Abstract
Background
Dental erosion (DE) is the chemical dissolution of tooth structure in the absence of bacteria when the environment is acidic (pH < 4.0). Recent studies indicate that low pH is the primary determinant of beverage erosive potential although citrate chelation of calcium ions may contribute to erosion at higher pH. The purpose of this study was to determine the erosive potential measured by the pH of commercially available beverages.
Methods
A total of 380 beverages were purchased from stores in Birmingham, Alabama, categorized (e.g. juices, sodas) and assessed for pH. An Accumet AR 15 pH meter was used to measure the pH of each beverage in triplicate immediately after opening at 25°C. The pH data were recorded as mean ± standard deviation.
Results
The majority (93%, 355/380) of beverages had a pH below 4.0 and 7% (25/380) had a pH ≥4. Relative beverage erosivity zones based on previous studies of apatite solubility in acid indicated: 39% (150/380) of the beverages were considered extremely erosive (pH <3.0); 54% (205/380) were considered erosive (pH 3.0 to 3.99); 7% (25/380) were considered minimally erosive (pH ≥4.0).
Conclusions
This comprehensive pH assessment of beverages available for human consumption found that the majority are potentially erosive to the dentition. This study will provide dental clinicians and hygienists information regarding the erosive potential (pH) of commercially available beverages.
Practical Implications
Specific dietary recommendations for the prevention of dental erosion may now be developed based on the patient’s history of beverage consumption.
Keywords: Erosive potential, commercial beverages, pH, dental erosion
INTRODUCTION
Sweetened beverage consumption has increased dramatically over the past 35 years in America with carbonated soft drinks being consumed the most frequently; children, teens, and young adults are the main consumers.1–3 In 1942 the annual production of soft drinks was about 60 12-ounce servings per person, and that number has increased almost 10-fold since 2005.4 Between 1999–2002 daily carbonated soft drink/fruit drink consumption by 13- to18-year-olds was 26 ounces, and the Center for Science in the Public Interest has reported that in 2004 total consumption of these drinks for every man, woman and child was approximately 68 gallons per year.4 The prevalence of dental erosion in the 21st century has also increased due to our enhanced preference for sweet and sour.5 The consumption of acidic beverages contributes to an erosive oral milieu, and should be of concern to the dental practitioner.6–9
The pH of commercial non-dairy beverages range from 2.1 (lime juice concentrate) to 7.4 (spring water).10 Commercially available beverages with a pH < 4.0 are potentially damaging to the dentition.11 Acids are added to beverages and compose a flavor profile giving the beverage a distinctive taste. Acids provide a tartness and tangy taste that helps to balance the sweetness of sugar present in the beverage; they are key factors in the taste of the beverage. Phosphoric acid is added to cola drinks to impart tartness, reduce growth of bacteria and fungi, and improve shelf-life. Citric acid, a substance naturally occurring in citrus drinks and added to many others, imparts a tangy flavor and functions as a preservative. Malic acid occurs naturally in apples, pears and cherries, and is added to many non-carbonated beverages such as fruit drinks, fortified juices, sports drinks and iced teas because it enhances the intrinsic flavor. Malic acid is added to artificially sweeten carbonated beverages to intensify taste and reduce the amount of other added flavorings. These additives give the beverage its distinctive sugar/acid signature taste.
Dental erosion is the irreversible acidic dissolution of surface tooth structure by chemical means in the absence of microorganisms. It primarily occurs when hydrogen ions [H+] interact with the surface flluoroapatite and hydroxyapatite crystals after diffusion through plaque-pellicle biofilm—a process termed proton-promoted dissolution.12 Erosion may initially progress through the enamel lamellae exposing dentinal tubules leading to dentinal sensitivity, however, with continuous erosive insult to the surface enamel, larger areas of the enamel-dentin junction will eventually become exposed, leading to enhanced sensitivity.7,13,14 As the oral cavity pH drops below 4.0, the tooth surface erodes and with each unit of decrease in pH there is a ten-fold increase in enamel solubility resulting in a 100 fold increase in enamel demineralization as the pH approaches 2.0 from 4.0.11 Importantly, the consumption of beverages with higher concentrations of available hydrogen ions (pH < 4.0) results in the immediate softening of the tooth surface that becomes quite susceptible to removal by abrasion and attrition.15
The frequent consumption of acidic beverages is a developing problem for children, teenagers, and adults. The dramatic increase in consumption of acid soft drinks, fruit juices, fruit drinks, sports drinks and carbonated beverages is now thought to be the leading cause of dental erosion observed among children and adolescents.16–18 A recent report of dental erosion in children indicates its prevalence may range from 10% to 80%.19 Deciduous teeth, having a thinner enamel layer, are more prone to rapid erosion into dentin leading to exposure of the dental pulp.19 It is evident that erosion causes many clinical problems with restorative treatment being necessary to replace lost tooth structure, eliminate dental pain and restore functional esthetics.
Previous investigations have indicated pH, not titratable acidity, is the critical determinant of beverage erosive potential.10,19–24 Citrate may also contribute to dental erosion by removing [Ca++] through ligand-promoted dissolution (chelation) at a higher pH approaching 6.12 The purpose of this study is to determine the hydrogen ion concentration (pH) of beverages including new products that are commercially available in stores, gas stations and vending machines. Information obtained from this study will enable dental care practitioners to make appropriate dietary beverage suggestions when counseling patients about the damaging effects of acid in drinks.
METHODS
Beverages were purchased from convenience stores, grocery stores, gas stations and vending machines in the Birmingham, Alabama area. A total of 380 beverages were studied and categorized. Groups included: waters and sport drinks (Table 1); juices and fruit drinks (Table 2); sodas (Table 3); energy drinks, teas and coffee (Table 4). An Accumet AR 15 pH meter (Fisher Scientific, Pittsburgh, PA) was used to measure the pH of each beverage in triplicate immediately after opening at 25 °C. The pH data were recorded as range and mean ± standard deviation. Nutritional information labels on the containers were used to determine the type of acid(s) added to the beverages.
Table 1.
pH of waters and sports drinks (pH ± standard deviation, n = 3).
| Active Water® Focus Dragonfruit | 2.82 ± 0.04 | Powerade® Zero – Orange | 2.93 ± 0.01 |
| Active Water® Power Strawberry Kiwi | 3.38 ± 0.03 | Propel® Berry | 3.01 ± 0.00 |
| Active Water® Vigor Triple Berry | 2.67 ± 0.01 | Propel® Grape | 3.10 ± 0.01 |
| Aquafina® regular | 6.11 ± 0.23 | Propel® Kiwi Strawberry | 3.17 ± 0.00 |
| Birmingham Municipal Water | 7.20 ± 0.05 | Propel® Lemon | 3.03 ± 0.00 |
| Clear American® (flavored water) Kiwi Strawberry | 3.70 ± 0.01 | S. Pelligrino® sparkling natural mineral water | 4.96 ± 0.09 |
| Clear American® (flavored water) Pomegranate Blueberry Acai | 3.24 ± 0.01 | Skinny Water® Acai Grape Blueberry | 3.81 ± 0.02 |
| Clear American® (flavored water) Tropical Fruit | 3.07 ± 0.01 | Skinny Water® Goji Fruit Punch | 3.67 ± 0.01 |
| Clear American® (flavored water) White Grape | 3.43 ±0.01 | Skinny Water® Rasberry Pomegranate | 3.68 ± 0.01 |
| Dasani® Grape | 3.05 ± 0.01 | Sobe Life Water® Acai Fruit Punch | 3.22 ± 0.01 |
| Dasani® Lemon | 3.03 ± 0.01 | Sobe Life Water® Blackberry Grape | 3.15 ± 0.01 |
| Dasani® Strawberry | 3.03 ± 0.01 | Sobe Life Water® Cherimoya Punch | 3.28 ± 0.00 |
| Dasani® regular | 5.03 ± 0.04 | Sobe Life Water® Strawberry Dragonfruit | 3.32 ± 0.01 |
| Gatorade® Frost Riptide Rush | 2.99 ± 0.01 | Sobe Life Water® Fuji Apple Pear | 3.53 ± 0.01 |
| Gatorade® Rain Lime | 3.19 ± 0.01 | Sobe Life Water® Mango Melon | 3.29 ± 0.01 |
| Gatorade® Rain Strawberry Kiwi | 3.17 ± 0.01 | Vidration Vitamin Enhanced Water® Defense Pomegranate Acai Blueberry | 2.92 ± 0.01 |
| Gatorade® Blueberry Pomegranate Low Calorie | 3.21 ± 0.01 | Vidration Vitamin Enhanced Water® Energy Tropical Citrus | 2.91 ± 0.01 |
| Gatorade® Fierce Grape | 3.05 ± 0.00 | Vidration Vitamin Enhanced Water® Multi V-Lemon Lime | 3.59 ± 0.01 |
| Gatorade® Fierce Melon | 3.05 ± 0.00 | Vidration Vitamin Enhanced Water® Recover Fruit Punch | 3.61 ± 0.01 |
| Gatorade® Fruit Punch | 3.01 ± 0.01 | Vitamin Water® Connect Black Cherry Lime | 2.96 ± 0.01 |
| Gatorade® Lemon Lime | 2.97 ± 0.01 | Vitamin Water® Dwnld Berry Cherry | 3.04 ± 0.01 |
| Gatorade® Orange | 2.99 ± 0.00 | Vitamin Water® Energy Tropical Citrus | 3.15 ± 0.01 |
| Gatorade® Rain Berry | 3.17 ± 0.01 | Vitamin Water® Essential orange orange | 3.23 ± 0.00 |
| Perrier® carbonated mineral water | 5.25 ± 0.10 | Vitamin Water® Focus Kiwi Strawberry | 3.04 ± 0.01 |
| Powerade® Fruit Punch | 2.77 ± 0.01 | Vitamin Water® Multi-V lemonade | 3.19 ± 0.01 |
| Powerade® Grape | 2.77 ± 0.01 | Vitamin Water® Power C Dragonfruit | 3.05 ± 0.00 |
| Powerade® Lemon Lime | 2.75 ± 0.01 | Vitamin Water® Revive Fruit Punch | 3.65 ± 0.01 |
| Powerade® Mountain Berry Blast | 2.82 ± 0.01 | Vitamin Water® Spark Grape Blueberry | 3.19 ± 0.01 |
| Powerade® Orange | 2.75 ± 0.02 | Vitamin Water® XXX Acai Blueberry Pomegranate | 2.98 ± 0.01 |
| Powerade® Sour Melon | 2.73 ± 0.00 | Vitamin Water® Zero Mega C Grape Rasberry | 3.05 ± 0.00 |
| Powerade® Strawberry Lemonade | 2.78 ± 0.01 | Vitamin Water® Zero Recoup Peach Mandarin | 3.01 ± 0.01 |
| Powerade® White Cherry | 2.81 ± 0.01 | Vitamin Water® Zero Rise Orange | 3.46 ± 0.00 |
| Powerade® Zero Grape | 2.97 ± 0.01 | Vitamin Water® Zero Squeezed Lemonade | 3.19 ± 0.00 |
| Powerade® Zero -Lemon Lime | 2.92 ± 0.00 | Vitamin Water® Zero XXX Acai Blueberry Pomegranate | 3.05 ± 0.01 |
| Powerade® Zero -Mixed Berry | 2.93 ± 0.01 | Vitamin Water® Zero-Go Go Mixed Berry | 3.08 ± 0.01 |
Red = extremely erosive, Yellow = erosive, Green = minimally erosive.
Table 2.
pH of (A) fruit juices, (B) fruit drinks (pH ± standard deviation, n = 3).
| A | |||
| Amp® Energy juice-Mixed Berry | 3.62 ± 0.01 | Ocean Spray® Pineapple Peach Mango Juice Blend | 3.64 ± 0.01 |
| Amp® Energy juice-Orange | 3.60 ± 0.01 | Ocean Spray® Ruby Red | 3.07 ± 0.01 |
| Barber's® Orange Juice | 3.81 ± 0.01 | Ocean Spray® Strawberry Kiwi Juice Cocktail | 2.90 ± 0.01 |
| Campbell's® Tomato Juice | 4.01 ± 0.01 | Ocean Spray® Orange Juice | 3.83 ± 0.01 |
| Dole® Pineapple Juice | 3.40 ± 0.01 | Simply® Apple | 3.67 ± 0.01 |
| Juicy Juice® Apple | 3.64 ± 0.01 | Simply® Orange Orange Juice | 3.78 ± 0.00 |
| Juicy Juice® Berry | 3.78 ± 0.01 | Tango® energy juice | 3.47 ± 0.00 |
| Juicy Juice® Sparkling Apple | 3.47 ± 0.01 | Tropicana®100% Juice-Apple Juice | 3.50 ± 0.02 |
| Juicy Juice® Sparkling Berry | 3.50 ± 0.01 | Tropicana® 100% Juice-Orange Juice | 3.80 ± 0.01 |
| Juicy Juice® Sparkling Orange | 3.49 ± 0.01 | Tropicana® Apple Orchard Style Juice | 3.57 ± 0.00 |
| Lemon Juice | 2.25 ± 0.01 | Tropicana® Grape Juice | 3.29 ± 0.01 |
| Minute Maid® Apple Juice | 3.66 ± 0.01 | Tropicana® Orange Juice (with Calcium) | 4.09 ± 0.01 |
| Minute Maid® Cranberry Apple Rasberry | 2.79 ± 0.01 | V8 Fusion® Cranberry Blackberry | 3.56 ± 0.01 |
| Minute Maid® Cranberry Grape | 2.71 ± 0.01 | V8Fusion® Pomegranate Blueberry | 3.66 ± 0.00 |
| Minute Maid® Natural Energy Mango Tropical | 3.34 ± 0.02 | V8 Fusion® Strawberry Banana | 3.66 ± 0.00 |
| Minute Maid® Natural Energy Pomegranate Berry | 3.33 ± 0.01 | V8 Splash® Berry Blend | 2.94 ± 0.01 |
| Minute Maid® Natural Energy Strawberry Kiwi | 3.40 ± 0.01 | V8 Splash® Strawberry Kiwi | 2.99 ± 0.01 |
| Minute Maid® Orange Juice | 3.82 ± 0.01 | V8 Splash® Tropical Blend | 2.93 ± 0.00 |
| Minute Maid® Pineapple Orange | 3.71 ± 0.01 | V8 Vegetable Juice® low sodium | 4.17 ± 0.01 |
| Minute Maid® Ruby Red Grapefruit Juice | 3.07 ± 0.03 | V8 Vegetable Juice® | 4.23 ± 0.01 |
| Naked® Blue Machine | 3.81 ± 0.01 | V8 Vegetable Juice Spicy Hot® | 4.19 ± 0.00 |
| Naked® Orange Mango | 3.75 ± 0.01 | Very Fine® Grapefruit Juice | 3.22 ± 0.03 |
| Naked® Protein Zone | 4.69 ± 0.01 | Welch's® Apple Juice | 3.57 ± 0.01 |
| Ocean Spray® Cranberry | 2.56 ± 0.00 | Welch's® Orange Juice | 3.73 ± 0.00 |
| Ocean Spray® Cran-Grape | 2.79 ± 0.01 | Welch's® 100% Grape Juice | 3.38 ± 0.00 |
| Ocean Spray®Cran-Pomegranate | 2.72 ± 0.01 | ||
| B | |||
| Barber's® Fruit Punch | 3.15 ± 0.00 | Minute Maid® Pink lemonade | 2.59 ± 0.00 |
| Barber's® Lemonade | 2.69 ± 0.00 | Mondo® (Legendary Berry) | 3.07 ± 0.01 |
| Barber's® Orange Drink | 2.96 ± 0.00 | Mondo® (primo punch) | 3.10 ± 0.01 |
| Bug Juice® Berry Raspberry | 2.99 ± 0.01 | Sesame Street® Elmo's Punch | 3.87 ± 0.01 |
| Bug Juice® Fruity Punch | 3.09 ± 0.00 | Simply® Lemonade | 2.61 ± 0.01 |
| Bug Juice® Grapey grape | 2.83 ± 0.00 | Snapple® Kiwi Strawberry | 2.77 ± 0.01 |
| Bug Juice® Leapin Lemonade | 3.06 ± 0.00 | Snapple® Mango Madness | 2.89 ± 0.01 |
| Bug Juice® Whistlin Watermelon | 3.40 ± 0.01 | Sobe® Black and Blueberry Brew | 2.69 ± 0.00 |
| CapriSun® Surfer Cooler | 3.08 ± 0.00 | Sobe® Citrus Energy | 2.63 ± 0.00 |
| Country Time® Lemonade | 2.72 ± 0.01 | Sobe® Fuji Apple Cranberry (Low Calorie) | 3.16 ± 0.01 |
| Crystal Lite® Fruit Punch | 2.96 ± 0.02 | Sobe® Orange Carrot | 3.34 ± 0.00 |
| Crystal Lite® Green Tea Rasberry Mix | 3.11 ± 0.02 | Sobe® Pina Colada | 3.25 ± 0.01 |
| Crystal Lite® Rasberry Ice | 2.77 ± 0.01 | Sobe® Power Fruit Punch | 2.43 ± 0.02 |
| Fuze® Banana Colada | 3.45 ± 0.03 | Sobe® Strawberry Banana | 2.62 ± 0.01 |
| Fuze® Blueberry Rasberry | 3.20 ± 0.01 | Sun Fresh® Lemonade | 2.68 ± 0.01 |
| Fuze® Green Tea-Honey and Ginseng | 3.28 ± 0.02 | Sunny D® Smooth | 2.92 ± 0.01 |
| Fuze® Orange Mango | 3.34 ± 0.02 | Sunny D® Tangy Original | 2.86 ± 0.01 |
| Fuze® Peach Mango | 3.53 ± 0.01 | Tahitian Treat® Fruit Punch | 3.01 ± 0.01 |
| Fuze® Strawberry Banana | 3.54 ± 0.01 | Tropicana® Cranberry Cocktail | 2.70 ± 0.01 |
| Fuze® Strawberry Guava | 3.55 ± 0.02 | Tropicana® Juice Beverage Cranberry | 2.59 ± 0.00 |
| Fuze® Strawberry Melon | 3.18 ± 0.01 | Tropicana® Juice Beverage-Grape | 2.58 ± 0.00 |
| Fuze® Tropical Punch | 3.17 ± 0.01 | Tropicana® Lemonade | 2.70 ± 0.01 |
| HiC® Tropical | 2.81 ± 0.03 | Tropicana Twister® Blue Rasberry Rush | 2.62 ± 0.00 |
| Jumex® Guava | 3.38 ± 0.02 | Tropicana Twister® Cherry Berry Blast | 2.63 ± 0.00 |
| Jumex® Mango | 3.41 ± 0.01 | Tropicana Twister® Orange Strawberry Banana Burst | 2.89 ± 0.01 |
| Jumex® Peach | 3.33 ± 0.02 | Tropicana Twister® Strawberry Kiwi Cyclone | 2.59 ± 0.01 |
| Jumex® Strawberry Banana | 3.68 ± 0.01 | TumE Yummies® Fruitabulous Punch | 3.35 ± 0.00 |
| Kool Aid® Burst (Tropical) | 3.07 ± 0.01 | TumE Yummies® Orangearific | 3.34 ± 0.01 |
| Kool Aid® Mix- Lemon Lime | Kool Aid® Mix- Lemon Lime | TumE Yummies® Soursational Rasberry | 3.18 ± 0.00 |
| Kool Aid® Mix- Pink Lemonade | 2.66 ± 0.01 | TumE Yummies® Very Berry Blue | 3.33 ± 0.00 |
| Kool Aid® Mix- Pink Lemonade | 2.66 ± 0.01 | TumE Yummies® Very Berry Blue | 3.33 ± 0.00 |
| Kool Aid® Mix- Tropical Punch | 2.69 ± 0.00 | Vitamin Stix® Dragonfruit Acai | 3.11 ± 0.01 |
| Kool Aid® Mix-Cherry | 2.71 ± 0.00 | Vitamin Stix® Passonfruit Citrus | 3.19 ± 0.01 |
| Kool Aid® Mix-Grape | 2.83 ± 0.01 | Vitamin Stix® Strawberry kiwi | 3.06 ± 0.01 |
| Kool Aid® Mix-Orange | 2.77 ± 0.01 | Welch's® Blueberry Kiwi Blast | 2.57 ± 0.01 |
| Little Hug® Grape | 3.09 ± 0.01 | Welch's® Cranberry | 2.59 ± 0.02 |
| Little Hug® Orange | 3.00 ± 0.01 | Welch's® Grape juice cocktail | 2.92 ± 0.01 |
| Minute Maid® Fruit Punch | 2.86 ± 0.00 | Welch's® Orange Pineapple | 3.20 ± 0.01 |
| Minute maid® Lemonade | 2.57 ± 0.01 | Welch's® Ruby Red Grapefruit Juice | 2.97 ± 0.01 |
| Minute Maid® Orangeade | 2.85 ± 0.00 | Welch's® Strawberry Kiwi | 3.03 ± 0.01 |
Red = extremely erosive, Yellow = erosive, Green = minimally erosive.
Table 3.
pH of sodas (pH ± standard deviation, n = 3).
| 7up® | 3.24 ± 0.02 | Hansen's® Cane Soda-Pomegranate | 2.55 ± 0.00 |
| 7up® Cherry | 2.98 ± 0.01 | Hawaiian Punch® (fruit Juicy Red) | 2.87 ± 0.01 |
| 7up® Diet | 3.48 ± 0.00 | IBC® Rootbeer | 4.10 ± 0.02 |
| A&W® Cream Soda | 3.86 ± 0.01 | Izze® Sparkling Blackberry | 3.28 ± 0.01 |
| A&W® Rootbeer | 4.27 ± 0.02 | Izze® Sparkling Clementine | 3.27 ± 0.01 |
| A&W® Rootbeer-Diet | 4.57 ± 0.00 | Izze® Sparkling Pomegranate | 3.01 ± 0.01 |
| Ale 8-One® | 3.13 ± 0.01 | Jolly Rancher® Orange | 2.88 ± 0.01 |
| Barq's® Rootbeer | 4.11 ± 0.02 | Jolly Rancher® Grape | 2.60 ± 0.01 |
| Boylan's® Black Cherry | 2.76 ± 0.02 | Jones® Blue Bubblegum | 2.99 ± 0.01 |
| Boylan's® Crème Soda | 4.17 ± 0.02 | Jones® Cream Soda | 3.04 ± 0.01 |
| Boylan's® Diet Black Cherry | 4.00 ± 0.01 | Jones® Green Apple Soda | 2.65 ± 0.01 |
| Boylan's® Diet Rootbeer | 4.05 ± 0.02 | Jones® M.F. Grape | 2.89 ± 0.02 |
| Boylan's® Grape | 2.91 ± 0.01 | Jones® Mandarin Orange | 2.93 ± 0.00 |
| Boylan's® Orange Cream | 3.59 ± 0.01 | Jones® Orange & Cream Soda | 2.79 ± 0.01 |
| Boylan's® Orange Soda | 3.22 ± 0.00 | Jones® Red Apple | 3.40 ± 0.02 |
| Boylan's® Original Birch Beer | 3.80 ± 0.00 | Jones® Root Beer | 3.42 ± 0.02 |
| Boylan's® Rootbeer | 4.01 ± 0.01 | Jones® Strawberry Lime | 2.81 ± 0.02 |
| Boylan's® Sugar Cane Cola | 2.54 ± 0.01 | Maine® Root Beer | 4.36 ± 0.02 |
| Buffalo Rock® Ginger Ale | 3.23 ± 0.01 | Mellow Yellow® | 3.03 ± 0.00 |
| Canada Dry® Club Soda | 5.24 ± 0.03 | Mountain Dew® (regular) | 3.22 ± 0.07 |
| Canada Dry® Ginger Ale | 2.82 ± 0.01 | Mountain Dew® Code Red | 3.27 ± 0.01 |
| Coca-Cola® Classic | 2.37 ± 0.03 | Mountain Dew® Diet | 3.18 ± 0.01 |
| Coca-Cola® Zero | 2.96 ± 0.03 | Mountain Dew® Voltage | 3.05 ± 0.01 |
| Coca-Cola® Caffeine Free Diet | 3.04 ± 0.01 | Mr. Pibb® xtra | 2.80 ± 0.01 |
| Coca-Cola® Caffeine Free | 2.34 ± 0.03 | Mug® Rootbeer | 3.88 ± 0.02 |
| Coca-Cola®Cherry | 2.38 ± 0.03 | Natural Brew Draft® Root Beer | 2.90 ± 0.00 |
| Coca-Cola® Cherry Zero | 2.93 ± 0.01 | Pepsi® | 2.39 ± 0.03 |
| Coca-Cola® Diet | 3.10 ± 0.05 | Pepsi®-Wild Cherry | 2.41 ± 0.03 |
| Coca-Cola® Lime Diet | 2.96 ± 0.03 | Pepsi Diet® | 3.02 ± 0.01 |
| Crush® Grape | 2.76 ± 0.01 | Pepsi Max® | 2.74 ± 0.01 |
| Crush® Orange | 2.87 ± 0.01 | Pepsi Max® Ceasefire | 2.70 ± 0.01 |
| Crush® Orange | 2.93 ± 0.03 | RC Cola® | 2.32 ± 0.02 |
| Dr Pepper® Diet Cherry | 3.32 ± 0.01 | Schweppes® Tonic Water | 2.54 ± 0.03 |
| Dr. Pepper® Cherry | 3.06 ± 0.02 | Sierra® Mist Diet | 3.31 ± 0.01 |
| Dr. Pepper® Diet | 3.20 ± 0.00 | Sierra® Mist | 3.09 ± 0.02 |
| Dr. Pepper® | 2.88 ± 0.04 | Sprite® | 3.24 ± 0.05 |
| Fanta® Grape (2 liter) | 2.67 ± 0.02 | Sprite® zero | 3.14 ± 0.01 |
| Fanta® Pineapple (2 liter) | 2.79 ± 0.02 | Sunkist® -Solar Fusion -Tropical Mandarin | 3.02 ± 0.01 |
| Fanta® Orange | 2.82 ± 0.02 | Sunkist® – Strawberry | 2.99 ± 0.01 |
| Fanta® Strawberry | 2.84 ± 0.01 | Sunkist® Diet | 3.49 ± 0.01 |
| Fresca® (1 liter) | 3.08 ± 0.01 | Sunkist® Orange | 2.98 ± 0.01 |
| Grapico® Diet | 3.04 ± 0.01 | Sunkist® Peach | 2.89 ± 0.01 |
| Grapico® | 2.77 ± 0.03 | Tab® | 2.72 ± 0.01 |
| Hansen's® Cane Soda-Black Cherry Diet | 3.47 ± 0.02 | Vault® | 2.77 ± 0.02 |
| Hansen's® Cane Soda-Cherry Vanilla Crème | 2.91 ± 0.01 | Vault® Red Blitz | 2.80 ± 0.01 |
| Hansen's® Cane Soda-Creamy Rootbeer Diet | 3.73 ± 0.01 | Vault® | 2.89 ± 0.03 |
| Hansen's® Cane Soda-Kiwi Strawberry | 2.59 ± 0.01 | Welch's® grape soda | 3.11 ± 0.02 |
| Hansen's® Cane Soda-Mandarin Lime | 2.57 ± 0.01 |
Red = extremely erosive, Yellow = erosive, Green = minimally erosive.
Table 4.
pH of (A) energy drinks, (B) teas and coffee (pH ± standard deviation, n = 3).
| A | |||
| 24:7® Energy Cherry Berry | 2.61 ± 0.01 | Monster® low carb | 3.60 ± 0.01 |
| 180 Blue® Orange Citrus Blast | 2.82 ± 0.00 | Monster® M-80 | 3.29 ± 0.00 |
| 180 Blue® with Acai | 2.82 ± 0.01 | Monster® MIXXD | 3.35 ± 0.00 |
| 5 hour energy® Berry | 2.81 ± 0.03 | Monster Hitman® energy shot | 3.44 ± 0.01 |
| 5 hour energy® extra strength | 2.82 ± 0.00 | Nitrous Monster® Anti-gravity | 3.64 ± 0.01 |
| 5 hour energy® Lemon-Lime | 2.81 ± 0.00 | Nitrous Monster® Killer B | 3.31 ± 0.00 |
| Amp energy® Elevate | 2.79 ± 0.01 | Nitrous Monster® Super Dry | 3.46 ± 0.00 |
| Amp energy® Overdrive | 2.78 ± 0.01 | No Fear® regular | 2.97 ± 0.02 |
| Amp energy® regular | 2.81 ± 0.01 | No Fear® sugar free | 3.06 ± 0.01 |
| Amp energy® sugar free | 2.86 ± 0.01 | NOS® fruit punch | 3.32 ± 0.00 |
| Crunk® Citrus | 3.20 ± 0.01 | NOS® grape | 3.27 ± 0.01 |
| Crunk® Energy Drink | 3.31 ± 0.01 | NOS® high performance energy drink | 3.31 ± 0.01 |
| Crunk® Grape Acai energy drink | 3.30 ± 0.01 | Nos® power shot | 3.03 ± 0.02 |
| Crunk® Low Carb Sugar Free | 3.34 ± 0.00 | Orange County Choppers® | 2.78 ± 0.02 |
| Drank® | 3.09 ± 0.01 | Purple Stuff Lean® | 2.87 ± 0.01 |
| Fuel Energy Shots® Lemon Lime | 3.97 ± 0.01 | Redbull® regular | 3.43 ± 0.01 |
| Fuel Energy Shots® Orange | 3.44 ± 0.01 | Redbull® shot | 3.25 ± 0.03 |
| Full Throttle® Blue Agave | 3.10 ± 0.01 | Redbull® sugar free | 3.39 ± 0.00 |
| Full Throttle® Citrus | 3.09 ± 0.01 | Redbull® sugar free shot | 3.28 ± 0.02 |
| Full Throttle® Red Berry | 3.08 ± 0.01 | Redline® Peach mango | 2.74 ± 0.02 |
| Hydrive® Blue Rasberry | 3.45 ± 0.01 | Redline® Princess exotic fruit | 2.85 ± 0.01 |
| Hydrive® Citrus Burst | 3.03 ± 0.01 | Redline® Triple Berry | 2.77 ± 0.01 |
| Hydrive® Lemon Lime | 3.42 ± 0.01 | Redline Xtreme® Grape | 3.23 ± 0.01 |
| Hydrive® Triple Berry | 3.15 ± 0.01 | Redline Xtreme® Triple Berry | 3.24 ± 0.01 |
| Jolt® Blue Bolt | 2.96 ± 0.00 | Redline Xtreme® Watermelon | 3.41 ± 0.00 |
| Jolt® Passion Fruit | 2.82 ± 0.01 | Rhinos® Energy Drink | 3.51 ± 0.01 |
| Jolt® Power Cola | 2.47 ± 0.01 | Rhinos® Sugar Free Energy Drink | 3.32 ± 0.01 |
| Jolt® Ultra: Sugar Free | 3.14 ± 0.00 | Rockstar® Energy Cola | 3.14 ± 0.01 |
| Killer Buzz® | 3.23 ± 0.01 | Rockstar® Energy Drink | 2.74 ± 0.01 |
| Killer Buzz® sugar free | 3.36 ± 0.00 | Rockstar® Juiced -energy + guava | 3.16 ± 0.01 |
| Meltdown® Energy Peach Mango | 2.77 ± 0.00 | Rockstar® Juiced -energy + juice-Mango Orange Passion | 3.05 ± 0.01 |
| Monster® Assault | 3.58 ± 0.01 | Rockstar® Punched (Energy + Punch) | 2.83 ± 0.01 |
| Monster® Energy | 3.48 ± 0.01 | Rockstar® Recovery | 2.84 ± 0.01 |
| Monster® Khaos | 3.47 ± 0.01 | Rockstar® Sugar Free | 3.15 ± 0.03 |
| B | |||
| Admiral® Iced Tea Green Tea | 3.72 ± 0.01 | Milos® No Calorie Famous Sweet Tea | 5.18 ± 0.03 |
| Admiral® Iced Tea Mango | 3.41 ± 0.00 | Nestea® Iced Tea with natural Lemon flavor | 2.94 ± 0.01 |
| Admiral® Iced Tea Rasberry | 2.94 ± 0.00 | Nestea® Red Tea pomegranate and passion fruit | 2.87 ± 0.01 |
| Admiral® Iced Tea Sweet Tea | 3.76 ± 0.01 | Red Diamond® Tea Fresh Brewed Sweet Tea | 5.04 ± 0.02 |
| Arizona® Diet Green tea + Ginseng | 3.29 ± 0.01 | Snapple® Diet Rasberry Tea | 3.39 ± 0.02 |
| Arizona® Iced Tea | 2.85 ± 0.03 | Snappl® Diet Peach Tea | 3.32 ± 0.01 |
| Lipton® Green Tea with Citrus | 2.93 ± 0.00 | Snapple® Peach Tea | 2.94 ± 0.01 |
| Lipton® Green Tea with Citrus-Diet | 2.92 ± 0.00 | Snapple® Rasberry Tea | 2.92 ± 0.00 |
| Milos® Famous Sweet Tea | 4.66 ± 0.02 | Starbucks® Medium Roast | 5.11 ± 0.05 |
Red = extremely erosive, Yellow = erosive, Green = minimally erosive.
RESULTS
All pH data is expressed as: range and mean ± standard deviation. Seventy waters and sports drinks had a pH range of 2.67 – 7.40 and a mean of 3.31 ± 0.77 (Table 1). Fifty-one juices had a pH range of 2.25 – 4.69 and a mean of 3.48 ± 0.47 (Table 2A). Seventy-eight fruit drinks had a pH range of 2.43 – 3.87 and a mean of 2.99 ± 0.31 (Table 2B). Ninety-five sodas had a pH range of 2.32 – 5.24 and a mean of 3.12 ± 0.52 (Table 3). Sixty-eight energy drinks had a pH range 2.47 – 3.97 and a mean of 3.13 ± 0.29 (Table 4A). Seventeen teas had a pH range of 2.85 – 5.18 and a mean of 3.48 ± 0.77; coffee had a pH of 5.11 (Table 4B). The majority of beverages tested had a pH < 4.0 (355/380 = 93%) (Tables 1–4). Relative beverage erosivity zones based on previous studies of apatite solubility in acid indicated: 39% (150/380) of the beverages tested were considered extremely erosive (pH < 3.0); 54% (205/380) were considered erosive (pH 3.0 to 3.99); 7% (25/380) were considered minimally erosive (pH ≥ 4.0)(Fig. 1). The most acidic beverages tested with a pH < 2.4 were: lemon juice (pH 2.25), RC Cola (pH 2.32), Coca-Cola Classic (2.37), Coca-Cola Cherry (pH 2.38), Pepsi (pH 2.39). Citric > phosphoric > malic acids were the most frequently added acids to the drinks.
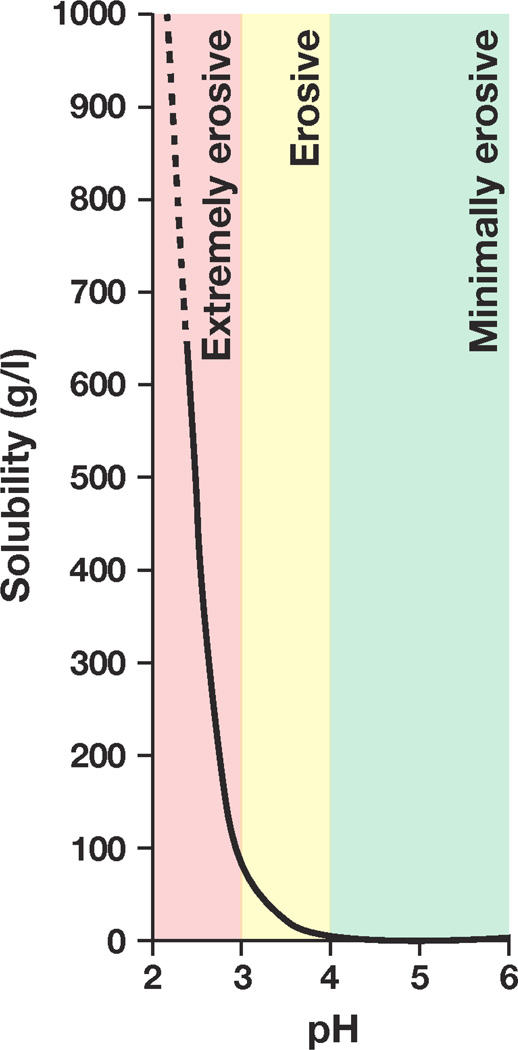
Fig 1.
Erosion zones based on theoretical solubility of apatite as a function of pH; adapted with permission from Larsen and Nyvad30
DISCUSSION
Previous laboratory studies have determined the pH of beverages for human consumption.6,10,22,24–28 Our study determined the pH of 380 beverages available to the American consumer and is the most comprehensive in terms of beverage numbers and diversity. Recently, there has been an increase in beverage diversity in the marketplace that probably accounts for the large number of beverages procured. Our results are consistent with reported beverage pH values by other investigators; for examples, we determined the pH of Coca Cola was 2.37 (Table 3) as compared to (2.46)21, (2.45)24, (2.48)25, (2.53)29, (2.39)22, (2.40)30, (2.49)31, (2.53)27, the pH of Schweppes Tonic Water was 2.54 (Table 3) as compared to (2.50)6, (2.48)30, the pH of Gatorade Lemon Lime was 2.97 (Table 1) as compared to (2.93)31, (2.95)26, (3.01)21, (2.90)10, (3.08)28, (3.17)24, (3.29)22, the pH of Pepsi was 2.39 (Table 3) as compared to (2.53)30, (2.36)22, (2.39)24, (2.30)10, (2.46)25, (2.53)27, and the pH of apple juice was 3.57 and 3.66 (Table 2A) as compared to (3.60)10, (3.41)24 and (3.60)32.
The pH of extrinsic solutions (dietary beverages) coming into contact with the dentition appears to be the major determinant of dental erosion; the hydrogen ion concentration [H+] or acidity, as measured in pH, is primarily responsible for the immediate dissolution and softening of surface tooth structure (erosive potential) by acidic beverages composed of weak acids, e.g. citric and phosphoric acid.10,12,19–24 The titratable acidity or buffer capacity, intrinsic to these acids, does not play as critical a role in dental erosion as pH due the limited time exposure the dentition has with ingested liquids during each drinking and swallowing episode.19,20,22,33,34 Therefore, pH or [H+] at the time of dental exposure is the important chemical parameter to assess when determining the erosive potential of beverages.
Teeth erode in the pH range of 2.0 to 4.0, although surface enamel starts to demineralize as the pH drops below 5.5 when the external milieu of the oral cavity becomes under-saturated for hydroxyapatite.35 Apatite solubility studies indicate a logarithmic increase in apatite solubility as pH drops under laboratory equilibrium conditions as can be seen in the solubility curve (Fig. 1).30,36 Apatite solubility above pH 4.0 is minimal; a drop of 1 unit to 3.0 results in a 10 fold increase in apatite solubility. Moreover, as pH drops from 3.0 to 2.0 there is an increase in apatite solubility that approaches 1000 g/l (Fig. 1). Based on the apatite solubility curve in Fig. 1, we propose that the chemical erosive potential of beverages be segregated into 3 zones: extremely erosive― pH < 3.0 (red); erosive―pH 3.0 to 3.99 (yellow); minimally erosive―pH ≥ 4.0 (green). The relative erosivity zones (extremely erosive, erosive, minimally erosive) of 380 beverages as determined by pH indicated: 39% (150/380) were extremely erosive (pH < 3.0); 54% (205/380) were erosive (pH 3.0 to 3.99); 7% (25/380) were minimally erosive (pH ≥ 4.0). Although apatite solubility as a function of pH is a continuum, the segregation of erosive potential into 3 discrete zones would be helpful to the dental clinician when providing a dietary guide of relative beverage erosivity to the patient. The prevailing paradigm for dental erosion remains: as the pH of the oral milieu decreases, the solubility of apatite on the tooth surface increases logarithmically.11
Dental erosion from beverages is primarily caused by either phosphoric acid and/or citric acid, and both are triprotic acids with 3 available [H+] enabling proton-promoted dissolution.12,37 Chelation or ligand-promoted dissolution by anionic citrate contributes to enamel demineralization by the removal of Ca++ at a higher pH range approaching 6.12 At the erosive pH 3 only 3% of citrate ions are appropriately ionized to chelate Ca++, indicating their contribution to the erosive process at this pH is minimal.38 However, if anionic citrate were to remain within the oral cavity for extended time intervals allowing the pH to rise to 6, chelation could play a contributing role in the erosive process; for example the eating of citrus fruits more than twice a day has been associated with dental erosion43. Nevertheless, high concentrations of [H+] reflected by low pH from citric and/or phosphoric acid result in undersaturation for both fluor- and hydroxyapatite leading to dental erosion. Hence, pH is the controlling parameter in determining the erosive potential of beverages.11,19–24
Knowledge of beverage pH is essential for the development of preventive strategies for patients with clinical erosion.7,39,40 The elimination of extremely erosive drinks (pH < 3.0), minimizing erosive drinks (pH 3.0 – 3.99), and substituting drinks with a (pH ≥ 4.0) would be prudent advice for the prevention of erosion. Fluoride does not prevent erosion since highly acidic environments solubilize fluorapatite and calcium fluoride.35,41,42 Xerostomic conditions exacerbate the erosive process due to lack of saliva essential for the dilution and buffering of [H+] in the oral cavity.43,44 The primary dentition of children is highly susceptible to the erosive process and low pH beverages should not be placed in a baby bottle, especially at sleep time when the mouth is xerostomic. Athletes may have decreased salivary flow rates due to dehydration from profuse sweating after prolonged, intense physical activity and should re-hydrate with water.45 Geriatric patients on medications with xerostomic side effects are vulnerable to erosion, and the exposure of cementum and dentin due to gingival recession allow for root demineralization and hypersensitivity from contact with erosive drinks.7,14,46 Obviously, saliva is an important ameliorating milieu for the abrogation of dental erosion by not only diluting and buffering extrinsic acids, but also providing the source of glycoproteins that coat the tooth surface as the protective acquired pellicle.20,43,44 However, when acidic beverage consumption is excessive, saliva may offer the dentition limited protection from erosion.47
CONCLUSION
Studies suggest that the pH or [H+] is the primary determinant of beverage erosive potential. The pH of 380 beverages was determined and assessed for relative erosivity. Relative beverage erosivity zones based on previous studies of apatite solubility in acid indicated: 39% (150/380) of the beverages tested were considered extremely erosive (pH < 3.0); 54% (205/380) were considered erosive (pH 3.0 to 3.99); 7% (25/380) were considered minimally erosive (pH ≥ 4.0). The most acidic beverages tested with a pH < 2.4 were: lemon juice (pH 2.25), RC Cola (pH 2.32), Coca-Cola Classic (2.37), Coca-Cola Cherry (pH 2.38), Pepsi (pH 2.39). Information obtained from this study will enable dental care practitioners to make appropriate dietary suggestions when counseling patients about the damaging dental effects of acid(s) in the beverages they drink.
ACKNOWLEDGEMENTS
This study was supported by Dr. Mary MacDougall and NIDCR training grant T32-DE017607. Ms. Momeni is a Dental Academic Research Training (DART) Predoctoral Fellow under NIDCR Institutional Grant#T-90 DE022736. We thank Mr. David Fisher, Medical Education and Design Services, The University of Alabama at Birmingham, Birmingham, AL for the creative design and production of the Tables. The authors also thank Karger AG, Basal, Switzerland for granting us copyright permission for the production of Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors report no conflicts of interest relevant to this article.
Disclosure. None of the authors reported any disclosures.
REFERENCES
- 1.Heller KE, Burt BA, Eklund SA. Sugared soda consumption and dental caries in the United States. J Dent Res. 2001;80(10):1949–1953. doi: 10.1177/00220345010800101701. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004;27(3):205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106(12):1992–2000. doi: 10.1016/j.jada.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MF. Liquid Candy: How Soft Drinks are Harming American’s Health. 2nd Ed. Washington, DC: Center for Science in the Public Interest; 2005. [Google Scholar]
- 5.Gambon DL, Brand HS, Veerman EC. Dental erosion in the 21st century: what is happening to nutritional habits and lifestyle in our society? Br Dent J. 2012;213(2):55–57. doi: 10.1038/sj.bdj.2012.613. [DOI] [PubMed] [Google Scholar]
- 6.Lussi A, Jaeggi T, Zero D. The role of diet in the aetiology of dental erosion. Caries Res. 2004;38(Suppl 1):34–44. doi: 10.1159/000074360. [DOI] [PubMed] [Google Scholar]
- 7.Zero DT, Lussi A. Erosion--chemical and biological factors of importance to the dental practitioner. Int Dent J. 2005;55(4 Suppl 1):285–290. doi: 10.1111/j.1875-595x.2005.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 8.Tahmassebi JF, Duggal MS, Malik-Kotru G, Curzon ME. Soft drinks and dental health: a review of the current literature. J Dent. 2006;34(1):2–11. doi: 10.1016/j.jdent.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Johansson AK, Omar R, Carlsson GE, Johansson A. Dental erosion and its growing importance in clinical practice: from past to present. Int J Dent. 2012 doi: 10.1155/2012/632907. Article ID 632907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seow WK, Thong KM. Erosive effects of common beverages on extracted premolar teeth. Aust Dent J. 2005;50(3):173–178. doi: 10.1111/j.1834-7819.2005.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 11.Larsen M. Erosion of Teeth. In: Fejerskov O, Kidd EAM, editors. Dental caries : the disease and its clinical management. 2nd ed. Oxford: Ames, Iowa: Blackwell Munksgaard; 2008. pp. 233–247. [Google Scholar]
- 12.Shellis RP, Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. Monogr Oral Sci. 2014;25:163–179. doi: 10.1159/000359943. [DOI] [PubMed] [Google Scholar]
- 13.Walker BN, Makinson OF, Peters MC. Enamel cracks. The role of enamel lamellae in caries initiation. Aust Dent J. 1998;43(2):110–116. doi: 10.1111/j.1834-7819.1998.tb06099.x. [DOI] [PubMed] [Google Scholar]
- 14.West N, Seong J, Davies M. Dentine hypersensitivity. Monogr Oral Sci. 2014;25:108–122. doi: 10.1159/000360749. [DOI] [PubMed] [Google Scholar]
- 15.Shellis RP, Addy M. Interaction between attrition,abrasion and erosion in tooth wear. Monogr Oral Sci. 2014;25:32–45. doi: 10.1159/000359936. [DOI] [PubMed] [Google Scholar]
- 16.Lussi A, Jaeggi T. Dental erosion in children. Monogr Oral Sci. 2006;20:140–151. doi: 10.1159/000093360. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho T, Lussi A, Jaeggi T, Gambon D. Erosive tooth wear in children. Monogr Oral Sci. 2014;25:262–278. doi: 10.1159/000360712. [DOI] [PubMed] [Google Scholar]
- 18.Murakami C, Oliveira LB, Sheiham A, et al. Risk indicators for erosive tooth wear in Brazilian preschool children. Caries Res. 2011;45(2):121–129. doi: 10.1159/000324807. [DOI] [PubMed] [Google Scholar]
- 19.Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55(4):358–367. doi: 10.1111/j.1834-7819.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- 20.Jensdottir T, Holbrook P, Nauntofte B, Buchwald C, Bardow A. Immediate erosive potential of cola drinks and orange juices. J Dent Res. 2006;85(3):226–230. doi: 10.1177/154405910608500304. [DOI] [PubMed] [Google Scholar]
- 21.Hara AT, Zero DT. Analysis of the erosive potential of calcium-containing acidic beverages. Eur J Oral Sci. 2008;116(1):60–65. doi: 10.1111/j.1600-0722.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane NJ, Cai F, Yuan Y, Reynolds EC. Erosive potential of beverages sold in Australian schools. Aust Dent J. 2009;54(3):238–244. doi: 10.1111/j.1834-7819.2009.01126.x. [DOI] [PubMed] [Google Scholar]
- 23.Barbour ME, Lussi A, Shellis RP. Screening and prediction of erosive potential. Caries Res. 2011;45(Suppl 1):24–32. doi: 10.1159/000325917. [DOI] [PubMed] [Google Scholar]
- 24.Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012;107(2):252–262. doi: 10.1017/S0007114511002820. [DOI] [PubMed] [Google Scholar]
- 25.von Fraunhofer JA, Rogers MM. Dissolution of dental enamel in soft drinks. Gen Dent. 2004;52(4):308–312. [PubMed] [Google Scholar]
- 26.von Fraunhofer JA, Rogers MM. Effects of sports drinks and other beverages on dental enamel. Gen Dent. 2005;53(1):28–31. [PubMed] [Google Scholar]
- 27.Jain P, Nihill P, Sobkowski J, Agustin MZ. Commercial soft drinks: pH and in vitro dissolution of enamel. Gen Dent. 2007;55(2):150–154. [PubMed] [Google Scholar]
- 28.Jain P, Hall-May E, Golabek K, Agustin MZ. A comparison of sports and energy drinks--Physiochemical properties and enamel dissolution. Gen Dent. 2012;60(3):190–197. [PubMed] [Google Scholar]
- 29.Attin T, Weiss K, Becker K, Buchalla W, Wiegand A. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005;11(1):7–12. doi: 10.1111/j.1601-0825.2004.01056.x. [DOI] [PubMed] [Google Scholar]
- 30.Larsen MJ, Nyvad B. Enamel erosion by some soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999;33(1):81–87. doi: 10.1159/000016499. [DOI] [PubMed] [Google Scholar]
- 31.Owens BM. The potential effects of pH and buffering capacity on dental erosion. Gen Dent. 2007;55(6):527–531. [PubMed] [Google Scholar]
- 32.Davis RE, Marshall TA, Qian F, Warren JJ, Wefel JS. In vitro protection against dental erosion afforded by commercially available, calcium-fortified 100 percent juices. J Am Dent Assoc. 2007;138(12):1593–1598. doi: 10.14219/jada.archive.2007.0109. [DOI] [PubMed] [Google Scholar]
- 33.Lagerlof F, Dawes C. The volume of saliva in the mouth before and after swallowing. J Dent Res. 1984;63(5):618–621. doi: 10.1177/00220345840630050201. [DOI] [PubMed] [Google Scholar]
- 34.Van Eygen I, Vannet BV, Wehrbein H. Influence of a soft drink with low pH on enamel surfaces: an in vitro study. Am J Orthod Dentofacial Orthop. 2005;128(3):372–377. doi: 10.1016/j.ajodo.2004.03.036. [DOI] [PubMed] [Google Scholar]
- 35.ten Cate JM, Larsen M, Paearce E, Fejerskov O. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd EAM, editors. Dental caries The disease and its clinical management. Oxford, UK: Blackwell Munksgaard Publishing Co.; 2008. pp. 209–231. [Google Scholar]
- 36.Larsen MJ. An investigation of the theoretical background for the stability of the calcium-phosphate salts and their mutual conversion in aqueous solutions. Arch Oral Biol. 1986;31(11):757–761. doi: 10.1016/0003-9969(86)90008-7. [DOI] [PubMed] [Google Scholar]
- 37.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69(11):722–724. [PubMed] [Google Scholar]
- 38.Barbour M, Lussi A. Erosion in relation to nutrition and the environment. Monogr Oral Sci. 2014;25:143–154. doi: 10.1159/000359941. [DOI] [PubMed] [Google Scholar]
- 39.Moynihan PJ. Dietary advice in dental practice. Br Dent J. 2002;193(10):563–568. doi: 10.1038/sj.bdj.4801628. [DOI] [PubMed] [Google Scholar]
- 40.Lussi A, Jaeggi T. Etiology and Risk Assessment. In: Lussi A, Jaeggi T, editors. Dental erosion diagnosis, risk assessment, prevention, treatment. London, UK: Quintessence Publishing Co.; 2011. pp. 37–53. [Google Scholar]
- 41.Larsen MJ. Prevention by means of fluoride of enamel erosion as caused by soft drinks and orange juice. Caries Res. 2001;35(3):229–234. doi: 10.1159/000047461. [DOI] [PubMed] [Google Scholar]
- 42.Larsen MJ, Richards A. Fluoride is unable to reduce dental erosion from soft drinks. Caries Res. 2002;36(1):75–80. doi: 10.1159/000057595. [DOI] [PubMed] [Google Scholar]
- 43.Jarvinen VK, Rytomaa II, Heinonen OP. Risk factors in dental erosion. J Dent Res. 1991;70(6):942–947. doi: 10.1177/00220345910700060601. [DOI] [PubMed] [Google Scholar]
- 44.Hara A, Zero D. The potential of saliva in protecting against dental erosion. Monogr Oral Sci. 2014;25:197–205. doi: 10.1159/000360372. [DOI] [PubMed] [Google Scholar]
- 45.Mulic A, Tveit A, Songe D, Sivertsen H, Skaare A. Dental erosive wear and salivary flow rate in physically active young adults. BMC Oral Health. 2012;12:8. doi: 10.1186/1472-6831-12-8. pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion--an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45(Suppl 1):2–12. doi: 10.1159/000325915. [DOI] [PubMed] [Google Scholar]
- 47.Dawes C. Salivary flow patterns and the health of hard and soft tissues. JADA. 2008;139(Suppl):18S–24S. doi: 10.14219/jada.archive.2008.0351. [DOI] [PubMed] [Google Scholar]