Abstract
BACKGROUND
Preterm birth remains a significant cause of neonatal morbidity and mortality. Women with a prior preterm birth are at risk for recurrent preterm birth. Treatment with 17-alpha-hydroxyprogesterone caproate has become standard-of-care for women with prior preterm birth to help reduce this risk. Factors that affect a woman’s decision to use this medication are largely unknown.
OBJECTIVE
The objective of our study was to investigate patient level barriers to 17-alpha-hydroxyprogesterone caproate use. We studied a cohort of women eligible for 17-alpha-hydroxyprogesterone caproate with the hypothesis that 17-alpha-hydroxyprogesterone caproate is underutilized and certain patient characteristics, such as obstetrical history, influence its use.
STUDY DESIGN
A cross-sectional study of all women seen at a specialty prematurity clinic from 2009 to 2013 was performed. Women with a singleton pregnancy were included if they had a prior spontaneous preterm birth. Chi-squared tests were performed for univariate analyses. Multivariable logistic regression was used to control for confounders.
RESULTS
243 women had 17-alpha-hydroxyprogesterone caproate recommended to them based on prior obstetrical history. There were 218 women with a pregnancy during our study period that were included in our analysis. 163 (74.7%) had documented 17-alpha-hydroxyprogesterone caproate use. Women were more likely to accept 17-alpha-hydroxyprogesterone caproate if they had a history of a second trimester loss only (OR 2.32, 1.17–4.58) or received recommendation for cerclage due to a short cervical length (OR 4.12, 1.55–10.99). Women with a prior full-term birth were less likely to accept 17-alpha-hydroxyprogesterone caproate (OR 0.48, 0.26–0.89), especially when the prior full-term birth was subsequent rather than prior to the preterm birth (OR 0.19, 0.08–0.47). Race, obesity, and insurance status did not impact 17-alpha-hydroxyprogesterone caproate use. There was no difference in the rate of spontaneous preterm birth between those who used and did not use 17-alpha-hydroxyprogesterone caproate (37.2 vs. 34.0%, p=0.7).
CONCLUSION
Past obstetric history impacted 17-alpha-hydroxyprogesterone caproate use. This study identifies biases regarding 17-alpha-hydroxyprogesterone caproate at the patient level and can be used to develop strategies to increase its use. However, the similarity in the spontaneous preterm birth rate between users and non-users highlights the importance of identifying specific populations where 17-alpha-hydroxyprogesterone caproate is and is not effective in preventing preterm birth.
Keywords: 17-alpha-hydroxyprogesterone caproate, acceptance, barriers, prematurity prevention program, preterm birth
INTRODUCTION
Preterm birth is a leading cause of immediate and long-term neonatal morbidity and mortality, posing a significant burden on health care resources. Despite the recent decrease in the rate of preterm delivery in the United States, preterm birth remains a noteworthy problem. (1) One of the strongest risk factors for preterm birth is a history of a prior preterm birth. (2) In 2003, the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (NICHD-MFMU) Network published a multicenter, randomized, placebo-controlled trial that demonstrated a 34% reduction in recurrent preterm delivery with the weekly administration of 17-alpha-hydroxyprogesterone caproate (17OHP-C) beginning at 16 to 20 weeks’ gestation in women with singleton pregnancies and a history of spontaneous preterm birth. (3) Since then, the American College of Obstetricians and Gynecologists (ACOG) has issued two Committee Opinions supporting and encouraging the use of 17OHP-C among eligible women. (4–5) The Society for Maternal-Fetal Medicine has also provided evidence-based clinical guidelines recommending both the use of the 17OHP-C as well as cervical cerclage placement for short cervix in women with a history of spontaneous preterm birth. (6–7)
Despite its known benefit in reducing the rate of recurrent preterm birth, there are many obstacles to ensuring the use of 17OHP-C in clinical practice. These barriers occur at the patient, provider, and health systems level. Patients may not perceive themselves at risk for preterm birth, may not know this intervention is available, or may be concerned regarding the risks and side effects of the medication. Providers may not be aware of the availability or effectiveness of this intervention. Lastly, at the health systems level, women may not be seeking prenatal care at an early enough gestational age to initiate the medication, may have difficulty in obtaining it and arranging weekly administration, or their insurance may not fully cover the medication. (8–9) In a survey of general obstetrician-gynecologists exploring attitudes regarding the use of 17OHP-C, respondents reported that they prescribed 17OHP-C to only 59% of eligible patients. (10) While evidence regarding the efficacy of 17OHP-C in reducing the risk of recurrent spontaneous preterm birth continues to mount, it is of critical importance to understand what biases affect its use in clinical practice. (9, 11)
To that end, the objective of our study was to investigate patient level barriers to 17OHP-C use. Patient characteristics that impact the use of 17OHP-C were evaluated among eligible women with the hypothesis that 17OHP-C is underutilized and certain factors, such as obstetrical history, influence its use.
MATERIALS AND METHODS
We performed a cross-sectional study of all women seen in our Prematurity Prevention Program from November 2009 through June 2013. Approval was obtained from the Institutional Review Board at the Hospital of the University of Pennsylvania prior to initiation of the study.
The Prematurity Prevention Program (PPP) was established in 2009 to allow focused counseling regarding possible interventions to reduce the risk of preterm birth among high-risk women. All women seen for consultation had their prior delivery records reviewed, when available, and a detailed history was taken. All records and obstetric history were reviewed by Maternal Fetal Medicine (MFM) subspecialists in order to determine whether the prior loss/delivery met the criteria for spontaneous preterm birth (sPTB) or second trimester loss as defined in prior studies. (3, 12) Standard counseling at our institution includes the recommendation for weekly 17OHP-C if the woman had a prior sPTB less than 37 weeks, as well as serial cervical length screening with cerclage recommendation for shortened cervix (≤15mm) if the prior sPTB was less than 34 weeks. (3, 12–14)
Women were included in our study if they had a prior sPTB between 16–36 6/7 weeks gestation and had 17OHP-C recommended to them at their PPP visit. Women were excluded from our study if they were seen for preconception counseling and did not have a pregnancy during the study period, if they were too advanced in gestation (≥24 weeks) to allow for initiation of 17OHP-C, and if there were no prenatal records available for review.
Our primary outcome for the study was the use of 17OHP-C as documented in the electronic medical record by: (1) the presence of 17OHP-C in the medication history and/or (2) notation of its use in any prenatal or ultrasound notes. If there was no documentation of 17OHP-C use or if there was specific mention that a woman did not use 17OHP-C, it was considered “not used.” Our secondary outcome for the study was sPTB (16–36 6/7 weeks). Spontaneous PTB was defined as spontaneous labor and delivery or spontaneous preterm premature rupture of membranes as has been defined in previous studies. (3, 12)
Data abstraction from the electronic medical record included women’s demographic information, obstetrical, gynecological, medical, and surgical history. Recommendations for 17OHP-C use and cervical length screening were also collected, as were delivery outcomes.
The sample size was fixed (n=316) based on the number of women seen in PPP during the study period. Based on the assumption that only 60% of eligible women used 17OHP-C (9), we would have at least 80% power to see the following differences: for exposure variables with a prevalence of 10%, we could detect a 2.25-fold difference in 17OHP-C use; 20% prevalence, a 1.25-fold difference; 40% prevalence, a 1.5-fold difference; and for 60% prevalence, a 1.5-fold difference.
Chi-squared tests were used to compare categorical variables in the univariate analysis, and Wilcoxon rank sum tests were used to compare continuous variables. Multivariable logistic regression was used to calculate odds ratios and adjust for confounders. The data were analyzed using Stata version 12.0 (College Station, TX). Statistical significance of p<0.05 was used.
RESULTS
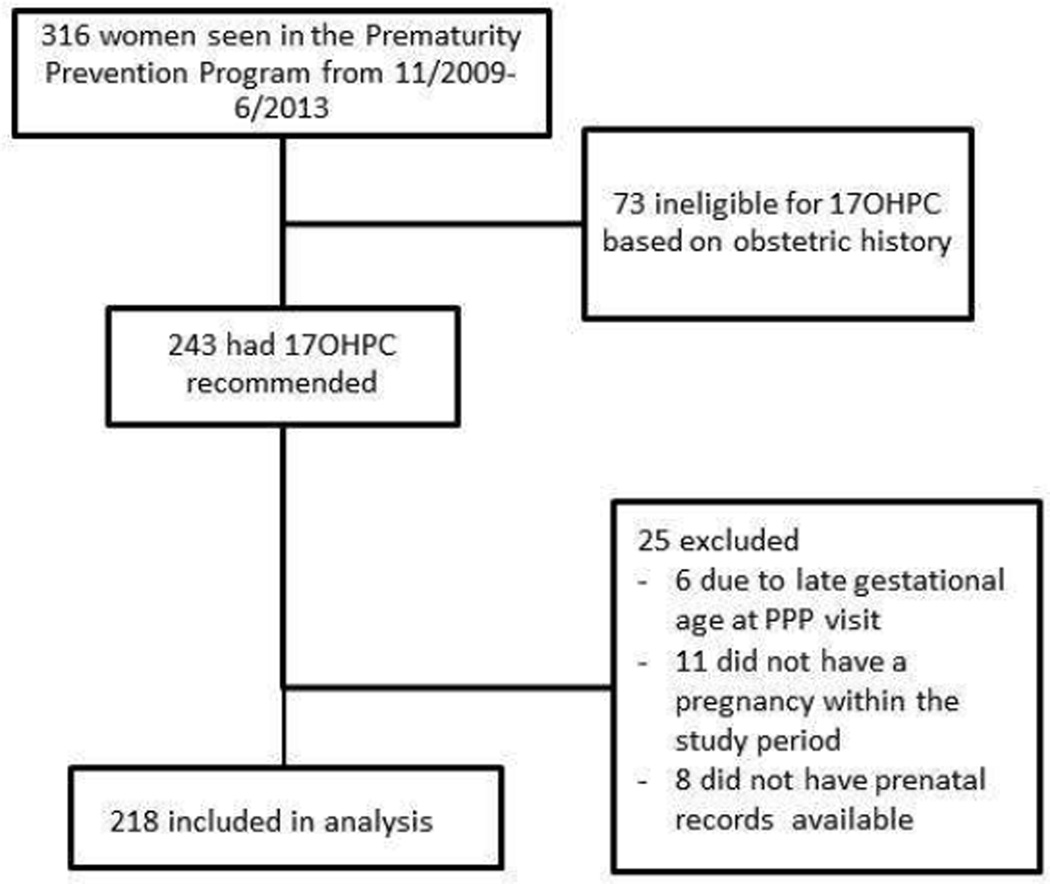
During the study period, 316 women were seen in PPP. Of those, 243 (76.9%) were eligible for 17OHP-C use based on prior obstetrical history and 100% of these women had 17OHP-C recommended to them. Among this group, 218 met inclusion criteria for our final analysis (Figure 1).
Figure 1.
Flow diagram of patient inclusion/exclusion criteria. Patient characteristics and 17OHP-C use.
Demographic information for the entire cohort is seen in Table 1. As noted, the majority of women in this study were African American (83%), had Medicaid insurance (61%), and were overweight or obese (65%).
Table 1.
Demographic information for our cohorta
| Characteristic | Cohort (n=218) |
|---|---|
| Maternal Ageb | 28.8 (±5.9) |
| Race | |
| African American | 82.6 |
| Caucasian | 11.5 |
| Other | 6.0 |
| BMI (kg/m2) | |
| <18.5 | 1.4 |
| 18.5–24.9 | 33.3 |
| 25–29.9 | 29.2 |
| >30 | 36.1 |
| Parityc | 1 [1–2] |
| Prior FTB | 44.5 |
| Insurance | |
| Private | 35.8 |
| Medicaid | 60.6 |
| None | 3.7 |
| Tobacco use | 11.9 |
| History of 17OHP-C use in prior pregnancy | 11.0 |
| Cerclage recommended based on short cervix | 23.9 |
BMI, body mass index. FTB, full-term birth. 17OHP-C, 17-alpha-hydroxyprogesterone caproate.
Data are presented as % unless otherwise indicated
Mean (±SD),
Median [Interquartile range]
There were 163 (74.7%) women who had documented use of 17OHP-C. Table 2 shows the effect of various demographic characteristics on 17OHP-C use. Race, body mass index, and insurance status had no impact on 17OHP-C use. While not statistically significant, women who used tobacco were 50% less likely to use 17OHP-C. The use of 17OHP-C in a prior pregnancy increased the odds of using 17OHP-C in a subsequent pregnancy. Among those eligible for cervical length screening in this pregnancy (12), women who had a cerclage recommended to them based on a short cervix had a 4-fold higher odds of using 17OHP-C than those who did not have a cerclage recommended to them (OR 4.12, 1.55–10.99). Women with a prior second trimester loss and no delivery ≥24 weeks had a 2-fold higher odds of using 17OHP-C (OR 2.32, 1.17–4.58).
Table 2.
Rate of use of 17OHP-C among various patient characteristics
| Characteristic | No 17OHP-C (n=55) |
17OHP-C used (n=163) |
OR | CI | p-value |
|---|---|---|---|---|---|
| African American race | 89.1 | 80.4 | 0.50 | 0.20–1.27 | 0.10 |
| BMI ≥30 | 38.2 | 35.4 | 0.89 | 0.47–1.67 | 0.70 |
| Medicaid insurance | 67.3 | 58.3 | 0.68 | 0.36–1.29 | 0.20 |
| Tobacco use | 18.2 | 9.8 | 0.49 | 0.21–1.15 | 0.10 |
| History of 17OHP-C use in prior pregnancy | 3.6 | 13.5 | 4.16 | 0.95–18.32 | 0.06 |
| Cerclage recommended based on short cervix | 9.1 | 28.8 | 4.12 | 1.55–10.99 | 0.01 |
| Prior second trimester loss only | 25.5 | 44.2 | 2.32 | 1.17–4.58 | 0.02 |
| Prior sPTB ≥24 weeks | 80.0 | 67.5 | 0.52 | 0.25–1.08 | 0.08 |
| Prior FTB | 58.2 | 39.9 | 0.48 | 0.26–0.89 | 0.02 |
17OHP-C, 17-alpha-hydroxyprogesterone caproate. OR, odds ratio. CI, 95% confidence interval. BMI, body mass index. sPTB, spontaneous preterm birth. FTB, full-term birth.
Women with a prior full-term birth (FTB) were half as likely to use 17OHP-C compared to women without a prior FTB (OR 0.48, 0.26–0.89). To investigate this further, we evaluated whether birth order of the FTB impacted our results. We found that women with a FTB as their most recent delivery were 80% less likely to use 17OHP-C compared to women whose FTB preceded their preterm birth (29.2 vs. 68.8%, OR 0.19, 0.08–0.47). When adjusting for confounders, including prior 17OHP-C use and recommendation for a cerclage, a prior FTB still conferred a decreased odds of 17OHP-C use (aOR 0.51, 0.27–0.97).
The overall rate of preterm birth in this cohort was 40.8%. The rate of sPTB in this cohort was 36.4%. There was no difference in the rate of sPTB between those who used and did not use 17OHP-C (37.2 vs. 34.0%, p=0.7). The gestational age of sPTB was not different between those who used and did not use 17OHP-C (32.5 [26.9–35.1] vs. 33.7 [18.9–34.9], p=0.4). The sPTB rate between women with and without a prior FTB was not statistically different (31.9 vs. 40.2%, p=0.2).
COMMENT
Effective utilization of 17OHP-C depends on factors at the patient, provider, and health systems level. In this study, we aimed to investigate specific characteristics at the patient level that are associated with the use of 17OHP-C among eligible women. We found that three-quarters of women eligible for 17OHP-C did, in fact, use 17OHP-C. A woman’s obstetric history influenced her use of 17OHP-C. In particular, women with a prior FTB are less likely to use 17OHP-C, especially if the FTB was the most recent delivery. Women with second trimester losses and no deliveries ≥24 weeks are also more likely to use 17OHP-C. Additionally, previous experience with 17OHP-C and the presence of a short cervix affected its use. There was no difference in the rate of sPTB between those that used and did not use 17OHP-C.
While many studies have looked at the efficacy of 17OHP-C in preventing preterm birth in different populations of women, there remains a gap in our understanding of hurdles that prevent its utilization. Rebarber and Ness surveyed general obstetrician-gynecologists and MFM subspecialists, respectively, regarding their use and perception of 17OHP- C. Eighty percent of general obstetrician-gynecologist respondents reported using 17OHP-C in their clinical practice, but admitted that they only prescribed it to 60% of eligible women, citing logistic and financial barriers as the most common reasons why not. (10) In contrast, 67% of MFM respondents reported using 17OHP-C, with non-users citing concerns about safety, efficacy, long-term fetal effects, and liability as reasons why not. MFM subspecialists who prescribed 17OHP-C estimated that 50% of patients offered progesterone accepted it, reporting the main reasons for patient refusal as: lack of insurance coverage, need for intramuscular injections, and concerns about risks. (8) There remains a dearth of information, however, examining acceptance rates of 17OHP-C from a patient’s perspective. While our study attempts to describe the population of women who accepted 17OHP-C, qualitative studies directly evaluating the patient’s perspective would help to further our understanding of acceptance rates.
Interestingly, we found no difference in the sPTB rate between women who used and did not use 17OHP-C. While it is important to note that recurrent sPTB rate was not our primary outcome and thus we were not powered to detect a different in this outcome, there are several factors that may explain this finding. First, this was not a randomized trial and therefore the groups that used and did not use 17OHP-C are inherently different. Women that elected not to use 17OHP-C may be a lower risk group, as they were more likely to have had a prior FTB and did not qualify for cerclage placement in their subsequent pregnancy. In that regard, if the women that used 17OHP-C were a higher risk group, the recurrent sPTB rate may have been even higher in the absence of 17OHP-C. This is a plausible explanation since other historical controls have a rate of sPTB in their control groups as high as 54%. (3) Another possible explanation is that 17OHP-C does not perform as well in “actual” clinical use as it has been shown to do in clinical trials. In addition, recent work has suggested that different races of women may respond to 17OHP-C dissimilarly. In a retrospective review of high-risk women receiving 17OHP-C, Timofeev et al. found that the rate of spontaneous recurrent PTB was higher in African Americans compared to Caucasians in their population (OR 2.1, 1.7–2.4). (15) This association remained after adjusting for other medical and psychosocial factors, and therefore the authors suggested a possible genetic or racial difference to the response to 17OHP-C. Caritis et al. investigated the pharmacokinetics of 17OHP-C in multifetal pregnancies and demonstrated higher clearance and lower concentrations of the drug in African American women compared with Caucasian women, again suggesting differential effects between races. (16) Manuck et al. demonstrated that single nucleotide polymorphisms in the human progesterone receptor affect a woman’s clinical response to 17OHP-C, finding a significant difference between the polymorphisms between African American and Caucasian populations. (17) While sPTB and recurrent sPTB is likely a multifactorial condition, it is possible that there may be an underlying genetic component contributing not only to the disease, but to the efficacy of the medication used to treat it. Future studies should focus on identifying specific populations where 17OHP-C is and is not effective in preventing PTB and the possible role of genetics within these populations.
Our study had several strengths. We examined a high-risk cohort of women seen in our Prematurity Prevention Program whose records and obstetric history were reviewed in detail, ensuring true eligibility of these women for 17OHP-C use. This group of women was derived from a single institution and counseling was performed by a select group of MFM subspecialists, lending to consistency in counseling and recommendations. Lastly, our study included a large proportion of African American women (83%), a population known to be at particularly high risk for preterm birth.
A limitation of our study was that it included a select group of women who were motivated to be seen in a specialty clinic for women at risk of preterm birth and may not be generalizable to other populations. In our study, 17OHP-C was recommended to all eligible women and we had a high utilization rate (75%). This may be attributed to the fact that women were counseled by a small number of MFM subspecialists in a specialized prematurity clinic whose main focus is PTB prevention. Therefore, these physicians may have increased comfort in discussing and prescribing 17OHP-C, as well as more experience and/or resources in obtaining and administering the medication as compared to general obstetrician-gynecologists or other advanced care providers that may perform this counseling in other institutions. Another limitation to our study is our reliance on chart documentation for 17OHP-C use. If there was no documentation of 17OHP-C use, it was assumed to have not been used. Relying solely on documentation in the chart may misrepresent the actual number of women that used, or intended to use, the medication.
Preterm birth remains a significant problem with regard to neonatal morbidity and mortality. While research into understanding its many etiologies and discovering new methods to reduce its impact is essential, we believe that it is also important to find ways to optimize implementation of currently proven strategies. Understanding the factors informing a woman’s decision to accept or reject 17OHP-C is one way of doing this. This study identifies biases regarding 17OHP-C use at the patient level and can be used to develop strategies to further increase its use. Given the fact that there was no difference in the sPTB rate between groups in this study, it is plausible that differences in susceptibility profiles between women can impact the populations in which 17OHP-C is most effective. Future studies should investigate whether there is a differential effect of 17OHP-C in certain populations of women. If 17OHP-C is found to be more effective in a specific subset of women, then strategies to increase its use among them would be critical.
Acknowledgments
Sources of Funding: This research was funded, in part, by a National Institute of Health career development award in Women’s Reproductive Health Research: K12-HD001265-14.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflict of interest.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. National vital statistics reports. 3. Vol. 59. Hyattsville, MD: National Center for Health Statistics; 2013. Births: preliminary data for 2012. [PubMed] [Google Scholar]
- 2.Spong CY. Prediction and prevention of recurrent spontaneous preterm birth. Obstet Gynecol. 2007;110:405–415. doi: 10.1097/01.AOG.0000275287.08520.4a. [DOI] [PubMed] [Google Scholar]
- 3.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2003;102:1115–1116. doi: 10.1016/j.obstetgynecol.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Society for Maternal Fetal Medicine Publications Committee. ACOG Committee Opinion No. 419. Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112:963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 6.Berghella V, Blackwell S, Andreson B, et al. Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–386. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Meis PJ. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105:1128–1135. doi: 10.1097/01.AOG.0000160432.95395.8f. [DOI] [PubMed] [Google Scholar]
- 8.Ness A, Dias T, Damus K, Burd I, Berghella V. Impact of the recent randomized trials on the use of progesterone to prevent preterm birth: a 2005 follow-up survey. Am J Obstet Gynecol. 2006;195:1174–1179. doi: 10.1016/j.ajog.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Sibai BM, Istwan NB, Palmer B, Stanziano GJ. Pregnancy outcomes of women receiving compounded 17 alpha-hydroxyprogesterone caproate for prophylactic prevention of preterm birth 2004 to 2011. Am J Perinatol. 2012;29:635–642. doi: 10.1055/s-0032-1311979. [DOI] [PubMed] [Google Scholar]
- 10.Rebarber A, Fox N, Klauser CK, Saltzman D, Roman AS. A national survey examining obstetrician perspectives on use of 17-alpha hydroxyprogestrone caproate post-US FDA Approval. Clin Drug Investig. 2013;33:571–577. doi: 10.1007/s40261-013-0099-4. [DOI] [PubMed] [Google Scholar]
- 11.Petrini JR, Callaghan WM, Klebanoff M, et al. Estimated effect of 17 alpha-hydroxyprogesterone caproate on preterm birth in the United States. Obstet Gynecol. 2005;105:267–272. doi: 10.1097/01.AOG.0000150560.24297.4f. [DOI] [PubMed] [Google Scholar]
- 12.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–375.e8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 14.Hassan SS, Roberto R, Berry SM, et al. Patients with an ultrasonographic cervical length ≤15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 15.Timofeev J, Singh J, Istwan N, Rhea D, Driggers RW. Spontaneous preterm birth in African-American and Caucasian women receiving 17α-hydroxyprogesterone caproate. Am J Perinatol. 2014;31:55–60. doi: 10.1055/s-0033-1334452. [DOI] [PubMed] [Google Scholar]
- 16.Caritis SN, Sharma S, Venkataramanan R, et al. Pharmacokinetics of 17- hydroxyprogesterone caproate in multifetal gestation. Am J Obstet Gynecol. 2011;205:d1–d8. doi: 10.1016/j.ajog.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manuck TA, Lai Y, Meis PJ, et al. Progesterone receptor polymorphisms and clinical response to 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2011;205:135.e1–135.e9. doi: 10.1016/j.ajog.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]