Abstract
Integration of the hypothalamic-pituitary-adrenal (HPA) axis and the limbic system through glucocorticoid signaling is imperative in initiating and regulating a suitable stress response following real or perceived threats. Dysfunction of these circuits that results in a persistent or inhibited glucocorticoid secretion can severely affect processing of stressful experiences and lead to risk for developing further psychiatric pathology. Exposure to toxic chemicals found in our environment, including pesticides, metals, and industrial compounds, have been shown to have significant impact on neurological health and disease. Indeed, studies have begun to identify the HPA axis and limbic system as potential targets of many of these environmental chemicals, suggesting a possible environmental risk for damage to the stress circuit and response to stressful stimuli. This review will focus on our current understanding of the impact exposure to environmental toxicants, including bisphenol A and lead, has on the synaptic physiology of the HPA axis and limbic system and how this contributes to an alteration in behavior output. Further, this discussion will provide a starting point to continue to couple novel toxicological and neurological approaches to elaborate our understanding of the influence of environmental chemicals on the stress response and pathology.
Keywords: Catecholamines, GABA, Glucocorticoids, Glutamate, Limbic, Pesticide
1. Introduction
In situations of physical or perceived adversity, a biological and physiological response is initiated that functions to ensure a context-relevant response that counteracts the challenge and allows an organism to adapt to future stressful encounters. In order for this to occur the body relies on several different, yet highly integrated neural circuits that work in concert to elicit an appropriate action (1). Under normal conditions, an intact stress circuit will initiate a response that is both physiologically and behaviorally aligned with magnitude and valence of the stressful experience. However, various malfunctions within the stress response circuit could lead to an aberrant response that is not suitable to the situation (2). While a maladaptive stress response certainly has implications for how someone navigates society and interpersonal interactions, a chronic disruption of this circuit can lead to pathological manifestations, including risk for depression and other psychiatric concerns (3).
It is well established that there are various environmental risk factors for alterations to both the centrally mediated hypothalamic-pituitary-adrenal (HPA) and the peripherally mediated sympatho-adrenomedullary (SAM) axis stress circuitry, including maternal care, psychological and physical traumas, socioeconomic status (SES), that can severely impact normal development and maintenance of the stress response (4–9). In addition to these concerns, we must also consider the neurological impact of exposure to environmental chemicals on the specific aspects of the stress circuit. These chemical specters encroach, unseen, at various entry points in our day to day lives, either through air pollution, contaminated food and water, or the inclusion of harmful chemicals into many of our consumer products, we are exposed to an extensive and diverse chemical cocktail. Thus, on any given day we are potentially exposed to hundreds of different chemicals, many of which are known to travel to the brain and affect neurological health. With these points in mind, this review will appreciate the most salient aspects of the HPA axis and stress circuitry and will continue this discussion in the context of environmental chemicals by highlighting specific targets of this circuit that have been shown to be altered by environmental exposures. It is hoped that this discussion will serve as a starting point, from which to initiate a greater appreciation for the environmental contribution to neurological disease and facilitate further in-depth investigations to uncover the impact of environmental chemicals in modulating the stress circuit and maladies related to its dysfunction.
2. Overview of the Central and Peripheral Stress Circuitry
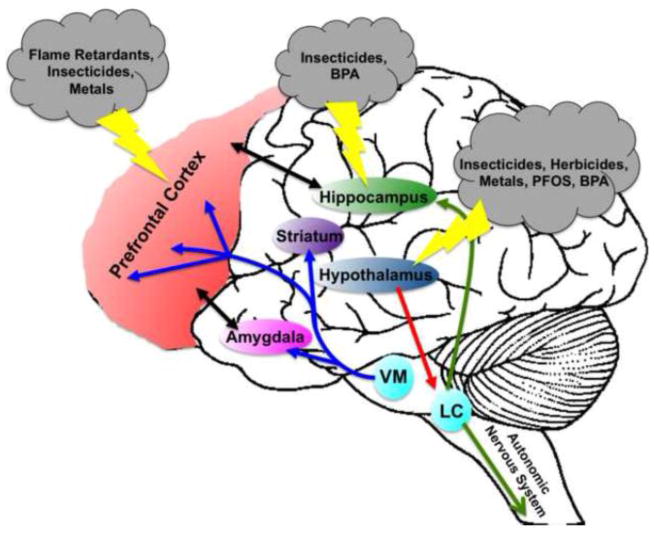
Mounting a response to a specific challenge requires equal input from both central as well as peripheral mediators of the stress circuitry. The peripheral stress response circuit is comprised of the SAM axis and is primarily tasked with integrating and transmitting viscero- and somatosensory stress stimuli. Stressful stimuli, such as visceral or somatic pain, loss of blood volume, or respiratory distress, activates sympathetic neurons in the spinal cord, which initiates the release of norepinephrine (NE) onto target organs (10). An elevation in NE stimulates an adaptive response to the stressor by increasing heart rate and respiration and mobilizing energy stores for use. Stressors that arise from the periphery are concurrently integrated with the central nervous system through ascending signals that synapse onto NE neuron populations in the locus coeruleus as well as other NE-releasing brainstem and medullary cell populations (11). These cells then transmit stress signals via projections to critical nuclei of the HPA axis and limbic system to further mediate homeostatic imbalance in the body. Thus, physical stressors as well as psychogenic stressors, such as a perceived threat or anticipated adversity are integrated and converge upon the hypothalamus, which initiates the secretion of corticotrophin-releasing hormone (CRH) from the periventricular nucleus (PVN) to the pituitary gland. In turn, adrenocorticotrophic hormone (ACTH) is sent to the adrenal gland in the periphery, stimulating the release of the glucocorticoids, cortisol (in humans) or corticosterone (in rodents) (12–14). These glucocorticoids signal through the glucocorticoid receptors (GR) and mineralcorticoid receptors (MR), which are located ubiquitously in the central and peripheral nervous systems (15) and serve as major mediators of the stress response. However, the location of these receptors in the limbic system, including the prefrontal cortex (PFC), hippocampus, and amygdala, make them especially important for responding to stressful stimuli. In addition to glucocorticoids, each of these regions is highly innervated and dependent upon glutamatergic, GABAergic, dopaminergic, and noradrenergic signaling in order to mediate the proper function of the stress pathway (Figure 1). Moreover, these circuits comprise an important feedback mechanism that communicates with the hypothalamus and serves to modulate glucocorticoid release and ultimately terminate the stress response (1, 16, 17).
Figure 1.
Localization of neurotransmitter circuits involved in the stress response and environmental toxicants that effect their function. The prefrontal cortex, amygdala, and hippocampus are the major nuclei of the limbic system that mediate the stress response through interactions with glucocorticoid and neuropeptides released centrally and peripherally. These interactions are further facilitated through dopaminergic projections (blue arrows) from the ventral tegmental area in the midbrain to the prefrontal cortex to assist with aspects of interpretation and memory of a stressful event. In addition, noradrenergic projections (green arrows) from the locus coeruleus in the brainstem to the hippocampus also participate in glutamate signaling and memory formation. An additional noradrenergic projection to the autonomic nervous system provides an important link between the peripheral and central mediators of the stress response. Signaling in the locus coeruleus is also regulated by corticotropin-releasing hormone (red arrow) sent from the paraventricular nucleus of the hypothalamus. Although simplified in this drawing, a complex reciprocal interaction (black arrows) between each of these anatomical regions provides additional regulation of the central stress response. Environmental toxicants have been shown to significantly impact the expression and function of specific neurotransmitter pathways in the stress circuit, with current research focused on the effects seen in the prefrontal cortex, hippocampus, and hypothalamus. Perturbations to neurotransmitter signaling can have severe consequences on multiple aspects of the stress circuit leading to maladaptive stress responses that may evolve into more serious neuropsychiatric disorders.
It is important to note that although this represents the conventional stress pathway, stress-induced signaling of CRH and to a lesser degree ACTH have additional functions and targets in the central and peripheral stress circuit as non-endocrine neuromodulators that are independent of CORT activation. In terms of CRH, extensive work has identified the localization and regulation of both CRH and the CRH receptor in the rodent brain (18–20). Given the role of CRH in the stress response, it is not surprising that dense populations of CRH neurons reside in many of the brain regions associated with the stress circuitry, including the cortex, hippocampus, and amygdala, as well as the PVN in the hypothalamus. Similarly, the CRH receptor is highly expressed in these same brain regions and can be activated by local CRH release or CRH projections from the PVN (21). Both scenarios contribute to the CRH-mediated regulation of behavioral responses to stress. While usually associated with perceived or impending threats, stress induces the release of CRH in areas like the hippocampus and amygdala, which then activates CRH receptors in these same regions to elicit an increase in anxiety behaviors as well as impair LTP in the hippocampus. Moreover, descending projections that release CRH to the LC in the brainstem, mediate stress-induced alterations in heart rate, blood pressure, and other autonomic outputs.
Although extensively and in many ways seamlessly integrated, regions of the limbic system involved in facilitating the stress response serve discrete functions that are imperative to a normal stress response. For example, the PFC serves an important function in decision making processes and working memory, as it relates to stressful events and plays a critical role in translating stressful events or information into action (22, 23). This is most important when the stressful situation is perceived or anticipated and the PFC must make a “value judgment” related to the magnitude of the threat. By comparing the current threat with prior stressful events an adequate physiological response is initiated. In order to accomplish this, the PFC relies on connectivity with the hippocampus and amygdala, as well as the ventral tegmental area (VTA), which sends projections to the PFC. While the hippocampus plays a critical role in learning and memory processes, the amygdala serves to integrate and consolidate emotionally salient memories for the expression of anxiety, both of which are regulated by glucocorticoids (24–26). Indeed, emotionally arousing experiences are better remembered, which allows us to recall emotional situations and apply them to future situations that are similar. Neuronal inputs, primarily from the LC to the hippocampus, may further contribute to enhance memory formation and consolidation for emotional events (27). Thus, the proper integration and functioning of these circuits is imperative to many important aspects of the stress response.
The underlying function of this circuit is extensively mediated by glutamatergic, GABAergic, dopaminergic, and noradrenergic responses and signaling following exposure to glucocorticoids and stress stimuli (1, 11, 16, 28, 29). Indeed, acute release of glucocorticoids facilitates an increase in glutamatergic output from the PFC, as seen by elevated glutamate release, in addition to upregulation and trafficking of NMDA and AMPA receptors to the plasma membrane of the postsynaptic neuron (30, 31). Similar rearrangements to glutamatergic signaling are also observed in the hippocampus and amygdala in response to glucocorticoids (32, 33). These alterations facilitate behavioral effects that serve to elaborate and strengthen the stress circuit and its adaptive responses by enhancing the formation and consolidation of emotionally salient memories (34–37). Catecholaminergic input from NE and DA circuits to the limbic system may underlie many of the alterations in glutamatergic signaling as well as the PFC response to stressful stimuli. In terms of the hippocampus, stress stimulates the release of NE from LC neurons onto the hippocampus. Elevated NE initiates phosphorylation of AMPA receptors and promotes the insertion of AMPA receptors into the postsynaptic membrane, which serves to increase glutamate signaling and long-term potentiation (LTP), which is critical to memory formation (27). Stressful stimuli and the increase in glucocorticoids have also been shown to increase the release of DA in the PFC. Interestingly, this stimulated release appears to be due to the interaction of glucocorticoids at the dopaminergic presynaptic terminal, rather than at the dopaminergic cell bodies in the VTA (29). Dopaminergic signaling in the PFC is involved in supplying the motivation and attention necessary to evaluate a stressful event and undertake the necessary behavioral response to relevant stimuli.
Integration of these limbic regions also provides an additional pathway for modulation of the stress response via direct and indirect interactions with the HPA axis, which provides a feedback mechanism to further monitor the release of glucocorticoids (1). The hypothalamus, especially the PVN is highly decorated with glucocorticoid receptors in order to provide a direct feedback pathway to monitor the levels of glucocorticoids in the system. Activation of these receptors in the PVN by glucocorticoids serves to inhibit or reduce glucocorticoid signaling. In contrast, feedback modulation of the HPA axis from the limbic system occurs via indirect mechanisms and pathways, as the projections from the PFC, hippocampus, and amygdala do not terminate directly on the PVN neurons (16, 38). In general, these pathways exert their modulation through intermediary connections that stimulate GABAergic synapses on the PVN and inhibit the HPA axis. Modulation of glucocorticoid signaling through feedback is important in maintaining safe or suitable levels of glucocorticoids in the brain (10). If this signal is disrupted due to pathology of this circuit, then a proper feedback will not occur and could contribute to a deleterious glucocorticoid signaling and stress response. Indeed, instances that limit or impair the ability of the feedback circuit to regulate glucocorticoid signaling can lead to elevated and persistent exposure to glucocorticoids, which has been shown to significantly affect the function of the stress response circuit as well as contribute to other neurological maladies, including depression and schizophrenia (3).
At each level of the limbic circuit, chronic stress can significantly alter neurotransmitter signaling and impair function of the circuit. Most notably, exposure to chronic stress leads to dendritic atrophy and spine loss in the PFC, affecting expression and function of glutamate receptors and signaling (39, 40). Specifically, persistent exposure to glucocorticoids reduces expression of various NMDA and AMPA receptor subunits, which causes impairments in performance during working memory tasks (41, 42). Stress-induced impairments in working memory may also be mediated by altered dopaminergic signaling through the mesocortical circuit (29). This projection serves an important feature in the stress response as it compares the magnitude of the current stressful event with similar past events in order to regulate an appropriate behavioral response to the situation. To execute these tasks the PFC requires precision in its DA signaling that has been best described as a U-shaped response, where in too much or too little DA can cause impairments in cognitive functions in the PFC (43). Thus, too much DA released onto the PFC as a result of chronic stress conditions could have further detrimental effects on working memory. In addition to alterations in neuronal complexity and function in the PFC, chronic stress also causes reductions in dendritic arborization in the hippocampus, leading to defects in spatial memory and other aspects of the cognitive process (44). In contrast to these reductions, chronic stress causes an increase in dendritic arborization in the amygdala. However, like the hippocampus, this alteration in the glutamatergic signaling results in the impairment of LTP and functions of memory consolidation in the amygdala (45, 46).
3. Environmental Chemicals and the Stress Response
Clearly, glucocorticoids play an imperative role in mediating many different aspects of a healthy stress response and are critical to ensuring that our stress response adapts and evolves to meet our needs. When these functions are disrupted, our ability to effectively navigate stressful situations is compromised. Early life experiences have been shown to significantly contribute to alterations in stress circuitry and a maladaptive stress response (4–7). For example, deficits in maternal care or traumatic events have been shown to affect the expression and function of key neural elements within the limbic system, which can modify the behavioral responses to stress. In addition to more psychologically based instigators, exposure to other exogenous elements, such as environmental chemicals are also risk factors for damage to the limbic system and contribute to alteration in various aspects of the stress circuit (Table 1).
Table 1.
Effects of Environmental Toxicant Exposure on the HPA Axis and Limbic Circuit
| Environmental Toxicant | Exposure | Effect on Stress Circuit | Reference |
|---|---|---|---|
|
| |||
| Insecticides | |||
|
| |||
| Hexachlorobenzene | Adult: 100 mg/kg for 5 days | Decreased plasma CORT | 64 |
|
| |||
| Lindane | Adult: | ||
| 1. >40 mg cumulative dose | Decreased plasma CORT | 65 | |
| 2. Single dose of 150 mg/kg | Decreased NE release in cortex | 85 | |
|
| |||
| Toxaphene | Adult: 1.2 mg/kg for 5 weeks | Decreased plasma CORT | 66 |
|
| |||
| Chlordane | Developmental: Single dose of 1 mg/pup (PND 4) | Decreased plasma CORT | 67 |
|
| |||
| Endosulfan | Developmental: 500 μg/kg through gestation and lactation | Decreased expression of cortical DAT and TH | 89 |
|
| |||
| Deltamethrin | Adult: Single dose of 10, 20, or 60 mg/kg | Increased plasma CORT and altered hippocampal glutamate and GABA release | 93 |
|
| |||
| Permethrin | Adult: Single dose of 10 mg/kg | Altered hippocampal glutamate and GABA release | 91, 92 |
| Developmental: 34 mg/kg PND 6–21 | Increase in NE release in frontal cortex and hippocampus | 95 | |
|
| |||
| Allethrin | Adult: Single dose of 10, 20, or 60 mg/kg | Altered hippocampal glutamate and GABA release | 93 |
|
| |||
| Cyhalothrin | Adult: Single dose of 10, 20, or 60 mg/kg | Altered hippocampal glutamate and GABA release | 93 |
|
| |||
| Herbicides | |||
|
| |||
| Atrazine | Adult: Single dose of 5, 25, 50, 75, 100, and 200 mg/kg | Increased plasma CORT and ACTH | 60–62 |
|
| |||
| Paraquat | Adult: Single dose of 25 μmol/kg | Increased plasma CORT | 63 |
|
| |||
| Industrial Chemicals | |||
|
| |||
| Polychlorinated Biphenyls (PCBs) | Adult: | ||
| 1. 10 mg/kg for 14 days | Altered glutamate signaling in the hippocampus | 107 | |
| 2. 10 mg/kg for 30 days | Altered glutamate signaling in the hippocampus | 108 | |
| Developmental: 1 mg/kg on GD 16 and 18 | Altered hippocampal GABA(B) receptor and NMDA receptors | 105 | |
|
| |||
| Polybrominated Diphenyl Ethers (PBDEs) | Adult: | ||
| 1. 0.1, 0.5, 1 mg/kg for 30 days | Decreased expression of NMDA receptors | 101 | |
| 2. 25 mg/kg for 30 days | Alterations in cortical glutamate and GABA receptors and transporter | 99 | |
| 3. 6.8 mg/kg once | Decreased expression of NMDA receptors and LTP | 102 | |
|
| |||
| Bisphenol A (BPA) | Developmental: | ||
| 1. 40 μg/kg/day | Increased plasma CORT. Decreased hippocampal GR and MR. Decreased AMPA and NMDA receptors in hippocampus and amygdala. Decreased expression of GAD 65/67 in amygdala | 125–127 | |
| 2. 500 μg/kg/day through gestation and lactation | Altered NE levels in frontal cortex and hippocampus | 129 | |
| 3. 500 μg/kg/day through gestation and lactation | Altered number of NE cells bodies in LC | 130 | |
|
| |||
| Perfluorooctanesulfonic Acid (PFOS) | Adult: | ||
| 1. 0.5–6.0 mg/kg for 28 days | Decreased plasma CORT, ACTH, and CRH | 77, 78 | |
| 2. 5, 20, or 40 mg/kg for 7 days | Increased plasma CORT | 75, 76 | |
| Developmental: 6 mg/kg on GD 12–16 | Decreased plasma CORT | 79 | |
|
| |||
| Metals | |||
|
| |||
| Lead (Pb2+) | Developmental: 50 or 150 mg/kg pre- or postnatal exposure | Altered plasma CORT. Altered hippocampal and cortical glutamate and GABA. Altered hippocampal GR. Decreased DA in cortex | 137–140 |
|
| |||
| Cadmium (Cd2+) | Adult: | ||
| 1. 1 mg/kg for 8 days | Decreased plasma CORT | 70, 71 | |
| 2. 30, 100, or 300 mg/kg for 35 days | Increased plasma CORT | 72 | |
| 3. 30, 150, or 300 mg/kg for 8 week | Decreased plasma CORT | 73 | |
| 4. 50 or 250 μg/kg for 70 days | Increased plasma CORT | 74 | |
Exposure to environmental chemicals has been shown to have severe effects on the development and function of the human brain. These compounds include pesticides used in both agricultural and residential settings to reduce the health effects of insects and increase food production. Additionally, industrial chemicals, such as solvents, metals, and flame-retardants, are introduced into many consumer products to enhance their production, function and utility. Exposure to these chemicals is common, occurring on a daily basis, either through ingestion of contaminated food and water, or inhalation of toxic chemicals both inside and outside of our homes and businesses. Alarmingly, many of these chemicals are able to travel to the brain and disrupt a multitude of neurological processes. Work over the last several decades has begun to associate many of these compounds with neurological deficits, including attention deficit hyperactivity disorder, Parkinson and Alzheimer disease, autism spectrum disorder, amyotrophic lateral sclerosis, as well as general decrements in learning and IQ and other psychomotor and neurobehavioral endpoints in children and adults (47–57). Further efforts have begun to uncover associations between many of these compounds and other psychiatric disorders, including schizophrenia, bipolar depression, which have been associated with pathological implications of chronic stress and glucocorticoid release (3, 58, 59).
Laboratory research has begun to elaborate upon these findings and identify the specific brain regions and neural circuitry that is being damaged and contributing to these neurological disorders. In short, these compounds have been found to alter various aspects of neurotransmitter function by either affecting the generation of an action potential by targeting the specific ion channels, creating deficits in neurotransmitter release by disrupting the presynaptic terminal or altering the response to each neurotransmitter by affecting postsynaptic receptor expression and function, and in many instances recapitulating the pathological and neurobehavioral features of the above mentioned diseases and disorders. The subsections that follow provide an initial appreciation of the influence exposure to specific environmental toxicants has on the function of the HPA axis as well as alterations in neurotransmitter signaling that are critically involved in mediating the stress response.
3.1 Environmental Toxicants and Glucocorticoids
Many of the pathways and brain regions that are altered by exposure to environmental chemicals, including the PFC, hippocampus, amygdala, and hypothalamus, have been implicated in facilitating the HPA axis stress response (Figure 1). More specifically, many of these compounds have been shown to affect the function of the HPA axis by increasing or decreasing the plasma levels of corticosterone (CORT) following exposure or damaging the expression and function of key neurotransmitter components that are critical to signaling in the limbic circuit. While changes in CORT levels certainly indicate a more direct alteration in HPA axis signaling, from the available studies, it is difficult to differentiate central versus peripheral effects of environmental toxicants, leading to HPA dysfunction. In addition to the HPA, disruption of neurotransmission within the limbic circuitry can also suggest points of interest that could be implicated in dysfunction of the stress response pathway.
Both herbicides and insecticides have been found to alter the levels of CORT in animal models of exposure, suggesting that exposure to these compounds could impact the normal function of the HPA axis. Atrazine is one of the most widely used herbicides in the United States to attenuate the growth and spread of nuisance plants by targeting and disrupting energy production. While atrazine has been specifically formulated to target components of plant physiology, its effects on various aspects of mammalian function are gaining wider appreciation and raising concern for its potential to affect human health. Adding to this concern is a series of studies that have found exposure of rats to a single dose of 5, 25, 50, 100, or 200 mg/kg of atrazine significantly elevates levels of both CORT and ACTH in the plasma of exposed animals (60–62). Similar elevations in CORT have also been recorded following exposure of rats to as single dose of 25 μmol/kg to the herbicide, paraquat (63). In contrast, exposure to various organochlorine insecticides, such as hexachlorobenzene, toxaphene, lindane, and chlordane have been found to reduce the basal plasma levels of CORT in exposed animal models (64–67). While a single exposure of 1 mg/kg chlordane to rats was enough to elicit these alterations in CORT, 1.2 mg/kg of toxaphene to rats for 5 weeks and a similar amount of lindane to mice were needed to cause reductions in CORT. Organochlorines exert their insecticidal effects through targeted disruption of several ion channels (sodium, potassium, calcium) involved in neurotransmission, in addition to blockade of the GABA(A) receptor, resulting in neuronal hyperexcitation and damage. As these channels are highly conserved across species, the same neuronal targets are affected in mammals, making exposure to organochlorine insecticides extremely dangerous to human health. While the manufacture and use of many of these compounds has been discontinued, measurable levels are still easily detectable in the environment and human tissue owing to organochlorines’ preferential deposition in fat and resistance to metabolism.
In addition to pesticides, we are also potentially exposed to elevated levels of metals through various exposure scenarios. Metals such as cadmium, manganese, arsenic, lead, and mercury, to name a few are routinely found in our food and water, as well as in the air we breathe. Moreover, our exposure to these elements can significantly increase above the normal population through occupational exposure or other activities. While the contribution of lead to dysregulation of the stress response circuit will be more fully appraised in subsequent sections, it is important to note that other metals have also been shown to alter aspects of the stress response, specifically levels of glucocorticoids. Of the common metals that we are routinely exposed to the most abundant body of work related to alterations in glucocorticoid signaling has been performed with cadmium. Exposure of the human population to cadmium generally occurs through food, where levels of cadmium in shellfish have been estimated at 1–2 mg/kg, while rice and wheat range from 10–150 μg/kg. Cadmium is also a major constituent of cigarette smoke, which can significantly increase body burden of this metal. Furthermore, occupational exposure to cadmium can also elevate body level, as it is a component or byproduct of metal manufacturing as well as paint production (68, 69). While numerous studies exist that evaluate the effect of cadmium exposure on glucocorticoid levels, the alterations elicited are variable and appear to be defined by the exposure paradigm used in each study, taking into account the concentration of cadmium given as well as the duration of exposure. For instance, while a single exposure to 0.3, 1.5, 3.0, or 6.0 mg/kg of cadmium did not appear to affect levels of CORT in the plasma of exposed animals, treatment with a similar concentration of 1 mg/kg of cadmium for 7 or 8 days did elicit a significant reduction in CORT levels (70, 71). As the concentration of cadmium administered increases it appears that at similar concentrations, duration of exposure may significantly contribute to alterations in CORT. For example, treatment with 30, 100, or 300 mg/kg cadmium for 35 days resulted in an increase in CORT levels (72). Yet, using similar concentrations of cadmium (30, 150, 300 mg/kg) for 8 weeks caused a reduction in plasma CORT concentrations (73). Finally, a study of particular interest exposed animals to 50 or 250 μg/kg cadmium for 70 days and found an increase in plasma CORT (74). This study is highlighted, as the concentrations of cadmium given are congruent with levels that the human population would feasibly be exposed to, especially if exposures are elevated through smoking or occupational settings.
Manufacturing and production of various consumer products is a major source of environmental toxicants. Of these compounds a significant amount of recent attention has been given to the impact of perfluorinated chemicals on the environment as well as human health. Of these compounds, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) has become the most recognizable and well studied, given their extensive use in nonstick or stain repellant consumer products, such as Teflon-coated cookware and stain resistant clothing and materials. Concern regarding these products is focused on their durability and persistence in the environment. Indeed, many of the same properties that make them so appealing to manufacturers and consumers, such as chemical stability also contribute to their accumulation and health concerns once they enter the environment and human body. These concerns are heightened given the fact that the biological half-life elimination of these chemicals is estimated to be 5 years, suggesting a chronic or persistent exposure scenario for the human population. The majority of studies that have appreciated the impact of perfluorinated chemicals on the stress pathway have done so from the standpoint of adult exposure to PFOS and subsequent assessment of plasma CORT levels and in some cases, determination of ACTH and CRH. While one study found elevations in CORT following exposure to 5, 20, or 40 mg/kg PFOS for 7 days, other studies that utilized lower doses of PFOS (0.5–6.0 mg/kg) for 28 days observed a significant reduction in CORT levels that were accompanied by similar reductions in ACTH and CRH (75–78). Related to these findings, appreciation of the effects of PFOS following a developmental exposure may also inform the potential impact of this compound on the stress circuitry. Using an abbreviated developmental exposure paradigm, treatment of pregnant mice with 6 mg/kg PFOS from gestational day 12–16 also caused a reduction in plasma CORT levels in the offspring (79).
3.2 Environmental Toxicants and Neurotransmitter Signaling
While changes in CORT provide a strong argument for the disruption of the HPA axis by environmental toxicants, additional endpoints should be appreciated when considering the potential contribution of specific toxicants to modifying the stress response (Figure 1). Previous sections of this review have already delineated the role of the glutamatergic, GABAergic, noradrenergic, and dopaminergic signaling pathways in the limbic circuit in mediating the stress response. With this in mind, it can be appreciated that alteration to the normal functioning of these pathways, which can either manifest as a change in neuronal activation, transmitter release or postsynaptic response to neuronal signaling, can have severe consequences on activation of the stress pathway. While not explicitly assessed in the context of evaluating the HPA axis and stress response, a variety of studies have contributed to our understanding of how exposure to specific environmental toxicants ultimately impacts the integrity and function of specific neuronal populations throughout the brain, including regions involved in mediating the stress response. In this regard, organochlorine and pyrethroid insecticides as well as chemicals with flame retardant properties provide an initial discussion point of how environmental compounds can affect specific neurotransmitter pathways and circuits, which could severely impact the interpretation and response to a stressful event. Similar to the discussion of alteration of glucocorticoid signaling following toxicant exposure, the majority of available data has focused on the impact of specific environmental toxicants on impairment of neurotransmission in the central nervous system, highlighting a gap in our general understanding of the impact of toxicants on the peripheral nervous system.
As discussed above, organochlorine insecticides exert their toxicity through targeting of specific neuronal components, leading to neuronal overactivation. Although all neurons rely on the movement of sodium and calcium ions for normal neurotransmission, assessment of the neurological effects of exposure to organochlorine insecticides has focused on alterations to monoamines, specifically dopamine and norepinephrine. Recording in vivo or from primary cultured neurons and brain slices exposed to the well-recognized organochlorine insecticides, DDT and lindane has found significant alterations to the release and metabolism of norepinephrine in these preparations (80–85). Most notably, 50 μM of lindane bath applied to hippocampal and cortical slices elicited a significant increase in NE release, while a single in vivo exposure to 150 mg/kg of lindane resulted in a reduction of NE in the cortex of exposed rats.
Organochlorine insecticides have also been extensively shown to alter the function of the dopamine system. These studies have primarily focus on damage to the nigrostriatal dopamine circuit in relation to Parkinson disease, whose pathology is uniquely driven by damage to the dopamine neurons and projections in this pathway (49, 86–88). Beyond the striatum, evidence is emerging that shows exposure to organochlorines can have significant effects on the mesocortical dopamine pathway. Using a developmental exposure to 500 μg/kg endosulfan throughout gestation and lactation, our group found a significant reduction in the expression of the dopamine transporter (DAT) and tyrosine hydroxylase (TH) in the frontal cortex of 4 month old male offspring (89).
Like organochlorine insecticides, pyrethroid insecticides are formulated to specifically target explicit aspects of the neuron, leading to its disruption and ultimate demise of the insect. In contrast to organochlorines, pyrethroids generally target the sodium channel, where they alter its conformation and function, resulting in an exaggerated influx of sodium ions during an action potential and a hyperexcitation of the neuron (90). The impact of these compounds on the glutamatergic and GABAergic transmitters systems has been extensively studied using both in vitro and in vivo models of neurotransmission (91, 92). Recording of glutamate and GABA release in the hippocampus of rats who have been administered increasing concentrations of various pyrethroid insecticides uncovered a dose and chemical dependent effect on these circuits. While a single dose of 10 and 20 mg/kg of allethrin elicited an increase in glutamate release and concomitant reduction in GABA release, a single dose of 60 mg/kg resulted in a reversal of these findings, with glutamate release being inhibited while GABA neurotransmission was increased. However, treatment with 10, 20, or 60 mg/kg cyhalothrin resulted in an increase in glutamate release and a reduction in GABA release at each dose (93). This differential effect on glutamate release was also observed when primary cultured hippocampal neurons were exposed to 10 μM deltamethrin or permethrin, two other pyrethroid insecticides (91, 92). While the precise mechanisms responsible for these dose and compound dependent alterations in glutamate and GABA signaling is unclear, it is important to note that pyrethroid insecticides can be stratified into Type I and Type II subclasses of compounds based on the presence of an α-cyano group to their chemical structure. Although both Type I and Type II compounds potently affect the function of the neuronal sodium channel, Type II compounds have also been shown to be more effective at stimulating calcium influx and inhibiting voltage-gated chloride channels, which could participate in a greater effect on glutamatergic and GABAergic signaling (94).
Similar to the organochlorine chemicals, assessment of the effects of pyrethroid insecticides on monoamine circuits of the brain have primarily been focused on dysregulation of dopamine signaling in the striatum, with minimal attention given to alterations in other monoaminergic circuits, although a single study did identify elevations in NE in both the PFC as well as the hippocampus following exposure to 34 mg/kg permethrin from postnatal day 6–21 (95).
Various aspects of neurotransmission and function are also targets of other environmental toxicants, including polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs). These compounds are synthetic chemicals manufactured and used in electrical equipment and furniture in order to provide thermal stability and reduce the flammability of these products. It is important to note, while various herbicides and insecticides are specifically formulated to target and disrupt select physiological functions, most industrial compounds were never intended to affect these functions and were not produced to have a discrete mechanism of action or defined target, either in the nervous system or any other organ system. However, extensive amounts of data collected over the last several decades have provided support to the idea that these compounds are readily found in our environment and our bodies and can have severe effects on the function of the nervous system (96–98). Work from our group has found exposure of mice to 30 mg/kg of PBDEs for 30 days significantly affects the expression of specific GABAergic and glutamatergic transporter and receptors in the frontal cortex, which could affect overall neurotransmitter signaling and response in this region (99). These findings are supported by work by Fonnum and Mariussen that demonstrated PBDEs to significantly inhibit the function of both GABA and glutamate transporters in isolated synaptosomes and synaptic vesicles treated with up to 20 μM PBDEs (100). Additionally, exposure to these compounds also affects learning and memory and has been shown to reduce the expression of several NMDA receptor subunits in the hippocampus of mice exposed to 0.1, 0.5, or 1 mg/kg of the PBDE isomer, BDE-47 for 30 days (101, 102). Similar results have been demonstrated with PCBs, where PCBs have been shown to inhibit the function of both the GABA and glutamate transporters in brain and shown to disrupt GABAergic and glutamatergic receptors and neuronal signaling in the frontal cortex, hippocampus and hypothalamus (103–108). Indeed, treatment of pregnant rats with 1 mg/kg PCBs on gestational day 16 and 18 caused significant alterations the GABA(B) receptor as well as the NMDA receptor subunits, NR2B and NR2C. In contrast, exposure of male rats to 10 mg/kg of the PCB mixture, Aroclor 1254 for 14 or 30 days severely disrupted glutamate signaling in the hippocampus and frontal cortex, respectively. In contrast to the GABA and glutamatergic circuit, a paucity of data exists that appraises the impact exposure to flame retardant compounds could have on catecholaminergic projections to specific regions of the limbic system. Interestingly, while we have conducted work with PBDEs and PCBs that investigated the impairment of the dopamine circuit in the striatum we did not detect alterations of the dopamine projections in the frontal cortex, when specifically evaluated.
4.0 Integrated Assessment of Toxicant-Induced Impairment of the Stress Response
The above studies provide initial information from which to hypothesize the potential contribution of environmental toxicants in influencing the stress response. However, as these data have not been integrated into a singular experimental approach in which alterations to explicit HPA axis endpoints, such as CORT have been paired with relevant neurobehavioral and neuropathological assessment a knowledge gap regarding the potential contribution of exposure to these compounds may play in modulating the stress response remains for many chemicals we are routinely exposed to. Although very few environmental chemicals have been evaluated in the context of the stress pathway and the stress response, two compounds, BPA and lead, have received a significant amount of attention and their effects on the neurotransmitter circuitry and glucocorticoid signaling in the HPA axis and augmentation of stress response have been assessed in multiple studies, providing a more comprehensive understanding of the influences of these compounds on the stress pathway.
4.1 Bisphenol A and Alterations to Stress Response
Bisphenol A (BPA) is a high volume synthetic chemical used extensively in the manufacture and production of polycarbonate plastic containers, as well as a major constituent of resin used to line food and beverage cans. Rather than being chemically integrated into the manufacturing process, BPA is considered an additive, allowing its presence in consumer goods to be extremely labile and prone to leach from the plastic product under situations of high heat. As a result, BPA is readily recorded in the environment, as well as human tissue, being found in amniotic fluid, breast milk, and plasma, making exposure to BPA a critical concern for the developing child (109–111). Although the current established threshold exposure to BPA has been set at 50 μg/kg-bw/day, several investigations have found BPA at concentrations much lower can effect endocrine signaling, suggesting a difficulty in truly establishing and regulating safe levels of BPA in the environment and in human tissue.
The dangers of BPA lie in its characterization as an estrogen receptor agonist, capable of mimicking estrogen-mediated cellular signaling (112, 113). These effects appear to be mediated by both a classical genomic signaling pathway, as well as a non-genomic signaling pathway, coincident with the localization of estrogen receptors in the nucleus, cytosol, and imbedded in the plasma membrane of cells. While the classical mechanism relies on dimerization of the estrogen receptor following activation, translocation to the nucleus and binding to estrogenic response elements on gene promoters, the non-genomic pathway stimulates gene expression through cellular signaling cascades induced by activation of plasma membrane estrogen receptors. Thus, exposure to BPA can create a milieu defined by persistent or hyperactivation of estrogen receptors, which can impact normal estrogenic signaling and cellular response.
The impact of exposure to BPA has raised concerns given the importance of estrogen and estrogen receptors in many aspects of neurodevelopment, including stimulation of proliferation and cellular differentiation, neurite outgrowth and branching, synaptogenesis, and neuronal survival (114, 115). Several studies have evaluated the effects of developmental BPA exposure and the impact this compound has on function of the HPA axis and the exposed offspring’s response to stressful situations. It is important to note that the majority of these studies were performed using concentrations of BPA that have been suggested to be in line or below the federal regulation for daily exposure to these compounds (50 μg/kg-bw/day). These are important considerations to keep in mind when evaluating behavioral and physiological endpoints in response to specific compounds, which may exceed environmentally relevant exposure levels.
The majority of these studies evaluated offspring that had been exposed to environmentally relevant levels of BPA during gestation and lactation, thus mimicking as closely as possible the route and duration of exposure that a normally developing human fetus would encounter. These studies then assessed the performance of both male and female offspring in well-established behavioral paradigms used to demonstrate an anxiety-like phenotype, such as elevated plus maze, locomotor activity, and light-dark test. Overwhelmingly, when tested, developmental exposure to BPA resulted in significant alterations in the stress pathway as shown by demonstrating an increase in anxiety-like behaviors in BPA exposed animals, compared with control. This phenotype manifested as less time spent in the center area during open field test, a reduced time spent in the open arm of the elevated plus maze, as well as less time spent in the light portion of the light-dark test (116–121). These findings are supported by recent epidemiological data that suggest exposure to BPA during key time points of neurodevelopment is associated with the development or increased expression of anxiety behaviors as well as behaviors that are considered depressive, which is an additional pathology associated with dysregulation of the HPA axis and stress pathway (122, 123).
Behavioral data from animal studies suggesting BPA-induced alterations in the stress response are corroborated by concurrent increases in plasma levels of CORT, and in some instances increases in ACTH and CRH in the offspring who have been developmentally exposed to BPA compared with control animals (116, 119, 121, 124). In some instances these elevations in CORT were also accompanied by changes in the expression of the GR and MR in various regions involved in the stress response. These findings appeared to be stratified by gender, with males and females demonstrating a differential alteration in these receptors. For example, a study by Panagiotidou et al., (124) found a reduction in GR and MR mRNA in both the hippocampus and the hypothalamus of female offspring. However, in contrast, male offspring only had a reduction in GR in the hippocampus.
Developmental exposure to BPA also affected select circuits that are imperative to normal function within the stress pathway. Several studies identified alterations in specific glutamatergic receptors, including the AMPA receptor subunit, mGluR1 and the NMDA receptor subunit NR1 expression being significantly reduced in the hippocampus and the amygdala following developmental exposure to BPA (125). Additionally, adolescent exposure to BPA caused a significant loss of spine density of pyramidal neurons in both the PFC and hippocampus (126, 127). In addition to alterations in glutamatergic signaling, multiple studies found developmental exposure to BPA also disrupted the expression and function of GABAergic signaling in the limbic system. Two studies found a reduction in the expression of GAD65 and GAD67, which are critical enzymes involved in the synthesis of GABA. Interestingly, these alterations were found to occur in the basolateral amygdala (BLA) of BPA exposed offspring. Furthermore, the reduction in GAD67 appeared to be resultant of an epigenetic modification caused by an increase of DNA methyltransferase1 and hypermethylation of the GAD67 promoter region, resulting in a reduction in the expression of the gene and protein (128). These findings are important as the GABAergic circuit within the BLA provides a critical inhibitory signal to glutamatergic neurons. A reduction in GABAergic signaling could feasibly cause a reduction in the inhibitory control of glutamatergic signaling and result in an increase in excitatory input sent from the BLA to the HPA axis. Additional dysfunction has been reported in the dopaminergic and noradrenergic systems following BPA exposure. Recent work from Ogi et al., (129) found gender-dependent alterations in levels of NE in the frontal cortex and hippocampus, where in, female offspring exposed to 500 μg/kg BPA during gestation and lactation showed an elevation in NE in these regions while male mice were spared. A similar gender difference was also found when quantification of NE cell bodies in the LC was conducted following the same exposure to BPA, with female mice had a reduction in NE neurons in the LC, while male mice showed an increase in NE neurons in this region. Interestingly, whether an elevation of reduction was observed, no change was seen in the density of NE fiber projections to the frontal cortex (130).
4.2 Developmental Lead Exposure and Stress Pathways
For decades we have recognized the deleterious effects of lead exposure, especially in young children who have been exposed during critical periods of brain development. Most significantly, these exposures have been associated with severe declines in intellectual capacity as well as other behavioral impairments, including reduced attention, increased impulsivity and risk for ADHD and schizophrenia (131–134). While substantial measures have been taken in order to reduce lead exposure in the human population and lowering the acceptable threshold of blood lead level to 10 μg/dl, more recent work has found that deficits in IQ may be even greater at significantly lower concentrations of lead (135). These data suggest that there may not be a neurologically safe level of lead that can be retained in the body, especially in children.
The pathological targets that underlie intellectual and behavioral abnormalities have primarily focused on lead-induced impairment of the glutamatergic neurotransmitter circuit, in addition to alterations to signaling within GABAergic neurons, especially in the hippocampus and frontal cortex. Through these studies investigators have pinpointed multiple cellular targets and pathways that are implicated in lead-induced deficits in learning. Overall, exposure to lead has been found to significantly affect various aspects of neurotransmission, through its interaction with both pre and postsynaptic elements of the glutamate and GABA system. In particular, lead has been shown to disrupt release of GABA and glutamate at the synapse by blocking calcium influx through voltage gated calcium channels. In addition, several vesicle-associated proteins that are implicit to synaptic vesicle release are also targeted by lead, including reductions in synaptophysin, synaptobrevin, and disruption of the calcium sensing vesicular protein, synaptotagmin (136). Postsynaptically, lead has been demonstrated to target the NMDA glutamate receptors, causing a reduction in NMDA receptor subunit N2A and further impeding glutamatergic signaling as an inhibitor of the NMDA receptors (136).
As these regions are intimately linked within the limbic circuit to the stress response, many studies have been performed to assess how exposure to lead during critical periods of neurodevelopment could affect these regions along with the function of the HPA axis in mediating relevant behavioral outputs. A large body of work related to these questions has been assembled and significantly contributes to our depth of understanding regarding how lead exposure can impact the stress response circuitry. In sum, these studies highlight the complexities of the neurological impact of lead, which appear to be mediated by duration and timing of exposure during development, gender, and neurochemical and behavioral endpoints evaluated. However, an interesting story emerges that clearly supports the negative effects of lead exposure on stress-related outcomes. Most notable, exposure to lead during development results in blood lead levels that are congruent with the governmental levels set as acceptable concentrations of lead in blood of children (approximate 10 μg/dl), suggesting good model validity for relevant human exposure to lead (137). Using this well-defined model of lead exposure, investigators routinely found alteration in basal plasma CORT levels that were dependent upon gender of the offspring as well as the level of lead exposure that was administered (137–140). Related to these alterations in CORT levels, offspring were also shown to have variations in expression of the GR in the hippocampus and when challenged with exposure to dexamethasone, offspring that were developmentally exposed to lead demonstrated an attenuated response as seen by fluctuations in CORT levels over 24 hours that were significantly different from control animals (139). In addition to these alterations, treatment with lead alone elicited a significant reduction in levels of dopamine in the frontal cortex (138). When adult offspring were further challenged with a stressful event, such as restraint, levels of CORT did not change relative to levels found in lead exposed offspring that did not receive restrain stress. In contrast, animals that did not receive lead showed a drastic influx of CORT following the restraint protocol. When combined, developmental exposure to lead and restraint stress as adults manifest as alterations in learning behavior as assessed by a fixed-interval reinforcement training paradigm (138, 140). These findings provide a clearer picture of the potential impact of developmental lead exposure on the function of the HPA axis and suggest these exposures may contribute to a stunted or attenuate stress response to stimuli and contribute to impairments in learning. As discussed previously, such alterations implicate additional concerns for future pathology related to a maladaptive HPA axis.
Exposure to environmental toxicants does not exist in isolation. Rather they occur in the context of exposure to other environmental chemicals and coincident with other environmental factors. One setting of particular concern is communities of low SES, as this population tends to have a higher concentration of families living in older housing that contains lead-based paint and other products and as a result children in these communities demonstrate the highest levels of lead in their bodies (141). Compounding this public health concern, this population is also subject to increased stress and incidence of disease that are associated with chronic or persistent stress (8, 9). Thus, multiple studies have been focused on addressing the concern of the impact of combined exposure to lead and stress on the function of the HPA axis and neurobehavior. Most notably, using a developmental exposure paradigm that combined pre and postnatal exposure to lead with maternal restraint stress administered at gestational day 16 and 17, investigators similarly found blood lead levels that aligned with threshold levels set by federal agencies (138–140, 142). As above, this exposure paradigm elicited a differential change in CORT levels that was dependent upon gender. While female offspring demonstrated a significant increase in plasma CORT following lead exposure, their CORT concentrations were attenuated when lead exposure was paired with maternal stress. These findings appear to mirror results from offspring that were developmentally exposed to lead followed by a postnatal stressor, again, indicting that when lead is paired with stress an attenuated or stunted response in the HPA axis is elicited. In conjunction with alterations in CORT levels, female offspring also demonstrated learning impairments that were similarly evident in animals that only received lead and animals that received a combined exposure to lead and maternal stress. Reiterating the complexities and heterogeneity of evaluating developmental lead exposure and stress, CORT levels in male rats did not show a change in either the lead only or combined lead and maternal stress exposure groups. Yet, while not as robust as their female counterparts, males also exhibited impairments in learning. The differential gender effects on CORT raises intriguing questions regarding the impact of the role of CORT in learning impairments, at least as assessed with these behavioral paradigms and implicates other factors that may be an additional mediator that needs to be considered.
5. Conclusions and Future Directions
Response to a physical or perceived stressor demands the highly orchestrated integration of multiple brain regions and neurotransmitter circuits in order to mediate the proper interpretation, response, and memory consolidation of these experiences that will serve as a template for future scenarios. Disruption of any of these working parts can have deleterious consequences, both neurologically as well as behaviorally for the sufferer. The importance and sensitivity of the HPA axis and limbic system has identified it as a potential target for alteration and disruption through various means. In addition to the impact of physical and psychological situations on the development and ultimate function of stress response circuit, we have also slowly begun to appreciate the effects of environmental toxicants to the function of the brain and the HPA axis and limbic system.
Unfortunately, in many ways we have fallen short in establishing the true impact of these chemical exposures to our neurological health. Several arguments can be made as to the underlying reason for this shortcoming, with the most simplistic answers concerning the sheer volume of chemicals that are being used, coupled with the lack of manpower to adequately evaluate their safety, that goes hand and hand with porous regulatory management. However, these do not absolve us from rigorously undertaking the task of determining the impact of environmental factors in neurological disease. Thus, given the importance of the stress response to our normal functioning in daily lives, it becomes paramount that we begin to create a richer understanding of the influence of environmental toxicants and their effect on the stress response circuit. As discussed in this review, a wealth of data illuminates the idea that exposure to these compounds could affect the normal functioning of the HPA axis and limbic system, resulting in alterations of the stress pathway from several different angles. Just as with psychological perturbations of this system, environmental exposures could manifest in dysfunction at various set points within the stress circuit, resulting in an inability to store and retrieve past stressful memories in the hippocampus and inability to properly interpret and perceive a stressful or threatening situation. Further, they could alter the feedback of glucocorticoids and potentiate a chronic over or underactivation of the stress circuit, which could contribute towards various pathological endpoints.
As seen from work performed with BPA and lead, we have developed a working template that integrates the most salient physiological and behavioral endpoints needed to provide a concise assessment of how other chemicals can affect the stress response. With this in mind we have set the stage to establish a way forward and advance our understanding of environmental toxicants and the stress response. However, a framework should be considered that would help to guide critical next steps in this process. Below I have outlined and briefly discussed three points that need to be appreciated as the field moves forward and progresses. By no means exhaustive, these points should serve to initiate discussion and be further extended in order to advance the field. First, we need to continue to elaborate our understanding of toxicant-induced disruption of the stress response by coupling strong toxicological approaches with well-established and progressive neuroscience techniques that will capitalize on the strengths of these two disciplines. In this regard, it is critical to be cognizant of environmentally relevant levels of exposure that generate relevant tissue concentrations of these compounds. Pairing these approaches with cutting edge biochemical and neurophysiological methodology, such as electrophysiology and optogenetics will provide a deeper understanding of the cellular and molecular pathways within the stress response circuit that are disrupted following exposure to environmental toxicants. Through these approaches we will be better positioned to address some of the major gaps in our understanding of the impact environmental toxicants have on the stress pathways. For example, devoting more attention to the impact exposure to environmental toxicants has on the function of the SAM axis in mediating the stress response. Additionally, delineating toxicant-induced alterations in CRH signaling that are independent and dependent on CORT release from the adrenal gland. Second, it is critical that we identify and direct research efforts towards the most environmentally salient chemicals. Given the volume of potential chemical exposures and our lack of understanding regarding their general toxicological functions, it is easy to get overwhelmed and lose sight of the most important chemical subjects. Determining which chemicals warrant the greatest effort can leverage current data from epidemiological and exposure science findings that will help identify chemicals and populations most severely affected by specific chemical compounds. This knowledge can help redirect the effort towards the most relevant chemicals and the most critical need. Third, as our understanding of the contribution chemical toxicants make to the risk of a dysfunctional stress response it will be important to integrate specific stress response endpoints into the chemical safety assessment and regulatory framework in order to ensure an initial evaluation of current and future newer for potential effects on the stress circuit. As the contribution of exposure to environmental contaminants continues to be recognized as exerting a larger and larger influence on our neurological health, elaboration of these perspectives will serve to stimulate our further understanding and growth in the field of neurotoxicology and stress circuitry.
Highlights.
HPA axis and limbic system function in parallel to mediate the stress response
Environmental toxicants have been shown to damage the HPA axis and limbic region
Chemicals cause alterations in glucocorticoids and neurotransmitter signaling
More work is needed to fill gaps in our understanding of chemicals in stress response
Acknowledgments
Financial support for this work was provided by a pilot grant from the Emory HERCULES Exposome Research Center P30 ES019776 (WMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cellular and molecular neurobiology. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herman JP. Neural control of chronic stress adaptation. Frontiers in behavioral neuroscience. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews. Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 4.Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American journal of psychiatry. 2001;158(4):575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- 5.Luecken LJ, Kraft A, Hagan MJ. Negative relationships in the family-of-origin predict attenuated cortisol in emerging adults. Hormones and behavior. 2009;55(3):412–417. doi: 10.1016/j.yhbeh.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews. Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 7.Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Development and psychopathology. 2010;22(1):165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biological psychiatry. 2000;48(10):976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 9.Pincus T, Callahan LF, Burkhauser RV. Most chronic diseases are reported more frequently by individuals with fewer than 12 years of formal education in the age 18–64 United States population. Journal of chronic diseases. 1987;40(9):865–874. doi: 10.1016/0021-9681(87)90186-x. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews. Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiological reviews. 2009;89(2):535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EO, Kamilaris TC, Chrousos GP, Gold PW. Mechanisms of stress: a dynamic overview of hormonal and behavioral homeostasis. Neuroscience and biobehavioral reviews. 1992;16(2):115–130. doi: 10.1016/s0149-7634(05)80175-7. [DOI] [PubMed] [Google Scholar]
- 13.Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8(4):383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dallman MF, et al. Regulation of ACTH secretion: variations on a theme of B. Recent progress in hormone research. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 15.Meaney MJ, Sapolsky RM, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. II. An autoradiographic study. Brain research. 1985;350(1–2):165–168. doi: 10.1016/0165-3806(85)90260-3. [DOI] [PubMed] [Google Scholar]
- 16.Myers B, Mark Dolgas C, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain structure & function. 2014;219(4):1287–1303. doi: 10.1007/s00429-013-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica... [et al.] 2012;45(4):292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Brunson KL, Muller MB, Cariaga W, Baram TZ. Immunocytochemical distribution of corticotropin-releasing hormone receptor type-1 (CRF(1))-like immunoreactivity in the mouse brain: light microscopy analysis using an antibody directed against the C-terminus. The Journal of comparative neurology. 2000;420(3):305–323. doi: 10.1002/(sici)1096-9861(20000508)420:3<305::aid-cne3>3.0.co;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korosi A, Baram TZ. The central corticotropin releasing factor system during development and adulthood. European journal of pharmacology. 2008;583(2–3):204–214. doi: 10.1016/j.ejphar.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacological reviews. 1991;43(4):425–473. [PubMed] [Google Scholar]
- 21.Chen Y, et al. Hippocampal corticotropin releasing hormone: pre- and postsynaptic location and release by stress. Neuroscience. 2004;126(3):533–540. doi: 10.1016/j.neuroscience.2004.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKlveen JM, et al. Role of prefrontal cortex glucocorticoid receptors in stress and emotion. Biological psychiatry. 2013;74(9):672–679. doi: 10.1016/j.biopsych.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiology of learning and memory. 1997;67(2):176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 25.Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. The European journal of neuroscience. 1997;9(1):76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 26.Roozendaal B, Quirarte GL, McGaugh JL. Stress-activated hormonal systems and the regulation of memory storage. Annals of the New York Academy of Sciences. 1997;821:247–258. doi: 10.1111/j.1749-6632.1997.tb48284.x. [DOI] [PubMed] [Google Scholar]
- 27.Morilak DA, et al. Role of brain norepinephrine in the behavioral response to stress. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29(8):1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature reviews. Neuroscience. 2012;13(1):22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nature reviews. Neuroscience. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hascup ER, et al. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. Journal of neurochemistry. 2010;115(6):1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuen EY, et al. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(33):14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karst H, Joels M. Corticosterone slowly enhances miniature excitatory postsynaptic current amplitude in mice CA1 hippocampal cells. Journal of neurophysiology. 2005;94(5):3479–3486. doi: 10.1152/jn.00143.2005. [DOI] [PubMed] [Google Scholar]
- 34.de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends in neurosciences. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 35.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24(6):1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roozendaal B, Portillo-Marquez G, McGaugh JL. Basolateral amygdala lesions block glucocorticoid-induced modulation of memory for spatial learning. Behavioral neuroscience. 1996;110(5):1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 37.Smeets T, et al. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology. 2009;34(8):1152–1161. doi: 10.1016/j.psyneuen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerqueira JJ, Taipa R, Uylings HB, Almeida OF, Sousa N. Specific configuration of dendritic degeneration in pyramidal neurons of the medial prefrontal cortex induced by differing corticosteroid regimens. Cerebral cortex. 2007;17(9):1998–2006. doi: 10.1093/cercor/bhl108. [DOI] [PubMed] [Google Scholar]
- 40.Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Journal of neurobiology. 2001;49(3):245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- 41.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(3):707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuen EY, et al. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73(5):962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Advances in pharmacology. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 44.Alfarez DN, Joels M, Krugers HJ. Chronic unpredictable stress impairs long-term potentiation in rat hippocampal CA1 area and dentate gyrus in vitro. The European journal of neuroscience. 2003;17(9):1928–1934. doi: 10.1046/j.1460-9568.2003.02622.x. [DOI] [PubMed] [Google Scholar]
- 45.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(15):6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braun JM, et al. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environmental health perspectives. 2014;122(5):513–520. doi: 10.1289/ehp.1307261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caudle WM, Guillot TS, Lazo CR, Miller GW. Industrial toxicants and Parkinson’s disease. Neurotoxicology. 2012;33(2):178–188. doi: 10.1016/j.neuro.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatcher JM, Pennell KD, Miller GW. Parkinson’s disease and pesticides: a toxicological perspective. Trends in pharmacological sciences. 2008;29(6):322–329. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malek AM, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neuro-degenerative diseases. 2014;14(1):31–38. doi: 10.1159/000355344. [DOI] [PubMed] [Google Scholar]
- 51.Rauh VA, et al. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(20):7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson JR, et al. Elevated serum pesticide levels and risk for Alzheimer disease. JAMA neurology. 2014;71(3):284–290. doi: 10.1001/jamaneurol.2013.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richardson JR, et al. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(5):1960–1972. doi: 10.1096/fj.14-260901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sagiv SK, Thurston SW, Bellinger DC, Altshul LM, Korrick SA. Neuropsychological measures of attention and impulse control among 8-year-old children exposed prenatally to organochlorines. Environmental health perspectives. 2012;120(6):904–909. doi: 10.1289/ehp.1104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shelton JF, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environmental health perspectives. 2014;122(10):1103–1109. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shelton JF, Hertz-Picciotto I, Pessah IN. Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environmental health perspectives. 2012;120(7):944–951. doi: 10.1289/ehp.1104553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Y, et al. Environmental risk factors and amyotrophic lateral sclerosis (ALS): a case-control study of ALS in Michigan. PloS one. 2014;9(6):e101186. doi: 10.1371/journal.pone.0101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beard JD, et al. Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environmental health perspectives. 2014;122(9):984–991. doi: 10.1289/ehp.1307450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for Schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2012;33(3):560–574. doi: 10.1016/j.neuro.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laws SC, et al. Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male Wistar rats. Toxicological sciences : an official journal of the Society of Toxicology. 2009;112(1):78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- 61.Fraites MJ, et al. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicological sciences : an official journal of the Society of Toxicology. 2009;112(1):88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- 62.Hotchkiss MG, Best DS, Cooper RL, Laws SC. Atrazine does not induce pica behavior at doses that increase hypothalamic-pituitary-adrenal axis activation and cause conditioned taste avoidance. Neurotoxicology and teratology. 2012;34(3):295–302. doi: 10.1016/j.ntt.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 63.Edmonds BK, Edwards GL. The area postrema is involved in paraquat-induced conditioned aversion behavior and neuroendocrine activation of the hypothalamic-pituitary-adrenal axis. Brain research. 1996;712(1):127–133. doi: 10.1016/0006-8993(95)01419-5. [DOI] [PubMed] [Google Scholar]
- 64.Lelli SM, Ceballos NR, Mazzetti MB, Aldonatti CA, San Martin de Viale LC. Hexachlorobenzene as hormonal disruptor--studies about glucocorticoids: their hepatic receptors, adrenal synthesis and plasma levels in relation to impaired gluconeogenesis. Biochemical pharmacology. 2007;73(6):873–879. doi: 10.1016/j.bcp.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 65.Lahiri P, Sircar S. Suppression of adrenocortical function in female mice by lindane (gamma-HCH) Toxicology. 1991;66(1):75–79. doi: 10.1016/0300-483x(91)90179-5. [DOI] [PubMed] [Google Scholar]
- 66.Mohammed A, Hallberg E, Rydstrom J, Slanina P. Toxaphene: accumulation in the adrenal cortex and effect on ACTH-stimulated corticosteroid synthesis in the rat. Toxicology letters. 1985;24(2–3):137–143. doi: 10.1016/0378-4274(85)90049-9. [DOI] [PubMed] [Google Scholar]
- 67.Rosecrans JA, Squibb RE, Jr, Johnson JH, Tilson HA, Hong JS. Effects of neonatal chlordecone exposure on pituitary-adrenal function in adult Fischer-344 rats. Neurobehavioral toxicology and teratology. 1985;7(1):33–37. [PubMed] [Google Scholar]
- 68.Jarup L, Berglund M, Elinder CG, Nordberg G, Vahter M. Health effects of cadmium exposure--a review of the literature and a risk estimate. Scandinavian journal of work, environment & health. 1998;24(Suppl 1):1–51. [PubMed] [Google Scholar]
- 69.Satarug S, Moore MR. Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental health perspectives. 2004;112(10):1099–1103. doi: 10.1289/ehp.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark JT, et al. Cadmium-induced sexual dysfunction does not involve increased hepatic metabolism of testosterone nor increased circulating levels of corticosterone. Physiology & behavior. 1994;56(5):975–981. doi: 10.1016/0031-9384(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 71.Nishiyama S, Nakamura K. Effect of cadmium on plasma aldosterone and serum corticosterone concentrations in male rats. Toxicology and applied pharmacology. 1984;76(3):420–425. doi: 10.1016/0041-008x(84)90346-6. [DOI] [PubMed] [Google Scholar]
- 72.Lall SB, Dan G. Role of corticosteroids in cadmium induced immunotoxicity. Drug and chemical toxicology. 1999;22(2):401–409. doi: 10.3109/01480549909017843. [DOI] [PubMed] [Google Scholar]
- 73.Jackl GA, Kollmer WE. The influence of exposure to different levels of cadmium on the secretion and biological halflife of corticosterone in the rat. The Science of the total environment. 1982;25(1):53–59. doi: 10.1016/0048-9697(82)90041-9. [DOI] [PubMed] [Google Scholar]
- 74.Der R, Yousef M, Fahim Z, Fahim M. Effects of lead and cadmium on adrenal and thyroid functions in rats. Research communications in chemical pathology and pharmacology. 1977;17(2):237–253. [PubMed] [Google Scholar]
- 75.Zheng L, Dong GH, Jin YH, He QC. Immunotoxic changes associated with a 7-day oral exposure to perfluorooctanesulfonate (PFOS) in adult male C57BL/6 mice. Archives of toxicology. 2009;83(7):679–689. doi: 10.1007/s00204-008-0361-3. [DOI] [PubMed] [Google Scholar]
- 76.Zheng L, et al. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS) Journal of immunotoxicology. 2011;8(1):30–38. doi: 10.3109/1547691X.2010.537287. [DOI] [PubMed] [Google Scholar]
- 77.Fuentes S, Colomina MT, Vicens P, Franco-Pons N, Domingo JL. Concurrent exposure to perfluorooctane sulfonate and restraint stress during pregnancy in mice: effects on postnatal development and behavior of the offspring. Toxicological sciences : an official journal of the Society of Toxicology. 2007;98(2):589–598. doi: 10.1093/toxsci/kfm121. [DOI] [PubMed] [Google Scholar]
- 78.Pereiro N, Moyano R, Blanco A, Lafuente A. Regulation of corticosterone secretion is modified by PFOS exposure at different levels of the hypothalamic-pituitary-adrenal axis in adult male rats. Toxicology letters. 2014;230(2):252–262. doi: 10.1016/j.toxlet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 79.Ribes D, Fuentes S, Torrente M, Colomina MT, Domingo JL. Combined effects of perfluorooctane sulfonate (PFOS) and maternal restraint stress on hypothalamus adrenal axis (HPA) function in the offspring of mice. Toxicology and applied pharmacology. 2010;243(1):13–18. doi: 10.1016/j.taap.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 80.Cristofol RM, Rodriguez-Farre E. Differential presynaptic effects of hexachlorocyclohexane isomers on noradrenaline release in cerebral cortex. Life sciences. 1991;49(15):1111–1119. doi: 10.1016/0024-3205(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 81.Cristofol RM, Rodriguez-Farre E. Modulation of noradrenaline release from hippocampal slices by hexachlorocyclohexane isomers. Effects of GABAergic compounds. Brain research. 1993;606(2):237–243. doi: 10.1016/0006-8993(93)90990-5. [DOI] [PubMed] [Google Scholar]
- 82.Cristofol RM, Rodriguez-Farre E. Hippocampal noradrenaline release is modulated by gamma- and delta-hexachlorocyclohexane isomers: which mechanisms are involved? European journal of pharmacology. 1994;252(3):305–312. doi: 10.1016/0014-2999(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 83.Cristofol RM, Rodriguez-Farre E, Sanfeliu C. Effects of gamma and delta hexachlorocyclohexane isomers on inositol phosphate formation in cerebral cortex and hippocampus slices from developing and adult rat. Neurotoxicology. 1993;14(4):451–458. [PubMed] [Google Scholar]
- 84.Hudson PM, Chen PH, Tilson HA, Hong JS. Effects of p,p′-DDT on the rat brain concentrations of biogenic amine and amino acid neurotransmitters and their association with p,p′-DDT-induced tremor and hyperthermia. Journal of neurochemistry. 1985;45(5):1349–1355. doi: 10.1111/j.1471-4159.1985.tb07199.x. [DOI] [PubMed] [Google Scholar]
- 85.Sunol C, Tusell JM, Gelpi E, Rodriguez-Farre E. Regional concentrations of GABA, serotonin and noradrenaline in brain at onset of seizures induced by lindane (gamma-hexachlorocyclohexane) Neuropharmacology. 1988;27(7):677–681. doi: 10.1016/0028-3908(88)90075-5. [DOI] [PubMed] [Google Scholar]
- 86.Caudle WM, Richardson JR, Wang M, Miller GW. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology. 2005;26(4):721–728. doi: 10.1016/j.neuro.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Richardson JR, et al. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20(10):1695–1697. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- 88.Richardson JR, et al. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology. 2008;29(5):855–863. doi: 10.1016/j.neuro.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilson WW, Onyenwe W, Bradner JM, Nennig SE, Caudle WM. Developmental exposure to the organochlorine insecticide endosulfan alters expression of proteins associated with neurotransmission in the frontal cortex. Synapse. 2014;68(11):485–497. doi: 10.1002/syn.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Soderlund DM, et al. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171(1):3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 91.Meyer DA, Shafer TJ. Permethrin, but not deltamethrin, increases spontaneous glutamate release from hippocampal neurons in culture. Neurotoxicology. 2006;27(4):594–603. doi: 10.1016/j.neuro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Meyer DA, Carter JM, Johnstone AF, Shafer TJ. Pyrethroid modulation of spontaneous neuronal excitability and neurotransmission in hippocampal neurons in culture. Neurotoxicology. 2008;29(2):213–225. doi: 10.1016/j.neuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Hossain MM, Suzuki T, Unno T, Komori S, Kobayashi H. Differential presynaptic actions of pyrethroid insecticides on glutamatergic and GABAergic neurons in the hippocampus. Toxicology. 2008;243(1–2):155–163. doi: 10.1016/j.tox.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Breckenridge CB, et al. Evidence for a separate mechanism of toxicity for the Type I and the Type II pyrethroid insecticides. Neurotoxicology. 2009;30(Suppl 1):S17–31. doi: 10.1016/j.neuro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 95.Nasuti C, et al. Effects of early life permethrin exposure on spatial working memory and on monoamine levels in different brain areas of pre-senescent rats. Toxicology. 2013;303:162–168. doi: 10.1016/j.tox.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 96.Tilson HA, Kodavanti PR. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology. 1998;19(4–5):517–525. [PubMed] [Google Scholar]
- 97.Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta bio-medica : Atenei Parmensis. 2008;79(3):172–183. [PubMed] [Google Scholar]
- 98.Fonnum F, Mariussen E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. Journal of neurochemistry. 2009;111(6):1327–1347. doi: 10.1111/j.1471-4159.2009.06427.x. [DOI] [PubMed] [Google Scholar]
- 99.Bradner JM, Suragh TA, Caudle WM. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology. 2013;312:48–55. doi: 10.1016/j.tox.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mariussen E, Fonnum F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochemistry international. 2003;43(4–5):533–542. doi: 10.1016/s0197-0186(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 101.Yan T, et al. Spatial learning and memory deficit of low level polybrominated diphenyl ethers-47 in male adult rat is modulated by intracellular glutamate receptors. The Journal of toxicological sciences. 2012;37(2):223–233. doi: 10.2131/jts.37.223. [DOI] [PubMed] [Google Scholar]
- 102.Dingemans MM, et al. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environmental health perspectives. 2007;115(6):865–870. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mariussen E, Morch Andersen J, Fonnum F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicology and applied pharmacology. 1999;161(3):274–282. doi: 10.1006/taap.1999.8806. [DOI] [PubMed] [Google Scholar]
- 104.Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters, dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159(1–2):11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- 105.Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicology and applied pharmacology. 2011;252(1):36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fernandes EC, et al. Activation and potentiation of human GABAA receptors by non-dioxin-like PCBs depends on chlorination pattern. Toxicological sciences : an official journal of the Society of Toxicology. 2010;118(1):183–190. doi: 10.1093/toxsci/kfq257. [DOI] [PubMed] [Google Scholar]
- 107.Hilgier W, Lazarewicz JW, Struzynska L, Frontczak-Baniewicz M, Albrecht J. Repeated exposure of adult rats to Aroclor 1254 induces neuronal injury and impairs the neurochemical manifestations of the NMDA receptor-mediated intracellular signaling in the hippocampus. Neurotoxicology. 2012;33(1):16–22. doi: 10.1016/j.neuro.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Struzynska L, Sulkowski G, Dabrowska-Bouta B. Aroclor 1254 selectively inhibits expression of glial GLT-1 glutamate transporter in the forebrain of chronically exposed adult rat. Toxicology. 2012;300(1–2):12–18. doi: 10.1016/j.tox.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 109.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reproductive toxicology. 2007;24(2):139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 110.Schonfelder G, et al. In utero exposure to low doses of bisphenol A lead to long-term deleterious effects in the vagina. Neoplasia. 2002;4(2):98–102. doi: 10.1038/sj.neo.7900212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun Y, et al. Determination of bisphenol A in human breast milk by HPLC with column-switching and fluorescence detection. Biomedical chromatography : BMC. 2004;18(8):501–507. doi: 10.1002/bmc.345. [DOI] [PubMed] [Google Scholar]
- 112.De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. Journal of environmental and public health. 2012;2012:713696. doi: 10.1155/2012/713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoon K, Kwack SJ, Kim HS, Lee BM. Estrogenic endocrine-disrupting chemicals: molecular mechanisms of actions on putative human diseases. Journal of toxicology and environmental health. Part B, Critical reviews. 2014;17(3):127–174. doi: 10.1080/10937404.2014.882194. [DOI] [PubMed] [Google Scholar]
- 114.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annual review of neuroscience. 1984;7:413–442. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 115.Montano MM, Welshons WV, vom Saal FS. Free estradiol in serum and brain uptake of estradiol during fetal and neonatal sexual differentiation in female rats. Biology of reproduction. 1995;53(5):1198–1207. doi: 10.1095/biolreprod53.5.1198. [DOI] [PubMed] [Google Scholar]
- 116.Zhou R, et al. Perinatal exposure to low-dose of bisphenol A causes anxiety-like alteration in adrenal axis regulation and behaviors of rat offspring: A potential role for metabotropic glutamate 2/3 receptors. Journal of psychiatric research. 2015;64:121–129. doi: 10.1016/j.jpsychires.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 117.Kubo K, et al. Exposure to bisphenol A during the fetal and suckling periods disrupts sexual differentiation of the locus coeruleus and of behavior in the rat. Neuroscience letters. 2001;304(1–2):73–76. doi: 10.1016/s0304-3940(01)01760-8. [DOI] [PubMed] [Google Scholar]
- 118.Kubo K, et al. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neuroscience research. 2003;45(3):345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 119.Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of bisphenol A. Neuroscience. 2010;167(3):741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 120.Patisaul HB, et al. Anxiogenic effects of developmental bisphenol A exposure are associated with gene expression changes in the juvenile rat amygdala and mitigated by soy. PloS one. 2012;7(9):e43890. doi: 10.1371/journal.pone.0043890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Diaz Weinstein S, Villafane JJ, Juliano N, Bowman RE. Adolescent exposure to Bisphenol-A increases anxiety and sucrose preference but impairs spatial memory in rats independent of sex. Brain research. 2013;1529:56–65. doi: 10.1016/j.brainres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 122.Harley KG, et al. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environmental research. 2013;126:43–50. doi: 10.1016/j.envres.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perera F, et al. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environmental health perspectives. 2012;120(8):1190–1194. doi: 10.1289/ehp.1104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Panagiotidou E, Zerva S, Mitsiou DJ, Alexis MN, Kitraki E. Perinatal exposure to low-dose bisphenol A affects the neuroendocrine stress response in rats. The Journal of endocrinology. 2014;220(3):207–218. doi: 10.1530/JOE-13-0416. [DOI] [PubMed] [Google Scholar]
- 125.Xu X, et al. Gestational and lactational exposure to bisphenol-A affects anxiety- and depression-like behaviors in mice. Hormones and behavior. 2012;62(4):480–490. doi: 10.1016/j.yhbeh.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 126.Bowman RE, et al. Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Hormones and behavior. 2015;69:89–97. doi: 10.1016/j.yhbeh.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bowman RE, Luine V, Khandaker H, Villafane JJ, Frankfurt M. Adolescent bisphenol-A exposure decreases dendritic spine density: role of sex and age. Synapse. 2014;68(11):498–507. doi: 10.1002/syn.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou R, Chen F, Chang F, Bai Y, Chen L. Persistent overexpression of DNA methyltransferase 1 attenuating GABAergic inhibition in basolateral amygdala accounts for anxiety in rat offspring exposed perinatally to low-dose bisphenol A. Journal of psychiatric research. 2013;47(10):1535–1544. doi: 10.1016/j.jpsychires.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 129.Ogi H, Itoh K, Ikegaya H, Fushiki S. Alterations of neurotransmitter norepinephrine and gamma-aminobutyric acid correlate with murine behavioral perturbations related to bisphenol A exposure. Brain & development. 2015 doi: 10.1016/j.braindev.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 130.Tando S, et al. Bisphenol A exposure disrupts the development of the locus coeruleus-noradrenergic system in mice. Neuropathology : official journal of the Japanese Society of Neuropathology. 2014;34(6):527–534. doi: 10.1111/neup.12137. [DOI] [PubMed] [Google Scholar]
- 131.Canfield RL, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. The New England journal of medicine. 2003;348(16):1517–1526. doi: 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lanphear BP, et al. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environmental health perspectives. 2005;113(7):894–899. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. Jama. 1996;275(5):363–369. [PubMed] [Google Scholar]
- 134.Guilarte TR. Prenatal lead exposure and schizophrenia: further evidence and more neurobiological connections. Environmental health perspectives. 2009;117(5):A190–191. doi: 10.1289/ehp.0800484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jusko TA, et al. Blood lead concentrations < 10 microg/dL and child intelligence at 6 years of age. Environmental health perspectives. 2008;116(2):243–248. doi: 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Neal AP, Guilarte TR. Molecular neurobiology of lead (Pb(2+)): effects on synaptic function. Molecular neurobiology. 2010;42(3):151–160. doi: 10.1007/s12035-010-8146-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cory-Slechta DA, Stern S, Weston D, Allen JL, Liu S. Enhanced learning deficits in female rats following lifetime pb exposure combined with prenatal stress. Toxicological sciences : an official journal of the Society of Toxicology. 2010;117(2):427–438. doi: 10.1093/toxsci/kfq221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cory-Slechta DA, Virgolini MB, Thiruchelvam M, Weston DD, Bauter MR. Maternal stress modulates the effects of developmental lead exposure. Environmental health perspectives. 2004;112(6):717–730. doi: 10.1289/ehp.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rossi-George A, Virgolini MB, Weston D, Cory-Slechta DA. Alterations in glucocorticoid negative feedback following maternal Pb, prenatal stress and the combination: a potential biological unifying mechanism for their corresponding disease profiles. Toxicology and applied pharmacology. 2009;234(1):117–127. doi: 10.1016/j.taap.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rossi-George A, Virgolini MB, Weston D, Thiruchelvam M, Cory-Slechta DA. Interactions of lifetime lead exposure and stress: behavioral, neurochemical and HPA axis effects. Neurotoxicology. 2011;32(1):83–99. doi: 10.1016/j.neuro.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pirkle JL, et al. Exposure of the U.S. population to lead, 1991–1994. Environmental health perspectives. 1998;106(11):745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Virgolini MB, Bauter MR, Weston DD, Cory-Slechta DA. Permanent alterations in stress responsivity in female offspring subjected to combined maternal lead exposure and/or stress. Neurotoxicology. 2006;27(1):11–21. doi: 10.1016/j.neuro.2005.05.012. [DOI] [PubMed] [Google Scholar]