycloserine on craving in cocaine-dependent individuals. Psychopharmacology (Berl) 2013;226:739–746. doi: 10.1007/s00213-011-2592-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann SG, Sawyer AT, Korte KJ, Smits JA. Is it Beneficial to Add Pharmacotherapy to Cognitive-Behavioral Therapy when Treating Anxiety Disorders? A Meta-Analytic Review. Int J Cogn Ther. 2009;2:160–175. doi: 10.1521/ijct.2009.2.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe N, Churchill R, Furukawa TA. Combination of psychotherapy and benzodiazepines versus either therapy alone for panic disorder: a systematic review. BMC psychiatry. 2007;7:18. doi: 10.1186/1471-244X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furukawa TA, Watanabe N, Churchill R. Psychotherapy plus antidepressant for panic disorder with or without agoraphobia: systematic review. Br J Psychiatry. 2006;188:305–312. doi: 10.1192/bjp.188.4.305. [DOI] [PubMed] [Google Scholar]
- 25.Otto MW, McHugh RK, Kantak KM. Combined pharmacotherapy and cognitive-behavioral therapy for anxiety disorders: Medication effects, glucocorticoids, and attenuated treatment outcomes. Clin Psychol Sci Pract. 2010;17:91–103. doi: 10.1111/j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE. Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol Psychiatry. 2013;73:1078–1086. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otto MW, Basden S, Leyro TM, McHugh RK, Hofmann SG. Clinical perspectives on the combination of d-cycloserine and CBT for the treatment of anxiety disorders. CNS Spectrums. 2007;12:51–61. doi: 10.1017/s1092852900020526. [DOI] [PubMed] [Google Scholar]
- 29.Quartermain D, Mower J, Rafferty MF, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- 30.Werner-Seidler A, Richardson R. Effects of D-cycloserine on extinction: consequences of prior exposure to imipramine. Biol Psychiatry. 2007;62:1195–7. doi: 10.1016/j.biopsych.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann SG, Otto MW. Cognitive behavior therapy for social anxiety disorder: Evidence–based and disorder-specific treatment techniques. New York, NY: Routledge; 2008. [Google Scholar]
- 32.Hope DA, Heimberg RG, Turk CL. Managing social anxiety: A cognitive-behavioral therapy approach, therapist guide. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 33.Otto MW, Pollack MH. Stopping anxiety medication (Therapist Guide) 2. New York, NY: Oxford University Press; 2009. [Google Scholar]
- 34.Barlow DH, Craske MG. Mastery of Your Anxiety and Panic: Therapist Guide. 2. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 35.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 36.Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48:675–679. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann SG, Smits JA, Rosenfield D, Simon N, Otto MW, Meuret AE, et al. D-Cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siegmund A, Golfels F, Finck C, Halisch A, Rath D, Plag J, et al. D-cycloserine does not improve but might slightly speed up the outcome of in-vivo exposure therapy in patients with severe agoraphobia and panic disorder in a randomized double blind clinical trial. J Psychiatr Res. 2011;45:1042–1047. doi: 10.1016/j.jpsychires.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 40.Otto MW, Tolin DF, Nations KR, Utschig AC, Rothbaum BO, Hofmann SG, et al. Five sessions and counting: considering ultra-brief treatment for panic disorder. Depress Anxiety. 2012;29:465–470. doi: 10.1002/da.21910. [DOI] [PubMed] [Google Scholar]
- 41.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Norberg MM, Gilliam CM, Villavicencio A, Pearlson GD, Tolin DF. D-cycloserine for treatment nonresponders with obsessive-compulsive disorder: A case report. Cogn Behav Pract. 2012;19:338–345. [Google Scholar]
- 43.Farrell LJ, Waters AM, Boschen MJ, Hattingh L, McConnell H, Milliner EL, et al. Difficult-to-treat pediatric obsessive-compulsive disorder: feasibility and preliminary results of a randomized pilot trial of D-cycloserine-augmented behavior therapy. Depress Anxiety. 2013;30:723–731. doi: 10.1002/da.22132. [DOI] [PubMed] [Google Scholar]
- 44.Otto MW, Moshier SJ, Kinner DG, Simon NM, Pollack MH, Orr SP. De novo fear conditioning across diagnostic groups in the affective disorders: evidence for learning impairments. Behav Ther. 2014;45:619–629. doi: 10.1016/j.beth.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, et al. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32:239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- 46.Dobkin RD, Rubino JT, Allen LA, Friedman J, Gara MA, Mark MH, et al. Predictors of treatment response to cognitive-behavioral therapy for depression in Parkinson’s disease. J Consult Clin Psychol. 2012;80:694–699. doi: 10.1037/a0027695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, et al. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. Am J Geriatr Psychiatry. 2007;15:680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- 48.D’Alcante CC, Diniz JB, Fossaluza V, Batistuzzo MC, Lopes AC, Shavitt RG, et al. Neuropsychological predictors of response to randomized treatment in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:310–317. doi: 10.1016/j.pnpbp.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 50.Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smits JA, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine enhancement of exposure therapy for social anxiety disorder: a randomized controlled trial. Biol Psychiatry. 2014;75:840–846. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, et al. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. Am J Psychiatry. 2014;171:1091–1098. doi: 10.1176/appi.ajp.2014.13101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kalisch R, Holt B, Petrovic P, De Martino B, Kloppel S, Buchel C, et al. The NMDA agonist D-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex. 2009;19:187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofmann SG. D-cycloserine for treating anxiety disorders: making good exposures better and bad exposures worse. Depress Anxiety. 2014;31:175–177. doi: 10.1002/da.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. Am J Psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parnas AS, Weber M, Richardson R. Effects of multiple exposures to D-cycloserine on extinction of conditioned fear in rats. Neurobiol Learn Mem. 2005;83:224–231. doi: 10.1016/j.nlm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 58.D’Souza DC, Gil R, Cassello K, Morrissey K, Abi-Saab D, White J, et al. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans. Biol Psychiatry. 2000;47:450–462. doi: 10.1016/s0006-3223(99)00133-x. [DOI] [PubMed] [Google Scholar]
- 59.Tart CD, Handelsman PR, Deboer LB, Rosenfield D, Pollack MH, Hofmann SG, et al. Augmentation of exposure therapy with post-session administration of D-cycloserine. J Psychiatr Res. 2013;47:168–174. doi: 10.1016/j.jpsychires.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mataix-Cols D, Turner C, Monzani B, Isomura K, Murphy C, Krebs G, et al. Cognitive-behavioural therapy with post-session D-cycloserine augmentation for paediatric obsessive-compulsive disorder: pilot randomised controlled trial. Br J Psychiatry. 2014;204:77–78. doi: 10.1192/bjp.bp.113.126284. [DOI] [PubMed] [Google Scholar]
- 61.Hofmann SG, Carpenter JK, Otto MW, Rosenfield D, Smits JAJ, Pollack MH. Dose timing of d-cycloserine to augment cognitive behavioral therapy for social anxiety: Study design and rationale. Contemp Clin Trial. 2015;43:223–230. doi: 10.1016/j.cct.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersson E, Hedman E, Enander J, Radu Djurfeldt D, Ljótsson B, Cervenka S, et al. d-Cycloserine vs placebo as adjunct to cognitive behavioral therapy for obsessive-compulsive disorder and interaction with antidepressants: A randomized clinical trial. JAMA Psychiatry. 2015;72:659–67. doi: 10.1001/jamapsychiatry.2015.0546. [DOI] [PubMed] [Google Scholar]
- 63.Nic Dhonnchadha BA, Szalay JJ, Achat-Mendes C, Platt DM, Otto MW, Spealman RD, et al. D-cycloserine deters reacquisition of cocaine self-administration by augmenting extinction learning. Neuropsychopharmacology. 2010;35:357–367. doi: 10.1038/npp.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine facilitates extinction of cocaine self-administration in rats. Synapse. 2011;65:938–944. doi: 10.1002/syn.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- 66.Myers KM, Carlezon WA., Jr D-cycloserine effects on extinction of conditioned responses to drug-related cues. Biol Psychiatry. 2012;71:947–955. doi: 10.1016/j.biopsych.2012.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, et al. D-Cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon JH, Newton TF, Haile CN, Bordnick PS, Fintzy RE, Culbertson C, et al. Effects of D-cycloserine on cue-induced craving and cigarette smoking among concurrent cocaine- and nicotine-dependent volunteers. Addict Behav. 2013;38:1518–1526. doi: 10.1016/j.addbeh.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Physicians’ Desk Reference Staff. Physicians’ Desk Reference 2006: Guide to Drug Interactions, Side Effects, and Indications. Montvale, NJ: Thompson PDR; 2006. [Google Scholar]
- 70.Watson BJ, Wilson S, Griffin L, Kalk NJ, Taylor LG, Munafo MR, et al. A pilot study of the effectiveness of D-cycloserine during cue-exposure therapy in abstinent alcohol-dependent subjects. Psychopharmacology (Berl) 2011;216:121–129. doi: 10.1007/s00213-011-2199-2. [DOI] [PubMed] [Google Scholar]
- 71.Hofmann SG, Huweler R, MacKillop J, Kantak KM. Effects of D-cycloserine on craving to alcohol cues in problem drinkers: preliminary findings. Am J Drug Alcohol Abuse. 2012;38:101–107. doi: 10.3109/00952990.2011.600396. [DOI] [PubMed] [Google Scholar]
- 72.Kamboj SK, Massey-Chase R, Rodney L, Das R, Almahdi B, Curran HV, et al. Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory study of the effects of D-cycloserine in heavy drinkers. Psychopharmacology (Berl) 2011;217:25–37. doi: 10.1007/s00213-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 73.Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A, et al. Effects of D-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology (Berl) 2015;232:2353–2362. doi: 10.1007/s00213-015-3882-5. [DOI] [PubMed] [Google Scholar]
- 74.MacKillop J, Few LR, Stojek MK, Murphy C, Malutinok S, Johnson F, et al. D-cycloserine to enhance extinction of cue-elicited craving for alcohol: A translational approach. Transl Psychiatry. 2015;5:e544. doi: 10.1038/tp.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinglass JE, Albano AM, Simpson HB, Wang Y, Zou J, Attia E, et al. Confronting fear using exposure and response prevention for anorexia nervosa: a randomized controlled pilot study. Int J Eat Disord. 2014;47:174–180. doi: 10.1002/eat.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levinson CA, Rodebaugh TL, Fewell L, Kass AE, Riley EN, Stark L, et al. D-Cycloserine facilitation of exposure therapy improves weight regain in patients with anorexia nervosa: a pilot randomized controlled trial. J Clin Psychiatry. 2015;76:e787–93. doi: 10.4088/JCP.14m09299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by D-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol. 1992;221:249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- 78.Monahan JB, Handelmann GE, Hood WF, Cordi AA. D-cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav. 1989;34:649–653. doi: 10.1016/0091-3057(89)90571-6. [DOI] [PubMed] [Google Scholar]
- 79.Thompson LT, Moskal JR, Disterhoft JF. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- 80.Otto MW, Basden SL, McHugh RK, Kantak KM, Deckersbach T, Cather C, et al. Effects of D-cycloserine administration on weekly nonemotional memory tasks in healthy participants. Psychother Psychosom. 2009;78:49–54. doi: 10.1159/000172620. [DOI] [PubMed] [Google Scholar]
- 81.Onur OA, Schlaepfer TE, Kukolja J, Bauer A, Jeung H, Patin A, et al. The N-methyl-D-aspartate receptor co-agonist D-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67:1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 82.Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 84.Klumpers F, Denys D, Kenemans JL, Grillon C, van der Aart J, Baas JM. Testing the effects of Δ9-THC and D-cycloserine on extinction of conditioned fear in humans. J Psychopharmacol. 2012;26:471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol Psychiatry. 2009;66:636–641. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, Yang MC, et al. D-cycloserine does not enhance exposure–response prevention therapy in obsessive–compulsive disorder. Int Clin Psychopharm. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- 87.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 88.Chasson GS, Buhlmann U, Tolin DF, Rao SR, Reese HE, Rowley T, et al. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48:675–9. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Nave AM, Tolin DF, Stevens MC. Exposure therapy, D-cycloserine, and functional magnetic resonance imaging in patients with snake phobia: a randomized pilot study. J Clin Psychiatry. 2012;73:1179–1186. doi: 10.4088/JCP.11m07564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.