Abstract
Introduction
Although perioperative chemotherapy improves survival in patients with resectable lung cancers, systemic recurrence remains common. Neoadjuvant chemotherapy permits response assessment and opportunity to switch treatment regimens. Response measured by fluorodeoxyglucose PET correlates better than CT with clinical outcomes. The NEOSCAN trial assessed PET-measured response rate to alternative chemotherapy in patients with a suboptimal PET response after 2 cycles of neoadjuvant chemotherapy.
Methods
This phase 2 study enrolled patients with resectable stage IB-IIIA lung cancers (primary tumor ≥2 cm and SUVpeak≥4.5). Patients had a pretreatment FDG PET/CT before 2 cycles of cisplatin (or carboplatin) + gemcitabine (squamous) or pemetrexed (adenocarcinomas) then repeat PET/CT. If SUVpeak in the primary tumor decreased by ≥35%, patients continued the initial chemotherapy. Individuals with <35% PET response were switched to vinorelbine + docetaxel. Post operative radiotherapy was recommended to all patients with positive N2 nodes. A Simon-optimal two stage design was used to evaluate the primary endpoint of a PERCIST-defined response rate to vinorelbine + docetaxel in previously non-responding patients.
Results
40 patients were enrolled. 15 patients (38%, 95% CI: 38–53%) had <35% decrease in SUVpeak and 13 received vinorelbine + docetaxel. The study met its primary endpoint with 10/15 (67%) PET metabolic responses to alternate therapy. Chemotherapy toxicities never precluded surgical exploration.
Conclusions
Utilizing FDG PET/CT to assess response and change preoperative chemotherapy in non-responding patients can improve radiographic measures of response. This adaptive approach can also be used to test new drugs, attempting to optimize perioperative chemotherapy to achieve better long term outcomes.
Keywords: Neoadjuvant therapy, non-small cell lung cancer, Adaptive clinical trial, 18FDG-PET
Introduction
Patients with resectable non-small-cell lung cancers (NSCLCs) are best treated with multimodality therapy, with the intent of cure. Despite this, 5-year overall survival rates even for the earliest stage NSCLCs remain poor: 72% with primaries ≤ 3 cm (T1a) and 55% with tumors > 3 cm (T1b).1 The majority of patients that die after NSCLC resection do so from distant relapse, hypothesized to be caused by micrometastatic disease present at surgery. Perioperative cisplatin-based chemotherapy (neoadjuvant or adjuvant) decreases the risk of death over surgery alone and is a standard-of-care.2
There are many advantages of neoadjuvant chemotherapy. First, a neoadjuvant approach prioritizes early treatment of micrometastatic disease. This accounts for a 5% absolute increase in survival over surgery alone.3 Second, a neoadjuvant approach provides a discovery platform for in vivo assessment of tumor response. Third, tissue available pre- and post treatment allows for translational studies and the utilization of surrogate endpoints. This is standard in the pathologic complete response assessment in early-stage breast cancers.4–7 Finally, neoadjuvant chemotherapy is better tolerated than adjuvant therapy.
We sought to apply these principles to the challenging subset of resectable NSCLCs that are radiographically resistant to standard induction therapy. We hypothesized that switching patients to an alternative, non-cross-resistant regimen could lead to measurable radiographic response if imaging after empirically selected platinum-based chemotherapy suggested insufficient in vivo chemosensitivity of the tumor (non-response).
Determining non-response to neoadjuvant chemotherapy in NSCLCs is challenging. Potential surrogate endpoints include radiologic response by computed tomography (CT) (e.g. RECIST8), scintigraphic response by positron emission tomography (PET) (e.g. PERCIST9), and pathologic response in the resection specimen. While pathologic response at surgery is the most robust surrogate of clinical outcomes,10, 11 it is not practical as an interim assessment to facilitate change in preoperative therapy. CT is the standard radiographic response assessment modality in advanced disease, but CT response to neoadjuvant chemotherapy in resectable NSCLC has not consistently correlated with clinical outcomes.12, 13
Functional imaging with PET/CT using the radiolabeled glucose analog fluorodeoxyglucose (FDG) is routinely obtained during evaluation of newly diagnosed NSCLCs. Unlike CT-based tumor response, the literature supports a strong predictive correlation between reduction in tumor FDG-avidity assayed by PET imaging after completion of neoadjuvant chemotherapy with clinical outcomes.12, 14 These series suggest an association between a reduction ≥50% in SUV at the completion of induction therapy with improved chance of pathologic response and/or more favorable patient outcomes. Many other studies in NSCLCs, have demonstrated that lack of a 35% improvement in SUV portends lack of pathologic response and/or poor prognosis.12, 14–17
We selected a <35% reduction in SUVpeak of the primary tumor between pretreatment and interim PET/CT to define non-response and select the intervention population assigned to an alternative chemotherapy. Based upon the literature, we felt that use of an FDG PET tumor SUV decrease of ≤35% (or increased tumor SUV) to define tumor non-response seems unlikely to misclassify patients who had meaningfully benefitted from the treatment received. The 35% tumor SUV change threshold is also used to dictate clinical care in early-stage esophageal cancer.18
We performed this phase 2 trial to test the hypothesis that patients who do not demonstrate a response to histology-guided neoadjuvant chemotherapy on PET imaging19 may benefit by switching to an alternative regimen. We chose vinorelbine and docetaxel as the alternative regimen as it does not mechanistically overlap with the original regimen, it has an exceptionally high response rates in clinical trials in metastatic NSCLC,20, 21 and it been tested in the adjuvant setting.22
Methods
Patients
All patients had pathologically confirmed stage IB–IIIA lung cancers, a Karnofsky performance status of ≥70%, and were deemed surgically resectable and medically operable by a thoracic surgeon at Memorial Sloan Kettering (MSK). Pre-treatment evaluation included chest CT, FDG-PET/CT, and magnetic resonance imaging of the brain. Mediastinal staging was performed at the discretion of the surgeon. Primary tumors were >2cm in size with demonstrated SUVmax >4.5 on PET. We excluded patients requiring insulin to minimize variables affecting tumor SUV.
Study Design
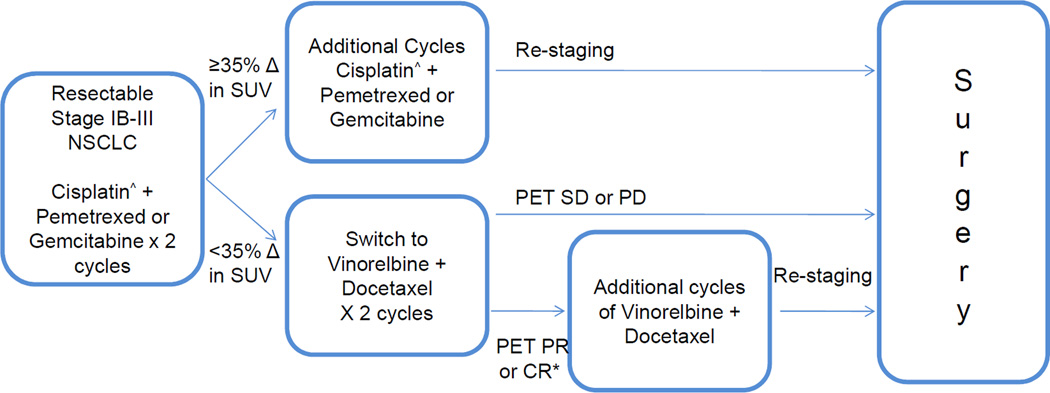
This was a single-institution phase 2 study. The schema is presented in Figure 1. Enrolled patients received 2 cycles of platinum-doublet chemotherapy and then underwent FDG-PET/CT and CT scans. Non-response to therapy was defined as <35% decrease (including an increase) in SUVpeak of the primary tumor on FDG-PET by modified PERCIST criteria. Those with non-response were treated with vinorelbine + docetaxel.
Figure 1.
Schema. *Primary endpoint – PERCIST response. ^Carboplatin was substituted for cisplatin in patients who were cisplatin ineligible
The primary endpoint for this study was PERCIST-defined PET response9 after 2 cycles of vinorelbine + docetaxel using the PET/CT performed after platinum-doublet therapy as a new baseline. Additional endpoints included: CT response, major pathologic response, pathologic downstaging, complete (R0) resection rate, disease-free survival (DFS), and overall survival (OS). Toxicity was recorded per CTCAE v4.0. This protocol was approved by the MSK IRB and all patients signed informed consent.
Preoperative Treatment
Patients with adenocarcinoma or non-small cell lung cancers, not otherwise specified (small biopsies in which squamous or adenocarcinoma features could not be certainly identified) received pemetrexed 500mg/m2 and cisplatin 75mg/m2 on day one of a 21 day cycle, for 2 cycles of therapy.19 Patients with squamous cell carcinomas received gemcitabine 1250mg/m2 (1000mg/m2 with carboplatin) on days one and eight of a 21 day cycle, and cisplatin 75mg/m2 on day eight of a 21 day cycle, for 2 cycles of therapy. Patients ineligible for cisplatin (grade ≥ 2 hearing impairment, GFR <60ml/min, grade ≥ 2 New York Heart Association congestive heart failure or patient refusal of cisplatin) received carboplatin AUC=5.
Patients with ≥35% decrease in primary tumor SUVpeak on PET after 2 cycles of therapy continued the initial regimen. Patients with <35% decrease (or increase) in SUVpeak were treated with vinorelbine 45mg/m2 + docetaxel 45mg/m2 on days one and 15 with pegylated filgrastim on days two and 16, of a 28 day cycle. The primary endpoint was assessed after 2 cycles of vinorelbine + docetaxel. After completing chemotherapy, patients underwent repeat body and brain imaging prior to surgery.
Surgery, Radiologic and Pathologic Analyses
The Discovery DSTE™ PET-CT imaging system (General Electric, USA) was used at MSK. Patients fasted ≥6 hours and blood glucose level was confirmed to be <200 mg/dl prior to radiotracer injection (400–488 MBq 18F-FDG). A CT for localization was acquired from skull vertex to proximal thighs. The CT data was acquired with standard helical imaging. PET imaging commenced after the CT, 55–65 minutes after the FDG tracer-injection. 3-D mode PET data acquisition was obtained for 3–5 minutes per bed position; 6–7 bed positions based on patient height.
Assessed by adapted PERCIST criteria9, tumor non-response was defined solely in the primary lung tumor by an increase in tumor SUVpeak or by a percent decrease in tumor SUVpeak of <35% accompanied by an absolute decrease of <0.8 SUV units. CT response was assessed by a thoracic radiologist (MSG) utilizing RECIST v1.1.8
Surgical exploration, resection, and mediastinal lymph node dissection occurred within 6 weeks of chemotherapy. Methods for pathologic response assessment have been previously described.23 The treatment effect was semi-quantitatively estimated in 10% increments by a dedicated thoracic pathologist (WDT).
Postoperative Therapy and Follow-up
Patients with persistent N2 disease at resection were referred for post-operative radiation. Patients were followed for disease recurrence with clinical history, physical examination, and CT scans of the chest performed every six months for two years from the date of surgery, and annually thereafter.2
Statistical Analysis
The primary endpoint was PET response by PERCIST9 (complete metabolic response + partial metabolic response) after 2 cycles of vinorelbine + docetaxel, using the PET scan obtained immediately before vinorelbine + docetaxel as a new baseline. A 10% response rate was considered negative, whereas a 30% response rate was positive. This was derived from the response rates to 2nd line chemotherapy in metastatic disease are generally <10% and doublet therapy should provideThe probability of type I and II error was 0.05 and 0.2, respectively. The Simon optimal two-stage design required >2/15 PET responses to vinorelbine + docetaxel in stage I to treat a maximum of 25 patients in stage II. Early stopping rules were defined as grade ≥4 adverse events occurring in ≥4/15 patients treated in stage 1 or ≥4/25 planned to receive vinorelbine + docetaxel. Exact 95% confidence intervals for response rates were included.
Major pathologic response was defined as ≥90% treatment effect in the resected specimen.23 Exploratory endpoints utilized PET and CT response as either categorical or continuous variables as percent change in SUV of primary tumor and percent change in sum of diameters of target lesions, respectively. The Wilcoxon rank sum test was used to compare PET and CT response after 2 and 4 cycles between those who did and did not experience major pathologic response. All statistical tests were 2-sided, and p-values < 0.05 were considered significant. All analyses were done using R version 3.0.1 including the Hmisc package.
Study support was provided by Eli Lilly & Co who had no part in the study design, conduct, results, interpretation nor reporting.
Results
Patient Characteristics
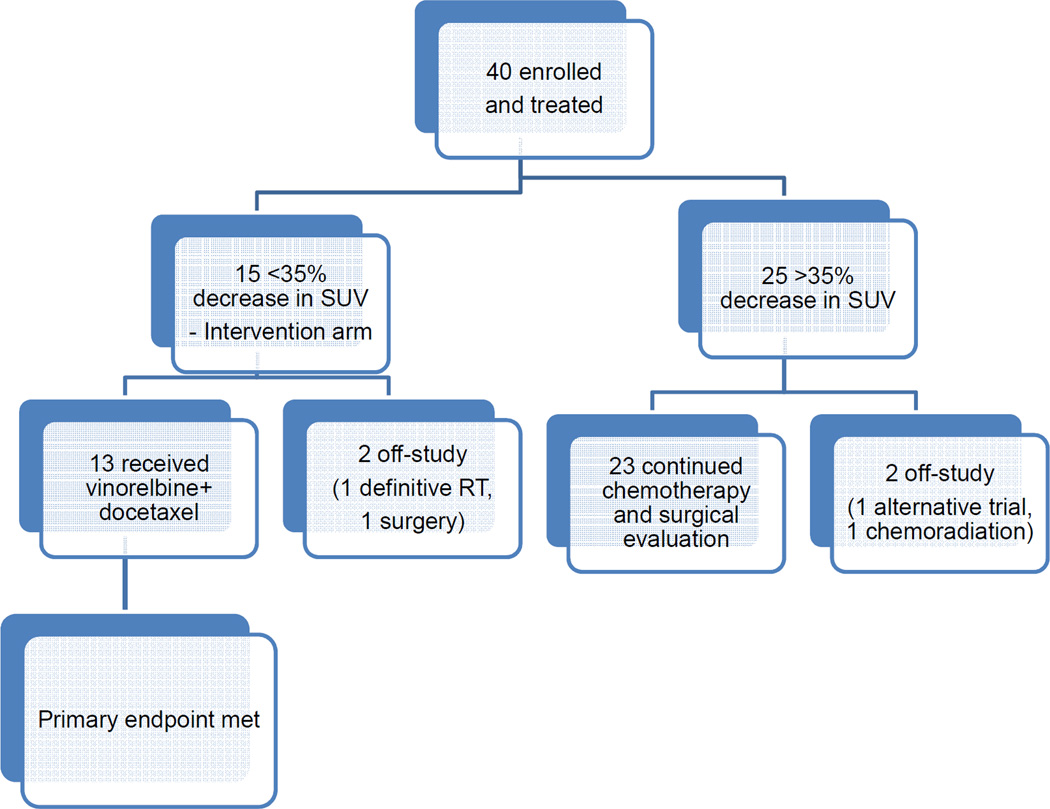
40 patients were enrolled between November 2011 and August 2014. Baseline patient characteristics are summarized in Table 1. There was no selection by sex but the study randomly accrued more than twice as many women as men, in keeping with the larger incidence of female patients with lung cancer referred to our institution. A consort diagram of the patients that were enrolled, treated and analyzed is depicted in Figure 2.
Table 1.
Patients
| Characteristic | N (% or range) |
|---|---|
| Male sex | 14 (35%) |
| Female sex | 36 (65%) |
| Age at diagnosis – Median (range) yrs | 63 (42𠄲77) |
| Cigarette Use | |
| Never | 2 (5%) |
| Former | 22 (55%) |
| Current | 16 (40%) |
| Preoperative Histology | |
| Squamous Cell Carcinomas | 7 (18%) |
| Adenocarcinomas | 29 (72%) |
| NSCLCs, not otherwise specified | 4 (10%) |
| Clinical Stage | |
| IB (T2N0) | 2 (5%) |
| IIB | 6 (15%) |
| T3N0 | 4 |
| T2N1 | 2 |
| IIIA | 32 (80%) |
| T1N2 | 2 |
| T2N2 | 16 |
| T3N2 | 14 |
Figure 2.
Consort flow diagram
Preoperative Chemotherapy
31 patients received pemetrexed, 23 with cisplatin and eight with carboplatin. Nine patients received gemcitabine, three with cisplatin and six with carboplatin. 15 patients had <35% decrease in SUV of primary tumor: 3 treated with carboplatin + gemcitabine and 12 treated with pemetrexed – 9 cisplatin and 3 carboplatin. 13 of 15 non-responding patients subsequently received vinorelbine + docetaxel.
Primary Endpoint- Response to ‘Switch’ Chemotherapy
25 (n=25/40, 63%, 95% confidence interval (CI) 46–77%) patients who received platinum doublet chemotherapy demonstrated a ≥35% decrease in SUVpeak of the primary tumor after the first 2 cycles of therapy. Of these 25, 23 patients continued chemotherapy and were reassessed for surgery. One pursued a different clinical trial and one chose to receive chemoradiation.
15 patients (n=15/40, 38%, 95% CI 23–54%) demonstrated <35% reduction in SUVpeak, and were assigned to receive vinorelbine + docetaxel. 13 patients received vinorelbine + docetaxel and 10/15 (intention to treat analysis, 67%, 95% CI 42 to 85%) had a partial (9) or complete (1) metabolic response per PERCIST. One patient chose to receive definitive chemoradiation instead of surgery and one proceeded immediately to surgery without additional chemotherapy.
The median reduction in SUVpeak of the primary tumor to vinorelbine + docetaxel therapy was 49% (range +34 to −71%). The PET and CT response data are summarized in Table 2. In individuals who did not switch (n=23), the median reduction in SUVpeak from baseline was 62% (range 36 to 87%) after 2 cycles. Using the second scan as the new baseline in these responding patients, the additional median reduction in SUVpeak with additional cycles of the same chemotherapy was 11% (range 149% increase to 75% reduction).
Table 2.
Radiologic Responses
|
PET Results (SUVpeak in primary tumor; PERCIST in all disease) |
Switch cohort | Continuation |
| >35% decrease after 2 cycles | 0/15 | 25/25 |
| >50% decrease after 2 cycles | 0/15 | 17/25 |
| >30% decrease from mid-treatment in switch group | 8/13 | |
| PERCIST PMR or CMR from mid-treatment in switch group (1oend) | 10/13 | |
| >50% decrease after all induction if <50% after 2 cycles | 7/13 | 1/8 |
| CT Results | Switch cohort | Continuation |
| PR after 2 cycles | 0/15 | 7/25 |
| PR after 4 cycles | 6/13 | 18/23 |
| PR from mid to post-treatment | 4/13 | 3/23 |
Toxicity, Surgery and Postoperative Course
There were no unexpected complications. Related non-hematologic toxicities that were grade 3 or 4 or met criteria for a severe adverse event are presented a supplementary table. There was one on-study death due to disease progression with fatal hemoptysis in a patient with that received 1 dose of vinorelbine + docetaxel but was not thrombocytopenic.
Of the 36 patients who continued per protocol with chemotherapy after initial scans, there were 29 R0 (73%), one R1 (3%) and two R2 (5%) resections. Four patients were unresectable, two identified at the time of surgery. Of the 13 patients who received vinorelbine + docetaxel, 12 patients went to surgery (one died from disease progression) and there were 11 R0 and one R1 resections.
There were no grade 3 to 5 operative complications. Intraoperative complications included one patient with transient asystole (grade 1). The median postoperative length of stay was five days, range two to nine days. Postoperative complications included one pneumothorax (grade 2), one prolonged air leak (grade 1), and one episode of atrial fibrillation.
Pathologic Response
Major pathologic response, defined as ≥90% treatment effect in the resected specimen, was demonstrated in six of the 36 (17%, 95% CI, 6–33%) assessable patients. This included 5/25 with initial PET response assigned to continue chemotherapy and 1/15 with suboptimal PET response assigned to switch chemotherapy. Of 29 patients with clinical N2 disease pre-treatment, six were downstaged to N0 and two were downstaged to N1. For all patients, in nonparametric analyses, response assessed by PET after 2 and 4 cycles of treatment correlated with major pathologic response (p=0.016 and p=0.034, respectively), but CT response did not.
Disease-free survival
The median follow-up for patients who have not recurred or died is 12 months from the date of surgery. Of the 16 patients who did not switch chemotherapy and underwent R0 resection, there have been seven recurrences. Of the 11 patients who received vinorelbine + docetaxel and underwent R0 resection, there have been three recurrences. 11 patients have died.
Discussion
None of the therapies demonstrated to improve survival in advanced lung cancers, including tyrosine kinase inhibitors and T-cell checkpoint inhibitors, have been thoroughly studied in the patients with resectable NSCLC. Most trials done mimic the adjuvant cisplatin-based studies administer interventions postoperatively. These studies allow no insight into the efficacy of the intervention short of waiting for recurrence or survival. While the substantial opportunity of adjuvant therapy to enhance curability justifies these long timelines and resources, better strategies to affect more cures in less time must be pursued.
This phase 2 study sought to determine whether PET-measured response to treatment may offer a "second neoadjuvant option" for those patients with resectable NSCLC who have insufficient response following what is considered the best, histology-selected chemotherapy.19 The definition of non-response was derived from the literature. The studies described above justify our selection of FDG PET tumor SUV decrease of ≤35% to define tumor non-response radiologically and appropriately as the threshold selected seems unlikely to misclassify patients who were truly benefitting.
A study in which changes in tumor FDG-avidity on PET after neoadjuvant chemotherapy did not stratify prognosis is worthy of discussion.24 This study differed from the others cited in its lack of standardization. Images were acquired with different methods, machines and between institutions. Without methodologic standardization, apparent changes in tumor FDG-avidity on serial PET scans may be artifactual, undermining accurate clinical correlations. PET response guidelines advise control of key methodologic variables when attempting to assay changes in FDG-avidity, as was observed in the otherwise concordant studies cited and our own trial.9
With our conservative definition of response/non-response, 38% of patients treated with platinum-doublet chemotherapy were assigned to the intervention arm of vinorelbine + docetaxel. These non-responders by PET had equally as underwhelming CT responses with no partial responses by RECIST (Table 2). The switch population safely and effectively received the potentially non-cross-resistant regimen of vinorelbine + docetaxel. Using the PET and CT scans obtained after 2 cycles of platinum-doublet therapy as a new baseline, 77% of the previously nonresponding patients experienced a partial or complete metabolic response by PET and 31% experienced partial response by RECIST. Toxicities were largely as expected. Operative outcomes were excellent with an 85% R0 resection rate in those who received vinorelbine + docetaxel and a 73% R0 resection rate in an intention-totreat analysis, including all enrolled patients and 80% R0 rate in those surgically explored. This R0 resection rate is comparable to randomized studies of preoperative chemotherapy that allow inclusion of patients with N2 disease.25, 26
While complete pathologic response correlates most robustly with survival, achievement of this endpoint in lung cancer is exceedingly rare. A modified response criterion in lung cancers, termed ‘major pathologic response’ and defined as <10% viable tumor cells in a resection specimen, occurs at a more clinically relevant frequency.27, 28 This endpoint has been demonstrated to fulfill Prentice’s criteria as a surrogate endpoint for overall survival with neoadjuvant chemotherapy in NSCLCs.27 This study had one complete pathologic response and six major pathologic responses.
This study met its primary endpoint of PET response to vinorelbine + docetaxel. It was not powered to determine if the study approach can increase the rate of pathologic response or prolong survival. Consistent with the literature, we demonstrated that PET response correlates better with pathologic response than CT response. Furthermore, PET response to a regimen was ascertained in most patients after 2 cycles with little additional scintigraphic response after further cycles of the same regimen. For future study, clinical outcomes may be superior if a threshold of benefit higher than the selected 35% change in SUV is utilized. A proposed 50% threshold based on data presented in Table 2 is worthy of further study.
Although there is limited experience with the neoadjuvant use of the pemetrexed-cisplatin regimen, the regimen is in widespread use and has been accepted as a consensus standard.2 We did not pursue neoadjuvant radiation at diagnosis or after poor initial response to chemotherapy. Radiation is inherently limited in its ability to control the micrometastatic disease that leads to the deadly systemic recurrences. Induction radiation has not been shown to improve outcomes beyond chemotherapy in the neoadjuvant setting but does add toxicity.26, 29 In contrast, there is substantial evidence that post operative radiation enhances survival post resection in patients with N2 disease.30, 31 We believe this data justifies a consultation with a radiation oncologist in all cases with N2 disease.
Beyond the details of the regimens utilized in this study and the choice of a specific threshold for switching therapy, it should be noted that this is the first use of this adaptive phase 2 study design in NSCLCs. This approach can effectively assess early response in the primary tumor as a measure of systemic benefit to allow for adaptive interventions. By intervening in non-responders, this design has the greatest chance of improving clinical outcomes while minimizing risk to the entire population (as is the approach in an adjuvant study). Utilizing a neoadjuvant platform is also a more efficient means of assessing new agents as comparable adjuvant approaches can take a decade to yield results. Access to sufficient amounts of resected tumor tissue after treatment will facilitate translational research by allowing for the correlation of clinical data with pathologic review and measurements of drug effects on targets in the tumor, while all obtained as part of standard care without additional resource utilization or risk to the patient.
In summary, we have demonstrated that patients with resectable NSCLCs that are not optimally responding to histology-guided neoadjuvant treatment can respond to an alternative induction regimen. This adaptive neoadjuvant approach can also be used to facilitate the study of new drugs. The usefulness of this approach can be further enhanced by a systematic assessment of major pathologic response in the resection specimens. Making full use of the opportunities presented in the neoadjuvant space may be able optimize the care of patients with resectable NSCLC and more rapidly detecting an efficacy signal for new interventions with the potential to enhance curability.
Supplementary Material
Acknowledgments
Funding: This study was funded, in part, by Eli Lilly and the Modell Fund for the Cure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors:
JEC, MD, WDT, RD and CGA contributed to the design of the study. All authors participated in study conduct, data collection, data analysis, manuscript writing and final approval.
Declarations of Interest: The authors report no related conflicts of interest.
References
- 1.Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147:747–752. doi: 10.1016/j.jtcvs.2013.10.001. Discussion 752-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NCCN Clinical Practice Guidelines in Oncology - Non-Small Cell Lung Cancer. 2015 Jan 30; Available at http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. [Google Scholar]
- 3.Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet. 2014;383:1561–1571. doi: 10.1016/S0140-6736(13)62159-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houssami N, Macaskill P, von Minckwitz G, et al. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 6.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30:3242–3249. doi: 10.1200/JCO.2011.39.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 8.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. Br J Cancer. 2006;94:1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012 doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pottgen C, Levegrun S, Theegarten D, et al. Value of 18F-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography in non-small-cell lung cancer for prediction of pathologic response and times to relapse after neoadjuvant chemoradiotherapy. Clin Cancer Res. 2006;12:97–106. doi: 10.1158/1078-0432.CCR-05-0510. [DOI] [PubMed] [Google Scholar]
- 13.William WN, Jr, Pataer A, Kalhor N, et al. Computed tomography RECIST assessment of histopathologic response and prediction of survival in patients with resectable non-small-cell lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2013;8:222–228. doi: 10.1097/JTO.0b013e3182774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dooms C, Verbeken E, Stroobants S, et al. Prognostic stratification of stage IIIA-N2 non-small-cell lung cancer after induction chemotherapy: a model based on the combination of morphometric-pathologic response in mediastinal nodes and primary tumor response on serial 18-fluoro-2-deoxy-glucose positron emission tomography. J Clin Oncol. 2008;26:1128–1134. doi: 10.1200/JCO.2007.13.9550. [DOI] [PubMed] [Google Scholar]
- 15.Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Potential use of FDGPET scan after induction chemotherapy in surgically staged IIIa-N2 non-small-cell lung cancer: a prospective pilot study. The Leuven Lung Cancer Group. Ann Oncol. 1998;9:1193–1198. doi: 10.1023/a:1008437915860. [DOI] [PubMed] [Google Scholar]
- 16.Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:8362–8370. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 17.Soussan M, Cyrta J, Pouliquen C, et al. Fluorine 18 fluorodeoxyglucose PET/CT volume-based indices in locally advanced non-small cell lung cancer: prediction of residual viable tumor after induction chemotherapy. Radiology. 2014;272:875–884. doi: 10.1148/radiol.14132191. [DOI] [PubMed] [Google Scholar]
- 18.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 19.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 20.Miller VA, Krug LM, Ng KK, et al. Phase II trial of docetaxel and vinorelbine in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:1346–1350. doi: 10.1200/JCO.2000.18.6.1346. [DOI] [PubMed] [Google Scholar]
- 21.Page RD, Smith FP, Geils GF, et al. Dose-dense vinorelbine and docetaxel with Filgrastim support in patients with advanced nonsmall cell lung carcinoma. Cancer. 2005;104:1956–1961. doi: 10.1002/cncr.21400. [DOI] [PubMed] [Google Scholar]
- 22.Chaft JE, Rekhtman N, Sima CS, et al. Phase II study of docetaxel and vinorelbine as adjuvant chemotherapy for resected non-small cell lung cancers. Cancer Chemother Pharmacol. 2013;72:931–934. doi: 10.1007/s00280-013-2263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15:e42–e50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanvetyanon T, Eikman EA, Sommers E, et al. Computed tomography response, but not positron emission tomography scan response, predicts survival after neoadjuvant chemotherapy for resectable non-small-cell lung cancer. J Clin Oncol. 2008;26:4610–4616. doi: 10.1200/JCO.2008.16.9383. [DOI] [PubMed] [Google Scholar]
- 25.Scagliotti GV, Pastorino U, Vansteenkiste JF, et al. Randomized Phase III Study of Surgery Alone or Surgery Plus Preoperative Cisplatin and Gemcitabine in Stages IB to IIIA Non-Small-Cell Lung Cancer. J Clin Oncol. 2012;30:172–178. doi: 10.1200/JCO.2010.33.7089. [DOI] [PubMed] [Google Scholar]
- 26.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
- 27.Hellmann MD, Chaft JE, William WN, Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. The lancet oncology. 2014;15:e42–e50. doi: 10.1016/S1470-2045(13)70334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pataer A, Kalhor N, Correa AM, et al. Histopathologic Response Criteria Predict Survival of Patients with Resected Lung Cancer After Neoadjuvant Chemotherapy. J Thorac Oncol. 2012;7:825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah AA, Berry MF, Tzao C, et al. Induction chemoradiation is not superior to induction chemotherapy alone in stage IIIA lung cancer. Ann Thorac Surg. 2012;93:1807–1812. doi: 10.1016/j.athoracsur.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Mikell JL, Gillespie TW, Hall WA, et al. Post-Operative Radiotherapy (PORT) is Associated with Better Survival in Non-Small Cell Lung Cancer with Involved N2 Lymph Nodes: Results of an Analysis of the National Cancer Data Base. J Thorac Oncol. 2014 doi: 10.1097/JTO.0000000000000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic n2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the national cancer data base. J Clin Oncol. 2015;33:870–876. doi: 10.1200/JCO.2014.58.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.