Abstract
The orexin/hypocretin (ORX) system plays a major role in motivation for natural and drug rewards. In particular, a number of studies have shown that ORX signaling through the orexin 1 receptor (OX1R) regulates alcohol seeking and consumption. Despite the association between ORX signaling and motivation for alcohol, no study to date has investigated what role the ORX system plays in alcohol dependence, an understanding of which would have significant clinical relevance. This study was designed to evaluate the effect of the highly selective OX1R antagonist GSK1059865 on voluntary ethanol intake in ethanol-dependent and control non-dependent mice. Mice were subjected to a protocol in which they were evaluated for baseline ethanol intake and then exposed to intermittent ethanol or air exposure in inhalation chambers. Each cycle of chronic intermittent ethanol (CIE), or air, exposure was followed by a test of ethanol intake. Once the expected effect of increased voluntary ethanol intake was obtained in ethanol dependent mice, mice were tested for the effect of GSK1059865 on ethanol and sucrose intake. Treatment with GSK1059865 significantly decreased ethanol drinking in a dose-dependent manner in CIE-exposed mice. In contrast GSK1059865 decreased drinking in air-exposed mice only at the highest dose used. There was no effect of GSK1059865 on sucrose intake. Thus, ORX signaling through the OX1R, using a highly-selective antagonist, has a profound influence on high levels of alcohol drinking induced in a dependence paradigm, but limited or no influence on moderate alcohol drinking or sucrose drinking. These results indicate that the ORX system may be an important target system for treating disorders of compulsive reward seeking such as alcoholism and other addictions in which motivation is strongly elevated.
Keywords: Alcohol, sucrose, mouse, hypothalamus, drinking, addiction
1. Introduction
The peptide orexin (ORX, also referred to as hypocretin; HCRT) is produced exclusively by a population of neurons located in the dorsomedial, perifornical, and lateral regions of the posterior hypothalamus (de Lecea et al., 1998; Sakurai et al., 1998). The precursor peptide prepro-orexin is cleaved into two peptide subtypes: ORX-A and ORX-B (also referred to as HCRT-1 and HCRT-2) (de Lecea et al., 1998; Sakurai et al., 1998). ORX has two known receptors. The orexin 1 receptor (OX1R) has high selectivity for ORX-A, whereas the orexin 2 receptor (OX2R) is approximately equally selective for ORX-A and ORX-B (Sakurai et al., 1998). Orexin has shown to play a major role in a wide range of behavioral control including regulation of sleep and arousal (Kilduff and Peyron, 2000; Mignot, 2004; Sakurai, 2007), stress and anxiety (Berridge et al., 2010; Bonaventure et al., 2015), feeding (Cason et al., 2010; Sakurai et al., 1998), and motivation (Aston-Jones et al., 2009). We and others have characterized the function of the ORX system broadly in the context of motivational activation (Mahler et al., 2014).
One of the major roles that ORX plays is in driving motivation for highly palatable or salient reinforcers (Aston-Jones et al., 2009; Aston-Jones et al., 2010; Borgland et al., 2009; Sakurai, 2014). In particular, ORX plays an important function in driving the seeking of drugs of abuse, including alcohol (Baimel et al., 2015; Brown and Lawrence, 2013; Lawrence, 2010; Mahler et al., 2012). The orexin peptide is increased in some animal models of alcohol drinking and decreased in others (Lawrence et al., 2006; Morganstern et al., 2010; Olney et al., 2015), and ORX neurons are activated following reinstatement of alcohol seeking (Dayas et al., 2008; Hamlin et al., 2007). Antagonism of the OX1R decreases alcohol drinking, notably in higher-drinking alcohol-preferring animals (Anderson et al., 2014; Moorman and Aston-Jones, 2009; Olney et al., 2015), decreases self-administration, progressive-ratio, and reinstatement of alcohol seeking (Jupp et al., 2011; Lawrence et al., 2006; Martin-Fardon and Weiss, 2014a; Richards et al., 2008). Critically, no study to date has examined the role of the ORX system in alcohol dependence. We and others have used a chronic intermittent exposure to alcohol method (CIE) to produce animal models of alcohol dependence (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). This is measured as dramatically elevated alcohol consumption, increased motivation for alcohol seeking, and alcohol seeking and taking that is resistant to paired punishment. Given that previous studies have demonstrated that the ORX system is particularly active in high drinking individuals, we hypothesized that the ORX system would be specifically involved in regulating alcohol drinking in alcohol-dependent animals and that the influence of ORX in these animals would be greater than in non-dependent individuals.
To test the role of the ORX system in drinking in alcohol-dependent individuals, we used the selective OX1R antagonist GSK1059865 to block the OX1R in alcohol dependent and non-dependent animals during alcohol and sucrose drinking. Previous studies, including those noted above, have shown a critical role of the OX1R in alcohol seeking and taking, typically using the OX1R antagonist SB-334867 (Brown and Lawrence, 2013; Lawrence, 2010). However, the effects of this compound have occasionally been mixed (Winrow and Renger, 2014), and there has been some concern that SB-334867 exhibits off-target effects that could confuse interpretations of results. Although SB-334867 exhibits ~50-fold selectivity for OX1R vs. OX2R (Haynes et al., 2000; Smart et al., 2001), Gotter and colleagues reported significant binding at a number of non-ORX targets, including the adenosine A2A and A3 receptors, the serotonin 5-HT2B and 5-HT2C receptors, and monoamine, norepinephrine, and adenosine transporters (Gotter et al., 2012). Also of note, SB-334867 did not alter acquisition or expression of alcohol-induced conditioned place preference (Voorhees and Cunningham, 2011). For these reasons, we tested the influence of the highly selective OX1R antagonist GSK1059865 (Gozzi et al., 2013; Merlo Pich and Melotto, 2014; Piccoli et al., 2012). This compound has slightly higher selectivity for OX1R vs. OX2R than SB-334867 (~79-fold) and displays none of the significant off-target binding shown by SB-334867 (Gotter et al., 2012). This compound has also been shown to decrease cocaine conditioned place preference and compulsive eating (though not homeostatic or hedonic eating), in the absence of any influence on arousal (Gozzi et al., 2013; Merlo Pich and Melotto, 2014; Piccoli et al., 2012). Based on these results, we predicted that highly selective OX1R antagonism would have minimal influences on hedonic alcohol or sucrose consumption, but would strongly impact dependence-based alcohol drinking, which has been shown to exhibit elements of compulsivity (Vendruscolo et al., 2012; Vendruscolo and Roberts, 2014).
2. Results
Blood ethanol concentration (BEC) registered during the CIE exposure cycle that preceded the evaluation of the effect of GSK1059865 on alcohol or sucrose intake were analyzed with ANOVA with GSK1059865 dose as between factor and cycle (6 vs.7) as repeated measure. The ANOVA did not indicate any difference in BEC between groups based on the dose of GSK1059865 treatment [F(3,28)=0.52] or the cycle of CIE exposure [F(1,28)=3.14]. The interaction between these factors also failed to reach significance [F(3,28)=0.01]. BEC values for cycle 6 were 222.8±17.6, 236.6±18.7, 232.8±18.7, and 216.3±20 for vehicle and 10, 25, 50 mg/kg doses of GSK1059865, respectively (values are mean ± SEM). BEC values for cycle 7 of CIE exposure were 244.9±12.9, 253.8±13.6, 255.1±13.6, and 239.4±14.6 for vehicle and 10, 25, 50 mg/kg doses of GSK1059865, respectively (values are mean ± SEM).
Preliminary analysis did not indicate any significant daily variations in voluntary alcohol or sucrose intake on testing days following cycle 6 [interaction between GSK dose and day, F(12,232)=0.85 and interaction between group, dose and day, F(12, 232)=0.28; both, p>0.05], indicating no significant development of tolerance to GSK1059865 during testing. Similarly, after cycle 7, there were no daily variations on ethanol intake for EtOH and CTL mice that received different doses of GSK1059865 (or vehicle) before drinking sucrose [GSK dose X day, F(12,232)=0.32 and the three-way interaction was F(12,232)=0.51; both, p>0.05]. Therefore, data were averaged over the five days of treatment for test cycles. These data were analyzed with ANOVA with Group (CTL, EtOH) and GSK1059865 (0, 10, 25, 50 mg/kg) as main factors. There were 7-9 mice assigned to each group and intake data were averaged over the test days. The analysis of the test cycle 5, prior to the test of GSK’s effect (Test 6), indicated a main effect of Group [F(1,63)=14.02, p<0.001] due to a higher ethanol intake of EtOH mice (3.41 ± 0.28 g/kg, Mean ± SEM) compared to CTL mice (2.74 ± 0.20 g/kg, Mean ± SEM), as expected.
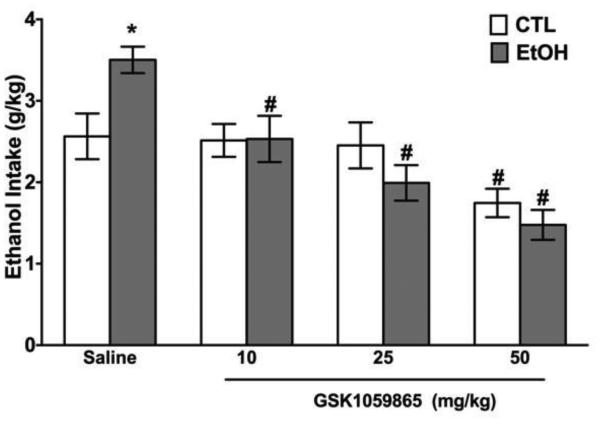
On test cycle 6, mice that were previously injected with vehicle were separated into four groups based on the dose of GSK1059865 they received before receiving access to ethanol. The analysis of this test indicated a significant interaction between Group and GSK1059865 treatment [F(3,58)=3.65, p<0.025]. Newman-Keuls’ post-hoc comparisons (alpha set at 0.05) indicated that EtOH mice that received vehicle consumed significantly more ethanol than CTL mice that also received vehicle (Figure 1). However, there was no difference between EtOH and CTL mice compared within each dose treatment for GSK1059865. This compound induced a significant decrease in ethanol intake in EtOH mice demonstrated by the fact mice receiving GSK1059865 (any dose) drank less ethanol than EtOH mice treated with vehicle. GSK1059865 also produced a significant decrease in intake of CTL mice but this effect was only observed after the administration of the highest dose tested (Figure 1).
Figure 1.
Voluntary ethanol intake (g/kg) for EtOH and CTL mice that received GSK1059865 treatment before drinking ethanol. * EtOH ≠ CTL group; # ≠ from vehicle treated group.
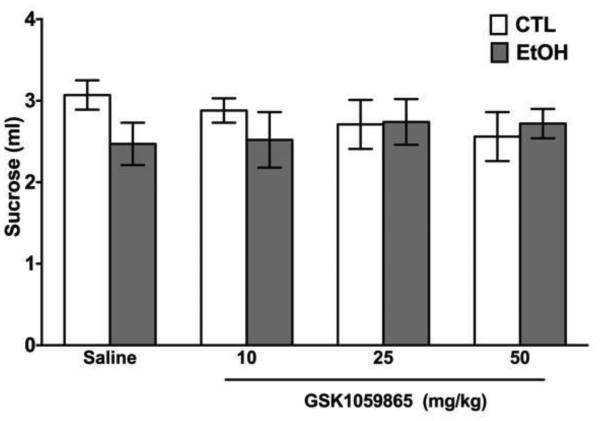
On the last test cycle, mice were given sucrose (5% w/v) instead of ethanol, vs. water. In this case, the ANOVA failed to indicate any significant effect of Group [F(1,58)=1.14], GSK1059865 [F(3,58)=0.09], or their interaction [F(3,58)=0.91] (Figure 2). This indicates that neither CIE exposure nor GSK1059865 administration has a significant effect on voluntary intake of sucrose.
Figure 2.
Voluntary sucrose intake (ml) for EtOH and CTL mice that received GSK1059865 treatment before drinking sucrose.
3. Discussion
Here we demonstrated for the first time that the ORX system plays a critical role, not just in the rewarding aspect of alcohol and other drugs of abuse, but specifically in dependence-related excessive alcohol drinking. As in previous studies, our results demonstrate that CIE exposure induces a significant increase in voluntary ethanol intake in mice, indicative of dependence-induced elevated drinking (Becker and Hale, 1993; Becker and Lopez, 2004; Griffin et al., 2009). The increase in voluntary alcohol intake due to CIE exposure obtained in this study is comparable to previous work that indicates that this increased amount of alcohol intake (consumed within 2-hr) results in significantly elevated blood and brain ethanol levels (Griffin et al., 2009; Lopez and Becker, 2004).
Treatment with the highly selective OX1R antagonist GSK1059865 significantly reduced voluntary ethanol intake in mice. Ethanol-dependent mice were more sensitive to the effect of GSK1059865 on ethanol intake than control non-dependent mice: lower doses of GSK1059865 that reduced ethanol intake in ethanol-dependent mice had no effect on the ethanol intake of control mice. In addition, the effect was selective to ethanol: treatment with GSK1059865 had no influence on voluntary intake of another highly-palatable solution (sucrose). The evaluation of GSK1059865’s effect on sucrose intake was done after the evaluation of its effects on alcohol intake. This raises the possibility that the lack of effect of this drug on sucrose intake can be related to a development of tolerance due to previous exposure. However, the lack of significant differences on daily values of alcohol or sucrose intake makes this alternative less likely. In sum, our data show that the main inhibitory effect of OX1R antagonism was on the elevated alcohol intake induced by chronic intermittent ethanol exposure.
These results are in line with a wide range of studies indicating that the ORX system is critically involved in stimuli or outcomes that produce strong degrees of motivated behavior (Borgland et al., 2009; Merlo Pich and Melotto, 2014). These studies led to our proposal that a major function of the ORX system is in motivational activation (Mahler et al., 2014). With respect to alcohol, we previously showed that OX1R antagonism using the compound SB-334867 reduced alcohol drinking and preference selectively in high alcohol-preferring rats with few effects on drinking in low alcohol-preferring rats (Moorman and Aston-Jones, 2009). Similarly, rats bred for high alcohol preference exhibit decreased alcohol seeking and consumption following orexin receptor blockade (Anderson et al., 2014; Dhaher et al., 2010; Jupp et al., 2011; Lawrence et al., 2006). Particularly relevant is work from our group and others showing that OX1R antagonism reduced binge-like ethanol drinking using a drinking-in- the-dark model in C57BL/6J mice (Anderson et al., 2014; Olney et al., 2015), indicating a significant influence of the ORX system on enhanced motivation as measured by binge-like ethanol consumption. Indeed, the ORX system also plays a major role in motivation for other drugs of abuse. Of note with respect to our findings, is the fact that, whereas OX1R antagonism had no effect on steady maintenance of FR1 cocaine self-administration (Smith et al., 2009), blockade at these receptors significantly reduced enhanced motivation for cocaine seen in studies using higher ratios (Hollander et al., 2012), progressive ratios (Espana et al., 2010), and behavioral economic-type threshold procedures (Bentzley and Aston-Jones, 2015; Espana et al., 2010). A similar inhibitory influence of OX1R antagonism has been seen for seeking of heroin (Smith and Aston-Jones, 2012), cannabinoids (Flores et al., 2014), nicotine (Hollander et al., 2008; Plaza-Zabala et al., 2013), and highly palatable food rewards (Borgland et al., 2009; Cason and Aston-Jones, 2013b; Cason and Aston-Jones, 2014; Choi et al., 2010; Kay et al., 2014; Sharf et al., 2010). OX1R antagonism decreases progressive ratio break points for alcohol in male (though not female) alcohol-preferring rats (Anderson et al., 2014; Jupp et al., 2011), and the effects on tasks requiring high effort for natural rewards such as sucrose have been mixed with some studies showing reductions but others showing no decreases, depending on the behavioral paradigm employed and the sex studied (Alcaraz-Iborra et al., 2014; Cason and Aston-Jones, 2013a; Cason and Aston-Jones, 2013b; Cason and Aston-Jones, 2014; Jupp et al., 2011). In line with our studies, Martin-Fardon and colleagues demonstrated that OX1R antagonism decreased discriminative stimulus-induced reinstatement of ethanol seeking (Martin-Fardon and Weiss, 2014a) and cocaine seeking (Martin-Fardon and Weiss, 2014b), but in neither case had an influence on reinstatement of natural reward seeking (a saccharin/glucose solution and sweetened condensed milk, respectively).
The results described above, along with our findings, support the proposal that the ORX system, particularly via the OX1R, is critically involved in high-levels of motivation for drugs and rewards. There are additional studies that demonstrate a role for the OX1R in regulating reward seeking behaviors more broadly, such as some of those described above. Of note, most of these studies have used the ORX1R antagonist SB-334867. Although this compound exhibits selectivity for the OX1R, particularly at lower doses, there have been some reports that the selectivity and/or stability of SB-334867 may be compromised at higher doses or depending on the preparation (Hollander et al., 2012; McElhinny et al., 2012; Merlo Pich and Melotto, 2014; Winrow and Renger, 2014). The compound used in the current study, GSK1059865, is highly selective for OX1R and exhibits high levels of OX1R occupancy (Bonaventure et al., 2015; Gozzi et al., 2011; Gozzi et al., 2013; Merlo Pich and Melotto, 2014; Piccoli et al., 2012). The compound has no influence on sleep/wake, in contrast with the highly selective OX2R antagonist JNJ10397049 (Gozzi et al., 2011; Piccoli et al., 2012) indicating that behavioral effects of OX1R antagonism are not related to sleep.
Because we did not measure blood alcohol levels in vehicle vs. GSK1059865-treated mice, we cannot rule out the possibility that the compound may have affected the metabolism of alcohol. A number of details mitigate this concern however. First, the fact that the compound had almost no effect on ethanol consumption in non-dependent animals, while not conclusive, suggests that non-specific effects on alcohol drinking were secondary to the overall motivational influences on alcohol drinking in CIE-treated animals. In addition, the fact that previous studies demonstrated that GSK1059865 decreased cocaine conditioned place preference and compulsive food eating further supports the idea that the prime influence of the compound is on motivational processes. However, neither of these details rule out the possibility that there may be an interaction between GSK1059865 and ethanol metabolism, and indicate that this may be a useful subject for future study.
Importantly, GSK1059865 at 10 and 30 mg/kg did not inhibit highly palatable food intake, but did inhibit compulsive eating in animals subjected to chronic stress and food restriction (Piccoli et al., 2012). These results strongly support the hypothesis, consistent with our results, that signaling via the OX1R regulates the elevated motivation seen in compulsive reward seeking but not hedonic or homeostatic reward seeking. In general, this series of findings underscores the role of the ORX system in motivational activation (Mahler et al., 2014) and compulsive behavior (Merlo Pich and Melotto, 2014), and indicates that ORX signaling through the OX1R may be particularly relevant for elevated reward-related motivation. Understanding ORX signaling, therefore, has significant clinical relevance, particularly in the case of diseases of motivation such as addiction or compulsive eating. Selective targeting of the OX1R may be an excellent treatment for many of the pathological, out-of-control aspects of these disorders, while sparing normal functions of reward and motivation.
4. Experimental Procedure
4.1. Subjects
Adult male C57BL/6J mice (25-30 g) purchased from Jackson Laboratories (Bar Harbor, ME) were used as subjects. All mice were individually housed with rodent food (Harland Teklad, Madison, WI) and water available ad libitum. Mice were maintained in an animal facility fully accredited by AAALAC, with automated control of temperature, humidity, and light cycles. Body weight was recorded weekly while mice were drinking but daily during CIE exposure cycles. Mice were housed under a 12-hr light/dark cycle (lights on at 0200 hr). All procedures were approved by the Institutional Animal Care and Use Committee and followed the NIH Guide for the Care and Use of Laboratory Animals (2011).
4.2 General study design and procedures
The general study procedure involved first establishing stable baseline levels of ethanol intake using a 2-hr limited access procedure (described below). Once stable ethanol intake was achieved mice were separated into EtOH and CTL groups and exposed to repeated cycles of chronic intermittent ethanol (CIE) exposure (described below) or control procedures, respectively. Precautions were taken to distribute mice into each group based on their baseline intake so that groups did not differ in ethanol intake before CIE or control exposure. Each cycle of CIE (or control) exposure was followed 72 hours later by a 5-day testing period for ethanol intake. This pattern of CIE exposure followed by an ethanol intake test period was repeated for 7 cycles. During baseline and the first 5 test cycles following CIE (or air) exposure, mice received vehicle (saline) injections (i.p.; 0.01 ml/g body weight) 30 minutes before drinking ethanol. On test cycles 6 and 7 mice received vehicle or GSK1059865 (10, 25, 50 mg/kg, generously donated by GlaxoSmithKline) before given access to ethanol 15% v/v (Test 6) or sucrose 5% w/v (Test 7) versus water. GSK1059865 was dissolved in saline and TWEEN 80 (0.5 % v/v) as vehicle. Doses were chosen based on those used in previous studies of this compound in which high selectivity and minimal hypnotic effects were observed at up to 30 mg/kg (Gozzi et al., 2011; Piccoli et al., 2012).
4.3 Ethanol drinking in a limited access situation
A modified sucrose fading procedure (Samson, 1986) was used to train mice to drink ethanol under a daily limited access schedule (2 hr/day). Mice started drinking a 10% ethanol and 5% sucrose solution (10%E/5%S). Then, the ethanol concentration was increased over consecutive days while sucrose was gradually removed from the solution. The schedule was as follows: 10%E/5%S, 12%E/5%S, 15%E/5%S, 15%E/2%S, 15%E/1%S, and 15%E/0%S. Mice were presented with each of these solutions for 2 days. The final concentration used for the remainder of the experiment was 15% ethanol without sucrose. Solutions were prepared daily by mixing ethanol (v/v) and sucrose (w/v) in deionized water and presented at room temperature. Thirty minutes before the start of the dark cycle, a 15 ml graduated tube containing 10 ml of an ethanol solution and a bottle containing water were introduced in the home cage and the regular water bottle was made available again. The position of the ethanol and water bottles was alternated daily. Mice were not food or water deprived at any time during the experiments. Ethanol bottles were read daily. The amount of ethanol consumed by each mouse was converted to g/kg based on the milliliters of ethanol consumed (± 0.1 ml) and body weight (± 0.1 g). As previously reported (Becker and Lopez, 2004), C57BL/6J mice will self-administer significant amounts of ethanol during the 2-hr limited access sessions, with amount consumed (g/kg) correlated with consequent blood ethanol concentration (BEC) attained at the end of the session. The amount of water ingested was minimal (between 0.0 and 0.1 ml) during the two hours of the limited access to alcohol or sucrose versus water. Intake values for water were not included in the analysis because these values are not affected by CIE exposure (Becker and Lopez, 2004).
4.4 CIE Exposure
Mice in the EtOH group received CIE vapor exposure in inhalation chambers (16 hr/day for 4 days) while control mice (CTL) were similarly handled, but exposed to air in control chambers. CIE exposure was administered in inhalation chambers according to procedures previously described (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). Briefly, mice were placed in Plexiglas inhalation chambers (60 × 36 × 60 cm) and exposed to ethanol vapor or uncontaminated air based on group assignment. Housing conditions were similar to those in the colony room. Ethanol was volatized by passing air through an air stone submerged in 95% ethanol. The ethanol vapor was mixed with fresh air and delivered to the inhalation chambers at a rate of 5 liters/min, which maintained the ethanol concentration in the chambers at 10-13 mg/l air. The ethanol concentration in the inhalation chamber was monitored daily. These conditions have been shown to yield stable blood ethanol concentrations (BEC) in C57BL/6J mice in the range of 175-225 mg/dl (Becker and Lopez, 2004; Griffin et al., 2009; Lopez and Becker, 2005). Before each chronic ethanol exposure cycle, intoxication was initiated in EtOH mice by administration of ethanol (1.6 g/kg), and BEC was stabilized by injection of the alcohol dehydrogenase inhibitor pyrazole (1 mmol/kg). Both ethanol and pyrazole were administered intraperitoneally (i.p.) in a volume of 0.02 ml/g body weight. CTL mice were similarly handled, but administered saline and pyrazole (i.p.) prior to being placed in control chambers that delivered only air (no ethanol vapor). Thus, all mice received the same number and timing of pyrazole injections prior to final removal from the inhalation chambers.
Highlights.
Orexin 1 receptor antagonism decreased ethanol vapor-enhanced ethanol drinking
Effects were dose-dependent
Orexin 1 receptor antagonism had minimal effects on drinking in air-treated controls
Orexin 1 receptor antagonism had no effect on sucrose drinking
Acknowledgements
Supported by PHS grants P50-AA010761, U01-AA014095, U01-AA020929, R21-DA032005, R37/R01-DA006214, UL1-RR029882 and VA Medical Research. We thank GlaxoSmithKline for providing GSK1059865. The authors declare no conflicts of interest regarding this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
MFL, DEM, GAJ, and HCB designed experiments. MFL collected data. MFL analyzed data in consultation with DEM and HCB. MFL and DEM wrote the manuscript. MFL, DEM, GAJ, and HCB edited the manuscript. All authors have approved the final version of the manuscript.
References
- Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I. Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: pharmacological and molecular evidence of orexin involvement. Behav Brain Res. 2014;272:93–9. doi: 10.1016/j.bbr.2014.06.049. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci. 2014;8:33. doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–21. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimel C, Bartlett SE, Chiou LC, Lawrence AJ, Muschamp JW, Patkar O, Tung LW, Borgland SL. Orexin/hypocretin role in reward: implications for opioid and other addictions. Br J Pharmacol. 2015;172:334–48. doi: 10.1111/bph.12639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Repeated episodes of ethanol withdrawal potentiate the severity of subsequent withdrawal seizures: an animal model of alcohol withdrawal "kindling". Alcohol Clin Exp Res. 1993;17:94–8. doi: 10.1111/j.1530-0277.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine- associated cues. Eur J Neurosci. 2015;41:1149–56. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, Vittoz NM. Hypocretin/orexin in arousal and stress. Brain Res. 2010;1314:91–102. doi: 10.1016/j.brainres.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman BT, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg T, Dugovic C. A selective orexin-1 receptor antagonist attenuates stress-induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015;352:590–601. doi: 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–25. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Curr Opin Neurobiol. 2013;23:467–72. doi: 10.1016/j.conb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, Aston-Jones G. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–28. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Attenuation of saccharin-seeking in rats by orexin/hypocretin receptor 1 antagonist. Psychopharmacology (Berl) 2013a;228:499–507. doi: 10.1007/s00213-013-3051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013b;226:155–65. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in female rats. Neuropharmacology. 2014;86:97–102. doi: 10.1016/j.neuropharm.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–7. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol- Seeking, in Alcohol-Preferring (P) Rats. J Addict Med. 2010;4:153–9. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin- orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–48. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry. 2014;75:499–507. doi: 10.1016/j.biopsych.2013.06.012. [DOI] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Turrini G, Piccoli L, Massagrande M, Amantini D, Antolini M, Martinelli P, Cesari N, Montanari D, Tessari M, Corsi M, Bifone A. Functional magnetic resonance imaging reveals different neural substrates for the effects of orexin-1 and orexin-2 receptor antagonists. PLoS One. 2011;6:e16406. doi: 10.1371/journal.pone.0016406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A. Differential effect of orexin-1 and CRF- 1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology. 2013;38:2120–30. doi: 10.1038/npp.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–80. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–36. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A. 2008;105:19480–5. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011;1391:54–9. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Kay K, Parise EM, Lilly N, Williams DL. Hindbrain orexin 1 receptors influence palatable food intake, operant responding for food, and food-conditioned place preference in rats. Psychopharmacology (Berl) 2014;231:419–27. doi: 10.1007/s00213-013-3248-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilduff TS, Peyron C. The hypocretin/orexin ligand-receptor system: implications for sleep and sleep disorders. Trends Neurosci. 2000;23:359–65. doi: 10.1016/s0166-2236(00)01594-0. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol- seeking in rats. Br J Pharmacol. 2006;148:752–9. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain Res. 2010;1314:124–9. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–96. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci. 2014;17:1298–303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. N-(2-methyl-6-benzoxazolyl)-N'-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol. 2014a;19:233–6. doi: 10.1111/j.1369-1600.2012.00480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport. 2014b;25:485–8. doi: 10.1097/WNR.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny CJ, Jr., Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett. 2012;22:6661–4. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- Merlo Pich E, Melotto S. Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Front Neurosci. 2014;8:26. doi: 10.3389/fnins.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E. Sleep, sleep disorders and hypocretin (orexin) Sleep Med. 2004;5(Suppl 1):S2–8. doi: 10.1016/s1389-9457(04)90001-9. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–86. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010;34:886–96. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39:21–9. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merlo-Pich E, Di Fabio R, Corsi M. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology. 2012;37:1999–2011. doi: 10.1038/npp.2012.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology. 2013;38:1724–36. doi: 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–17. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–81. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The role of orexin in motivated behaviours. Nat Rev Neurosci. 2014;15:719–31. doi: 10.1038/nrn3837. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–60. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Sabido-David C, Brough SJ, Jewitt F, Johns A, Porter RA, Jerman JC. SB-334867- A: the first selective orexin-1 receptor antagonist. Br J Pharmacol. 2001;132:1179–82. doi: 10.1038/sj.bjp.0703953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, See R, Aston-Jones G. Orexin / hypocretin signaling at the OX1 receptor regulates cue- elicited cocaine-seeking. Europ. J. Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin / hypocretin 1 receptor antagonist reduces heroin self- administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid- dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Roberts AJ. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol. 2014;48:277–86. doi: 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology (Berl) 2011;214:805–18. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. Br J Pharmacol. 2014;171:283–93. doi: 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]