Abstract
Deficits in verbal learning and memory are a prominent feature of neurocognitive function in HIV-infected women, and are associated with high levels of perceived stress. To understand the neurobiological factors contributing to this stress-related memory impairment, we examined the association between stress, verbal memory, and brain volumes in HIV-infected women. Participants included 38 HIV-infected women (Mean age=43.9 years) from the Chicago Consortium of the Women’s Interagency HIV Study (WIHS). Participants underwent structural magnetic resonance imaging (MRI) and completed standardized measures of verbal learning and memory, and stress (Perceived Stress Scale-10; PSS-10). Brain volumes were evaluated in a priori regions of interest, including the medial temporal lobe (MTL) and prefrontal cortex (PFC). Compared to HIV-infected women with lower stress (PSS-10 scores in lower two tertiles), HIV-infected women with higher stress, (scores in the top tertile) performed worse on measures of verbal learning and memory and showed smaller volumes bilaterally in the parahippocampal gyrus, superior frontal gyrus, middle frontal gyrus, and inferior frontal gyrus (p’s<0.05). Reduced volumes in the inferior frontal gyrus, middle frontal gyrus, and superior frontal gyrus (all right hemisphere) were negatively associated with verbal learning and memory performance. Prefrontal cortical atrophy is associated with stress-related deficits in verbal learning and memory in HIV-infected women. The time course of these volume losses in relation to memory deficits has yet to be elucidated, but the magnitude of the volumetric differences between women with higher versus lower stress suggests a prolonged vulnerability due to chronic stress and/or early life trauma.
Keywords: stress, memory, brain volume, HIV, women
Introduction
HIV-infected individuals often experience acute and chronic stress including childhood trauma, adult sexual assault, physical violence, transactional sex, unemployment, poverty, and single parenting (Brief et al, 2004; Cohen et al, 2000). In the Women’s Interagency HIV Study (WIHS), 31% of HIV-infected women report being victims of childhood sexual abuse, 66% report a history of domestic violence, and 21% report recent domestic violence (Cohen et al, 2000). Stressful life events in HIV-infected individuals are associated with higher morbidity and mortality (Evans et al, 1995; Evans et al, 1997; Leserman, 2003a; Leserman, 2003b; Leserman et al, 2002; Leserman et al, 2005) and worse cognitive outcomes, including decreased executive functioning, attention, and processing speed in HIV-infected men (Pukay-Martin et al, 2003) and decreased verbal memory in HIV-infected women (Rubin et al, 2015). Recently, we demonstrated in a large cohort study (n=1499) that higher levels of perceived stress were associated with deficits in verbal memory in HIV-infected women, but not HIV-uninfected women (Rubin et al, 2015). The association between elevated levels of perceived stress and verbal memory deficits was particularly strong among HIV-infected women whose viral loads were high (≥10,000 copies/ml)(Rubin et al, 2015). The association between stress and memory performance in HIV-infected women may be sex-specific as previous studies in HIV-infected men demonstrate that acute stressful life events are associated with worse executive function, attention, and processing speed, but not memory (Pukay-Martin et al, 2003). The neurobiological factors linking stress to memory deficits in HIV-infected women are unexplored but, when elucidated can provide insights into neural circuits that can be targeted in future intervention studies.
The negative association between perceived stress and verbal memory in HIV-infected women may reflect the combined deleterious effects of stress and HIV infection on medial temporal (i.e., hippocampus, parahippocampus) and prefrontal brain areas (i.e., anterior cingulate, middle frontal, and inferior frontal gyrus) that subserve verbal memory (Buckner et al, 2000; Dickerson and Eichenbaum, 2010). Converging evidence from animal and human studies demonstrate that these brain areas are particularly vulnerable to uncontrollable stress and excess cortisol , a steroid hormone released by the adrenal glands in response to stress (Amat et al, 2005; Arnsten, 2009; Kavushansky et al, 2006). Cortisol binds to glucocorticoid receptors, which are abundant in medial temporal and prefrontal regions (Diorio et al, 1993; Magarinos et al, 1987; McEwen et al, 1986; Meaney and Aitken, 1985; Sanchez et al, 2000). Psychological stress and elevated glucocorticoid levels disrupt long-term potentiation, suppress neuronal excitability, and cause apoptosis and atrophy in the hippocampus (Alderson and Novack, 2002; McEwen, 2007; McEwen and Sapolsky, 1995). In the medial prefrontal cortex chronic stress and corticosteroid-induced stress can cause dendritic shortening and atrophy (McEwen, 2007). It is likely because of these neurotoxic effects that pharmacological administration or suppression of glucocorticoids impacts performance on cognitive tasks dependent on the hippocampus and prefrontal cortex in healthy individuals (Henckens et al, 2011; Lupien et al, 1999). Neuroimaging findings in the WIHS also link alterations in hippocampal functioning to verbal memory deficits (Maki et al, 2009). Specifically, HIV-infected women show decreased hippocampal activation during encoding of words compared to HIV- women, and this underactivity predicts lower verbal memory performance (Maki et al, 2009).
Taken together, previous findings suggest that the medial temporal lobe (MTL) and prefrontal cortex (PFC) are brain regions that are both critical to verbal memory performance and particularly vulnerable to the negative effects of stress. Thus, the aim of the present study was to examine the association of perceived stress and brain areas critical for verbal memory in HIV-infected women using structural magnetic resonance imaging (MRI). We hypothesized that high levels of perceived stress would be associated with decreased MTL and PFC volume and that these reductions would be associated with worse verbal learning and memory performance.
Methods
Participants
Participants were women enrolled in the Chicago WIHS Consortium during semiannual visits in 2010–2011. The WIHS is a longitudinal, multisite study of women living with HIV (Bacon et al, 2005; Barkan et al, 1998). One hundred and ninety-nine women were approached by WIHS staff about the study and asked about willingness to participant because they met the following inclusionary criteria: age 21 to 60 years; spoke and read English fluently; and completed greater than 8 years of formal education. Eighty-four interested women completed a phone screen which covered numerous exclusionary criteria (listed below) with UIC study personnel. Of the 84 women, five women were no longer interested in participating and 17 women were ineligible due to the following exclusion criteria: history of dementia (n=1); uncontrolled diabetes (n=2); closed head injury with loss of consciousness (n=0); open head injury of any kind (n=0); vision impairment (n=1); seizure disorder (n=0); current pregnancy (n=1); self-reported diagnosis of psychosis (n=2); history of any clinical AIDS-defining disorders (n=0); currently hospitalization (n=1); endocrine/systemic disease (n=0); and current use of psychiatric medication known to influence cognition (n=2). Additional MRI exclusionary criteria included metal in the body (n=5), claustrophobia (n=1), or weight greater than 250 pounds (due to dimensions of the scanner)(n=1). Sixty-two women were scheduled for a visit to UIC and consented. Following informed consent, six women were withdrawn due to: 1) antipsychotic medication (n=1), metal in body (n=2), weight greater than 250 lbs (n=1), claustrophobia (n=1), and multiple failed toxicology screens (n=1). Fifty-six women (39 HIV-infected) completed the imaging study. One additional HIV-infected woman was excluded from analysis because she was currently using antidepressant which was revealed after the imaging protocol. Thus, for the present study, participants were 38 HIV-infected women who completed neurocognitive testing (and self-report stress measures) and participated in a structural MRI study at the University of Illinois at Chicago within 3 months on average (M=3.18, SD=1.78) of the neurocognitive testing. Brain volume changes are not expected to occur within that time frame.
Measures
Perceived Stress Scale (PSS-10)
The PSS-10 (Cohen et al, 1983; Cohen and Williamson, 1988) is a widely used self-report instrument measuring the degree to which personal situations in the previous month are appraised as stressful. Items assess the degree to which respondents have found their lives unpredictable (e.g., How often have you been upset because of something that happened unexpectedly?), uncontrollable (e.g., How often have you felt that you were unable to control the important things in your life?), and overloaded (e.g., How often have you felt difficulties were piling up so high that you could not overcome them?). Each of the 10 items was rated on a five-point Likert scale (0=never, 1=almost never, 2=sometimes, 3=fairly often, 4=very often). A total score was computed by summing item responses, with higher scores indicating greater perceived stress (scores range from 0 to 40). A Cronbach Alpha of 0.88 in the present study indicated excellent internal consistency. Consistent with our WIHS-wide epidemiologic study in 1505 women (Rubin et al, 2015), we categorized perceived stress as lower when PSS-10 scores were <18 (bottom two tertiles) and higher when PSS-10 scores were ≥18 (top tertile).
PTSD Checklist-Civilian version (PCL-C)
The PCL-C (Weathers et al, 1991) is a 17-item self-report measure of the DSM-IV symptoms of PTSD. The PCL-C queries about symptoms (re-experiencing, avoidance, hyperarousal) in relation to “stressful experiences”. Five of the items assess re-experiencing trauma symptoms (e.g., nightmares or flashbacks concerning the trauma), seven assess avoidance symptoms (e.g., avoidance of thoughts or feelings about the trauma) and five items assess hyperarousal symptoms (e.g., difficulty concentrating, trouble falling or staying asleep). A total symptom severity score (range = 17–85) was obtained by summing the scores from each of the 17 items. A Cronbach Alpha of 0.90 in the present study indicated excellent internal consistency.
Center for Epidemiological Studies Depression (CES-D) scale
The CES-D (Radloff, 1977) is a 20-item self-report measure of depressive symptoms. The CES-D has excellent reliability and validity and is commonly used in studies in HIV-infected cohorts including studies of HIV-infected women (Cook et al, 2002; Maki et al, 2012; Rubin et al, 2011). A total symptom severity score (range =0- 60) was obtained by reverse scoring the appropriate items and summing the scores from each of the 20 items.
Primary Cognitive Outcome Measure
Substance use history
Substance use history for crack and cocaine use was ascertained by a modified version of the Kreek-McHugh-Schluger-Kellogg scale (Kellogg et al, 2003).
Verbal Learning and Memory
The Hopkins Verbal learning Test (HVLT)(Benedict et al, 1998) is a verbal learning and memory task. A total of twelve words from three semantic categories are read aloud during each of three learning trials and the participant is asked to recall the list after each of the learning trials and again after a 20–25 minute delay. A yes/no recognition trial consisting of 12 targets, 6 semantic distractors, and 6 unrelated distractors follow. To assess verbal learning and memory (primary outcomes) we created two composite indices which are consistent with our previous publications (Rubin et al, 2015; Valcour et al, 2015). In the present sample, z-scores were first computed for individual tests from raw scores and then averaged to create each composite index. The verbal learning domain score was calculated by averaging z-scores for Trial 1 (single trial learning) and for the total words recalled across each of three learning trials (total learning). The verbal memory domain score was calculated by averaging z-scores for the number of words recalled after a 25-minute delay (delayed recall) and percent retention (delayed recall/maximum score on trial 2 or 3). Secondary outcomes included recognition, retrieval, and clustering. Recognition scores were calculated by subtracting the number of false positives (incorrectly responding “yes” to at word not presented) from the number of hits (correctly responding “yes” to a word that was presented). A retrieval index (Woods et al, 2005) was computed by subtracting the total number of words recalled during delayed recall from the number of correct words recalled during recognition. A semantic clustering domain score was calculated by averaging z-scores for semantic clustering on Trial 1, clustering across the three learning trials, and clustering on the delayed free recall trial. Semantic clustering is an executive functioning strategy where words belonging to a semantic category are grouped or “clustered” together to enhance performance on a word list memory test (e.g., recalling “scarf,” “socks,” and “khakis” followed by “soda” and “coffee”)(Delis et al, 1988; Stricker et al, 2002).
Secondary Cognitive Outcome Measures
Secondary cognitive outcome measures were included to determine the specificity of findings to the primary outcome of verbal learning and memory. Attention/Concentration. Attention and concentration were assessed with Trials 1 and 2 of the Stroop test (Comalli et al, 1962), Trail Making Test part A, and the control/attention condition from the Letter-Number Sequence (LNS) test from the Wechsler Adult Intelligence Scale (WAIS IV). Executive Functioning. Executive functioning was assessed with Trial 3, the color-word condition (interference) of the Stroop Test, Trail Making Test part B, and the working memory condition of LNS. Psychomotor Speed. Psychomotor speed was assessed with the Symbol Digit Modalities Test. Verbal Fluency Verbal fluency as assessed with a letter (generate words in response to the letters F, A, and S) and category fluency task (generate words in response to the semantic category of animals). For each of the secondary cognitive domains, z-scores were first computed for individual tests from raw scores in the present study sample and then averaged to create each composite index. Tests were grouped into cognitive domains consistent with our previous publications (Rubin et al, 2015; Valcour et al, 2015).
Structural Magnetic Resonance Imaging
Scanning was performed using a General Electric 3.0-Tesla SignaHDx scanner (General Electric Healthcare, Waukesha, WI) at the University of Illinois at Chicago. Structural imaging was acquired using a T1-weighted 3-dimensional inversion recovered fast spoiled gradient echo sequence [repetition time (TR)/echo time (TE)/ inversion recovery (IR)] = 13.8/4.3/300, flip angle = 25 degrees, 120 slices). To extract volumetric data for each subject, we used FreeSurfer (version 5.1.0 available at http://surfer.nmr.mgh.harvard.edu/), a fully automated, atlas-based segmentation software package that generates an individualized anatomical label map, based on an atlas composed of manually traced reference scans (Fischl et al, 2002; Fischl et al, 2004). The two a priori regions of interest included the MTL and PFC given their role in stress and/or verbal memory. In the MTL we extracted volumetric data in the hippocampus, parahippocampal region (parahippocampal gyri, entorhinal cortex), and amygdala. In the frontal lobe, we extracted volumetric data in the superior frontal, middle frontal, and inferior frontal gyri (BA44, BA45), orbital frontal cortex, and anterior cingulate. The reliability and validity of automated volumetry, particularly subcortical structures, has previously been demonstrated in HIV-infected individuals (Dewey et al, 2010). All brain volumes were corrected for the total intracranial volume (TIV) which is derived from the sum of grey matter, white matter, and cerebral spinal fluid (Ances et al, 2012; Labate et al, 2010). Specifically, all cortical volumes (mm3) were divided by the TIV (mm3) and multiplied by 1000. There were 6 outliers across all brain volumes of interest (<1% of the data), and these values were winsorized so that they were equivalent to the next highest/lowest volume within their respective groups.
Statistical Analysis
Differences between groups (HIV-infected higher stress; HIV-infected lower stress) in demographic characteristics were examined using independent t-tests for continuous variables and Chi-square tests for categorical variables. Group differences (HIV-infected higher stress; HIV-infected lower stress) in brain volumes and verbal learning and memory (HVLT primary outcome measures) were examined using multivariable linear regression with age as a covariate. Significance was defined as p<0.05 (two-sided). Cohen’s d effect sizes (small effect = 0.2; medium effect = 0.5; large effect = 0.8) were calculated using pooled standard deviations and estimated means adjusted for age (Cohen, 1992). Multivariable linear regressions were then used to examine the behavioral correlates of brain volumes that were both significantly associated with perceived stress (higher vs. lower) at p<0.05. In these analyses, verbal learning and memory was the outcome, brain volume was the primary predictor, and age was the covariate. Similar analyses were conducted on secondary outcome measures to examine the specificity of findings to the primary outcome measure. All analyses were performed using SAS (version 9.4, SAS Institute Inc, Cary, NC).
Results
Participants were 27 to 59 years of age (M=43.89, SD=6.88) and 97% were African American. The mean CD4 lymphocyte count was 564 (range, 5–1791), 13% of participants had AIDS-defining (<200) CD4 counts, 79% were on combination antiretroviral therapy (cART), and 68% were on cART with ≥95% adherence (self-reported usage of prescribed medication). Current plasma viral load was undetectable for 58%. The prevalence of elevated depressive symptoms was 26% (defined by a Center for Epidemiologic Studies Depression Scale score ≥16), elevated post-traumatic symptom burden was evident in 26% (PTSD Checklist-Civilian Version score ≥44), and 84% reported ever having experienced abuse (sexual, physical violence, or domestic coercion). The pattern of substance use (i.e., peak use, frequency of use) did not significantly differ between women with higher versus lower perceived stress (Supplemental Table 1). Women with higher perceived stress had lower CD4 count than women with lower perceived stress (p=0.02; Table 1). On the HVLT, women with higher perceived stress performed worse than women with lower perceived stress on the verbal memory domain and its indices as well as on the delayed clustering score (p’s<0.05;Table 2). Controlling for hepatitis C virus and ever or current use of cocaine did not alter these findings. There were no significant differences between women with high and low perceived stress on secondary outcome measures of attention/concentration, executive functioning, psychomotor speed, or verbal fluency (Table 3).
Table 1.
Demographic and clinical characteristics for HIV-infected women as a function of perceived stress.
| Perceived Stress |
|||
|---|---|---|---|
| Variables | Lower (n=20) n (%) |
Higher (n=18) n (%) |
p-value |
| Socio-demographic factors | |||
| Age, M (SD) | 43.00 (6.14) | 44.89 (7.67) | 0.40 |
| WRAT-R, M (SD) | 86.95 (19.69) | 83.17 (16.89) | 0.53 |
| Years of Education, M (SD) | 13.22 (2.33) | 12.14 (2.26) | 0.15 |
| Race/ethnicity | |||
| African American | 20 (100) | 17 (94) | 0.29 |
| Caucasian | - | 1 (6) | |
| Risky health behaviors | |||
| Currently smoking | 10 (50) | 7 (39) | 0.50 |
| Recent1 use | |||
| Alcohol | 0.91 | ||
| Abstainer | 11 (55) | 11 (61) | |
| Not heavy | 6 (30) | 5 (28) | |
| Heavy | 3 (15) | 2 (11) | |
| Marijuana | 3 (15) | 3 (17) | 0.89 |
| Crack | 1 (5) | 4 (22) | 0.12 |
| Cocaine | 1 (5) | - | 0.34 |
| Heroin | 1 (5) | 3 (17) | 0.25 |
| Ever use | |||
| Marijuana | 13 (65) | 15 (83) | 0.21 |
| Crack/Cocaine | 12 (60) | 13 (72) | 0.43 |
| Psychological profile | |||
| Perceived stress (PSS-10), M (SD) | 10.85 (3.96) | 22.00 (3.74) | <0.001 |
| Depressive symptoms (CES-D≥16) | 3 (15) | 7 (39) | 0.09 |
| PTSD (PCL-C≥44) | 1 (5) | 9 (50) | 0.002 |
| PTSD (PCL-C≥44)/Depressive symptoms (CES-D≥16) | 0.17 | ||
| No/No | 14 (70) | 2 (11) | |
| Yes/No | 3 (15) | 9 (50) | |
| No/Yes | 3 (15) | 0 (0) | |
| Yes/Yes | 0 (0) | 7 (39) | |
| Negative life events | |||
| Abuse ever (sexual, violence, or domestic) | 18 (90) | 14 (78) | 0.31 |
| Sex abuse | 9 (45) | 8 (44) | 0.97 |
| Violence | 16 (80) | 14 (78) | 0.87 |
| Domestic coercion | 12 (60) | 11 (61) | 0.94 |
| Clinical characteristics | |||
| Hepatitis C virus antibody (HCV) | 3 (15) | 6 (33) | 0.19 |
| Nadir CD4 count (cells/µl), M (SD) | 325 (226) | 287 (177) | 0.56 |
| CD4 Count (cells/µl) | 0.02 | ||
| > 500 | 14 (74) | 5 (28) | |
| ≥ 200 and < 500 | 3 (16) | 10 (55) | |
| < 200 | 2 (10) | 3 (17) | |
| Viral Load (HIV RNA (cp/ml)) | 0.24 | ||
| Undetectable | 14 (70) | 8 (44) | |
| < 10,000 | 4 (20) | 5 (28) | |
| ≥ 10,000 | 2 (10) | 5 (28) | |
| Medication Use | 0.83 | ||
| No cART or cART+ < 95% medication adherence | 6 (30) | 6 (33) | |
| cART+ ≥ 95% medication adherence | 14 (70) | 12 (67) | |
Note.
”Recent” refers to within 6 months of the most recent WIHS visit; WRAT-R = Wide Range Achievement Test Standard Score; CES-D= Center for Epidemiologic Studies Depression Scale; PSS-10=Perceived Stress Scale; PCL-C=PTSD Checklist-Civilian Version; PSS-10 correlated with CES-D, r=0.50, p<0.001; PSS-10 correlated with PCL-C, r=0.79, p<0.001; CES-D correlated with PCL-C, r=0.44, p=0.006. Heavy alcohol use = >7 drinks per week or > 4 drinks at a sitting; Undetectable=<48copies/ml; cART = combination antiretroviral therapy.
Table 2.
Cognitive profile on the Hopkins Verbal Learning Test (HVLT) as a function of perceived stress.
| Perceived Stress |
|||||
|---|---|---|---|---|---|
| Domain | Lower (n=20) M (SE) |
Higher (n=18) M (SE) |
β | p-value | Cohen d (95%CI) |
| Verbal learning domain z-score | 0.13 (0.13) | −0.14 (0.22) | −0.14 | 0.38 | −0.29 (−0.93 to 0.35) |
| Trial 1 | 6.26 (0.37) | 5.83 (0.40) | −0.13 | 0.43 | −0.26 (−0.90 to 0.38) |
| Total Learning | 24.23 (0.96) | 22.97 (1.02) | −0.14 | 0.37 | −0.30 (−0.94 to 0.34) |
| Verbal memory domain z-score | 0.37 (0.19) | −0.41 (0.20) | −0.42 | 0.005 | −1.05 (−1.72 to −0.37) |
| Delay free recall | 8.87 (0.53) | 7.03 (0.56) | −0.36 | 0.02 | −0.86 (−1.52 to −0.19) |
| Retention | 92.61 (4.24) | 78.04 (4.47) | −0.36 | 0.02 | −0.85 (−1.51 to −0.18) |
| Retrieval index | 2.21 (0.44) | 4.21 (0.46) | −0.45 | 0.002 | −1.21 (−1.90 to −0.51) |
| Recognition | 10.10 (0.56) | 10.45 (0.59) | 0.07 | 0.67 | 0.14 (−0.50 to 0.78) |
| Clustering domain z-score | 0.29 (0.16) | −0.32 (0.17) | −0.38 | 0.01 | −0.91 (−1.58 to −0.24) |
| Trial 1 cluster score | 1.71 (0.23) | 1.15 (0.25) | −0.25 | 0.09 | −0.57 (−1.22 to 0.07) |
| Immediate total cluster score | 7.49 (0.83) | 5.29 (0.88) | −0.28 | 0.07 | −0.63 (−1.28 to 0.02) |
| Delay cluster score | 3.89 (0.51) | 2.01 (0.54) | −0.38 | 0.01 | −0.92 (−1.59 to −0.25) |
Note. All models control for age. CI=Confidence Interval. Z-scores were first computed for individual tests from raw scores in the present study sample and then averaged to create each composite index.
Table 3.
Scores on the secondary cognitive outcome measures as a function of lower versus higher perceived stress.
| Perceived Stress |
|||||
|---|---|---|---|---|---|
| Domain z-scores | Lower (n=20) M (SE) |
Higher (n=18) M (SE) |
β | p-value | Cohen d (95%CI) |
| Attention/concentration | 0.10 (0.16) | −0.10 (0.16) | −0.13 | 0.37 | −0.31 (−0.95 to 0.33) |
| Executive functioning | 0.004 (0.15) | −0.04 (0.16) | −0.06 | 0.69 | −0.54 (−1.19 to 0.11) |
| Psychomotor speed | 0.17 (0.20) | −0.18 (0.20) | −0.17 | 0.23 | −0.42 (−1.06 to 0.23) |
| Verbal fluency | 0.22 (0.20) | −0.21 (0.19) | −0.25 | 0.13 | −0.54 (−1.19 to 0.11) |
Note. All models control for age. CI=Confidence Interval. Z-scores were first computed for individual tests from raw scores in the present study sample and then averaged to create each composite index.
Medial temporal region
HIV-infected women with higher perceived stress (i.e., PSS-10 scores in the top tertile) compared to HIV-infected women with lower perceived stress showed smaller volumes bilaterally in the parahippocampal gyri after controlling for age (Table 4/Supplementary Figure 1). The association remained significant in both hemispheres after controlling for HIV-related disease characteristics including CD4 nadir (or current CD4 count) and HIV viral load (p’s<0.05). Again, controlling for hepatitis C virus and cocaine use did not alter these findings. To determine the specificity of this association, we examined the association depressive symptoms and PTSD symptom burden (both assessed continuously due to the small proportion of participants meeting elevated symptoms) with parahippocampal gyri volumes; higher PTSD symptom scores were marginally associated with smaller volumes in the right parahippocampal gyrus (β=−0.28, p=0.05). Perceived stress group was not associated with volumetric differences in the hippocampus, entorhinal cortex, or amygdala (p’s>0.11). However, higher PTSD symptom scores were also marginally associated with smaller volumes in the left hippocampus (β=−0.31, p=0.05) and left amygdala (β=−0.29, p=0.06). CES-D scores did not correlate with brain volumes in the medial temporal region.
Table 4.
Ratio of Volume/Total intracranial volume (TICV) and effect size for a priori regions of interest as a function of perceived stress.
| Volume (Estimated Mean (SE),mm3/TICV) x1000 |
|||||
|---|---|---|---|---|---|
| Perceived Stress |
|||||
| Region | Lower (n=20) M (SE) |
Higher (n=18) M (SE) |
β | p-value | Cohen d (95%CI) |
| Left Hemisphere | |||||
| Medial temporal region | |||||
| Hippocampus | 4.07 (0.19) | 3.80 (0.20) | −0.15 | 0.35 | −0.31 (−0.95 to 0.33) |
| Para-hippocampal region | |||||
| Parahippocampal gyrus | 2.38 (0. 11) | 1.90 (0.12) | −0.42 | 0.003 | −0.98 (−1.65 to −0.30) |
| Entorhinal cortex | 1.37 (0.09) | 1.40 (0.10) | 0.03 | 0.85 | 0.06 (−0.57 to 0.70) |
| Amygdala | 1.34 (0.05) | 1.22 (0.05) | −0.25 | 0.11 | −0.54 (−1.19 to 0.10) |
| Prefrontal region | |||||
| Superior frontal gyrus | 20.28 (0.53) | 18.49 (0.56) | −0.32 | 0.02 | −0.83 (−1.50 to −0.17) |
| Middle frontal gyrus | 20.22 (0.61) | 17.94 (0.65) | −0.37 | 0.01 | −0.92 (−1.59 to −0.25) |
| Inferior frontal gyrus | |||||
| BA44 | 4.05 (0.13) | 3.58 (0.14) | −0.36 | 0.01 | −0.90 (−1.57 to −0.23) |
| BA45 | 5.42 (0.15) | 5.22 (0.15) | −0.14 | 0.36 | −0.31 (−0.95 to 0.33) |
| Anterior cingulate | 3.75 (0.17) | 3.39 (0.18) | −0.21 | 0.15 | −0.49 (−1.13 to 0.16) |
| Orbital frontal cortex | 10.42 (0.25) | 10.67 (0.26) | 0.10 | 0.49 | 0.23 (−0.41 to 0.87) |
| Right Hemisphere | |||||
| Medial temporal region | |||||
| Hippocampus | 3.78 (0.19) | 3.82 (0.20) | 0.02 | 0.88 | 0.05 (−0.59 to 0.68) |
| Para-hippocampal region | |||||
| Parahippocampal gyrus | 2.06 (0.08) | 1.80 (0.08) | −0.31 | 0.03 | −0.79 (−1.45 to −0.12) |
| Entorhinal cortex | 1.33 (0.08) | 1.27 (0.08) | −0.08 | 0.62 | −0.16 (−0.80 to 0.47) |
| Amygdala | 1.38 (0.05) | 1.28 (0.06) | −0.21 | 0.19 | −0.45 (−1.09 to 0.20) |
| Prefrontal region | |||||
| Superior frontal gyrus | 20.48 (0.59) | 18.11 (0.62) | −0.38 | 0.006 | −1.03 (−1.71 to −0.35) |
| Middle frontal gyrus | 21.00 (0.57) | 18.62 (0.60) | −0.39 | 0.004 | −1.08 (−1.76 to −0.40) |
| Inferior frontal gyrus | |||||
| BA44 | 6.07 (0.19) | 5.27 (0.20) | −0.39 | 0.004 | −1.09 (−1.77 to −0.41) |
| BA45 | 8.10 (0.24) | 7.71 (0.25) | −0.16 | 0.29 | −0.38 (−1.02 to 0.26) |
| Anterior cingulate | 3.37 (0.14) | 3.48 (0.15) | 0.08 | 0.60 | 0.18 (−0.46 to 0.81) |
| Orbital frontal cortex | 10.65 (0.31) | 10.49 (0.32) | −0.52 | 0.72 | −0.12 (−0.76 to 0.52) |
Note. All models control for age. TICV= Total intracranial volume. CI=Confidence Interval.
Parahippocampal gyri volumes were also not significantly associated with HVLT performance (p’s>0.35). However, smaller volume in the left hippocampus was associated with worse performance on the verbal memory composite after controlling for age (β=−0.31, p=0.04). Among measures comprising the composite index, smaller left hippocampal gyrus volume was associated with decreased percent retention (β=−0.34, p=0.03) and was marginally associated with delayed free recall (β=−0.29, p=0.06).
Prefrontal cortex
HIV-infected women with higher perceived stress compared to HIV-infected women with lower perceived stress showed smaller volumes bilaterally in the inferior frontal gyrus (BA44), middle frontal gyri, and superior frontal gyri after controlling for age (Table 4/Supplementary Figure 1). The associations remained significant in both hemispheres after controlling for HIV-related disease characteristics including CD4 nadir (or current CD4 count) and HIV viral load (p’s<0.05). Furthermore, controlling for hepatitis C virus and cocaine use did not alter these findings. Although depressive symptoms were not significantly associated with brain volumes in these regions, greater PTSD symptom burden was associated with smaller volumes in the right inferior frontal gyrus (β=−0.33, p=0.02), left middle frontal gyrus (β=−0.36, p=0.01), and right superior frontal gyrus (β=−0.34, p=0.02). Perceived stress was not associated with volumetric loss in the anterior cingulate or the orbital frontal cortex (p’s>0.15). PTSD symptom burden and depressive symptoms were also not associated with these brain regions.
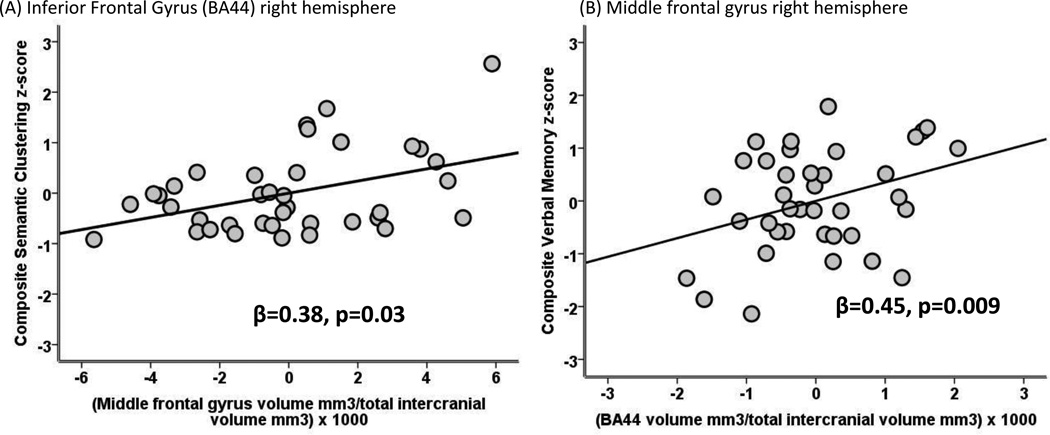
Smaller inferior frontal gyrus (BA44) volume in the right hemisphere (β=0.38, p=0.02; Figure 1), but not the left hemisphere (β=0.25, p=0.12) was significantly associated with worse performance on the verbal memory composite after controlling for age. Among the measures comprising the composite index, smaller inferior frontal gyrus (BA44) volume in the right hemisphere was associated with decreased percent retention (β=0.41, p=0.01) and was marginally associated with worse delayed free recall (β=0.32, p=0.05). Smaller inferior frontal gyrus (BA44) volume in the right hemisphere was also associated with the worse performance on the retrieval index (β=0.38, p=0.02). Smaller right middle frontal gyrus volume was significantly associated with decreased scores on the composite clustering index (β=0.45, p=0.004; Figure 1) and specifically with a worse Trial 1 clustering score (β=0.34, p=0.03), total learning clustering score (β=0.39, p=0.02), and delayed clustering score (β=0.38, p=0.02). The right superior frontal gyrus was also associated with the retrieval index (β=0.36, p=0.03). Although not quite significant, there was a trend for the right middle frontal gyrus to relate to the retrieval index (β=0.28, p=0.09). The same pattern of associations was evident after controlling for current CD4 count and HIV viral load; similar pattern was also evident after controlling for Hepatitis C virus and cocaine use.
Figure 1.
Partial plot from the multivariable linear regression analysis examining the association between: (A) inferior frontal gyrus (BA44) right hemisphere volume and the verbal memory composite z-score after controlling for age and (B) Middle frontal gyrus volume and semantic clustering composite z-score.
Note. The same pattern of associations was seen between inferior frontal gyrus (BA44) in the right hemisphere and percent retention (p=0.01) and delayed free recall (p=0.05) as well as the retrieval index (p=0.02). The same pattern of associations were seen between the middle frontal gyrus in the right hemisphere and all clustering scores (p’s<0.05)
Discussion
To our knowledge, this is the first study to examine links among measures of stress, regional brain volume, and cognitive performance in HIV-infected women. Consistent with our previous study of 1505 women (Rubin et al, 2015), higher perceived stress was associated with worse verbal memory performance. Here, we built on this previous finding by showing that high perceived stress is specifically associated with prefrontal-based aspects of verbal memory performance, namely memory retrieval and semantic clustering. Furthermore, among HIV-infected women, higher levels of perceived stress were associated with smaller volumes bilaterally in the MTL (parahippocampal gyri) and prefrontal cortex regions (superior, middle, and inferior frontal gyri), brain regions critical for verbal memory performance. Notably, volume loss in the prefrontal cortex, but not in the MTL, was associated with decreased verbal memory performance. The particular aspect of memory function that was associated with prefrontal volume loss differed by region, but associations were right-hemisphere dominant. Specifically, right inferior frontal gyrus (BA44) was associated with verbal memory, right middle frontal gyrus was associated with semantic clustering, and right superior frontal gyrus was associated with retrieval. Women with higher levels of perceived stress also had lower CD4 counts, and lower CD4 counts were associated with worse memory performance. Controlling for HIV-related disease characteristics did not change the pattern of results. Overall, these findings implicate structural alterations in the prefrontal cortex as one explanatory factor for our earlier behavioral findings in the WIHS where we demonstrated that stress is negatively associated with verbal memory only in the context of HIV(Rubin et al, 2015).
The pattern of associations between perceived stress and structural volumes strongly suggests PTSD and/or anxiety disorders among the HIV-infected women. The composition of our sample was comprised of a high proportion of women who have experienced abuse (83%) and who self-reported symptoms of PTSD (26%). Previous studies of HIV-uninfected women indicate that increasing cumulative exposure to adverse life events as well as PTSD and other stress-related disorders are associated with structural alterations in the MTL and prefrontal cortex (Ansell et al, 2012; Bremner, 2007; Li et al, 2014). Inconsistent with these previous studies (Ansell et al, 2012; Bremner, 2007; Li et al, 2014) we did not find significant associations between higher perceived stress and decreased hippocampal, amygdala, and anterior cingulate volumes. It is important to note; however, that we did find small to moderate effect sizes for these brain structures with the largest being the amygdala (Cohen’s d=0.54 left hemisphere; Cohen’s d=0.45 right hemisphere) followed by the anterior cingulate (Cohen’s d=0.49 left hemisphere). One possibility is that the neural correlates of stress may be more prefrontal than temporal-dependent in HIV-infected women compared to HIV-uninfected women. Our data also suggest that these structures, particularly the left hippocampus and amygdala, may be more important for PTSD, albeit we only found trends, rather than perceived stress. Regardless, larger sample sizes are needed to detect these associations.
Our findings in HIV-infected women are consistent with previous studies of healthy individuals demonstrating that higher levels of perceived stress are associated with smaller volumes in the parahippocampus (Li et al, 2014; Papagni et al, 2011). The parahippocampus has been implicated in not only memory consolidation but also in the detection of stress-related stimuli, emotion regulation, and emotion perception (Bremner, 2007; Hahn et al, 2012; Lai, 2014; Phillips et al, 2008; Sakamoto et al, 2005). Of note, however, there was no association between volume of the parahippocampal gyrus and verbal memory suggesting that volume loss in this region may not be a factor linking perceived stress to verbal memory deficits in HIV-infected women.
We also demonstrated that higher levels of stress were associated with smaller prefrontal cortex volume in the superior frontal, middle frontal, and inferior frontal gyri, particularly in the right hemisphere. Notably, there were regional variations in the specific aspects of memory function associated with prefrontal volume loss. Specifically, lower volumes in the right inferior frontal gyrus were associated with lower scores in the verbal memory domain, particularly with lower scores on individual measure of retention (significant) and delayed recall (trend). Lower volumes in the right superior (significant) and right middle frontal gyrus (trend) were associated with lower scores on the retrieval index. Lower volumes in the right middle frontal gyrus were associated with lower scores on the clustering composite scores, and with all of the individual scores comprising that composite score.
Neuroimaging studies in healthy individuals implicate these prefrontal brain regions in verbal memory and other episodic memory functions (Buckner et al, 2000; Demb et al, 1995; Dickerson and Eichenbaum, 2010; Wiggs et al, 1999). In comparison to HIV-uninfected individuals, HIV-infected individuals demonstrate an altered pattern of prefrontal activation during episodic memory tasks including decreased recruitment of the right inferior and middle frontal gyrus (Castelo et al, 2006), and decreased PFC activation during recognition trials (Meyer et al, 2014). Executive functions are mediated by the prefrontal cortex and play an important, facilitative role in memory retrieval. Among these functions, inhibition and cognitive control processes are dependent on the right inferior frontal gyrus (Aron et al, 2004; Brass et al, 2005; Levy and Anderson, 2002), whereas semantic clustering is dependent on the right dorsolateral prefrontal cortex (DLPFC), which lies in the middle frontal gyri (Long et al, 2010). The DLPFC is also active during post-retrieval processing which involves the monitoring and evaluation of information once it is retrieved (Achim and Lepage, 2005; Hayama and Rugg, 2009; Henson et al, 1999). Broadly, our right hemisphere-dominant findings for retrieval and recall are consistent with the hemispheric encoding/retrieval asymmetry (HERA) model whereby brain regions tend to be right lateralized during retrieval (Tulving et al, 1994). Structural vulnerability in the prefrontal cortex, especially in the inferior and middle frontal gyri, may contribute to verbal memory deficits in HIV-infected women who experience stress due in part to this regions role in cognitive control and inhibition of irrelevant information.
Due to the cross-sectional nature of this investigation it is not possible to determine the temporal pattern of these changes. The magnitude of the difference in volumes between higher and lower stressed women suggests a potential longstanding vulnerability due to chronic elevations in perceived stress and/or early life trauma, which are common in HIV-infected women. Early life trauma is highly prevalent in HIV-infected women, and one possibility is that a history of trauma may be a basis behind the relationships among high perceived stress, memory, and structural brain abnormalities. Early life trauma is associated with HIV and with enduring functional and structural abnormalities in prefrontal cortex as well as memory deficits, due in part to lasting alterations in the hypothalamic-pituitary-adrenal (HPA) axis (Lupien et al, 2007; Lupien et al, 2009); HIV infection may compound these brain vulnerabilities leading to memory dysfunction. Stress may exacerbate the effects of HIV on brain structure; basic science studies show that glucocorticoids exacerbate the negative effects of gp120 protein on function and structure of hippocampal and cortical tissue (Yusim et al, 2000). HIV infection may also serve to deplete cognitive reserve mechanisms (Stern, 2002). PFC volumes are associated with perceived stress regardless of HIV status; yet, stress is only associated with memory deficits in HIV-infected and not uninfected women in the WIHS. Our results suggest that lower PFC volumes may be a neural mechanism underlying the stress and memory impairment association that occurs in HIV-infected women but not uninfected women. Therefore, we speculate that HIV-infected women may be able to compensate for stress-induced lower PFC volumes by recruiting alternate neural networks or employing other cognitive strategies in order to maintain normal memory performance. Notably, a lack of a relationship between hippocampal volume and memory performance in healthy, adults has been demonstrated previously (Wirth et al, 2013). Alternatively, cognitive impairment or lower brain reserve capacity may interfere with an individuals’ capacity to use top-down cognitive control resources to manage stress, which in turn could result in higher perceived stress scores. Thus, it may not be that stress is directly contributing to poor memory performance or to brain structural changes. Rather, it could be that the perceived stress scale is capturing an aspect of cognitive control (e.g., feeling easily overwhelmed) that is limited due to prefrontal compromise. Arguing against this interpretation is the specificity of findings with verbal learning and memory; there was no association between stress and measures of attention, executive function, psychomotor speed, or verbal fluency. Finally, HIV-related brain vulnerabilities might lead to memory deficits in HIV-infected women which in turn might lead to stress. The effects of HIV and stress on brain regions subserving memory function are likely multidirectional (Lupien et al, 1998). Additional studies are needed to disentangle these relationships in HIV-infected women.
The present study has several limitations in addition to the cross-sectional design noted earlier. We did not have a large enough uninfected at-risk comparison group (n=17, only 5 with high perceived stress) and thus could not investigate the interactive effects of HIV and stress on brain structure. However, findings from our WIHS-wide behavioral study (Rubin et al, 2015) indicate that stress was negatively associated with verbal memory only in the context of HIV; thus, we focused this study on HIV-infected women only. Finally, larger samples are needed to demonstrate the reliability of these findings.
In sum, high perceived stress may exacerbate the negative effect of HIV infection on memory performance which may be associated with loss of frontal lobe volume. The time course of these volume losses in relation to memory and stress has yet to be determined. Future studies should address whether behavioral and brain effects are modifiable through interventions to lower stress. A greater understanding of the neurobiological factors linking stress to memory deficits in HIV-infected women will ultimately help to identify neural mechanisms that should be targeted for treatment thus leading to better mental health and functional outcomes such as medication adherence. More broadly, our results underscore the importance of taking mental health factors into account when conducting research studies of cognition in HIV-infected women, as well as when making a diagnosis of HIV-associated Neurocognitive Disorders (HAND), as these risk factors are prevalent in women.
Highlights.
HIV-infected women with higher stress performed worse on measures of verbal memory compared to HIV-infected women with lower stress.
HIV-infected women show smaller volumes bilaterally in the temporal and frontal cortices.
Prefrontal cortical atrophy is associated with stress-related deficits in verbal learning and memory in HIV-infected women.
Acknowledgments
Study Funding: Data in this manuscript were collected by the Chicago site of the Women’s Interagency HIV study (WIHS) and is funded by National Institute of Allergy and Infectious Diseases (NIAID) grant U01 A1034993 (PI: Mardge Cohen, M.D.) and co-funded by National Cancer Institute and National Institute of Drug Abuse. L. Rubin’s effort was supported by grant number 1K01MH098798-01 from the National Institute of Mental Health (NIMH) and grant number K12HD055892 from the National Institute of Child Health and Human Development (NICHD), and the National Institutes of Health Office of Research on Women’s Health (ORWH).This grant was also supported by the Chicago Developmental Center for AIDS Research (D-CFAR), an NIH funded program (P30 AI 082151), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NCCAM). V. Meyer’s effort on this project was supported by the National Institute on Drug Abuse (1F31DA028573). E. Sundermann’s effort on this project was supported by the National Institute of Mental Health (1F31MH083537).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achim AM, Lepage M. Dorsolateral prefrontal cortex involvement in memory post-retrieval monitoring revealed in both item and associative recognition tests. Neuroimage. 2005;24:1113–1121. doi: 10.1016/j.neuroimage.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Alderson AL, Novack TA. Neurophysiological and clinical aspects of glucocorticoids and memory: a review. J Clin Exp Neuropsychol. 2002;24:335–355. doi: 10.1076/jcen.24.3.335.987. [DOI] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59:469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test - Revised: Normative Data and Analysis of Inter-Form and Test-Retest Relability. The Clinical Neuropsychologist. 1998:12. [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends Cogn Sci. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Neuroimaging in posttraumatic stress disorder and other stress-related disorders. Neuroimaging Clin N Am. 2007;17:523–538. doi: 10.1016/j.nic.2007.07.003. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brief DJ, Bollinger AR, Vielhauer MJ, Berger-Greenstein JA, Morgan EE, Brady SM, Buondonno LM, Keane TM. Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care. 2004;(16 Suppl 1):S97–S120. doi: 10.1080/09540120412301315259. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Logan J, Donaldson DI, Wheeler ME. Cognitive neuroscience of episodic memory encoding. Acta Psychol (Amst) 2000;105:127–139. doi: 10.1016/s0001-6918(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cohen M, Deamant C, Barkan S, Richardson J, Young M, Holman S, Anastos K, Cohen J, Melnick S. Domestic violence and childhood sexual abuse in HIV-infected women and women at risk for HIV. Am J Public Health. 2000;90:560–565. doi: 10.2105/ajph.90.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, editors. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, Palacio H, Richardson J, Wilson T, Young M. Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr. 2002;30:401–409. doi: 10.1097/00042560-200208010-00005. [DOI] [PubMed] [Google Scholar]
- Delis DC, Freeland J, Kramer JH, Kaplan E. Integrating clinical assessment with cognitive neuroscience: construct validation of the California Verbal Learning Test. J Consult Clin Psychol. 1988;56:123–130. doi: 10.1037//0022-006x.56.1.123. [DOI] [PubMed] [Google Scholar]
- Demb JB, Desmond JE, Wagner AD, Vaidya CJ, Glover GH, Gabrieli JD. Semantic encoding and retrieval in the left inferior prefrontal cortex: a functional MRI study of task difficulty and process specificity. J Neurosci. 1995;15:5870–5878. doi: 10.1523/JNEUROSCI.15-09-05870.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey J, Hana G, Russell T, Price J, McCaffrey D, Harezlak J, Sem E, Anyanwu JC, Guttmann CR, Navia B. Reliability and validity of MRI-based automated volumetry software relative to auto-assisted manual measurement of subcortical structures in HIV-infected patients from a multisite study. Neuroimage. 2010;51:1334–1344. doi: 10.1016/j.neuroimage.2010.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Tamul K, Liao D, van der Horst CM, Hall CD, Folds JD, et al. Stress-associated reductions of cytotoxic T lymphocytes and natural killer cells in asymptomatic HIV infection. Am J Psychiatry. 1995;152:543–550. doi: 10.1176/ajp.152.4.543. [DOI] [PubMed] [Google Scholar]
- Evans DL, Leserman J, Perkins DO, Stern RA, Murphy C, Zheng B, Gettes D, Longmate JA, Silva SG, van der Horst CM, Hall CD, Folds JD, Golden RN, Petitto JM. Severe life stress as a predictor of early disease progression in HIV infection. Am J Psychiatry. 1997;154:630–634. doi: 10.1176/ajp.154.5.630. [DOI] [PubMed] [Google Scholar]
- Hahn TT, McFarland JM, Berberich S, Sakmann B, Mehta MR. Spontaneous persistent activity in entorhinal cortex modulates cortico-hippocampal interaction in vivo. Nat Neurosci. 2012;15:1531–1538. doi: 10.1038/nn.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayama HR, Rugg MD. Right dorsolateral prefrontal cortex is engaged during post-retrieval processing of both episodic and semantic information. Neuropsychologia. 2009;47:2409–2416. doi: 10.1016/j.neuropsychologia.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckens MJ, van Wingen GA, Joels M, Fernandez G. Time-dependent corticosteroid modulation of prefrontal working memory processing. Proc Natl Acad Sci U S A. 2011;108:5801–5806. doi: 10.1073/pnas.1019128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: a functional MRI test of the monitoring hypothesis. Brain. 1999;122(Pt 7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Kavushansky A, Vouimba RM, Cohen H, Richter-Levin G. Activity and plasticity in the CA1, the dentate gyrus, and the amygdala following controllable vs. uncontrollable water stress. Hippocampus. 2006;16:35–42. doi: 10.1002/hipo.20130. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Labate A, Cerasa A, Aguglia U, Mumoli L, Quattrone A, Gambardella A. Voxel-based morphometry of sporadic epileptic patients with mesiotemporal sclerosis. Epilepsia. 2010;51:506–510. doi: 10.1111/j.1528-1167.2009.02310.x. [DOI] [PubMed] [Google Scholar]
- Lai CH. Patterns of cortico-limbic activations during visual processing of sad faces in depression patients: a coordinate-based meta-analysis. J Neuropsychiatry Clin Neurosci. 2014;26:34–43. doi: 10.1176/appi.neuropsych.12060143. [DOI] [PubMed] [Google Scholar]
- Leserman J. The effects of stressful life events, coping, and cortisol on HIV infection. CNS Spectr. 2003a;8:25–30. doi: 10.1017/s1092852900023439. [DOI] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. 2003b;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, Perkins DO, Folds JD, Evans DL. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Leserman J, Whetten K, Lowe K, Stangl D, Swartz MS, Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Li H, Li W, Wei D, Chen Q, Jackson T, Zhang Q, Qiu J. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage. 2014;92C:1–7. doi: 10.1016/j.neuroimage.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Long NM, Oztekin I, Badre D. Separable prefrontal cortex contributions to free recall. J Neurosci. 2010;30:10967–10976. doi: 10.1523/JNEUROSCI.2611-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: a dose-response study in humans. Behav Neurosci. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Somoza G, De Nicola AF. Glucocorticoid negative feedback and glucocorticoid receptors after hippocampectomy in rats. Horm Metab Res. 1987;19:105–109. doi: 10.1055/s-2007-1011753. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: a preliminary study. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Cohen M, Golub ET, Greenblatt RM, Young M, Schwartz RM, Anastos K, Cook JA. Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause. 2012;19:1215–1223. doi: 10.1097/gme.0b013e318255434d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McEwen BS, De Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. [3H]Dexamethasone binding in rat frontal cortex. Brain Res. 1985;328:176–180. doi: 10.1016/0006-8993(85)91340-x. [DOI] [PubMed] [Google Scholar]
- Meyer VJ, Little DM, Fitzgerald DA, Sundermann EE, Rubin LH, Martin EM, Weber KM, Cohen MH, Maki PM. Crack cocaine use impairs anterior cingulate and prefrontal cortex function in women with HIV infection. J Neurovirol. 2014;20:352–361. doi: 10.1007/s13365-014-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress. 2011;14:227–232. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukay-Martin ND, Cristiani SA, Saveanu R, Bornstein RA. The relationship between stressful life events and cognitive function in HIV-infected men. J Neuropsychiatry Clin Neurosci. 2003;15:436–441. doi: 10.1176/jnp.15.4.436. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rubin LH, Cook JA, Grey DD, Weber K, Wells C, Golub ET, Wright RL, Schwartz RM, Goparaju L, Cohan D, Wilson ML, Maki PM. Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J Womens Health (Larchmt) 2011;20:1287–1295. doi: 10.1089/jwh.2010.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Weber KM, Cohen MH, Martin E, Valcour V, Milam J, Anastos K, Young MA, Alden C, Gustafson DR, Maki PM. The association of perceived stress and verbal memory is greater in HIV-infected versus HIV-uninfected women. J Neurovirol. 2015;21:422–432. doi: 10.1007/s13365-015-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H, Fukuda R, Okuaki T, Rogers M, Kasai K, Machida T, Shirouzu I, Yamasue H, Akiyama T, Kato N. Parahippocampal activation evoked by masked traumatic images in posttraumatic stress disorder: a functional MRI study. Neuroimage. 2005;26:813–821. doi: 10.1016/j.neuroimage.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Young LJ, Plotsky PM, Insel TR. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, Baldo JV, Delis DC. New semantic and serial clustering indices for the California Verbal Learning Test-Second Edition: background, rationale, and formulae. J Int Neuropsychol Soc. 2002;8:425–435. doi: 10.1017/s1355617702813224. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FI, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour V, Rubin LH, Tien P, Anastos K, Young M, Mack W, Cohen M, Golub ET, Crystal H, Maki PM. Human immunodeficiency virus (HIV) modulates the associations between insulin resistance and cognition in the current combination antiretroviral therapy (cART) era: a study of the Women’s Interagency HIV Study (WIHS) J Neurovirol. 2015;21:415–421. doi: 10.1007/s13365-015-0330-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Huska JA, Keane TM. PCL-C for DSM-IV. Division NCfPBS. Boston: 1991. [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37:103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, Rabinovici GD, Jagust WJ. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70:1512–1519. doi: 10.1001/jamaneurol.2013.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Scott JC, Dawson MS, Morgan EE, Carey CL, Heaton RK, Grant I Group HIVNRC. Construct validity of Hopkins Verbal Learning Test-Revised component process measures in an HIV-1 sample. Arch Clin Neuropsychol. 2005;20:1061–1071. doi: 10.1016/j.acn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Yusim A, Franklin L, Brooke S, Ajilore O, Sapolsky R. Glucocorticoids exacerbate the deleterious effects of gp120 in hippocampal and cortical explants. J Neurochem. 2000;74:1000–1007. doi: 10.1046/j.1471-4159.2000.0741000.x. [DOI] [PubMed] [Google Scholar]