Abstract
Response inhibition deficits are widely believed to be at the core of Attention-Deficit Hyperactivity Disorder (ADHD). Several studies have examined neural architectural correlates of ADHD, but research directly examining structural correlates of response inhibition is lacking. Here we examine the relationship between response inhibition as measured by a Go/No Go task, and cortical surface area and thickness of the caudal inferior frontal gyrus (cIFG), a region implicated in functional imaging studies of response inhibition, in a sample of 114 young adults with and without ADHD diagnosed initially during childhood. We used multiple linear regression models to test the hypothesis that Go/No Go performance would be associated with cIFG surface area or thickness. Results showed that poorer Go/No Go performance was associated with thicker cIFG cortex, and this effect was not mediated by ADHD status or history of substance use. However, independent of Go/No Go performance, persistence of ADHD symptoms and more frequent cannabis use were associated with thinner cIFG. Go/No Go performance was not associated with cortical surface area. The association between poor inhibitory functioning and thicker cIFG suggests that maturation of this region may differ in low performing participants. An independent association of persistent ADHD symptoms and frequent cannabis use with thinner cIFG cortex suggests that distinct neural mechanisms within this region may play a role in inhibitory function, broader ADHD symptomatology, and cannabis use. These results contribute to Research Domain Criteria (RDoC) by revealing novel associations between neural architectural phenotypes and basic neurobehavioral processes measured dimensionally.
Keywords: response inhibition, MRI, cortical surface area, ADHD persistence, cannabis use, alcohol use
Recent initiatives such as the Research Domain Criteria (RDoC; Insel et al. 2010) highlight the need to focus on dimensional behavioral and biological phenotypes that underlie multiple overlapping disorders. Response inhibition, one such phenotype, is defined as the ability to inhibit a prepotent response to a stimulus or event, stop an ongoing response pattern, and control interference from competing stimuli or events (Barkley 1997a; 1999). Deficits in response inhibition have been observed in several disorders, including anxiety and mood disorders (Wright et al. 2014), schizophrenia (Ethridge et al. 2014), and substance use disorders (Groman et al. 2009). However, response inhibition is most notably disrupted in hyperactive and combined subtypes of Attention-Deficit/Hyperactivity Disorder (ADHD; Barkley 1997b). ADHD is one of the most commonly diagnosed neurodevelopmental disorders in childhood, affecting approximately 5% of school-age children (Faraone et al. 2003; Polanczyk et al. 2007), It is believed to persist into adulthood in about 50% of children who are diagnosed with the disorder (Simon et al. 2009), and estimates of its prevalence in adulthood are 3% to 5% in nonclinical individuals (Biederman 2005) and as much as 16.8% in psychiatric outpatient settings (Almeida Montes et al. 2007). Therefore, understanding the neural correlates of response inhibition is of great interest. The purpose of the current study is to examine whether cortical surface area and thickness of the inferior frontal gyrus (IFG), a region that has been linked to response inhibition in the functional imaging literature, covary with response inhibition performance in adults who vary widely in their inhibitory functioning (i.e., a combined sample of participants who were diagnosed with ADHD in childhood and those who were not), independent of their diagnostic status. A secondary aim of this study is to determine whether any noted associations are linked to response inhibition per se, or whether they are mediated by the high rates of substance use in these individuals.
A behavioral task commonly used to measure response inhibition is the Go/No Go task, a type of continuous performance test (Conners et al. 2003; Rosvold et al. 1956). This task requires the participant to respond repeatedly to non-target stimuli (e.g., all letters except “X”), and then, on infrequent “No Go” trials, inhibit that prepotent response when the target stimulus (e.g., the letter “X”) appears. Several performance dimensions are measured with this task, including sustained attention over the course of the task, tendency to miss non-target stimuli (i.e., omission errors), and tendency to respond on “No Go” trials (i.e., commission errors). Early studies have demonstrated that the Go/No Go task is sensitive to ADHD symptomatology (Trommer et al. 1988) and response to stimulant medication (Trommer and Hoeppner 1991) in children, and more recently, Tamm and colleagues (2013) showed that young adults who were diagnosed with ADHD in childhood made significantly more commission errors on the Go/No Go task than comparison subjects.
A large functional imaging literature using the Go/No Go and other similar tasks implicates fronto-striatal neural circuitry in response inhibition processes (Chambers et al. 2009; Stevens et al. 2007). Early studies have shown activation on this task that is distributed across the dorsolateral prefrontal cortex, the orbitofrontal cortex, and the caudate nucleus, and it has been hypothesized that these regions support various functional demands of this task from response inhibition itself to working memory, error processing, and voluntary movement, respectively (Casey et al. 1997; Liddle et al. 2001; Menon et al. 2001). The cerebellum has also been linked to performance (Liddle et al. 2001; Menon et al. 2001), presumably do to its role in motor response execution. More recent studies have zeroed in on the IFG as playing a critical role in cognitive control and response inhibition (Aron et al. 2007; 2014). Functional magnetic resonance imaging (fMRI) studies have linked performance on the Go/No Go task to functional responses in the IFG specifically in adults with ADHD (Dibbets et al. 2009; Epstein et al. 2007; Sebastian et al. 2012). However, evidence for structural correlates of response inhibition in adults with ADHD is limited, particularly with regard to the IFG.
To our knowledge, no studies have examined the structural correlates of Go/No Go task performance in adults with a history of ADHD. However, a number of structural MRI (sMRI) studies have examined correlates of ADHD in adults. A series of reports by Almeida Montes and colleagues has shown that adults with ADHD have reduced cortical thickness in the right superior frontal cortex (Almeida Montes et al. 2010) and right frontoparietal cortex (Almeida Montes et al. 2012), and that the severity of their ADHD symptoms correlates negatively with thickness in these regions. These reports also reveal reduced grey matter volume in the caudate nucleus (Almeida Montes et al. 2010) and, specifically in women, reduced grey matter density in the right cerebellum (Almeida Montes et al. 2011) in these individuals. Other studies have found decreased volume and/or cortical thickness in the orbitofrontal, dorsolateral, and anterior and posterior cingulate regions (Cubillo and Rubia 2010; Makris et al. 2007). The only study to find thinner cortex specifically in part of the IFG (i.e., pars opercularis) among participants with ADHD (Batty et al. 2010) found this in children and adolescents rather than adults. Another study showed thinner cortex in a large cluster that included parietal, temporal, and posterior frontal regions (including parts of the IFG) roughly corresponding to the dorsal attentional network and limbic areas in adults who were diagnosed with ADHD in childhood and in those with persistent adult ADHD (Proal et al. 2011). Interestingly, these authors noted that the children with ADHD performed more poorly on a Go/No Go task, but they did not directly test the relationship between Go/No Go performance and thickness of the IFG. Yet another sMRI study found that thinning in PFC regions was specifically related to persistence of ADHD symptoms over time, especially inattentive symptoms (Shaw et al. 2013). The associations with persistence of symptoms highlighted in the latter two studies suggest that understanding neurocognitive phenotypes that either differ as a function of persistence, or alternatively, persist even after controlling for this factor, may be of particular interest.
The primary aim of the current study was to examine the association between response inhibition and cortical surface area and thickness in the IFG, independent of the presence or persistence of ADHD. Indeed, an important limitation of the studies noted above is that they did not employ methods that carefully differentiate cortical thickness and surface area and examine each separately. This is likely an important distinction because these two cortical phenotypes are conflated in measures of cortical volume and they follow different developmental trajectories (Brown et al. 2012). They also appear to be influenced by different cellular mechanisms and genetic factors (Chen et al. 2012; Panizzon et al. 2009). Consistent with RDoC, investigating these two phenotypes independent of diagnostic status allows us to examine the correlates of the underlying dimensional phenotype by taking advantage of the wide variability in inhibitory functioning, while also ensuring that any noted findings are not driven simply by the diagnosis per se.
Due to the high rates of substance use in young adults with poor inhibitory functioning and/or ADHD, a secondary aim of the current study was to determine whether apparent structural correlates of response inhibition are really attributable to substance use. Adolescents and adults with ADHD are up to twice as likely as their peers to use illicit substances, including cannabis (Faraone et al. 2007) and alcohol (Molina et al. 2007). Long-term cannabis use in youth has been shown to impair executive functions, including response inhibition (Gruber et al. 2012; Lisdahl and Price 2012; Pope and Yurgelun-Todd 1996; Solowij et al. 2002), placing ADHD users at potentially higher risk and creating a potential confound in the current literature. Adolescent abstainers with a history of cannabis use who showed no differences in performance on a Go/No Go task still showed increased processing effort during inhibition trials in a wide range of cortical regions (Tapert et al. 2007). Alcohol abuse has also been shown to impact neuropsychological functioning broadly (Brown et al. 2000), and has been associated with altered frontal and parietal circuitry (Tapert et al. 2004). Thus cannabis and/or alcohol use could at least partially account for the observed associations between executive functioning and neural architecture in adolescents and adults with ADHD, although few studies have examined their influence on response inhibition and IFG structure in adults with and without ADHD.
With regard to our first aim, we hypothesized that variability in performance on the Go/No Go task would be associated with variability in relative cortical surface area (regionalization) and/or cortical thickness in the IFG. Importantly, we predicted that, consistent with a direct relationship between these phenotypes, the association would remain after controlling for childhood ADHD diagnosis and persistence of adult ADHD symptoms. In other words, we hoped to establish that the association between IFG architecture and inhibitory functioning would not be accounted for by mediating factors related to ADHD diagnosis per se. While thinner cortex has been observed in individuals with ADHD in a number of regions, it has also been associated with more mature brains in developmental studies (Brown et al. 2012; Sowell et al. 2004; Squeglia et al. 2013; Tamnes, Østby, Fjell, et al. 2010; Wierenga et al. 2014). Since poor response inhibition has been associated with both ADHD and less mature brains, we remained agnostic regarding the directions of the hypothesized associations with cortical metrics. We also predicted that significant structural correlates of response inhibition would not be mediated by substance use.
Methods
Participants
Participants in the current study were recruited from the longitudinal follow-up to the Multimodal Treatment of ADHD (MTA) study. This sample originally consisted of 579 children ages 7 to 9 with ADHD combined type (M = 8.5 years, SD = 0.8 years), 465 of whom were male (80%), who were followed over the course of a 14-month randomized clinical trial of four treatment strategies; medication management, behavior therapy, combined medication management and behavior therapy, and community comparison. The recruitment strategy and diagnostic procedures for these participants were detailed by the (MTA Cooperative Group 1999). Briefly, participants were included if they met DSM-IV criteria for ADHD Combined Type using the Diagnostic Interview Schedule for Children (DISC), parent report, version 3.0. Exclusion criteria were limited to conditions or environmental situations that would have prevented full participation in the study. Common comorbid diagnoses were not excluded unless they were serious enough to require separate treatment that could confound results. In addition, a local normative comparison group (LCNG; n = 289) was recruited at 24 months to reflect the demographics of the ADHD sample. Both of these groups were followed longitudinally at years 3, 6, 8, 10, 12, 14, and 16.
Participants for the current study were recruited from those undergoing the year 14 or 16 follow-up visits. Each group in the current sample was enriched for cannabis use based on year 14 or 16 substance use ratings on the Substance Use Questionnaire (Molina and Pelham 2003; Molina et al. 2013). A total of 129 participants were recruited for neuroimaging and neuropsychological assessment. This sample was comprised of 88 participants who carried a childhood ADHD diagnosis (44 of whom were cannabis users), and 41 LNCG participants (20 of whom were cannabis users). Of these, 122 participants had neuroimaging data that passed quality control, and 119 of these also completed the Go/No Go task. Four of these participants were excluded due to lack of information regarding current ADHD status as described below. One was excluded due to inconsistencies between self- and observer-reports of cannabis and alcohol use. Written informed consent was completed by all participants prior to initiation of procedures.
The final sample of 114 participants (94 males) ranged in age from 21 to 27 years (M = 24.6, SD = 1.26). By self-report, the sample was 62.3% Caucasian (n = 71), 17.5 % African American (n = 20), 7.9% Hispanic/Latino (n = 9), 2.6% Asian (n = 3), 7.0% mixed race/ethnicity, and 2.6% Other (n = 3). Seventy-eight of these participants carried a childhood diagnosis of ADHD, and 53 participants from this ADHD group met criteria for persistence of ADHD symptoms into adulthood. Additional demographic information for this sample is provided by ADHD diagnostic group in Table 1.
Table 1.
Current demographics and descriptive statistics
| ADHD |
||||
|---|---|---|---|---|
| Persistent (n= 53) |
Desistant (n = 25) |
LNCG (n = 36) |
Statistical Test |
|
| Age | 24.7 (1.27) | 25.0 (1.12) | 24.1 (1.22) | F(2, 111) = 4.3328, p = .0154 |
| Gender | χ2 (2) = 1.087, p = .5806 | |||
| Male | 44 (83.0%) | 22 (88.0%) | 28 (77.8%) | |
| Female | 9 (17.0%) | 3 (12.0%) | 8 (22.2%) | |
| Ethnicity | χ2 (10) = 4.977, p = .8927 | |||
| Caucasian | 32 (60.4%) | 16 (64.0%) | 23 (63.9%) | |
| African American | 11 (20.8%) | 4 (16.0%) | 5 (13.9%) | |
| Hispanic/Latino | 3 (5.7%) | 1 (4.0%) | 5 (13.9%) | |
| Asian | 2 (3.8%) | 1 (4.0%) | 0 | |
| Mixed | 4 (7.6%) | 2 (8.0%) | 2 (5.6%) | |
| Other | 1 (1.9%) | 1 (4.0%) | 1 (2.7%) | |
| Socioeconomic Status | 4.11 (0.61) | 4.08 (0.76) | 4.09 (0.78) | F(2, 110) = 0.0261, p = .9742 |
| Full Scale IQ Estimate | 102 (14.1) | 105 (14.9) | 109 (23.0) | F(2, 109) = 1.5977, p = .2071 |
| ADHD Medication Use | χ2 (4) = 4.153, p = .3857 | |||
| No | 48 (90.6%) | 24 (96.0%) | 36 (100%) | |
| Yes, some of the time | 1 (1.9%) | 0 | 0 | |
| Yes, most of the time | 4 (7.5%) | 1 (4.0%) | 0 | |
| Past Year Cannabis Use | 5.96 (4.64) | 4.56 (4.64) | 5.72 (4.65) | F(2, 111) = 0.7996, p = .4521 |
|
Past Year Binge Alcohol Use |
3.51 (2.46) | 4.08 (2.52) | 3.78 (2.67) | F(2, 111) = 0.4418, p = .6440 |
|
% Go/No Go Commission Errors |
51.3 (20.3) | 40.0 (24.3) | 32.3 (16.5) | F(2, 111) = 9.8876, p = .0001 |
LNCG, local normative comparison group; Socioeconomic status was rated on a scale from 1 to 6, 1 = eighth grade or less, 2 = some high school, 3 = high school graduate or GED, 4 = some college or post-high school, 5 = college graduate, 6 = advanced graduate or professional degree; two participants were missing current full scale IQ estimates, and one participant did not report socioeconomic status.
Measures
Young Adult ADHD Persistence
Current ADHD status was used as a covariate in the primary analyses for the current study. Participants who were originally diagnosed with ADHD in childhood were classified in adulthood as either “persistent” or “desistant” based on self- and/or parent-report data from the Conners’ Adult ADHD Rating Scale (CAARS; Conners et al. 2002) at either year 12, 14, or 16 of the primary MTA study. Participants were classified as “persistent” if they had either a self-report or a parent-report (or both) suggestive of persistent symptoms as defined by having at least 4 symptoms in at least one domain (i.e., inattentive, hyperactive/impulsive) that were endorsed at a level 2 (“pretty much, often”) or 3 (“very much, very frequently”) response. They were classified as “desistant” if they had both a self-report and a parent-report, and neither was suggestive of symptom persistence. Further details regarding this persistence measure can be found in Sibley et al. (in preparation). For the purpose of covarying for “ADHD status” in the current study, this variable was turned into a three level ordinal measure in which the lowest level was the comparison group who were never diagnosed with ADHD in childhood. The ADHD desistant group was the intermediate level, and the ADHD persistent group was the highest level. Four participants were excluded because they could not be classified in terms of ADHD persistence, as they only had one report measure (self- or parent-report, but not both), and that measure did not indicate persistence.
Substance Use Measures
In order to test for mediating effects of substance use in the current study, cannabis and alcohol use over the past year were quantified using participants’ self-reports on the Substance Use Questionnaire (Molina et al. 2013; Molina and Pelham 2003). The cannabis use measure was the response to an item estimating the frequency of cannabis use in the past year (e.g., once a month, once a week, once a day, etc). The alcohol measure was the response to an item estimating the frequency with which the participant drank five or more drinks in a single session (i.e., binge drinking).
Go/No Go Response Inhibition Task
The Go/No Go task is a response inhibition measure that is based on the Conners Continuous Performance Task (Conners and Staff 2004). It requires participants to respond, by pressing the spacebar, to a variety of non-target stimuli (i.e., individually presented letters on a screen) while inhibiting their response to a specific target stimulus (the letter “X”). Target and non-target stimuli appear individually on the computer screen for 250 milliseconds followed by presentation of a fixation cross for the duration of the interstimulus interval. Participants completed 360 trials. A ratio of 10% target stimuli and 90% non-target stimuli was maintained. Based on findings from Tamm et al. (2013) that ADHD and LNCG participants differed significantly on percent of commission errors on the Go/No Go task, this was chosen as our Go/No Go measure of interest.
Neuroimaging Procedure
High-resolution anatomical MPRAGE T1-weighted images (TR/TE/TI=2170/5.56/1100ms, 160 sagittal slices, TH=1.2mm, in-plane resolution=1×1mm) were acquired along with T2-weighted images (TR/TE=6440/67ms) co-planar to the functional acquisitions. Pulse sequence parameters used across scanner manufacturers and models were optimized for equivalence in contrast properties and consistency in image-derived quantitative measures.
All data included in the current study underwent detailed evaluation to assess the quality and accuracy of the brain measures. Standardized quality control procedures were followed for both raw data and data at various processing stages. This included visual inspection ratings by trained imaging technicians and automated quality control algorithms, both testing general image characteristics as well as aspects specific to each imaging modality, such as contrast properties, registrations, and artifacts from motion and other sources.
Morphometric analysis of structural MRI data was performed using a specialized processing stream that is based upon FreeSurfer, with additional corrections and analyses developed at the UC San Diego Multimodal Imaging Laboratory. Briefly, Freesurfer yields a semi-automated surface-based analysis in which images are pre-processed for spatial (Talairach) and signal intensity normalization; brain tissues are segmented by labeling white matter, gray matter, and subcortical and cerebellar regions, and volumes are calculated (Dale et al. 1999); outer gray matter and white matter boundaries are identified to define the cortical surface and converted to a mesh of over 150,000 tessellated vertices to allow point-to-point surface measures; and cortical thickness (in millimeters) is measured as the distance between corresponding vertices of the white matter and gray matter surfaces (Fischl and Dale 2000).
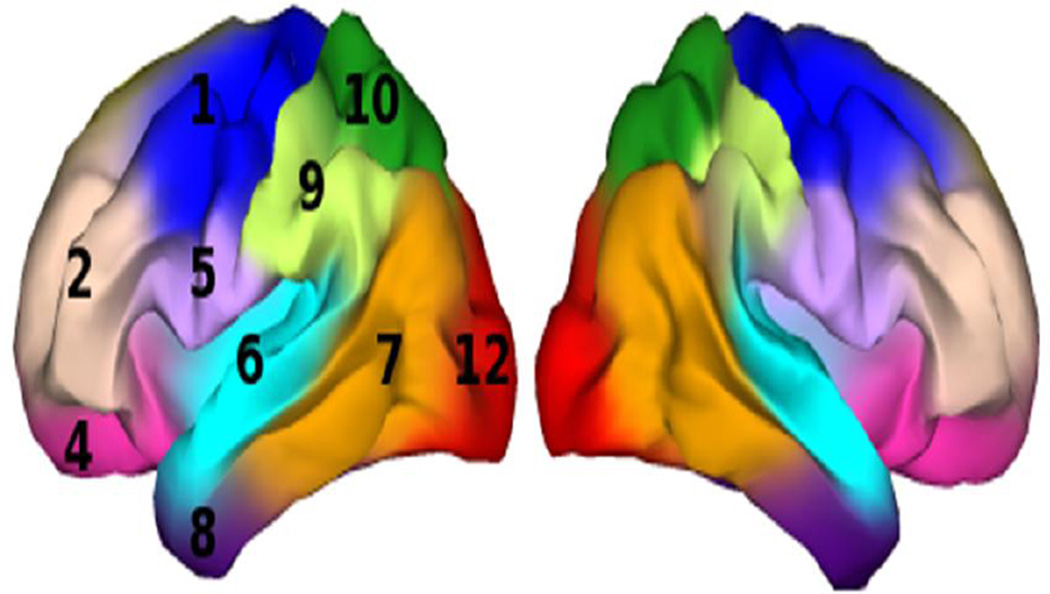
The IFG region of interest (ROI) examined here was defined with a novel genetically-informed cortical parcellation scheme (Chen et al. 2012). These authors used a fuzzy clustering method to analyze the matrix of genetic correlations among vertex-wise estimates of cortical surface expansion in a sample of 406 monozygotic and dizygotic twins. This clustering method resulted in a human brain atlas based solely on genetically informative data. Using silhouette coefficients, these authors determined that the optimal genetic clustering solution included 12 parcels that are largely bilaterally symmetric, each corresponding closely to meaningful structural and functional brain regions. For the current study, partial membership-weighted averages of voxel expansion factors were computed for the cluster labeled 5 by Chen et al. (Fig. 1), a cluster that subtends pars opercularis and some of pars triangularis of the IFG. This parcel was chosen because it corresponds with the caudal IFG region most frequently linked in previous studies to inhibitory function and ADHD-related functional abnormalities, and because there is evidence that individual differences in the relative surface area of this region are influenced by genetic factors that are distinct from genetic factors influencing relative surface area of other regions. Hereafter we refer to this ROI as caudal IFG (cIFG).
Fig. 1.
Genetically-informed fuzzy clusters, adapted from Chen et al., 2012; caudal inferior frontal gyrus (cIFG) is the cluster in light purple labeled “5”
Statistical Analyses
The two primary hypotheses for the current study were that variability in Go/No Go commission errors would predict variability in 1) relative cortical surface area (surface regionalization) of cIFG and 2) cortical thickness of cIFG, independent of ADHD status. These hypotheses were tested using multiple linear regression models to estimate the effects of Go/No Go performance on each cortical phenotype separately, in each case controlling for childhood ADHD status and persistence of adult ADHD. Because imaging data were collected at 6 different sites, scanner was entered as a covariate of no interest in each of these models. Because cortical architecture is known to vary with age and gender (Wierenga et al. 2014), we entered age and gender as additional covariates in all models. Finally, the regionalization hypothesis (hypothesis 1) involved using total cortical surface area as a covariate in the model in order to test for associations with surface area of the cIFG ROI relative to the entire cortex. Because there were two primary hypotheses, a bonferroni-corrected p-value of .025 was required for significance of the Go/No Go effect in these ROI analyses. Significant results were followed up with secondary multiple linear regression analyses to determine whether findings were mediated by substance use (recent cannabis and binge alcohol use). All ROI analyses were performed using the JMP 11 statistical package. Preliminary analyses also examined the possible mediating effects of ethnicity, socioeconomic status, IQ, and current medication use. However, these variables were left out of the primary analyses because they did not approach significance, and their inclusion would have reduced the sample size and the power to detect hypothesized effects. In secondary analyses we also examined whether results differed by hemisphere. They did not, so results are presented for the bilateral cIFG ROI.
Results
Demographics
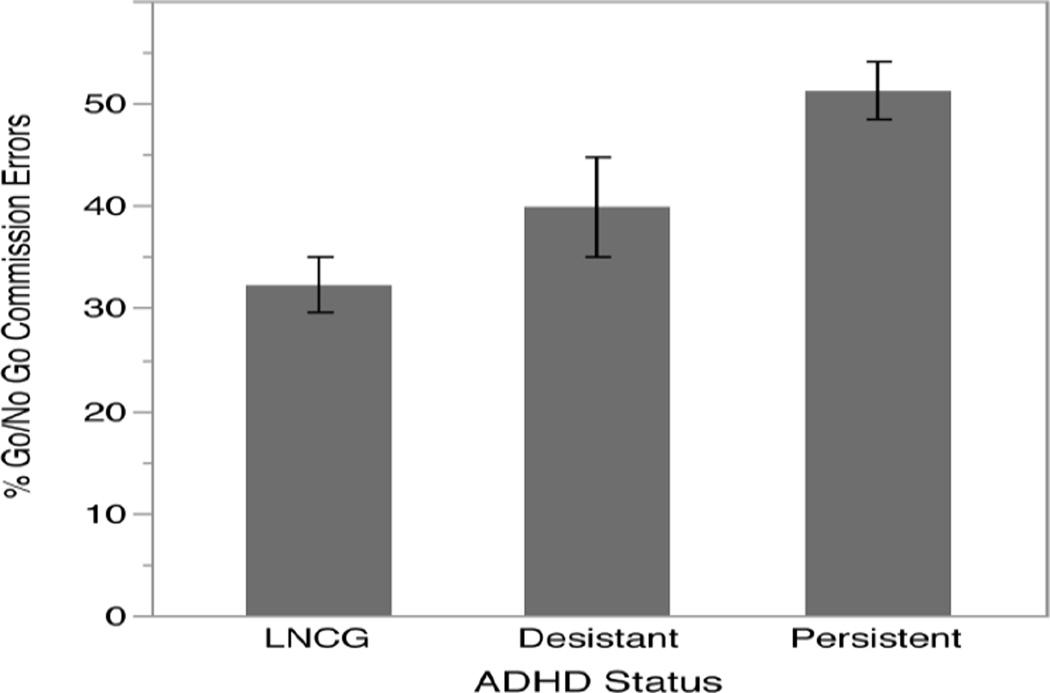
Table 1 provides demographic information and descriptive statistics for each ADHD diagnostic group, as well as statistical tests (i.e., ANOVA or Chi-Square) of the differences between groups. The groups were not statistically different in terms of gender, ethnicity, socioeconomic status, IQ, current medication use, or cannabis use or binge drinking over the past year. The groups differed statistically in terms of age, with LNCG participants being significantly younger than both the persistent ADHD participants (t = 2.86, p < 0.01) and the desistant ADHD participants (t = 2.02, p < 0.05). The ADHD persistent and desistant groups were not significantly different in age. The groups also differed significantly in terms of their percentage of commission errors on the Go/No Go task (Fig. 2). These group differences persisted in an ANCOVA controlling for age and gender (F = 12.5702, p < .001). Desistant participants made significantly more errors than LNCG participants (t = 2.28, p < .05) and significantly fewer errors than persistent participants (t = 2.06, p < .05).
Fig. 2.
Percentage of Go/No Go commission errors by ADHD status; error bars reflect one standard error. LNCG, local normative comparison group
Primary Analyses
In order to test the hypothesis that Go/No Go performance was significantly associated with relative surface area of the cIFG, we ran a linear regression model predicting cIFG surface area from percent Go/No Go commission errors while covarying for scanner, age, gender, and ADHD persistence status (Table 2). Importantly, we also included total surface area as a covariate because we were interested in the effect of Go/No Go performance on cIFG surface area relative to the entire cortex (regionalization of the cIFG). Results of the effect of gender on surface area approached significance (t = 1.90, p < .10), but there was no significant effect of Go/No Go performance or ADHD persistence. Consequently, no follow-up analyses were conducted on cortical surface area.
Table 2.
Models predicting cortical surface area and thickness from % Go/No Go commission errors
| Term | B | SE | t | p |
|---|---|---|---|---|
| cIFG Surface Area (relative to total area) | ||||
| Persistence (Group F = 1.9486, p = .1506) | ||||
| [Desistant – LNCG] | −0.0125 | 0.0064 | −1.95 | 0.0539 |
| [Persistent – Desistant] | 0.0074 | 0.0057 | 1.30 | 0.1980 |
| Gender | 0.0064 | 0.0033 | 1.90 | 0.0603 |
| Age | 0.0048 | 0.0019 | 2.52 | 0.0132 |
| Total Surface Area | 2.78e-6 | 1.71e-7 | 16.22 | <.0001 |
| % Go/No Go Commission Errors | −7.07e-5 | 0.0001 | −0.61 | 0.5459 |
| cIFG Thickness | ||||
| Persistence (Group F = 3.2118, p = .0443) | ||||
| [Desistant – LNCG] | −0.0249 | 0.0293 | −0.85 | 0.3964 |
| [Persistent – Desistant] | −0.0380 | 0.0259 | −1.47 | 0.1459 |
| Gender | 0.0146 | 0.0131 | 1.11 | 0.2687 |
| Age | 0.0014 | 0.0087 | 0.16 | 0.8720 |
| % Go/No Go Commission Errors | 0.0016 | 0.0005 | 3.09 | 0.0026 |
LNCG, local normative comparison group
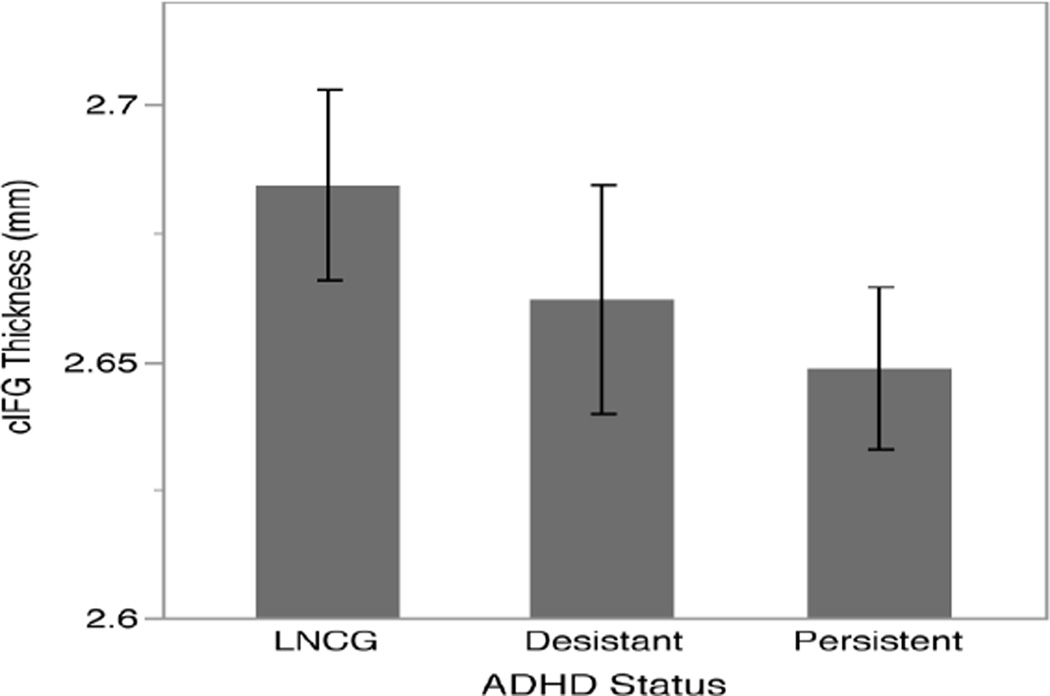
We ran a similar model predicting cortical thickness in the cIFG (Table 2). Results indicated a significant association between Go/No Go performance and cIFG thickness (t = 3.09, p < .01), independent of ADHD status, such that more commission errors (poorer performance) were associated with thicker cIFG. ADHD status was also a significant predictor of cIFG thickness (p < .05), but, surprisingly the effect associated with persistent ADHD was in the opposite direction from the Go/No Go performance results. Although we did not hypothesize a direction for the relationship between Go/No Go performance and cIFG thickness, we did expect that continuing to have ADHD symptoms (i.e., persistent ADHD) would show a similar relationship to cIFG thickness as making more Go/No Go commission errors, particularly given the highly significant differences in commission errors between ADHD groups. However, while more Go/No Go commission errors were associated with thicker cortex, ADHD persistence was associated with thinner cortex when both factors were modeled simultaneously. To examine these opposing effects separately, we tested two reduced models, one with Go/No Go performance but leaving out ADHD status, and one with ADHD status leaving out Go/No Go performance. Without ADHD status in the model, Go/No Go performance was still significantly associated with cIFG thickness in the positive direction (t = 2.18, p < .05). Without Go/No Go performance in the model, the effect of ADHD status no longer approached significance (Group F = 0.8582, p > .40). Figure 3 shows mean thickness of the cIFG for each group. We further sought to determine whether the direction and/or strength of the association between Go/No Go performance and cIFG thickness depends on childhood or current ADHD status. We added a performance by group interaction to the primary thickness model, but this interaction was not significant (Group F = 1.0525, p > .30).
Fig. 3.
Thickness of the inferior frontal gyrus by ADHD status; error bars reflect one standard error. LNCG, local normative comparison group
Secondary Analyses
Substance Use
We next wanted to determine whether the association between Go/No Go performance and cIFG thickness was mediated by substance use. To do this, we added past-year use of both cannabis and alcohol to the previous model. Neither of these substance use variables was a significant predictor of cIFG thickness, nor did either mediate the association between Go/No Go performance and cIFG thickness, which remained significant (t = 3.26, p < .01).
IFG Specificity
Results thus far have indicated a significant association between Go/No Go performance and cortical thickness in the cIFG, independent of ADHD status and substance use. However we were also interested in whether Go/No Go performance was specifically or disproportionately related to cIFG thickness, or whether it related to thickness more globally. We therefore tested the same model predicting global cortical thickness. These results indicated a significant association (t = 2.08, p < .05) such that more commission errors predicted thicker cortex globally. None of the other variables in this model were significant predictors of global thickness. Because Go/No Go performance was a significant predictor of cortical thickness globally, we decided to include the latter as a covariate in the previous model predicting cIFG thickness to see if there was still a disproportionately larger effect in the cIFG. The results of this model are shown in Table 3. The Go/No Go effect on cIFG thickness remained significant (t = 2.65, p < .01) despite the inclusion of global thickness. The ADHD status effect (Group F = 4.4827, p < .05) and the cannabis use effect (t = −2.12, p < .05) were also significant in this model. Thus, poorer Go/No Go performance was related to disproportionately thicker cortex in the cIFG, while ADHD status and cannabis use were related to disproportionately thinner cortex in the cIFG.
Table 3.
Substance use mediation models
| Term | B | SE | t | p |
|---|---|---|---|---|
| cIFG Thickness | ||||
| Persistence (Group F = 3.3828, p = .037 8) | ||||
| [Desistant – LNCG] | −0.0286 | 0.0288 | −0.99 | 0.3236 |
| [Persistent – Desistant] | −0.0357 | 0.0256 | −1.39 | 0.1662 |
| Gender | 0.0059 | 0.0134 | 0.44 | 0.6628 |
| Age | 0.0007 | 0.0086 | 0.09 | 0.9323 |
| Past Year Cannabis Use | −0.0037 | 0.0023 | −1.62 | 0.1087 |
| Past Year Binge Alcohol Use | −0.0054 | 0.0041 | −1.30 | 0.1954 |
| % Go/No Go Commission Errors | 0.0017 | 0.0005 | 3.26 | 0.0015 |
| cIFG Thickness (relative to global thickness) | ||||
| Persistence (Group F = 4.4827, p = .0137) | ||||
| [Desistant – LNCG] | −0.0130 | 0.0165 | −0.79 | 0.4343 |
| [Persistent – Desistant] | −0.0281 | 0.0146 | −1.92 | 0.0575 |
| Gender | −0.0075 | 0.0077 | −0.97 | 0.3340 |
| Age | −0.0001 | 0.0049 | −0.03 | 0.9766 |
| Global Thickness | 0.9688 | 0.0671 | 14.45 | <.0001 |
| Past Year Cannabis Use | −0.0028 | 0.0013 | −2.12 | 0.0362 |
| Past Year Binge Alcohol Use | −0.0035 | 0.0024 | −1.48 | 0.1417 |
| % Go/No Go Commission Errors | 0.0008 | 0.0003 | 2.65 | 0.0094 |
LNCG, local normative comparison group
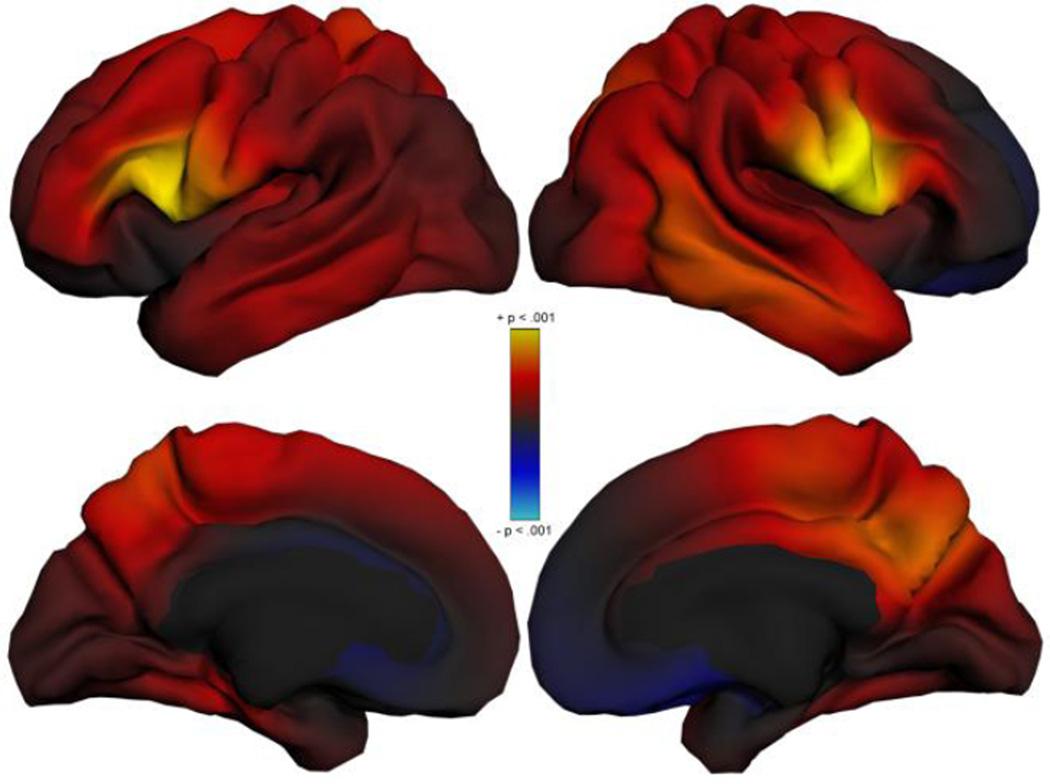
Because our analyses indicated significant Go/No Go effects on cortical thickness globally, we produced exploratory vertex maps showing the anatomical distribution of these thickness effects across the cortex, controlling for scanner, age, gender, ADHD status, cannabis use, and alcohol use (Fig. 4). This was done using a version of the PING Data Exploration Portal (Bartsch et al. 2014) that was developed for the MTA neuroimaging study. These maps show that Go/No Go performance effects are strongest (yellow) in the cIFG bilaterally, also stretching into the inferior motor and sensory strips in the right hemisphere.
Fig. 4.
Exploratory vertex maps showing the effect of % Go/No Go Commission Errors on cortical thickness; model covaries for scanner, age, gender, cannabis use, alcohol use, and persistence level. Areas in yellow are associated with p < .001 (uncorrected)
Discussion
The current study is, to our knowledge, the first to examine the relationship between response inhibition (as measured by percentage of commission errors on the Go/No Go task) and both cortical surface area and thickness in the same cohort. Based on previous work on the neural correlates of response inhibition (Aron 2007; Chambers et al. 2009; Dibbets et al. 2009; Epstein et al. 2007; Sebastian et al. 2012; Stevens et al. 2007), we were particularly interested in associations with surface area and thickness in the caudal inferior frontal gyrus (cIFG), which we defined using a novel genetically-informed, data-driven cortical parcellation scheme (Chen et al. 2012). These associations were investigated in a cohort of young adults who were either diagnosed with ADHD in childhood, or recruited as part of a typically developing local normative comparison group. The ADHD group was further classified as either persistent or desistant in their ADHD symptomatology. Given this composition, the sample was enriched for the full range of inhibitory functioning. The sample was, by design, also enriched for cannabis use, allowing us to test whether substance use mediates any observed associations between task performance and cortical surface area or thickness.
Our primary finding in this study was that, independent of childhood or current ADHD, poorer response inhibition was associated with thicker cortex in the inferior frontal gyrus. This is perhaps not surprising given the developmental trajectories of response inhibition and cortical thinning. Many studies have shown that older adolescents and young adults perform better and more efficiently than children on response inhibition tasks, and that inhibitory processes have a protracted course of maturation (Johnstone et al. 2007; 2005; Jonkman 2006; Tamm et al. 2002). Structural MRI studies demonstrate that typical development is characterized by a fairly linear trajectory of cortical thinning that extends at least into early adulthood (Brown et al. 2012; Sowell et al. 2004; Tamnes, Østby, Fjell, et al. 2010; Wierenga et al. 2014). It may therefore follow that less mature inhibitory control might be associated with an alteration of the process of cortical thinning (i.e., thicker cortex) in young adults, particularly in those regions mediating response inhibition. In addition to the specific association we hypothesized between Go/No Go performance and cIFG thickness, we also found a weaker association with our measure of global (mean) cortical thickness. This is consistent with the possibility that effects extend beyond our region of interest. However, the exploratory map suggests that the effect size peaks in the cIFG region, and indeed the effect in the cIFG ROI persists when global thickness is a covariate in the model. While the specific associations observed here between Go/No Go performance and cIFG thickness have not been reported previously, this general trend is consistent with previous findings that better and/or faster maturing cognitive performance, including on inhibitory tasks, was associated with thinner cortex in adolescents (Squeglia et al. 2013; Tamnes, Østby, Walhovd, et al. 2010).
Seemingly at odds with this result, however, is our finding that, independent of task performance, ADHD status in these young adults was modestly associated with thinner cortex in the cIFG. ADHD has been associated with thinner cortex in many areas, including the dorsolateral prefrontal cortex, orbitofrontal cortex, anterior and posterior cingulate cortex, and the temporo-occipito-parietal junction (Makris et al. 2007; Shaw et al. 2006), particularly with regard to the persistence of inattentive symptoms (Shaw et al. 2013). There is one study in adults that notes thinner cortex in the IFG associated with ADHD (Proal et al. 2011), and one additional study in children reporting this same association (Batty et al. 2010). Here, an ADHD association with decreased thickness only reaches significance if one controls for the association with Go/No Go task performance in the same model. Therefore, it appears that our findings suggesting thinner cortex in the cIFG associated with ADHD diagnosis only when controlling for the level of inhibitory control, but thicker cIFG cortex in participants with poor inhibitory control, reveal two distinct factors relating to cIFG thickness in ADHD, one more strongly associated with response inhibition than the other. Interestingly, one fMRI study found that, during a controlled version of a Stop Signal Task, the right IFG was recruited in response to the stimulus regardless of whether an inhibitory response or any motor response at all was required (Hampshire et al. 2010), and the authors interpreted this as evidence for the role of rIFG in attention to and detection of salient stimuli. This is consistent with a prominent theory of attention suggesting that the cIFG, particularly in the right hemisphere, is crucial for directing attention toward relevant and/or salient environmental cues (Corbetta and Shulman 2002). However, other investigators (Swann et al. 2012) provide evidence from electrocorticography suggesting that the rIFG also contributes to “stopping”, i.e., inhibition, per se. Thus, possibly, processes altering the normal course of cortical thinning in cIFG give rise to inhibitory deficits, and distinct neural processes resulting in decreased thickness of cIFG cortex contribute to other functions of IFG affected in ADHD. These findings may reflect the underlying behavioral heterogeneity of the ADHD symptom presentation and suggest that, consistent with findings by (Shaw et al. 2013), thinning in ADHD may be more related to the inattentive components of this presentation. Whatever the cause, these data provide limited support for a general trend in the literature of associations between ADHD and thinner cortex. However, they provide stronger support for an association between poorer response inhibition and thicker cIFG cortex.
The association between Go/No Go performance and cIFG thickness was not mediated by cannabis use or alcohol binge drinking over the previous year in the current sample. Alcohol use was not a significant predictor of cIFG thickness, and the effect of cannabis use was only significant after global cortical thickness was added to the model as a covariate. Other studies of adolescent substance use have noted decreased cortical thickness to be associated with heavier cannabis use in areas surrounding the cIFG (e.g., caudal middle frontal gyrus, insula, paracentral lobule), but not specifically in the cIFG (Jacobus et al. 2014; Lopez-Larson et al. 2011). Given the fact that cannabis effects on the cIFG were not included in our primary hypotheses, and these effects are only observed in a model controlling for global thickness, Go/No Go performance, and ADHD status in the current study, this finding needs to be replicated in future studies.
Cortical surface regionalization of the cIFG was not related to Go/No Go performance in the current study. Although we find no association here, this does not necessarily mean that there is no relationship between inhibitory functioning and surface regionalization. Cortical surface area and thickness are influenced by different genetic factors and cellular mechanisms (Chen et al. 2012; Panizzon et al. 2009), and the time courses of their development are different. While cortical thinning occurs fairly linearly throughout development, surface area development is characterized by early expansion of the cortex followed by contraction that is heterochronous across different regions (Brown and Jernigan 2012). It is possible that these distinct cortical phenotypes, as well as the factors that drive them, relate to behavior in different ways and at different times in development. This type of age-dependent association was recently reported between anxiety and both surface area and thickness in a sample of 287 typically developing children and adolescents ranging in age from 7 to 20. Anxiety was most strongly related to both cortical phenotypes in the youngest children (Newman et al. 2015). Given that the current sample is comprised of young adults, the possibility of a surface area effect earlier in development should not be dismissed.
The current study has a number of strengths and limitations. Most notably, this study was conducted in a large subset of participants from the MTA study. As such, participants were well characterized, both prospectively with regard to childhood diagnoses, and with respect to current symptom persistence, past year substance use, and neuropsychological functioning. We were therefore able to examine the relationship between response inhibition and cortical structure within this context, controlling for ADHD status and testing for possible mediation effects of recent substance use. We also used state-of-the-art image processing procedures that allowed us to differentiate cortical surface area from cortical thickness rather than using traditional volumetrics that conflate these distinct aspects of cortical architecture. A limitation of the current study is that, despite being conducted within a larger longitudinal study of individuals diagnosed with ADHD in childhood, imaging data were only acquired at one time point in early adulthood. We were therefore unable to examine this association within a developmental context.
The current study begins to address questions raised by the RDoC initiative (Insel et al. 2010) by examining associations between neural architectural phenotypes and basic neurobehavioral processes that a) cut across disorders, and b) can be measured dimensionally. Furthermore, these results highlight the importance of such an approach by showing that the conclusions reached in such a study (i.e., poor response inhibition is associated with thicker cortex) may differ from those that would be reached by a study of diagnostic group differences (i.e., a disorder that is assumed to reflect poor inhibition is associated with thinner cortex). However, much additional work is needed in this area. As noted above, it will be crucial to examine these associations within a neurodevelopmental framework to see if the nature of these relationships differs with age (e.g., changes in the specific cortical phenotype or the direction of the association at different points in development). Such information could provide invaluable insights into the neurobiological underpinnings of a variety of disorders, and ultimately help to improve both pharmacological and behavioral treatment options.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (J.N.E., grant number HHSN271200800009C, T.L.J., grant number RC2 DA029475); and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (T.L.J., grant number R01 HD061414 and R01 HD075489). The content presented herein is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Anders M. Dale: Anders M. Dale is a founder of and holds equity interest in CorTechs Labs, La Jolla, CA and serves on its scientific advisory board. The terms of this arrangement have been reviewed and approved by UC San Diego, in accordance with its conflict of interest policies.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- Almeida Montes LG, Alcántara HP, García RBM, la Torre, De LB, Acosta DÁ, Duarte MG. Brain Cortical Thickness in ADHD: Age, Sex, and Clinical Correlations. Journal of Attention Disorders. 2012 doi: 10.1177/1087054711434351. 1087054711434351. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Hernández García AO, Ricardo-Garcell J. ADHD prevalence in adult outpatients with nonpsychotic psychiatric illnesses. Journal of Attention Disorders. 2007;11(2):150–156. doi: 10.1177/1087054707304428. [DOI] [PubMed] [Google Scholar]
- Almeida Montes LG, Ricardo-Garcell J, Barajas De La Torre LB, Prado Alcántara H, Martínez García RB, Fernández-Bouzas A, Avila Acosta D. Clinical correlations of grey matter reductions in the caudate nucleus of adults with attention deficit hyperactivity disorder. Journal of Psychiatry & Neuroscience : JPN. 2010;35(4):238–246. doi: 10.1503/jpn.090099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida Montes LG, Ricardo-Garcell J, la Torre, De LB, Alcántara HP, García RBM, Acosta DÁ, Bouzas AF. Cerebellar gray matter density in females with ADHD combined type: a cross-sectional voxel-based morphometry study. Journal of Attention Disorders. 2011;15(5):368–381. doi: 10.1177/1087054710366421. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2007;13(3):214–228. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. The Journal of Neuroscience. 2007;27(14):3743–3752. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences. 2014;18(4):177–185. doi: 10.1016/j.tics.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Barkley RA. ADHD and the Nature of Self-Control. 1997a [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997b;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Response inhibition in Attention-Deficit Hyperactivity Disorder. Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:177–184. [Google Scholar]
- Bartsch H, Thompson WK, Jernigan TL, Dale AM. A web-portal for interactive data exploration, visualization, and hypothesis testing. Frontiers in Neuroinformatics. 2014;8:25. doi: 10.3389/fninf.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ. Cortical gray matter in attention-deficit/hyperactivity disorder: a structural magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):229–238. doi: 10.1016/j.jaac.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a selective overview. Biological Psychiatry. 2005;57(11):1215–1220. doi: 10.1016/j.biopsych.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: Effects of protracted alcohol use. Alcoholism, clinical and experimental research. 2000;24(2):164–171. [PubMed] [Google Scholar]
- Brown TT, Jernigan TL. Brain development during the preschool years. Neuropsychology review. 2012;22:313–333. doi: 10.1007/s11065-012-9214-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TT, Kuperman JM, Chung Y, Erhart M, Mccabe C, Hagler DJ, et al. Neuroanatomical assessment of biological maturity. Current biology : CB. 2012;22:1693–1698. doi: 10.1016/j.cub.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Trainor RJ, Orendi JL, Schubert AB, Nystrom LE, Giedd JN, et al. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. Journal of cognitive neuroscience. 1997;9(6):835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and biobehavioral reviews. 2009;33(5):631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Chen C-H, Gutierrez ED, Thompson W, Panizzon MS, Jernigan TL, Eyler LT, et al. Hierarchical genetic organization of human cortical surface area. Science. 2012;335(6076):1634–1636. doi: 10.1126/science.1215330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK, Staff M. Conners' Continuous Performance Test II (CPT II V. 5). North Tonawanda. San Antonio, TX: 2004. [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Conners' Adult ADHD Rating Scales. 2002 [Google Scholar]
- Conners C, Epstein J, Angold A, Klaric J. Continuous performance test performance in a normative epidemiological sample. Journal of Abnormal Child Psychology. 2003;31(5):555–562. doi: 10.1023/a:1025457300409. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cubillo A, Rubia K. Structural and functional brain imaging in adult attention-deficit/hyperactivity disorder. Expert review of neurotherapeutics. 2010;10(4):603–620. doi: 10.1586/ern.10.4. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dibbets P, Evers L, Hurks P, Marchetta N, Jolles J. Differences in feedback- and inhibition-related neural activity in adult ADHD. Brain and cognition. 2009;70(1):73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. Journal of Child Psychology and Psychiatry. 2007;48(9):899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill S, Keefe RSE, et al. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–113. [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE, Petty C. Substance use among ADHD adults: Implications of late onset and subthreshold diagnoses. The American Journal on Addictions. 2007;16:24–34. doi: 10.1080/10550490601082767. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, Jentsch JD. Poor response inhibition: At the nexus between substance abuse and attention deficit/hyperactivity disorder. Neuroscience and biobehavioral reviews. 2009;33(5):690–698. doi: 10.1016/j.neubiorev.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenc A, Killgore WDS. Age of onset of marijuana use impacts inhibitory processing. Neuroscience letters. 2012;511(2):89–94. doi: 10.1016/j.neulet.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50(3):1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of studies on alcohol and drugs. 2014;75(5):729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Dimoska A, Smith JL, Barry RJ, Pleffer CB, Chiswick D, Clarke AR. The development of stop-signal and Go/No Go response inhibition in children aged 7- 12 years: Performance and event-related potential indices. International Journal of Psychophysiology. 2007;63(1):25–38. doi: 10.1016/j.ijpsycho.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Johnstone SJ, Pleffer CB, Barry RJ, Clarke AR, Smith JL. Development of inhibitory processing during the Go/No Go task. Journal of Psychophysiology. 2005;19(1):11–23. [Google Scholar]
- Jonkman LM. The development of preparation, conflict monitoring and inhibition from early childhood to young adulthood; a Go/Nogo ERP study. Brain research. 2006;1097(1):181–193. doi: 10.1016/j.brainres.2006.04.064. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of response inhibition. Human brain mapping. 2001;12(2):100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society : JINS. 2012;18(4):678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural brain research. 2011;220(1):164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Valera EM, Bush G, Kaiser J, Kennedy DN, et al. Cortical thinning of the attention and executive function networks in adults with Attention-Deficit/Hyperactivity Disorder. Cerebral Cortex. 2007;17(6):1364–1375. doi: 10.1093/cercor/bhl047. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human brain mapping. 2001;12(3):131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112(3):497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Eugene Arnold L, Swanson JM, Pelham WE, Hechtman L, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(3):250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, Thompson AL, Marshal MP. Attention-Deficit/Hyperactivity Disorder risk for heavy drinking and alcohol use disorder is age specific. Alcoholism, clinical and experimental research. 2007;31(4):643–654. doi: 10.1111/j.1530-0277.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Cooperative Group. A 14-Month randomized clinical trial of treatment strategies for Attention-Deficit/Hyperactivity Disorder. Archives of general psychiatry. 1999;56(12):1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Newman E, Thompson WK, Bartsch H, Hagler DJ, Chen C-H, Brown TT, et al. Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Structure and Function. 2015:1–13. doi: 10.1007/s00429-015-1085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19(11):2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima M, Horta B, Biederman J, Rohde L. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164(6):942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Pope HG, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. Journal of the American Medical Association. 1996;275(7):521–527. [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. Brain gray matter deficits at 33-year follow-up in adults with attention-deficit/hyperactivity disorder established in childhood. Archives of general psychiatry. 2011;68(11):1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Sarason I, Bransome ED, Beck LH. A continuous performance test of brain damage. Journal of consulting psychology. 1956;20(5):343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Sebastian A, Gerdes B, Feige B, Klöppel S, Lange T, Philipsen A, et al. Neural correlates of interference inhibition, action withholding and action cancelation in adult ADHD. Psychiatry research. 2012;202(2):132–141. doi: 10.1016/j.pscychresns.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with Attention-Deficit/Hyperactivity Disorder. Archives of general psychiatry. 2006;63(5):540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Greenstein D, de Rossi P, Sharp W. Trajectories of cerebral cortical development in childhood and adolescence and adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;74(8):599–606. doi: 10.1016/j.biopsych.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. The British Journal of Psychiatry. 2009;194(3):204–211. doi: 10.1192/bjp.bp.107.048827. [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association. 2002;287(9):1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. The Journal of Neuroscience. 2004;24(38):8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. Early Adolescent Cortical Thinning Is Related to Better Neuropsychological Performance. Journal of the International Neuropsychological Society. 2013;19(09):962–970. doi: 10.1017/S1355617713000878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behavioural brain research. 2007;181(1):12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann NC, Cai W, Conner CR, Pieters TA, Claffey MP, George JS, et al. Roles for the pre-supplementary motor area and the right inferior frontal gyrus in stopping action: Electrophysiological responses and functional and structural connectivity. NeuroImage. 2012;59(3):2860–2870. doi: 10.1016/j.neuroimage.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. Journal of the American Academy of Child and Adolescent Psychiatry. 2002;41(10):1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20(3):534–548. doi: 10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Walhovd KB, Westlye LT, Due-Tønnessen P, Fjell AM. Neuroanatomical correlates of executive functions in children and adolescents: A magnetic resonance imaging (MRI) study of cortical thickness. Neuropsychologia. 2010;48(9):2496–2508. doi: 10.1016/j.neuropsychologia.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcoholism, clinical and experimental research. 2004;28(10):1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SPA, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology. 2007;194(2):173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommer BL, Hoeppner J. The go-no go test in attention deficit disorder is sensitive to methylphenidate. Journal of Child Psychology and Psychiatry. 1991 doi: 10.1177/0883073891006001s13. [DOI] [PubMed] [Google Scholar]
- Trommer BL, Hoeppner JA, Lorber R, Armstrong KJ. The go-no-go paradigm in attention deficit disorder. Annals of neurology. 1988;24(5):610–614. doi: 10.1002/ana.410240504. [DOI] [PubMed] [Google Scholar]
- Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87(C):120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, Schachar R. Response inhibition and psychopathology: a meta-analysis of go/no-go task performance. Journal of Abnormal Psychology. 2014;123(2):429–439. doi: 10.1037/a0036295. [DOI] [PubMed] [Google Scholar]