Abstract
Sleep:wake cycles are known to be disrupted in people with neurodegenerative disorders. These findings are now supported by data from animal models for some of these disorders, raising the question of whether the disrupted sleep/circadian regulation contributes to the loss of neural function. As circadian rhythms and sleep consolidation also break down with normal aging, changes in these may be part of what makes aging a risk factor for disorders like Alzheimer's disease. Mechanisms underlying the connection between circadian/sleep dysregulation and neurodegeneration remain unclear, but several recent studies provide interesting possibilities. While mechanistic analysis is underway, it is worth considering treatment of circadian/sleep disruption as a means to alleviate symptoms of neurodegenerative disorders.
Keywords: circadian rhythms, sleep, neurodegeneration, aging, Parkinson's, Alzheimer's, Huntington's
Could age-associated changes in circadian rhythms and sleep contribute to neurodegenerative disorders?
Aging is associated with decreased circadian rhythmicity of behaviors including sleep. Age impacts sleep timing, duration, and consolidation, such that overall sleep decreases and also tends to be more fragmented in the elderly. Sleep disruption - be it shorter sleep duration or poorer sleep quality - is correlated with worsened cognitive performance [1–4]. Aging brings with it a myriad of changes to the brain that may also impact cognitive performance, so it is difficult to isolate the specific effects of sleep and circadian changes. Nevertheless, there is a large and growing body of literature which links changes that occur in the sleep and circadian systems -- at the molecular, circuit, and behavioral levels -- with normal aging, and with diseases of aging such as neurodegenerative disease.
Aging of central and peripheral circadian systems
Introduction to the circadian system
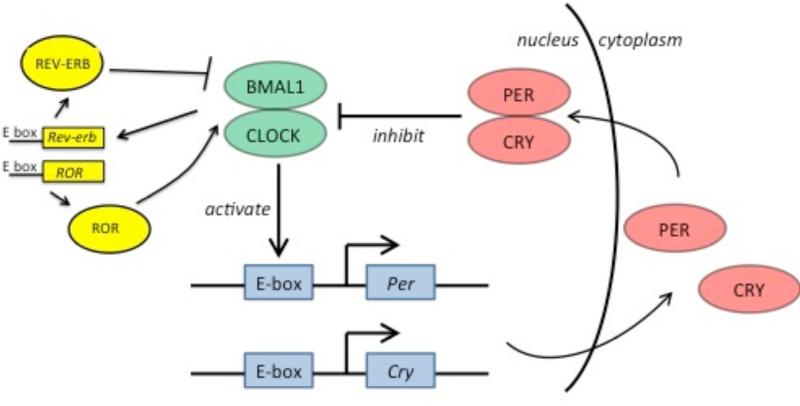
The molecular circadian oscillator consists of transcriptional-translational negative feedback loops [5,6]. In the major loop, the transcription factors BMAL1 and CLOCK heterodimerize during the early circadian day and induce transcription of genes with promoters containing circadian E-box elements. Among these genes are their own negative feedback repressors, PERIOD (Per) and CRYPTOCHROME (Cry) (Figure 1). During the early circadian night, PER and CRY form complexes and repress BMAL1-CLOCK-mediated transcription, thus downregulating their own expression. PER and CRY degrade throughout the night, releasing negative regulation on BMAL1 and CLOCK and enabling the start of a new circadian day. In an interlocked loop, BMAL1-CLOCK target the expression of nuclear receptors rev-erbα and ROR, which, in turn, inhibit and activate respectively the transcription of BMAL1 to produce cycling of BMAL1 mRNA. Many other genes also have E-box or REV-ERB/ROR regulatory sequences, so the circadian cycle imposes rhythmic expression on genes involved in many aspects of cellular function [7]. These lead to tissue and circuit-level oscillations, which ultimately generate overt rhythms of physiology and behavior.
Figure 1. The circadian clock.
BMAL1 and CLOCK are transcription factors that heterodimerize and bind to E-box element-containing promoters, including promoters for Per and Cry. PER and CRY form a complex that inhibits BMAL1/CLOCK. In an interlocked loop, BMAL1 and CLOCK target nuclear receptors, REV-ERB and ROR, which feedback to negatively or positively regulate BMAL1 transcription respectively
The suprachiasmatic nucleus (SCN) is the central pacemaker of the mammalian circadian system. The SCN contains a heterogeneous population of neuron types [8], including vasoactive intestinal peptide (VIP)-producing neurons in the ventral core and vasopressin (AVP) cells in the dorsal shell. Explanted SCN is capable of maintaining robust circadian rhythmicity for many days in vitro [9]. Many other peripheral tissues (e.g. liver, lung, skeletal muscle) are also rhythmic in vitro, but with oscillations that are much less robust and do not persist as long [9], suggesting that the periphery contains damped oscillators that are entrained by the central SCN.
The role of the SCN as master circadian pacemaker was established by seminal studies using orthotopic SCN transplantation. SCN-lesioned hamsters have permanently disrupted circadian rhythms, but those rhythms can be restored by implantation of brain grafts containing fetal SCN [10]. Critical follow-up studies using mutant hamster strains with short circadian periods demonstrated that the period of circadian rhythm is determined by the genotype of the transplant SCN rather than the host [11], establishing the primacy of the SCN in the circadian system. Although the SCN signals circadian information to other brain regions via direct wiring -- see below discussion on the circuitry relevant to sleep and arousal regions -- these transplant studies are powerful evidence that SCN signaling is at least in part indirect (e.g. via peptidergic signaling). Transplanted SCN does form some synaptic connections with the host brain post-transplant, but it is unlikely to fully recapitulate the endogenous wiring pattern.
Changes in the circadian system seen in normal aging
Since the SCN signals both directly and indirectly to numerous brain regions, clock function and aging may be linked by pathology at the level of the SCN, SCN projections, and SCN secreted signals. In addition, clock activity in other brain regions and in peripheral tissues may change with age.
Although rest:activity rhythms are clearly impaired with age, the core clock in the SCN appears to remain relatively robust despite some age-related changes in expression of individual clock proteins [12–14]. Data are mixed as to whether aging impairs SCN entrainment to light [15,16]. On the other hand, it is clear that peripheral oscillators dampen with age, whether from loss of intrinsic clock function or decline in entraining signals from the SCN, and could contribute to the aging process [17]. Although changes in clock gene expression are still not causally linked to pathology associated with aging, we note that several clock targets are relevant to much of this pathology. For instance, REV-ERBα modulates expression of genes in several metabolic and inflammatory pathways [18–21]. Also, sirtuins are involved in clock regulation and also in the control of aging [14].
Although the SCN is relatively resistant to age at the level of the molecular clock, it undergoes significant age-related degradation at the network level. The total number of SCN neurons is unchanged with aging, but elderly rats have significantly fewer vasopressinergic cells [22], a change that likely has consequences for downstream signaling. A greater proportion of SCN cells are silent in older animals in vitro [23] and SCN neurons display decreased circadian phase coherence with age [24]. This results in desynchronization [24] and decreased amplitude of electrical activity rhythms [25] at the network level. Together these are predicted to reduce the strength of output rhythms and could account for attenuation of behavioral rhythms in older animals.
Age-associated changes in the circadian system have also been examined in Drosophila. As in mammals, the central brain clock is robust in old flies, although the rest:activity rhythm is impaired, most likely due to deficits in output [26]. However, oscillations in body clocks are considerably dampened, most likely from loss of clock function as peripheral clocks in Drosophila contain their own photoreceptors and so do not rely upon entraining signals from the central clock in the presence of light:dark cycles. This raises the question of whether such dampening contributes to the aging process or is merely a consequence of it. Interestingly, Drosophila clock mutants, period and timeless (orthologs of mammalian Per and Cry), are less sensitive to lifespan-extending effects of dietary restriction (DR) [27]. Together with the observation that DR strengthens circadian oscillations in peripheral tissues, these findings suggest that clocks, at least those in peripheral tissues, are relevant for determination of lifespan under some conditions.
Aging of the sleep system
Introduction to the sleep system
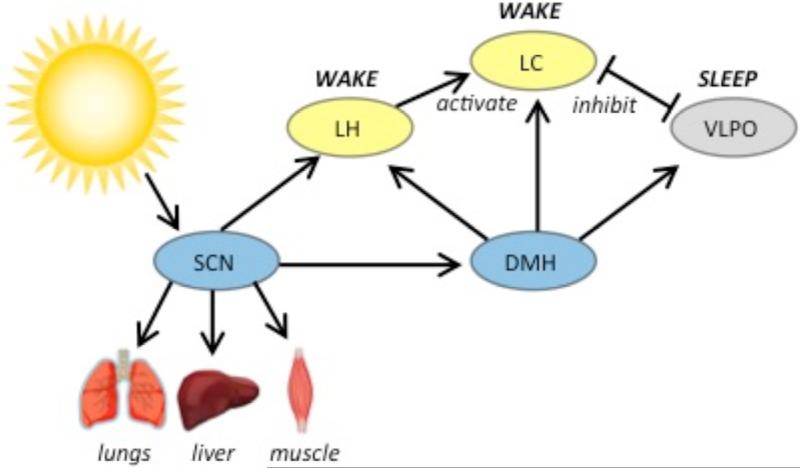
Sleep and wakefulness are regulated by multiple brain regions, including the ventrolateral preoptic area (VLPO) in the anterior hypothalamus, the hypocretin (orexin) neurons in the lateral hypothalamus, and the locus coeruleus (LC) in the pons (Figure 2). The VLPO contains inhibitory (galaninergic and GABAergic) neurons that are active during sleep [28] and targeted VLPO lesions result in profoundly disrupted sleep [29]. The hypocretins are neuropeptides [30] expressed in neurons that fire most during active waking and are almost silent during sleep [31]. Activation of these neurons promotes wakefulness [32,33]. Hypocretinergic neurons send dense excitatory projections to multiple nuclei including the LC [34], a noradrenergic nucleus that also promotes arousal [35]. Both VLPO and hypocretinergic neurons send projections to the brainstem, where REM sleep in particular is regulated [36,37]. In the ‘flip-flop’ model of sleep and wakefulness, the sleep-active VLPO and the wake-active monoaminergic nuclei (including the LC) mutually inhibit each other, creating rapid transition between sleep and wake states; the wake-active hypocretin neurons reinforce the arousal system and stabilize the switch [38].
Figure 2. Sleep and circadian brain circuitry in mammals.
The SCN is the central circadian pacemaker, entraining other brain regions as well as peripheral tissues. The SCN projects directly to the wake-promoting LH, which contains hypocretin neurons, and to the DMH. The DMH projects broadly to sleep and arousal centers. Abbreviations: SCN: suprachiasmatic nucleus; DMH: dorsomedial hypothalamus; LH: lateral hypothalamus; LC: locus coeruleus; VLPO: ventrolateral preoptic area
Since sleep is a strongly circadian behavior, it is expected that the SCN regulates sleep and arousal brain regions. However, the SCN sends only sparse projections to hypocretin neurons [39], and no direct projections to the LC [40] or to the VLPO [41,42], suggesting a polysynaptic signaling pathway instead. Anatomic tracing studies point to the dorsomedial hypothalamus (DMH) as a nexus of this polysynaptic circuit. The DMH is a region notable for receiving inputs from most major nuclei and areas of the hypothalamus [43] including from the SCN [43–45]. The DMH in turn projects to the LC [40], to the VLPO [42,45], and to hypocretin neurons in the lateral hypothalamus [45]. Lesions of the DMH eliminate circadian changes in LC activity [40] and reduce circadian rhythms of wake-sleep, feeding, locomotor activity, and corticosteroid secretion [45] confirming its critical role in the circuit.
Changes in the sleep system seen in normal aging
Normal aging is associated with significant changes in sleep. Elderly adults exhibit less total sleep time, poorer sleep efficiency, increased sleep latency, increased nighttime awaking, and excessive daytime sleepiness with increased daytime naps [46–49]. Similar age-associated changes have also been shown in animal models spanning non-human primates [50], rodents [25,51–55], and flies [56].
Normal aging is also associated with changes in neural sleep circuitry; hypocretin neurons and their projections appear to be particularly altered with age. Several studies have demonstrated decreased hypothalamus hypocretin levels with aging in mice [57,58], although rhesus macaques do not display age-related decrease in numbers of hypocretin neurons in the lateral hypothalamus [59]. A recent study also found that hypocretin expression levels decline with aging in humans [60]. Hypocretin neurons may undergo additional impairments aside from changes in hypocretin expression levels: for instance, older mice reportedly have increased endoplasmic reticulum stress and dyshomeostasis in wake-active hypocretin neurons [53]. Hypocretin signaling pathways are also altered with age, with declining levels of hypocretin receptor expression [61], and degradation of hypocretinergic projections [59,62].
Sleep and circadian disruptions are common in neurodegenerative disease
Disrupted sleep and circadian rhythms are common in people with neurodegenerative disease. Since these diseases disproportionately affect the elderly, who may also have other medical conditions and take multiple medications, it can be complicated to determine how much observed behavior is specifically due to the neurodegenerative disease process. Nevertheless, a large and growing body of clinical studies supports the fact that neurodegenerative diseases are associated with sleep and circadian disruption.
Alzheimer's Disease
Alzheimer's Disease (AD) is a chronic neurodegenerative disease with clinical symptoms of progressive short-term memory loss, disorientation, mood swings, loss of motivation, and behavioral changes. Pathologically, it is characterized by the presence of extracellular amyloid plaques (made of β-amyloid peptides) and by intracellular neurofibrillary tangles (made of tau) within the brain [63]. AD progresses through preclinical pathologic stages before disease burden reaches threshold for being clinically detectable [64]; sleep pathology is seen even In cognitively normal individuals with β-amyloid deposition, suggesting that the presence of β-amyloid impacts sleep architecture [65].
AD patients have sleep-wake cycle and rest-activity dysfunction [66,67]. Increased daytime sleepiness is correlated with greater functional impairment [68], and is an independent risk factor (controlling for age) for the presence of dementia [69]. Circadian core-body temperature cycling also appears to be disrupted in AD [66,70] although AD patients do appear to retain robust rhythmic daily cortisol profiles [71,72]. A study by Cermakian and colleagues found that although patients with AD still have clock gene expression in multiple brain regions (bed nucleus of the stria terminalis (BNST), cingulate cortex, and pineal gland), there is a loss of typical phase coherence within regions, and of phase relationships between regions [73].
Circadian disruption in AD patients is likely from disruption within clock circuits and from deficits in outputs. AD patients exhibit significant neuronal loss in the SCN [74–76] and analysis of actigraphy data and post-mortem brain tissue analysis from the same human population of AD subjects shows a correlation between circadian rhythm amplitude of motor activity and SCN neuronal loss [77]. However, flies expressing β-amyloid have circadian behavioral disruption [78] despite intact cell number and oscillations of the central clock, pointing to additional disruption at the level of clock outputs. Consistent with this, in postmortem analysis of brains from patients with AD, neurofibrillary degeneration in the hypothalamus was primarily observed in cortical projection neurons [79].
Parkinson's Disease
Parkinson's disease (PD) is a neurodegenerative disorder clinically characterized by motor symptoms including resting tremor, rigidity, and slow movement (bradykinesia); in later stages, the disease can also lead to cognitive decline and to behavioral and emotional problems. Pathologically, PD is characterized by loss of midbrain dopaminergic neurons and by abnormal accumulation of the protein alpha-synuclein into intraneuronal inclusions called Lewy bodies [80]. PD also involves destruction of multiple wake-active neuronal populations [81], including a decrease in the number of hypothalamic hypocretin neurons [82,83].
Sleep disturbances are among the most morbid and prevalent non-motor symptoms of PD, with nearly two-thirds of PD patients reporting some form of sleep symptoms [84]. Multiple sleep disorders are associated with PD, including REM behavior disorder (RBD), excessive daytime somnolence, insomnia, and restless leg syndrome [84,85]. Among these, RBD, in which the skeletal muscle atonia that typically occurs during REM sleep is lost, enabling complex motor activity to occur during dreams, is the most specific to PD.
Sleep abnormalities in PD are progressive over time and therefore correlate with disease duration [86]. Destruction of sleep architecture also correlates with presence of hallucinations in PD patients [87], but not with degree of motor impairment [86,87]. PD patients also have disrupted cortisol and melatonin regulation [72,88–90], suggesting a generalized circadian dysfunction beyond sleep-wake dysregulation. Consistent with this, peripheral (leukocyte) BMAL1 levels are decreased in PD patients [91].
Transgenic mouse models of PD have been employed to investigate the relationship between PD and sleep pathology. Alpha-synuclein overexpressing (ASO) transgenic mice have circadian deficits evidenced by fragmented and reduced locomotor activity that progress with age. SCN neuron firing is dampened in these mice, suggesting a weakened circadian output [92]. Another transgenic mouse line, MitoPark, exhibits circadian rhythms that are much more vulnerable to environmental insult, with profound disruption of locomotor rhythms in a constant darkness or constant light exposure paradigm [93].
Observational studies following patients with known RBD demonstrate that a large majority go on to develop PD or a related disorder such as dementia with Lewy Bodies [94,95]. This suggests that RBD can be seen as a prodromal phase of PD, with a mean interval of ~14 years (range 5-29 years) between RBD and clinical PD onset. This temporal delay between sleep pathology and clinical PD onset is not well understood, but may relate to findings from postmortem pathologic analysis, which suggests that PD neurodegenerative disease progresses often undetected over many years through multiple presymptomatic phases until becoming clinically apparent [96]. Interestingly, the disease progression is often topographically predictable: pathology first begins in the brainstem and olfactory system, then progresses to include the substantia nigra, and finally involving the neocortex [96]. Consistent with this, sleep disorders such as excessive daytime somnolence [97] and RBD [94] often predate onset of the canonical motor symptoms of PD.
Huntington's Disease
Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder involving problems with mood and cognition, as well as general loss of coordination and abnormal involuntary writhing movements termed chorea. The disease is caused by expansion of a CAG triplet repeat within the Huntingtin gene, which results in a different form of the Huntingtin protein. This aberrant protein damages cells in the brain through mechanisms that, although not fully understood, include the creation of protein aggregates that form inclusion bodies within cells.
Patients with HD have progressive disordered sleep, with reduced sleep efficiency and total sleep duration [98,99]. Symptoms may include insomnia, RBD, advanced sleep phase, and reduced REM sleep [100]. Asymptomatic abnormalities in sleep architecture are detectable even early in the disease process [101]. Transgenic HD model mice (R6/2 mice) also exhibit sleep disturbances, corresponding with dysregulated expression of mPer2 and mBmal1 in the SCN [102]. Interestingly, the SCN from these mice demonstrates normal circadian gene expression and electrophysiological output in vitro, suggesting that the abnormality is due to disrupted SCN afferents, as opposed to an intrinsic SCN disruption [103]. Indeed, these mice have reduced VIP expression in the SCN, despite preserved total SCN cell count [104]. Human patients with HD show significant loss of vasopressin, oxytocin, and hypocretin neurons [105,106].
What are the causal links between circadian dysfunction and neurodegenerative disease?
Neurodegenerative diseases correlate clinically with disruption of sleep and circadian rhythms, but correlation does not prove causation, nor does it inform the direction of causation. Does neurodegenerative disease cause sleep and circadian dysfunction, or does sleep and circadian dysfunction promote neurodegeneration? Or, since these possibilities are not mutually exclusive, are both true? Answering these questions requires posing a number of additional ones: Does circadian dysfunction make the brain more vulnerable to neurodegenerative disease processes, for instance via oxidative stress or via dysregulation of metabolism? Is sleep protective against neurodegeneration and, conversely, does lack of sleep potentiate neurodegeneration?
Circadian dysfunction leads to neuronal damage: mechanical insights
Do circadian genes contribute to the onset and progression of neurodegeneration? One link is found in Presenilin-2, a protein that is involved in cleaving of amyloid precursor protein and mutations in which are a major cause of autosomal dominant hereditary cases of AD [107]. The Presenilin-2 gene contains several upstream E-boxes, and is under circadian control, with direct gene activation by both CLOCK and BMAL1 [108]. Evidence from Drosophila suggests another link: SPAGHETTI (SPAG) is a regulator of the circadian kinase DOUBLETIME (DBT) and affects aggregation of Huntingtin [109]. SPAG protects DBT from degradation, stabilizing its expression [110]. Reduction of either DBT or SPAG activates the caspase DRONC, and leads to DRONG-dependent Tau cleavage with increased Tau toxicity and neurodegeneration.
More generally, oxidative stress is hypothesized to be a feature shared across neurodegenerative disorders, and may contribute to protein misfolding and aggregation. Multiple studies link circadian disruption, aging, and oxidative stress. Drosophila with a null mutation in the period gene showed less resilience to oxidative stress, with increased oxidative damage and increased mortality [111]. These flies also had increased neuronal degeneration relative to age-matched controls, perhaps as a result of impaired stress defense. Double mutant flies containing both the period null mutant and a mutation in sniffer, which on its own leads to an oxidative stress and neurodegenerative phenotype, displayed accelerated neuronal degeneration and reduced lifespans [112]. In another Drosophila study, the breakdown of sleep:wake cycles seen with aging was also seen with increased oxidative stress, suggesting that age-associated accumulation of oxidative damage may contribute to sleep:wake cycle disruption [56].
Several studies have investigated aging and oxidative stress in Bmal1−/− mice. These mice have a premature aging phenotype, including accelerated reduction of bone and muscle mass, less subcutaneous fat, decreased hair growth, development of cataracts, and significantly reduced lifespan [113]. Reactive oxygen species (ROS) accumulation was significantly higher in Bmal1−/−mice, and correlated with age-dependent pathology in specific tissues. Therefore the authors concluded that the mechanism of the early aging in Bmal1−/− mice is their increased stress response. In support of this conclusion, the same authors later showed that supplementing these Bmal1−/− mice with the antioxidant N-acetyl-L-cysteine led to a partial rescue of their decreased lifespan and age-dependent pathology [114]. Musiek and colleagues found increased astrocytosis in Bmal1−/− mice relative to age-matched controls [115]. Bmal1−/− mice also showed increased markers of neuronal oxidative damage. Since mice with brain specific Bmal1 deletion (NestinCre+;Bmal1f/f mice) also had increased astrocytosis despite grossly normal locomotor circadian rhythms and sleep:wake cycling, the authors concluded that the brain phenotype is due to increased oxidative damage as opposed to sleep:wake disturbance or systemic circadian mechanisms [115]. Given that BMAL1 is a transcriptional activator, loss of it could produce phenotypes due to downregulation of target genes as opposed to loss of their cycling. Thus, it is unclear if phenotypes of Bmal1−/− mice can be attributed to disrupted circadian function.
Metabolic changes may mediate effects of circadian/sleep disruption on neurodegenerative disorders
Metabolic disruption is correlated with neurodegenerative disorders, in particular AD. Clinically, insulin resistance is associated with increased risk of developing AD [116], and childhood obesity is not only a risk factor for diabetes, it may also increase the risk of developing cognitive impairment at later life stages [117]. Further evidence comes from studies of apolipoprotein E (APOE), an important regulator of lipid metabolism expressed mainly in brain astrocytes and liver. The APOE ε4 allele impairs mitochondrial function [118], which may contribute to insulin resistance and metabolic defects [119], and is also a major risk factor for AD [120]. Young APOE ε4 carriers have dyslipidemia and reduced glucose metabolism in the same brain areas as older subjects with AD. Taken together, these data suggest that peripheral metabolic dysfunction contributes to the development of AD-associated neuropathology.
Most metabolic activity is under circadian control, and loss of circadian clocks has been associated with cellular and system-wide deficits in metabolism [121,122]. Sleep loss also has profound effects on metabolism [123–125], which include an increase in markers of insulin resistance. Given these findings, it is tempting to speculate that circadian/sleep disruption confer susceptibility to AD through their regulation of metabolism.
Sleep promotes clearance of metabolites from the brain
Multiple studies have shown that β-amyloid levels in the brain have a diurnal variation. A study trending hourly measurements of CSF β-amyloid levels in human subjects revealed significant circadian patterns, with β-amyloid concentrations correlating inversely with the amount of sleep [126]. In a rodent model, interstitial fluid levels of β-amyloid in the brain also fluctuate with the normal sleep-wake cycle [127]. The diurnal variation of β-amyloid levels suggests that β-amyloid may be cleared from the brain during sleep. Consistent with this, sleep deprivation leads to significantly elevated interstitial fluid levels of β-amyloid [128,129] and increased plaque formation [128]. Conversely, enhancing sleep reduces β-amyloid deposition [129].
Recent breakthrough work by Xie and colleagues may provide mechanistic insight into how β-amyloid is cleared from the brain's interstitial space during sleep [130]. Using in vivo two-photon imaging in mice, they demonstrate that sleep is associated with improved efficiency of the so-called glymphatic system (a waste clearance pathway), with significant (>60%) increase in the volume of the interstitial space, leading to increased exchange of CSF with interstitial fluid and more efficient clearance of β-amyloid [130].
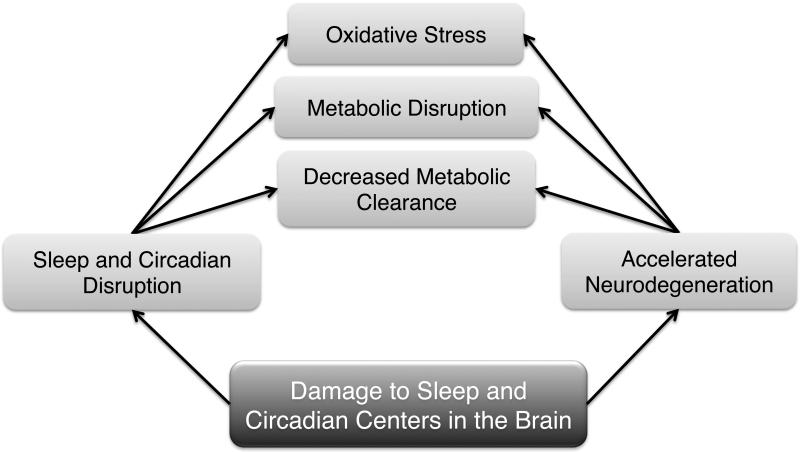
Across humans, rodents and Drosophila, plaque formation leads to reduced and fragmented sleep [65,127,129]. This suggests a positive feedback loop between sleep disruption and β-amyloid deposition, which could play a key role in the pathogenesis of AD (Figure 3). It will be important to investigate whether there is a similar process linking other aggregating proteins (e.g. α-synuclein and Huntingtin) to sleep pathology.
Figure 3. Possible causal links between neurodegenerative disorders and disruptions in sleep and circadian rhythms.
Sleep and circadian disruption may lead to oxidative damage, metabolic disruption, and decreased clearance of metabolites such as β-amyloid. These may accelerate neurodegeneration. Neurodegeneration may also impact brain centers that control sleep and circadian behavior.
Concluding Remarks and Future directions
It is clear that circadian and sleep dysregulation constitute a significant part of the phenotype associated with several neurodegenerative disorders, perhaps more so with some (e.g. PD) than with others. While mechanisms linking the two processes still need to be elucidated, recent studies have implicated specific proteins. Going forward, it will be important to dissect the relevant molecular and cellular pathways and also to establish causality, i.e. do circadian/sleep disruptions indeed increase susceptibility to neurodegeneration, and vice versa (see also Outstanding Questions). However, in the meantime, it is worth considering the circadian and sleep phenotypes for diagnostic and therapeutic purposes.
Sleep and circadian regulation as a therapeutic tool
Regulation of sleep and circadian behavior in patients with neurodegenerative disease is a promising therapeutic strategy that warrants further attention. These therapies are notably low-cost, non-invasive and with relatively low side-effect profiles. In pilot studies, bright light therapy to strengthen circadian rhythms shows small but significant therapeutic benefit in PD patients [131,132]. Pharmaceutical and non-pharmaceutical therapies to treat sleep disturbances in patients with AD have shown promise [133,134]. Among patients with the apoE (APOE) ε4 allele, a known risk factor for developing AD, those with better sleep consolidation had attenuated effects of genotype on developing AD, cognitive decline, and on neurofibrillary tangle pathology on autopsy [135]. Pharmacological imposition of sleep in transgenic HD mice reversed the SCN clock gene dysregulation and slowed cognitive decline based on behavioral testing [103].
Genetic investigation into the relationship between sleep and neurodegeneration using human narcolepsy
Narcolepsy is a sleep disorder characterized by excessive daytime sleepiness and by episodes of cataplexy (motor paralysis). While most cases are sporadic, some cases are familial, enabling linkage analysis [136]. Narcolepsy is thought to be caused by a loss of hypocretin-producing neurons [137], with some data supporting a broader neurodegenerative process underlying symptom onset [138]. Human narcolepsy therefore poses an opportunity to extend genetic investigation into the relationship between sleep and neurodegeneration.
Focus on sleep hygiene as a public health issue
If promoting sleep and circadian rhythms is therapeutic, then it stands to reason that the converse may also be true, and disruption of sleep and circadian rhythms may have detrimental effects on health. Indeed, hamsters subjected to chronic phase advances of the light:dark cycle (i.e. experimental jet lag) had impaired performance on memory tasks, and were observed to have reduced hippocampal neurogenesis [139]. In humans, studies of airline employees show that chronic jet lag is associated with impaired performance on memory tasks and with temporal lobe atrophy [140,141]. If sleep disruption is re-conceptualized as a public health hazard, then multiple policies -- ranging from noise and light pollution to irregular shift schedules for low-wage workers -- would be implicated.
Sleep and circadian dysregulation as a screening tool to identify at-risk populations
A major obstacle in treatment of neurodegenerative disease is that the disease is generally at a significantly advanced stage by the time it is diagnosed. Early (or “prodromal”) sleep manifestations of neurodegenerative disease present a tantalizing possibility for earlier detection, and potentially more effective therapeutic intervention. Among otherwise asymptomatic elderly adults, those with high sleep fragmentation or decreased circadian activity have increased risk of going on to develop cognitive decline or dementia [142,143]. Excessive daytime sleepiness often predates the onset of motor symptoms in PD [97]. Patients with RBD may be a useful population for research in treatments to modify PD progression, and are a patient population to monitor carefully for developing symptoms of PD.
As our knowledge of sleep and circadian disruption in early disease states becomes more sophisticated, and as we develop therapeutic strategies to prevent progression of early disease, screening patients for sleep and circadian disruption -- just as we now routinely screen for diabetes, hypertension, or hyperlipidemia -- will become an increasingly important part of primary care.
Outstanding questions.
To what extent is there a causal relationship between sleep/circadian disruption and neurodegenerative disorders?
Do effects of circadian and sleep dysfunction on metabolism confer susceptibility to neurodegenerative disorders?
Can symptoms of neurodegenerative disorders be alleviated by improving sleep cycles?
Trends.
Sleep and circadian dysfunction are increasingly being associated with neurodegenerative disorders. Earlier studies with human subjects are now supported by findings in genetic models.
Circadian and sleep circuits are damaged in disorders like Huntington's and Parkinson's Disease, and disrupted sleep/circadian rhythms may exacerbate disease pathology. Thus, these two processes may feed back on each other.
Metabolic factors may provide a mechanistic link between circadian rhythms, sleep and neurodegenerative disorders
Sleep and circadian dysregulation could provide a screening tool to identify populations at risk for neurodegenerative disorders
Acknowledgements
AS is a recipient of an Ellison Senior Scholar Award in Aging and an Investigator of the HHMI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyata S, et al. Poor sleep quality impairs cognitive performance in older adults. J. Sleep Res. 2013;22:535–541. doi: 10.1111/jsr.12054. [DOI] [PubMed] [Google Scholar]
- 2.Goel N, et al. Circadian Rhythms, Sleep Deprivation, and Human Performance. 1st edn. 119Elsevier Inc.; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goel N, et al. Neurocognitive Consequences of Sleep Deprivation. Semin. Neurol. 2009;29:320–339. doi: 10.1055/s-0029-1237117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim J, Dinges DF. Sleep Deprivation and Vigilant Attention. Ann. N. Y. Acad. Sci. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 5.Hardin PE, Panda S. Circadian timekeeping and output mechanisms in animals. Curr. Opin. Neurobiol. 2013;23:724–731. doi: 10.1016/j.conb.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Sehgal A. Speed control: Cogs and gears that drive the circadian clock. Trends Neurosci. 2012;35:574–585. doi: 10.1016/j.tins.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Honma S, et al. Suprachiasmatic nucleus: cellular clocks and networks. 1st edn. 199Elsevier B.V.; 2012. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki S, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science (80−. ) 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 10.Lehman MN, et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J. Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ralph MR, et al. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 12.Kolker DE, et al. Aging Alters Circadian and Light-Induced Expression of Clock Genes in Golden Hamsters. J. Biol. Rhythms. 2003;18:159–169. doi: 10.1177/0748730403251802. [DOI] [PubMed] [Google Scholar]
- 13.Wyse C. a., Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
- 14.Chang HC, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153:1448–1460. doi: 10.1016/j.cell.2013.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai M, et al. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J. Neurosci. Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- 16.Davidson AJ, et al. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol. Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki S, et al. Effects of aging on central and peripheral mammalian clocks. Proc. Natl. Acad. Sci. U. S. A. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs JE, et al. The nuclear receptor REV-ERB α mediates circadian regulation of innate immunity through selective regulation of in fl ammatory cytokines. Pnas. 2011;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morioka N, et al. Stimulation of nuclear receptor REV-ERBs regulates tumor necrosis factor-induced expression of proinflammatory molecules in C6 astroglial cells. Biochem. Biophys. Res. Commun. 2016;469:151–157. doi: 10.1016/j.bbrc.2015.11.086. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, et al. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014;192:407–17. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 21.Duez H, Staels B. Rev-erbα gives a time cue to metabolism. FEBS Lett. 2008;582:19–25. doi: 10.1016/j.febslet.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Roozendaal B, et al. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- 23.Nygård M, et al. Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Res. Bull. 2005;65:149–154. doi: 10.1016/j.brainresbull.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Farajnia S, et al. Evidence for Neuronal Desynchrony in the Aged Suprachiasmatic Nucleus Clock. J. Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura TJ, et al. Age-related decline in circadian output. J. Neurosci. 2011;31:10201–10205. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo W, et al. Old flies have a robust central oscillator but weaker behavioral rhythms that can be improved by genetic and environmental manipulations. Aging Cell. 2012;11:428–438. doi: 10.1111/j.1474-9726.2012.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katewa SD, et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab. 2015 doi: 10.1016/j.cmet.2015.10.014. DOI: 10.1016/j.cmet.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherin JE, et al. Activation of ventrolateral preoptic neurons during sleep. Science (80−. ) 1996;271:216–219. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 29.Lu J, et al. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J. Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MG, et al. Discharge of Identified Orexin/Hypocretin Neurons across the Sleep-Waking Cycle. J. Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamantidis AR, et al. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carter ME, et al. Sleep Homeostasis Modulates Hypocretin-Mediated Sleep-to-Wake Transitions. J. Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carter ME, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, et al. A putative flip–flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- 37.Weber F, et al. Control of REM sleep by ventral medulla GABAergic neurons. Nature. 2015 doi: 10.1038/nature14979. DOI: 10.1038/nature14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saper CB, et al. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 39.Abrahamson EE, et al. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 40.Aston-Jones G, et al. A neural circuit for circadian regulation of arousal. Nat. Neurosci. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 41.Novak CM, Nunez A. a. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuroreport. 2000;11:93–96. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- 42.Chou TC, et al. Afferents to the ventrolateral preoptic nucleus. J. Neurosci. 2002;22:977–990. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson RH, Swanson LW. Organization of inputs to the dorsomedial nucleus of the hypothalamus: A reexamination with Fluorogold and PHAL in the rat. Brain Res. Rev. 1998;27:89–118. doi: 10.1016/s0165-0173(98)00010-1. [DOI] [PubMed] [Google Scholar]
- 44.Watts a G., Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- 45.Chou TC, et al. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J. Neurosci. 2003;23:10691–702. doi: 10.1523/JNEUROSCI.23-33-10691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Redline S, et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 47.Moraes W, et al. Effects of aging on sleep structure throughout adulthood: A population-based study. Sleep Med. 2014;15:401–409. doi: 10.1016/j.sleep.2013.11.791. [DOI] [PubMed] [Google Scholar]
- 48.Huang YL, et al. Age-associated difference in circadian sleep-wake and rest-activity rhythms. Physiol. Behav. 2002;76:597–603. doi: 10.1016/s0031-9384(02)00733-3. [DOI] [PubMed] [Google Scholar]
- 49.Carskadon M. a., et al. Sleep fragmentation in the elderly: Relationship to daytime sleep tendency. Neurobiol. Aging. 1982;3:321–327. doi: 10.1016/0197-4580(82)90020-3. [DOI] [PubMed] [Google Scholar]
- 50.Zhdanova IV, et al. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J. Biol. Rhythms. 2011;26:149–159. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]
- 51.Valentinuzzi VS, et al. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am. J. Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- 52.Van Gool W. a, et al. Age-related changes in circadian sleep-wakefulness rhythms in male rats isolated from time cues. Brain Res. 1987;413:384–387. doi: 10.1016/0006-8993(87)91034-1. [DOI] [PubMed] [Google Scholar]
- 53.Naidoo N, et al. Endoplasmic reticulum stress in wake-active neurons progresses with aging. Aging Cell. 2011;10:640–649. doi: 10.1111/j.1474-9726.2011.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiromani PJ, et al. Compensatory sleep response to. 12 h wakefulness in young and old rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R125–133. doi: 10.1152/ajpregu.2000.278.1.R125. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, et al. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, Fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 56.Koh K, et al. A Drosophila model for age-associated changes in sleep:wake cycles. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawai N, et al. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci. Lett. 2010;468:51–55. doi: 10.1016/j.neulet.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 58.Brownell SE, Conti B. Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci. Lett. 2010;472:29–32. doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Downs JL, et al. Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol. Aging. 2007;28:1286–1295. doi: 10.1016/j.neurobiolaging.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 60.Hunt NJ, et al. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol. Aging. 2015;36:292–300. doi: 10.1016/j.neurobiolaging.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 61.Terao A, et al. Age-related decline in hypocretin (orexin) receptor. 2 messenger RNA levels in the mouse brain. Neurosci. Lett. 2002;332:190–194. doi: 10.1016/s0304-3940(02)00953-9. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J-H, et al. Age-related changes in hypocretin (orexin) immunoreactivity in the cat brainstem. Brain Res. 2002;930:206–211. doi: 10.1016/s0006-8993(02)02240-0. [DOI] [PubMed] [Google Scholar]
- 63.Bloom GS. Amyloid-β and Tau. JAMA Neurol. 2014;71:505. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 64.Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging. 1995;16:271–278. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 65.Ju Y-ES, et al. Sleep quality and preclinical Alzheimer disease. JAMA Neurol. 2013;70:587–93. doi: 10.1001/jamaneurol.2013.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Satlin a, et al. Circadian locomotor-activity and core-body temperature rhythms in Alzheimers-disease. Neurobiol. Aging. 1995;16:765–771. doi: 10.1016/0197-4580(95)00059-n. [DOI] [PubMed] [Google Scholar]
- 67.Van Someren EJW, et al. Circadian rest-activity rhythm disturbances in Alzheimer's disease. Biol. Psychiatry. 1996;40:259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, et al. Daytime Sleepiness and Functional Impairment in Alzheimer Disease. Am. J. Geriatr. Psychiatry. 2007;15:620–626. doi: 10.1097/JGP.0b013e3180381521. [DOI] [PubMed] [Google Scholar]
- 69.Merlino G, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Med. 2010;11:372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 70.Lee JH, et al. Twenty-Four-Hour Rhythms of Sleep-Wake Cycle and Temperature in Alzheimer ’ s Disease. J. Neuropsychiatr. 2004;6:192–198. doi: 10.1176/jnp.16.2.192. [DOI] [PubMed] [Google Scholar]
- 71.Hatfield CF, et al. Disrupted daily activity/rest cycles in relation to daily cortisol rhythms of home-dwelling patients with early Alzheimer's dementia. Brain. 2004;127:1061–1074. doi: 10.1093/brain/awh129. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann A, et al. Twenty-Four Hour Cortisol Release Profiles in Patients With Alzheimer's and Parkinson's Disease Compared to Normal Controls: Ultradian Secretory Pulsatility and Diurnal Variation. Neurobiol. Aging. 1997;18:285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 73.Cermakian N, et al. Circadian clock gene expression in brain regions of Alzheimer 's disease patients and control subjects. J. Biol. Rhythms. 2011;26:160–170. doi: 10.1177/0748730410395732. [DOI] [PubMed] [Google Scholar]
- 74.Stopa EG, et al. Pathologic evaluation of the human suprachiasmatic nucleus in severe dementia. Journal of neuropathology and experimental neurology. 1999;58:29–39. doi: 10.1097/00005072-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Zhou JN, et al. VIP neurons in the human SCN in relation to sex, age, and Alzheimers-disease. Neurobiol. Aging. 1995;16:571–576. doi: 10.1016/0197-4580(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 76.Swaab DF, et al. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342:37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- 77.Wang JL, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann. Neurol. 2015;78:317–322. doi: 10.1002/ana.24432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen K-F, et al. The central molecular clock is robust in the face of behavioural arrhythmia in a Drosophila model of Alzheimer's disease. Dis. Model. Mech. 2014;7:445–458. doi: 10.1242/dmm.014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saper CB, German DC. Hypothalamic pathology in Alzheimer's disease. Neurosci. Lett. 1987;74:364–370. doi: 10.1016/0304-3940(87)90325-9. [DOI] [PubMed] [Google Scholar]
- 80.Schulz-Schaeffer WJ. The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stern AL, Naidoo N. Wake-active neurons across aging and neurodegeneration: a potential role for sleep disturbances in promoting disease. Springerplus. 2015;4 doi: 10.1186/s40064-014-0777-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fronczek R, et al. Hypocretin (orexin) loss in Parkinson's disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 83.Thannickal TC, et al. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Barone P, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov. Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 85.Chaudhuri KR, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: The NMSQuest study. Mov. Disord. 2006;21:916–923. doi: 10.1002/mds.20844. [DOI] [PubMed] [Google Scholar]
- 86.Diederich N, et al. Progressive sleep “destructuring” in Parkinson's disease. A polysomnographic study in. 46 patients. Sleep Med. 2005;6:313–318. doi: 10.1016/j.sleep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 87.Whitehead DL, et al. Circadian rest-activity rhythm is altered in Parkinson's disease patients with hallucinations. Mov. Disord. 2008;23:1137–1145. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- 88.Breen DP, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–95. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bordet R, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin. Neuropharmacol. 2003;26:65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Videnovic A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson's disease. JAMA Neurol. 2014;71:463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cai Y, et al. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson's disease. Eur. J. Neurol. 2010;17:550–554. doi: 10.1111/j.1468-1331.2009.02848.x. [DOI] [PubMed] [Google Scholar]
- 92.Kudo T, et al. Circadian dysfunction in a mouse model of Parkinson's disease. Exp. Neurol. 2011;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 93.Fifel K, Cooper HM. Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson's disease. Neurobiol. Dis. 2014;71:359–369. doi: 10.1016/j.nbd.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 94.Iranzo A, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 95.Schenck CH, et al. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 96.Braak H, et al. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 97.Abbott RD, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 98.Goodman a. O.G., Barker R. a. How vital is sleep in Huntington's disease? J. Neurol. 2010;257:882–897. doi: 10.1007/s00415-010-5517-4. [DOI] [PubMed] [Google Scholar]
- 99.Aziz NA, et al. Sleep and circadian rhythm alterations correlate with depression and cognitive impairment in Huntington's disease. Parkinsonism Relat. Disord. 2010;16:345–350. doi: 10.1016/j.parkreldis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 100.Arnulf I, et al. Rapid Eye Movement Sleep Disturbances in Huntington Disease. Arch. Neurol. 2008;65:482–488. doi: 10.1001/archneur.65.4.482. [DOI] [PubMed] [Google Scholar]
- 101.Goodman AOG, et al. Asymptomatic sleep abnormalities are a common early feature in patients with Huntington's disease. Curr. Neurol. Neurosci. Rep. 2011;11:211–217. doi: 10.1007/s11910-010-0163-x. [DOI] [PubMed] [Google Scholar]
- 102.Morton a J., et al. Disintegration of the sleep-wake cycle and circadian timing in Huntington's disease. J. Neurosci. 2005;25:157–163. doi: 10.1523/JNEUROSCI.3842-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pallier PN, et al. Pharmacological Imposition of Sleep Slows Cognitive Decline and Reverses Dysregulation of Circadian Gene Expression in a Transgenic Mouse Model of Huntington's Disease. J. Neurosci. 2007;27:7869–7878. doi: 10.1523/JNEUROSCI.0649-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fahrenkrug J, et al. Decreased VIP and VPAC2 Receptor Expression in the Biological Clock of the R6/2 Huntington's Disease Mouse. J. Mol. Neurosci. 2007;31:139–148. doi: 10.1385/jmn/31:02:139. [DOI] [PubMed] [Google Scholar]
- 105.Gabery S, et al. Changes in key hypothalamic neuropeptide populations in Huntington disease revealed by neuropathological analyses. Acta Neuropathol. 2010;120:777–788. doi: 10.1007/s00401-010-0742-6. [DOI] [PubMed] [Google Scholar]
- 106.Aziz A, et al. Hypocretin and Melanin-Concentrating Hormone in Patients with Huntington Disease. Brain Pathol. 2008;18:474–483. doi: 10.1111/j.1750-3639.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levy-lahad AE, et al. Candidate Gene for the Chromosome. 1 Familial Alzheimer's Disease Locus. Science (80−. ) 2015;269 doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 108.Bélanger V, et al. The circadian regulation of Presenilin-2 gene expression. Chronobiol. Int. 2006;23:747–766. doi: 10.1080/07420520600827087. [DOI] [PubMed] [Google Scholar]
- 109.Zhang S, et al. A genomewide RNA interference screen for modifiers of aggregates formation by mutant huntingtin in drosophila. Genetics. 2010;184:1165–1179. doi: 10.1534/genetics.109.112516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Means JC, et al. Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy. PLOS Genet. 2015;11:e1005171. doi: 10.1371/journal.pgen.1005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krishnan N, et al. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging (Albany. NY) 2009;1:937–948. doi: 10.18632/aging.100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krishnan N, et al. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012;45:1129–35. doi: 10.1016/j.nbd.2011.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kondratov RV, et al. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kondratov RV, et al. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging (Albany. NY) 2009;1:979–987. doi: 10.18632/aging.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Musiek ES, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Willette A. a., et al. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle–Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;53792:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luciano R, et al. Biomarkers of Alzheimer Disease, Insulin Resistance, and Obesity in Childhood. Pediatrics. 2015;135:1074–1081. doi: 10.1542/peds.2014-2391. [DOI] [PubMed] [Google Scholar]
- 118.Huang Y, Mahley RW. Apolipoprotein E: Structure and function in lipid metabolism, neurobiology, and Alzheimer's diseases. Neurobiol. Dis. 2014;72:3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Szendroedi J, et al. The role of mitochondria in insulin resistance and type. 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011;8:92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 120.Lane-Donovan C, et al. More than Cholesterol Transporters: Lipoprotein Receptors in CNS Function and Neurodegeneration. Neuron. 2014;83:771–787. doi: 10.1016/j.neuron.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J. Intern. Med. 2015;277:513–527. doi: 10.1111/joim.12347. [DOI] [PubMed] [Google Scholar]
- 122.Maury E, et al. Circadian disruption in the pathogenesis of metabolic syndrome. Diabetes Metab. 2014;40:338–346. doi: 10.1016/j.diabet.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 123.Weljie AM, et al. Oxalic acid and diacylglycerol 36:3 are cross-species markers of sleep debt. Proc. Natl. Acad. Sci. 2015;112:2569–2574. doi: 10.1073/pnas.1417432112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davies SK, et al. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. 2014;111:10761–10766. doi: 10.1073/pnas.1402663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Taheri S, et al. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang Y, et al. Effects of Age and Amyloid Deposition on A Dynamics in the Human Central Nervous System. Arch. Neurol. 2012;69:51–58. doi: 10.1001/archneurol.2011.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Roh JH, et al. Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of - Amyloid in Mice with Alzheimer's Disease Pathology. Sci. Transl. Med. 2012;4:150ra122–150ra122. doi: 10.1126/scitranslmed.3004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kang J-E, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (80-. ) 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tabuchi M, et al. Sleep Interacts with Ab to Modulate Intrinsic Neuronal Excitability Article Sleep Interacts with Ab to Modulate Intrinsic Neuronal Excitability. Curr. Biol. 2015;25:1–11. doi: 10.1016/j.cub.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science (80-. ) 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rutten S, et al. Bright Light Therapy in Parkinson's Disease: An Overview of the Background and Evidence. Parkinsons. Dis. 2012;2012:1–9. doi: 10.1155/2012/767105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Paus S, et al. Bright light therapy in Parkinson's disease: A pilot study. Mov. Disord. 2007;22:1495–1498. doi: 10.1002/mds.21542. [DOI] [PubMed] [Google Scholar]
- 133.Wu Y-H, Swaab DF. Disturbance and strategies for reactivation of the circadian rhythm system in aging and Alzheimer's disease. Sleep Med. 2007;8:623–636. doi: 10.1016/j.sleep.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 134.Peter-Derex L, et al. Sleep and Alzheimer's disease. Sleep Med. Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 135.Lim ASP, et al. Sleep modifies the relationship of APOE to the risk of Alzheimer disease and neurofibrillary tangle pathology. JAMA Neurol. 2013;70:1544–51. doi: 10.1001/jamaneurol.2013.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hor H, et al. A missense mutation in myelin oligodendrocyte glycoprotein as a cause of familial narcolepsy with cataplexy. Am. J. Hum. Genet. 2011;89:474–479. doi: 10.1016/j.ajhg.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Crocker a., et al. Concomitant loss of dynorphin, NARP, and orexin in narcolepsy. Neurology. 2005;65:1184–1188. doi: 10.1212/01.wnl.0000168173.71940.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siegel JM, et al. Neuronal degeneration in canine narcolepsy. J. Neurosci. 1999;19:248–257. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gibson EM, et al. Experimental “Jet Lag” Inhibits Adult Neurogenesis and Produces Long-Term Cognitive Deficits in Female Hamsters. PLoS One. 2010;5:e15267. doi: 10.1371/journal.pone.0015267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cho K, et al. Chronic jet lag produces cognitive deficits. J. Neurosci. 2000;20:RC66. doi: 10.1523/JNEUROSCI.20-06-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cho K. Chronic “jet lag” produces temporal lobe atrophy and spatial cognitive deficits. Nat.Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 142.Lim ASP, et al. Sleep Fragmentation and the Risk of Incident Alzheimer's Disease and Cognitive Decline in Older Persons. Sleep. 2013;36:1027–1032. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tranah GJ, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann. Neurol. 2011;70:722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]