Abstract
Diabetes mellitus and obesity, which is a major risk factor in the development of type 2 diabetes mellitus, have reached epidemic proportions worldwide including the United States. The current statistics and forecasts, both short- and long-term, are alarming and predict severe problems in the near future. Therefore, there is a race for developing new compounds, discovering new receptors or finding alternative solutions to prevent and/or treat the symptoms and complications related to obesity and diabetes mellitus. It is well demonstrated that members of the transient receptor potential (TRP) superfamily play a crucial role in a variety of biological functions both in health and disease. In the recent years, transient receptor potential vanilloid type 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) were shown to have beneficial effects on whole body metabolism including glucose homeostasis. TRPV1 and TRPA1 have been associated with control of weight, pancreatic function, hormone secretion, thermogenesis and neuronal function; which suggest a potential therapeutic value of these channels. This review summarizes recent findings regarding TRPV1 and TRPA1 in association with whole body metabolism with emphasis on obese and diabetic conditions.
Keywords: TRPV1, TRPA1, diabetes, obesity, metabolism, glucose homeostasis
Introduction
During the past decades the prevalence of obesity and diabetes mellitus significantly increased worldwide including the United States. Statistics from the Centers for Disease Control and Prevention (CDC) revealed that more than one third of the adults are obese in the United States (CDC, Division of Nutrition, Physical Activity and Obesity). Given the continuous increase in prevalence since the 1970s, forecasts suggest further significant increase in obesity prevalence and estimate that by 2030 more than 50 % of the United States population will be obese (1). Obesity is a major risk factor for a variety of diseases including cardiovascular diseases and diabetes mellitus. The National Diabetes Statistics Report 2014 by CDC estimated that approximately 9.3 % of the United States population has diabetes mellitus. The number of new cases increased by 1.7 million within couple of years and these data predict a severe and growing problem. The increasing prevalence of metabolic diseases is not restricted to economically developed countries, since dramatic elevation in the rates of obesity and diabetes mellitus has been seen in developing countries. Currently available solutions for improving obese and diabetic conditions and preventing further complications include life style changes, mainly decreasing calorie intake and increasing energy expenditure; bariatric surgeries that result in weight loss and remission of type 2 diabetes mellitus in some cases; and pharmacological interventions. While these solutions and treatments are available, there is a continuous race for developing new therapeutic interventions for proper maintenance of glucose homeostasis.
Members of the transient receptor potential (TRP) superfamily have been shown to have numerous biological functions and TRP channels became potential drug targets for a variety of pathophysiological condition (2). Indeed, recent findings suggest that some of the TRP channels are involved in the regulation of whole body metabolism and could have therapeutic value. This review focuses on current data available on two TRP channels, TRPV1 and TRPA1 and on their role in the regulation of whole body metabolism including glucose homeostasis with emphasis on obese and diabetic conditions.
Dietary and topical interventions in obese and diabetic conditions
Transient receptor potential vanilloid type 1
Among the TRP channels, up to date the role of transient receptor potential vanilloid type 1 (TRPV1) in the regulation of metabolism and energy homeostasis is the most established (3). TRPV1 is a nonselective cation channel, which could be activated by exogenous ligands (e.g., capsaicin) and by endogenous ligands, the endovanilloids (4). In general, TRPV1 has been linked to the development and progression of diabetes mellitus, both type 1 and type 2 diabetes mellitus (5–8). The link between diabetes mellitus and TRPV1 is proposed at multiple levels, which include control of appetite and weight (9–11), regulation of pancreatic function (5, 12–14), secretion of glucagon-like peptide-1 (GLP-1) (8, 15), modulation of the adiponectin (16) and leptin signaling (17), interaction between sensory nerves and pancreas (5, 18), and control of the autonomic nervous system (ANS) (3, 19, 20).
There is growing amount of data associating TRPV1 with weight regulation, suggesting that it has a role in the control of weight and energy homeostasis; although, in some cases the results are contradictory. Beneficial effects of dietary capsaicin, the pungent ingredient of red pepper that is an exogenous agonist of TRPV1 have been shown both in human and animal studies (9–11, 21). These positive effects were mainly associated with increased satiety, reduced food intake, increased activity of the sympathetic nervous system, and effects on thermogenesis. These and some of the contradictory findings were recently reviewed somewhere else in more detail (2, 3), and here we focus on beneficial effects of dietary and topical interventions during obese and diabetic conditions.
Dietary capsaicin has been shown to reduce obesity-induced inflammation, insulin resistance and hepatic steatosis in obese mice fed a high-fat diet (22). Dietary capsaicin (0.015 %) was supplied for a ten-week period and resulted in lower fasting glucose levels, lower insulin and leptin levels, improved glucose tolerance compared to the obese control group without capsaicin treatment. These beneficial effects were associated with attenuation of inflammation in the liver and adipose tissue, which would be favorable in obesity-related metabolic syndrome (22). Furthermore, the improvement of glucose intolerance induced by obesity is crucially important for preventing the development of type 2 diabetes.
Similar beneficial results were found in diabetic KKAy mice following the same 0.015 % dietary capsaicin administration (16). In this study the authors observed that three-week dietary capsaicin administration significantly reduced fasting glucose, insulin, triglyceride levels and inflammation; while adiponectin and its receptor, the AdipoR2 increased, which was associated with increased fatty acid oxidation (16). It has to be noted that in this study the observed capsaicin-dependent beneficial metabolic effects were independent of loss of weight or adiposity, and the authors suggested that the upregulation of the adiponectin system could be behind their findings.
Topical application of capsaicin in obese mice also had beneficial effects on whole body metabolism (23). The effect of topical capsaicin (0.075 %) in pre- and post-obese mice was investigated. The cream was topically applied for seven to eight weeks, depending on the used experimental paradigm. The capsaicin treated mice weight less and had less mesenteric and epididymal adipose tissue with reduced-size lipid droplets (23). These findings were accompanied with lower plasma glucose, cholesterol and triglyceride levels. The decreased adiposity was associated with lower expression levels of TNF-α and IL-6 and increased expression of adipokines and genes related to lipid metabolism including adiponectin, PPARs, UCP2, lipoprotein lipase and many more (23).
Interestingly, another finding demonstrated that the combination of exercise and capsinoids, the non-pungent capsaicin analogues, supplementation increases energy expenditure and more importantly additively suppresses diet-induced obesity (24). Diet induced obese mice were subjects of exercise, capsinoids or exercise combined with capsinoids interventions. Capsinoids in combination with exercise additively suppressed body weight gain most likely due to decreased epididymal, mesenteric and perirenal fat accumulation (24). The combination of the interventions resulted in decreased glucose, insulin, leptin and cholesterol levels. Increased whole body oxygen consumption and fat oxidation was observed in addition to the reduced total lipid and triglyceride levels, which was consistent with improvement of diet-induced hepatic steatosis (24). Furthermore, exercise combined with capsinoids increased cAMP levels and activated PKA in brown adipose tissue. The findings of this study add to the previous observations, where only TRPV1 agonist or exercise was used as treatment. Here, it has been demonstrated that the combination of exercise and capsinoids have an additive anti-obesity effect, which may suggest increased efficacy for overturning obesity and contributing to the prevention of type 2 diabetes mellitus.
A recent research article used hyperinsulinemic-euglycemic clamp experiments on wild-type and TRPV1 knockout mice fed with high-fat diet to conduct metabolic measurements (17). TRPV1 knockout mice gained more weight and were heavier following a five week high-fat diet than wild-type mice on high-fat diet. The weight gain was accompanied with increased whole body fat mass. High-fat diet TRPV1 knockout mice had increased food intake and decreased physical activity (17). The hyperinsulinemic-euglycemic clamp studies revealed that TRPV1 knockout mice were more insulin resistant following high-fat diet than their wild-type controls. High-fat diet treated TRPV1 knockout mice displayed a reduction in insulin-stimulated glucose uptake in the white and brown adipose tissue, and also had significantly higher leptin levels, while adiponectin levels did not change. Leptin failed to suppress food intake in high-fed diet TRPV1 knockout mice and there was impaired leptin signaling in the hypothalamus, indicating leptin resistance and dysfunctional leptin signaling in TRPV1 knockout mice (17). Furthermore, the authors conducted longitudinal metabolic studies comparing three and nine month old mice and found that TRPV1 deficiency causes leptin resistance and further increases obesity and insulin resistance (17). Together, the metabolic studies revealed that TRPV1 knockout mice develop a more severe insulin resistance and TRPV1 plays a major role in regulating glucose metabolism and hypothalamic leptin signaling. These recent findings are in contrast with a previous study by Motter and Ahern (25), where the authors demonstrated that TRPV1 knockout mice are resistant to diet-induced obesity. The contradictory findings can originate from the experimental design including the length of the studies. On the other hand, both studies suggest that TRPV1 has a significant role controlling metabolism; however, future investigations are necessary to determine the underlying mechanisms and the contribution of TRPV1, which might depend on the circumstances including composition of the diet, length of the dietary treatment, age of the animals and many other factors.
In summary, the majority of animal model studies were consistent with previous human studies demonstrating potential beneficial effects of capsaicin and capsaicinoid consumption or topical application on whole body metabolism (26, 27) (Table 1). The current assumptions for mechanisms underlying these effects are still debated and may include peripheral and/or central mechanisms. Dietary capsaicin is rapidly absorbed in the body (28). Capsaicin could be detected in the blood 10 minutes after ingestion and the levels are maintained up to 90 min (13). Capsaicin is lipophilic and is likely to cross the blood brain barrier, therefore, it may directly affect the central nervous system; while it can reduce visceral fat and inflammation through stimulating the adiponectin system (16, 22, 23), increase energy expenditure/thermogenesis via the sympathetic nervous system (24, 29), and interact with leptin signaling (17), or glucagon-like peptide-1 secretion (8); however, more detailed studies are needed to entirely delineate these potentially important findings.
Table 1.
Beneficial effects of TRPV1 and TRPA1 agonist administration in obese and/or diabetic conditions.
| Capsaicin administration (TRPV1) |
Cinnamon, cinnamaldehyde, AITC administration (TRPA1) |
|---|---|
| Decreased weight | Decreased weight |
| Lower glucose levels | Lower glucose levels |
| Lower insulin levels | Improved glucose tolerance |
| Improved glucose tolerance | Improved lipid levels |
| Improved lipid levels | Less fat accumulation |
| Increased adiponectin signaling | Improved hepatic steatosis |
| Less adipose tissue | Increased thermogenesis |
| Reduced inflammation | |
| Improved hepatic steatosis | |
| Increased thermogenesis | |
Transient receptor potential ankyrin 1
Many members of the TRP superfamily are activated by herbal compounds, and could be used as alternative therapies. In the case of TRPV1, capsaicin and capsinoids are prospective dietary supplements as described above, while other natural compounds activating TRP channels are recently receiving more attention. A potentially interesting anti-diabetic alternative is cinnamon treatment, which was investigated in clinical trials (30, 31). Cinnamon as a spice is very well known and used almost in every culture. Cinnamon is obtained from the bark of trees, which belong to the Cinnamomun genus. In a study, type 2 diabetic patients received cinnamon or placebo treatment for sixty days and plasma glucose and lipid profile were analyzed (32). Cinnamon treatment resulted in decreased fasting glucose levels, triglyceride and cholesterol levels compared to the placebo group (32). A moderate glucose lowering effect was found in another study conducted on type 2 diabetic patients (33). In contrast, there are studies suggesting no difference following cinnamon treatment (34). The controversy regarding effects of cinnamon undoubtedly exists and further studies required determining the patient population which could benefit from cinnamon supplementation. Detailed review of findings regarding cinnamon interventions in diabetes mellitus can be found in other papers (35, 36).
Despite the controversy about the effect of cinnamon the mechanism of action is under investigation. There are postulated mechanisms underlying the effects of cinnamon interventions including inactivation of insulin receptor (37), and enhancement of glucose uptake through increasing the amount of insulin receptor beta, insulin substrate 1, and glucose transporter 4 (GLUT4) (38, 39). Proliferator-activated receptors (PPARs) and anti-inflammatory effects of cinnamon were also proposed as potential mechanisms (40, 41).
Cinnamon extracts contain many different ingredients and one of the main ingredients is cinnamaldehyde, which largely contributes to the unique flavor and odor of the spice, and it is a potent agonist of transient receptor potential A1 (TRPA1). TRPA1 is a nonselective cation channel with high permeability to Ca2+ and Na+, and up to date the only member of its group. Cinnamaldehyde and TRPA1 has been associated with insulin secretion (42), glucagon-like peptide-1 secretion (43), ghrelin secretion (44), improvement of insulin sensitivity in the brain and lowering liver fat (45).
Besides cinnamaldehyde, allyl isothiocyanate (AITC), which is responsible for the pungent taste of mustard, horseradish and wasabi, is a potent TRPA1 agonist. The effect of dietary AITC (250 mg/kg) was evaluated in high-fat induced insulin resistant conditions (46). The investigations revealed that AITC increased glucose uptake, improved insulin signaling and improved mitochondrial function in vitro. In vivo evaluation demonstrated that AITC supplementing the high-fat diet resulted in less weight gain and organ hypertrophy compared to high-fat diet only. These findings were associated with improved dyslipidemia and smaller adipocyte size. AITC supplementation also reduced high-fat diet induced hepatic steatosis. Furthermore, the high-fat diet induced hyperglycemia, hyperinsulinemia and increased hemoglobin A1C levels were markedly improved, in some case reversed in the AITC intervention group. Glucose tolerance also improved and AITC treatment normalized the expression of glucose metabolism-related genes (46). These data suggest that AITC treatment, likely through TRPA1 activation had beneficial effect on glucose uptake and improved impaired insulin signaling. The protective effect against insulin resistance was associated with increased mitochondrial activity; however, further studies needed to determine the details of TRPA1 contribution.
Taken together, emerging data suggest that dietary supplements including capsaicin, cinnamaldehyde in cinnamon, and AITC, which are potent activators of TRPV1 or TRPA1 likely will have beneficial effects in obese and/or type 2 diabetic patients and also in patients suffering from lipid disorders (2, 3, 47, 48); however, the mechanisms underlying the effects including peripheral (e.g., modulation of insulin secretion) and/or central (e.g., alteration of neuronal activity) effects needed to be further investigated.
The influence of TRPV1 and TRPA1 on autonomic circuits
Glucose homeostasis are largely regulated by the autonomic nervous system (ANS) (49, 50). The ANS consists of the sympathetic and parasympathetic nervous system. Dysfunction of the ANS has been linked to metabolic abnormalities, development of diabetes mellitus and cardiovascular diseases (19, 51–53). The activity of the ANS is modulated by preautonomic neurons in the autonomic centers of the brain, and the activity of these preautonomic neurons is controlled by neuromodulators, hormones and nutrients. These neurons are part of a specific central network receiving information about the status of the peripheral organs, which are involved in metabolism. This central network contains hypothalamic and brainstem circuits, which are highly interconnected (53). It is known that hyperglycemia alters vagus nerve activity, induces insulin release (54, 55), and modulation of hypothalamic circuits influences glucose levels (56). Capsaicin injection into the fourth ventricle of rats eliminated the suppression of sham feeding induced by cholecystokinin, while subcutaneous capsaicin did not have effect. These findings indicate that capsaicin sensitive cells around the fourth ventricle are involved in the suppression of sham feeding by some of the intestinal nutrients (57). TRPV1 expression in the hypothalamus was down-regulated in high-fat diet treated mice, while dietary capsaicin increased TRPV1 expression levels (58). Capsaicin treatment in high-fat diet mice enhanced the expression of anorexic genes (e.g., urocortin, peptide YY) in the hypothalamus compared to the high-fat diet alone, whereas the expression of orexigenic genes was decreased (58). Here, we give and overview of the cellular effects of TRPV1 and TRPA1 activation on neuronal activity in autonomic areas of the brain.
In the past decades numerous studies have been demonstrated the expression of TRPV1 in the brain including in the autonomic centers. TRPV1 expression has been shown in the hypothalamus, which is well known in the regulation of feeding behavior, energy and glucose homeostasis. In particular, TRPV1 expression was determined in paraventricular nucleus (PVN), dorsomedial hypothalamus (DMH), and lateral hypothalamus (LH) (59–61). TRPV1 expression was also revealed in the brainstem including the dorsal vagal complex, which has major role in the regulation of vagal output to the subdiaphragmatic organs and thus controls gastric function, liver glucose production, insulin secretion.
Activation of TRPV1 has been shown to facilitate synaptic inputs (62–65), which ultimately modulate neuronal activity, and thereby TRPV1 has the potential to alter the activity of ANS in order to modulate metabolism. In the brainstem, activation of TRPV1 with capsaicin increased action potential independent inhibitory postsynaptic currents (64). Importantly, the enhancement of GABA release caused by TRPV1-dependent increase in glutamate release demonstrated heterosynaptic facilitation of synaptic inputs to neurons of the dorsal motor nucleus of the vagus (DMV). Activation of TRPV1 also increased the frequency of glutamatergic postsynaptic currents (64, 66). Regulation of DMV neurons by TRPV1-dependent excitatory neurotransmission was further investigated in hyperglycemic conditions (65). Patch-clamp slice electrophysiology was conducted using control and hyperglycemic mice and demonstrated a robust increase of glutamate release to DMV neurons following TRPV1 activation in intact mice. This TRPV1-dependent enhancement of glutamatergic neurotransmission was reduced in the DMV of hyperglycemic mice (65), without change in TRPV1 protein expression. Recently, possible interaction between TRPV1 and leptin receptor expressing DMV neurons was shown. Activation of TRPV1 enhanced excitatory neurotransmission to a subpopulation of leptin receptor expressing DMV neurons, including stomach-related DMV neurons, whereas the overall inhibitory neurotransmission was not affected (67). These results let us speculate that TRPV1 has the potential to modulate the effect of leptin on food intake.
Within the hypothalamus the PVN is a major, integrative autonomic center. The PVN contains multiple types of neurons and preautonomic neurons could be identified by retrograde viral labeling. Pseudorabies virus 152 (PRV–152) was used to identify organ-related neurons in the PVN, and it has been demonstrated that TRPV1 controls excitatory neurotransmission in stomach- and liver-related PVN neurons (20, 68). Interaction between glucocorticoid and TRP signaling was revealed by demonstrating that dexamethasone biphasically modulates excitatory neurotransmission in a subset of stomach-related PVN neurons. The modulation of glutamate release involved TRPV1 and TRPV4 and also cannabinoid type 1 receptors (68). This recent publication is also intriguing because it demonstrated multiphasic modulation of glutamate inputs in preautonomic PVN neurons and showed the complexity of the regulation of ANS.
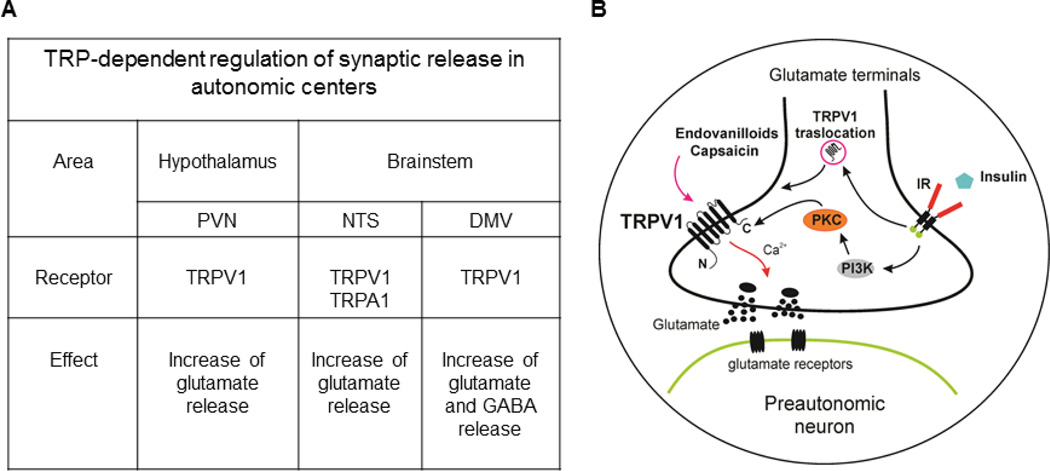
Administration of NMDA into the PVN increased plasma glucose levels in rodents (56). Glucose level is largely modulated by hepatic glucose production; however, there is limited information about the synaptic regulation of liver-related PVN neurons. Retrograde viral labeling with PRV-152 was used to identify liver-related PVN neurons within the brain-liver circuit in control, streptozotocin (STZ)-treated type 1 diabetic and insulin-treated type 1 diabetic mice. Our study demonstrated TRPV1-dependent regulation of preautonomic, liver-related PVN neurons in control conditions (20). The TRPV1-dependent excitation diminished in a similar manner as shown in DMV (65); whereas, insulin-treatment, both in vivo and in vitro, restored TRPV1 activity in a PI3 kinase/PKC-dependent manner (20). TRPV1 translocation from the cytosol to the plasma membrane and phosphorylation of TRPV1 was stimulated in the hypothalamus of type 1 diabetic mice (20) (Fig. 1). The above described functional plasticity of central TRPV1 in the hypothalamus and brainstem suggests altered autonomic outflow in hyperglycemic mice, which likely contributes to metabolic dysfunction via altering excitability of preautonomic neurons regulating pancreatic, liver and gastrointestinal function.
Figure 1.
Synaptic regulation of preautonomic neurons in the hypothalamus and brainstem. A: Autonomic centers known to be regulated by TRPV1 and TRPA1. B: Schematics of TRPV1-dependent synaptic regulation of preautonomic neurons. Activation of TRPV1 leads to glutamate release and insulin has the ability to modulate TRPV1-dependent glutamate release through PI3 kinase/PKC and translocation-dependent mechanisms. PVN: paraventricular nucleus of the hypothalamus; NTS: nucleus tractus solitarii; DMV: dorsal motor nucleus of the vagus; IR: insulin receptor.
On the other hand, there is less information on the cellular effects of TRPA1 in the autonomic centers. In general, activation of TRPA1 produces Ca2+ influx followed by excitation of neurons. Similarly to TRPV1, TRPA1 is activated by pungent compounds including AITC and cinnamaldehyde (69, 70). In the periphery, TRPA1 is involved in thermosensation and mechanosensation (71); however, far less is known about the role of TRPA1 in the central nervous system. Recent observations indicate that TRPA1 is functionally expressed in a variety of brain areas and is able to modulate neuronal activity (72, 73). Electrophysiological studies conducted in magnocellular neurons of the supraoptic nucleus of the hypothalamus demonstrated that exogenous agonists of TRPA1 (e.g., AITC, cinnamaldehyde) enhance glutamate release (72). Both AITC and cinnamaldehyde increased the frequency of miniature excitatory postsynaptic currents without altering the amplitude indicating that TRPA1 is located in presynaptic terminals and modulate glutamate release to neurons in the supraoptic nucleus (72). Modulation of synaptic inputs in brainstem neurons was also determined. AITC application increased glutamate release on mechanically dispersed neurons of the nucleus tractus solitarii (NTS) (73). The NTS as part of the vago-vagal reflex integrates visceral information and transmits the processed information to the DMV; therefore, TRPA1 has the potential to modulate the vagal outflow to the subdiaphragmatic organs.
In addition, TRPA1 was recently identified as a molecular sensor for oxidative stress (e.g., hydrogen peroxide, superoxide and products of lipid peroxidation) (74). This suggests that TRPA1 may be the crucial link between oxidative stress, inflammation, altered neuronal activity, and a critical mechanism underlying vagal dysfunction and thus could contribute to metabolic dysregulation; however, further detailed studies are required to delineate this scenario.
In summary, TRPV1 and TRPA1 modulate neuronal activity at the level of hypothalamus and brainstem, though the role of TRPV1 in the regulation of preautonomic neurons is more established. Modulation of excitatory and/or inhibitory neurotransmission ultimately leads to increased or decreased activity of neurons, which can lead to increase or decrease of the ANS. Increased sympathetic and decreased parasympathetic activity is already associated with metabolic syndrome (51), therefore, determining the unique role of TRPV1 and TRPA1 in the regulation of preautonomic neurons may lead to the development of new therapeutic approaches using the brain-periphery route to maintain proper metabolism.
TRPV1 and TRPA1 in the pancreas
Proper insulin secretion in pancreatic beta cells and adequate response of the body to insulin is necessary to avoid the development of diabetes mellitus. TRPV1 is well known to have influence on pancreatic function and insulin secretion both in humans and animals (5, 13). Insulin secretion is increased following TRPV1 activation with capsaicin in vitro and in vivo (14). In addition, TRPV1 is expressed on sensory nerves innervating the islets, and TRPV1 has been proposed to play a significant role in islet destruction, development of inflammation and type 1 diabetes mellitus (5, 7, 18). Therefore, TRPV1 can have multiple effects on pancreatic function by affecting insulin secretion and/or modulating the sensory nerves. Further details about the potential role of TRPV1 in the pancreas can be found in previous reviews (3, 6, 18).
TRPA1 was also shown to be expressed in pancreatic beta cells and in rat insulinoma RINm5F cells (42, 75). Incubation of pancreatic islets with the TRPA1 agonist, cinnamaldehyde increased insulin secretion (76). Moreover, different methods including immunostaining and RT-PCR revealed expression of TRPA1 in pancreatic beta cells; whereas, there was no TRPA1 expression in alpha cells (75). Activation of TRPA1 with a variety of agonists including AITC caused Ca2+ influx and insulin release both in a pancreatic beta cell line and in primary cultured pancreatic beta cells. This TRPA1-dependent insulin release was prevented with antagonist of TRPA1. The authors also proposed a very compelling idea that suggests that TRPA1 and ATP-dependent K+ channels work synergistically together to increase insulin release. Furthermore, it has been demonstrated that glibenclamide, a widely used sulfonylurea working through ATP-dependent K+ channel, is an agonist of TRPA1 (77). Based on these recent findings the role of TRPA1 and its potential for glucose management or its negative effects on the pancreas has to be investigated in more detail.
TRPV1, TRPA1 and secretion of ghrelin and glucagon-like peptide-1
Dietary cinnamaldehyde has been shown to effect the function of the gastrointestinal tract including gut motility (78), gastric emptying (79), and cholecystokinin secretion. A recent finding investigated the effect of TRPA1 on ghrelin secretion (44). Ghrelin belongs to the group of orexigenic hormones and it is mainly produced in the stomach and small bowel. Cinnamaldehyde effect on gut physiology and ghrelin secretion was investigated in detail (44). TRPA1 expression has recently been described in the gut as a sensor in enterochromaffin cells for controlling gastrointestinal functions via serotonin signaling (78, 79). Expression of TRPA1 was also determined in the stomach (44) with high expression in the pyloric part. Then mouse ghrelinoma 3-1 (MGN3-1) cells was used to determine the effect of cinnamaldehyde on TRPA1, insulin receptor and ghrelin gene expression and on ghrelin secretion. Cinnamaldehyde upregulated TRPA1 and insulin receptor gene expression, while it decreased ghrelin secretion in MGN3-1 cells (44). The reduction of ghrelin secretion was prevented by a TRPA1 antagonist. The study also confirmed that administration of a single dose of cinnamaldehyde (250 mg/kg body weight) decreased food intake. Gastric emptying was also significantly slower following cinnamaldehyde treatment, while this effect was absent in TRPA1 deficient mice (44). Furthermore, chronic cinnamaldehyde administration reduced cumulative weight gain and reduced body fat mass in high-fat diet induced obese mice. Mice with chronic cinnamaldehyde treatment showed a larger glucose reduction during oral glucose tolerance test, but without change in plasma insulin levels. These findings indicate improved insulin sensitivity in response to glucose load (44), and also consistent with previous findings showing that ghrelin is involved in the regulation of glucose homeostasis (80). Together, the findings discussed above support the idea that TRPA1 has anti-hyperglycemic effects, and underlying mechanisms might involve modulation of ghrelin secretion and enhancement of insulin sensitivity.
The gut hormone glucagon-like peptide-1 (GLP-1) is a major player in the control of glucose metabolism via altering insulin secretion and the gut-brain-periphery axis. Furthermore, the relatively new anti-diabetic therapies (e.g., sitagliptin, linagliptin) are based on prolonging the life time of GLP-1; therefore, alternative therapies influencing GLP-1 levels could be useful in diabetic patients. Capsaicin has been shown to increase GLP-1 levels in human subjects, which demonstrates a possible interaction between TRPV1 and GLP-1 (15). This was supported by animal studies demonstrating that TRPV1 is present in GLP-1 expressing intestinal cells and TRPV1 stimulates GLP-1 release (8).
Recently, TRPA1 gene was identified in enteroendocrine L-cells and functional expression of TRPA1 in L-cells was determined (43). The TRPA1 agonist, AITC increased intracellular Ca2+ and this response was inhibited with a TRPA1 antagonist. AITC increased GLP-1 secretion independently of glucose concentration and was prevented by a TRPA1 antagonist. Moreover, AITC administration by gavage or administration into the duodenum increased plasma GLP-1 concentration (43); however, the effect was preserved in TRPA1 knockout mice suggesting existence of other pathways for increasing GLP-1 secretion. In contrast, a recent study did not find difference in GLP-1 levels following activation of TRPA1 with methyl syringate, while increased peptide YY levels, decreased food intake and gastric emptying was observed (81). Taken together, a variety of findings suggest that TRPV1 and TRPA1 may have potential for modulating ghrelin and/or GLP-1 secretion.
TRPV1 and TRPA1 in adipose tissue
Expression of multiple TRP channels has been shown in adipose tissue and they may play a role in adipogenesis. Mice kept on high-fat and capsaicin diet for three months showed alteration in anorectic, orexigenic and energy expenditure related genes in white adipose tissue (WAT) compared to high-fat diet and standard diet controls (58). Capsaicin supplementation resulted in “browning” genotype in WAT and increased genes related to thermogenesis and mitochondrial function in brown adipose tissue (BAT) (58). Ingestion of the TRPA1 agonist cinnamaldehyde has been shown to reduce visceral adipose tissue in mice fed a high-fat high-sucrose diet (82). The dietary TRPA1 agonist resulted in decreased mesenteric adipose tissue, and a decreasing trend was observed in perirenal and epididymal adipose tissue weights. Another study demonstrated alteration of genes following cinnamaldehyde treatment and showed upregulation of glucose transporter genes (GLUT1, GLUT8, GLUT12) and Cpt1a in white adipose tissue, which suggests increased fatty acid oxidation (44).
In addition to WAT, brown adipose tissue has a significant impact on whole body metabolism and it has been proposed as a potential target for obesity and metabolic disorders (83). BAT is rich in mitochondria and densely innervated with sympathetic nerves. Thermogenesis in BAT is directly controlled by the sympathetic nerves, and increase of uncoupling protein 1 (UCP1) expression is known after norepinephrine release from the sympathetic nerves; therefore, increase of UPC1 is a marker for sympathetic activity in BAT.
A number of studies revealed the effects of TRPV1 and TRPA1 agonists on thermogenesis in interscapular BAT of rats and mice (84, 85). Capsaicin administration resulted in increased whole body energy expenditure and rise in BAT thermogenesis. TRPV1 activation was shown to elevate UCP1 content in BAT of mice fed high-fat high-sucrose diet, while visceral fat accumulation was decreased (86). On the other hand, analysis of genes expression in BAT following chronic treatment with cinnamaldehyde demonstrated upregulation of acetyl-CoA synthetase 4 (ACSL4), which activates fatty acids involved in beta-oxidation. Increased UCP1 protein levels were found in interscapular BAT in cinnamaldehyde treated mice fed a high-salt high-sucrose diet (82). Taken together, the studies suggest that activation of TRP channels decreases visceral fat in high-fat diet conditions and stimulates BAT heat production; however, there are numerous questions, which need to be answered in order to understand the role of TRPV1 and TRPA1 in white and brown adipose tissue.
Conclusions
Whole body metabolism is regulated at multiple levels including peripheral and central mechanisms. There is solid evidence demonstrating TRPV1 and TRPA1 expression in the entire body and their potential beneficial effects on whole body metabolism including glucose metabolism. Activation of TRPV1 and TRPA1 with their agonists have multiple effects, which include control of body weight, satiety, secretion of hormones, regulation of adipocytes, modulation of thermogenesis and alteration of neuronal activity. To delineate and differentiate the site of action (periphery or central), the underlying mechanisms or the combination of mechanisms, the potential applicable doses and routes require future studies. However, the currently available studies demonstrate that TRP channels have potential therapeutic value for improving obese and diabetic conditions.
Acknowledgements
The authors acknowledge funding support from the National Institutes of Health (R01 DK099598 for AZs and HL122829 for AVD).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Reference list
- 1.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Nilius B, Szallasi A. Transient receptor potential channels as drug targets: from the science of basic research to the art of medicine. Pharmacol Rev. 2014;66:676–814. doi: 10.1124/pr.113.008268. [DOI] [PubMed] [Google Scholar]
- 3.Zsombok A. Vanilloid receptors--do they have a role in whole body metabolism? Evidence from TRPV1. J Diabetes Complications. 2013;27:287–292. doi: 10.1016/j.jdiacomp.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Der Stelt M, Di Marzo V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 5.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, Driver JP, Serreze D, Salter MW, Dosch HM. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 6.Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Tsui H, Paltser G, Chan Y, Dorfman R, Dosch HM. 'Sensing' the link between type 1 and type 2 diabetes. Diabetes Metab Res Rev. 2011;27:913–918. doi: 10.1002/dmrr.1279. [DOI] [PubMed] [Google Scholar]
- 8.Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, Ma L, Zhao Z, Liu D, Zhu Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986;116:1272–1278. doi: 10.1093/jn/116.7.1272. [DOI] [PubMed] [Google Scholar]
- 10.Westerterp-Plantenga MS, Smeets A, Lejeune MP. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int J Obes (Lond) 2005;29:682–688. doi: 10.1038/sj.ijo.0802862. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M, Tremblay A. Effects of red pepper on appetite and energy intake. Br J Nutr. 1999;82:115–123. [PubMed] [Google Scholar]
- 12.Gram DX, Ahren B, Nagy I, Olsen UB, Brand CL, Sundler F, Tabanera R, Svendsen O, Carr RD, Santha P, Wierup N, Hansen AJ. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- 13.Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J Med Assoc Thai. 2009;92:108–113. [PubMed] [Google Scholar]
- 14.Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321:219–225. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- 15.Smeets AJ, Westerterp-Plantenga MS. The acute effects of a lunch containing capsaicin on energy and substrate utilisation, hormones, and satiety. Eur J Nutr. 2009;48:229–234. doi: 10.1007/s00394-009-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang JH, Tsuyoshi G, Le Ngoc H, Kim HM, Tu TH, Noh HJ, Kim CS, Choe SY, Kawada T, Yoo H, Yu R. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. J Med Food. 2011;14:310–315. doi: 10.1089/jmf.2010.1367. [DOI] [PubMed] [Google Scholar]
- 17.Lee E, Jung DY, Kim JH, Patel PR, Hu X, Lee Y, Azuma Y, Wang HF, Tsitsilianos N, Shafiq U, Kwon JY, Lee HJ, Lee KW, Kim JK. Transient receptor potential vanilloid type-1 channel regulates diet-induced obesity, insulin resistance, and leptin resistance. FASEB J. 2015;29:3182–3192. doi: 10.1096/fj.14-268300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsui H, Razavi R, Chan Y, Yantha J, Dosch HM. 'Sensing' autoimmunity in type 1 diabetes. Trends Mol Med. 2007;13:405–413. doi: 10.1016/j.molmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Carnethon MR, Golden SH, Folsom AR, Haskell W, Liao D. Prospective investigation of autonomic nervous system function and the development of type 2 diabetes: the Atherosclerosis Risk In Communities study, 1987–1998. Circulation. 2003;107:2190–2195. doi: 10.1161/01.CIR.0000066324.74807.95. [DOI] [PubMed] [Google Scholar]
- 20.Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependent regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes. 2012;61:1381–1390. doi: 10.2337/db11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063–1070. doi: 10.1161/01.RES.0000262653.84850.8b. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Goto T, Han IS, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity (Silver Spring) 2010;18:780–787. doi: 10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- 23.Lee GR, Shin MK, Yoon DJ, Kim AR, Yu R, Park NH, Han IS. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity (Silver Spring) 2013;21:115–122. doi: 10.1002/oby.20246. [DOI] [PubMed] [Google Scholar]
- 24.Ohyama K, Nogusa Y, Suzuki K, Shinoda K, Kajimura S, Bannai M. A combination of exercise and capsinoid supplementation additively suppresses diet-induced obesity by increasing energy expenditure in mice. Am J Physiol Endocrinol Metab. 2015;308:E315–E323. doi: 10.1152/ajpendo.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloomer RJ, Canale RE, Shastri S, Suvarnapathki S. Effect of oral intake of capsaicinoid beadlets on catecholamine secretion and blood markers of lipolysis in healthy adults: a randomized, placebo controlled, double-blind, cross-over study. Lipids Health Dis. 2010;9:72. doi: 10.1186/1476-511X-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts K, Shenoy R, Anand P. A novel human volunteer pain model using contact heat evoked potentials (CHEP) following topical skin application of transient receptor potential agonists capsaicin, menthol and cinnamaldehyde. J Clin Neurosci. 2011;18:926–932. doi: 10.1016/j.jocn.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res. 2010;131:682–691. [PubMed] [Google Scholar]
- 29.Ono K, Tsukamoto-Yasui M, Hara-Kimura Y, Inoue N, Nogusa Y, Okabe Y, Nagashima K, Kato F. Intragastric administration of capsiate, a transient receptor potential channel agonist, triggers thermogenic sympathetic responses. J Appl Physiol. 2011;1985;110:789–798. doi: 10.1152/japplphysiol.00128.2010. [DOI] [PubMed] [Google Scholar]
- 30.Lu T, Sheng H, Wu J, Cheng Y, Zhu J, Chen Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr Res. 2012;32:408–412. doi: 10.1016/j.nutres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Davis PA, Yokoyama W. Cinnamon intake lowers fasting blood glucose: meta-analysis. J Med Food. 2011;14:884–889. doi: 10.1089/jmf.2010.0180. [DOI] [PubMed] [Google Scholar]
- 32.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–3218. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 33.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanschoonbeek K, Thomassen BJ, Senden JM, Wodzig WK, van Loon LJ. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J Nutr. 2006;136:977–980. doi: 10.1093/jn/136.4.977. [DOI] [PubMed] [Google Scholar]
- 35.Chase CK, McQueen CE. Cinnamon in diabetes mellitus. Am J Health Syst Pharm. 2007;64:1033–1035. doi: 10.2146/ajhp060538. [DOI] [PubMed] [Google Scholar]
- 36.Rafehi H, Ververis K, Karagiannis TC. Controversies surrounding the clinical potential of cinnamon for the management of diabetes. Diabetes Obes Metab. 2012;14:493–499. doi: 10.1111/j.1463-1326.2011.01538.x. [DOI] [PubMed] [Google Scholar]
- 37.Imparl-Radosevich J, Deas S, Polansky MM, Baedke DA, Ingebritsen TS, Anderson RA, Graves DJ. Regulation of PTP-1 and insulin receptor kinase by fractions from cinnamon: implications for cinnamon regulation of insulin signalling. Horm Res. 1998;50:177–182. doi: 10.1159/000023270. [DOI] [PubMed] [Google Scholar]
- 38.Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch Biochem Biophys. 2007;459:214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- 39.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 40.Huang B, Yuan HD, Kim do Y, Quan HY, Chung SH. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J Agric Food Chem. 2011;59:3666–3673. doi: 10.1021/jf104814t. [DOI] [PubMed] [Google Scholar]
- 41.Qin B, Dawson H, Polansky MM, Anderson RA. Cinnamon extract attenuates TNF-alpha-induced intestinal lipoprotein ApoB48 overproduction by regulating inflammatory, insulin, and lipoprotein pathways in enterocytes. Horm Metab Res. 2009;41:516–522. doi: 10.1055/s-0029-1202813. [DOI] [PubMed] [Google Scholar]
- 42.Numazawa S, Takase M, Ahiko T, Ishii M, Shimizu S, Yoshida T. Possible involvement of transient receptor potential channels in electrophile-induced insulin secretion from RINm5F cells. Biol Pharm Bull. 2012;35:346–354. doi: 10.1248/bpb.35.346. [DOI] [PubMed] [Google Scholar]
- 43.Emery EC, Diakogiannaki E, Gentry C, Psichas A, Habib AM, Bevan S, Fischer MJ, Reimann F, Gribble FM. Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes. 2015;64:1202–1210. doi: 10.2337/db14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Camacho S, Michlig S, de Senarclens-Bezencon C, Meylan J, Meystre J, Pezzoli M, Markram H, le Coutre J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci Rep. 2015;5:7919. doi: 10.1038/srep07919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sartorius T, Peter A, Schulz N, Drescher A, Bergheim I, Machann J, Schick F, Siegel-Axel D, Schurmann A, Weigert C, Haring HU, Hennige AM. Cinnamon extract improves insulin sensitivity in the brain and lowers liver fat in mouse models of obesity. PLoS One. 2014;9:e92358. doi: 10.1371/journal.pone.0092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn J, Lee H, Im SW, Jung CH, Ha TY. Allyl isothiocyanate ameliorates insulin resistance through the regulation of mitochondrial function. J Nutr Biochem. 2014;25:1026–1034. doi: 10.1016/j.jnutbio.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Jiao L, Zhang X, Huang L, Gong H, Cheng B, Sun Y, Li Y, Liu Q, Zheng L, Huang K. Proanthocyanidins are the major anti-diabetic components of cinnamon water extract. Food Chem Toxicol. 2013;56:398–405. doi: 10.1016/j.fct.2013.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Xu YC, Guo FJ, Meng Y, Li ML. Anti-diabetic effects of cinnamaldehyde and berberine and their impacts on retinol-binding protein 4 expression in rats with type 2 diabetes mellitus. Chin Med J (Engl) 2008;121:2124–2128. [PubMed] [Google Scholar]
- 49.Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann N Y Acad Sci. 2010;1212:114–129. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- 50.Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, D'Alessio D. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Licht CM, Vreeburg SA, van Reedt Dortland AK, Giltay EJ, Hoogendijk WJ, DeRijk RH, Vogelzangs N, Zitman FG, de Geus EJ, Penninx BW. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95:2458–2466. doi: 10.1210/jc.2009-2801. [DOI] [PubMed] [Google Scholar]
- 52.Wulsin LR, Horn PS, Perry JL, Massaro JM, D'Agostino RB. Autonomic Imbalance as a Predictor of Metabolic Risks, Cardiovascular Disease, Diabetes, and Mortality. J Clin Endocrinol Metab. 2015;100:2443–2448. doi: 10.1210/jc.2015-1748. [DOI] [PubMed] [Google Scholar]
- 53.Zsombok A, Smith BN. Plasticity of central autonomic neural circuits in diabetes. Biochim Biophys Acta. 2009;1792:423–431. doi: 10.1016/j.bbadis.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laury MC, Takao F, Bailbe D, Penicaud L, Portha B, Picon L, Ktorza A. Differential effects of prolonged hyperglycemia on in vivo and in vitro insulin secretion in rats. Endocrinology. 1991;128:2526–2533. doi: 10.1210/endo-128-5-2526. [DOI] [PubMed] [Google Scholar]
- 55.Ahren B, Sundkvist G, Mulder H, Sundler F. Blockade of muscarinic transmission increases the frequency of diabetes after low-dose alloxan challenge in the mouse. Diabetologia. 1996;39:383–390. doi: 10.1007/BF00400669. [DOI] [PubMed] [Google Scholar]
- 56.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yox DP, Stokesberry H, Ritter RC. Fourth ventricular capsaicin attenuates suppression of sham feeding induced by intestinal nutrients. Am J Physiol. 1991;260:R681–R687. doi: 10.1152/ajpregu.1991.260.4.R681. [DOI] [PubMed] [Google Scholar]
- 58.Baboota RK, Murtaza N, Jagtap S, Singh DP, Karmase A, Kaur J, Bhutani KK, Boparai RK, Premkumar LS, Kondepudi KK, Bishnoi M. Capsaicin-induced transcriptional changes in hypothalamus and alterations in gut microbial count in high fat diet fed mice. J Nutr Biochem. 2014;25:893–902. doi: 10.1016/j.jnutbio.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O'Donnell D, Nicoll RA, Shah NM, Julius D, Basbaum AI. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci. 2011;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 61.Zsombok A, Gao H, Miyata K, Issa A, Derbenev AV. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Res. 2011;1398:30–39. doi: 10.1016/j.brainres.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doyle MW, Bailey TW, Jin YH, Andresen MC. Vanilloid receptors presynaptically modulate cranial visceral afferent synaptic transmission in nucleus tractus solitarius. J Neurosci. 2002;22:8222–8229. doi: 10.1523/JNEUROSCI.22-18-08222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peters JH, McDougall SJ, Fawley JA, Smith SM, Andresen MC. Primary afferent activation of thermosensitive TRPV1 triggers asynchronous glutamate release at central neurons. Neuron. 2010;65:657–669. doi: 10.1016/j.neuron.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derbenev AV, Monroe MJ, Glatzer NR, Smith BN. Vanilloid-mediated heterosynaptic facilitation of inhibitory synaptic input to neurons of the rat dorsal motor nucleus of the vagus. J Neurosci. 2006;26:9666–9672. doi: 10.1523/JNEUROSCI.1591-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zsombok A, Bhaskaran MD, Gao H, Derbenev AV, Smith BN. Functional plasticity of central TRPV1 receptors in brainstem dorsal vagal complex circuits of streptozotocin-treated hyperglycemic mice. J Neurosci. 2011;31:14024–14031. doi: 10.1523/JNEUROSCI.2081-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anwar IJ, Derbenev AV. TRPV1-dependent regulation of synaptic activity in the mouse dorsal motor nucleus of the vagus nerve. Front Neurosci. 2013;7:238. doi: 10.3389/fnins.2013.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zsombok A, Jiang Y, Gao H, Anwar IJ, Rezai-Zadeh K, Enix CL, Munzberg H, Derbenev AV. Regulation of leptin receptor-expressing neurons in the brainstem by TRPV1. Physiol Rep. 2014;2 doi: 10.14814/phy2.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boychuk CR, Zsombok A, Tasker JG, Smith BN. Rapid Glucocorticoid-Induced Activation of TRP and CB1 Receptors Causes Biphasic Modulation of Glutamate Release in Gastric-Related Hypothalamic Preautonomic Neurons. Front Neurosci. 2013;7:3. doi: 10.3389/fnins.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 70.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yokoyama T, Ohbuchi T, Saito T, Sudo Y, Fujihara H, Minami K, Nagatomo T, Uezono Y, Ueta Y. Allyl isothiocyanates and cinnamaldehyde potentiate miniature excitatory postsynaptic inputs in the supraoptic nucleus in rats. Eur J Pharmacol. 2011;655:31–37. doi: 10.1016/j.ejphar.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 73.Sun B, Bang SI, Jin YH. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport. 2009;20:1002–1006. doi: 10.1097/WNR.0b013e32832d2219. [DOI] [PubMed] [Google Scholar]
- 74.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao DS, Zhong L, Hsieh TH, Abooj M, Bishnoi M, Hughes L, Premkumar LS. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PLoS One. 2012;7:e38005. doi: 10.1371/journal.pone.0038005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anand P, Murali KY, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem Biol Interact. 2010;186:72–81. doi: 10.1016/j.cbi.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 77.Babes A, Fischer MJ, Filipovic M, Engel MA, Flonta ML, Reeh PW. The anti-diabetic drug glibenclamide is an agonist of the transient receptor potential Ankyrin 1 (TRPA1) ion channel. Eur J Pharmacol. 2013;704:15–22. doi: 10.1016/j.ejphar.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 78.Nozawa K, Kawabata-Shoda E, Doihara H, Kojima R, Okada H, Mochizuki S, Sano Y, Inamura K, Matsushime H, Koizumi T, Yokoyama T, Ito H. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A. 2009;106:3408–3413. doi: 10.1073/pnas.0805323106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. TRPA1 agonists delay gastric emptying in rats through serotonergic pathways. Naunyn Schmiedebergs Arch Pharmacol. 2009;380:353–357. doi: 10.1007/s00210-009-0435-7. [DOI] [PubMed] [Google Scholar]
- 80.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Kahn SE, Cummings DE, Tschop MH, D'Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim MJ, Son HJ, Song SH, Jung M, Kim Y, Rhyu MR. The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One. 2013;8:e71603. doi: 10.1371/journal.pone.0071603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tamura Y, Iwasaki Y, Narukawa M, Watanabe T. Ingestion of cinnamaldehyde, a TRPA1 agonist, reduces visceral fats in mice fed a high-fat and high-sucrose diet. J Nutr Sci Vitaminol (Tokyo) 2012;58:9–13. doi: 10.3177/jnsv.58.9. [DOI] [PubMed] [Google Scholar]
- 83.Poher AL, Altirriba J, Veyrat-Durebex C, Rohner-Jeanrenaud F. Brown adipose tissue activity as a target for the treatment of obesity/insulin resistance. Front Physiol. 2015;6:4. doi: 10.3389/fphys.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshida T, Yoshioka K, Wakabayashi Y, Nishioka H, Kondo M. Effects of capsaicin and isothiocyanate on thermogenesis of interscapular brown adipose tissue in rats. J Nutr Sci Vitaminol (Tokyo) 1988;34:587–594. doi: 10.3177/jnsv.34.587. [DOI] [PubMed] [Google Scholar]
- 85.Masamoto Y, Kawabata F, Fushiki T. Intragastric administration of TRPV1, TRPV3, TRPM8, and TRPA1 agonists modulates autonomic thermoregulation in different manners in mice. Biosci Biotechnol Biochem. 2009;73:1021–1027. doi: 10.1271/bbb.80796. [DOI] [PubMed] [Google Scholar]
- 86.Iwasaki Y, Tamura Y, Inayoshi K, Narukawa M, Kobata K, Chiba H, Muraki E, Tsunoda N, Watanabe T. TRPV1 agonist monoacylglycerol increases UCP1 content in brown adipose tissue and suppresses accumulation of visceral fat in mice fed a high-fat and high-sucrose diet. Biosci Biotechnol Biochem. 2011;75:904–909. doi: 10.1271/bbb.100850. [DOI] [PubMed] [Google Scholar]