Abstract
Hippocampus has an extended developmental trajectory, with refinements occurring in the trisynaptic circuit until adolescence. While structural change should suggest a protracted course in behavior, some studies find evidence of precocious hippocampal development in the first postnatal year and continuity in memory processes beyond. However, a number of memory functions, including binding and relational inference, can be cortically supported. Evidence from the animal literature suggests that tasks often associated with hippocampus (visual paired comparison, binding of a visuomotor response) can be mediated by structures external to hippocampus. Thus, a complete examination of memory development will have to rule out cortex as a source of early memory competency. We propose that early memory must show properties associated with full function of the trisynaptic circuit to reflect “adult-like” memory function, mainly (1) rapid encoding of contextual details of overlapping patterns, and (2) retention of these details over sleep-dependent delays. A wealth of evidence suggests that these functions are not apparent until 18–24 months, with behavioral discontinuities reflecting shifts in the neural structures subserving memory beginning approximately at this point in development. We discuss the implications of these observations for theories of memory and for identifying and measuring memory function in populations with typical and atypical hippocampal function.
Keywords: Memory development, Hippocampus, Cortex, Sleep, Atypical populations
1. Introduction
Research examining the development of children's memory has often demonstrated that infants and young children show early competencies in memory function, remembering some items and associations across long term delays. For instance, 2-month-old infants can remember a specific mobile for as long as 2 weeks if encoding occurs across three 6-min sessions (Rovee-Collier, 1999), and 5–6 month olds remember a face they encode for 2 min up to 2 weeks later (Fagan, 1973). Such findings have been attributed to an early-maturing hippocampus (Rovee-Collier, 1997) or the functions this structure may first subserve in its developmental course (e.g., Richmond and Nelson, 2009). However, early competency is in contrast to children's delayed explicit verbal memory for everyday events, which slowly develops, emerging in an immature form after 24 months and undergoing continued refinement until 7 years (Peterson et al., 2011, Rubin, 2000) and beyond (Ghetti and Bunge, 2012, Ghetti et al., 2010). For instance, although some young children can remember a small number of salient events they experience before 24 months, children retain more memories with greater detail after this age (Peterson et al., 2011), consistent with a demarcation between early- and late-developing memories. Prior to 18–24 months most children fail to form lasting, everyday memories they can consciously recollect (but see Bauer, 2015). This is often referred to as the a period of “childhood amnesia”. Consistent with such findings some have suggested that early and late developing memories may reflect development of separate memory systems, including an implicit and explicit system (Schacter and Moscovitch, 1984). Nadel and Zola-Morgan (1984) first attributed the lack of episodic detail in young children's memories to the late trajectory of hippocampal development, suggesting that it would not be until this structure was fully developed that children would be able to show robust episodic recall. Given rapidly emerging knowledge of the development of the hippocampus and the surrounding cortex, researchers have begun to theorize how disparate memory processes may map onto changes in these neural structures (Bachevalier, 2014, Lavenex and Banta Lavenex, 2013, Olson and Newcombe, 2014). Here we expand on recent findings in behavioral memory development and how these results may reflect the development of the medial temporal lobe (MTL) versus hippocampus.
In adults, an established body of research supports the existence of distinct learning and memory systems in the brain, e.g., the basal ganglia reward system supporting procedural memory (Knowlton et al., 1996), and the MTL supporting episodic and semantic memory (Eichenbaum and Cohen, 2001, Nadel and Hardt, 2011, Tulving, 1972). There are also distinct roles for substructures within these systems. Within the MTL, for instance, perirhinal cortex [PRC] supports object recognition, parahippocampal cortex [PHC] supports scene recognition, and the hippocampus supports relational memory in its capacity to bind information from PRC and PHC (Eichenbaum and Cohen, 2001, Diana et al., 2007). The subfields of hippocampus (CA fields 1–4 and dentate gyrus) are also thought to serve specific functions. Work from animal and human neuroscience has shown that the neurons of CA3 are specialized to perform pattern completion, the dentate gyrus (DG) supports pattern separation, and CA1 has been linked to representation of space and temporal sequence over repeated exposures (Bakker et al., 2008, Gilbert et al., 2001, Nakashiba et al., 2008). The subfields of the hippocampus have different retention functions as does cortex. Memories supported by CA3 and DG neurons form rapidly in as little as one exposure in contrast to CA1, which requires repeated exposures for memory formation (Nakashiba et al., 2008). Memories are also thought to emerge gradually in networks of cortical neurons (McClelland et al., 1995) supported by architectures with shallow retention profiles that require extended or repeated exposure for long-term memory retention.
An important fact for memory development is that some of the MTL circuitry has a protracted period of development, with the functions of the PRC developing early, CA1 volumes developing substantially over the first two years, albeit at different rates based on layer input origination, and CA3 and the DG volumes developing the latest both in human and primate development (Bachevalier, 2014, Lavenex and Banta Lavenex, 2013). These varied developmental trajectories led these authors to propose that maturation of these substructures should reflect the emergence of different memory processes in development. A challenge for this proposal is how to reconcile this protracted view of development with recent reports of early memory function in tasks known to elicit hippocampal processing in adults, such as relational binding of a face to a scene (Richmond and Nelson, 2009, Richmond et al., 2004, Chong et al., 2015), memory for spatial relations between objects in a display (Richmond et al., 2015), remembering temporal relations between events in a scene (Barr et al., 1996, Bauer et al., 2003), relational inference (Rovee-Collier and Giles, 2010), demonstrations of context effects (Richmond et al., 2004, Edgin et al., 2014), and better retention after sleep than after a similar period of wakefulness (Friedrich et al., 2015, Seehagen et al., 2015).
Researchers have long noted early and late stages of memory development (Carver and Bauer, 2001, Jabés and Nelson, 2015, Mullally and Maguire, 2014, Nelson, 1995, Piaget, 1973, Schacter and Moscovitch, 1984) placing the emergence of the “late” stage at about 9 months in human children. However, this proposal is inconsistent with evidence on brain development that exists in the literature that we also review (e.g., Bachevalier, 2014, Lavenex and Banta Lavenex, 2013). Our unique proposal is that 18–24 months of age reflects a major milestone in hippocampal development and its connections to cortex when circuitry among key hippocampal subfields and neocortical–hippocampal connections should be mature enough to support sleep neural replay. Before this time we propose that memory function is mostly supported by cortical structures characterized by an incremental learning profile with memories established through repeated exposure, inflexible representations and shallow retention profiles. In comparison, hippocampal memories are established rapidly in a couple exposures, objects and contexts are linked in memory but are also maintained separately, and retention profiles are robust, supported by neural replay during sleep. Consistent with proposals by Bachevalier (2014), Lavenex and Banta Lavenex (2013) and Olson and Newcombe (2014), it is only after basic circuitry is established among the subfields of the hippocampus that we should see more advanced hallmarks of memory function associated with relational binding, spatial relations, temporal order, and the binding of items in scenes.
In the ensuing pages, we briefly review development of MTL anatomy. Next using examples from typical and atypical populations, we re-interpret several examples of early memory function in light of MTL development. We go on to propose unique behavioral signatures that should emerge with basic maturity of hippocampal circuitry as well as methods for investigating these signatures behaviorally with typical and atypical populations. Finally, we point to new issues and questions that arise from mapping memory development more closely to the development of different learning and memory structures.
We focus here on episodic memory development supporting retrieval of memories of specific learning events that are functionally and anatomically separate from memories supported by procedural habit systems, such as memories formed using conjugate mobile reinforcement which are nondeclarative in nature, likely engaging the basal ganglia and cerebellum (see Bauer, 2007, Jabés and Nelson, 2015, Nelson, 1995, Schacter and Moscovitch, 1984 for similar arguments).
2. Anatomical development of MTL
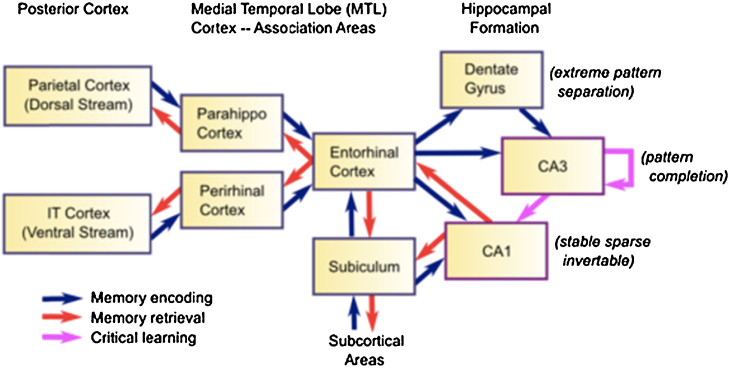
Encompassing the amygdala and hippocampus, the MTL is surrounded by perirhinal and parahippocampal cortices, with entorhinal cortex connecting hippocampal and cortical structures (see Fig. 1). Critically, regions of the MTL and subfields of the hippocampus and their connectivity develop at different rates (Bachevalier, 2014, Jabés and Nelson, 2015, Lavenex and Banta Lavenex, 2013). Some patterns of local neural firing in the MTL develop early in rat models, with hippocampal CA1 place cells, which fire in response to an organism's position in the environment, emerging at postnatal day 16 (P16), and grid cells in entorhinal cortex developing at P20, substantially earlier than once thought (Wills et al., 2010). While glucose utilization and the number and density of synapses in most of the hippocampus are also adult-like by 6 months of age in humans (Seress and Ábrahám, 2008), the DG undergoes protracted development with rapid rates of neurogenesis at 8–16 months and achievement of adult like-morphology by 12–15 months (Bauer, 2007). Slow pruning of synapses to adult levels occurs after 4–5 years in DG (Bauer, 2007, Eckenhoff and Rakic, 1991). Myelination of hippocampus and its subfields also follows a protracted course (Arnold and Trojanowski, 1996), continuing to be modified into adolescence, with the DG showing the latest time frame to reach maturity (Ábrahám et al., 2010).
Fig. 1.
The hippocampus acts as an index, encoding information from across the brain. The Dentate Gyrus, and CA3 are part of the trisynaptic circuit, which develops slowly. Mature circuitry is needed to support sharp-wave CA3 to CA1 ripple activity and mature sleep neural replay.
Figure reprinted from O’Reilly et al. (2012).
In adults, information converging on hippocampus from PRC and PHC via entorhinal cortex [ERC] takes two routes through the HIPP, a short route through the monosynaptic circuit with bidirectional ERC ←→ CA1 connections and a longer route through the trisynaptic circuit (containing DG, CA3). While the short route is available in early infancy with CA1 mature by 2 years of age (Jabés and Nelson, 2015, Lavenex and Banta Lavenex, 2013), it is not until after 18–24 months that DG mossy fibers and CA3 Schaffer collaterals may acquire sufficient maturity for trisynaptic communication from DG and CA3 to the monosynaptic circuit of CA1 and between the hippocampus, parahippocampal gyrus containing PRC and PHC, and neocortex (Ábrahám et al., 2010, Eckenhoff and Rakic, 1991, Lavenex and Banta Lavenex, 2013), structures involved in the formation and retrieval of declarative memories (Nyberg et al., 1996).
A seminal study showed that the use of spatial context to guide search for objects emerged in a rudimentary fashion at 24 months in humans, suggesting this as the age at which allocentric, or map-like, representations of space using distal cues could first be used to guide memory for an object's location (Newcombe et al., 1998), a key hallmark of hippocampal function (O’Keefe and Nadel, 1978). Other studies have documented 18–24 months as an important transition point for flexible single trial learning, including data to suggest that this is the first point at which children can generalize learned imitation sequences across contexts or remember an object separate from its learning context after a single trial of learning (Robinson and Pascalis, 2004, Meltzoff, 1995). While hippocampal substrates supporting some forms of memory are available earlier in development (e.g., CA1), others are not available until later (e.g., DG/CA3), with substantial continued development between 2 and 5 years and beyond (Lavenex and Banta Lavenex, 2013, Ghetti and Bunge, 2012).
Further examination of the behavioral correlates of the monosynaptic short-route and CA1 connections vs. the trisynaptic circuit in transgenic animal models suggested that this route may drive incremental learning of the spatial environment (i.e., Morris Water Maze) without influence of the trisynaptic circuit, whereas the trisynaptic circuit helps to support one trial learning, pattern separation, and spatial tuning of CA1 cells (Nakashiba et al., 2008). In particular, while processing in DG and CA3 can affect CA1, the bidirectional monosynaptic circuit connecting CA1 and entorhinal cortex does not directly affect DG and CA3. These functional dissociations, coupled with differing rates of development of the two hippocampal circuits, could lead to the early emergence of some memory functions, including early developing competency in tasks with many repetitions or prolonged exposure, but later emergence for the rapid acquisition of fine discriminations between overlapping patterns. Stepping back, the latter set of functions encompasses much of what we consider episodic memory for the day to day events of our lives, as most of what we experience and remember occurs in similar spatial environments often with the same players, just subtle variations in the sequence or timing of activity.
In contrast, some cortices adjoining the hippocampus develop early. The PRC at the top of the ventral visual pathway retains memory for objects across 10-s delays in nonhuman primates as young as two weeks of age (Bachevalier et al., 1993). PRC is capable of polymodal binding (Murray et al., 2007), and thus is a candidate for supporting early memory function. PHC, supporting scene memory with input from the dorsal pathway, develops much later (Lavenex and Banta Lavenex, 2013, Golarai et al., 2007). Interestingly, entorhinal cortex, with bi-directional connections between the parahippocampal region (PRC and PHC) and the hippocampus, can form new visuomotor associations without hippocampal input once the basic parameters of a task are acquired (Yang et al., 2014). Yang et al. first trained rhesus macaques to associate one of three objects on a computer screen with a specific location on the screen requiring the animal to touch a specific location. Although it took the animals hundreds of trials to learn the basic task, once they achieved sufficient training they formed new associations between novel objects and the three locations within 30 trials. New learning was impaired when the entorhinal cortex was pharmacologically inactivated but not after inactivation of the hippocampus, demonstrating the contribution of entorhinal cortex to such learning. Layers in entorhinal cortex develop at different rates, with medial entorhinal cortex showing early mature grid cell activity in rats (Wills et al., 2010), and neuronal soma size in superficial layers reaching adult levels by 12 months in humans, potentially supporting early memory function.
Such findings suggest that memory development should proceed in stages with behavior at a particular point in time aligning with the maturation of the underlying learning systems, however much memory research has argued for the idea that memory is in place early in development and develops in a continuous, unvariegated fashion (Rovee-Collier and Cuevas, 2009, Rovee-Collier and Giles, 2010). We propose in the next section that we may reconcile findings associated with these different views of memory development by connecting different forms of learning and memory more closely with maturation of underlying brain systems. Further, based on the studies reviewed here we conclude that many of the memory behaviors attributed to precocious hippocampal development may be actually supported in structures external to the hippocampus. It is only through full development of the trisynaptic circuit and its connections with the cortex that adult-like episodic memory will emerge. Given that trisynaptic circuit refinement is unlikely prior to 18–24 months, much of early memory is likely mediated by the parahippocampus or by the short monosynaptic route into the hippocampus. These assertions have implications for our understanding of infant memory competency and the assessment of memory functions in typical and atypical populations. Mainly, hippocampal dysfunction may be expressed behaviorally at later stages of development, as the cortex may be able to support much of early memory development.
3. State of the field based on behavioral research
Infants form memories from before birth with repeated experience (DeCasper and Spence, 1986, DeCasper et al., 1994), and memory function improves through adolescence (Brainerd et al., 2004, Ghetti and Angelini, 2008, Hayne, 2004), but development of distinct MTL properties has not been often studied in human infants and toddlers. This is partly due to challenges of conducting fMRI of deep subcortical structures in young, awake children (due to noise, isolation, and motion artifact). A second reason is historical, based on theoretical commitments made early in infant memory research to the idea that declarative memory was in place early in development, improving continuously thereafter (see Hayne, 2004 for a review).
These conclusions stemmed largely from the use of “filters” for determining underlying memory function from behavioral data (Schacter and Moscovitch, 1984, Hayne, 2004). The amnesia filter identifies declarative memory if participants perform well on a task that amnesiacs, with hippocampal injury, cannot perform (see Squire and Schacter, 2002 for review). A similar logic holds for the use of parameter filters, which had its roots in process dissociation approaches for determining the difference between explicit and implicit memory (Jacoby, 1991). Here a task is thought to tap declarative knowledge if behavior exhibits characteristics of typical adult declarative but not implicit memory such as context specificity, vulnerability to interference, improvement with increased study time and/or levels of processing, and serial position effects (Hayne, 2004, Schacter and Moscovitch, 1984; see (Rovee-Collier, 1997, Table 3 for a full list). That infants pass these filters led to the conclusion that a rudimentary form of declarative memory was available as early as 6 months of age (Barr et al., 1996), developing from there in a continuous manner (Rovee-Collier, 1997, Rovee-Collier and Cuevas, 2009).
However, many of these conclusions were drawn from evidence obtained with the conjugate mobile reinforcement task used by Rovee-Collier that taps procedural memory versus memory supported by the MTL, with procedural memories strongly linked to the stimulus cue encoded initially. In contrast, declarative memories for episodic details and facts form rapidly (in adults in one trial, e.g., Bakker et al., 2008) and are expressed through recall and recognition (Bachevalier, 2014). In contrast, procedural memories take time to encode (exposures exceed 6 min in the conjugate mobile paradigm) and are expressed through performance. There is general agreement that more flexible forms of memory, supported by the MTL, begin emerging by 8–9 months with the field generally assuming that hippocampal signatures of memory emerge from this point forward (Bauer, 2007, Jabés and Nelson, 2015, Nelson, 1995).
We argue here that memory is far from unitary. Improvements in the precision of lesion studies (e.g., Heuer and Bachevalier, 2011, Pascalis et al., 2009) and imaging in human adults (e.g., Bakker et al., 2008, Ranganath, 2010, Staresina et al., 2011) demonstrate that MTL supports diverse forms of memory. For instance, visual-paired comparison (VPC), which reveals object memory in a preference for a novel object over a familiar one after a delay, was long thought hippocampally mediated (Heuer and Bachevalier, 2011). But, human infants exhibit robust performance on this task with neonates exhibiting a novelty effect after a 2-min delay (Pascalis and de Schonen, 1994), 3 month olds sustaining a delay of 24 h (Pascalis et al., 1998) and 6 month olds a delay of 2 weeks (Fagan, 1973). This was taken at the time as evidence of hippocampal-mediated memory function in infancy (Hayne, 2004, Robinson and Pascalis, 2004). However, recent studies in nonhuman primates (Heuer and Bachevalier, 2011, Zeamer et al., 2010, Zeamer et al., 2015) with ablations targeted to specific MTL structures (e.g., hippocampus or PRC exclusively) reveal that object memory is heavily supported by the PRC (see Jabés and Nelson, 2015 for the proposal that some function could be supported in the subiculum of the hippocampus). Although hippocampus acts as an index, binding the elements of an event (e.g., object, scene) so they can be recognized together or separately (Brown and Aggleton, 2001, Davachi, 2006, Eichenbaum et al., 1994), the parahippocampal region supports memory in a unitized, or fused form, such that elements of events cannot be retrieved separately or recognized in new contexts (Graf and Schacter, 1989, Quamme et al., 2007). Given the underdevelopment of the trisynaptic circuit in infancy and rapid rates of neurogenesis in the DG that may impede retention (Josselyn and Frankland, 2012), the functions of DG pattern separation and CA3 pattern completion are not expected to be available until after infancy as evidenced by the protracted development of hippocampal function in early and middle childhood (e.g., Ghetti and Bunge, 2012, Ofen, 2012, Olson and Newcombe, 2014). As such, the late emergence of the trisynaptic circuit has ramifications for views of memory development. A few examples from the literature help to make this point.
3.1. Deferred imitation
Deferred imitation of sequences of events is taken as an index of declarative memory function based on amnesia and parameter filters (Hayne, 2004). Infants as young as 6 months see a novel 3-action sequence performed with a puppet where an experimenter removes a glove containing a hidden jingle bell from a puppet, shakes the glove, and replaces it (Barr et al., 1996; see also Bauer et al., 1998). The ability to reproduce the experimenter's actions after a delay is taken as evidence of declarative memory. While the number of actions infants imitate is greater than baseline, or spontaneous production of the target actions, infants rarely imitate more than one action at 12 months of age and do not produce two-action sequences of arbitrarily related actions reliably until 22 months (Bauer et al., 1998). Arbitrarily related actions are supported by memory of temporal order only whereas causally related actions are constrained logically (e.g., in deferred imitation one cannot shake the glove before removing it). Thus retrieval may be aided by semantic knowledge acquired outside of the experiment.
Although 6- and 12-month-old infants show better performance if given 6 encoding trials instead of 3 (Barr et al., 1996), they rarely produce 2-action sequences. Bauer et al. (1998) reported that 16 month olds retained arbitrarily-related actions over a 2-week delay if they saw a sequence demonstrated 6 times across two separate encoding sessions (3 demonstrations in each session), but there were multiple exposures that may have recruited cortical learning.
Jabés and Nelson (2015) point to CA1 as possibly supporting early memory function given its early development, reaching adult volumes by 24 months. CA1 involvement is consistent with demonstrations that infants do not transfer performance to a new spatial context or puppet before 12 months and the idea that CA1 supports unitized representations. Another candidate is entorhinal cortex, which supports learning of visuomotor associations (Yang et al., 2014). Finally, given that the PRC is thought to conjoin information from different sensory cortices (e.g., objects, features of objects, and sounds) (Murray et al., 2007) and hippocampal connectivity matures so late, retention of individual actions could be supported by PRC in the youngest infants with later-emerging memory for sequentially-ordered pairs of actions supported by increased hippocampal connectivity.
Retention of temporal order is informative because high-resolution imaging implicates the trisynaptic pathway (Hsieh et al., 2014, Schapiro et al., 2012), which also supports long-term retention after sleep neural replay as we develop below. Children are not reported in the literature to retain briefly presented sequences of arbitrarily related actions over a two-week delay until 28 months of age (Bauer et al., 1998).
3.2. Faces on scenes (relational binding)
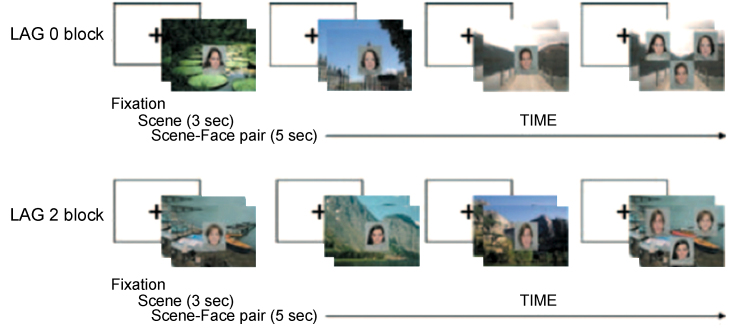
A second example comes from Richmond and Nelson (2009) who adapted a paradigm recruiting hippocampus in adults (Hannula and Ranganath, 2009). The faces-on-scenes paradigm promotes hippocampal binding of input from different parahippocampal cortices. At encoding subjects view a landscape for 3 s before a face is superimposed at the center of the scene. Adults tested on displays containing the original face in a different location on the scene along with two faces previously paired with other scenes, spend more time fixating the correctly-paired face over the other faces compared to trials in which 3 familiar faces occur on an incorrect scene, with fMRI implicating hippocampal binding. Richmond and Nelson replicated the behavioral effect with 9 month olds (see Fig. 2) leading them to conclude that an automatic form of weak hippocampal binding is in place at this age, but immaturity of the trysynaptic circuit raises another possibility. Because PRC is known for conjoining featural details, it may have conjoined the elements of the display in a rudimentary, unitized form with looking at test captured by a change in the location of the face on the scene that was not supported by enough featural detail to disrupt memory altogether. This interpretation is consistent with Richmond and Power (2014) who replicated the effects of Richmond and Nelson (2009) in 6- but not 12-month-olds (see also Chong et al., 2015). The null effect for the older age in Richmond and Power could have arisen from increased PRC development reflecting a large enough discrepancy between the test display and the unitized memory of the encoding display to disrupt performance altogether. The 6- and 9-month-old infants’ precocious relational memory measured through eye movements is unlikely to originate from the hippocampus given that children fail to show preferential eye movements on the faces-on-scenes task at age 4 years across all trials (Koski et al., 2013).
Fig. 2.
Examples of Lag 0 and Lag 2 match blocks from Richmond and Nelson (2009). Three study trials preceded a probe trial with the position of the match face counterbalanced across trials.
Figure reprinted from Richmond and Nelson (2009).
3.3. Spatial relations reflected in looking behavior
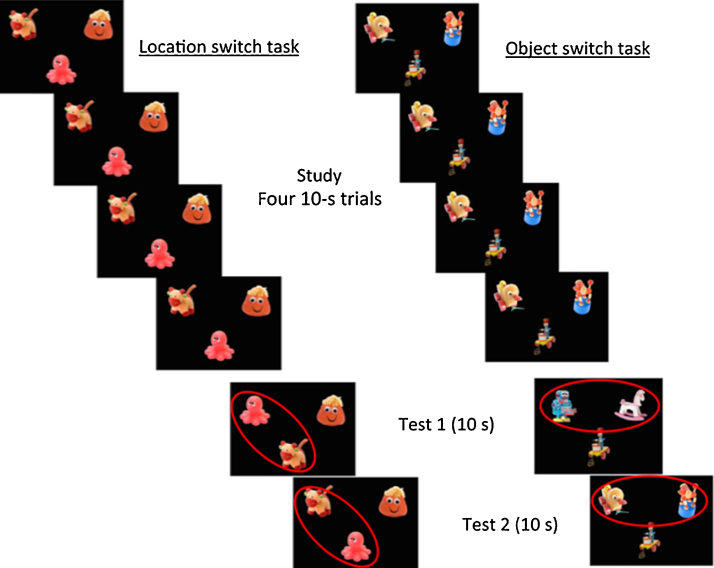
Recently, Richmond et al. (2015) tested memory for spatial relations among objects in a 2D visual display in 9-, 18-, and 27-month-old children. Remembering where things are relative to each other is an important factor in spatial navigation (Banta Lavenex et al., 2006). Richmond et al. used visual displays instead of 3D spatial contexts given that children do not show evidence of using allocentric cues to retrieve objects in real space until about 20 months (Newcombe et al., 1998). Infants viewed 3 objects on a black computer screen during four 10-s familiarization trials. In an immediate test of object recognition, two novel objects took the place of two previously viewed objects on each of two 10-s test trials (see the object switch task in Fig. 3). In the test of spatial-relations two of the objects switched positions on the screen relative to each other (see the location switch task in Fig. 3). Although 18- and 27-month-old children looked longer at the test stimuli in the test of spatial relations none of the three age groups increased looking time on the tests of object recognition making it difficult to know how to interpret these data. In contrast infant rhesus macaques with hippocampal lesions showed robust object memory (Bachevalier and Nemanic, 2008, Blue et al., 2013). Richmond et al. habituated 9 month olds to the familiarization stimuli in a second experiment, reasoning that perhaps the youngest age group needed more time to encode the stimuli. After accumulating 30–35 s on average of familiarization time (similar to the encoding time in Bachevalier and Nemiac and Blue et al. and twice that of Experiment 1 in Richmond et al.) the infants dishabituated to the test scenes of both conditions. Richmond et al. took dishabituation in the test of spatial relations as evidence of hippocampal mediation given that infant, juvenile, and adult nonhuman primates with hippocampal lesions fail at a similar task (Bachevalier and Nemanic, 2008, Blue et al., 2013). However, the array of objects on the screen was more similar to a condition that reflected an egocentric frame of reference that rhesus macaques with hippocampal lesions passed by the time they were juveniles (see Bachevalier and Nemanic, 2008, Blue et al., 2013). Human infants may have increased looking time at test by treating the objects in the test display as egocentric instead of allocentric cues given that human infants can use egocentric information to guide search (Vasilyeva and Lourenco, 2012). Alternately, given evidence that CA1 can support incremental spatial learning (Nakashiba et al., 2008) it is possible that CA1 may support detection of the novel spatial relations at test with sustained memory for the configuration of objects dependent on sufficient exposureas exhibited in the performance gains of 9 month olds who accumulated greater amounts of exposure in Experiment 2.
Fig. 3.
Left panel: The location switch condition from Richmond et al. (2015) meant to tap spatial relational memory. Note that the position of two of the objects was switched at test. Right panel: The object recognition condition (object switch) where two objects were replaced with novel ones. The manipulated positions were counterbalanced across trials; the red ovals were absent during stimulus presentation.
Figure reprinted from Richmond et al. (2015).
3.4. Preconditioning of relational memory
There are some data suggesting that very young infants can exhibit “binding” of memories with 6 month olds forming associations for items that never directly appeared together, but were linked through preconditioning in previous learning sessions (Mullally and Maguire, 2014, Rovee-Collier and Giles, 2010). In these studies infants learned in several phases: (1) first, through initial exposure to simultaneously paired stimuli (stimulus A and B), which could occur for several hours over a number of days, (2) a training phase in which stimulus A is paired with another memory task (i.e., mobile conjugate or deferred imitation), and (3) a transfer task in which memory performance is tested in conjunction with the untrained stimulus (stimulus B). In Barr et al. (2003), for instance, 6-month-old infants received pre-exposure to two hand puppets over a period of 2 days. They were then trained on a deferred imitation sequence with one puppet with transfer to the untrained puppet tested at 24 h. Infants completed the first action in the sequence on the untrained puppet at a rate higher than baseline (puppet C was also tested which was never seen before).
These results could be interpreted as evidence for flexible associative memory and hippocampal binding (as in Mullally and Maguire, who suggest this as evidence for episodic recall), but the developmental trajectory of these effects would suggest otherwise. Specifically, Cuevas et al. (2009) (cited in Rovee-Collier and Giles, 2010) tested infants’ ability to associate the untrained puppet to the sequence after various delays. Infants 6–9 months showed imitation above baseline 2 but not 3 weeks later, but 12 and 15 month olds could not retain the association even after a one-day delay. At 18 months the ability to transfer these associations reappeared. These findings mirror those in which young rats (e.g., P8) will form very strong associative memories that are not retained in older animals (P21). However, it is of importance that this ability was not present if the A and B stimuli were presented sequentially in time instead of simultaneously, suggesting that the animals formed an inflexible, and possibly perceptual representation that guided transfer (Cheslock et al., 2003). Other results point to transitions in infancy in the developmental time-frame in which children are able to transfer knowledge to sequentially versus simultaneously presented stimuli, suggesting that the infant's memory for these events and binding may follow a complicated trajectory in the first year.
In total, these results suggest infant memory includes a period of robust associative learning that is not continuously maintained and therefore should not be equated to later emerging memory functions. These transitions may help to explain some other developmental discontinuities in binding functions, such as those documented in Richmond and Nelson (2009) and Richmond and Power (2014) showing binding of faces onto background scenes in young infants that disappear at 12 months (Richmond and Power, 2014). The findings documented in this section, in concert with the other findings we have reviewed above, cause us to caution the interpretation of these early “associative” memories for evidence of adult episodic learning and the continuous development of memory systems. More work is needed to examine the neural basis of strong associative memories, mediated we suggest by structures external to the hippocampus as their presence in the rat is apparent before the emergence of mature hippocampal function (at P8 as in Cheslock et al., 2003).
3.5. Context effects on object recognition
A final example comes from tasks assessing context effects on object recognition. Sensitivity to context has been posed as a parameter filter to determine if a task taps declarative memory in infants and young children (Schacter and Moscovitch, 1984). The use of this filter was based on findings that conditioning or implicit memories are often expressed without influence of the environmental context. In fact, Nadel et al. (1985) hypothesized that much of early memory would be context independent, as the hippocampus would not have the maturity to support the configural binding of the elements of the memory. In contrast, some studies have demonstrated context effects on similar memory tasks in young infants and adults (e.g., visual paired comparison, Richmond et al., 2004), a finding that led researchers to propose continuity in declarative memory across a wide age range.
However, not all studies have shown consistent effects of context on object recognition. In particular, Edgin et al. (2014) have demonstrated that context effects on object recognition show a u-shaped trajectory from 3 years to adulthood. Specifically, children <4.5 years and participants with Down syndrome, an intellectual disability affecting declarative and hippocampal development, showed clear decrements in performance when an item was removed from its original learning context (a 2-dimensional background scene). However, older children did not demonstrate these effects and were equally good at recognizing an object in and out of the original encoding context. Of interest, participants older than 13 years and adults did show the often replicated and robust effects of context on object recognition (Hayes et al., 2007), suggesting a later developing shift in the mechanisms underlying context effects. From these results, Edgin and colleagues suggested that the context effects measured in early development and adulthood likely tapped different underlying neural systems, with early developing effects possibly resulting from unitized and inflexibly bound representations based in the cortex.
Other studies demonstrate similar transitions emphasizing the role of a functional hippocampus in these developmental shifts. Robinson and Pascalis (2004) tested toddlers’ recognition of objects presented on colored backgrounds and found that it was not until 18–24 months that the children showed recognition of the object outside its original learning context. Pascalis et al. (2009) also found that rhesus macaques with hippocampal lesions were not able to recognize an object when a background context changed, a finding suggesting that the animals were binding an object to its context, but via mechanisms external to the hippocampus. Therefore, as these studies show, an examination of behavior at isolated points in development may lead to incorrect conclusions regarding the nature of memory processes across development.
In sum, we have argued that children may rely on the parahippocampus or the monosynaptic circuit of the hippocampus to support memory in infancy. We have cited findings from the animal literature supporting our assertions but until we can develop technologies for high-resolution functional imaging of deep subcortical structures in human infants our evidence is indirect. In lieu of these methods we propose sleep manipulations as a behavioral methodology that should reflect refinement of the trisynaptic circuit and the earliest emergence of episodic memory formation by 18–24 months. We have come to this conclusion based on evidence from the animal literature and evidence from our own work and the work of others on sleep-dependent memory consolidation in infants and young children as we spell out in the next section.
4. Emergence of a hippocampal signature of memory function
The integrity of hippocampal function is apparent after sleep-dependent delays in adults, and the mechanisms driving sleep-dependent memory consolidation can help guide predictions for similar developmental discontinuity in infant's long-term consolidation. Active Systems Consolidation (Diekelmann and Born, 2010), a prominent theory of sleep consolidation, based on complementary learning systems theory (McClelland et al., 1995) assumes that information is simultaneously encoded in the cortex and hippocampus during learning with hippocampus rapidly forming indices to cortex that provide a unique spatial and temporal context for later retrieval. In contrast, memories gradually integrate in cortex through repeated encodings and/or through sleep. In adult sleep three types of synchronized brain oscillations integrate memory into cortical stores (Diekelmann and Born, 2010, Moelle et al., 2002): high frequency, synchronous sharp-wave ripples reflecting neural replay of awake experience (Wilson and McNaughton, 1994) arise in CA3 and CA1 pyramidal cells (Chrobak and Buzsaki, 1994); sleep spindles, short high-frequency (9–15 Hz), thalamo-cortical oscillations reflect communication between brain regions (Anders et al., 1971); and, slow waves, high amplitude, low frequency 1–4.5 Hz oscillations originating in neocortex coordinate the activity of sharp-wave ripples and spindles (Coons and Guilleminault, 1982, Moelle et al., 2002). These synchronized oscillations are thought to reactivate hippocampal–cortical connections repeatedly during sleep, thus contributing to cortical strengthening and consolidation. Sleep spindles and slow wave activity correlate with memory retention in preschool age children and adults (e.g., Kurdziel et al., 2013, Tamminen et al., 2010). It is thus through sleep that hippocampal circuitry may support memory retrieval based on a single learning experience. Although sharp-wave ripples are one of the earliest oscillations to occur in development (Buzsaki, 2006), we argue that there cannot be mature CA1 replay and active systems consolidation until connectivity between CA3 and CA1 is sufficiently mature for the sharp-wave ripple activity originating in CA3 to propagate to CA1. Although rudimentary CA1 activity may propagate to cortex during infancy oscillations between hippocampus and cortex may be immature as reflected by the fact that children do not exhibit mature default network activity, thought to reflect intrinsic connectivity of the hippocampus with other memory systems, until about 2 years of age (Gao et al., 2009). Although basic trisynaptic circuitry is still forming before 24 months (Seress and Ábrahám, 2008), we propose that it may begin to support functional neural replay with sharp-wave ripple propagation to cortex by 18–24 months. Our proposal is supported by vastly different behavioral outcomes for sleep in infants and toddlers as we outline here.
4.1. Sleep and memory in infants
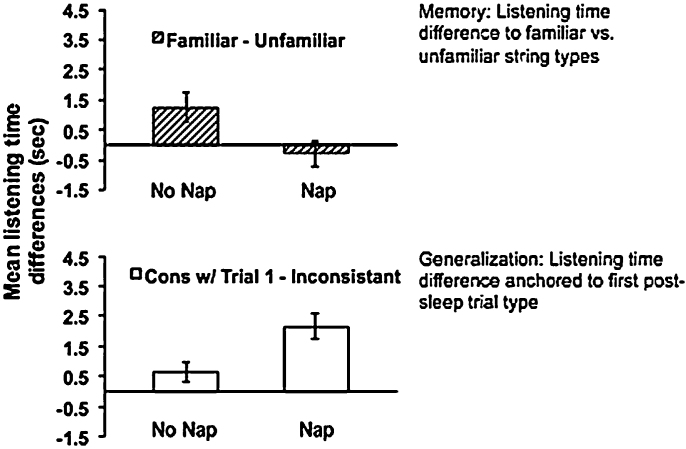
Gómez et al. (2006) were the first to report effects of sleep on memory in infants in an experimental design. Fifteen month olds heard an artificial language with rules linking the first and third word in 3-word sentences such that sentences beginning with vot ended in jic or beginning with pel ended with rud (e.g., vot-wadim-jic, pel-kicey-rud, vot-kicey-jic……). Nap and wakefulness groups heard their training language for 15 min while playing quietly at home. Four hours later infants listened to legal sentences (vot-jic/pel-rud) versus illegal ones that violated the first-third-word rule (vot-rud/pel-jic). No-nap infants listened longer to legal than to illegal sentences suggesting that they remembered the specific words instantiating the rules (see Fig. 4). Nap infants instead listened longer on average to sentences of the first post-sleep trial type (the first sentence-type they heard at test whether legal or illegal). In other words, infants abstracted a rule while asleep they were then able to map onto similar strings. Hupbach et al. (2009) used the same design as Gómez et al. but tested children 24 h instead of 4 h later with the same result for the nap group who slept in the 4-h interval following training. The no-nap group who slept later in the day, but not within 4 h of training showed no retention. Thus, unlike adults who can retain learning for nighttime consolidation, 15 month olds appear to need to sleep soon after learning to retain information the next day. The fact that sleep required re-learning based on exposure to a particular trial type suggests that memory for the specific word dependencies decreased across sleep. However, the rhythmic properties of the stimuli that were identical for each string may have reminded infants of the predictive relationship between words in sentences that helped them detect new predictive rules in similar sentences.
Fig. 4.
Mean listening time differences for 15 month olds from Gómez et al. (2006), 4 h after familiarization in Nap and No-Nap conditions. Nap infants who slept in the interval between familiarization and test showed a significant difference on the abstraction measure. Infants who stayed awake retained a specific memory.
Figure reprinted from Gómez and Edgin (2015).
Sleep effects are also reported in younger 6 month olds who show many of the EEG sleep characteristics of adults, including slow wave activity reflecting the deepest stage of sleep (Anders et al., 1971, Coons and Guilleminault, 1982, Ednick et al., 2009). Seehagen et al. (2015) tested 6- and 12-month-old infants over a 24-h period after deferred imitation. Consistent with cortical learning requiring multiple exposures for encoding, 6 month olds required 6 exposures to retain on average one of the 3 causally related actions. Twelve month olds needed 3 exposures but also retained an average of one action in comparison to no-nap infants who performed at equal levels to the nap infants at a 4-h delay but worse than nap infants after 24 h. That nap and wakefulness performance did not differ at the 4-h delay is consistent with equal retention of non-hippocampal object (or item) recognition across short delays containing wakefulness or sleep (Inostroza et al., 2013, van der Helm et al., 2011).
Our own work with 6.5 month olds suggests that although sleep may protect new learning from interference, it does not stabilize learning to any great degree (Newman-Smith et al., submitted for publication). Infants heard 4 bisyllabic words strung together in random order without pauses for 8 min before a period of sleep. In prior studies of statistical learning (e.g., Saffran et al., 1996, Thiessen and Saffran, 2003) infants listen longer to words than part-words because of the higher conditional probabilities for syllables occurring within words, versus the lower probabilities occurring between syllables between words (part words). There was an interaction of test block (first vs. second) and trial type (word vs. part-word) such that infants discriminated words and part words in the first test-block but not in the second. While slow-wave activity correlated with Block 1 performance, memory for words did not survive exposure to the interfering part-words, as there was no discrimination in Block 2. The fragility of the memory at this age is underscored by the fact that the test occurred on average about 64 min after training. Interestingly, all infants showed greater posterior than anterior slow wave activity, but those with more anterior activity showed greater discrimination, consistent with existing literature showing a posterior to anterior shift of slow-wave activity across development (Jenni et al., 2004, Kurth et al., 2010). We took our results to demonstrate a relationship between cortical maturation and learning, but not sleep and memory consolidation at this young age.
4.2. Sleep and memory in preschool children
While sleep shows little benefit in 6.5 month olds with fragile patterns of learning, sleep has more robust effects in children age 2.5 and older. Kurdziel et al. (2013) taught 3- to 5-year-old children the location of pictures on 9-item (<44 months) and 12-item grids (≥44 months) to 75% accuracy. Children recalled the mappings 5.5 and 24 h later after wakefulness or sleep delays. Retention was high for children at 5.5 and 24 h if they napped after encoding but worse at 5.5 and 24 h for children who did not nap. The loss at 5.5 h was greater in habitual nappers (≥5 naps/week) than in children who were taking 2 or fewer naps per week.
For preschoolers exposed to more challenging learning scenarios, napping is essential for retention of learning. Three and a half year olds who slept after learning two new words across 3 different stories (12 exposures to each of two new nouns) remembered the words more accurately after the nap 2.5 h, 24 h, and 7 days later compared to nonhabitual nappers who did not sleep and who napped on average less than 3 times per week (Williams and Horst, 2014). In our own work, verb-learning 3 year olds exposed to just 4 instances of each of 2 new action-label mappings (e.g., blicking) generalized the verb to a new actor 24 h later only if they napped within 4 h even if they were no longer napping habitually, demonstrating the importance of naps for retention of learning in preschool-age children (Sandoval et al., submitted for publication).
Perhaps the most striking findings of sleep-dependent consolidation in preschoolers comes from our work with 2.5 year olds who heard three labels, each paired once with three different-colored instances of an object, each time on a different colored background (Fig. 5; Werchan and Gómez, 2014) for a total of 9 training trials (three for each of three new nouns). Children, tested immediately or 4 h later after a nap or wakefulness delay, had to select new instances of the labeled object on a new background in a 4-alternative forced-choice test. Children, performed at chance levels on immediate test and after the sleep delay, but chose the new instance with 80% accuracy after the wakefulness delay. This pattern of performance led us to suggest that sleep consolidated the encoding instances and their details (the different object and background colors) separately, preventing integration of the instances and generalization after sleep. In contrast, children forgot the details from familiarization over the wakefulness period, resulting in generalization of the newly learned nouns 4 h later at test.
Fig. 5.
Children in Werchan and Gómez (2014) saw three training exemplars (a) and a distractor (b). At test, children selected from a novel category exemplar, the distractor, a novel object, and a familiar object (c).
Figure reprinted from Werchan and Gómez (2014).
Compare the effects of Werchan and Gómez to new noun learning in infants. Friedrich et al. (2015) tested 9- to 16-month-old infants after nap/wakefulness delays on retention and generalization. Consistent with a cortical profile of encoding infants had 8 exposures to each object-label or category-label mapping. Object-label mappings consisted of the same picture paired with the same label 8 times. Category mappings consisted of slight variations of smaller features on a distinctively shaped object paired with the same label 8 times. The absence of a behavioral measure makes it difficult to compare degree of retention with existing studies, but in contrast to Werchan and Gómez who found improvement after wakefuless but not after sleep, the pattern of ERP signatures of word learning and generalization occurred for the sleep group only. That the N400 effect correlated with spindle activity suggested a specific contribution of sleep, but one we suggest reflects cortical memory, not sleep-neural replay supported by trisynaptic circuitry. A positive correlation between spindle activity and an ERP signature does not necessarily indicate hippocampal involvement. Sleep spindles are generated by thalami-cortical oscillations that correlate with but are not proven to be causally related to hippocampal sharp-wave ripple activity. In fact the findings are exactly what we would predict were we to test infants in the paradigm used by Werchan and Gómez: in contrast to preschoolers who generalized after wakefulness but not sleep, infants should generalize after sleep but not wakefulness, but the effects should be short-lived. Consistent with the proposal that the infant brain does not support sleep neural replay and long-term retention of a brief learning experience, deferred imitation did not sustain a 2-week delay at 14 months despite the fact that retention correlated with an Nc ERP (with similar timing characteristics to that reported for the N400 in Friedrich et al.) at a 30-min delay (Nordqvist et al., 2015). However, if neural replay is immature in infants, what contributes to enhanced performance? We discuss two candidate theories below.
4.3. Theories of sleep consolidation in infants
In Gómez and Edgin (2015) we argued that these noted inconsistencies in patterns of sleep-dependent consolidation in early childhood may arise through different sleep processes supported by the brains of infants and preschoolers. We proposed that hippocampal maturation involving more mature connections within the hippocampus and cortex should permit sleep neural replay and the emergence of behaviors with signatures of trisynaptic function by 18 and 24 months. Before this time, sleep-dependent effects should mostly reflect cortical consolidation (Aton et al., 2009, Tononi and Cirelli, 2014) of the type we discuss next.
The synaptic homeostasis hypothesis is one such theory of cortical consolidation (SHY; Tononi and Cirelli, 2014). By this view synaptic energy accumulates across the brain during wakefulness, but decreases during sleep with the effect of preserving the strongest cortical connections, and does not appear to require the hippocampus. Strengthening of newly formed synapses in hippocampal-immature animals also occurs during sleep through a cortical process of LTP (Aton et al., 2009). Like SHY, however, this process should be greater for learning that is encoded strongly through repeated exposures. These predicted effects are consistent with those we find in our infant generalization studies where retention of the more frequent details of a learning experience appears greater than for the less frequent word dependencies, permitting generalization (Gómez et al., 2006, Hupbach et al., 2009).
Thus far we have presented findings consistent with the proposal that a distinct stage of memory development reflecting more mature hippocampal function emerges between 18 and 24 months, almost a year later than predicted in other theories. Our primary departure from these theories is that before 18 months learning should be primarily cortical, and should not bear signatures of trisynaptic memory function. We propose several novel predictions stemming from this view (Gómez and Edgin, 2015).
5. A view of late hippocampal development: predictions
One prediction is that children relying on cortical networks should require many more exposures for learning and retain less. An excellent example comes from word learning. Bergelson and Swingley (2012) report that by 6–9 months of age children know the meanings of many common words, knowledge that must certainly emerge through massive exposure. If learning were mediated by rapid-learning trisynaptic circuitry we would expect 6 month olds to have much larger vocabularies and they do not. Consistent with the proposal that cortical learning requires many exposures compared to learning supported by trisynaptic circuitry (McClelland et al., 1995), 13 month olds in an experimental setting required 9 exposures to a single word-referent pairing for retention (Woodward et al., 1994). In contrast a well-documented increase in the rate of word learning occurs between 18 and 24 months in children (Goldfield and Reznick, 1990). This increase coincides with emerging trisynaptic connectivity and also reflects individual differences noted for the timing of the word spurt in different children. The hippocampus is highly vulnerable to insult (Lowenstein et al., 1992), often reflecting individual differences in learning, consistent with the wide range of individual variation noted for word learning in typical and atypical groups, including infants born prematurely (Foster-Cohen et al., 2007). A recent study reported a positive correlation between expressive vocabulary and hippocampal volume in typically-developing preschool age children (Lee et al., 2015).
A second prediction is that sleep should contribute to greater accuracy in children with more mature trisynaptic circuitry. Cortical learning (before 18–24 months) should be incremental or should reflect less forgetting than in a wakefulness condition, consistent with our findings of greater generalization after sleep in infancy and greater fidelity of memory in toddlers (Gómez et al., 2006, Hupbach et al., 2009, Werchan and Gómez, 2014). Sleep findings in infants and young children are still scarce, but in distinguishing our discontinuous view of memory development from continuous theories (e.g., Rovee-Collier and Cuevas, 2009, Rovee-Collier and Giles, 2010), it will be important to test younger and older children in experiments with the same tasks before and after sleep. With more exposures for younger children, we predict encoding to the same performance levels across development. However sleep after learning should produce qualitatively more precise and more robust memorial outcomes in children 18–24 months and older aided by sleep neural replay. These patterns should be greatest for tasks tapping trisynaptic function such as those supporting retention of temporal order, allocentric spatial relations, pattern separation, and binding of information from different cortical pathways (e.g., objects of the ventral stream with contexts from the dorsal stream).
A third prediction is that children with compromised hippocampal development and function such as premature children and children with Down syndrome (Pennington et al., 2003) should exhibit fairly robust cortical learning, but limitations in learning signatures of trisynaptic function involving more precise, single trial acquisition, episodic detail, and greater retention after sleep versus wake. Such children should need more exposures to reach criterion levels of immediate memory performance and they should retain less after a delay. These memory deficits are present in patients with developmental amnesia due to hippocampal damage (i.e., patient “Jon” who was born at 26 weeks gestational age; Gardiner et al., 2008). Further, mouse models of Down syndrome show a pattern of worse retention after a 24 h delay for equally encoded information compared to wild-type mice (Smith et al., 2014). In our own work with children with Down syndrome of an age to have some maturity of hippocampal function (albeit compromised) we find larger vocabularies for children with better sleep and smaller vocabularies in children with obstructive sleep apnea and fragmented sleep (Breslin et al., 2014, Edgin et al., in press). A strong prediction that we are presently testing is that degree of developmental hippocampal compromise in these populations should predict the precision of memories and their retention functions over sleep and wake delays. Another prediction is that the extended trajectory of hippocampal development should relate to a later onset of memory difficulties in these populations. It has been noted that the hippocampal patient's difficulties are not always recognized until the school age years (Gadian et al., 2000), and other recent studies have suggested greater memory impairments measured in later vs. early development in Down syndrome (Roberts and Richmond, 2014).
6. Conclusions
We have reviewed a number of findings supporting the idea of separate cortical and hippocampal learning systems supporting learning and memory across early and later stages of infancy and childhood. We suggest that some of the findings reported for children 18 months and younger that are attributed to trisynaptic function might instead entail CA1 or cortical learning. If so, if memory were tested after a delay, instead of immediately after encoding, we would expect some preservation across sleep delays of the more frequently occurring features from encoding but not for the less frequent ones. We would also expect loss of detail compared to learning that is consolidated through sleep neural replay.
It is clear from the data presented here that understanding the nature of memory functions in infants and young children cannot always be mapped onto the functions as tested in adults. Rarely do infants receive tasks in the same manner, as encoding exposures are often repeated over a number of trials. We make a strong claim that we should not consider a task to require mature trisynaptic circuit function in young children until it is (1) learned in a couple trials and retained over a sleep delay, and (2) requires the separation of overlapping patterns including some details of spatial or temporal context. Functional neuroimaging of deep subcortical structures within the MTL and hippocampus is difficult in young children, but recent approaches have utilized the analysis of sleep states, and future studies could potentially use these techniques in conjunction with memory reactivation paradigms to test some of our assumptions. For now, our own approach is to use the same task in conjunction with sleep manipulations before and after the 18–24 month transition in human children. We predict a nonmonotonic change in the function reflecting the number of exposures necessary for retaining a new memory over a delay before and after this transition. More critically, in children 18 months and younger sleep will not have the same benefit for memories encoded with the same number of exposures required for children 24 months and older. Sleep may result in less forgetting in young infants but it will not contribute to the fidelity of a memory or to hallmarks of memory function, such as better pattern separation, as it will in older children. We predict qualitative differences in retention across this transition using a range of converging operations. We are in the process of conducting such studies.
Although we have focused specifically on cortical and hippocampal learning systems, there are other learning systems in the brain that may relate to memory development. One important system includes the basal ganglia, which are highly responsive to reward and function as a pattern separator with respect to selecting motor actions to perform (O’Reilly et al., 2012). Another learning system is the cerebellum that is highly attuned to error between motor output and sensory expectations (O’Reilly et al., 2012, Shadmehr et al., 2010, Wolpert et al., 2011). Both the cerebellum and basal ganglia may be implicated in skilled activity and are proposed to underlie conjugate mobile reinforcement learning (Bauer, 2007, Nelson, 1995, Jabés and Nelson, 2015). Indeed, these systems appear to be available early (see Rovee-Collier, 1997 for a review of behavioral functions likely to be supported by these learning systems), and they appear to have vastly different retention functions than those supporting episodic and semantic memory. Additionally, cytoarchitecture differs between different cortical areas with potential for retention of different forms of information. Although we would expect learning and memory profiles to be similar in these extra-hippocampal regions, their relative development will surely affect the memory function of learning systems.
Along these lines, we expect a complex interplay between the functions of these memory systems such that the hippocampus, cortex, and other learning systems will mutually interact to influence the development and functional specialization of each region. From the interactive specialization perspective posited by Johnson and colleagues (Johnson, 2001, Johnson et al., 2002), the functions of brain regions are not static from birth, but the development of a certain region may cause a reorganization of function in other areas. One example here is that development of prefrontal cortex is essential for coordinating neural signatures of hippocampal sleep neural replay with cortical oscillations, affecting subsequent retention. Patterns of neural replay in the hippocampus are then likely to evolve as prefrontal cortex undergoes its extended trajectory of development. Further, this account of neural development leaves open the possibly that some rudimentary functions of the early developing hippocampus may shift and undergo reorganization as cortical and trisynaptic circuit inputs mature. In total, this viewpoint argues for an extended course of hippocampal development based on the interactions between this region and other important learning systems in the brain.
One last note is in order. We mean in no way to imply that the hippocampus is fully mature by 18–24 months. Investigators (see Olson and Newcombe, 2014 for a review) chart major changes in memory development in preschool age children and older involving the emergence of explicit recognition (Drummey and Newcombe, 1995), accurate memory of the source of newly-learned information (Drummey and Newcombe, 2002, Riggins, 2014), relational memory linking objects and contexts (Riggins, 2014, Sluzenski et al., 2006), and development of spatial memory (Newcombe et al., 1998; see also Ribordy et al., 2013). Of course, development does not end in the preschool years (the upper age we have discussed in this review). Work by Ghetti and colleagues points to an extended trajectory of medial temporal lobe development that extends past the middle-school years and beyond, including refinements in hippocampus’ role in relational memory in children as old as 14 years (Ghetti and Bunge, 2012, Ghetti et al., 2010). In the same tradition, we propose a more extended view of memory development in children's earliest months and years.
Acknowledgments
Work on this paper was supported by the National Science Foundation (BCS-1052887) and the National Institutes of Health (R03HD073417) to RLG. The Lumind Research Down Syndrome Foundation, the Bill and Melinda Gates Foundation (OPP1119381), Fondation Jérôme Lejeune, the National Institutes of Health (R01HD07434601A1), and the Arizona Alzheimer's Consortium funded JE.
References
- Ábrahám H., Vincze A., Jewgenow I., Veszprémi B., Kravják A., Gömöri É., Seress L. Myelination in the human hippocampal formation from midgestation to adulthood. Int. J. Dev. Neurosci. 2010;28(5):401–410. doi: 10.1016/j.ijdevneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Anders T., Emde R., Parmelee A. Technique and Criteria for Scoring of State of Sleep and Wakefulness in Newborn Infants. UCLA Brain Information Service, NINDS Neurological Information Network; Los Angeles, CA: 1971. A manual of standardized terminology. [Google Scholar]
- Arnold S.E., Trojanowski J.Q. Human fetal hippocampal development. I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J. Comp. Neurol. 1996;367(2):274–292. doi: 10.1002/(SICI)1096-9861(19960401)367:2<274::AID-CNE9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Aton S.J., Seibt J., Dumoulin M., Jha S.K., Steinmetz N., Coleman T., Frank M.J. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J. The development of memory from a neurocognitive and comparative perspective. In: Bauer P.J., Fivush R., editors. vol. 1. Wiley-Blackwell; 2014. pp. 285–308. (Handbook on the Development of Children's Memory). [Google Scholar]
- Bachevalier J., Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Brickson M., Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. Neuroreport. 1993;4:77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Bakker A., Kirwan C.B., Miller M.I., Stark C.E.L. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banta Lavenex P., Amaral D.G., Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J. Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R., Marrott H., Rovee-Collier C. The role of sensory preconditioning in memory retrieval by preverbal infants. Anim. Learn. Behav. 2003;31(2):111–123. doi: 10.3758/bf03195974. [DOI] [PubMed] [Google Scholar]
- Barr R., Dowden A., Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behav. Dev. 1996;19:159–170. [Google Scholar]
- Bauer P.J., Hertsgaard L.A., Dropik P., Daly B.P. When even arbitrary order becomes important: developments in reliable temporal sequencing of arbitrarily ordered events. Memory. 1998;6:165–198. doi: 10.1080/741942074. [DOI] [PubMed] [Google Scholar]
- Bauer P.J. Lawrence Erlbaum Associates; Mahwah, NJ: 2007. Remembering the Times of Our Lives: Memory in Infancy and Beyond. [Google Scholar]
- Bauer P.J. Complementary processes account of the development of childhood amnesia and a personal past. Psychol. Rev. 2015;122(2):204–231. doi: 10.1037/a0038939. [DOI] [PubMed] [Google Scholar]
- Bauer P.J., Wiebe S.A., Carver L.J., Waters J.M., Nelson C.A. Developments in long-term explicit memory late in the first year of life behavioral and electrophysiological indices. Psychol. Sci. 2003;14(6):629–635. doi: 10.1046/j.0956-7976.2003.psci_1476.x. [DOI] [PubMed] [Google Scholar]
- Bergelson E., Swingley D. At 6–9 months, human infants know the meanings of many common nouns. Proc. Natl. Acad. Sci. 2012;109:3253–3258. doi: 10.1073/pnas.1113380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blue S.N., Kazama A.M., Bachevalier J. Development of memory for spatial locations and object/place associations in infant rhesus macaques with and without neonatal hippocampal lesions. J. Int. Neuropsychol. Soc. 2013;19:1–12. doi: 10.1017/S1355617713000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd C.J., Holliday R.E., Reyna V.F. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Dev. 2004;75:505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Breslin J., Spanò G., Bootzin R., Anand P., Nadel L., Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev. Med. Child Neurol. 2014;56:657–664. doi: 10.1111/dmcn.12376. [DOI] [PubMed] [Google Scholar]
- Brown M.W., Aggleton J.P. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Oxford University Press; 2006. Rhythms of the Brain. [Google Scholar]
- Carver L.J., Bauer P.J. The dawning of a past: the emergence of long-term explicit memory in infancy. J. Exp. Psychol. Gen. 2001;130(4):726. [PubMed] [Google Scholar]
- Cheslock S.J., Varlinskaya E.I., High J.M., Spear N.E. Higher order conditioning in the newborn rat: effects of temporal disparity imply infantile encoding of simultaneous events. Infancy. 2003;4(2):157–176. [Google Scholar]
- Chrobak J., Buzsaki G. Selective activation of deep layer (V–VI) retrohippocampal neurons during hippocampal sharp waves in the behaving rat. J. Neurosci. 1994;14:6160–6171. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons S., Guilleminault C. Development of sleep-wake patterns and non-rapid eye movements sleep stages during the first six months of life in normal infants. Pediatrics. 1982;69:793–798. [PubMed] [Google Scholar]
- Chong H.J., Richmond J.L., Wong J., Qiu A., Rifkin-Graboi A. Looking behavior at test and relational memory in 6-month-old infants. Infancy. 2015;20(1):18–41. [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- DeCasper A.J., Spence M.J. Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav. Dev. 1986;9:133–150. [Google Scholar]
- DeCasper A.J. Fetal reactions to recurrent maternal speech. Infant Behav. Dev. 1994;17:159–164. [Google Scholar]
- Diana R.A., Yonelinas A.P., Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diekelmann S., Born J. The memory function of sleep. Nat. Rev. Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Drummey A.B., Newcombe N. Remembering versus knowing the past: children's explicit and implicit memories for pictures. J. Exp. Child Psychol. 1995;59:549–565. doi: 10.1006/jecp.1995.1025. [DOI] [PubMed] [Google Scholar]
- Drummey A.B., Newcombe N.S. Developmental changes in source memory. Dev. Sci. 2002;5(4):502–513. [Google Scholar]
- Eckenhoff M.F., Rakic P. A quantitative analysis of synaptogenesis in the molecular layer of the dentate gyrus in the rhesus monkey. Dev. Brain Res. 1991;64:129–135. doi: 10.1016/0165-3806(91)90216-6. [DOI] [PubMed] [Google Scholar]
- Edgin J.O., Spano G., Kawa K., Nadel L. Remembering things without context: development matters. Child Dev. 2014;85(4):1491–1502. doi: 10.1111/cdev.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin, J.O., Tooley, U., Demara, B., Anand, P., Spanò, G., in press. Sleep quality, language development, and autism symptoms in preschool-age children with Down syndrome. [DOI] [PMC free article] [PubMed]
- Ednick M., Cohen A., McPhail G., Beebe D., Simakajomboon N., Amin R. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep. 2009;32:1449–1458. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H.E., Cohen N.J. Oxford University Press; New York: 2001. From Conditioning to Conscious Recollection: Memory Systems of the Brain. [Google Scholar]
- Eichenbaum H., Otto T., Cohen N.J. Two functional components of the hippocampal memory system. Behav. Brain Sci. 1994;17:449–518. [Google Scholar]
- Fagan J.F. Infants’ delayed recognition memory and forgetting. J. Exp. Child Psychol. 1973;16(3):424–450. doi: 10.1016/0022-0965(73)90005-2. [DOI] [PubMed] [Google Scholar]
- Foster-Cohen S., Edgin J.O., Champion P., Woodward L. Early language development in children born very premature. J. Child Lang. 2007;34:655–675. doi: 10.1017/s0305000907008070. [DOI] [PubMed] [Google Scholar]
- Friedrich M., Wilhelm I., Born J., Friederici A.D. Generalization of word meaning during infant sleep. Nat. Commun. 2015 doi: 10.1038/ncomms7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadian D.G., Aicardi J., Watkins K.E., Porter D.A., Mishkin M., Vargha-Khadem F. Developmental amnesia associated with early hypoxic–ischaemic injury. Brain. 2000;123(3):499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Gao W., Zhu H., Giovanello K.S., Smith J.K., Shen D., Gilmore J.H., Lin W. Evidence on the emergence of the brain's default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner J.M., Brandt K.R., Baddeley A.D., Vargha-Khadem F., Mishkin M. Charting the acquisition of semantic knowledge in a case of developmental amnesia. Neuropsychologia. 2008;46:2865–2868. doi: 10.1016/j.neuropsychologia.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., Angelini L. The development of recollection and familiarity in childhood and adolescence: Evidence from the dual-process signal detection model. Child Dev. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S., Bunge S. Neural changes underlying the development of episodic memory during middle childhood. Dev. Cogn. Neurosci. 2012;2:381–395. doi: 10.1016/j.dcn.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S., DeMaster D.M., Yonelinas A.P., Bunge S.A. Developmental differences in medial temporal lobe function during memory encoding. J. Neurosci. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P.E., Kesner R.P., Lee I. Dissociating hippocampal subregions: a double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Golarai G., Ghahremani D.G., Whitfield-Gabrieli S., Reiss A., Eberhardt J.L., Gabrieli J.D., Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat. Neurosci. 2007;10(4):512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield B.A., Reznick J.S. Early lexical acquisition: rate, content, and the vocabulary spurt. J. Child Lang. 1990;17:171–183. doi: 10.1017/s0305000900013167. [DOI] [PubMed] [Google Scholar]
- Gómez R.L., Bootzin R.R., Nadel L. Naps promote abstraction in language-learning infants. Psychol. Sci. 2006;17:670–674. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Gómez R.L., Edgin J.O. Sleep as a window into early neural development: shifts in sleep-dependent memory formation across early childhood. Child Dev. Perspect. 2015;9:183–189. doi: 10.1111/cdep.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf P., Schacter D.L. Unitization and grouping mediate dissociations in memory for new associations. J. Exp. Psychol. Learn. Mem. Cogn. 1989;15:930–940. doi: 10.1037//0278-7393.15.1.3. [DOI] [PubMed] [Google Scholar]
- Hannula D.E., Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S.M., Nadel L., Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–889. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayne H. Infant memory development: implications for childhood amnesia. Dev. Rev. 2004;24:33–73. [Google Scholar]
- Heuer E., Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behav. Neurosci. 2011;125:137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh L.T., Gruber M.J., Jenkins L.J., Ranganath C. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A., Gómez R.L., Bootzin R.R., Nadel L. Nap-dependent learning in infants. Dev. Sci. 2009;12:1007–1012. doi: 10.1111/j.1467-7687.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Inostroza M., Binder S., Born J. Sleep-dependency of episodic-like memory consolidation in rats. Behav. Brain Res. 2013;237:15–22. doi: 10.1016/j.bbr.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Jabés A., Nelson C.A. 20 years after “The ontogeny of human memory: a cognitive neuroscience perspective”. Where are we? Int. J. Behav. Dev. 2015 [Google Scholar]
- Jacoby L.L. A process dissociation framework: separating automatic from intentional uses of memory. J. Mem. Lang. 1991;30(5):513–541. [Google Scholar]
- Jenni O.G., Borbély A.A., Achermann P. Development of the nocturnal sleep electroencephalogram in human infants. Am. J. Physiol. 2004;286(3):R528–R538. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- Johnson M.H. Functional brain development in humans. Nat. Rev. Neurosci. 2001;2(7):475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Halit H., Grice S.J., Karmiloff-Smith A. Neuroimaging of typical and atypical development: a perspective from multiple levels of analysis. Dev. Psychopathol. 2002;14(3):521–536. doi: 10.1017/s0954579402003073. [DOI] [PubMed] [Google Scholar]
- Josselyn S.A., Frankland P.W. Infantile amnesia: a neurogenic hypothesis. Learn. Mem. 2012;19:423–433. doi: 10.1101/lm.021311.110. [DOI] [PubMed] [Google Scholar]
- Knowlton B.J., Mangels J.A., Squire L.R. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Koski J., Olson I.R., Newcombe N.S. Tracking the eyes to see what children remember. Memory. 2013;21:396–407. doi: 10.1080/09658211.2012.735241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdziel L., Duclos K., Spencer R.M. Sleep spindles in midday naps enhance learning in preschool children. Proc. Natl. Acad. Sci. 2013;110:17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth S., Ringli M., Geiger A., LeBourgeois M., Jenni O.G., Huber R. Mapping of cortical activity in the first two decades of life: a high-density sleep electroencephalogram study. J. Neurosci. 2010;30(40):13211–13219. doi: 10.1523/JNEUROSCI.2532-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Nordahl C.W., Amaral D.G., Lee A., Solomon M., Ghetti S. Assessing hippocampal development and language in early childhood: Evidence from a new application of the automatic segmentation adapter tool. Hum. Brain Mapping. 2015 doi: 10.1002/hbm.22931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein D.H., Thomas M.J., Smith D.H., McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12(12):4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland J.L., McNaughton B.L., O’Reilly R.C. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Meltzoff A.N. What infant memory tells us about infantile amnesia: long-term recall and deferred imitation. J. Exp. Child Psychol. 1995;59(3):497–515. doi: 10.1006/jecp.1995.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelle M., Marshall L., Gais S., Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J. Neurosci. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally S.L., Maguire E.A. Learning to remember: the early ontogeny of episodic memory. Dev. Cogn. Neurosci. 2014;9:12–29. doi: 10.1016/j.dcn.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray E.A., Bussey T.J., Saksida L.M. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nadel L., Hardt O. Update on memory systems and processes. Neuropsychopharmacology. 2011;36(1):251–273. doi: 10.1038/npp.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L., Willner J., Kurz E.M. Cognitive maps and environmental context. In: Balsam P.D., Tomie A., editors. Context and Learning. Erlbaum; Hillsdale, NJ: 1985. pp. 385–406. [Google Scholar]
- Nadel L., Zola-Morgan S. Infantile amnesia: a neurobiological perspective. In: Moscovitch M., editor. Infant Memory: Its Relation to Normal and Pathological Memory in Humans and Other Animals. Springer; New York: 1984. pp. 145–171. [Google Scholar]
- Nakashiba T., Young J.Z., McHugh T.J., Buhl D.L., Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science. 2008;319(5867):1260–1264. doi: 10.1126/science.1151120. [DOI] [PubMed] [Google Scholar]
- Nelson C.A. The ontogeny of human memory: a cognitive neuroscience perspective. Dev. Psychol. 1995;31:723–738. [Google Scholar]
- Newcombe N., Huttenlocher J., Drummey A.B., Wiley J.G. The development of spatial location coding: place learning and dead reckoning in the second and third years. Cogn. Dev. 1998;13:185–200. [Google Scholar]
- Newman-Smith, K., Werchan, D., Goldstein, M., Sweeney, L., Nadel, L., Bootzin, R.R., Gomez, R.L., submitted for publication. Quantitative analysis of sleep and learning; insights into cortical development and retention abilities in infants.
- Nordqvist E., Rudner M., Johansson M., Lindgren M., Heimann M. The relationship between deferred imitation, associative memory and communication in 14-month-old children. Behavioral and electrophysiological indices. Front. Psychol. 2015;6:260. doi: 10.3389/fpsyg.2015.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Mclntosh A.R., Houle S., Nilsson L.G., Tulving E. Activation of medial temporal structures during episodic memory retrieval. Nature. 1996;380:715–717. doi: 10.1038/380715a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J.O., Nadel L. Clarendon Press; Oxford: 1978. The Hippocampus as a Cognitive Map. [Google Scholar]
- Ofen N. The development of neural correlates for memory formation. Neurosci. Biobehav. Rev. 2012;36:1708–1717. doi: 10.1016/j.neubiorev.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson I.R., Newcombe N.S. Binding together the elements of episodes: Relational memory and the developmental trajectory of the hippocampus. In: Bauer P.J., Fivush R., editors. vol. 1. Wiley-Blackwell; 2014. pp. 285–308. (Handbook on the Development of Children's Memory). [Google Scholar]
- O’Reilly R.C., Munakata Y., Frank M.J., Hazy T.E. PediaPress; 2012. Computational Cognitive Neuroscience. [Google Scholar]
- Pascalis O., Hunkin N.M., Bachevalier J., Mayes A.R. Change in background context disrupts performance on visual paired comparison following hippocampal damage. Neuropsychologia. 2009;47(10):2107–2113. doi: 10.1016/j.neuropsychologia.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Pascalis O., de Haan M., Nelson C.A., de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. J. Exp. Psychol. Learn. Mem. Cogn. 1998;24:249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Pascalis O., de Schonen S. Recognition memory in 3- to 4-day-old human neonates. Neuroreport. 1994;5:1721–1724. doi: 10.1097/00001756-199409080-00008. [DOI] [PubMed] [Google Scholar]
- Pennington B.F., Moon J., Edgin J., Stedron J., Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74(1):75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Peterson C., Warren K.L., Short M.M. Infantile amnesia across the years: a 2-year follow-up of children's earliest memories. Child Dev. 2011;82(4):1092–1105. doi: 10.1111/j.1467-8624.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Piaget J., Inhelder . Basic Books; New York: 1973. Memory and Intelligence. [Google Scholar]
- Quamme J.R., Yonelinas A.P., Norman K.A. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17:192–200. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Ribordy F., Jabès A., Banta Lavenex P., Banta Lavenex P. Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cognit. Psychol. 2013;66(1):1–29. doi: 10.1016/j.cogpsych.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Richmond J., Sowerby P., Colombo M., Hayne H. The effect of familiarization time, retention interval, and context change on adults’ performance in the visual paired comparison task. Dev. Psychobiol. 2004;44(2):146–155. doi: 10.1002/dev.10161. [DOI] [PubMed] [Google Scholar]
- Richmond J., Nelson C.A. Relational memory during infancy: evidence from eye-tracking. Dev. Sci. 2009;12:549–556. doi: 10.1111/j.1467-7687.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- Richmond J.L., Power J. Age-related differences in memory expression during infancy: using eye-tracking to measure relational memory in 6- and 12-month-olds. Dev. Psychobiol. 2014;56(6):1341–1351. doi: 10.1002/dev.21213. [DOI] [PubMed] [Google Scholar]
- Richmond J.L., Zhao J.L., Burns M.A. J. Exp. Child Psychol. 2015;130:79–91. doi: 10.1016/j.jecp.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Dev. Psychol. 2014;50(2):449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts L.V., Richmond J.L. Preschoolers with Down syndrome do not yet show the learning and memory impairments seen in adults with Down syndrome. Dev. Sci. 2014;18(3):404–419. doi: 10.1111/desc.12225. [DOI] [PubMed] [Google Scholar]
- Robinson A.J., Pascalis O. Development of flexible visual recognition memory in human infants. Dev. Sci. 2004;7:527–533. doi: 10.1111/j.1467-7687.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C. Dissociations in infant memory: rethinking the development of implicit and explicit memory. Psychol. Rev. 1997;104:467–498. doi: 10.1037/0033-295x.104.3.467. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C. The development of infant memory. Curr. Dir. Psychol. Sci. 1999;8(3):80–85. [Google Scholar]
- Rovee-Collier C., Cuevas K. Multiple memory systems are unnecessary to account for infant memory development: an ecological model. Dev. Psychol. 2009;45(1):160–174. doi: 10.1037/a0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovee-Collier C., Giles A. Why a neuromaturational model of memory fails: exuberant learning in early infancy. Behav. Process. 2010;83(2):197–206. doi: 10.1016/j.beproc.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.C. The distribution of early childhood memories. Memory. 2000;8(4):265–269. doi: 10.1080/096582100406810. [DOI] [PubMed] [Google Scholar]
- Saffran J., Aslin R., Newport E. Statistical learning by eight-month-old infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Sandoval, M., LeClerc, J., Gómez, R.L., submitted for publication. Napping in the preschool years: when sleep maturation outpaces memory development.
- Schapiro A.C., Kustner L.V., Turk-Browne N.B. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Curr. Biol. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D.L., Moscovitch M. Infants, amnesics, and dissociable memory systems. In: Moscovitch M., editor. Infant Memory. Plenum; New York: 1984. pp. 173–216. [Google Scholar]
- Seehagen S., Konrad C., Herbert J.S., Schneider S. Timely sleep facilitates declarative memory consolidation in infants. Proc. Natl. Acad. Sci. 2015 doi: 10.1073/pnas.1414000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L., Ábrahám H. Pre- and postnatal morphological development of the human hippocampal formation. In: Nelson C.A., Luciana M., editors. Handbook of Developmental Cognitive Neuroscience. 2nd ed. The MIT Press; Cambridge, MA: 2008. pp. 187–209. [Google Scholar]
- Shadmehr R., Smith M., Krakauer J. Error correction, sensory prediction, and adaptation in motor control. Annu. Rev. Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Sluzenski J., Newcombe N.S., Kovacs S.L. Binding, relational memory, and recall of natu- ralistic events: A developmental perspective. J. Exp. Psychol. Learn. Mem. Cogn. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Smith G.K., Kesner R.P., Korenberg J.R. Dentate gyrus mediates cognitive function in the Ts65Dn/DnJ mouse model of down syndrome. Hippocampus. 2014;24:354–362. doi: 10.1002/hipo.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L.R., Schacter D.L., editors. Neuropsychology of Memory. 3rd ed. Guilford Press; New York: 2002. [Google Scholar]
- Staresina B., Duncan K., Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J. Neurosci. 2011;31:8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J., Payne J.D., Stickgold R., Wamsley E.J., Gaskell M.G. Sleep spindle activity is associated with the integration of new memories and existing knowledge. J. Neurosci. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiessen E., Saffran J. When cues collide: use of stress and statistical cues to word boundaries by 7- to nine-month-old infants. Dev. Psychol. 2003;39:706–716. doi: 10.1037/0012-1649.39.4.706. [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. vol. 381 (e402) Academic; London: 1972. p. 4. (Episodic and Semantic Memory. 1. Organization of Memory). [Google Scholar]
- Vasilyeva M., Lourenco S.F. Development of spatial cognition. Wiley Interdiscip. Rev. Cogn. Sci. 2012;3:349–362. doi: 10.1002/wcs.1171. [DOI] [PubMed] [Google Scholar]
- van der Helm E., Gujar N., Nishida M., Walker M.P. Sleep-dependent facilitation of episodic memory details. PLoS ONE. 2011;6:e27421. doi: 10.1371/journal.pone.0027421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werchan D.M., Gómez R.L. Wakefulness (not sleep) promotes generalization of word learning in 2.5-year-old children. Child Dev. 2014;85:429–436. doi: 10.1111/cdev.12149. [DOI] [PubMed] [Google Scholar]
- Wills T.J., Cacucci F., Burgess N., O’Keefe J. Development of the hippocampal cognitive map in preweanling rats. Science. 2010;328(5985):1573–1576. doi: 10.1126/science.1188224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.E., Horst J.S. Goodnight book: sleep consolidation improves word learning via storybooks. Front. Psychol. 2014;5:184. doi: 10.3389/fpsyg.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.A., McNaughton B.L. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wolpert D., Diedrichsen J., Flanagan J. Principles of sensorimotor learning. Nat. Neurosci. Rev. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- Woodward A.L., Markman E.M., Fitzsimmons C.M. Rapid word learning in 13- and 18-month-olds. Dev. Psychol. 1994;30:553–566. [Google Scholar]
- Yang T., Bavley R.L., Fomalont K., Blomstrom K.J., Mitz A.R., Turchi J., Rudebeck P.H., Murray E.A. Contributions of the hippocampus and entorhinal cortex to rapid visuomotor learning in rhesus monkeys. Hippocampus. 2014;24:1102–1111. doi: 10.1002/hipo.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A.E., Heuer E., Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus monkeys with and without neonatal hippocampal lesions. J. Neurosci. 2010;30:9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A., Richardson R.L., Weiss A.R., Bachevalier J. The development of object recognition memory in rhesus macaques with neonatal lesions of the perirhinal cortex. Dev. Cogn. Neurosci. 2015;11:31–41. doi: 10.1016/j.dcn.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]