Abstract
Epigenetic regulation of the placental glucocorticoid receptor gene (NR3C1) was investigated as a mechanism underlying links between maternal smoking during pregnancy (MSDP) and infant neurobehavior in 45 mother-infant pairs (49% MSDP-exposed; 52% minorities; ages 18-35). The NICU Network Neurobehavioral Scale was administered 7 times over the first postnatal month; methylation of placental NR3C1 was assessed via bisulfite pyrosequencing. Increased placental NR3C1 methylation was associated with increased infant attention and self-regulation, and decreased lethargy and need for examiner soothing over the first postnatal month. A causal steps approach revealed that NR3C1 methylation and MSDP were independently associated with lethargic behavior. Although preliminary, results highlight the importance of epigenetic mechanisms in elucidating pathways to neurobehavioral alterations from MSDP.
Despite ongoing public health campaigns, between 10-30% of infants born in the United States are exposed to maternal smoking during pregnancy (MSDP). Infants born to poor, less educated, and underserved mothers show disproportionately higher rates of exposure (Tong et al., 2013). MSDP-exposed infants are increased risk for low birth weight, preterm delivery, and increased length of neonatal intensive care unit stays (Adams, Melvin, Raskind-Hood, Joski, & Galactionova, 2011; Dietz et al., 2010). In older children and adolescents, exposure to MSDP has been associated with disorders of attention, disruptive behavior and conduct as well as smoking and nicotine dependence (Buka, Shenassa, & Niaura, 2003; Langley, Rice, van den Bree, & Thapar, 2005; Wakschlag, Leventhal, Pine, Pickett, & Carter, 2006).
In an effort to delineate early pathways to long-term child behavioral outcomes, an emerging body of literature examined effects of MSDP on behavioral regulation in the first postnatal month. These studies highlighted differing effects of MSDP in the early (postnatal days 1-5) versus later (days 10-30) neonatal periods consistent with the possibility of a nicotine withdrawal syndrome in the early neonatal period but alterations in regulatory processes in the later neonatal period. Specifically, studies of the early neonatal period found increased hypertonicity, irritability, excitability, and more stress-abstinence signs in MSDP-exposed versus unexposed neonates (Godding et al., 2004; Law et al., 2003; Mansi et al., 2007; Stroud, Paster, Goodwin, et al., 2009). Neonatal stress-abstinence signs include alterations in autonomic, neurologic, gastro-intestinal, skin, and state regulatory functions (Hudak & Tan, 2012). Studies of the later neonatal period revealed MSDP effects on self-regulation, attention, need for external soothing or handling, and arousal--potentially portending longer-term differences in attention and regulation (Espy, Fang, Johnson, Stopp, & Wiebe, 2011; Stroud, Paster, Papandonatos, et al., 2009; Yolton et al., 2009).
Although associations between MSDP and both long and short-term behavioral outcomes have been replicated across assessment measures and samples, little is known regarding biological pathways linking MSDP with behavioral outcomes. We propose that MSDP acts as an intrauterine stressor which alters the maternal-placental-fetal neuroendocrine milieu and “programs” alterations in offspring neurobehavior via glucocorticoid pathways. Supporting this proposal, preclinical models have revealed associations between prenatal nicotine exposure and alterations in maternal glucocorticoids and offspring hypothalamic-pituitary-adrenal (HPA) stress response (Chen et al., 2007; Poland et al., 1994), Human studies have confirmed increased cortisol in the cord blood of MSDP-exposed offspring (McDonald et al., 2006; Varvarigou, Petsali, Vassilakos, & Beratis, 2006), associations between MSDP and infant cortisol stress response (Schuetze, Lopez, Granger, & Eiden, 2008; Stroud, Papandonatos, Rodriguez, et al., 2014), and perinatal glucocorticoid programming of nicotine dependence in adult offspring (Stroud, Papandonatos, Shenassa, et al., 2014).
Because alterations in glucocorticoid pathways from perturbations in the prenatal environment are persistent and predict adult disease in animal models, a growing theoretical literature has highlighted the importance of epigenetic mechanisms in transmitting perinatal insults from mother to fetus (Babenko, Kovalchuk, & Metz, 2015; Meaney, Szyf, & Seckl, 2007). Epigenetic mechanisms involve alterations to DNA that influence gene expression without altering the nucleotide sequence. Methylation is a stable epigenetic mechanism that involves activation or suppression of gene expression through effects on transcription factor binding. It involves the addition of a methyl group to a cytosine molecule residing prior to a guanine molecule connected by a phosphodiester bond (CpG sites).
In animal models, pre and early postnatal stress and maternal care have been associated with consistent and persistent (through adulthood) alterations in hippocampal GR and HPA hyper-reactivity (Meaney, 2001). In models of postnatal maternal care, changes in hippocampal GR are mediated by differential methylation of the promoter region of the hippocampal GR gene (exon 17 of the NR3C1 promoter) which alters binding of the transcription factor nerve growth factor inducible protein A (NGFI-A) (Meaney et al., 2007; Weaver et al., 2004). A small number of human studies have also highlighted the importance of methylation of the human analog of this same NR3C1 promoter (exon 1F; homologous to rat exon 17; contains the NGFI-A binding site) in mediating altered neural and behavioral outcomes following prenatal and early life adversity (McGowan et al., 2009; Oberlander et al., 2008; Radtke et al., 2011; Tyrka, Price, Marsit, Walters, & Carpenter, 2012).
Prior studies of prenatal adversity (Mulligan, D'Errico, Stees, & Hughes, 2012; Oberlander et al., 2008; Radtke et al., 2011) measured NR3C1 promoter methylation in offspring cord and peripheral blood. The placenta is a unique metabolic and endocrine organ at the maternal-fetal interface that maintains and regulates stages of gestation, nutrient delivery, and endocrine and immune communication between mother and fetus. It also serves as a barrier to protect the fetus from infection, toxins, and xenobiotic substances. The crucial role of the placenta in sustaining fetal brain development via protection from environmental perturbations and adaptations to the maternal environment has been highlighted (Bonnin & Levitt, 2011). Thus, investigation of epigenetic alterations in the placenta may reveal a “footprint” of in utero exposures as well as potential pathways linking environmental insults and neurobehavioral outcomes (Hogg, Price, Hanna, & Robinson, 2012; Suter & Aagaard, 2012).
Two prior studies have demonstrated associations between methylation of the placental NR3C1 promoter and a measure of neonatal neurobehavior at a single time point (Bromer, Marsit, Armstrong, Padbury, & Lester, 2013; Conradt, Lester, Appleton, Armstrong, & Marsit, 2013). However, despite evidence for profound changes in neural and neurobehavioral development over the first postnatal month (Coyle et al., 2012; Dubois et al., 2014; Lagercrantz, Hanson, Ment, & Peebles, 2010) and evidence for differing effects of MSDP on neonatal neurobehavior in the early and later postnatal periods, we know of no studies investigating methylation of the placental NR3C1 promoter in relation to multiple longitudinal measures of infant neurobehavior over the first postnatal month. The present study is an epigenetic substudy of an intensive, prospective longitudinal study of MSDP and neonatal neurobehavioral development. We investigated epigenetic regulation of the placental NR3C1 promoter as a mechanism underlying associations between MSDP and evolution of neonatal neurobehavior over the first postnatal month. The present analyses are the first to investigate placental NR3C1 methylation in relation to evolution of neonatal neurobehavior. Therefore analyses were exploratory and directional hypotheses were not proposed.
METHOD
Participants
Participants were English-speaking, primarily low-income mothers from a racially and ethnically diverse sample who completed the epigenetic substudy of a prospective study of MSDP and infant neurobehavior over the first postnatal month (Stroud, Papandonatos, Rodriguez, et al., 2014). Participants were recruited from obstetrical offices, health centers, and community postings in southern New England between 2007 and 2010. Pregnant women were enrolled if they were ages 18-40, had no illicit drug use besides marijuana (meconium confirmed), or serious medical conditions (e.g., pre-eclampsia, severe obesity). Infants were healthy singletons born >36 weeks gestational age (GA) with no congenital anomalies or serious medical complications. All participants provided written informed consent; procedures were reviewed and approved by Women and Infants’ and Lifespan Hospitals’ Institutional Review Boards.
Sixty-three pregnant women ages 18-35 completed the epigenetic substudy. Of these, 4 were excluded for regular drug use, 2 for serious maternal medical conditions, and 2 for delivery <36 weeks. Participants were assigned to the smoking or control group based on maternal report of smoking during at least 2 trimesters and or positive saliva or meconium bioassays. Ten participants who quit smoking prior to 28 weeks gestation were also excluded. The final sample (n = 45) included 22 smokers and 23 biochemically-verified controls.
Procedures
Maternal interviews
Women completed 2-4 comprehensive interview sessions (M=3.6) between 24 and 42 weeks gestation. Each session included the Timeline Follow Back (TLFB) interview--a calendar and anchor-based assessment of smoking, alcohol and drug use over pregnancy and three months prior to conception (Robinson, Sobell, Sobell, & Leo, 2014), and a socioeconomic status (SES) interview from which education, occupation, income, and Hollingshead four-factor index of SES were extracted (Gottfried, 1985). Caffeine consumption and hours of environmental tobacco smoke (ETS) exposure were assessed using detailed interviews covering each trimester. Mothers were also interviewed regarding their health and pregnancy history, and symptoms of depression (Hamilton, 1960). Saliva for cotinine verification of smoking status was also collected.
Delivery
Placental tissue was collected from the n=45 subsample (M=1.2 hours after delivery) for extraction of DNA for NR3C1 methylation analysis. Meconium was collected for 3 days post-birth to verify maternal report of MSDP and other drug use.
Infant neurobehavior over the first postnatal month
The NICU Network Neurobehavioral Scale (NNNS) (Lester, Tronick, & Brazelton, 2004) was utilized to assess infant neurobehavior over the first month and administered by certified examiners blind to MSDP status. The NNNS is a comprehensive assessment of neurobehavioral performance designed to reveal subtle differences in substance-exposed infants (Coyle et al., 2012; Law et al., 2003). The NNNS begins with a pre-examination observation, followed by neurologic and behavioral components (Lester et al., 2004). The exam includes exposure to auditory, visual, social and non-social stimuli and lasts approximately 30 minutes and involves mild stress as the infant is observed and handled during periods of sleep, awake, crying, and non-crying states. Subscales of focus for the study include: Lethargy, Attention, Self-Regulation, and Handling (the need for external soothing of the infant to maintain a quiet alert state). The NNNS was administered up to 7 times (M=6) over the first postnatal month at days 0 (M=7 hours), 1, 2, 3-4, 5, 11, and 31. Saliva samples were collected at the time of the NNNS and assayed for cotinine to determine infant exposure to nicotine via ETS and or breast milk.
Bioassays
Saliva cotinine
Mother and infant saliva samples were frozen until analysis of cotinine (biomarker for nicotine levels) (Jarvis, Tunstall-Pedoe, Feyerabend, Vesey, & Saloojee, 1987) by Salimetrics LLC using highly-sensitive enzyme immunoassay (HS EIA) with intra and inter-assay coefficients of variation of 6.4% and 6.6%.
Meconium
Meconium was analyzed for nicotine and cannabinoid markers, opiates, cocaine, and amphetamines via EMIT screens, tandem liquid chromatography mass spectrometry or gas chromatography mass spectroscopy confirmation. Samples from all participants in the final sample were negative for cocaine, opiates, and amphetamines. Samples with nicotine markers ≥10 ng/g or cannabinoid markers ≥40 ng/g were considered positive for nicotine or cannabinoids.
Placental NR3C1 promoter methylation
Placental tissue that was free from maternal decidua was excised and placed immediately in RNAlater solution (Life Technologies, Grand Island, NY) then stored at 4° C. Placenta samples were removed from RNAlater after at least 72 hours then blotted dry, snap-frozen in liquid nitrogen, pulverized to homogeneity, and stored at −80° C until analysis. Placental genomic DNA was extracted using the QIAmp DNA Mini kit (Qiagen, Inc.), and assessed for quantity and quality using a ND-1000 Spectophotometer (Nanodrop, DE). DNA samples were sodium bisulfite modified using the EZ DNA Methylation Kit (Zymo Research, CA). Degree of methylation at the NR3C1 promoter region was examined via quantitative pyrosequencing (Dupont, Tost, Jammes, & Gut, 2004; Oberlander et al., 2008) using the PyroMark MD Pyrosequencing System. The region analyzed encompasses exon 1F (human homologue of rat exon 17) and contains 13 CpGs. Reactions were performed in triplicate; averaged, and SDs for individual sites calculated. Any sample with SD>3% was re-analyzed. Sodium bisulfite-modified, fully-methylated referent positive control and fully-unmethylated (whole genome amplified) negative control DNA (Qiagen, Valencia, CA) were included with each batch.
Potential confounders
Potential confounders of the association between MSDP and NNNS outcomes included maternal age, race and ethnicity (% Non-Hispanic White), Hollingshead SES (low SES ≥ 4), gravida, paritymaternal medical conditions, (e.g., gestational hypertension, gestational diabetes), maternal medications (steroids, antidepressants), weight gain over pregnancy, maternal depressive symptoms (structured interview) (Hamilton, 1960), maternal alcohol use (interview) (Robinson et al., 2014), maternal cannabis use (interview, meconium), maternal caffeine use and environmental tobacco smoke (ETS) exposure (structured interview), infant ETS exposure (infant saliva cotinine), infant sex, delivery mode, GA at birth, small for GA (SGA; birth weight <10th percentile for GA), Apgar (medical chart review), and infant feeding method (breast vs. bottle) (maternal report).
Statistical analysis
NNNS subscales exhibiting significant skewness (Lethargy, Handling) were symmetrized via a logarithmic transformation. Longitudinal trajectories of the four NNNS subscales (Lethargy, Attention, Self-Regulation, Handling) were then analyzed via Generalized Least Squares in Splus 8.2 (TIBCO Spotfire Inc., 2010), assuming heteroscedastic variances by MSDP group, and a continuous AR(1) correlation structure. Each potential confounder was tested individually in relation to MSDP group and then in relation to specific NNNS subscales. Two-sample t-, Mann-Whitney, Chi-square, and Fisher's tests were then utilized to assess associations of MSDP group with potential confounders (Table 1). Potential confounders showing significant bivariate associations with both MSDP and individual NNNS outcomes (p's < .05) were included as covariates in the corresponding normal linear regression models (ETS exposure for Lethargy and Attention, breast-feeding for Self-regulation) (Table 3).
Table 1.
Maternal and Infant Characteristics for the Full Sample and by Maternal Smoking Group
| Full Sample (n=45) Mean (SD)/% | Smokers (n=22) Mean (SD)/% | Controls (n=23) Mean (SD)/% | |
|---|---|---|---|
| Maternal Characteristics | |||
| Maternal age (years) | 25 (5) | 24 (4) | 26 (5) |
| Race/Ethnicity (% Non-Hispanic White) | 48% | 50% | 45% |
| Low SESa | 41% | 59% | 23%* |
| Parity | 1 (2) | 1 (2) | 1 (2) |
| Weight gain (pounds)b | 27 (14) | 30 (15) | 26 (13) |
| Alcohol Use (>1 drink/week) | 2% | 5% | 0% |
| Marijuana Use (>1 joint/week or meconium)c | 2% | 5% | 0% |
| ETS Exposure (hours per week)d | 11 (18) | 21 (22) | 1 (2)*** |
| Caffeine Use (>200 mg/day caffeine)e | 18% | 33% | 4%* |
| Gestational Medical Conditionf | 9% | 14% | 4% |
| Maternal Depressiong | 2% | 5% | 0% |
| Infant Characteristics | |||
| Sex (% male) | 49% | 41% | 57% |
| Delivery Mode (% vaginal delivery) | 80% | 77% | 83% |
| Gestational age at birth (weeks) | 39 (1) | 39 (1) | 40 (1) |
| Small for gestational ageh | 7% | 9% | 4% |
| Apgar score, 5 minutes | 9 (0.3) | 9 (0) | 9 (0.4) |
| Any breast-feedingi | 64% | 45% | 83%* |
| ETS Exposure: saliva cotinine (ng/ml)j | 8 (17) | 15 (24) | 1 (2)*** |
Based on a score of 4 or 5 on the Hollingshead Index.
Weight gain in pounds between pre-pregnancy and 35±1 weeks.
>1 joint per week or meconium positive for marijuana metabolites.
Hours of environmental tobacco smoke (ETS) exposure per week measured by structured interview.
Equivalent of 2 cups of coffee per day.
e.g., gestational hypertension, gestational diabetes.
Score of 16 or more Hamilton Depression Rating Scale (Hamilton, 1960).
Birthweight < 10th percentile for gestational age.
Percentage of infants who were breast-fed only or who were breast-fed and bottle fed.
ETS exposure measured by infant saliva cotinine (ng/ml).
p < .05.
** p < .01.
p < .001.
Table 3.
NR3C1 Promoter Methylation and Maternal Smoking in relation to Infant Neurobehavior over the First Postnatal Month, measured by the NICUNetwork Neurobehavioral Scale (NNNS)
| Model 1 |
Model 2 |
Model 3 |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Predictors | B | SE | P | B | SE | P | β | SE | P |
| Lethargya,e | |||||||||
| Intercept | 2.406 | 0.163 | <.001 | 2.480 | 0.169 | <.001 | 2.569 | 0.168 | <.001 |
| Feeding Timee,f | -0.069 | 0.028 | .015 | -0.067 | 0.028 | .018 | -0.050 | 0.028 | .079 |
| Infant Agee,g | -0.109 | 0.018 | <.001 | -0.107 | 0.017 | <.001 | -0.107 | 0.018 | <.001 |
| ETSh | 0.004 | 0.002 | .021 | 0.002 | 0.002 | .213 | - | - | - |
| Maternal Smoking | 0.154 | 0.056 | .007 | 0.159 | 0.059 | .007 | - | - | - |
| NR3C1 CpG 3 | - | - | - | -0.039 | 0.019 | .037 | -0.055 | 0.018 | .002 |
| NR3C1 CpG 4 | - | - | - | 0.000 | 0.025 | .990 | -0.027 | 0.024 | .264 |
| Attentionb | |||||||||
| Intercept | 2.843 | 0.608 | <.001 | 2.607 | 0.63 | <.001 | 2.000 | 0.646 | .002 |
| Feeding Timee,f | 0.311 | 0.104 | .003 | 0.303 | 0.104 | .004 | 0.271 | 0.108 | .013 |
| Infant Agee,g | 0.283 | 0.065 | <.001 | 0.281 | 0.065 | <.001 | 0.295 | 0.068 | <.001 |
| ETSh | -0.012 | 0.006 | .044 | -0.009 | 0.006 | .149 | - | - | - |
| Maternal Smoking | -0.798 | 0.210 | <.001 | -0.776 | 0.227 | .001 | - | - | - |
| NR3C1 CpG 3 | - | - | - | 0.093 | 0.070 | .185 | 0.149 | 0.069 | .031 |
| NR3C1 CpG 4 | - | - | - | 0.047 | 0.091 | .603 | 0.193 | 0.088 | .030 |
| Self Regulationb | |||||||||
| Intercept | 4.897 | 0.339 | <.001 | 4.724 | 0.354 | <.001 | 4.397 | 0.335 | <.001 |
| Feeding Timee,f | 0.065 | 0.057 | .253 | 0.054 | 0.057 | .338 | 0.059 | 0.057 | .301 |
| Infant Agee,g | 0.096 | 0.035 | .007 | 0.091 | 0.035 | .009 | 0.089 | 0.035 | .012 |
| Breast-feeding | -0.351 | 0.109 | .001 | -0.351 | 0.119 | .003 | - | - | - |
| Maternal Smoking | -0.181 | 0.103 | .082 | -0.115 | 0.115 | .318 | - | - | - |
| NR3C1 CpG 3 | - | - | - | 0.101 | 0.037 | .007 | 0.079 | 0.037 | .033 |
| NR3C1 CpG 4 | - | - | - | 0.015 | 0.055 | .790 | 0.072 | 0.048 | .138 |
| Handlingd, e | |||||||||
| Intercept | 1.085 | 0.274 | <.001 | 1.158 | 0.284 | <.001 | 1.253 | 0.278 | <.001 |
| Feeding Timee,f | 0.042 | 0.046 | .367 | 0.037 | 0.046 | .422 | 0.038 | 0.046 | .403 |
| Infant Agee,g | -0.028 | 0.030 | .353 | -0.027 | 0.030 | .375 | -0.027 | 0.030 | .375 |
| Maternal Smoking | 0.153 | 0.076 | .045 | 0.112 | 0.084 | .182 | - | - | - |
| NR3C1 CpG 3 | - | - | - | 0.019 | 0.027 | .491 | 0.015 | 0.027 | .573 |
| NR3C1 CpG 4 | - | - | - | -0.061 | 0.043 | .158 | -0.083 | 0.040 | .038 |
Note. Model 1 includes Maternal Smoking and relevant covariates but not NR3C1 methylation. Model 2 includes NR3C1 methylation, Maternal Smoking, and relevant covariates. Model 3 includes NR3C1 methylation but not Maternal Smoking. B's represent values when all variables in each model are entered simultaneously.
NICU Network Neurobehavioral Scale (NNNS) Lethargy is a measure of infant lethargic behavior or low levels of motor, state and physiologic reactivity.
NNNS Attention is a measure of orientation to animate and inanimate auditory and visual stimuli.
NNNS Self-Regulation measures the infant's capacity to organize activity, physiology, and state and response to positive and negative stimuli
NNNS Handling is a measure of need for intervention from the NNNS examiner to soothe the infant and assist the infant in maintaining a quiet, alert state.
Natural log transformed.
Time since Feeding: measured in minutes.
Infant Age: measured in hours.
Maternal Environmental Tobacco Smoke (ETS): hours of maternal perinatal ETS exposure per week measured by structured interview.
A causal steps approach (Baron & Kenny, 1986) was utilized to explore NR3C1 methylation as a mediator of the link between MSDP and infant neurobehavior over the first postnatal month. This requires significant associations between: a) predictor (MSDP) and outcome (each of the four NNNS subscale), b) predictor (MSDP) and mediator (NR3C1 methylation), and c) mediator and outcome adjusted for the predictor, as well as demonstrating: d) attenuation of the predictor-outcome association, when the putative mediator is included in the model. Causal step (b) relied on previously described Mann-Whitney rank-based tests to determine associations between MSDP and methylation of the NR3C1 promoter across all 13 CpG sites (Stroud, Papandonatos, Rodriguez, et al., 2014); results are summarized in Table 2. The remaining causal steps utilized longitudinal regression modeling with MSDP and NR3C1 methylation as between subjects’ factors, and infant age at NNNS as the primary time scale for measuring longitudinal change in neurobehavior over the first postnatal month. Models also controlled for time since feeding, an important determinant of infant neurobehavior, to sharpen inferences for MSDP associations (Table 3).
Table 2.
Methylation of Placental NR3C1 Promoter CpG Sites by Maternal Smoking Group
| NR3C1 CpG site | MSDP-exposed % methylation Mean (SE) | Unexposed % methylation Mean (SE) |
|---|---|---|
| CpG 1 | 2.09 (.46) | 1.50 (.19) |
| CpG 2 | .85 (.37) | .51 (.10) |
| CpG 3 | 1.51 (.32) | 2.07 (.22)* |
| CpG 4 | .87 (.19) | 2.34 (.75)** |
| CpG 5 | 1.18 (.37) | 1.07 (.21) |
| CpG 6 | 2.5 (.28) | 2.47 (.26) |
| CpG 7 | 6.80 (.51) | 6.59 (.51) |
| CpG 8 | 3.97 (.42) | 4.08 (.45) |
| CpG 9 | 3.22 (.49) | 3.43 (.43) |
| CpG 10 | 4.04 (.51) | 4.36 (60) |
| CpG 11 | 3.17 (.68) | 4.20 (.67) |
| CpG 12 | 2.29 (.32) | 2.73 (.35) |
| CpG 13 | 1.54 (.38) | 1.77 (.34) |
Note.
p < .05
p < .01.
RESULTS
Sample characteristics
Descriptive statistics for the overall sample and stratified by MSDP group are presented in Table 1. Pregnant smokers were more likely to be low socio-economic status (59% vs. 23%, p < .05), to endorse greater perinatal ETS exposure (M's=21 vs. 1 hour per week, p < .001), and to report high caffeine use (>200 mg/day; 33% vs. 4%, p < .05). MSDP-exposed infants were less likely to be breast-fed (45% vs. 83%, p < .05) and had higher saliva cotinine levels (M's = 15 vs. 1 ng/ml, p < .01).
Associations between MSDP group and evolution of infant neurobehavior (NNNS subscales) over first postnatal month
After control for subscale-specific confounders, significant positive associations emerged for Lethargy (p = .007) and Handling (p = .045), and a negative association emerged for Attention (p < .001). A trend association emerged between MSDP and Self-Regulation (p = .082).
Role of NR3C1 promoter methylation in associations between MSDP and evolution of infant neurobehavior
We previously described methylation of placental NR3C1 across all CpG sites by MSDP group (Stroud, Papandonatos, Rodriguez, et al., 2014) with results summarized in Table 2. MSDP was associated with attenuated methylation at sites CpG 3 and CpG 4. The low percentage and range of methylation values for CpG 3 and 4 is notable but consistent with prior human studies (Conradt et al., 2013; Mulligan et al., 2012; Oberlander et al., 2008; Radtke et al., 2011).
Table 3 presents longitudinal regression models for the four NNNS subscales (Lethargy, Attention, Self-regulation, Handling) including estimates for three types of effects: 1) MSDP effects on NNNS, 2) joint MSDP and NR3C1 effects on NNNS, and 3) NR3C1 effects on NNNS. NR3C1 longitudinal analyses focused on CpG3 and CpG4 sites based on significant associations with MSDP group and findings from identical regions in a human study and homologous regions in preclinical studies (Oberlander et al., 2008; Weaver et al., 2004). All models included infant age at NNNS (time scale) and time since feeding (a priori covariate). Models with MSDP include subscale-specific confounders (ETS for Lethargy and Attention, breast-feeding for Self-regulation). Prior to entry into regression models, we investigated associations between MSDP, ETS and NR3C1 CpG 3 and CpG 4. Pearson correlations were all <.4 suggesting no evidence for multicollinearity.
NNNS Lethargy
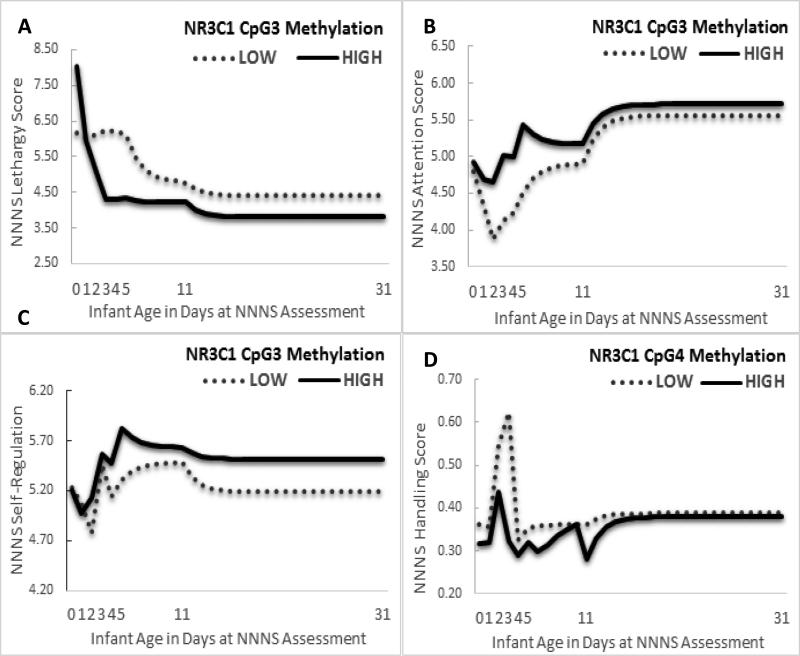
As shown in Model 1 (Table 3), MSDP was associated with 17%, (95% CI [10%, 23%]) increased lethargy over the first postnatal month (β = .154, SE = .056, p =.007). Addition of NR3C1 methylation in Model 2 revealed significant effects of NR3C1 CpG 3 (p = .037) but not CpG 4 methylation, with no attenuation of the MSDP coefficient (p=.007). Model 2 results suggest independent effects of MSDP and NR3C1 methylation on NNNS Lethargy, but no mediation of the MSDP-Lethargy link by NR3C1 methylation. Maternal perinatal ETS exposure, confounder of the MSDP-Lethargy association, was also associated with increased lethargy over the first postnatal month (β = .004, SE = .002, p = .021); each hour increase in daily ETS increased lethargy by 2.8% [0.1%, 5.7%]. Addition of NR3C1 in Model 2 deflated the ETS coefficient by one half (β = .002, SE = .002, p=.213) providing suggestive evidence of mediation of an ETS-Lethargy link by NR3C1 CpG 3 methylation. When NR3C1 methylation was evaluated on its own in Model 3 (Figure 1a), increased CpG3 methylation was associated with decreased Lethargy over the first postnatal month (β = −.055, SE = .018, p = .002); each percentile increased methylation decreased lethargy by 5% [4%, 7%].
Figure 1.
Associations between epigenetic regulation of placental NR3C1 (CpG 3 and 4) on the evolution of infant neurobehavior over the first postnatal month (a) NR3C1 CpG 3 Methylation and NICU Network Neurobehavioral Scale (NNNS) Lethargy, (b) NR3C1 CpG 3 methylation and NNNS Attention, (c) NR3C1 CpG 3 methylation and NNNS Self-Regulation, (d) NR3C1 CpG 4 methylation and NNNS Handling.
The NICU Network Neurobehavioral Scale (NNNS) was administered up to 7 times over the first postnatal month at days 0 (M=7 hours), 1, 2, 3-4, 5, 11, and 31. Although age was modeled in hours and NNNS Lethargy and NNNS Handling in the logarithmic scale for statistical analyses, results are presented by day of life with raw mean NNNS values for ease of visual display. NR3C1 CpG 3 and CpG 4 methylation were modeled continuously including time-varying covariates in the statistical analyses but are presented by High and Low methylation based on a median split of unadjusted methylation values for ease of visual display.
NNNS Attention
In Model 1, MSDP was associated with high magnitude decreases in infant attention over the first postnatal month (β = −.798, SE = .210, p < .001). Addition of NR3C1 methylation in Model 2 revealed no significant effects of either CpG3 or CpG4 methylation, but significant effects of MSDP (p < .001), highlighting effects of MSDP, but not NR3C1 pathways. Maternal ETS exposure was also associated with decreased attention over the first postnatal month (β = −.012, SE = .006, p = .044). In Model 3 (Figure 1b), increased NR3C1 methylation was associated with increased attention over the first postnatal month at both CpG3 (β = .149, SE = .069, p = .031) and CpG4 sites (β = .193, SE = .088, p = .030).
NNNS Self-Regulation
In Model 1, MSDP was associated with a trend towards decreasing infant self-regulation levels over the first postnatal month (β = −.181, SE = .103, p = .082). Addition of NR3C1 in Model 2 revealed a significant effect of NR3C1 CpG3 methylation on NNNS Self-Regulation (β = .101, SE = .037, p = .007), while the MSDP coefficient was deflated by one third (β = −.115, SE = .115, p = .318). Results provide suggestive evidence of mediation of the trend MSDP-Self Regulation link by NR3C1 CpG 3 methylation. NR3C1 CpG 4 methylation did not add significantly to Model 2 (p=.790). In Model 3 (Figure 1c), increased NR3C1 methylation was associated with increased Self-Regulation over the first postnatal month at CpG3 (β = .079, SE = .037, p = .033) but not CpG4.
NNNS Handling
In Model 1, MSDP was associated with a 17% [8%, 26%] increased need for external handling over the first month of life (β = .153, SE = .076, p = .045). Although addition of NR3C1 methylation in Model 2 deflated the MSDP coefficient by 27% (β = .112, SE = .084, p = .182), results do not provide evidence of mediation of the MSDP-Attention link by NR3C1 because there were no significant effects of NR3C1 methylation on NNNS Handling in Model 2 (p = .158). In Model 3 (Figure 1d), increased NR3C1 methylation was associated with decreased Handling scores over the first postnatal month (β = −.083, SE = .040, p = .038); each percentile increase in NR3C1 CpG 4 methylation was associated with an 8% [1, 15%] decrease in need for Handling.
DISCUSSION
We previously investigated associations between maternal smoking during pregnancy (MSDP) and epigenetic regulation of placental NR3C1 promoter in relation to infant cortisol stress response over the first postnatal month (Stroud, Papandonatos, Rodriguez, et al., 2014). In this study, we explored the role of epigenetic regulation of placental NR3C1 as a mechanism underlying links between MSDP and infant neurobehavior over the first postnatal month. We found significant associations between NR3C1 methylation and positive neurobehavioral development (decreased lethargy, increased attentiveness, increased self-regulatory behaviors, and decreased need for examiner soothing to maintain a quiet alert state). In causal steps models, MSDP and NR3C1 methylation independently influenced lethargic behaviors, with no evidence for mediation. Persistent associations with MSDP but not NR3C1 methylation emerged for infant attention.
The primary limitation of the present study is the small sample size and low power, especially given the complex multivariate mediation modeling and multiple NNNS subscales analyzed. Thus, results should be considered preliminary. Although epigenetic regulation of NR3C1 was associated with differences in offspring behavior, replication of observed patterns and direction of effects in additional larger samples is needed and is currently underway by our group. Nonetheless, significant associations between MSDP, placental NR3C1 methylation and infant neurobehavior highlight placental epigenetic pathways as an important area for future research.
The key strength of the present study is the longitudinal and detailed characterization of maternal and infant phenotypes. MSDP was measured prospectively by calendar-based interview with two types of biochemical verification (saliva and meconium), (non)use of illicit drugs was confirmed by meconium bioassay, numerous potential confounders were measured by detailed interview and or medical chart review, infant neurobehavior was measured up to seven times (M=6) over the first postnatal month, and placental samples for epigenetic analyses were collected and preserved immediately following delivery. The racially and ethnically diverse and low socio-economic status sample and consistent and persistent tobacco exposure in the MSDP-exposed group (at least 2 trimesters) represent additional strengths of the study.
An important innovation in the present study is focus on placental tissue to investigate the influence of the perinatal environment on offspring behavior. The placenta is unique in serving as a metabolic, endocrine, and immune organ whose existence emerges and dissipates with the pregnancy and which serves as the vehicle for communication of environmental signals between mother and fetus. Moreover, the placenta's expression profile has been described as similar in complexity to the human brain. Indeed, the placenta has been referred to as “the third brain” (Yen, 1994). Given the noted tissue specificity of the epigenome, it is possible that epigenetic signatures of the placenta may show greater association with those in the developing fetal brain than other available peripheral tissues (Yen, 1994). Finally, the placenta is unique in its availability for research study; it is often discarded within a short time after delivery unless pathological examination is indicated. Thus, the development of well-designed studies of the placental epigenome in relation to perinatal exposures and offspring behavioral outcomes represents a fertile area for future behavioral epigenetic research (Knopik, Maccani, Francazio, & McGeary, 2012; Lester, Conradt, & Marsit, 2014).
The present study is the first to investigate associations between placental NR3C1 methylation and longitudinal measures of infant neurobehavior. In models that did not include MSDP, we found significant associations between increased NR3C1 methylation and adaptive neurobehavioral development (decreased Lethargy, increased Attention, increased Self-Regulation, and decreased need for Handling). For example, each one-percentile increase in placental NR3C1 promoter methylation was associated with an 8% decrease in need for external soothing (Handling) and a 5% decrease in Lethargy. Overall increasing NR3C1 CpG 3 and CpG 4 methylation were associated with a profile of an alert, engaged infant with self-regulatory and self-soothing skills. Results complement prior studies in confirming that despite low overall and range of methylation levels for NR3C1 exon 1F, small absolute differences in its’ methylation have meaningful functional consequences for infant neurobehavior and stress response (Conradt et al., 2013; Oberlander et al., 2008). In closely related work (Stroud, Papandonatos, Rodriguez, et al., 2014), we found negative associations between MSDP and infant baseline and stress reactive cortisol over the first postnatal month but positive associations between NR3C1 CpG 3 methylation and infant baseline cortisol and infant cortisol response to the NNNS. Results from this related study suggest links between placental NR3C1 methylation with adaptive neuroendocrine regulation, complementing our present finding of associations with more adaptive neurobehavioral regulation. Positive associations between NR3C1 methylation and adaptive neurobehavioral and neuroendocrine profiles over the first postnatal month and studies revealing inverse associations between NR3C1 methylation and GR expression in the placenta (Bromer et al., 2013; Filiberto et al., 2011) suggest a compensatory mechanism to protect the fetus from potential maternal glucocorticoid overload.
Results are also consistent with Bromer et al (2013), who found positive associations between NR3C1 (CpG 7) methylation and improved newborn neurobehavioral outcomes including quality of movement, stress, and habituation to environmental stimuli. However, our results contrast with Bromer et al. (2013) who found associations between overall NR3C1 methylation (across CpG sites) and decreased infant attention, and Conradt et al. (2013), who found interactions between maternal depressed mood and increased placental NR3C1 methylation (CpG 2) were associated with less adaptive newborn neurobehavior (decreased self-regulation and increased hypotonia and lethargy). Contrasting results may be related to the focus of prior studies on neurobehavior at one time point (first days of life). Given profound normative neurobehavioral and neural development over the first postnatal month, our focus on associations between NR3C1 and longitudinal measures of infant neurobehavior may have led to differential findings. Moreover, prior studies focused on different CpG sites (CpG 2, 7, and mean methylation across CpG sites) from the present study (CpG 3 and 4). Finally, our prior studies focused on a less racially and ethnically diverse sample selected for gestational age at birth versus the current diverse sample selected for smoking status during pregnancy. It is possible that links between NR3C1 and neurobehavior may differ based on race and ethnicity and perinatal exposures. Future studies are needed to reconcile differential findings across studies.
In analyses previously presented by our group (Stroud, Papandonatos, Rodriguez, et al., 2014), MSDP was associated with attenuated methylation of NR3C1 CpG 3 and CpG 4. Differential methylation in these sites has been demonstrated in prior human studies of prenatal adversity (Mulligan et al., 2012; Oberlander et al., 2008; Radtke et al., 2011). Furthermore, homologous sites were differentially methylated in preclinical studies of maternal care (Oberlander et al., 2008; Weaver et al., 2004). Associations between MSDP and methylation of placental NR3C1 highlight the importance of glucocorticoid regulation pathways in elucidating effects of MSDP and other perinatal insults and offer translation of preclinical and theoretical models of perinatal stress to humans (Meaney et al., 2007). We also found independent associations between NR3C1methylation and MSDP in influencing lethargic behavior over the first postnatal month. Results suggest additive influences of MSDP and NR3C1 on infant lethargy and highlight the possibility that other perinatal factors besides MSDP may influence NR3C1 methylation. With respect to infant attention, positive associations with NR3C1 were outweighed by MSDP in multivariate models. Results highlight the strength of associations between MSDP and infant attention, but suggest other pathways besides NR3C1 may be involved. Future studies are needed to replicate patterns of associations between MSDP, NR3C1 and newborn neurobehavior. Associations between NR3C1 methylation and both MSDP and infant neurobehavioral development complement preclinical studies showing effects of prenatal nicotine on maternal glucocorticoids and offspring HPA stress response (Chen et al., 2007; Poland et al., 1994), and human studies showing effects of MSDP on maternal and fetal (cord blood) glucocorticoids and offspring HPA stress response, including effects on offspring cortisol in the current subsample (Schuetze et al., 2008; Stroud, Papandonatos, Rodriguez, et al., 2014; Stroud, Papandonatos, Shenassa, et al., 2014; Varvarigou et al., 2006). Results extend prior research through investigating placental glucocorticoid pathways linking MSDP and infant neurobehavior (versus cortisol response).
Exposure to MSDP was associated with increases in both lethargy and need for external soothing (Handling), as well as decreases in attention over the first postnatal month. Associations between MSDP and lethargy and attention are consistent with some previous studies of MSDP effects on neonatal neurobehavior. Specifically, our results complement prior cross-sectional and longitudinal studies of the later neonatal period (postnatal days 10-30), which have shown alterations in attentional and regulatory processes in exposed infants, including measures of attention and self-regulation, as well as increased need for external soothing (Espy et al., 2011; Stroud, Paster, Papandonatos, et al., 2009; Yolton et al., 2009). Our results with respect to MSDP and infant neurobehavior are inconsistent with prior studies of the immediate neonatal period (postnatal days 1-5), in which MSDP-exposed infants showed signs of abstinence, irritability, and increased muscle tone consistent with a withdrawal process (Godding et al., 2004; Law et al., 2003; Mansi et al., 2007; Stroud, Paster, Goodwin, et al., 2009). Although our study bridged the immediate and later neonatal periods, our focus on main effects of MSDP may have obscured dissipating effects of MSDP on signs of withdrawal.
We acknowledge several additional limitations of our study. We did not investigate associations between NR3C1 methylation and GR gene expression in the present sample. In addition, pregnant smokers and controls differed on several potential confounders including socioeconomic status, environment tobacco smoke exposure, and caffeine consumption. Although relevant confounders statistically controlled, it remains possible that associations with epigenetic pathways were not due to MSDP only.
Conclusions
We previously revealed associations between MSDP, methylation of the placental NR3C1 promoter, and infant cortisol regulation (Stroud, Papandonatos, Rodriguez, et al., 2014). In the present study, increases in site-specific methylation of the NR3C1 promoter in the placenta were associated with positive infant neurobehavioral development over the first postnatal month including decreased lethargy and need for external soothing and increased attention and self-regulation. Results also revealed placental NR3C1 methylation and MSDP as independent, additive pathways in relation to infant lethargy. Results add to a growing body of literature highlighting glucocorticoid programming as important mechanism underlying associations between MSDP and offspring behavioral alterations. Results also highlight placental epigenetic and glucocorticoid pathways as an important area for future research in relation to long-term outcomes from MSDP.
Acknowledgments
Preparation of this manuscript was supported by the National Institutes of Health (R01 DA019558 and R01 DA031188 to L.R.S.) and the Flight Attendant Medical Research Institute Clinical Innovator Award to L.R.S. We gratefully acknowledge the families who contributed to this study and the Maternal-Infant Studies Laboratory staff for their assistance with data collection. We also thank Polly Gobin for her assistance with data management. We are also grateful to Cheryl Boyce and Nicolette Borek, Program Officers, for their support of this work and this field.
Contributor Information
Laura R. Stroud,
George D. Papandonatos,
Amy L. Salisbury,
Maureen G. Phipps,
Marilyn A. Huestis,
Raymond Niaura, .
James F. Padbury,
Carmen Marsit, .
Barry Lester, .
REFERENCES
- Adams EK, Melvin CL, Raskind-Hood C, Joski PJ, Galactionova E. Infant delivery costs related to maternal smoking: An update. Nicotine & Tobacco Research. 2011;13:627–637. doi: 10.1093/ntr/ntr042. doi:10.1093/ntr/ntr042. [DOI] [PubMed] [Google Scholar]
- Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neuroscience and Biobehavioral Reviews. 2015;48C:70–91. doi: 10.1016/j.neubiorev.2014.11.013. doi:10.1016/j.neubiorev.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bonnin A, Levitt P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience. 2011;197:1–7. doi: 10.1016/j.neuroscience.2011.10.005. doi:10.1016/j.neuroscience.2011.10.005 S0306-4522(11)01179-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer C, Marsit CJ, Armstrong DA, Padbury JF, Lester B. Genetic and epigenetic variation of the glucocorticoid receptor (NR3C1) in placenta and infant neurobehavior. Developmental Psychobiology. 2013;55:673–683. doi: 10.1002/dev.21061. doi:10.1002/dev.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: A 30-year prospective study. American Journal of Psychiatry. 2003;160:1978–1984. doi: 10.1176/appi.ajp.160.11.1978. [DOI] [PubMed] [Google Scholar]
- Chen M, Ting W, Zhang-xiu L, Xiao-liang P, Ying-Hong F, Hui W. Nicotine-induced prenatal overexposure to maternal glucocorticoid and intrauterine growth retardation in rat. Experimental and Toxicological Pathology. 2007;59:245–251. doi: 10.1016/j.etp.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Conradt E, Lester BM, Appleton AA, Armstrong DA, Marsit CJ. The roles of DNA methylation of NR3C1 and 11beta-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics. 2013;8:1321–1329. doi: 10.4161/epi.26634. doi:10.4161/epi.26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle MG, Salisbury AL, Lester BM, Jones HE, Lin H, Graf-Rohrmeister K, Fischer G. Neonatal neurobehavior effects following buprenorphine versus methadone exposure. Addiction. 2012;107(Suppl 1):63–73. doi: 10.1111/j.1360-0443.2012.04040.x. doi:10.1111/j.1360-0443.2012.04040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the US. American Journal of Preventive Medicine. 2010;39:45–52. doi: 10.1016/j.amepre.2010.03.009. doi:10.1016/j.amepre.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, Hertz-Pannier L. The early development of brain white matter: A review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. doi:10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Dupont JM, Tost J, Jammes H, Gut IG. De novo quantitative bisulfite sequencing using the pyrosequencing technology. Analytical Biochemistry. 2004;333:119–127. doi: 10.1016/j.ab.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Espy KA, Fang H, Johnson C, Stopp C, Wiebe SA. Prenatal tobacco exposure: Developmental outcomes in the neonatal period. Developmental Psychology. 2011;47:153–156. doi: 10.1037/a0020724. doi:10.1037/a0020724 2010-22322-001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, Marsit CJ. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics. 2011;6:566–572. doi: 10.4161/epi.6.5.15236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godding V, Bonnier C, Fiasse L, Michel M, Longueville E, Lebecque P, Galanti L. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates? Pediatric Research. 2004;55:645–651. doi: 10.1203/01.PDR.0000112099.88740.4E. [DOI] [PubMed] [Google Scholar]
- Gottfried AW. Measures of socioeconomic status in child development research: Data and recommendations. Merrill-Palmer Quarterly. 1985;31:85–92. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg K, Price EM, Hanna CW, Robinson WP. Prenatal and perinatal environmental influences on the human fetal and placental epigenome. Clinical Pharmacology and Therapeutics. 2012;92:716–726. doi: 10.1038/clpt.2012.141. doi:10.1038/clpt.2012.141. [DOI] [PubMed] [Google Scholar]
- Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–560. doi: 10.1542/peds.2011-3212. doi:10.1542/peds.2011-3212. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, Vesey C, Saloojee Y. Comparison of tests used to distinguish smokers from nonsmokers. American Journal of Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS, Maccani MA, Francazio S, McGeary JE. The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Development and Psychopathology. 2012;24:1377–1390. doi: 10.1017/S0954579412000776. doi:10.1017/S0954579412000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H, Hanson MA, Ment LR, Peebles DM, editors. The newborn brain: Neuroscience and clinical applications. Cambridge University Press; Cambridge, U.K.: 2010. [Google Scholar]
- Langley K, Rice F, van den Bree MB, Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatrica. 2005;57:359–371. [PubMed] [Google Scholar]
- Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- Lester BM, Conradt E, Marsit CJ. Are epigenetic changes in the intrauterine environment related to newborn neurobehavior? Epigenomics. 2014;6:175–178. doi: 10.2217/epi.14.9. doi:10.2217/epi.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–667. [PubMed] [Google Scholar]
- Mansi G, Raimondi F, Pichini S, Capasso L, Sarno M, Zuccaro P, Paludetto R. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Pediatric Research. 2007;61:257–261. doi: 10.1203/pdr.0b013e31802d89eb. doi:10.1203/pdr.0b013e31802d89eb. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, Ohlsson A. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG: An International Journal of Obstetrics & Gynaecology. 2006;113:1289–1295. doi: 10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neuroscience. 2009;12:342–348. doi: 10.1038/nn.2270. doi:10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends in Molecular Medicine. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mulligan CJ, D'Errico NC, Stees J, Hughes DA. Methylation changes at NR3C1 in newborns associate with maternal prenatal stress exposure and newborn birth weight. Epigenetics. 2012;7:853–857. doi: 10.4161/epi.21180. doi:10.4161/epi.2118021180 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- Poland RE, Lutchmansingh P, Au D, Edelstein M, Lydecker S, Hsieh C, McCracken JT. Exposure to threshold doses of nicotine in utero: I. Neuroendocrine response to restraint stress in adult male offspring. Life Sciences. 1994;55:1567–1575. doi: 10.1016/0024-3205(94)00318-1. [DOI] [PubMed] [Google Scholar]
- Radtke KM, Ruf M, Gunter HM, Dohrmann K, Schauer M, Meyer A, Elbert T. Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Translational Psychiatry. 2011;1:e21. doi: 10.1038/tp.2011.21. doi:10.1038/tp.2011.21 tp201121 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychology of Addictive Behaviors. 2014;28:154–162. doi: 10.1037/a0030992. doi:10.1037/a0030992. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Developmental Psychobiology. 2008;50:819–834. doi: 10.1002/dev.20334. doi:10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, Marsit CJ. Maternal smoking during pregnancy and infant stress response: Test of a prenatal programming hypothesis. Psychoneuroendocrinology. 2014;48:29–40. doi: 10.1016/j.psyneuen.2014.05.017. doi:10.1016/j.psyneuen.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Papandonatos GD, Shenassa E, Rodriguez D, Niaura R, Lewinn KZ, Buka SL. Prenatal glucocorticoids and maternal smoking during pregnancy independently program adult nicotine dependence in daughters: A 40-year prospective study. Biological Psychiatry. 2014;75:47–55. doi: 10.1016/j.biopsych.2013.07.024. doi:S0006-3223(13)00679-3 [pii] 10.1016/j.biopsych.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Goodwin MS, Shenassa E, Buka S, Niaura R, Lipsitt LP. Maternal smoking during pregnancy and neonatal behavior: A large-scale community study. Pediatrics. 2009;123:e842–848. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Paster RL, Papandonatos GD, Niaura R, Salisbury AL, Battle C, Lester B. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. Journal of Pediatrics. 2009;154:10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Aagaard K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Epigenomics. 2012;4:115–118. doi: 10.2217/epi.12.7. doi:10.2217/epi.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIBCO Spotfire Inc . SPLUS 8.2 for Solaris/Linux User's Guide. TIBCO Software, Inc; Seattle, Wa: 2010. [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, England LJ. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2013;62:1–19. [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood adversity and epigenetic modulation of the leukocyte glucocorticoid receptor: Preliminary findings in healthy adults. PLoS One. 2012;7:e30148. doi: 10.1371/journal.pone.0030148. doi:10.1371/journal.pone.0030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. Journal of Perinatal Medicine. 2006;34:466–470. doi: 10.1515/JPM.2006.091. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: The case of prenatal smoking and disruptive behavior. Child Development. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Meaney MJ. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Yen SS. The placenta as the third brain. The Journal of Reproductive Medicine. 1994;39:277–280. [PubMed] [Google Scholar]
- Yolton K, Khoury J, Xu Y, Succop P, Lanphear B, Bernert JT, Lester B. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicology and Teratology. 2009;31:356–363. doi: 10.1016/j.ntt.2009.07.004. doi:10.1016/j.ntt.2009.07.004 S0892-0362(09)00143-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]