Abstract
Background
A history of preeclampsia is an independent risk factor for cardiac events and stroke. Changes in vasculature structure that contribute to these associations are not well understood.
Objective
The aim of this study was to quantify coronary artery calcification, a known risk factor for cardiac events, in a prospective cohort of women with and without histories of preeclampsia.
Study Design
Women without prior cardiovascular events (40 with and 40 without histories of preeclampsia, matched for parity and age at index birth) were recruited from a large population based cohort of women who were residents of Olmsted County, MN and who had delivered between 1976 and 1982. A computed tomography was performed to measure coronary artery calcification in Agatston Units. All pregnancy histories and covariates were confirmed by review of the medical records. Current clinical variables were assessed at the time of imaging. Differences between women with and without histories of preeclampsia were examined using chi-square tests and t-tests; coronary artery calcification, in particular, was compared as a categorical and ordinal variable, with a chi-squared test and with Wilcoxon 2-sample tests and ordinal logistic regression, as appropriate.
Results
Mean age (standard deviation) at imaging was 59.5 (± 4.6) years. Systolic and diastolic blood pressures, hyperlipidemia, and current diabetes status did not differ between women with and without histories of preeclampsia. However, the frequencies of having a current clinical diagnosis of hypertension (60% v. 20%, p < 0.001) and higher body mass index in kg/m2 [29.8 (25.9, 33.7) vs. 25.3 (23.1, 32.0), median (25th–75th percentile), p = 0.023] were both greater in the women with histories of preeclampsia compared to those without. The frequency of a CAC score > 50 Agatston units was also greater in the preeclampsia group (23% v. 0%, p=0.001). Compared to women without preeclampsia, the odds of having a higher coronary artery calcification score was 3.54 (1.39 – 9.02) times greater in women with prior preeclampsia without adjustment, and 2.61 (0.95 – 7.14) times greater after adjustment for current hypertension. After adjustment for body mass index alone, the odds of having a higher coronary artery calcification based on a history of preeclampsia remained significant, 3.20 (1.21, 8.49).
Conclusion
In this first prospective cohort study with confirmation of preeclampsia by medical record review, a history of preeclampsia is associated with an increased risk of coronary artery calcification more than 30 years after affected pregnancies, even after controlling individually for traditional risk factors. A history of preeclampsia should be considered in risk assessment when initiating primary prevention strategies to reduce cardiovascular disease in women. Among women with histories of preeclampsia, the presence of coronary artery calcifications may be able to identify those at a particularly high cardiovascular risk, and should be the subject of future studies.
Indexing words: cardiovascular disease, hypertension, pregnancy, preeclampsia
Introduction
Cardiovascular disease (CVD) is the leading cause of death in women. A history of preeclampsia is a sex-specific independent risk factor for cardiac events and stroke.1–4 The importance of documenting a history of adverse events in pregnancy, such as preeclampsia, is now highlighted in guidelines for the prevention of stroke and coronary artery disease. 5, 6
It is important to understand the pathophysiologic processes linking preeclampsia to subsequent CVD risk, given the strong association between preeclampsia and future adverse cardiovascular outcomes. Conventional risk factors, including obesity and chronic hypertension, are shared by both conditions. 7 Other non-traditional risk factors that are shared by preeclampsia and adverse CVD outcomes include increased levels of C-reactive protein and homocysteine. 8, 9
Coronary artery calcification (CAC), as measured in Agatston units (AU) by computed tomography, is an important biomarker of cardiovascular disease. The extent of CAC strongly correlates with a higher risk for an adverse cardiac event even in asymptomatic individuals 10 : for a CAC > 100, the relative risk for a cardiac event in the next 4 years is greater than 9. 11 The American College of Cardiology/AHA guidelines from both 2010 and 2013 stated that measurement of CAC may help to define risk in those persons at low to intermediate risk based on Framingham scoring.12 CAC measurement may be beneficial in assessing risk of CVD for women in particular as the Framingham Risk score typically underestimates female CVD risk.
A systematic study of the relationship between a history of preeclampsia, as defined by accepted clinical criteria, and CAC quantification in asymptomatic women has not been undertaken. The aim of this study was to quantify and compare CAC in age- and parity-matched women with and without histories of preeclampsia, and to establish whether this association was independent of traditional CVD risk factors. We postulated that CAC would be higher in women with histories of preeclampsia and could better stratify cardiovascular risk in these women.
Material and Methods
The Rochester Epidemiology Project (REP) medical records-linkage system was used to identify, from a larger population based cohort, 40 age- and parity-matched women with and without histories of preeclampsia who then were recruited to undergo imaging, examination and laboratory assessment.
The REP medical records-linkage system was established in 1966 to capture all health care information for the entire population of Olmsted County, MN, USA. 13–15 The REP now encompasses the medical records of a population with an estimated 6.3 million person-years of experience; only 2% of the county residents have denied access to the use of their medical records for research.
HICDA (Hospital Adaptation of the International Classification of Diseases Adapted) are analogous to ICD-9 codes. Nine hundred-ninety women who delivered between 1976 and 1982 and identified using the REP had HICDA codes that might be indicative of a possible hypertensive pregnancy disorder (see supplement for specific codes). The medical records of these women were fully abstracted for demographic and clinical information at the time of each pregnancy, including date of birth, ethnicity, race, education, marital status, family history, blood pressures, weight, laboratory values (e.g., creatinine, proteinuria, liver enzymes), medications, tobacco, alcohol, pregnancy complications, seizures, persistent headache, epigastric pain, coma, chronic hypertension, diabetes, stroke, cardiac disease, renal disease, hepatic disease, autoimmune disorder, admission to the intensive care unit, postpartum depression, and hyperreflexia.
A potential exposure was confirmed as preeclampsia if a woman had at least one preeclamptic pregnancy between 1976–1982 and met the standard definition: 1) two or more blood pressure readings of a systolic blood pressure (SBP) > 140 mm Hg or a diastolic blood pressure (DBP) >90 mm Hg that occur at least 4 hours apart after 20 weeks gestation and 2) new onset proteinuria, as defined by a urine dipstick 1+, or proteinuria ≥0.300 g /24 hours, or a protein/creatinine ratio equivalent to ≥ 0.3 g per 24 hours. Emergency room visits were not included in the assessment.
Inclusion and Exclusion criteria
A woman had to be a resident of Olmsted County, MN when delivering a baby from a pregnancy lasting >20 weeks (live birth or stillbirth) between January 1, 1976 and December 31, 1982 to be eligible for the present study. She also had to be a current resident of Olmsted County, MN, to have had a documented clinical visit within the last 2 years, and to live within 120 miles of Olmsted County at the time of the current study in order to be available for in-person visits. The primary focus of the study was to understand the potential vascular damage and mechanisms that place women with histories of preeclampsia at risk of subsequent CVD. Therefore, all women, regardless of their pregnancy outcomes, were excluded with a medical-record confirmed clinical diagnosis of the following conditions: myocardial infarction, congestive heart failure, stroke, dementia, any cancer (with the exception of non-melanoma skin cancer), autoimmune disease (e.g., multiple sclerosis, lupus), and neurological conditions (e.g., epilepsy).
Identification of women with preeclamptic pregnancies for current study
Seventy-seven eligible women from the population based cohort described above with confirmed previous preeclampsia were sent letters describing the study and the contact information for the study coordinator. The study coordinator attempted to contact the woman by phone if she did not respond within two weeks. Of the 77 women contacted with confirmed histories of preeclampsia, 25 (32%) refused, 7 (9%) did not respond (i.e., did not respond to the initial letter and we did not contact them further because an eligible woman had been identified), and 5 (6%) were found to be ineligible after screening for additional medical conditions not identified in the medical record. We continued until we identified 40 women with histories of preeclampsia.
Identification of women with normotensive pregnancies
For each woman with a confirmed history of preeclampsia, potential age- and parity-matched women were identified without any of the possible hypertensive pregnancy codes. Women were sequentially contacted and recruited in order to obtain one woman with a history of normotensive pregnancies that was matched for each of the 40 with histories of preeclampsia. Ultimately, 104 women were contacted. Of these, 18 refused (17%), 8 (8%) did not respond, 5 (5%) wanted to participate but another matched control already had been recruited, and 33 did not respond after the letters, but were not contacted further as a control had already been found. The medical records of these women were fully abstracted for demographic and clinical information, as described previously. All protocols were approved by the Mayo Clinic Institutional Review Board (PR10-005198-05) and all individuals gave written informed consent.
Assessment of Outcomes
Contemporary medical record review
The following demographic and clinical data were obtained from review of the medical records and patient interviews at the time of assessment of outcomes: age, body mass index (BMI), SBP, DBP, anti-hypertensive and-lipid lowering medication use, tobacco use (current, ever or never), family history of heart disease, and chart abstracted and physician confirmed diagnoses of hypertension, hyperlipidemia and diabetes mellitus.
Assessment of coronary artery calcification (CAC)
CAC images were obtained using a 64 detector computed tomography scanner (Siemens Sensation 64, Siemens Medical Solutions, Forcheim, Germany), with a scan configured to cover the heart as described previously. 16, 17 Calcium scoring was performed using the Agatston scoring method. 18
Traditional risk factors
The diagnosis of hypertension was confirmed if a prior diagnosis and/or use of prescription anti-hypertensive medication were confirmed upon medical record review, or if a SBP≥ 140 mm Hg or DBP≥ 90 mm Hg was documented in the medical records on 2 separate occasions. Smoking was defined as never, past (>1 year ago) and current (including within the last 12 months). The diagnosis of dyslipidemia was confirmed if one or more of the following criteria were met: use of lipid-lowering drugs or laboratory measurements revealing a total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, or HDL ≤ 50 mg/dL. Diabetes mellitus was diagnosed by HgA1c≥ 6.5% and a fasting glucose > 126 mg/dL, or a physician diagnosis in the past, with or without current glucose-lowering agents.
Blood collection
Blood was collected from an antecubital vein after an overnight fast from participants using a 21 gauge needle and placed into appropriate laboratory tubes. Biochemical parameters were measured by standard methods on a Hitachi 911 Chemistry Analyzer (Roche Diagnostics, Indianapolis IN), at Mayo Clinic Clinical Laboratories, Rochester, MN)
Statistics
Distributions of continuous variables were summarized with means and standard deviations or medians and 25th to 75th interquartile range (IQR) depending on normality of distribution, while categorical variables were reported as counts and percentages. Differences between groups with and without histories of preeclampsia were tested using the t-test or Wilcoxon rank sum test for continuous and ordinal variables, and the chi-square test for categorical variables. Due to a heavily asymmetric distribution, the proportional odds model was used to analyze CAC as an ordinal outcome variable based only on its rank values to test the hypothesis that a history of preeclampsia is associated with increasing CAC independent of potentially confounding factors. This model combines the results of binary logistic regressions on CAC performed at all possible thresholds for “positivity”, thus providing the ability to control for multiple variables while retaining the robustness of a rank-based analysis, and avoiding the loss of the quantitative CAC information. Confounding factors included as adjusting covariates in proportional odds modeling, both individually and together, were current hypertension status (binary- yes or no) and BMI (in kg/m2), as a continuous variable. All analyses were carried out with SAS statistical programming (Version 9.4, SAS Institute Inc, Cary, NC). An alpha level of 0.05 was used to judge statistical significance.
Results
The mean age of the study participants at the time of delivery was 24 years old. Prior to their index pregnancies, women with preeclampsia, compared to those with normotensive pregnancies, had higher BMI, expressed as median (IQR: 23.9 [21.3, 26.1] versus 21.0 [20.0, 24.3], p=0.012). The rates of gestational diabetes were similar between the groups, affecting 2 of 40 women in each group (p=1.00). The mean age of the study participants at the time of imaging was 59.5 (± 4.6) years. There were no statistically significant differences between groups in systolic and diastolic blood pressures, hyperlipidemia and diabetes status at the time of imaging. However, BMI (29.8 [25.9, 33.7] vs 25.3 [23.1, 32.0], p = 0.023) was higher in women with histories of preeclampsia compared to those women with histories of normotensive pregnancies, respectively. The frequency of a current clinical diagnosis of hypertension (HTN) (60% v. 20%, p < 0.001) was greater in the preeclampsia group (Table 1).
Table 1.
Baseline characteristics of women with histories of normotensive and preeclamptic pregnancies at the time of recruitment
| Variable x | History of Normotensive Pregnancy (n=40) |
History of Preeclampsia (n=40) |
p-value |
|---|---|---|---|
| Age at study consent (years) | 59.7±4.5 | 59.4±4.8 | 0.782 |
| Age at 1st live birth (years) | 24.3±3.4 | 24.2±3.7 | 0.903 |
| Time since 1st live birth (years) | 34.5 (33.6, 36.7) | 34.9 (32.9, 36.7) | 0.564 |
| Self-identified Caucasian race | 39 (98%) | 40 (100%) | 1.00x |
| Parity | 2.7±0.8 | 2.8±0.9 | 0.895 |
| Education: | 0.219x | ||
| . High school or less | 3 (8%) | 6 (15%) | |
| . Some college | 21 (53%) | 22 (55%) | |
| . College graduate or higher | 16 (40%) | 12 (30%) | |
| Employment Status | 0.180 | ||
| . Employed | 22 (56%) | 29 (73%) | |
| . Retired | 14 (36%) | 7 (18%) | |
| . Unemployed/homemaker/other | 3 (8%) | 4 (10%) | |
| Marital Status: | 0.785 | ||
| . Married/living as married | 31 (78%) | 32 (80%) | |
| . Separated/divorced/widowed | 9 (23%) | 8 (20%) | |
| Tobacco Use: | 0.209 | ||
| . Never | 21 (53%) | 28 (70%) | |
| . Past | 15 (38%) | 8 (20%) | |
| . Current | 4 (10%) | 4 (10%) | |
| Family History of Heart Disease | 23 (58%) | 26 (65%) | 0.491 |
| Systolic blood pressure (mm Hg) | 131.4±20.6 | 131.8±14.9 | 0.911 |
| Diastolic blood pressure (mm Hg) | 75.8±10.7 | 78.2±9.6 | 0.287 |
| BMI (kg/m2) | 25.3 (23.1, 32.0) | 29.8 (25.9, 33.7) | 0.023 |
| Total cholesterol (mg/dL) | 204.5 (182.0, 222.5) | 189.5 (168.0, 215.0) | 0.095 |
| LDL Cholesterol (mg/dL) | 123.0 (99.7, 136.4) | 106.1 (87.9, 124.3) | 0.087 |
| HDL Cholesterol (mg/dL) | 64.0 (50.5, 76.5) | 54.5 (41.0, 69.5) | 0.054 |
| Triglycerides (mg/dL) | 97.5 (72.0, 123.5) | 108.0 (85.0, 163.0) | 0.078 |
| Creatinine (mg/dL) | 0.75±0.10 | 0.77±0.15 | 0.495 |
| Calcium (mg/dL) | 9.4±0.4 | 9.4±0.3 | 0.863 |
| Fasting glucose (mg/dL) | 95.5 (91.0, 101.5) | 98.0 (91.5, 109.5) | 0.151 |
| Insulin (µIU/mL) | 4.6 (3.3, 6.0) | 7.1 (4.7, 14.8) | <0.001 |
| Hemoglobin A1C (%) | 5.5±0.4 | 5.7±1.3 | 0.555 |
| Anti-hypertensive meds | 5 (13%) | 23 (58%) | <.001 |
| Lipid-lowering meds | 5 (13%) | 10 (25%) | 0.152 |
| Hypertension | 8 (20%) | 24 (60%) | <.001 |
| Hyperlipidemia | 29 (73%) | 32 (80%) | 0.431 |
| Diabetes mellitus | 2 (5%) | 4 (10%) | 0.414 |
| Framingham Risk Score | 14.2±3.2 | 14.7±2.9 | 0.465 |
Continuous variables with normal distribution are reported as mean ± SD and are tested for a difference between groups with a t-test; non-normally distributed variables are presented as median (IQR) and compared with a Wilcoxon rank sum test. Categorical variables are summarized with counts and percentages, and are tested for a group difference using the chi-square test. Group difference assessed with Fisher’s exact test due to low frequencies; ordinal categories tested between groups based on Cochran-Armitage test for trend
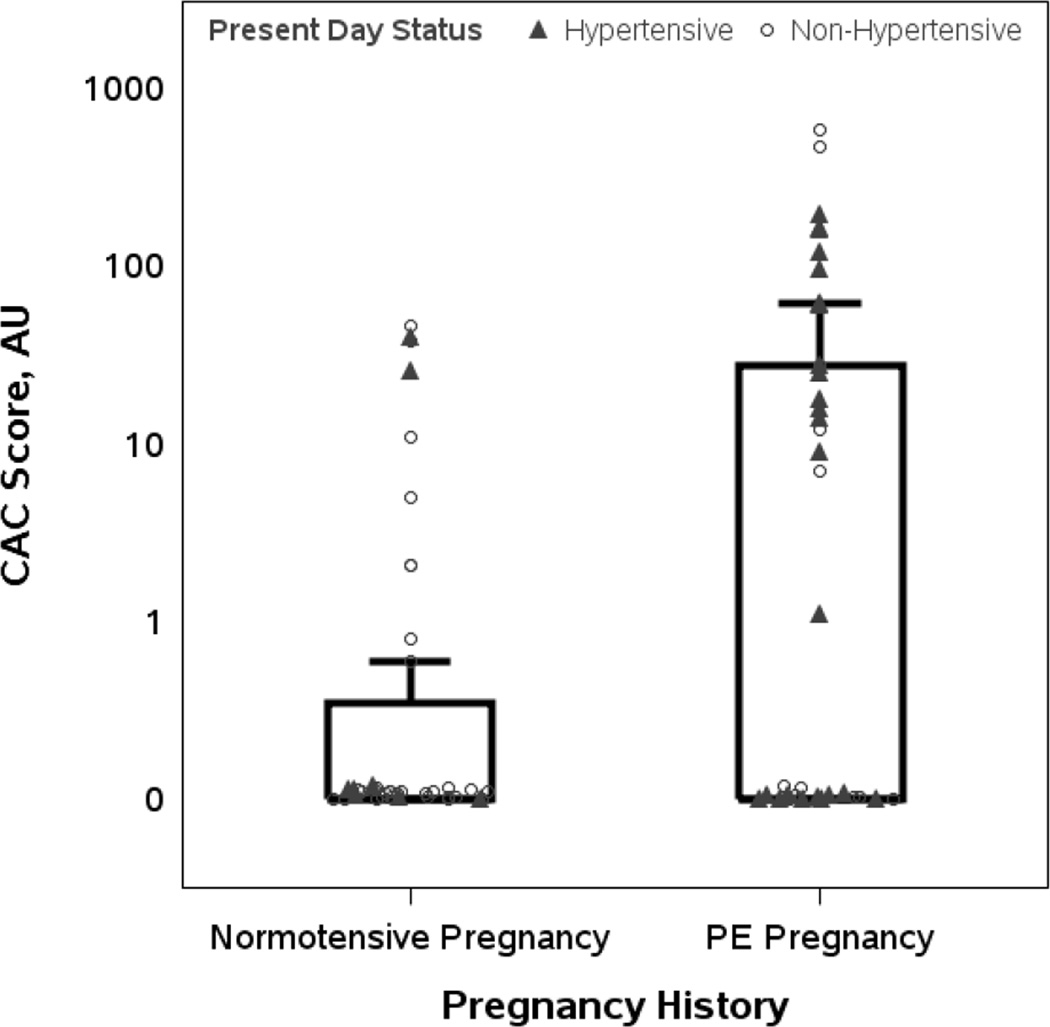
All recruited women except one (with a history of preeclampsia) underwent CAC measurements. A majority of women (n = 50) among the 79 women in whom CAC was measured, had CAC scores of zero: 30 of 40 women (75%) with normotensive pregnancies and 20 of 39 women (51%) with histories of preeclampsia. The mean (± SD) and median (IQR) CAC score was 4.3 ±11.7 AU and 0.0 AU (0.0, 0.3) in women with histories of normotensive pregnancies, and 54.1±126.3 AU and 0.0 AU (0.0, 28.0) in women with histories of preeclampsia (p = 0.007). The number of women with a CAC score > 0 was 10 (25%) in the normotensive pregnancy group versus 19 (49%) in the preeclampsia pregnancy group (p = 0.029). The frequency of a CAC score > 50 AU was greater in the preeclampsia group (23% v. 0%, p=0.001) compared with the normotensive pregnancy group (Figure 1). Compared to those with histories of normotensive pregnancy, the odds of a higher CAC score was 3.54 (1.39 – 9.02) times greater in women with prior preeclampsia without adjustment for present day hypertension, and 2.61 (0.95 – 7.14) times greater after adjustment. Adjustment for present day BMI alone reduced the OR, but the increased odds of CAC based on a history of preeclampsia remained significant. Modeling adjusting for both covariates showed an expected continued reduction in the OR; nevertheless, the trend of increased risk of higher CAC remained (Table 2).
Figure 1. Distribution of CAC scores in subjects with and without a history of preeclampsia stratified by present day hypertension.
Individual CAC scores (normotensive pregnancy, n=40; preeclamptic pregnancy, n=39) are plotted on a logarithmic scale. Triangles represent women with current hypertension and circles indicate those without current hypertension. The interquartile range (i.e., 25th to 75th percentiles) is indicated by the box, with the upper whisker the largest non-outlier. The mean (± SD) and median (25th–75th percentiles) CAC score were 4.3 (±11.7) AU and 0.0 (0.0, 0.3) AU, respectively, in women with a history of normotensive pregnancy and 54.1 (±126.3) and 0.0 (0.0, 28.0) AU, respectively, in women with a history of preeclampsia (P = 0.007). The number of women with a CAC score > 0 was 10 (25%) in the normotensive pregnancy group, compared to 19 (49%) in the preeclampsia group (P = 0.029). The frequency of CAC score > 50 AU was greater in the preeclampsia group (23% v. 0%, P=0.001).
Table 2.
Unadjusted and adjusted odds ratios for having a higher CAC score due to a history of preeclampsia
| Adjusting Model: | Effect of Preeclampsia OR 95% CI x [P-value] |
|---|---|
| None (unadjusted) | 3.54 (1.39, 9.02) [0.008] |
| BMI (kg/m2) | 3.20 (1.21, 8.49) [0.019] |
| Hypertension, chart-abstracted | 2.61 (0.95, 7.14) [0.062] |
| BMI + Hypertension | 2.48 (0.86, 7.19) [0.093] |
Odds of a higher CAC score for women with a history of preeclampsia (n=39) vs. normotensive pregnancy (n=40) using ordinal logistic regression
Comment
This is the first prospective cohort study that utilized review of the medical record to confirm the diagnosis of preeclampsia by accepted clinical criteria which demonstrated that a history of preeclampsia is associated an increased risk of CAC more than 3 decades after pregnancy in asymptomatic women without prior CVD events. The association between preeclampsia and CAC, in part, could be due to conventional CVD risk factors common to both processes. Indeed, women with preeclampsia had higher BMI and more clinical diagnoses of chronic hypertension at the time of imaging. However, the association between a history of preeclampsia and CAC was only partially attenuated by adjustment for these covariates. Available data indicate that obesity and blood pressure trajectories in the 3rd – 5th decades correlate positively with higher CAC scores later in life. 19, 20 As obesity and hypertension are more prevalent and with sooner onset in women with histories of hypertensive pregnancy disorders, 3 it is possible that the longer duration of exposure to these variables contributes to the increased CAC risk.
Adjustment for BMI alone diminished the impact that a history of preeclampsia had on the presence of CAC, but the odds remained statistically significant; current hypertension modified the OR of positive CAC just enough that the lower confidence interval barely crossed one. An increase in sample size or ascertainment at a later age would likely have allowed the odds ratios to remain significant. The trend toward increased risk for CAC persisted, even with adjustment for both variables, suggesting that mechanisms that accelerate deposition of calcium other than traditional risk factors at the time of CAC assessment were involved in a subset of women with preeclampsia.
CAC is an important subclinical marker for future cardiovascular events in asymptomatic individuals and correlates significantly with age. A positive CAC score is rare among women < 55 years of age, 21 as in the women in the current study, but, when present, its extent positively correlates with rates of myocardial infarction, cardiac arrest and long term mortality in asymptomatic patients. 22, 23
Our results extend the findings of previous studies by validating the association between a history of preeclampsia and CAC, while confirming the diagnosis of preeclampsia based on accepted clinical criteria. High blood pressure in pregnancy was associated with a 57% increase in risk of having a positive CAC in a Dutch cohort.24 However, ascertainment of having had a hypertensive disorder in pregnancy was based on a single question (self-reported), rather than a validated survey, determined decades after pregnancy (mean age 66.8 years), with no confirmation by review of medical records. A study from the United States reported the age-adjusted odds ratio for a positive CAC was OR 2.72 (1.37–5.41) in 498 women in their 60s, of whom 52 women screened positive for a history of preeclampsia based on a validated survey. 25
The major strength of the present study is the accurate assessment of exposure to preeclampsia in a prospective cohort. Confirmation of exposure based on accepted clinical criteria rather than relying on subject recall is important because even the best survey tools only have 80% sensitivity, resulting in significant misclassification bias, especially with three decades or more having elapsed since pregnancy. 26 The positive predictive value of patient recall of disease only averages 50%, given the prevalence of preeclampsia in the population. 27 Another strength of this study is that it controls for the possible vascular effects of pregnancy per se, as subjects were matched for parity.
This study reflects Olmsted County, Minnesota, which is primarily white and non-Hispanic. Therefore, these results may not generalize to women of other ethnicities and races, especially as the risks of preeclampsia, hypertension, and CAC vary with ethnicity. 28 Future studies in more diverse populations are needed.
Women with histories of preeclamptic pregnancies demonstrated higher BMI prior to their index pregnancies compared to those with normotensive pregnancies, which may be indicative of an early pro-atherosclerotic predisposition. However, the number of women with clinical obesity (BMI>30) was not different between the groups (3 in normotensive and 4 in preeclamptic group). No significant difference was seen in the rates of either gestational diabetes or current diabetes mellitus between the groups. However, women with histories of preeclamptic pregnancies compared to those without such histories demonstrated a less favorable metabolic state, as shown by increased serum insulin levels and trends in fasting blood glucose and hemoglobin A1C levels. We did not observe elevated rates of diabetes mellitus in the preeclampsia group. A possible explanation is our recruitment criteria which excluded women with prior cardiovascular events, and thus likely those with higher rates of diabetes mellitus. The role of CAC scoring in long term CVD risk assessment is evolving. Currently, modalities such as the CAC and high sensitivity C-reactive protein (hsCRP) are suggested for evaluating “intermediate risk” individuals when there is uncertainty about the role for lipid-lowering agents. 6, 29 A CAC score ≥ 300 or ≥ the age, sex and ethnicity adjusted 75th percentile, justifies upward risk revision and initiation of statin therapy. 30
Therefore, CAC testing may provide additional information in low risk women. A positive CAC score was found in 32% of women judged to be “low risk” by Framingham Risk Score, and a score ≥300 conferred an adjusted hazard ratio of 8.3 (2.3–30.0) for coronary heart disease. 31
Measurement of CAC may be particularly useful in women with histories of preeclampsia as both CAC and a history of preeclampsia convey an increased risk for CVD, and traditional risk factor scoring systems such as the Framingham criteria perform less well in females. 6 A positive CAC can prompt primary prevention interventions, such as the use of diet, exercise, weight management, and lipid-lowering medications to reduce risk. The association between a history of preeclampsia and CAC seems to be mediated, at least in part, through a diagnosis of hypertension. Therefore, our data support current recommendations that women with histories of preeclampsia undergo close follow up for timely hypertension diagnosis and treatment, which may decrease the burden of cardiovascular disease in women later in life.
As a trend toward an increased risk of CAC due to preeclampsia persists after adjustments for individual covariates of hypertension and obesity, further study of CAC positive women with histories of preeclampsia may better delineate the mechanisms through which such a history increases risk, independent of traditional risk factors. The role of CAC screening in women with histories of preeclampsia should be explored in future studies.
Supplementary Material
Acknowledgments
This research was funded, in part, by a grant from the NIH P50 AG044170, UL1 RR024150, a Mayo Clinic Clinical and Translational Science Award, the Department of Surgery and the Mayo Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no conflicts of interest.
This paper was presented as an oral presentation at the 36th Annual Meeting – The Pregnancy Meeting; Atlanta, Georgia; February 2016.
References
- 1.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and metaanalysis. Bmj. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 3.Garovic VD, Bailey KR, Boerwinkle E, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens. 2010;28:826–833. doi: 10.1097/HJH.0b013e328335c29a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by Women's Health Care Providers of Long-Term Cardiovascular Disease Risk After Preeclampsia. Obstet Gynecol. 2015;125:1287–1292. doi: 10.1097/AOG.0000000000000856. [DOI] [PubMed] [Google Scholar]
- 5.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–1588. doi: 10.1161/01.str.0000442009.06663.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnussen EB, Vatten LJ, Smith GD, Romundstad PR. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol. 2009;114:961–970. doi: 10.1097/AOG.0b013e3181bb0dfc. [DOI] [PubMed] [Google Scholar]
- 8.Brown CM, Turner ST, Bailey KR, et al. Hypertension in pregnancy is associated with elevated C-reactive protein levels later in life. J Hypertens. 2013;31:2213–2219. doi: 10.1097/HJH.0b013e3283642f6c. discussion 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White WM, Turner ST, Bailey KR, et al. Hypertension in pregnancy is associated with elevated homocysteine levels later in life. Am J Obstet Gynecol. 2013;209:454 e1–454 e7. doi: 10.1016/j.ajog.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Genereux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol. 2014;63:1703–1714. doi: 10.1016/j.jacc.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, C-reactive protein, and atherosclerotic cardiovascular disease events: the St. Francis Heart Study. J Am Coll Cardiol. 2005;46:158–165. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 12.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 13.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ, 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ., 3rd History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic proceedings. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayachandran M, Litwiller RD, Lahr BD, et al. Alterations in platelet function and cell-derived microvesicles in recently menopausal women: relationship to metabolic syndrome and atherogenic risk. J Cardiovasc Transl Res. 2011;4:811–822. doi: 10.1007/s12265-011-9296-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harman SM, Black DM, Naftolin F, et al. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: a randomized trial. Ann Intern Med. 2014;161:249–260. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Mallikethi-Reddy S, Rubenfire M, Jackson LA, Brook RD. Coronary artery calcium in hypertension: a review. J Am Soc Hypertens. 2015;9:993–1000. doi: 10.1016/j.jash.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. Jama. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. Jama. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budoff MJ, Mohlenkamp S, McClelland R, et al. A comparison of outcomes with coronary artery calcium scanning in unselected populations: the Multi-Ethnic Study of Atherosclerosis (MESA) and Heinz Nixdorf RECALL study (HNR) J Cardiovasc Comput Tomogr. 2013;7:182–191. doi: 10.1016/j.jcct.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-Term Prognosis After Coronary Artery Calcification Testing in Asymptomatic Patients: A Cohort Study. Ann Intern Med. 2015;163:14–21. doi: 10.7326/M14-0612. [DOI] [PubMed] [Google Scholar]
- 24.Sabour S, Franx A, Rutten A, et al. High blood pressure in pregnancy and coronary calcification. Hypertension. 2007;49:813–817. doi: 10.1161/01.HYP.0000258595.09320.eb. [DOI] [PubMed] [Google Scholar]
- 25.Cassidy-Bushrow AE, Bielak LF, Rule AD, et al. Hypertension during pregnancy is associated with coronary artery calcium independent of renal function. J Womens Health (Larchmt) 2009;18:1709–1716. doi: 10.1089/jwh.2008.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diehl CL, Brost BC, Hogan MC, et al. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008;198:e11–e13. doi: 10.1016/j.ajog.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt) 2013;22:37–47. doi: 10.1089/jwh.2012.3740. [DOI] [PubMed] [Google Scholar]
- 28.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 29.Yeboah J, Polonsky TS, Young R, et al. Utility of Nontraditional Risk Markers in Individuals Ineligible for Statin Therapy According to the 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines. Circulation. 2015;132:916–922. doi: 10.1161/CIRCULATIONAHA.115.016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raggi P, Callister TQ, Cooil B, et al. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 31.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as "low risk" based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.