Abstract
Objective
To evaluate children with Crohn’s disease for inverse relationships between systemic inflammatory cytokines and sex hormone regulation in the context of anti-TNF-α therapy.
Study design
An observational study design was used to assess sex hormone and gonadotropin levels at the time of initiation of anti-TNF-α therapy, and 10 weeks and 12 months later in 72 adolescents (Tanner stage 2–5) with Crohn’s disease. Mixed-model linear regression was used to evaluate relationships between hormone levels, systemic inflammation and DXA whole body fat mass Z-scores over the study interval.
Results
Sex hormone Z-scores increased significantly over the 10 week induction interval: testosterone Z-scores in males increased from a median of −0.36 to 0.40 (p<0.05) and estradiol Z-scores in females increased from −0.35 to −0.02 (p<0.01). In mixed model regression, the pediatric Crohn’s disease activity index score, cytokine levels and measures of inflammation were significantly and negatively associated with sex hormone Z-scores, and with luteinizing hormone and follicle stimulating hormone levels, adjusted for sex and Tanner stage. Sex hormone and gonadotropin levels were not associated with BMI or fat mass Z-scores.
Conclusions
Crohn’s disease is associated with delayed maturation, and initiation of anti-TNF-α therapy was associated with significant and rapid increases in sex hormone and gonadotropin levels, in associated with improvements in disease activity and measures of inflammation. These data are consistent with preclinical studies of the effects of inflammation on sex hormone regulation.
Keywords: inflammation, gonadotropin, puberty, inflammatory bowel disease
Chronic inflammatory diseases such as Crohn's disease are associated with delays in the onset and progression of puberty,1, 2 with potential sequelae including poor linear growth,3, 4 impaired bone accrual,5, 6 and decreased quality of life.7, 8 Animal studies have demonstrated that introduction of inflammatory cytokines and other inflammatory mediators resulted in decreased release of luteinizing hormone (LH) from the pituitary gland.9 Furthermore, induction of colitis resulted in lower testosterone and estradiol levels compared with pair-fed controls,10–12 with levels of gonadotropins that did not demonstrate the normal feedback increase in response to low levels of sex hormones. As such, the suppression of sex hormones in animal models of inflammation appeared to be centrally-mediated and thus hypogonadotropic in nature. In addition, mice with colitis had longer delay of puberty than seen in mice who were food restricted, suggesting that this delay was due to factors besides body fat and levels of the adipokine leptin—both known to play a permissive role in sex hormone production.10–14 In mice with experimental colitis, treatment with a monoclonal antibody against TNF-α resulted in partial normalization of estrogen production as evidenced by earlier vaginal opening, an estrogen-dependent marker of pubertal progression in murine models.15
Studies of children and adolescents with Crohn's disease reported delays in bone age,16, 17 breast development,18 menarche,17–19 testicular enlargement,18 and the pubertal growth spurt16, 20 - delays that persisted despite improvements in disease treatment.2 Prior studies of the impact of Crohn’s disease therapy on sex hormone levels in adolescents with Crohn’s disease are limited to a case report in a male and a series in adolescents enrolled at a highly variable interval after starting anti-TNF-α theray.7, 21 Therefore, the regulation of sex hormones in pubertal children with Crohn’s disease remains understudied.
This study assessed changes in sex hormone levels and biomarkers of inflammation in a cohort of pubertal and post-pubertal adolescents with Crohn's disease following initiation of treatment with infliximab, a monoclonal antibody against TNF-α. Our hypothesis was that prior to anti-TNF-α treatment, males and females have testosterone and estradiol levels below the reference ranges, respectively, and without compensatory increases in gonadotropin levels. We further hypothesized that sex hormone levels would increase following treatment with anti-TNF-α treatment, and reductions in measures of inflammation (more so than increases in measures of body fat) would be associated with greater increases in sex hormones. These data may offer insights into the central regulation of sex hormone production and pubertal progression in the setting of chronic inflammatory disease.
METHODS
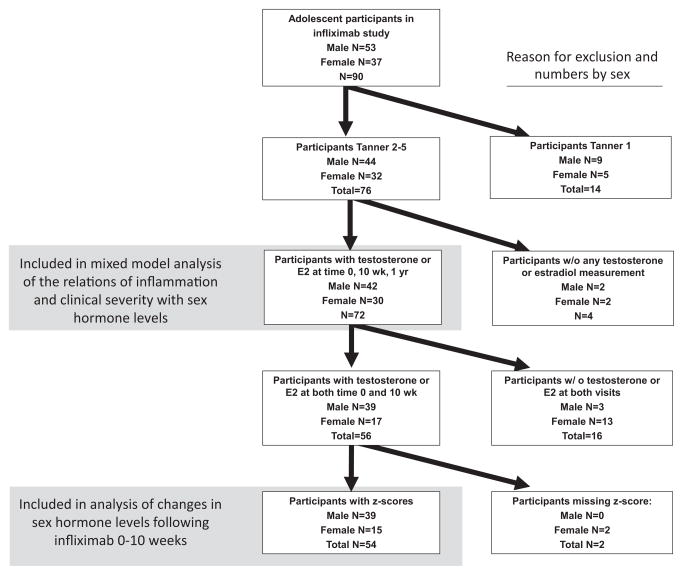
This study is ancillary to a prior prospective cohort study of bone and mineral metabolism in 90 children and adolescents, ages 5 to 21 years, enrolled at the time of initiation of anti-TNF-α therapy at the Children’s Hospital of Philadelphia (CHOP).22, 23 Participants were excluded for prior anti-TNF-α therapy or medical illness or therapies unrelated to Crohn disease that could potentially affect bone, nutrition or growth. Study visits were completed at the time of the first anti-TNF-α infusion (baseline visit), and 10 weeks, 6 months and 12 months later. The study protocol was approved by the CHOP Institutional Review Board. Informed consent was obtained from participants ≥ 18 years of age, and a parent or guardian of participants < 18 years. Assent was obtained from participants 7 to 18 years of age. The informed consent covered the ancillary measures described here. Short–term changes in mineral metabolism have beenreported, demonstrating significant increases in parathyroid hormone (PTH) and 1,25(OH)2 vitamin D levels in association with improvements in disease activity22 and decreases in cytokine and C-reactive protein (CRP) levels. Improvements in disease activity over the first 10 weeks were associated with gains in height, trabecular bone mineral density and cortical structure over the 12 month study interval.23 This study of sex hormones and maturation is limited to participants that were Tanner stages 2 through 5 at enrollment. Of the 76 that were eligible according to Tanner stage, 72 had at least one specimen available for ancillary measures.
Disease characteristics, medications, and anthropometry were recorded at each visit. Height was measured using a stadiometer and weight with a digital scale. Height and body mass index (BMI, kg/m2) were converted to sex-specific Z-scores relative to age using national growth charts.24 Tanner stage was ascertained by self-assessment questionnaire at baseline and 12 month visits.25 For the 10-week visit, participants were classified at being at the same Tanner stage as at baseline. Females were asked if they had started their menstrual period; however, the regularity of menses was not assessed. Disease activity was assessed using the Pediatric Crohn’s Disease Activity Index (PCDAI) based on symptoms (30%), physical examination (30%), laboratory variables (20%), and growth (20%), with scores ranging from 0–100.26 Disease activity was categorized as none (1–10), mild (11–30), and moderate to severe (> 30).
At the baseline and 12 month visit, whole body DXA scans were obtained using a Hologic Delphi densitometer (Bedford, MA) with a fan beam in the array mode. The results for whole body fat mass, excluding the head, were converted to sex- and race-specific Z-scores relative to age, based on our 921 healthy reference children and adolescents, as previously described.6 Referent children also performed self-assessment of Tanner stage as described above.25
Laboratory studies at each visit included hematocrit, erythrocyte sedimentation rate (ESR; mm/h), and serum C-reactive protein (CRP, mg/L) and albumin (g/dL) concentrations, analyzed using standard methods in the clinical laboratory. Serum TNF-α and IL-6 were measured by the Luminex platform and the human cytokine six-plex high-sensitivity antibody bead kit (Millipore) with a sensitivity of 0.08 and 0.10 pg/mL and interassay coefficient of variation (CV) of 8.3% and 7%, respectively.22
Testosterone and estradiol were tested at Quest Diagnostics (Madison, NJ) using an ultrasensitive liquid chromatography-tandem mass spectrometry (LC/MS/MS) assay with sensitivity down to 1 ng/dL for testosterone and 2 pg/mL for estradiol. For testosterone, the intraassay CVs were 7.1 and 10.5 and the interassay CV’s were 7.6 and 10.8 at testosterone levels of 9.6 and 43.7 ng/mL, respectively. For estradiol, the intraassay CV’s were 15.3 and 10.4 and the interassay CV’s were 7.7–15.3% and 9.9–14.0% at estradiol levels of 10 and 200 pg/mL. Testosterone and estradiol values were converted to Z-scores based on the mean and standard deviation of reference values from Quest (Table I; available at www.jpeds.com). For cases where the lower limit of 2 SD’s below the mean dropped below 0, Z-score was calculated as Z-score = [LOG10(measured value)-LOG10(reference mean)]/(reference SD/reference mean).27 For testosterone, the Z-scores were Tanner-stage-specific. For females, the estradiol reference data were available according to age range (years 1–9, 10–11, 12–14, and 15–17 years). Because of the high proportion with delayed maturation (55% of females), Z-scores were generated using bone age, including the baseline bone age for baseline and 10-week estradiol levels and the 1-year bone age for the 1-year estradiol levels. LH and follicle stimulating hormone (FSH) were analyzed at the University of Virginia Center for Research in Reproduction Ligand Core Laboratory using chemiluminescence. Manufacturer, assay sensitivity, and intra- and interassay CVs for LH and FSH measurements had sensitivities of 0.1 and 0.05 IU/L, respectively, with intraassay CVs of 1.9–3.2% and interassay CVs 4.8–6.6%.28 Bone age radiographs were obtained at baseline and 1 year and read on a clinical basis by a pediatric radiologist who was aware of the participants’ age but not Tanner stage.
Table 1. Mean and standard deviation for testosterone and estradiol for z-score calculations.
Testosterone values in boys by Tanner stage and estradiol values for girls by age for the liquid chromatography/mass spectroscopy tests used. (Adapted from Quest Diagnostics, Madison, NJ, http://www.questdiagnostics.com/testcenter/testguide.action?dc=TS_Testosterone_LCMSMS, http://www.questdiagnostics.com/testcenter/BUOrderInfo.action?tc=30289&labCode=SJC).
| Testosterone in males (ng/mL) | ||
|---|---|---|
| Tanner stage | Mean* | St. Dev.* |
| I | 2.7 | 1.04 |
| II | 36.7 | 53 |
| III | 160.5 | 95 |
| IV | 201.5 | 133.7 |
| V | 367 | 154 |
| Estradiol in females (pg/mL) | ||
| Age (y) | Mean* | St. Dev.* |
| 1 – 9 | 4.2 | 3.9 |
| 10 – 11 | 13.5 | 18.4 |
| 12 –14 | 44.1 | 36.4 |
| 15 – 17 | 43.9 | 64.9 |
Calculated using 2 pg/mL for all values below sensitivity
Statistical analyses
Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA). Results are expressed as mean (SD) if normally-distributed, and median (interquartile range, IQR) if non-normally distributed. The non-normally distributed variables were natural-log transformed for all subsequent analyses. Changes over time were assessed using paired t-tests. To assess the relationship of sex hormone and gonadotropin levels with BMI, body fat mass, PCDAI score and measures of inflammation, we used a mixed model approach that takes into account intra-individual correlations over the three study visits. To assess whether baseline use of other medications was associated with change in sex hormones from 0–10 weeks, regression analysis was performed for each of the other medications assessed (glucocorticoids, aminosalicylates, mercaptopurine-azathioprine and methotrexate), adjusted for baseline Tanner stage. In all analyses, a p-value < 0.05 was considered statistically significant.
RESULTS
A total of 72 participants were Tanner stage 2 through 5 at enrollment and had at least one measurement of sex hormones (Figure 1; available at www.jpeds.com). Participants are described in Table II. Of these, 54 (39 males and 15 females) had measures of sex hormones at baseline and 10 weeks and (for females) had bone age information to enable calculation of bone-age-specific estradiol Z-scores. Age, disease severity and baseline sex hormone levels were similar between the Tanner 2 – 5 participants and those included in the longitudinal models (data not shown). Age-for-Tanner-stage for adolescents with Crohn’s disease was delayed compared with 627 healthy referent participants measured in the same laboratory29; using linear regression adjusted for sex, adolescents with Crohn’s disease at Tanner stages 2, 3 and 4 were 2.00, 1.27, and 1.43 years older, respectively (all p < 0.0001).
Figure 1.
online: Flow chart of participants numbers for included analyses, with reason for exclusion.
Table 2.
Participant Characteristics.
| Total N | 72 |
| Age, years median (intraquartile range) [overall range] | 15.1 (7.4–21.9) |
| Age, mean (SD) | 15.1 (2.6) |
| Age, n (%), years | |
| 5–9 | 2 (2.8) |
| 10–14 | 32 (44.4) |
| 15–21 | 38 (52.8) |
| Sex, n (%) | |
| Male | 42 (58.3) |
| Female | 30 (41.7) |
| Duration of Disease, years median (intraquartile range) [overall range] | 1.4 (0.4, 3.9) [0.02–12.8] |
| Tanner Stage, n (%) | |
| 2 | 17 (23.6) |
| 3 | 18 (25.0) |
| 4 | 22 (30.6) |
| 5 | 15 (20.8) |
| Menstruating female, N (percent) | 21/34 (61.8) |
| Age of menarche, mean (SD) years | 12.5 (1.3) |
| Bone age, mean (SD), years | 14.3 (2.4) |
| Bone age, years median (intraquartile range) [overall range] | 14.3 (12.7, 16.0) [5.0, 19.0] |
| Bone age delay, mean (SD), years | −0.28 (1.1) |
| Bone age delay, years median (intraquartile range) [overall range] | −0.25 (−1.0, 0.5) [−2.8, 2.3] |
| Location of Disease, n (%) | |
| Ileal | 4 (5.6) |
| Colonic | 24 (28.5) |
| Ileocolonic | 50 (69.4) |
| Iso upper | 61 (84.7) |
| Perianal | 27 (37.5) |
| Medications, n (%): | |
| On anti-TNF-α agent | 85 (100.0) |
| On glucocorticosteroids | 23 (31.9) |
| On aminosalicylates | 61 (84.7) |
| On mercaptopurine-azathioprine | 21 (29.2) |
| On methotrexate | 14 (19.4) |
The PCDAI scores, laboratory variables, anthropometry measures and fat mass data are summarized in Table III at baseline, 10 weeks and 12 months in the 72 participants who were Tanner 2–5 with at least one sex hormone measure. Disease activity and serum measures of inflammation decreased significantly over the first 10 weeks following initiation of anti-TNF-α treatment, as previously described.22, 23 At the 12 month visit, these markers remained below baseline level, though in paired t-tests, the 12 month levels of TNF-α and CRP were significantly higher than at 10 weeks. Compared with baseline, height, weight, BMI and fat mass Z-scores were significantly greater at 12 months.
Table 3. Change in anthropometry and inflammatory markers over time.
Data shown are for the 72 children Tanner 2–5 with at least one sex hormone measure.
| Baseline | 10 weeks | 52 Weeks | |
|---|---|---|---|
| Height z-score † | −0.45 (1.03) | −0.41 (1.05) | −0.32 (1.01) **, ## |
| Weight z-score † | −0.356 (1.04) | −0.02 (0.98) ** | 0.13 (1.11) **, # |
| BMI z-score † | −0.17 (1.07) | 0.22 (0.97) ** | 0.33 (0.98) ** |
| Subtotal fat mass z-score | 0.30 (0.91) | 0.58 (0.89) * | |
| PCDAI# | 25 (15, 37.5) | 10 (5, 17.5) ** | 10 (2.5, 15) ** |
| PCDAI (%) | |||
| 0–10 (no disease) | 21.7 | 56.9 | 70.2 |
| 11–30 (mild disease) | 34.8 | 35.4 | 24.6) |
| >30 (moderate to severe disease) | 43.5 | 7.7 | 5.3 |
| Inflammatory markers# | |||
| IL-6, pg/mL | 11.7 (5.68, 22.8) | 5.0 ** (2.6, 9.6) | 5.1 ** (2.5, 10.3) |
| TNF-α, pg/mL | 7.1 (4.9, 10.2) | 1.6 ** (1.0, 2.6) | 2.6 ** # (0.9, 6.8) |
| C-reactive protein | 1.2 (0.5, 2.5) | 0.5 ** (0.3, 0.5) | 0.5 ** # (0.3, 0.8) |
| ESR, mm/hr | 21 (12, 39) | 9 ** (4, 14) | 11 ** (5, 17) |
mean (SD)
median (intraquartile range), log-transformed values used for comparisons
Comparisons: Different from baseline value using paired t-test:
p<0.05;
p<0.0001.
Different from 10-week value using paired t-test:
p<0.01,
p<0.001.
Table IV (available at www.jpeds.com) summarizes sex hormone and gonadotropin levels according to Tanner stage and sex from the 76 participants with at least one sex hormone measure. Among these 16 females and 34 males had sex hormone measurements at baseline, 10 weeks and 1 year; 7 females and 6 males had measures at two of these time points; and 7 females and 2 males had measures at 1 time point only. Participants had a baseline Tanner stage median (intraquartile range) of 3 (2, 4) for males and 4 (3, 4) for females and at 1 year of age this was progressed to 4 (3, 5) for males and 4 (4, 5) for females (p<0.0001). Adolescents in Tanner stages 2–4 (ie, those able to advance in Tanner stage) had a median increase of 1 (0, 1) Tanner stages. Overall there were increases in testosterone, estradiol, LH and FSH values from baseline to 10 weeks and 1 year (all p < 0.01), assessed as paired t-tests on log-transformed data. Using univariate linear regression, Z-scores for testosterone in males and estradiol in females were not associated with the degree of delay in bone age (data not shown).
Table 4. Changes in raw values of sex hormones over time following treatment with infliximab.
Median and intraquartile ranges are shown. Data reflect all participants included in the mixed model analysis, including those who were missing data at individual time points. Paired t-tests were performed for those with levels at either baseline and 10-week or baseline and 1-year, accounting for instances where the median level for a group decreases but the paired t-test reveals an increase in hormone level over the time interval.
| Tanner stage (number, percent) | Testosterone (ng/mL) | LH (mIU/mL) | FSH (mIU/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1-year | Baseline n=41 | 10-week# n=40 | 1-year# n=35 | Baseline n=37 | 10-week# n=36 | 1-year# n=35 | Baseline n=34 | 10-week# n=34 | 1-year# n=31 | |
| Males | |||||||||||
| Tanner 2 | 12 (23.5) | 5 (11) | 4.5 (4.0, 7.5) | 15.5* (6.5, 55.5) | 76 ** (12, 142) | 0.3 (0.2, 0.5) | 1.05 (0.7, 1.6) | 1.7 * (0.7, 2.1) | 1.1 (0.3, 1.2) | 1.8 (1.3, 2.7) | 1.5 (0.8, 3.1) |
| Tanner 3 | 10 (19.6) | 9 (19.5) | 71 (4, 189) | 139.5 (31–198) | 100 (68, 230) | 0.9 (0.2, 6) | 2.3 (0.8, 5.3) | 4.0 (0.8, 5.0) | 3.6 (0.9, 7.1) | 3.4 (1.6, 7.2) | 2.6 (1.2, 2.9) |
| Tanner 4 | 11 (21.6) | 6 (13.0) | 246 (155, 595) | 402 (276, 630) | 409 (386, 461) | 3.15 (1.9, 4.0) | 2.7 (2.0, 3.7) | 3.0 (2.6, 4.1) | 4.2 (2.1, 8.6) | 2.6 (1.7, 5.9) | 4.2 (2.1, 4.5) |
| Tanner 5 | 9 (17.6) | 17 (37.0) | 442 (143, 522) | 556 (734, 523) | 520.5 (378, 647) | 3.9 (2.0, 5.0) | 4.8 (4.0, 7.6) | 3.4 (2.0, 5.7) | 2.8 (1.2, 6.7) | 5.9 (1.8, 7.7) | 4.5 (1.2, 5.4) |
| Females | Estradiol (pg/mL) | LH (mIU/mL) | FSH (mIU/mL) | ||||||||
| n=22 | n=25 | n=22 | n=18 | n=21 | n=20 | n=18 | n=21 | n=18 | |||
| Tanner 2 | 5 (14.7) | 3 (11.1) | 2.5 (2.0, 6.0) | 38 (6, 41) | 48 (11, 80) | 1.0 (0.1, 1.8) | 2.0 (1.1, 4.0) | 3.1 (0.3, 8.7) | 1.45 (0.2, 2.7) | 2.2 (0.9, 4.2) | 3.8 (1.4, 5.1) |
| Tanner 3 | 8 (23.5) | 4 (14.8) | 21 (2, 59.5) | 21 (15, 25) | 45 (30, 56) | 4.5 (1.1, 14.5) | 4.1 (3.6, 6.8) | 6.0 (4.7, 9.5) | 3.9 (1.9, 6.0) | 4.7 (3.9, 5.5) | 5.2 (4.1, 6.2) |
| Tanner 4 | 11 (32.4) | 9 (33.3) | 38 (6.5, 74.5) | 34 * (15, 71) | 61 (13, 129) | 1.75 (0.1, 4.0) | 6.7 (3.6, 8.7) | 3.2 (1.2, 4.3) | 1.45 (0.2, 6.0) | 3.2 (2.8, 4.3) | 2.9 (1.1, 4.0) |
| Tanner 5 | 6 (17.6) | 10 (37.0) | 49.5 (28.0, 129) | 70.5 (25.5, 143.0) | 30 (7, 67) | 5.7 (3.3, 19.4) | 4.8 (1.5, 14.6) | 5.1 (4.1, 5.6) | 4.5 (2.9, 4.7) | 4.6 (4.0, 5.3) | 5.7 * (4.6, 5.8) |
Values according to the participant’s baseline Tanner stage.
Comparisons: Higher than baseline value using a paired t-test on log-transformed values:
p<0.05;
p<0.01.
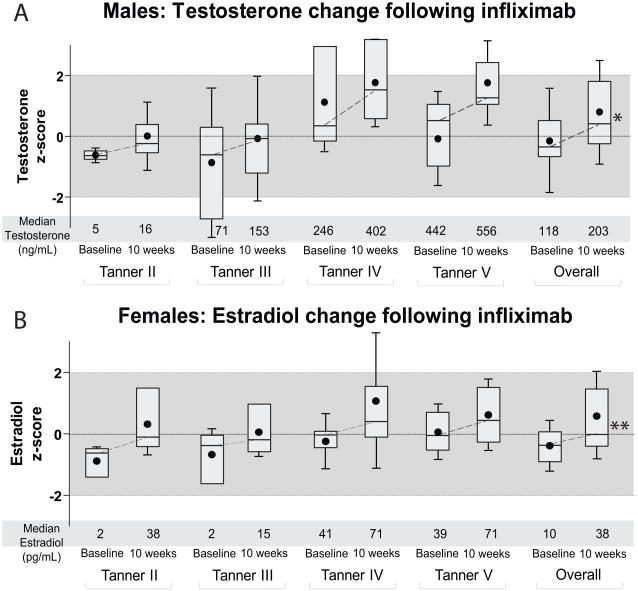
Figure 2 shows Z-scores for testosterone in males and estradiol in females by Tanner stage in the 54 participants with sex hormone Z-score results at both baseline and 10 weeks. During the 10 week induction interval, testosterone Z-scores increased significantly in males (p < 0.05) and estradiol Z-scores increased significantly in females (p < 0.01) in Tanner stages 2 through 5 combined. Results were similar when estradiol Z-scores were calculated based on age and not bone age (data not shown). Overall, testosterone levels in males were 53% and 42% higher at 10 weeks and 12 months, compared with baseline, respectively. Estradiol levels in females were 132% and 90% greater at 10 weeks and 12 months, compared with baseline, respectively. Baseline use of any of the other Crohn’s disease medications was not associated with change in sex hormone Z-score (data not shown).
Figure 2. Box Plots of Testosterone and Estradiol Z-scores at Baseline and 10-weeks after Infliximab.
Median and intraquartile range (with dot for mean and whiskers for standard deviation) for Tanner-stage specific testosterone Z-scores (A) and bone-age-specific estradiol Z-scores (B) among participants Tanner 2–5, both by individual Tanner stage and overall group. Corresponding median values for raw measures are shown below. Differences in t-test of log-transformed values: * p < 0.05; **p < 0.01.
Table V displays the results of mixed-model regression analysis of the relationship of sex hormone Z-scores and gonadotropins with BMI Z-score, total fat mass and markers of disease status and inflammation over the 12 month study interval. Testosterone and estradiol Z-scores were inversely and significantly associated with PCDAI score, cytokine levels and ESR. Testosterone Z-scores were also negatively associated with CRP levels; estradiol Z-scores had a similar direction of association but wider standard error and were not significantly associated with CRP. LH and FSH levels were inversely associated with PCDAI score and TNF-α levels, adjusted for sex and Tanner stage. Sex hormone Z-scores and gonadotropin levels were not related to BMI Z-scores or fat mass Z-scores over the course of treatment.
Table 5. Mixed model assessment of the effect of inflammation and adiposity on sex hormone levels.
Data shown are for univariate models assessing relations between inflammatory and adiposity markers at each time point on sex hormone and gonadotropin levels among the 72 participants in Tanner stage 2–5 with at least one sex hormone measurement.
| Testosterone z-score (males) | Estradiol z-score (females) | LH*# | FSH*# | |||||
|---|---|---|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| PCDAI | −0.028 (0.007) | <0.001 | −0.025 (0.010) | 0.017 | −0.021 (0.006) | 0.008 | −0.013 (0.005) | 0.027 |
| IL-6* | −0.387 (0.136) | 0.006 | −0.254 (0.104) | 0.021 | −0.133 (0.066) | 0.163 | −0.067 (0.061) | 0.548 |
| TNF-α* | −0.203 (0.085) | 0.020 | −0.141 (0.068) | 0.048 | −0.219 (0.068) | 0.001 | −0.141 (0.059) | 0.019 |
| ESR* | −0.357 (0.172) | 0.041 | −0.310 (0.114) | 0.011 | −0.092 (0.085) | 0.388 | −0.092 (0.054) | 0.255 |
| CRP* | −0.342 (0.149) | 0.025 | −0.159 (0.184) | 0.340 | −0.256 (0.114) | 0.027 | −0.250 (0.096) | 0.014 |
| BMI-z | 0.151 (0.150) | 0.317 | 0.248 (0.161) | 0.133 | 0.049 (0.102) | 0.525 | −0.033 (0.082) | 0.856 |
| Fat mass z-score | −0.072 (0.210) | 0.735 | 0.042 (0.280) | 0.842 | −0.104 (0.123) | 0.623 | −0.151 (0.100) | 0.810 |
natural log used to produce normality
p values after adjustment for current Tanner stage and sex
DISCUSSION
This study examined changes in sex hormone and gonadotropin levels following initiation of anti-TNF-α therapy in adolescents with Crohn’s Disease. Improvements in disease activity and cytokine levels during induction therapy were associated with rapid and significant increases in sex hormone and gonadotropin levels, independent of BMI or fat mass. These data suggest systemic inflammation suppresses gonadotropin-stimulated production of sex hormones, consistent with preclinical studies.9–12, 15
Prior to treatment with anti-TNF-α treatment, the adolescents in the study had mean Z-scores of testosterone and estradiol of −0.36 and −0.35, respectively, that increased to 0.4 and −0.02, respectively over 10 weeks of anti-TNF-α therapy. Although increases in sex hormones are expected during pubertal progression, over a 10-week period we observed 53–132% increases in levels—which were still overall in the normal range for Tanner stage or bone age. In the case of testosterone, this far surpasses expected gains for males in this age range, expected to be approximately 112 ng/dL per year between the ages of 12–16 years.30 Indeed, among participants with data for all three time points, there were slightly lower levels of testosterone and estradiol at 1 year compared with 10 weeks, suggesting against increases in sex hormones based only on the passage of time. It is notable that TNF-α levels were higher at the 1-year visit compared with 10 weeks, supporting the potential for some rebound of inflammatory suppression of sex hormone production.
Suppression of sex hormone levels in inflammatory disease has been felt to be at the level of gonadotropin regulation.9–12, 15 Consistent with this concept, we found levels of LH and FSH that were not in the hypergonadotropic range at baseline. Release of LH and FSH is under the control of gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus. Over the past several years there has been much progress in the description of the regulation of neurotransmitters that strongly affect the function of GnRH neurons—and thus affect gonadotropin-driven sex hormone production. These neurotransitters include kisspeptin, neurokinin B and dynorphin (secreted from “KNDy” neurons in the arcuate nucleus of the hypothalamus),31 with the recent addition of MKRN3 as an upstream suppressor of puberty.32 However, despite these discoveries into direct regulators of GnRH, the overall regulation of gonadotropins in pubertal progression remains to be described. The relationships we found linking gonadotropin and sex hormone levels with systemic inflammatory factors may implicate a role of inflammatory cytokines in this regulation among individuals with inflammatory disease.
One well-described influence on gonadotropin release is leptin, an adipokine secreted in increasing proportion to the amount of fat mass.33 Low levels of leptin are associated with lower sex hormone levels,13 potentially as a protection against female reproduction in a setting of low energy stores.14 Although leptin levels were not available in our participants, we assessed for relationships between BMI Z-score and fat mass Z-scores with sex hormone levels to evaluate for the potential role of body fat in the regulation of sex hormones following anti-TNF-α treatment. We found that neither BMI Z-score nor fat mass Z-score were related to levels of sex hormones or gonadotropins over the course of this study, potentially indicating that short-term changes in adiposity may not be as strong of a predictor of short-term changes in sex hormone regulation. Clearly, more detailed evaluations are required.
The regulation of gonadotropins and estradiol in females becomes more complex with the cycling of these levels as part of the menstrual cycle, when over a short period of time levels of LH and FSH peak to several fold above baseline, with concurrent increase in estradiol. The changes in these levels account for the wide normal ranges of estradiol in later Tanner stages (see Table 4; online) and the difficulty in determining an expected increase in estradiol levels over the course of time. A normal pattern of cycling is dependent on multiple factors, including the presence of adequate fat mass.13, 14 We did not have data regarding the timing of the last menstrual period; however it is unlikely that there were systematic differences in the timing of the study visit relative to participant menstrual cycle at the baseline or 10 week visit. Rather, this imprecision likely introduced measurement error. Had the study design included measures of sex hormones during the follicular phase, then we likely would have observed even stronger association with disease activity and laboratory variables. More data are needed among young women with Crohn’s disease regarding the influence of anti-inflammatory therapy on overall menstrual cycle regulation.
This study had other additional limitations. As opposed to an observational nature of this type, a randomized controlled trial would have afforded additional specificity to the conclusions regarding relationships between anti-TNF-α treatment and sex hormone regulation. Due to limited specimen availability following completion of the primary study, we were only able to evaluate the change in sex hormone Z-scores in a subset (71%) of Tanner 2–5 adolescents. However, this subset did not differ from the cohort of adolescents without Z-scores in terms of age or sex hormone levels. We relied on a validated survey of child self-assessment of puberty instead of investigator examination, potentially leading to misclassification of pubertal stage. We used the baseline Tanner stage assessment at 10 weeks, potentially missing an interval progression of Tanner stage between these time points. Bone age assessments were performed only on a clinical basis, and the pediatric radiologist was not blinded to the child’s age, potentially biasing toward less bone age delay. Finally, hormone levels were measured at random times of day (sex hormone levels are highest in the morning) and random times of the menstrual cycle (estradiol levels peak in the luteal phase); however, as detailed above, this likely results in an underestimation of the strength of our associations. However, this study also had multiple strengths, including prospective follow of adolescents with Crohn’s disease at the initiation of a potent anti-inflammatory treatment.
In conclusion, adolescents with Crohn’s disease exhibited an increase in levels of testosterone, estradiol, LH and FSH in short-term follow-up after initiation of anti-TNF-α treatment, with sex hormone and gonadotropin levels that were related to measures of disease severity and systemic inflammation. These data corroborate basic science studies demonstrating effects of systemic inflammation on the regulation of sex hormone production. These findings may have implications in the clinical care of chronic inflammatory disease regarding the delay of puberty and related sequelae.
Acknowledgments
Supported by National Institutes of Health (K23 DK082012 [to M.T.], K23 DK093556 [to M.D.], K24 DK076808 [to M.L.], K08 HD060739 [to M.D.]); the Clinical and Translational Science Award (UL1RR024134 and UL1TR000003); the Penn Joint Center for Inflammatory Bowel Diseases; and the University of Virginia Children’s Hospital. The authors declare no conflicts of interest.
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- CV
coefficient of variation
- DXA
Dual-energy X-ray absorptiometry
- ESR
erythrocyte sedimentation rate
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- PCDAI
Pediatric Crohn’s Disease Activity Index
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ballinger AB, Savage MO, Sanderson IR. Delayed puberty associated with inflammatory bowel disease. Pediatr Res. 2003;53(2):205–210. doi: 10.1203/01.PDR.0000047510.65483.C9. [DOI] [PubMed] [Google Scholar]
- 2.DeBoer MD, Denson LA. Delays in puberty, growth, and accrual of bone mineral density in pediatric Crohn's disease: despite temporal changes in disease severity, the need for monitoring remains. J Pediatr. 2013;163(1):17–22. doi: 10.1016/j.jpeds.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham BP, Mehta S, El-Serag HB. Natural history of pediatric-onset inflammatory bowel disease: a systematic review. J Clin Gastroenterol. 2012;46(7):581–589. doi: 10.1097/MCG.0b013e318247c32f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawczenko A, Ballinger AB, Savage MO, Sanderson IR. Clinical features affecting final adult height in patients with pediatric-onset Crohn's disease. Pediatrics. 2006;118(1):124–129. doi: 10.1542/peds.2005-2931. [DOI] [PubMed] [Google Scholar]
- 5.Robinson RJ, Iqbal SJ, Al-Azzawi F, Abrams K, Mayberry JF. Sex hormone status and bone metabolism in men with Crohn's disease. Aliment Pharmacol Ther. 1998;12(1):21–25. doi: 10.1046/j.1365-2036.1998.00271.x. [DOI] [PubMed] [Google Scholar]
- 6.Thayu M, Denson LA, Shults J, Zemel BS, et al. Determinants of changes in linear growth and body composition in incident pediatric Crohn's disease. Gastroenterology. 2010;139(2):430–438. doi: 10.1053/j.gastro.2010.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeBoer MD, Barnes BH, Stygles NA, Sutphen JL, Borowitz SM. Changes in inflammation and QoL after a single dose of infliximab during ongoing IBD treatment. J Pediatr Gastroenterol Nutr. 2012;54(4):486–490. doi: 10.1097/MPG.0b013e3182382ee3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyams J, Crandall W, Kugathasan S, et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology. 2007;132(3):863–873. doi: 10.1053/j.gastro.2006.12.003. quiz 1165–1166. [DOI] [PubMed] [Google Scholar]
- 9.Nappi RE, Rivest S. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neuronal system in cycling female rats: an evaluation at the transcriptional level. Endocrinology. 1997;138(4):1374–1384. doi: 10.1210/endo.138.4.5044. [DOI] [PubMed] [Google Scholar]
- 10.Azooz OG, Farthing MJ, Savage MO, Ballinger AB. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Regul Integr Comp Physiol. 2001;281(5):R1483–1491. doi: 10.1152/ajpregu.2001.281.5.R1483. [DOI] [PubMed] [Google Scholar]
- 11.DeBoer MD, Li Y, Cohn S. Colitis causes delay in puberty in female mice out of proportion to changes in leptin and corticosterone. J Gastroenterol. 2010;45(3):277–284. doi: 10.1007/s00535-009-0192-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeBoer MD, Li Y. Puberty is delayed in male mice with dextran sodium sulfate colitis out of proportion to changes in food intake, body weight, and serum levels of leptin. Pediatr Res. 2011;69(1):34–39. doi: 10.1203/PDR.0b013e3181ffee6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):458–464. doi: 10.1097/MED.0b013e3282f1cfdc. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi IS. Leptin and the onset of puberty: insights from rodent and human genetics. Semin Reprod Med. 2002;20(2):139–144. doi: 10.1055/s-2002-32505. [DOI] [PubMed] [Google Scholar]
- 15.DeBoer MD, Steinman J, Li Y. Partial normalization of pubertal timing in female mice with DSS colitis treated with anti-TNF-α antibody. J Gastroenterol. 2012;47(6):647–654. doi: 10.1007/s00535-012-0542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood HC, Cohen LE, Lee JJ. Late adolescent linear growth pattern in pediatric-onset inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53(3):246–249. doi: 10.1097/MPG.0b013e31821898ae. [DOI] [PubMed] [Google Scholar]
- 17.Gupta N, Lustig RH, Kohn MA, Vittinghoff E. Menarche in pediatric patients with Crohn's disease. Dig Dis Sci. 2012;57(11):2975–2981. doi: 10.1007/s10620-012-2235-z. [DOI] [PubMed] [Google Scholar]
- 18.Brain CE, Savage MO. Growth and puberty in chronic inflammatory bowel disease. Baillieres Clin Gastroenterol. 1994;8(1):83–100. doi: 10.1016/s0950-3528(06)80020-5. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson A, Sedgwick DM. Juvenile onset inflammatory bowel disease: height and body mass index in adult life. Bmj. 1994;308(6939):1259–1263. doi: 10.1136/bmj.308.6939.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mason A, Malik S, Russell RK, Bishop J, McGrogan P, Ahmed SF. Impact of inflammatory bowel disease on pubertal growth. Horm Res Paediatr. 2011;76(5):293–299. doi: 10.1159/000329991. [DOI] [PubMed] [Google Scholar]
- 21.Fujimura Y, Honda K, Sato I, et al. Remarkable improvement of growth and developmental retardation in Crohn's disease by parenteral and enteral nutrition therapy. Intern Med. 1992;31(1):39–43. doi: 10.2169/internalmedicine.31.39. [DOI] [PubMed] [Google Scholar]
- 22.Augustine MV, Leonard MB, Thayu M, et al. Changes in vitamin D-related mineral metabolism after induction with anti-tumor necrosis factor-α therapy in Crohn's disease. J Clin Endocrinol Metab. 2014;99(6):E991–998. doi: 10.1210/jc.2013-3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin LM, Thayu M, Baldassano RN, et al. Improvements in Bone Density and Structure during Anti-TNF-α Therapy in Pediatric Crohn's Disease. J Clin Endocrinol Metab. 2015;100(7):2630–2639. doi: 10.1210/jc.2014-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 25.Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc. 1980;9(3):271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 26.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991;12(4):439–447. [PubMed] [Google Scholar]
- 27.Kreyszig E. Applied Mathematics. 4. New York, NY: Wiley Press; 1979. [Google Scholar]
- 28.McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab. 2009;94(1):56–66. doi: 10.1210/jc.2008-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostoufi-Moab S, Ginsberg JP, Bunin N, Zemel B, Shults J, Leonard MB. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27(4):760–769. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Peddada SD, Malina RM, Rogol AD. Longitudinal assessment of hormonal and physical alterations during normal puberty in boys. VI. Modeling of growth velocity, mean growth hormone (GH mean), and serum testosterone (T) concentrations. Am J Hum Biol. 2000;12(6):814–824. doi: 10.1002/1520-6300(200011/12)12:6<814::AID-AJHB9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 31.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. doi: 10.1056/NEJMoa1302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan JL, Matarese G, Shetty GK, et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci U S A. 2006;103(22):8481–8486. doi: 10.1073/pnas.0505429103. [DOI] [PMC free article] [PubMed] [Google Scholar]