Abstract
Precision medicine is a rapidly evolving area in the treatment of gynecologic malignancies. Advances in sequencing technology have resulted in an increasing wealth of data regarding the genomic characteristics of ovarian, endometrial, and cervical cancers. These vast new datasets of information have led to novel insights into potential vulnerabilities and therapeutic targets for these cancers. However, unraveling the complex molecular changes within cancer cells to determine how best to attack these targets and to identify effective biomarkers of response remains a significant challenge. In this article, we review the current status of biomarker-driven targeted therapy in gynecologic malignancies.
Keywords: Gynecologic cancer, biomarkers, personalized medicine, targeted therapy, precision medicine
Introduction
Molecular characterization of tumors and the tumor microenvironment has become a leading area of research and development in cancer medicine. The hallmarks of cancer as described by Hanahan and Weinberg provide a roadmap to guide the development of targeted therapy against the key survival capabilities of cancer cells(1). As more data emerge, there is a movement towards leveraging this molecular information to not only identify potential therapeutic targets but also biomarkers of response to targeted therapies. Early successes in biomarker-driven therapies, such as the efficacy of imatinib in bcr-abl chronic myelogenous leukemia(2) or erlotinib in EGFR mutant lung cancer(3, 4) have shaped our approach towards targeted therapy development. However, as our knowledge has grown, it has become clear that the complexity of advanced and recurrent gynecologic malignancies will likely not succumb to single agent therapy. Thus, understanding the robustness of cancer and the intricate signaling networks that drive tumorigenesis and resistance to therapy is paramount(5).
In the field of gynecologic malignancies, recent years have seen an explosion in the wealth of available molecular data. The Cancer Genome Atlas project (TCGA) has published genomic data on both high-grade serous ovarian cancers as well as endometrial cancers(6, 7), and a broad molecular characterization of cervical cancers has also been performed(8). These investigations provide new insight into the molecular underpinnings of gynecologic malignancies, but growing awareness of the complexity of cancer genomics also leads to new challenges in selecting the best therapeutic approach in the clinical setting. In this article, we review recent developments regarding use of biomarkers to rationally select targeted or biologic therapies in gynecologic malignancies (Figure 1). A thorough discussion of the rational development and validation of potential predictive biomarkers is beyond the scope of this article but may be found in the excellent review by de Gramont and colleagues(9).
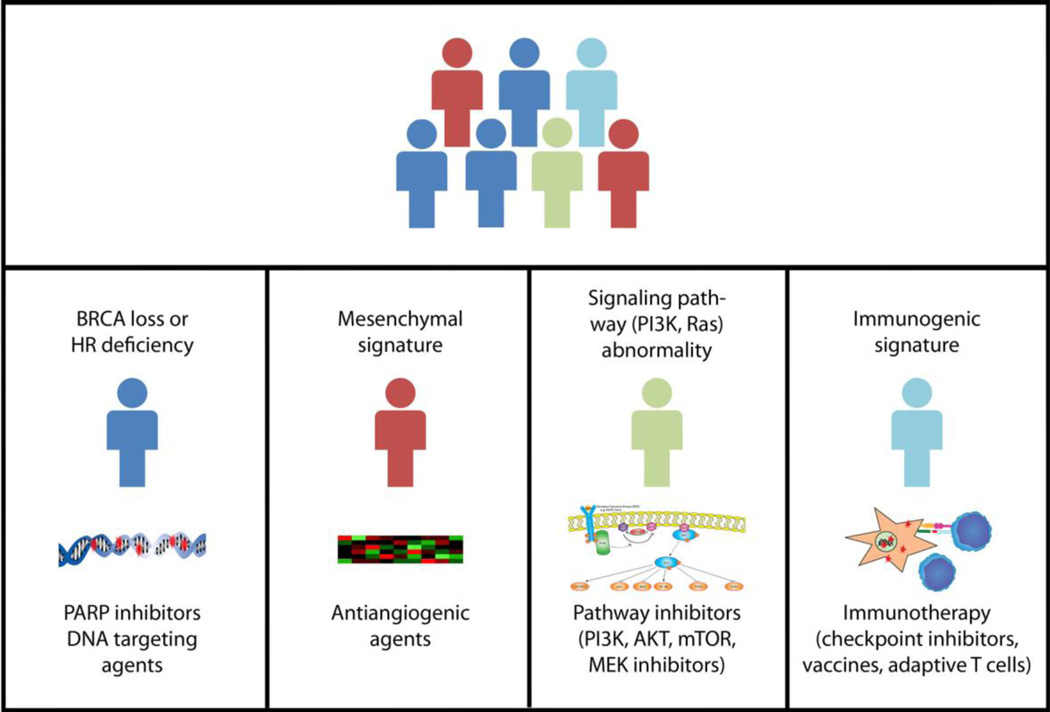
Figure 1.
A hypothetical approach to biomarker driven therapy in gynecologic malignancies. While traditionally these cancers would have been grouped together as one type of malignancy (top panel), various molecular signatures could place each cancer in a different molecular category (represented by a different color; bottom panels). These molecular biomarkers could then be used to identify agents to be preferentially used to treat cancers within the given molecular category. Currently, in gynecologic malignancies, the only validated biomarker for targeted therapy treatment is BRCA mutation for PARP inhibitors.
Poly-ADP-ribose polymerase (PARP) Inhibition
Genomic instability has long been a target of standard chemotherapeutic agents that cause DNA damage or interfere with DNA repair. More recently, targeted agents which impair DNA repair pathways have been investigated in gynecologic malignancies, the most notable of these being drugs that inhibit the action of PARP. PARP-1 plays a pivotal role in the DNA damage response(10) and is essential in the repair of single-strand DNA breaks. When single-strand DNA breaks are not repaired, the cell becomes more dependent upon double-strand break repair mechanisms, such as homologous recombination (HR), leading to the potential for increased toxicity of PARP inhibitors (PARPi’s) in cells that are deficient in HR. This synthetic lethality between PARP inhibition and HR deficiency was demonstrated in two landmark papers in 2005 describing that cells deficient in BRCA2, a key component of the HR pathway, were vulnerable to the effects of PARPi’s(11, 12).
Subsequent clinical studies confirmed activity of PARPi’s in BRCA-deficient tumors, most notably in ovarian cancers. A Phase 2 study of olaparib in women with BRCA-related ovarian cancer demonstrated a response rate of 33%(13). Similarly, a Phase 2 study of veliparib, found a response rate of 26% in women with BRCA-related ovarian cancer(14). Further, olaparib monotherapy as a maintenance therapy following platinum-based chemotherapy for platinum-sensitive recurrence significantly increased progression-free survival, especially in women with a BRCA mutation(15, 16). These findings led to approval of olaparib in the United States in the setting of BRCA-related ovarian cancer that has received at least three prior lines of therapy, and in Europe as a maintenance therapy following response to platinum-therapy after platinum-sensitive recurrence. Further Phase 3 trials of olaparib as maintenance therapy in BRCA-related ovarian cancer, in both the upfront (SOLO1; NCT01844986) or platinum-sensitive recurrent (SOLO2; NCT01874353) setting have completed accrual and are awaiting results.
The approval of olaparib for BRCA-related ovarian cancer marks the first therapy approved for gynecologic malignancies with an associated biomarker. However, growing evidence suggests that responses to PARPi’s will not be limited to BRCA-related tumors alone, as PARPi’s can also exhibit synthetic lethality with HR deficiency independent of loss of BRCA function. Data from TCGA reported that nearly 50% of high-grade serous ovarian carcinomas harbor a genomic alteration in at least one gene associated with HR7), while a separate report detailed that 31% of ovarian carcinomas have a deleterious germline or somatic mutation in one or more of 13 HR genes(17). Indeed, in a Phase 2 trial, Gelmon and colleagues observed a response rate to olaparib of 24% in women without a germline BRCA mutation(18). Similarly, in the ARIEL2 study, the activity of the PARP inhibitor rucaparib was examined in women with recurrent ovarian cancer(19). All women on this study underwent a biopsy prior to proceeding to treatment. Based upon genomic signature, women were classified as having biomarker negative, BRCA-mutant, or BRCA-like tumors, defined as tumors that were BRCA-wild type but had evidence of genome-wide loss of heterozygosity. The study found that women with BRCA-mutant tumors had a response rate of 69% and median PFS of 9.4 months, while response rates and median PFS were 30% and 7.1 months and 13% and 3.7 months for BRCA-like and biomarker-negative tumors, respectively(20).
The results from ARIEL2 not only underscore the power of BRCA loss as a biomarker of response to PARP inhibitors, but also suggest that a BRCA-like signature may identify another population of patients who will also derive benefit. The response rate of 30% (compared to 69% for BRCA-mutant tumors) suggests that further refining of this signature may be necessary. Multiple studies to investigate the activity in PARPi’s in non-BRCA-selected populations and to identify a signature of response are ongoing, including further expansion of the ARIEL2 study as well as a single-agent study of niraparib (QUADRA: NCT02354586) in women who have received at least three prior lines of chemotherapy for ovarian cancer. Additional studies to explore the activity of PARPi’s as maintenance therapy in the non-BRCA-mutant population (ARIEL3, NCT01968213; NOVA, NCT01847274) are also ongoing and should generate further insights into biomarkers of activity.
While the predominant focus of PARPi development has been concentrated in ovarian and breast cancers, activity of these drugs has also been demonstrated in other malignancies with BRCA1/2 loss(21). However, the role of PARPi’s in non-ovarian gynecologic malignancies remains unclear. Data from TCGA suggested that a portion of endometrial cancers share many molecular characteristics with high-grade serous ovarian and triple-negative breast cancers, including high copy number alterations and high rates of TP53 mutation(22). In contrast to ovarian and breast cancers, however, the rates of BRCA1 and BRCA2 mutation or epigenetic silencing in endometrial cancer are low. Pre-clinically, a separate rationale for activity of PARPi’s in endometrial cancer exists, as PTEN-deficient cells are more sensitive to the effects of PARPi’s(23, 24), and PTEN alterations are very common in endometrial cancer. One case of a woman who derived benefit from olaparib for 8 months in the setting of a metastatic endometrial endometrioid cancer that was found to have loss of PTEN but no BRCA1/2 mutation has been reported(25). Results of ongoing studies evaluating the utility of PARP inhibition and exploring biomarkers of response and resistance in endometrial cancer are eagerly anticipated (NCT02208375; NCT02127151). Similarly, pre-clinical data have supported potential synergy between PARPi’s in combination with chemotherapy or with radiation in cervical cancer cell lines(26); early stage trials to evaluate these combinations have been initiated.
Anti-angiogenic agents
Targeting angiogenesis has been widely explored in gynecologic malignancies. In ovarian cancer, activity has been seen with anti-angiogenics as single-agents in the recurrent setting(27–30) as well as in combination with chemotherapy in both initial treatment(31–33) and in the setting of disease recurrence(34–37). Of note, the benefit of adding anti-angiogenics to chemotherapy has been primarily in increasing progression-free survival, with only one study preliminarily reporting a statistically significant increase in overall survival(35). Similarly, anti-angiogenics have been studied in endometrial cancer, where single agent activity has been demonstrated (38) and Phase 2 studies suggest added activity in combination with chemotherapy(39, 40). In cervical cancer, the GOG240 study demonstrated the addition of bevacizumab to chemotherapy in the setting of advanced or recurrent disease improved both progression-free and overall survival(41). However, to date, investigational efforts have not identified a reproducible biomarker of response to anti-angiogenic agents.
Efforts to study predictive biomarkers to anti-angiogenic agents in large randomized studies have yielded mixed results. In 2014, two studies reported the correlation of molecular subtypes of ovarian cancer with outcomes from the ICON7 study, which randomized women to receive either carboplatin and paclitaxel or carboplatin, paclitaxel, and bevacizumab followed by bevacizumab maintenance in first-line therapy for newly diagnosed ovarian cancer. In one study, unsupervised hierarchical clustering was performed on gene expression data from 265 patients enrolled to the ICON7 study in Scotland and identified three major subgroups, two of which had upregulation of angiogenic genes, and one of which had decreased expression of angiogenic genes and upregulation of immune genes(42). In this study, it was observed that the immune signature had a more favorable prognosis; however, it appeared that the additional of bevacizumab to chemotherapy conveyed a detrimental effect in terms of both progressionfree and overall survival in this subset. In the second study, gene expression data from 380 patients enrolled to ICON7 from Germany were generated and classified into the four TCGA subclassifications(43). While patients in all subgroups derived some benefit from the addition of bevacizumab, patients with the mesenchymal subtype benefited most. Molecular subtyping of samples from the GOG-218 study, which also investigated the effects of adding bevacizumab to upfront chemotherapy, have not been reported; however, exploration of immunohistochemistry (IHC) signatures found that tissue VEGF-A expression and microvascular density, as measured by CD31 IHC correlated with the degree of benefit derived from the addition of bevacizumab(44). In cervical cancer, exploratory studies from GOG-240 have suggested a correlation between pre-treatment circulating tumor cells (CTCs) and decline of CTCs during treatment with a lower risk of death upon addition of bevacizumab(45).
The findings from these studies highlight the importance and challenges of identifying reproducible predictive biomarkers for anti-angiogenic response. The possibility that certain subsets of patients may experience decreased survival with addition of an anti-angiogenic to therapy makes further validation of these findings critical. However, while both analyses from ICON7 utilized a molecular approach, it is unclear to what extent the identified molecular subgroups in the two studies overlap. Thus, it will be important in future studies to consider exploring biomarker signatures that can be reproduced and validated in broader sample sets.
Phosphoinositide 3-kinase (PI3K) pathway
It is well established that the PI3K pathway is a key player in tumorigenesis, impacting cell survival, growth, and avoidance of apoptosis. Constitutive activation of this pathway in gynecologic malignancies can occur through mutation and/or aberration of receptor tyrosine kinases and major pathway nodes, including, but not limited to PTEN, PI3KCA, PI3KR1, and AKT(46–53) (Table 1). There are myriad novel agents in development that target this pathway; however, results of early studies in gynecologic malignancies have been modest.
Table 1.
Proportion of molecular aberrations in the PI3K pathway by tumor type UNK, unknown
| Molecular Aberration |
Endometrial | Ovarian | Cervical | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Endometrioid | Non- endometrioid |
High Grade Serous |
Low Grade Serous |
Clear Cell |
Endometrioid | Mucinous | Squamous | Adenocarcinoma | |
| PTEN protein loss |
80 | 5 | 45 | rare | 24 | rare | 13 | 3.6 | |
| PTEN mutation |
43 | 0–11 | rare | <1 | 3– 20 |
5–33 | 2 | 6 | rare |
| PI3KCA mutation |
30–40 | 20 | rare | 2 | 20– 34 |
20 | 13 | 14–48 | 14–25 |
| PI3KCA amplification |
2–4 | 46 | 68 | 20 | 20 | 20 | UNK | UNK | UNK |
| AKT mutation |
2–3 | 0 | rare | rare | UN K |
UNK | UNK | UNK | UNK |
| PI3KR1 mutation |
43 | 12 | UNK | UNK | UN K |
UNK | UNK | UNK | UNK |
| ARID1A mutation |
34 | 0 | UNK | UNK | 29 | 32 | UNK | UNK | UNK |
| IGF-IR over- expression |
75 | rare | 25 | 75 | UN K |
UNK | UNK | UNK | UNK |
Endometrial cancer arguably has the highest number of PI3K pathway aberrations found in any solid tumor; thus, this cancer type has been a primary focus of investigators studying these agents in gynecologic malignancies. To date, the majority of studies have consisted of evaluation of agents targeting single nodes, including mTORC1, AKT, and PI3K. Few objective responses have been observed, especially among patients that have been previously treated with cytotoxic chemotherapy (54–58). Similar to findings in other solid tumors, it would appear that inhibiting only one node along the pathway is not sufficient to inhibit tumor growth. No doubt this is related to the complexity of the PI3K pathway, including feedback loops and crosstalk between pathways. One example among many is the compensatory activation of AKT seen after inhibition of mTORC1(59).
Several investigators have attempted to identify potential biomarkers of response to PI3K-directed therapies from the wealth of potential markers. Thus far, there has been little correlation between aberrations in the pathway and response to therapy. In a study of temsirolimus in recurrent endometrial cancer, modest activity was found in tumors independent of PTEN status(54). The National Cancer Institute of Canada (NCIC) clinical trials group analyzed archival tumor samples from 96 women treated with single agent mTORC1 inhibitors (temsirolimus, ridaforalimus). Although mutations in pathway nodes and aberrations such as PTEN loss were encountered, there was no correlation observed between molecular findings and response to therapy(60). Conversely, Meyer and colleagues sought to determine if there was an association between marker expression and resistance to single agent everolimus therapy. Although there was no one marker that predicted resistance, the combination of KRAS mutation and expression of phosphorylated s6, a downstream marker of PI3K pathway activity, were associated with nonresponse to therapy(61). Of note, the majority of the molecular analyses have been performed on archival tissue from primary diagnosis, and it is unknown how much the findings from the primary archival tumor will reflect the molecular profile in the recurrent setting. The use of concurrent biopsies holds promise to refine utility of molecular aberrations to predict response to therapy.
The PI3K pathway may also be of importance in ovarian cancer, especially as a mechanism of resistance to paclitaxel and carboplatin(62). Initial findings of PI3KCA amplification and PTEN loss led to excitement for mTORC1 inhibition in this cancer. Similar to endometrial cancer, single agent temsirolimus yielded response rates of only 9% and a 6 month PFS of 24%, and this agent was not further explored(63). To assess the ability to impact chemoresistance, a completed phase Ib/II trial (NCT00756847) evaluated the combination of XL147, a PI3K inhibitor, with paclitaxel and carboplatin in patients with advanced solid tumors. Preliminary results suggested the combination was well tolerated and induced tumor regression in a heavily pretreated patient population. Favorable responses in ovarian cancer led to a dose expansion in this tumor type although final results have not yet been presented(64).
Although initial explorations of PI3K pathway inhibitors in ovarian cancer were in all histologies, molecular data reveal that these agents may be more successful in the rare subtypes. Activation of the PI3K pathway in low grade serous ovarian cancer occurs through expression of insulin-like growth factor receptor (IGF-R). King and colleagues reported high IGF-1 expression and upregulation of phosphorylated AKT in response to IGF-1 treatment. This effect was abrogated by treatment with an AKT inhibitor (MK-2206) and IGF-1R knockdown(65). Clear cell and endometrioid ovarian cancers have a high proportion of mutations in PI3KCA and PTEN, as well as PTEN protein loss and PI3KCA amplification(66, 67). Thus far, these treatments have primarily been explored in expansions of phase I studies, however, a Phase III study combining temsirolimus with paclitaxel and carboplatin for the treatment of advanced clear cell ovarian cancer has recently completed accrual (NCT01196429).
Not surprisingly, single agent studies of PI3K pathway-directed agents in cervical cancer have also demonstrated limited success despite a moderate proportion of tumors with PIK3CA mutation and PTEN protein loss(68, 69). Although a stable disease rate of 58% was achieved with temsirolimus in the phase II setting, only one of 33 evaluable patients with cervical cancer had an objective response. No association was found between PI3K pathway abnormalities including PTEN and PI3KCA expression, copy number and methylation and clinical benefit on this trial(70).
Retrovirus-associated DNA sequences (Ras)/v-raf 1 murine leukemia viral oncogene homolog 1 (Raf) Pathway
The Ras/raf pathway plays a critical role in the regulation of cellular survival, proliferation, motility and avoidance of apoptosis(71). Further, this pathway has been implicated in targeted therapy resistance, including agents targeting EGFR and PI3K(72). Activation of the Ras/Raf pathway can result from mutations in RAS, RAF or MEK(71). Similar to the PI3K pathway, activation of upstream receptor tyrosine kinases can also stimulate pathway activity. Clinically, MEK inhibitors (MEKi’s) have been explored in the most depth for gynecologic tumors. Predictors of response to MEK inhibition may be extrapolated from work in other solid tumors. Thus far, activating mutations in BRAF render tumors highly sensitive to MEK inhibition in melanoma and KRAS or NRAS mutation may also affect response(73–76).
Of the gynecologic cancers, low grade serous and mucinous ovarian cancers have the highest rates of Ras/Raf pathway aberrations. KRAS mutations can be seen in up to 40% of low grade and 60% of mucinous tumors(77–81). BRAF mutations are more rare, occurring in approximately 5% of low grade and 10% of mucinous tumors(77–80). To date, MEKi’s have not been explored in mucinous ovarian cancer, likely due to the rare nature of this diagnosis. A phase II GOG trial of selumetinib, a MEK inhibitor, for low grade serous ovarian cancer, found promising activity, with 15% of patients achieving an objective response and 65% with stable disease(80). Based on these results, two large phase III studies have been activated to evaluate the efficacy of MEKi in comparison to standard of care therapy in low grade serous ovarian cancer (GOG281, NCT02101788; MILO NCT01849874).
Farley and colleagues explored potential molecular correlates of response to selumetinib therapy. Thirty four patients had adequate tissue for molecular analysis, which revealed no correlation between KRAS, BRAF, or NRAS mutation and response. Of note, 82% of the specimens were from primary tumors and not recurrent metastatic disease immediately prior to treatment. These data suggest that other cell signaling pathways can activate the Ras/Raf pathway independent of mutation status and may be responsible for resistance to MEKi therapy.
Endometrial cancer is known to have a high prevalence of KRAS mutations, with up to 30% in endometrioid and up to 10% in nonendometrioid histology types(82). Given this prevalence, the GOG recently reported a Phase II trial of selumetinib in recurrent endometrial cancer. Results were modest, achieving an objective response rate of 6% and a stable disease rate of 26%(83) (Coleman). These modest results have led to a series of combination therapies with relevant pathways to attempt to maximize clinical impact.
On the Horizon
P53
P53 mutation is arguably the most prevalent molecular aberration noted across advanced solid tumors. Among gynecologic malignancies, the proportion with p53 mutation can range from a low of 5% (early grade endometrioid endometrial cancer) to a high of 98% (high grade serous ovarian cancer)(7, 84). Until recently, this mutation was largely thought to be “undruggable”, however, several agents are currently in development which may yield improved clinical response for tumors bearing a p53 mutation. The Wee1 inhibitor, AZD1775, acts on a key regulator of the G2 cell cycle checkpoint, which is essential for DNA damage repair among tumors with dysfunctional p53. This agent has been evaluated in combination with standard paclitaxel and carboplatin in the setting of recurrent platinum-sensitive ovarian cancer, demonstrating improved response rate and progression free survival in the setting of minimal additional toxicity(85). No clinical studies have been reported to indicate which patients may garner the most benefit. In addition to Wee1 inhibitors, there are myriad molecules under evaluation that appear to reactivate mutant p53 or restore the activity of wildtype p53(86). The development of concurrent biomarkers that predict response to therapy will be essential, as each unique p53 mutation in gynecologic malignancies may have varying impact on tumorigenesis and ultimately, the clinical utility of these agents.
Immunotherapy
Immune evasion and escape has long been known to be a key factor in tumorigenesis and malignant potential(1). There are an extraordinary number of immunotherapy agents currently being explored(87); however, clear characterization of biomarkers that will identify cancers against which tumor immunotherapy might be most active has been elusive. PD-L1 expression has been linked to response to PD-1/PD-L1 immune checkpoint blockade(88, 89). However, significant responses have been observed even in patients whose tumors are classified as PD-L1 “negative”(89–91). In gynecologic malignancies, experience with immunomodulatory agents remains limited. Early Phase 1 trials of nivolumab, avelumab, and pembrolizumab have suggested response rates of 11 to 15% in otherwise unselected recurrent ovarian cancers(92–94). However, a recent study suggested that BRCA-mutated ovarian cancers are associated with markers of higher immunogenicity through an increased higher CD8+/ CD4+ ratio of tumor-infiltrating lymphocytes and higher peritumoral T cells, raising the question of whether these tumors might also be more responsive to immunotherapy(95). In endometrial cancer, POLE hypermutated tumors and tumors with microsatellite instability (MSI) have similarly been linked with increased tumor-infiltrating and peritumoral lymphocytes, while also having higher expression of PD-L1(96). MSI may be of particular interest in endometrial cancer, as previous studies have suggested that the presence of mismatch repair deficiency is linked to response to PD-1 blockade(97). These insights into factors linked to tumor immunogenicity may provide valuable information as immune-based therapies move forward in development in gynecologic cancers. Immunotherapies are also an active area of exploration in cervical cancer, where adoptive T-cell therapy in nine women with metastatic cervical cancer resulted in two complete responses and one partial response, with the two complete responses ongoing at 15 and 22 months.
Combination Targeted Therapy
Given the results presented above, it seems clear that targeting multiple nodes across multiple pathways may be necessary for success in gynecologic malignancies. This is supported by the low objective response rates in molecularly aberrant tumors to “appropriately targeted therapy”. Even among targeted therapies with higher objective responses, e.g. PARPi’s in BRCA-mutant ovarian cancer, universal responses have not been observed. These findings support the presence of underlying resistance and/or cross-talk that tempers the impressive responses seen in the in vitro setting. This rationale has led to the exploration of numerous cross-pathway combinations in gynecologic malignancies, including PI3K/MEK, PARP/anti-angiogenic, PARP/PI3K, PARP/immunotherapy, and PI3K/hormonal. Although more promising clinical results have been reported, success of many of these combinations has been limited due to excessive toxicity and difficulty achieving biological relevant doses of each agent. Further, rational selection of effective combinations has not yet been optimized.
Summary
The rapid advance of molecular discovery in gynecologic malignancies has led to increased excitement and enthusiasm regarding the promises of targeted therapies. A greater understanding of the molecular drivers of these malignancies will be critical to fully harnessing the potential of biomarker-driven therapies. While the ability to rapidly gather genomic information on an individual tumor is now providing us with unprecedented access to molecular data, our ability to interpret these findings to greatest effect continues to develop.
Despite marked abnormality in a pathway, clinical impact of targeted therapy can be limited. There are numerous reasons behind this phenomenon. Given the vast landscape of genomic alterations within cancer cells, it is not always easy to dissect out “driver” alterations that are critical to the cancer cell’s survival from “bystander” or “passenger” alterations that occur only in the context of the genomic instability of the cancer cell. In some areas, we have begun to learn how to identify these critical dependencies, and the identification of BRCA1/2 mutation and potentially HR deficiency as biomarkers of response to PARPi’s in ovarian cancer has been a marked advance in the field. However, in other areas, such as with PI3K signaling in endometrial cancer, additional work is needed to delineate when a given cancer with an alteration in the pathway of interest might truly be vulnerable to an agent targeting that pathway. The challenges of identifying biomarkers that can accurately predict the response to a given targeted therapy are not unique to gynecologic malignancies. As part of a goal to expand the ability to practice precision medicine, the National Cancer Institute (NCI) has launched the NCI-MATCH trial. In this study, the patient’s tumor is molecularly tested, and then patients are “matched” with an appropriately targeted therapy based upon the tumor’s molecular characteristics, if one is available.
Other considerations complicate the development of biomarker-driven therapies, such as the development of tumor resistance. Whether innate, acquired, or even adaptive, it is essential to identify mechanisms of resistance, as this information may guide regimen design or provide insights into how to avoid or overcome the development of resistance. Additionally, tumor heterogeneity must be considered, as it is possible that a biopsy in one site may not represent the molecular milieu of other tumor sites. Thus, it is possible that one set of tumor sites may harbor different molecular vulnerabilities than others. Indeed, future clinical trials of targeted therapies incorporating tissue/serum collection and planned exploration of biomarkers will be critical to advance our understanding of how best to select biomarker-driven therapies in gynecologic malignancies as well as all solid tumors.
Highlights.
Applications for targeted therapy in gynecologic cancers are growing rapidly
Resistance to targeted therapy may be innate, acquired, or adaptive
Translational endpoints will be essential to maximize response to targeted therapy
Acknowledgments
K12 CA088084 K12 Calabresi Scholar Award (SW)
NIH 2P50 CA098258-06 SPORE in Uterine Cancer (SW)
NIH 2P50CA083639 SPORE in Ovarian Cancer (SW)
Conflict of Interest
Dr. Liu has served as a consultant for Genentech/Roche and AstraZeneca, and is the site PI on trials sponsored by Genentech/Roche, AstraZeneca, Boston Biomedical, Atara Biotherapeutics, and Merrimack Pharmaceuticals. Dr. Westin serves as a consultant for Roche and AstraZeneca and receives research support from AstraZeneca, Novartis, and Critical Outcomes Technology, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 4.Tsao MS, Sakurada A, Cutz JC, Zhu CQ, Kamel-Reid S, Squire J, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. N Engl J Med. 2005;353(2):133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 5.Westin JR. Busting robustness: using cancer's greatest strength to our advantage. Future Oncol. 2015;11(1):73–77. doi: 10.2217/fon.14.49. [DOI] [PubMed] [Google Scholar]
- 6.Getz G, Gabriel SB, Cibulskis K, Lander E, Sivachenko A, Sougnez C, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ojesina AI, Lichtenstein L, Freeman SS, Pedamallu CS, Imaz-Rosshandler I, Pugh TJ, et al. Landscape of genomic alterations in cervical carcinomas. Nature. 2014;506(7488):371–375. doi: 10.1038/nature12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gramont A, Watson S, Ellis LM, Rodon J, Tabernero J, de Gramont A, et al. Pragmatic issues in biomarker evaluation for targeted therapies in cancer. Nat Rev Clin Oncol. 2015;12(4):197–212. doi: 10.1038/nrclinonc.2014.202. [DOI] [PubMed] [Google Scholar]
- 10.Weaver AN, Yang ES. Beyond DNA Repair: Additional Functions of PARP-1 in Cancer. Front Oncol. 2013;3:290. doi: 10.3389/fonc.2013.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 12.Helleday T, Bryant HE, Schultz N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle. 2005;4(9):1176–1178. doi: 10.4161/cc.4.9.2031. [DOI] [PubMed] [Google Scholar]
- 13.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 14.Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–391. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 16.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 17.Pennington KP, Walsh T, Harrell MI, Lee MK, Pennil CC, Rendi MH, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. The lancet oncology. 2011;12(9):852–861. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 19.McNeish IA, Oza AM, Coleman RL, Scott CL, Konecny GE, Tinker A, et al. Results of ARIEL2: A Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. J Clin Oncol. 2015;33 (suppl; abstr 5508) [Google Scholar]
- 20.Swisher E, Oza AM, Coleman RL, Scott C, Lin K, Dominy E, et al. Tumor BRCA mutation or high genomic LOH identify ovarian cancer patients likely to respond to rucaparib: Interim results for ARIEL2 clinical trial. Gyn Onc. 2015;138(supplement 1):4. [Google Scholar]
- 21.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33(12):1397–1406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO molecular medicine. 2009;1(6–7):315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2(53) doi: 10.1126/scitranslmed.3001538. 53ra75. [DOI] [PubMed] [Google Scholar]
- 25.Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, et al. Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol. 2011;8(5):302–306. doi: 10.1038/nrclinonc.2011.42. [DOI] [PubMed] [Google Scholar]
- 26.Shunkwiler L, Ferris G, Kunos C. Inhibition of Poly(ADP-Ribose) Polymerase Enhances Radiochemosensitivity in Cancers Proficient in DNA Double-Strand Break Repair. Int J Mol Sci. 2013;14(2):3773–3785. doi: 10.3390/ijms14023773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33):5165–5171. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 28.Cannistra SA, Matulonis UA, Penson RT, Hambleton J, Dupont J, Mackey H, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 29.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33):5601–5606. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirte H, Lheureux S, Fleming GF, Sugimoto A, Morgan R, Biagi J, et al. A phase 2 study of cediranib in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: a trial of the Princess Margaret, Chicago and California Phase II Consortia. Gynecol Oncol. 2015;138(1):55–61. doi: 10.1016/j.ygyno.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 31.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 32.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 33.du Bois A, Huober J, Stopfer P, Pfisterer J, Wimberger P, Loibl S, et al. A phase I open-label dose-escalation study of oral BIBF 1120 combined with standard paclitaxel and carboplatin in patients with advanced gynecological malignancies. Ann Oncol. 2010;21(2):370–375. doi: 10.1093/annonc/mdp506. [DOI] [PubMed] [Google Scholar]
- 34.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ledermann JA, Raja FA, Embleton A, Rustin GJS, Jayson G, Kaye SB. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: results of the ICON6 trial. Eur J Cancer. 2013;49 (Suppl 3: LBA 10) [Google Scholar]
- 36.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Kristensen G, Sorio R, et al. AURELIA: A randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)- resistant ovarian cancer (OC) J Clin Oncol. 2012;30 doi: 10.1200/JCO.2013.51.4489. (suppl; abstr LBA5002^) [DOI] [PubMed] [Google Scholar]
- 37.Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. A phase III randomized controlled clinical trial of carboplatin and paclitaxel alone or in combination with bevacizumab followed by bevacizumab and secondary cytoreductive surgery in platinum-sensitive, recurrent ovarian, peritoneal primary and fallopian tube cancer (Gynecologic Oncology Group 0213) Gyn Onc. 2015;137(Supplement 1):3–4. [Google Scholar]
- 38.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, et al. Phase II Trial of Bevacizumab in Recurrent or Persistent Endometrial Cancer: A Gynecologic Oncology Group Study. J Clin Oncol. 2011;29(16):2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aghajanian C, Filiaci VL, Dizon DS, Carlson JW, Powell MA, Secord AA, et al. A randomized phase II study of paclitaxel/carboplatin/bevacizumab, paclitaxel/carboplatin/temsirolimus and ixabepilone/carboplatin/bevacizumab as initial therapy for measurable stage III or IVA, stage IVB or recurrent endometrial cancer, GOG-86P. J Clin Oncol. 2015;33 (suppl; abstr 5500) [Google Scholar]
- 40.Lorusso D, Ferrandina G, Colombo N, Pignata S, Salutari V, Maltese G, et al. Randomized phase II trial of carboplatin-paclitaxel (CP) compared to carboplatin-paclitaxel-bevacizumab (CP-B) in advanced (stage III–IV) or recurrent endometrial cancer: The MITO END-2 trial. J Clin Oncol. 2015;33 doi: 10.1016/j.ygyno.2019.10.013. (suppl; abstr 5502) [DOI] [PubMed] [Google Scholar]
- 41.Tewari KS, Sill MW, Long HJ, 3rd, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gourley C, McCavigan A, Perren T, Paul J, Michie CO, Churchman M, et al. Molecular subgroup of high-grade serous ovarian cancer (HGSOC) as a predictor of outcome following bevacizumab. J Clin Oncol. 2014;32 (5s (Suppl; abstr 5502)) [Google Scholar]
- 43.Winterhoff BJN, Kommoss S, Oberg AL, Wang C, Riska SM, Konecny GE, et al. Bevacizumab and improvement of progression-free survival (PFS) for patients with the mesenchymalmolecular subtype of ovarian cancer. J Clin Oncol. 2014;32 5s (suppl; abstr 5509)) [Google Scholar]
- 44.Birrer MJ, Choi YJ, Brady MF, Mannel RS, Burger RA, Wei W, et al. Retrospective analysis of candidate predictive tumor biomarkers (BMs) for efficacy in the GOG-0218 trial evaluating front-line carboplatin–paclitaxel (CP) ± bevacizumab (BEV) for epithelial ovarian cancer (EOC) J Clin Oncol. 2015;33 (suppl; abstr 5505) [Google Scholar]
- 45.Tewari KS, Sill M, Monk BJ, Penson RT, Lankes HA, Leitao MM, et al. Impact of circulating tumor cells (CTCs) on overall survival among patients treated with chemotherapy plus bevacizumab for advanced cervical cancer: An NRG Oncology/Gynecologic Oncology Group study. Gyn Onc. 2015;137(Supplement 1):12. [Google Scholar]
- 46.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 47.Bartlett JM. Biomarkers and patient selection for PI3K/Akt/mTOR targeted therapies: current status and future directions. Clin Breast Cancer. 10(Suppl 3):S86–S95. doi: 10.3816/CBC.2010.s.017. [DOI] [PubMed] [Google Scholar]
- 48.Oda K, Stokoe D, Taketani Y, McCormick F. High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res. 2005;65(23):10669–10673. doi: 10.1158/0008-5472.CAN-05-2620. [DOI] [PubMed] [Google Scholar]
- 49.Bigsby RM, Li AX, Bomalaski J, Stehman FB, Look KY, Sutton GP. Immunohistochemical study of HER-2/neu, epidermal growth factor receptor, and steroid receptor expression in normal and malignant endometrium. Obstet Gynecol. 1992;79(1):95–100. [PubMed] [Google Scholar]
- 50.Dutt A, Salvesen HB, Greulich H, Sellers WR, Beroukhim R, Meyerson M. Somatic mutations are present in all members of the AKT family in endometrial carcinoma. Br J Cancer. 2009;101(7):1218–1219. doi: 10.1038/sj.bjc.6605301. author reply 20-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci U S A. 2008;105(25):8713–8717. doi: 10.1073/pnas.0803379105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J Clin Oncol. 2006;24(29):4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 53.Shoji K, Oda K, Nakagawa S, Hosokawa S, Nagae G, Uehara Y, et al. The oncogenic mutation in the pleckstrin homology domain of AKT1 in endometrial carcinomas. Br J Cancer. 2009;101(1):145–148. doi: 10.1038/sj.bjc.6605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol. 2011;29(24):3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray-Coquard I, Favier L, Weber B, Roemer-Becuwe C, Bougnoux P, Fabbro M, et al. Everolimus as second- or third-line treatment of advanced endometrial cancer: ENDORAD, a phase II trial of GINECO. British journal of cancer. 2013;108(9):1771–1777. doi: 10.1038/bjc.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slomovitz BM, Lu KH, Johnston T, Coleman RL, Munsell M, Broaddus RR, et al. A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma. Cancer. 2010 doi: 10.1002/cncr.25515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Myers AP, Broaddus R, Makker V, Konstantinopoulos P, Drapkin R, Horowitz NS, et al. Phase II, two-stage, two-arm, PIK3CA mutation stratified trial of MK-2206 in recurrent endometrial cancer. J Clin Oncol. 2013;31 doi: 10.1002/ijc.32783. (suppl; abstr 5524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsoref D, Welch S, Lau S, Biagi J, Tonkin K, Martin LA, et al. Phase II study of oral ridaforolimus in women with recurrent or metastatic endometrial cancer. Gynecol Oncol. 2014;135(2):184–189. doi: 10.1016/j.ygyno.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 59.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackay HJ, Eisenhauer EA, Kamel-Reid S, Tsao M, Clarke B, Karakasis K, et al. Molecular determinants of outcome with mammalian target of rapamycin inhibition in endometrial cancer. Cancer. 2013 doi: 10.1002/cncr.28414. [DOI] [PubMed] [Google Scholar]
- 61.Meyer LA, Slomovitz BM, Djordjevic B, Westin SN, Iglesias DA, Munsell MF, et al. The search continues: looking for predictive biomarkers for response to mammalian target of rapamycin inhibition in endometrial cancer. Int J Gynecol Cancer. 2014;24(4):713–717. doi: 10.1097/IGC.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, et al. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23(34):5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 63.Behbakht K, Sill MW, Darcy KM, Rubin SC, Mannel RS, Waggoner S, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123(1):19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Traynor AM, Kurzrock R, Bailey HH, Attia S, Scheffold C, van Leeuwen B, et al. A phase I safety and pharmacokinetic (PK) study of the PI3K inhibitor XL147 (SAR245408) in combination with paclitaxel (P) and carboplatin (C) in patients (pts) with advanced solid tumors. J Clin Oncol. 2010;28 15s (suppl; abstr 3078) (15s (suppl; abstr 3078)) [Google Scholar]
- 65.King ER, Zu Z, Tsang YT, Deavers MT, Malpica A, Mok SC, et al. The insulin-like growth factor 1 pathway is a potential therapeutic target for low-grade serous ovarian carcinoma. Gynecol Oncol. 2011;123(1):13–18. doi: 10.1016/j.ygyno.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazzoletti M, Broggini M. PI3K/AKT/mTOR inhibitors in ovarian cancer. Curr Med Chem. 2010;17(36):4433–4447. doi: 10.2174/092986710794182999. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Helland A, Holm R, Kristensen GB, Borresen-Dale AL. PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat. 2005;25(3):322. doi: 10.1002/humu.9316. [DOI] [PubMed] [Google Scholar]
- 68.Zagouri F, Sergentanis TN, Chrysikos D, Filipits M, Bartsch R. Molecularly targeted therapies in cervical cancer. A systematic review. Gynecol Oncol. 2012;126(2):291–303. doi: 10.1016/j.ygyno.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Wright AA, Howitt BE, Myers AP, Dahlberg SE, Palescandolo E, Van Hummelen P, et al. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer. 2013;119(21):3776–3783. doi: 10.1002/cncr.28288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tinker AV, Ellard S, Welch S, Moens F, Allo G, Tsao MS, et al. Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. A trial of the NCIC Clinical Trials Group (NCIC CTG IND 199) Gynecol Oncol. 2013;130(2):269–274. doi: 10.1016/j.ygyno.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 71.Ahn NG, Nahreini TS, Tolwinski NS, Resing KA. Pharmacologic inhibitors of MKK1 and MKK2. Methods in enzymology. 2001;332:417–431. doi: 10.1016/s0076-6879(01)32219-x. [DOI] [PubMed] [Google Scholar]
- 72.Abrams SL, Steelman LS, Shelton JG, Wong EW, Chappell WH, Basecke J, et al. The Raf/MEK/ERK pathway can govern drug resistance, apoptosis and sensitivity to targeted therapy. Cell Cycle. 2010;9(9):1781–1791. doi: 10.4161/cc.9.9.11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dummer R, Robert C, Chapman PB, Sosman JA, Middleton M, Bastholt L, et al. AZD6244 (ARRY- 142886) vs temozolomide (TMZ) in patients (pts) with advanced melanoma: An open-label, randomized, multicenter, phase II study. J Clin Oncol. 2008;26 Suppl; abstr 9033. [Google Scholar]
- 74.Patel SP, Lazar AJ, Mahoney S, Vaughn C, Gonzalez N, Papadopoulos NE, et al., editors. Clinical responses to AZD6244 (ARRY-142886)-based combination therapy stratified by gene mutations in patients with metastatic melanoma. 2010 ASCO Molecular Markers. 2010 doi: 10.1002/cncr.27790. [DOI] [PubMed] [Google Scholar]
- 75.Friday BB, Adjei AA. Advances in targeting the Ras/Raf/MEK/Erk mitogen-activated protein kinase cascade with MEK inhibitors for cancer therapy. Clin Cancer Res. 2008;14(2):342–346. doi: 10.1158/1078-0432.CCR-07-4790. [DOI] [PubMed] [Google Scholar]
- 76.Chapman MS, Miner JN. Novel mitogen-activated protein kinase kinase inhibitors. Expert Opin Investig Drugs. 2011;20(2):209–220. doi: 10.1517/13543784.2011.548803. [DOI] [PubMed] [Google Scholar]
- 77.Wong KK, Tsang YT, Deavers MT, Mok SC, Zu Z, Sun C, et al. BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol. 2010;177(4):1611–1617. doi: 10.2353/ajpath.2010.100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grisham RN, Iyer G, Garg K, DeLair D, Hyman DM, Zhou Q, et al. BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer. 2013;119(3):548–554. doi: 10.1002/cncr.27782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tsang YT, Deavers MT, Sun CC, Kwan SY, Kuo E, Malpica A, et al. KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol. 2013;231(4):449–456. doi: 10.1002/path.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Farley J, Brady WE, Vathipadiekal V, Lankes HA, Coleman R, Morgan MA, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 82.Koul A, Willen R, Bendahl PO, Nilbert M, Borg A. Distinct sets of gene alterations in endometrial carcinoma implicate alternate modes of tumorigenesis. Cancer. 2002;94(9):2369–2379. doi: 10.1002/cncr.10498. [DOI] [PubMed] [Google Scholar]
- 83.Coleman R, Sill M, Thaker PH, De Geest K, Street D, McGuire W, et al. A phase II evaluation of AZD6244, a selective MEK-1/2 inhibitor in the treatment of recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. Gynecol Oncol. 2013;130(1):e12–e13. doi: 10.1016/j.ygyno.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalifa MA, Mannel RS, Haraway SD, Walker J, Min KW. Expression of EGFR, HER-2/neu, P53, and PCNA in endometrioid, serous papillary, and clear cell endometrial adenocarcinomas. Gynecol Oncol. 1994;53(1):84–92. doi: 10.1006/gyno.1994.1092. [DOI] [PubMed] [Google Scholar]
- 85.Oza A, Weberpals JI, Provencher DM, Grischke E-M, Hall M, Uyar D, et al. An international, biomarker-directed, randomized, phase II trial of AZD1775 plus paclitaxel and carboplatin (P/C) for the treatment of women with platinum-sensitive, TP53-mutant ovarian cancer. J Clin Oncol. 2015;33 doi: 10.1158/1078-0432.CCR-20-0219. (suppl; abstr 5506) [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Sun Y. Targeting p53 for Novel Anticancer Therapy. Transl Oncol. 2010;3(1):1–12. doi: 10.1593/tlo.09250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith EL, Zamarin D, Lesokhin AM. Harnessing the immune system for cancer therapy. Curr Opin Oncol. 2014;26(6):600–607. doi: 10.1097/CCO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 88.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 90.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 91.Motzer RJ, Rini BI, McDermott DF, Redman BG, Kuzel TM, Harrison MR, et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J Clin Oncol. 2015;33(13):1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015 Sep 8; doi: 10.1200/JCO.2015.62.3397. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 93.Disis ML, Patel MR, Pant S, Infante JR, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: A phase Ib, open-label expansion trial. J Clin Oncol. 2015;33 (suppl; abstr 5509) [Google Scholar]
- 94.Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. J Clin Oncol. 2015;33 (suppl; abstr 5510) [Google Scholar]
- 95.Strickland K, Howitt BE, Rodig SJ, Ritterhouse L, D'Andrea AD, Matulonis U, et al. Tumor infiltrating and peritumoral T cells and expression of PD-L1 in BRCA1/2-mutated high grade serous ovarian cancers. J Clin Oncol. 2015;33 (suppl abstr 5512) [Google Scholar]
- 96.Howitt BE, Sholl LM, Ritterhouse L, Watkins JC, Rodig SJ, Strickland K, et al. Association of POLE-mutated and MSI endometrial cancers with an elevated number of tumor-infiltrating and peritumoral lymphocytes and higher expression of PD-L1. J Clin Oncol. 2015;33 doi: 10.1001/jamaoncol.2015.2151. (suppl; abstr 5511) [DOI] [PubMed] [Google Scholar]
- 97.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]