Abstract
Staphylococcus aureus is a bacterial pathogen that frequently infects the skin, causing lesions and cell destruction through its primary virulence factor, alpha-toxin. Here we show that interferon gamma (IFN-γ) protects human keratinocytes from cell death induced by staphylococcal alpha toxin. We find that IFN-γ prevents alpha toxin binding and reduces expression of the alpha toxin receptor, ADAM10. We determine that the mechanism for IFN-γ mediated resistance to alpha toxin involves the induction of autophagy, a process of cellular adaptation to sublethal damage. We find that IFN-γ potently stimulates activation of the primary autophagy effector, LC3. This process is dependent upon up-regulation of Apolipoprotein L1 (ApoL1). Depletion of ApoL1 by siRNA significantly increases alpha toxin induced lethality and inhibits activation of LC3. We conclude that IFN-γ plays a significant role to protect human keratinocytes from the lethal effects of staphylococcal alpha toxin through ApoL1 induced autophagy.
INTRODUCTION
Staphylococcus aureus infections are frequently observed in the chronic inflammatory skin disease of atopic dermatitis (AD) (Boguniewicz and Leung, 2011; Leung and Guttman-Yassky, 2014). The major virulence factor produced by S. aureus is alpha toxin (Bubeck Wardenburg et al., 2007), a pore forming toxin that causes cell lysis (Prevost et al., 2001). In AD skin, a number of cytokines are known to be deregulated (Gittler et al., 2012). The T-helper type 2 (Th2) cytokines IL-4 and IL-13 are over-expressed (Gittler et al., 2012; Hamid et al., 1994; Hamid et al., 1996), resulting in increased susceptibility of keratinocytes to alpha toxin induced cell death (Brauweiler et al., 2014). AD patients prone to S. aureus skin infections can also be deficient in IFN-γ production (Machura et al., 2008). However, the role of IFN-γ in protection of primary human keratinocytes from staphylococcal alpha toxin has not been previously explored.
In monocytes, IFN-γ confers protection from damage caused by bacterial pathogens through a process known as autophagy (Gutierrez et al., 2004). During autophagy, endocytosed bacteria and cellular products are engulfed and isolated in a double-membrane vesicle that is subsequently targeted to the lysosome for elimination (Gutierrez et al., 2004; Levine and Kroemer, 2008). However, the role of IFN-γ induced autophagy in protection of keratinocytes from bacterial pathogens is not known. The induction of autophagy involves a series of sequential steps (Levine and Klionsky, 2004) that lead to activation of light chain 3 (LC3) (Kabeya et al., 2000). LC3 undergoes complex C-terminal proteolytic and lipid modifications, which cause it to translocate from the cytosol to the autophagosomal membrane (Kabeya et al., 2000), targeting the contents for destruction (Levine and Kroemer, 2008).
Although potentially important in pathogen resistance, IFN-γ induced genes that regulate autophagy have not been well described. IFN-γ signaling events culminate in activation of the transcription factor, Signal Transducer and Activator of Transcription 1 (STAT1) (Saha et al., 2010). In the current study, we find that STAT1 potently induces expression of Apolipoprotein L1 (ApoL1), a protein known to be involved in autophagy (Wan et al., 2008). We therefore explored the role of ApoL1 in IFN-γ mediated resistance to alpha toxin induced keratinocyte death and determined its role in mediating autophagy.
RESULTS
IFN-γ protects from alpha toxin induced keratinocyte death
Since cytokine expression has been shown to be deregulated in AD skin, we determined the effects of AD associated cytokines on alpha toxin induced cell death. Primary human keratinocytes were treated with media alone or with IFN-γ, IFN-α, IL-4/IL-13, IL-17, IL-22, or IL-25 for 24 hours. Alpha toxin was then added for an additional 24 hours and cell death was determined by lactic dehydrogenase (LDH) release. We found that primary human keratinocytes were highly sensitive to alpha toxin (Fig. 1a). A striking protection from alpha toxin induced cell-death was induced by pre-treatment with IFN-γ (p< 0.001). Modest protection was also observed with IFN-α. Pre-treatment with the cytokines, IL-17, IL-22, and IL-25 had negligible effects on cellular sensitivity to alpha toxin, while the Th2 cytokines, IL-4/IL-13 increased cell death. Therefore, of the panel of cytokines, IFN-γ was most significantly able to protect against alpha toxin induced lethality.
Figure 1. IFN-γ blocks staphylococcal alpha toxin induced keratinocyte death.
(a) Primary human keratinocytes were cultured in the presence of media, IFN-γ, IFN-α, IL-17, IL-22, IL-4/IL-13 or IL-25 for 24 hours. 100 ng/ml of alpha toxin was then added, and cell death was measured by LDH release. (b) Keratinocytes were incubated with the indicated concentrations of IFN-γ for 24 hours and then 100 ng/ml alpha toxin was added or (c) cells were harvested and analyzed for expression of phospho-STAT1. Data (a and b) are mean ± SEM, n = 3. *P<0.05; **P<0.01; ***P<0.001 (as compared to the cells grown in medium without cytokines).
We then evaluated the dose dependence of IFN-γ on protection from alpha toxin. We determined that IFN-γ induced protection was dose dependent (Fig. 1b), with the maximal protection achieved at 25 ng/ml. Since the downstream events induced by IFN-γ are known to be mediated by the transcription factor STAT1, we next determined the relationship between STAT1 activation and extent of IFN-γ protection from the alpha toxin induced cell death. We found that treatment with 25 ng/ml IFN-γ caused maximal phosphorylation of STAT1 and that the degree of IFN-γ induced STAT1 phosphorylation directly correlated with keratinocyte protection from alpha toxin (Fig. 1c).
IFN-γ mediated protection requires STAT1
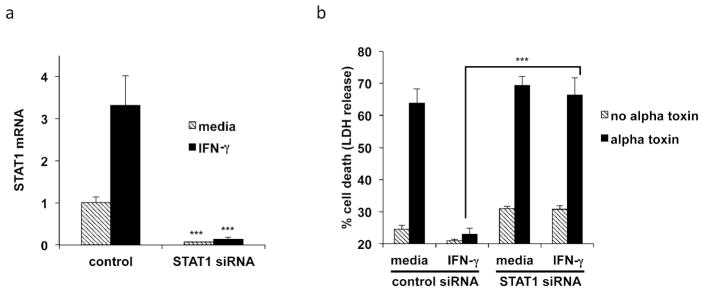
We used siRNA directed against STAT1 to determine its role in IFN-γ induced protection from alpha toxin. STAT1 siRNA transfection significantly inhibited STAT1 mRNA expression in keratinocytes (Fig. 2a). Keratinocytes transfected with control siRNA and treated with IFN-γ were shown to be protected from alpha toxin induced cell death (Fig. 2b). In marked contrast, keratinocytes transfected with STAT1 siRNA and treated with IFN-γ became very sensitive to alpha toxin. The increase in cell death induced in STAT1 knockdown cells was significant (p < 0.001). We concluded that STAT1 is an essential component in IFN-γ mediated protection of keratinocytes from alpha toxin.
Figure 2. IFN-γ mediated protection from alpha toxin is dependent upon STAT1.
(a) Primary keratinocytes were transfected with control (non-targeting) or STAT1 siRNA. Transfected cells were then treated with media or IFN-γ. Gene expression was analyzed by real-time PCR for STAT1. (b) Cells treated as above were subsequently incubated with 100 ng/ml alpha toxin and cell death was measured by LDH release. Data are mean ± SEM, n = 3. ***P<0.001
IFN-γ protects from IL-4/IL-13 mediated increases in alpha toxin induced cell death
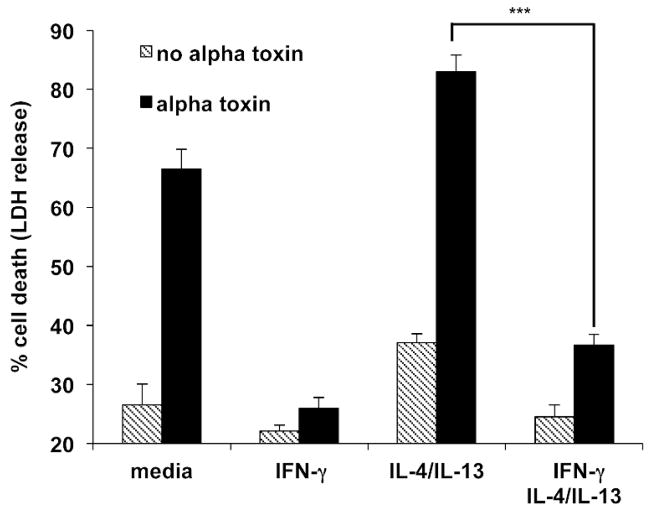
We recently demonstrated that IL-4 and IL-13 enhance keratinocyte sensitivity to alpha toxin (Brauweiler et al., 2014). Also, previous studies have shown interplay between the Th2 cytokines, IL-4/IL-13, and IFN-γ (Dickensheets and Donnelly, 1999; Galizzi et al., 1988). We were therefore interested in determining whether IFN-γ could block the increased alpha toxin-induced cell death mediated by Th2 cytokines. Primary keratinocytes were treated with IL-4/IL-13 alone and in combination with IFN-γ. As shown in Fig. 3, treatment of keratinocytes with IFN-γ decreased alpha toxin induced cell death. The data in Fig. 3 also demonstrate that IFN-γ could reverse IL-4/IL-13 mediated increases in alpha toxin lethality. A statistically significant decrease in cell death (p < 0.001) was observed by co-treatment of IFN-γ with IL-4/IL-13 compared to treatment with IL-4/IL-13 alone.
Figure 3. IFN-γ protects from IL-4/13 mediated increases in alpha toxin induced cell death.
Primary keratinocytes were treated with media, IFN-γ, IL-4/IL-13 or IFN-γ in combination with IL-4/IL-13. 100 ng/ml alpha toxin was then added for 24 hours, and cell death was measured by LDH release. Data are mean ± SEM, n = 3. ***P<0.001
IFN-γ reduces alpha toxin binding and reduces ADAM10 protein levels
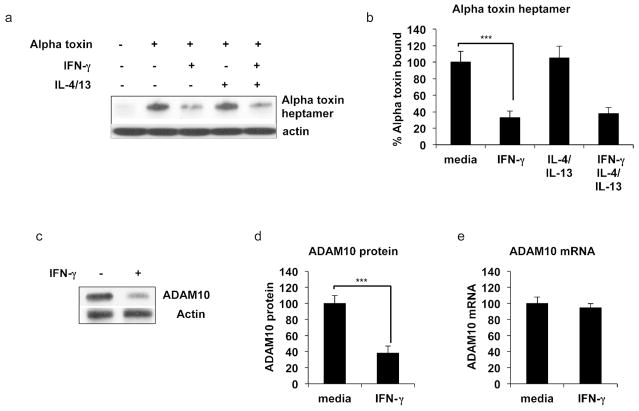
A critical step in alpha toxin induced pore formation is the binding of alpha toxin to the cell surface. After initial attachment, alpha toxin oligerimerizes into a stable heptamer (Song et al., 1996). Since keratinocytes treated with IFN-γ were protected from alpha toxin induced lethality, we thought that it was plausible that IFN-γ treatment reduced alpha toxin binding and heptamer formation. We therefore determined the levels of alpha toxin heptamer bound to keratinocytes that were grown in the presence and absence of IFN-γ. Fig. 4a and b show that cells treated with IFN-γ bound significantly less alpha toxin compared to cells grown in media. In contrast, IL-4/IL-13 treatment had minimal effects on alpha toxin binding (Fig. 4a and b).
Figure 4. IFN-γ reduces alpha toxin binding to keratinocytes and reduces ADAM10 protein levels.
(a) Primary keratinocytes were treated with media, IFN-γ, IL-4/IL-13 or IFN-γ in combination with IL-4/13. 50 ng/ml alpha toxin was then added for 2 hours, and cells were harvested and analyzed by western blot for alpha toxin heptamer formation. (b) Quantitation of alpha toxin binding to keratinocytes under the various cytokine conditions. (c) Cells treated with media or IFN-γ for 24 hours were harvested and analyzed for expression of ADAM10 protein. (d) Quantitation of ADAM10 protein and (e) mRNA levels in the presence and absence of IFN-γ. Data are mean ± SEM, n = 3. ***P<0.001
Since the amount of heptamer bound to the cell depends on the number of alpha toxin receptors on the cell surface, we examined levels of the alpha toxin receptor, a disintegrin and metalloproteinase 10 (ADAM10) (Wilke and Bubeck Wardenburg, 2010). Keratinocytes were treated with media or IFN-γ for 24 hours, and ADAM10 expression was determined. Fig. 4c and d show that IFN-γ reduced the amount of ADAM10 protein. Prior studies have shown that IL-4/IL-13 treatment did not affect levels of ADAM10 (Brauweiler et al., 2014). In fig. 4e, we examined levels of ADAM10 mRNA. We found that mRNA levels of ADAM10 were unchanged by IFN-γ treatment, suggesting that IFN-γ affected ADAM10 protein turnover, a process reflective of autophagy.
IFN-γ augments alpha toxin induced autophagy
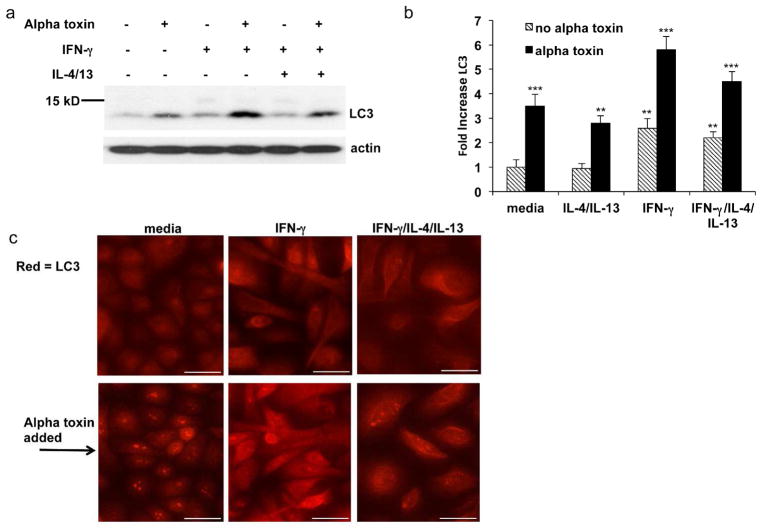
To determine whether IFN-γ induces autophagy in keratinocytes, we assessed LC3 activation. Figs. 5a and b show that alpha toxin itself is a prominent inducer of LC3. LC3 protein levels increased over 3-fold during the 2 hour incubation with alpha toxin. Furthermore, pretreatment with IFN-γ caused further increases in LC3 expression, a process amplified by alpha toxin. We also determined the effects of IL-4/IL-13 treatment on LC3 expression. In contrast to IFN-γ, IL-4/IL-13 did not induce expression of LC3. We found that the increase in LC3 induced by IFN-γ was relatively unaffected by co-treatment with Th2 cytokines (Fig. 5b). In Fig. 5c, we examined localization of LC3 under various cytokine conditions. After 24 hours of cytokine pre-treatment, cells were treated with alpha toxin for 2 hours or remained untreated. Alpha toxin caused a unique re-localization and coalescence of LC3. Notably, LC3 coalescence was not prominent in the cells treated with IFN-γ.
Figure 5. IFN-γ augments alpha toxin induced autophagy by increasing LC3 levels and modulating LC3 localization.
Primary keratinocytes were treated with media or the indicated cytokines for 24 hrs. The indicated cells were then treated with 100 ng/ml alpha toxin for an additional 2 hours. (a) Cells were harvested and levels of the autophagy marker, LC3, were determined by Western blot. (b) Quantitation of LC3 protein levels after pre-treatment with the indicated cytokines followed by further treatment with media or alpha toxin. (c) Immuno-fluorescence microscopy of LC3 protein. Scale bar = 50 μm. Increases in LC3 (b) are mean ± SEM, n = 3. **P<0.01; ***P<0.001 (as compared to the cells grown in medium alone).
IFN-γ protects from alpha toxin induced keratinocyte death through ApoL1 induced autophagy
To further explore the mechanism for IFN-γ mediated autophagy, we examined a published gene array analysis of keratinocytes treated with IFN-γ (Nograles et al., 2008) to determine genes that were both highly induced by IFN-γ and involved in autophagy. ApoL1 has been previously described to play a role in autophagy (Wan et al., 2008), and we observed a 50-fold increase in ApoL1 expression in IFN-γ treated keratinocytes (Fig. 6a). Furthermore, we demonstrated that IFN-γ mediated induction of ApoL1 was STAT1 dependent, as STAT1 siRNA prevented induction of ApoL1 mRNA by IFN-γ (Fig. 6b), (p < 0.001). We also examined the effect of Th2 cytokines on IFN-γ induced ApoL1 expression and found that ApoL1 induction was not significantly affected by co-treatment of IFN-γ with Th2 cytokines (supplemental fig 1).
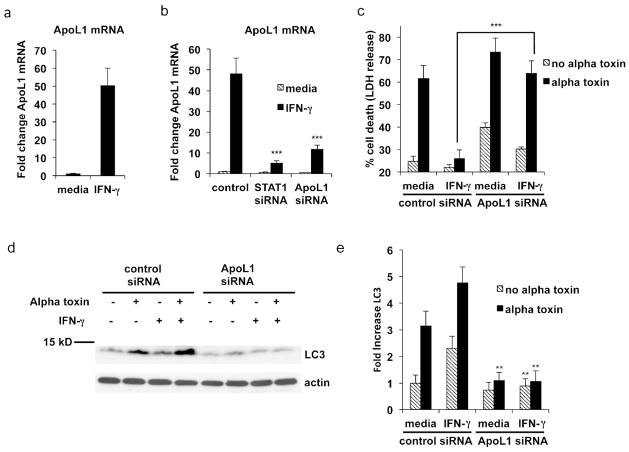
Figure 6. IFN-γ induced ApoL1 mediates autophagy and protects from alpha toxin induced cell death.
(a) Primary keratinocytes were treated with media or IFN-γ for 24 hours. ApoL1 gene expression was analyzed by RT-PCR. (b) Primary keratinocytes transfected with control, STAT1 or ApoL1 siRNA were treated with media or IFN-γ for 24 hours. ApoL1 gene expression was analyzed by RT-PCR. (c) Cells transfected with control or ApoL1 siRNA and treated with IFN-γ as described above were subsequently treated with 100ng/ml alpha toxin for 24 hours. Cell death was measured by LDH release. (d) ApoL1 or control transfected cells were treated with media or IFN-γ as indicated and were subsequently treated with alpha toxin for 2 hours. Cells were lysed and LC3 protein expression was analyzed. (e) LC3 protein level quantitation. Data are mean ± SEM, n = 3. **P<0.01; ***P<0.001 (as compared to control siRNA).
To determine whether ApoL1 plays a role in mediating protection from alpha toxin induced by IFN-γ, we transfected control and ApoL1 siRNA into primary keratinocytes, and treated the cells with media or IFN-γ. Fig. 6b shows the knockdown of ApoL1. Strikingly, we found that IFN-γ mediated protection from alpha toxin was highly dependent on expression of ApoL1. Fig. 6c shows that IFN-γ was no longer able to protect from alpha toxin in cells transfected with ApoL1 siRNA compared to control siRNA transfected cells (p < 0.001). We therefore conclude that ApoL1 plays a critical role in resistance to alpha toxin mediated cell death.
We also examined the consequences of ApoL1 knockdown on autophagy as determined by expression of the marker LC3. In cells treated with control siRNA, significant increases in LC3 expression were observed following treatment with IFN-γ (Fig. 6d and e). Fig. 6d and e also show that the IFN-γ mediated induction of LC3 is ablated in ApoL1 siRNA treated cells (p < 0.01). These results demonstrate that IFN-γ mediated induction of ApoL1 plays a critical role in LC3 activation.
DISCUSSION
In this study, we show that IFN-γ protects primary human keratinocytes from staphylococcal alpha toxin induced cell death. We determined that IFN-γ induced resistance to alpha toxin results in activation of the primary effector of autophagy, LC3. We screened a previously published array analysis of IFN-γ treated keratinocytes (Nograles et al., 2008) for genes related to autophagy, and found that IFN-γ significantly induced the autophagy effector, ApoL1. Knockdown of ApoL1 gene expression by siRNA ablated the IFN-γ induced resistance of keratinocytes to alpha toxin. Furthermore, levels of LC3 were severely depleted in keratinocytes transfected with ApoL1 siRNA, demonstrating a crucial role for ApoL1 in autophagy and protection from alpha toxin induced cell death. To our knowledge, this is the first evidence that autophagy and ApoL1 induced by IFN-γ play an essential role in resistance to staphylococcal alpha toxin in human keratinocytes.
The cellular function of ApoL1 is becoming better elucidated. In addition to the role in activation of LC3 demonstrated here, ApoL1 has also been shown to be involved in endocytosis and trafficking to the lysosome, as well as degradation of cellular and pathogenic proteins (Taylor et al., 2014).
To better assess the biological role of autophagy in S. aureus infection, mice have been generated which are genetically deficient in the autophagy regulator, Atg16L1 (Maurer et al., 2015), a known activator of LC3. Atg16L deficient mice have significantly increased lethality in response to lung infection induced by S. aureus, thus demonstrating a critical biological role for autophagy in resistance to staphylococcal infection.
Interestingly, our data shown here supports the novel concept that alpha toxin itself plays a role in autophagy. Alpha toxin increases LC3 levels, however, it also causes profoundly altered LC3 localization. This change in localization does not occur when cells are protected with IFN-γ. It seems plausible that by altering LC3 localization, alpha toxin may subvert its destruction.
In our studies, we also compared the effects of IFN-γ versus IFN-α in protection from alpha toxin induced cell death. IFN-α, although protective in bronchial epithelial cells (Lizak and Yarovinsky, 2012), was significantly less protective than IFN-γ in keratinocytes (Fig. 1a). Furthermore, IFN-α did not induce ApoL1 expression (supplemental fig 2) to the same extent as IFN-γ. Since epithelial cells robustly respond to IFN-α, it is probable that IFNs have different effects on different cell types.
In addition to functioning as an inducer of autophagy, we find that IFN-γ can antagonize the effects of the Th2 cytokines, IL-4 and IL-13. IFN-γ blocks IL-4/IL-13 mediated increases in alpha toxin induced keratinocyte death. Previous studies have attributed antagonism between IFN-γ and IL-4/IL-13 to effects on gene transcription (Schindler et al., 1995). IFN-γ activates gene transcription through STAT1. In contrast, Th2 effects are mediated through the transcription factor, STAT6 (Travagli et al., 2004). We therefore examined whether IFN-γ could modulate STAT6 induced gene expression. Supplemental Figure 3a shows that expression of a known target of STAT6, Serpin B3 (Sivaprasad et al., 2015; Song et al., 2012), is potently blocked by IFN-γ. However, STAT6 phosphorylation is unaffected by IFN-γ (Supplemental 3b). Since STAT1 and STAT6 can bind similar target DNA sequences, it has been proposed that STAT1 may function to either block STAT6 binding (Schindler et al., 1995) or interfere with recruitment of critical co-factors (Dickensheets and Donnelly, 1999), thus inhibiting IL-4/IL-13 induced gene transcription. Recent studies have also shown that IFN-γ also induces chromatin remodeling and histone acetylation (Qiao et al., 2013). It remains plausible that altered chromatin structure may also interfere with STAT6 transcription. Since blockade of IL-4/IL-13 function has recently been shown to be a viable treatment for reducing S. aureus infection and disease (Beck et al., 2014), a better understanding of the protective effects mediated by IFN-γ may result in potential new therapies to block the effects of Th2 cytokines and improve the outcome of S. aureus exacerbated AD.
Each year, in the United States alone, approximately 500,000 infections and 20,000 deaths are caused by S. aureus (Klevens et al., 2007). Since alpha toxin is the major staphylococcal virulence factor, it seems plausible that a better knowledge of the factors that modulate alpha toxin induced lethality may help to reduce disease. Our current study demonstrates that IFN-γ treated keratinocytes are resistant to S. aureus alpha toxin induced cell death. We find that the resistance induced by IFN-γ is mediated through expression of STAT1 and ApoL1. Further characterization of molecular events that protect from alpha toxin induced lethality will facilitate development of new approaches for treatment of S. aureus infections.
MATERIALS AND METHODS
Keratinocyte cell culture
Primary human keratinocytes (Cascade Biologics; Grand Island, NY) were grown in serum-free keratinocyte growth medium (EpiLife; Cascade Biologics), with 1% human keratinocyte growth supplement (Cascade Biologics), 0.06 mM CaCl2, and antibiotics. Cells were grown on collagen matrix coated tissue culture plasticware. IL-4 and IL-13 cytokines were used at 50 ng/ml each. All other cytokines were used at 25ng/ml except when otherwise indicated. All cytokines were from R&D systems (Minneapolis, MN). For LDH assays, keratinocytes were plated at 20,000 per well in a 96 well plate and were allowed to adhere overnight before treatment. Keratinocytes were treated with cytokines or media for 24 hours before treatment with 100 ng/ml alpha toxin (Sigma; St. Louis, MO) for 24 hours. LDH release was determined using the Cyto-Tox One Kit from Promega (Madison, WI) according to the manufacturer’s instructions.
Quantitative RT-PCR
Total RNA was isolated using RNeasy Mini Kits (Qiagen, Inc.; Valencia, CA) according to the manufacturer’s protocol. One microgram of RNA was reverse transcribed using the Qiagen Quantiscript kit according to manufacturers protocol. RT-PCR was performed and analyzed by the dual-labeled fluorogenic probe method by using an ABI Prism 7300 sequence detector (Applied Biosystems; Foster City, CA). Primers and probes for human Actin, STAT1, Serpin B3, ApoL1 and ADAM10 were purchased from Applied Biosystems. Amplification reactions were performed in MicroAmp optical plates (Applied Biosystems) in a 25-μL volume as previously described (Howell 2007). All reactions were normalized to beta actin.
siRNA transfection
Second-passage keratinocytes of 50–60% confluence were transfected according to the manufacturer’s instructions using Lipofectamine 2000 (Invitrogen) with 20 μM non-targeting, ApoL1, or STAT1 Smartpool siRNA (Dharmacon) in antibiotic free media.
Immunoblot analysis
For analysis of alpha toxin binding and heptamerization, keratinocytes were treated with alpha toxin for two hours, washed with PBS, and harvested in hypotonic lysis buffer with 1% Triton X-100, containing protease inhibitor (Complete, Roche). Cellular debris was pelleted by centrifugation, clarified lysates were resuspended in Laemmli buffer and proteins resolved on a 5–15% gradient gel (Biorad). (The alpha-toxin heptamer is stable during electrophoresis.) Transferred proteins were blotted with anti-alpha toxin antibody (Sigma), and detected by enhanced chemiluminescence (Amersham). Scanned images were quantitated with Image J software. For immunoblotting Actin (Sigma), ADAM10 (Millipore), phospho-STAT1 (Cell Signaling) and LC3 (Abcam), cells were harvested, lysed and probed as described above.
Cell staining and microscopy
Immunofluorescence staining was performed using keratinocytes grown on coverslips. Cells were left untreated or treated with the indicated cytokines for 24 hours prior to addition of alpha toxin for 2 hours. Cells were then washed, fixed with paraformaldehyde and permeabilized briefly with .5% Triton X-100. After blocking with BSA, sections were incubated with primary LC3 antibody (Abcam; Cambridge, MA) for 2 hours at room temperature. Secondary Cy-3 antibody (Jackson labs; West Grove, PA) was added for 1 hour. Images were taken with a Leica Microscope at 40x magnification using SlideBook software.
Statistical analyses
All statistical analysis was conducted using Graph Pad Prism. Comparisons of expression levels were performed using analysis of variance (ANOVA) techniques and Student’s t tests as appropriate.
Supplementary Material
Acknowledgments
The authors wish to acknowledge The Edelstein Family Foundation for their generous support of this work. This research was supported by NIH grants R01 AR41256 and The Atopic Dermatitis Research Network (NIH/NIAID contract NIH/NIAID HHSN272201000020C). This research was also supported in part by Colorado Clinical and Translational Sciences Institute (CCTSI), and in part by Colorado Grant UL1RR025780 from NCRR/NIH and UL1 TR000154 from NIH/NCATS.
Abbreviations
- AD
Atopic Dermatitis
- ADAM10
A Disintegrin and Metalloproteinase 10
- ApoL1
Apolipoprotein L1
- IL
Interleukin
- IFN
Interferon
- LC3
Light Chain 3
- LDH
Lactate dehydrogenase
- RT-PCR
Real Time PCR
- STAT1
Signal Transducer and Activator of Transcription 1
- Th2
T Helper type 2
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauweiler AM, Goleva E, Leung DY. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6) J Invest Dermatol. 2014;134:2114–21. doi: 10.1038/jid.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Bae T, Otto M, et al. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–6. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- Dickensheets HL, Donnelly RP. Inhibition of IL-4-inducible gene expression in human monocytes by type I and type II interferons. J Leukoc Biol. 1999;65:307–12. doi: 10.1002/jlb.65.3.307. [DOI] [PubMed] [Google Scholar]
- Galizzi JP, Cabrillat H, Rousset F, et al. IFN-gamma and prostaglandin E2 inhibit IL-4-induced expression of Fc epsilon R2/CD23 on B lymphocytes through different mechanisms without altering binding of IL-4 to its receptor. J Immunol. 1988;141:1982–8. [PubMed] [Google Scholar]
- Gittler JK, Shemer A, Suarez-Farinas M, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez MG, Master SS, Singh SB, et al. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94:870–6. doi: 10.1172/JCI117408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid Q, Naseer T, Minshall EM, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–31. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. Embo J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134:769–79. doi: 10.1016/j.jaci.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–77. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizak M, Yarovinsky TO. Phospholipid scramblase 1 mediates type i interferon-induced protection against staphylococcal alpha-toxin. Cell Host Microbe. 2012;11:70–80. doi: 10.1016/j.chom.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machura E, Mazur B, Golemiec E, et al. Staphylococcus aureus skin colonization in atopic dermatitis children is associated with decreased IFN-gamma production by peripheral blood CD4+ and CD8+ T cells. Pediatr Allergy Immunol. 2008;19:37–45. doi: 10.1111/j.1399-3038.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- Maurer K, Reyes-Robles T, Alonzo F, 3rd, et al. Autophagy mediates tolerance to Staphylococcus aureus alpha-toxin. Cell Host Microbe. 2015;17:429–40. doi: 10.1016/j.chom.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nograles KE, Zaba LC, Guttman-Yassky E, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008;159:1092–102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost G, Mourey L, Colin DA, et al. Staphylococcal pore-forming toxins. Curr Top Microbiol Immunol. 2001;257:53–83. doi: 10.1007/978-3-642-56508-3_4. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Giannopoulou EG, Chan CH, et al. Synergistic activation of inflammatory cytokine genes by interferon-gamma-induced chromatin remodeling and toll-like receptor signaling. Immunity. 2013;39:454–69. doi: 10.1016/j.immuni.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Jyothi Prasanna S, Chandrasekar B, et al. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Schindler U, Wu P, Rothe M, et al. Components of a Stat recognition code: evidence for two layers of molecular selectivity. Immunity. 1995;2:689–97. doi: 10.1016/1074-7613(95)90013-6. [DOI] [PubMed] [Google Scholar]
- Sivaprasad U, Kinker KG, Ericksen MB, et al. SERPINB3/B4 contributes to early inflammation and barrier dysfunction in an experimental murine model of atopic dermatitis. J Invest Dermatol. 2015;135:160–9. doi: 10.1038/jid.2014.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song KJ, Ahn HJ, Nam HW. Anti-apoptotic effects of SERPIN B3 and B4 via STAT6 activation in macrophages after infection with Toxoplasma gondii. Korean J Parasitol. 2012;50:1–6. doi: 10.3347/kjp.2012.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Hobaugh MR, Shustak C, et al. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–66. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- Taylor HE, Khatua AK, Popik W. The innate immune factor apolipoprotein L1 restricts HIV-1 infection. J Virol. 2014;88:592–603. doi: 10.1128/JVI.02828-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli J, Letourneur M, Bertoglio J, et al. STAT6 and Ets-1 form a stable complex that modulates Socs-1 expression by interleukin-4 in keratinocytes. J Biol Chem. 2004;279:35183–92. doi: 10.1074/jbc.M403223200. [DOI] [PubMed] [Google Scholar]
- Wan G, Zhaorigetu S, Liu Z, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–9. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke GA, Bubeck Wardenburg J. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A. 2010;107:13473–8. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.