Abstract
Objective
To determine the rate of sinusitis complicating upper respiratory tract illnesses (URIs) in children. We prospectively identified the clinical, virologic, and epidemiologic characteristics of URIs in a population of 4- to 7-year-old children followed for 1 year.
Study design
This was an observational cohort study in 2 primary care pediatric practices in Madison, Wisconsin. Nasal samples were obtained during 4 asymptomatic surveillance visits and during symptomatic URIs. A polymerase chain reaction-based assay for 9 respiratory viruses was performed on nasal samples. A diagnosis of sinusitis was based on published criteria.
Results
Two hundred thirty-six children ages 48-96 months were enrolled. A total of 327 URIs were characterized. The mean number of URIs per child was 1.3 (range 0-9) per year. Viruses were detected in 81% of URIs; rhinovirus (RV) was most common. Seventy-two percent of URIs were resolved clinically by the 10th day. RV-A and RV-C were detected more frequently at URI visits; RV-B was detected at the same rate for both asymptomatic surveillance visits and URI visits. Sinusitis was diagnosed in 8.8% of symptomatic URIs. Viruses were detected frequently (33%) in samples from asymptomatic children.
Conclusions
Sinusitis occurred in 8.8% of symptomatic URIs in our study. The virus most frequently detected with URIs in children was RV; RV-A and RV-C detection but not RV-B detection were associated with illness. Viruses, especially RV, are detected frequently in asymptomatic children. Most URIs have improved or resolved by the 10th day after onset. Children experienced a mean of 1.3 URIs per year, which was lower than expected.
Keywords: ADV, Adenovirus; CoV, Coronavirus; EV, Enterovirus; FLU, Influenza virus; GEE, Generalized estimating equation; hBoV, Human bocavirus; hMPV, Human metapneumovirus; PIV, Parainfluenza virus; RSV, Respiratory syncytial virus; RV, Rhinovirus; URI, Upper respiratory tract illness
Upper respiratory tract illness (URI) is the most common infectious disease of childhood and one of the most frequent reasons that parents seek medical care.1 The first characterization of URI symptoms using modern virologic techniques was in the 1960s in young adults.2 The frequency of URI in children and the nature and duration of respiratory symptoms has received increasing attention in the current era of molecular virology.3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 Historically, children have been reported to experience an average of 6-8 acute respiratory illnesses per year.16 However, the number of URIs and the particular viruses involved vary with age, season, geography, and attendance at out-of-home childcare. Previous studies have evaluated URIs in: (1) birth cohorts of children at high risk for atopy11, 12, 17, 18; or (2) cross-sectional studies during a single respiratory season.4, 5, 7, 15, 17 Only a few studies have prospectively followed substantial numbers of young children longitudinally, both when well and during symptomatic URI.18, 19, 20
Although the frequency of acute otitis media as a complication of viral URI has been reported, there has been relatively little systematic investigation of the rate of sinusitis as a complication of URI. The single recent study investigating this question was performed in children 6-36 months of age.9 In this age group, acute otitis media is a frequent early complication of URI (30%-37%) that leads to antimicrobial treatment that, in turn, may prevent the subsequent development of acute bacterial sinusitis.9, 19 To determine the rate of sinusitis complicating URI in children less susceptible to the confounding effects of otitis media, we prospectively studied the clinical, virologic, and epidemiologic characteristics of URIs in a population of 4- to 7-year-old children in an observational cohort study.
Methods
Healthy children ages 48-96 months were recruited in a rolling fashion from 2 primary care pediatric practices in Madison, Wisconsin and followed for 1 year. Families of eligible children received information about the study by mail approximately 2 weeks before their next scheduled well-child visit. Parental interest in the study was queried by the office nurse. If interest was expressed, the study coordinator provided additional information and invited the parent to participate in the study. Children were excluded from the study if they had an underlying condition reported by the parent or noted in the medical record, likely to alter the natural history of URI, including congenital or acquired immunodeficiency, craniofacial abnormalities, cystic fibrosis, allergic rhinitis, or a previous episode of chronic sinusitis. Written, informed consent was obtained and assent also was obtained from older children (≥7 years of age). Subjects received a small stipend for participation. The study was approved by the University of Wisconsin Institutional Review Board. Demographic information was obtained by the study nurse. Responses to race/ethnicity questions were self-reported by the subject's parent.
Nasal samples were obtained at entry and during 4 surveillance visits (February, April, September, and December) when children were asymptomatic as verified by the study nurses. Parents were instructed to call the study nurse at the first sign of a URI, which was defined as at least 48 hours of respiratory symptoms including nasal congestion, nasal discharge, or cough. Nasal samples were obtained on day 3-4 of illness by the study nurse, and a recovery sample was obtained on day 15. A clinical assessment at the time of the visit assured that symptoms reflected illness confined to the upper respiratory tract. A symptom survey was filled out on day 3-4 and subsequently by telephone on days 7, 10, and 15.21 The survey inquired about fever, nasal symptoms, cough, headache, irritability, facial pain, facial swelling, activity, sleep, and impaired appetite. If a symptom was present initially, a score of 2 was assigned. If it was absent the score was 0. If a symptom became more severe, less severe, or stayed the same, +1, −1 or 0, respectively, was added to the previous score for each symptom. The URI was considered resolved if the score was ≤2. In addition, parents were asked to record any missed days of childcare, school, or work because of respiratory illness in their child on a calendar provided for that purpose.
Each URI was classified as either an uncomplicated viral URI or sinusitis. The diagnosis of sinusitis was based on one of the following clinical criteria: (1) persistent symptoms—respiratory symptoms, including nasal discharge or cough or both, that lasted more than 10 days and were not improving (symptom score at 10 days ≥50% of highest score); (2) severe symptoms—a combination of purulent (thick, colored, and opaque) nasal discharge plus temperature >39°C for at least 72 hours; or (3) worsening symptoms—sudden onset of respiratory symptoms or fever after apparent improvement, usually beyond the sixth day of illness.22
Samples of nasal mucus were obtained using an established nose blowing technique.18, 23 Saline was sprayed into each nostril and then blown into a plastic “baggie.” Two milliliters of a solution containing buffered saline (pH 7.4) along with 0.5% gelatin was added to the baggie; the contents were transferred to a sterile tube, processed, and frozen.
Virus Identification
Diagnostic virology was performed on nasal samples by multiplex polymerase chain reaction (Respiratory Multicode Assay; EraGen Biosciences, Madison, Wisconsin; or Respiratory Viral Panel; Luminex Co, Austin, Texas) to test for the following viruses: respiratory syncytial virus (RSV, groups A and B), rhinovirus (RV ∼160 known types), parainfluenza virus (PIV; 1, 2, 3, 4a, and 4b), influenza virus (FLU; A, B, and C), adenovirus (ADV; B, C, and E), coronavirus (CoV; 229E, NL63, OC43, HK, and SARS), enterovirus (EV), human bocavirus (hBoV), and human metapneumovirus (hMPV; A and B). Nasal specimens also were analyzed by partial sequencing to determine which RV types were present, and to differentiate closely related EVs from RVs.24
Statistical Analyses
The number of URIs per subject was transformed to the log(x+1) scale to obtain a more symmetric distribution for estimating the mean. The result was transformed back to the original scale. Percentages of URI and surveillance visits with virus (or RV species) present were calculated using the generalized estimating equation (GEE) approach to logistic regression for clustered binomial data where the clusters are subjects. GEE also was used to estimate the proportion of visits each calendar month in which each virus was present. Models with a term for visit type were used to compare percentages between surveillance and URI visits. P values and CIs were calculated using a normal approximation.
GEE analysis with a random effect for subject was used on data from the URI visits to estimate the percentage of URIs with various symptoms, complications, and symptom recovery rates. The same approach was used to estimate the proportion of URIs that resulted in missed school and work days and the effect of the presence of viruses on the proportion of URIs with fever. Mean days missed from work and school and mean duration of fever were estimated using a linear mixed effects model with a random effect for patient. Mean symptom scores were estimated for URIs with only RV present and without RV present using a mixed effects model with random effects for subject and for URI within subject and fixed effects for day and RV. The interaction term between RV and day was not significant. Symptom scores were transformed to the log(x+2) before analysis to obtain approximately symmetrically distributed residuals. Estimated mean symptom scores were transformed back to the original scale.
Results
Invitations to participate were sent to families of 3763 patients from 2 pediatric clinics; 367 families were not solicited, as screening disclosed an underlying medical condition. From February 2012 to March 2015, 236 subjects were enrolled. A subgroup of 119 children has completed the 1-year study; 58 subjects withdrew, and 59 children are still being followed. All 236 subjects are the cohort in whom the characteristics of URIs are described. The subgroup of 119 children who completed the study was used to calculate the frequency of URIs per subject per year. The demographic characteristics of enrolled subjects are shown in Table I .
Table I.
Subject demographics (n = 236)
| Age (y) | |
| Mean | 5.1 |
| Range | 4.0-7.9 |
| Female | 46% |
| Race | |
| American Indian or Alaska Native | 1% |
| Asian | 5% |
| African American | 8% |
| Other | 5% |
| Unknown or not reported | 1% |
| Caucasian | 80% |
| Ethnicity | |
| Hispanic | 7% |
| Attends day care/school | 83% |
| Tobacco exposure | 3% |
| Maternal education level | |
| Graduate/professional | 36% |
| College degree | 34% |
| Some college | 16% |
| Vocational/technical | 6% |
| High school or less | 6% |
| Grade school | 1% |
| Not reported | 1% |
| Public insurance (Medicaid) | 18% |
Virology and Frequency of URI
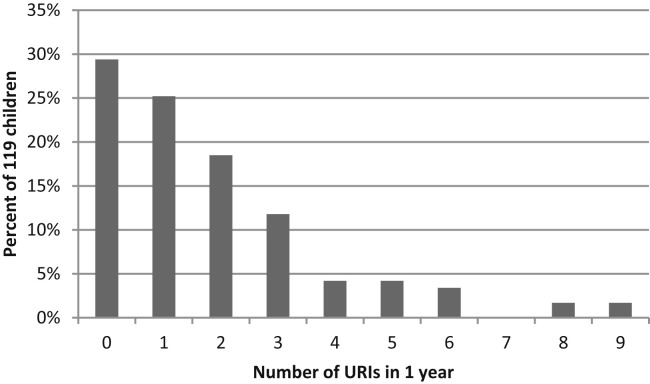
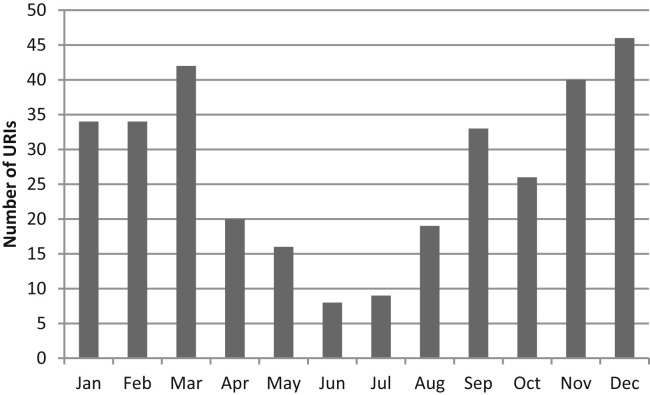
The mean number of URIs per child was 1.3 per year (range 0-9) for the 119 subjects who completed 1 year of follow-up. Of these, 29% had no documented URIs and 15% had at least 4 URI episodes in a year (Figure 1; available at www.jpeds.com). URIs occurred year-round but were most prevalent in the late fall and winter months (Figure 2; available at www.jpeds.com).
Figure 1.
Number of URIs per subject in a 1-year period (n = 119).
Figure 2.
Number of URIs (n = 327) by month in 236 children.
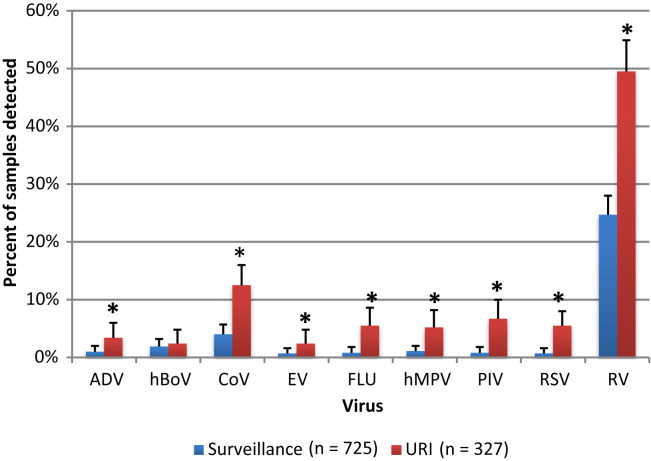
One or more respiratory viruses were detected in 266 (81%) of 327 URIs in 236 study subjects. The frequency of detection of the viruses in URI visits is shown in Figure 3 . RV was the most commonly detected virus, occurring in 161 of 327 (49%) URIs. Multiple viruses were detected in 12% of nasal specimens during URI: 36 (11%) specimens had 2 viruses, 2 specimens (<1%) had 3 viruses, and 1 specimen (<1%) had 4 viruses detected.
Figure 3.
Respiratory viruses detected in nasal specimens from 725 asymptomatic surveillance visits and 327 URI visits from 236 study subjects. The detection rates shown include samples with both single and multiple viruses detected. ORs and 95% CIs (shown in parentheses) for the difference in detection of each virus between URI and surveillance specimens: ADV 3.6 (1.4-9.3), hBoV 1.3 (0.5-3.1), CoV 3.4 (2.1-5.7), EV 3.6 (1.2-11.1), FLU 7.0 (2.8-17.8), hMPV 4.9 (2.1-11.5), PIV 8.7 (3.5-21.5), RSV 8.4 (3.1-22.8), and RV 3.0 (2.3-3.9). There were 242 viruses detected in surveillance visits and 266 viruses in URI visits. ∗P < .001 for an OR different than 1.
Polymerase chain reaction for respiratory viruses was also performed on nasal samples obtained at 725 asymptomatic surveillance visits in 236 study subjects. The distribution of viruses in these visits is also shown in Figure 3. One or more viruses were detected in 242 (33%) surveillance samples with 2 viruses detected in 17 (2%) samples. RV was detected most frequently in 179 of 725 (25%) well children.
The ORs and 95% CIs (shown in parentheses) for the difference in detection of each virus between URI and surveillance specimens were as follows: ADV 3.6 (1.4-9.3), hBoV 1.3 (0.5-3.1), CoV 3.4 (2.1-5.7), EV 3.6 (1.2-11.1), FLU 7.0 (2.8-17.8), hMPV 4.9 (2.1-11.5), PIV 8.7 (3.5-21.5), RSV 8.4 (3.1-22.8), and RV 3.0 (2.3-3.9). All viruses, except hBoV, were detected significantly more often during URI visits compared with surveillance visits. Of all the viruses assayed, PIV, FLU, and RSV were the most likely to be associated with URI rather than asymptomatic surveillance visits. The types of CoVs, FLU, RSV, and PIV detected are shown in Table II (available at www.jpeds.com).
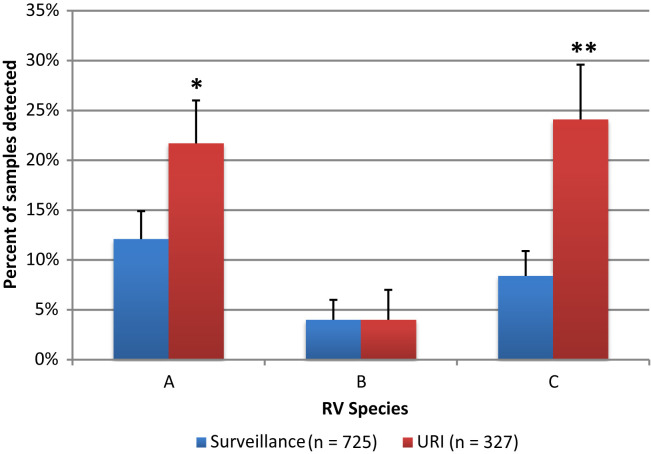
Speciation and subtyping were performed on all detected RVs. There were 76 different subtypes of RV detected in the 161 RV-positive samples obtained from symptomatic patients. The number of unique RV types per subject ranged between 0 and 9. The rate of detection of the 3 species of RV is depicted in Figure 4 . RV-A and RV-C were significantly more likely to be detected at URI than at asymptomatic surveillance visits (P < .001). There was no significant difference in the rate of RV-B according to visit type.
Figure 4.
RV species detected in nasal specimens (n = 179) in 725 surveillance (n = 725) vs 327 URI visits (n = 327). ORs for the comparison between surveillance and URI visits for each species: RV-A 2.0 (1.4-2.8,∗P < .001), RV-B 0.9 (0.5-1.7, P = Not Significant), RV-C 3.5 (2.3-5.1, ∗∗P < .001).
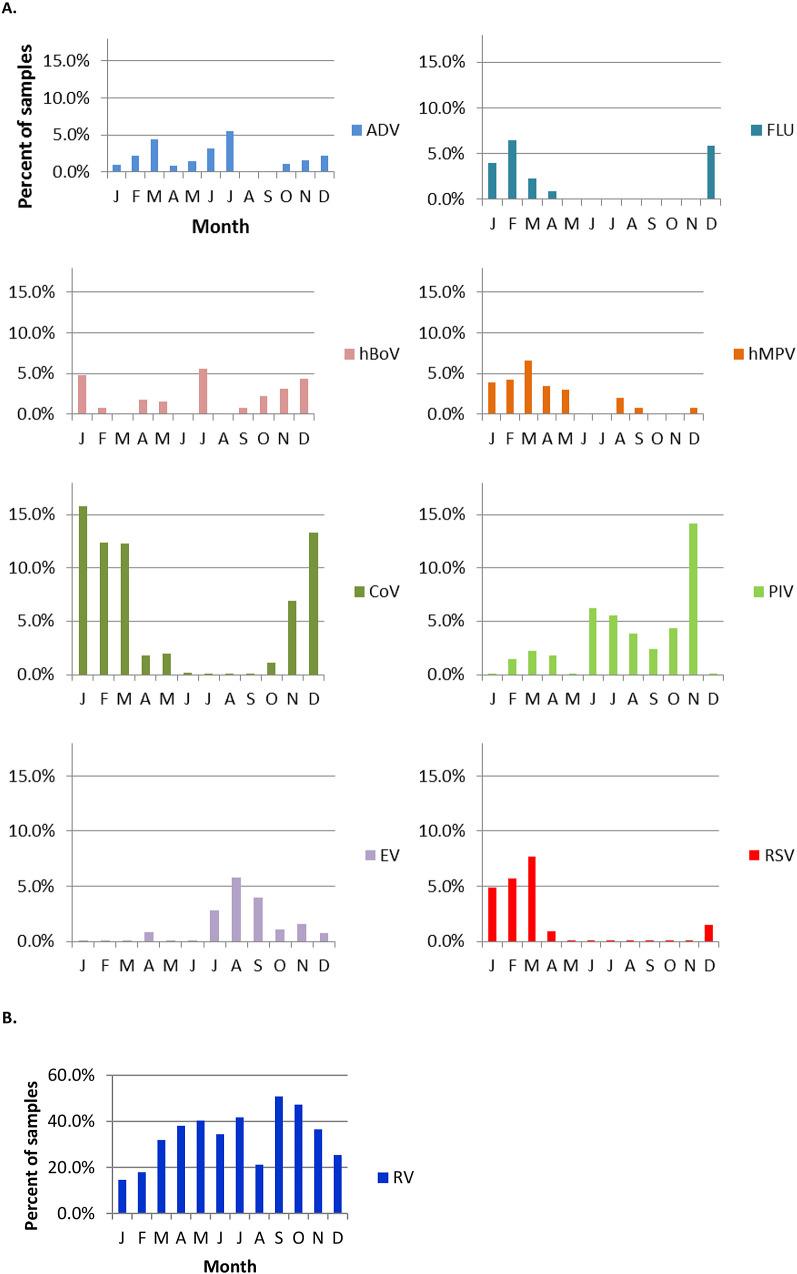
The distribution of viruses detected at all visits by month is shown in Figure 5 . A winter predominance was noted for the detection of FLU, CoV, and RSV. PIV and EV were detected more frequently during the summer and fall and hMPV in winter and spring. RV was found continuously throughout the year with a predilection for spring and fall, and hBoV occurred sporadically.
Figure 5.
Detection of virus by month in 725 surveillance samples and 327 URI samples collected over three respiratory seasons from 236 subjects. A, ADV, hBoV, CoV, EV, FLU, hMPV, PIV, and RSV. B, RV (different scale). Because the number of samples per month varied, the data are presented as the percent of samples (surveillance + URI) in which a particular virus was detected.
Symptoms
Nasal congestion and nasal discharge were the most common symptoms reported by parents in 89% of 327 URIs, followed by daytime cough (75%), night time cough (64%), sore throat (26%), vomiting (9%), diarrhea (4%), and rash (3%). Fever was reported by parental history in 21% of URI episodes; the average reported temperature in febrile URIs was 38.1°C. Fever was most likely to be present in URIs associated with FLU (53%, OR 4.9, 95% CI 2-12, P < .001) and less likely to be present in URIs with RV (12%, OR 0.33 CI 0.2-0.6 P < .001). The mean duration of fever for subjects reporting fever was 1.5 days (range 1-5).
URI symptoms peaked on the third day of illness. By the 10th and 15th day of illness, 72% and 94%, respectively, of subjects had resolution of URI symptoms. Mean symptom scores from URIs associated with RV were significantly lower than scores from URIs with all other non-RV viruses with a day 3 symptom score of 7.7 (CI 6.7-8.7) for RV vs a score of 8.8 (CI 7.8-9.9) for non-RV viruses (P < .005).
Impact and Complications
URIs resulted in missed school or day care 21% of the time; the mean number of days missed for affected children was 1 (range 0-12 days). Likewise, a parent or caregiver missed work because of the child's URI 11% of the time; the mean number of days missed was 1 (range 0-12 days) for affected caregivers.
URIs resulted in a medically attended visit 24% of the time. Of 327 episodes of symptomatic URI, 29 (8.8%, CI 6.1%-12.6%) met predefined criteria for sinusitis, which was the most common complication. The 29 episodes of sinusitis occurred in 24 children: 1 episode each in 22 children, 2 episodes each in 2 children, and 3 episodes in 1 child. Fifteen episodes of sinusitis met criteria for presentation with persistent symptoms and 14 for worsening symptoms. An antibiotic was prescribed for complications of URI in 10.8% of all symptomatic URIs. Acute otitis media was diagnosed by the patient's primary physician in 5.8% of episodes of symptomatic URI. Other complications of episodes of URI included wheezing in 1.7%, pneumonia in 0.9%, and group A streptococcal pharyngitis in 0.9%.
Discussion
Our goal was to determine the frequency and viral etiology of symptomatic URIs and their complications, specifically sinusitis, in children ages 4-7 years. The unique features of this study include prospective follow-up of preschool/young school age children for 1 year, quarterly nasal sampling when children were well, and inclusion of 3 consecutive respiratory seasons, thereby dampening the effect of year-to-year variation in prevalence of different respiratory viruses. This age group was specifically chosen to determine the rate of sinusitis complicating URI because the frequency of URI is high and the risk of acute otitis media is considerably lower than during the first 3 years of life. Using these methods, viruses were identified in 81% of symptomatic URIs. RV infections were most common, and there was evidence of differential virulence among viruses, even between RV species. Using predefined stringent criteria, sinusitis complicated 8.8% of URIs.
URIs resulted in frequent medically attended visits for substantial rates of secondary infection as well as time lost from work or childcare. We determined sinusitis to be the most common complication of URI in this age group. Our estimated rate of sinusitis is similar to other studies examining sinusitis as a complication of URI in different populations of children. Wald et al25 examined 0- to 3-year-old children attending childcare and determined the rate of sinusitis following URI to be 6.5%-13%. Marom et al9 reported a rate of 8% in a population of 6- to 35-month-old children who were followed prospectively. In both of these studies, the rate of sinusitis that was reported may have been blunted by the frequency of acute otitis media that is usually managed with antibiotics, which may have prevented the development of sinusitis. Ueda et al,26 basing the diagnosis of sinusitis on radiographic findings, found the rate of sinusitis to be 6.7% in 2- to 15-year-olds with URI symptoms. Two other observational studies of children with URI found rates of 8.3%-17.3% in children presenting to clinic with respiratory symptoms.27, 28
Our study determined the mean number of URIs per child per year. Although there was variation in our data with children acquiring from 0-9 URIs per year, the average of 1.3 was lower than expected. A classic study done in the 1950s, the Cleveland Illness in the Home study, found a rate of 6-7 “common respiratory diseases” per child per year in this age group.16 This difference may be explained by changes in societal structure as reflected by an increase in childcare attendance by very young children. Early communal experiences may result in children developing more illnesses earlier in life and fewer illnesses in later years. Three other recent studies provide contradictory results. Revai et al29 and Byington et al20 found a rate of approximately 0.5 illnesses per month in children less than five years of age. These children were followed in a similar fashion to children in our investigation but were younger. In a household surveillance study, Monto et al8 reported a rate of 0.6 URIs per respiratory season in children 5-17 years old. Although children in the Monto study were only followed during the respiratory virus season, it is safe to say that children had fewer than 1 acute respiratory illness per year. These differences almost certainly relate to variation by age group, season, and geography.
Viruses were detected in 81% of episodes of symptomatic URI; RV was detected most often, followed in frequency by CoV and FLU. Viruses, particularly RVs, were also detected frequently at asymptomatic surveillance visits, which have been reported previously.13, 14, 20, 30, 31, 32, 33
Our results show that there are differences in clinical symptoms according to the viral cause of URI. For example, as is well known, fever was seen more often in association with FLU and less often with RV illnesses. In addition, the probability of symptomatic illness was related to RV species. The risk of URI was greatest with RV-C and then RV-A, but RV-B was not significantly associated with illness, which is in agreement with previous findings in infants.11 RVs were most prevalent in the spring and fall but were detected continuously throughout the year, whereas most other viruses had a more seasonal pattern.
Symptom scores were followed closely during URIs; scores peaked on day 3 and 72% of episodes were improved or resolved by the 10th day after onset. Pappas et al34 studied URIs in school-aged children and found that 40% of children were symptomatic through day 9, although most were improving. Likewise, similar to our results, a study of children seeking care for URI in the United Kingdom had a rate of persistent symptoms of 7% at 2 weeks.35
This study has several limitations which may limit generalizability. First, by design, the children included in this study were of a fairly narrow age group (4-7 years), as this is the age with the peak incidence of clinical episodes of sinusitis. URIs in infants and older children may have different characteristics and different viral antecedents.9 Second, the population studied was largely Caucasian and well-educated, reflecting the demographics of our Midwestern city. Third, the actual rate of URIs per child may be somewhat higher than we reported. Our study coordinators called families each month to encourage reporting of URIs and samples often were obtained at home visits for the convenience of the participants. Despite these efforts, it is possible that some URIs, particularly those of mild severity, might have been missed. Studies relying on self-report of URIs can underestimate the frequency of illnesses.12 Lastly, nasal rather than nasopharyngeal samples were used to identify viruses, which may have overestimated the rate of RV and underestimated the role of PIV, hMPV, FLU, and RSV.
This study provides a comprehensive and detailed clinical and virologic characterization of URIs in children 4-7 years of age. The rate of sinusitis as a complication of URI, not previously described prospectively in this important age group, has been meticulously delineated in this study. We have also documented that most URIs are improving or resolved by the 10th day after onset of symptoms, lending support to national guidelines for differentiating self-limited URI from sinusitis.22 Additional investigation of the role of viruses in the pathogenesis of acute bacterial sinusitis is necessary.
Footnotes
Funded by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (R01 AI097172). J.G. is supported by the National Institutes of Health, GlaxoSmithKline and Merck Inc; and serves as a consultant for GlaxoSmithKline, Johnson & Johnson, Merck Inc, Medimmune, Boehringer Ingelheim, Gilead, and Genentech. S.L. is supported by Broad Foundation, Janssen Inc, Sloan Foundation, and Pfizer Inc; received personal fees from Janssen, Boston Consulting Group, and Regeneron; has filed for or holds patents for reductive prodrug cancer chemothera (Stan449-PRV), combination antibiotic and antibody therapy for the treatment of Pseudomonas aeruginosa illnesses with royalties paid to KaloBios Inc, use of Lactobacillus sakei and other lactic acid bacteria as a therapeutic strategy for chronic rhinosinusitis, and the use of PhyloChip as a diagnostic and prognostic clinical tool pending. The other authors declare no conflicts of interest.
Appendix.
Table II.
Virus subtypes detected at surveillance (n = 725) and URI (n = 327) samples
| Surveillance samples number (%) | URI samples number (%) | |
|---|---|---|
| CoV | ||
| OC43 | 12 (40) | 23 (53) |
| NL63 | 9 (30) | 14 (33) |
| HK | 7 (23) | 1 (4) |
| 229E | 2 (7) | 4 (9) |
| Total | 30 (100) | 43 (100) |
| FLU | ||
| FLU A | 3 (50) | 10 (56) |
| FLU B | 3 (50) | 8 (44) |
| FLU C | 0 (0) | 0 (0) |
| Total | 6 (100) | 18 (100) |
| RSV | ||
| RSV A | 1 (20) | 7 (39) |
| RSV B | 4 (80) | 11 (61) |
| Total | 5 (100) | 18 (100) |
| PIV | ||
| PIV-1 | 1 (17) | 3 (14) |
| PIV-2 | 3 (50) | 9 (41) |
| PIV-3 | 2 (33) | 8 (36) |
| PIV-4 | 0 (0) | 2 (9) |
| Total | 6 (100) | 22 (100) |
References
- 1.Fendrick A.M., Monto A.S., Nightengale B., Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163:487–494. doi: 10.1001/archinte.163.4.487. [DOI] [PubMed] [Google Scholar]
- 2.Gwaltney J.M., Jr., Hendley J.O., Simon G., Jordan W.S., Jr. Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA. 1967;202:494–500. [PubMed] [Google Scholar]
- 3.Martin E.T., Fairchok M.P., Stednick Z.J., Kuypers J., Englund J.A. Epidemiology of multiple respiratory viruses in childcare attendees. J Infect Dis. 2013;207:982–989. doi: 10.1093/infdis/jis934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leekha S., Irish C.L., Schneider S.K., Fernholz E.C., Espy M.J., Cunningham S.A. Viral detection using a multiplex polymerase chain reaction-based assay in outpatients with upper respiratory infection. Diagn Microbiol Infect Dis. 2013;75:169–173. doi: 10.1016/j.diagmicrobio.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivaska L., Niemela J., Heikkinen T., Vuorinen T., Peltola V. Identification of respiratory viruses with a novel point-of-care multianalyte antigen detection test in children with acute respiratory tract infection. J Clin Virol. 2013;57:136–140. doi: 10.1016/j.jcv.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nokso-Koivisto J., Pyles R.B., Miller A.L., Patel J.A., Loeffelholz M., Chonmaitree T. Viral load and acute otitis media development after human metapneumovirus upper respiratory tract infection. Pediatr Infect Dis J. 2012;31:763–766. doi: 10.1097/INF.0b013e3182539d92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhedin S., Lindstrand A., Rotzen-Ostlund M., Tolfvenstam T., Ohrmalm L., Rinder M.R. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. 2014;133:e538–e545. doi: 10.1542/peds.2013-3042. [DOI] [PubMed] [Google Scholar]
- 8.Monto A.S., Malosh R.E., Petrie J.G., Thompson M.G., Ohmit S.E. Frequency of acute respiratory illnesses and circulation of respiratory viruses in households with children over 3 surveillance seasons. J Infect Dis. 2014;210:1792–1799. doi: 10.1093/infdis/jiu327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marom T., Alvarez-Fernandez P.E., Jennings K., Patel J.A., McCormick D.P., Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. Pediatr Infect Dis J. 2014;33:803–808. doi: 10.1097/INF.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa L.F., Queiroz D.A., Lopes da Silveira H., Bernardino Neto M., de Paula N.T., Oliveira T.F. Human rhinovirus and disease severity in children. Pediatrics. 2014;133:e312–e321. doi: 10.1542/peds.2013-2216. [DOI] [PubMed] [Google Scholar]
- 11.Lee W.M., Lemanske R.F., Jr., Evans M.D., Vang F., Pappas T., Gangnon R. Human rhinovirus species and season of infection determine illness severity. Am J Respir Crit Care Med. 2012;186:886–891. doi: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gern J.E., Pappas T., Visness C.M., Jaffee K.F., Lemanske R.F., Togias A. Comparison of the etiology of viral respiratory illnesses in inner-city and suburban infants. J Infect Dis. 2012;206:1342–1349. doi: 10.1093/infdis/jis504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Zalm M.M., Wilbrink B., van Ewijk B.E., Overduin P., Wolfs T.F., van der Ent C.K. Highly frequent infections with human rhinovirus in healthy young children: a longitudinal cohort study. J Clin Virol. 2011;52:317–320. doi: 10.1016/j.jcv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Chonmaitree T., Alvarez-Fernandez P., Jennings K., Trujillo R., Marom T., Loeffelholz M.J. Symptomatic and asymptomatic respiratory viral infections in the first year of life: association with acute otitis media development. Clin Infect Dis. 2015;60:1–9. doi: 10.1093/cid/ciu714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmerman R.K., Rinaldo C.R., Nowalk M.P., Gk B., Thompson M.G., Moehling K.K. Influenza and other respiratory virus infections in outpatients with medically attended acute respiratory infection during the 2011-12 influenza season. Influenza Other Respir Viruses. 2014;8:397–405. doi: 10.1111/irv.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dingle J., Badjer G., Jordan W. Western Reserve University; Cleveland, OH: 1964. Illness in the Home: A study of 25,000 illnesses in a group of Cleveland families. [Google Scholar]
- 17.Kusel M.M., de Klerk N.H., Holt P.G., Kebadze T., Johnston S.L., Sly P.D. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 18.Olenec J.P., Kim W.K., Lee W.M., Vang F., Pappas T.E., Salazar L.E. Weekly monitoring of children with asthma for infections and illness during common cold seasons. J Allergy Clin Immunol. 2010;125:1001–1006.e1. doi: 10.1016/j.jaci.2010.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chonmaitree T., Revai K., Grady J.J., Clos A., Patel J.A., Nair S. Viral upper respiratory tract infection and otitis media complication in young children. Clin Infect Dis. 2008;46:815–823. doi: 10.1086/528685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byington C.L., Ampofo K., Stockmann C., Adler F.R., Herbener A., Miller T. Community surveillance of respiratory viruses among families in the Utah better identification of germs-longitudinal viral epidemiology (BIG-LoVE) Study. Clin Infect Dis. 2015;61:1217–1224. doi: 10.1093/cid/civ486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald E., Nash D., Eickhoff J. Effectiveness of Amoxicillin-clavulanate potassium in the treatment of acute bacterial sinusitis in children: a double-blind, placebo-controlled trial. Pediatrics. 2009;124:9–15. doi: 10.1542/peds.2008-2902. [DOI] [PubMed] [Google Scholar]
- 22.Wald E.R., Applegate K.E., Bordley C., Darrow D.H., Glode M.P., Marcy S.M. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132:e262–e280. doi: 10.1542/peds.2013-1071. [DOI] [PubMed] [Google Scholar]
- 23.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bochkov Y.A., Grindle K., Vang F., Evans M.D., Gern J.E. Improved molecular typing assay for rhinovirus species A, B, and C. J Clin Microbiol. 2014;52:2461–2471. doi: 10.1128/JCM.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wald E.R., Guerra N., Byers C. Upper respiratory tract infections in young children: duration of and frequency of complications. Pediatrics. 1991;87:129–133. [PubMed] [Google Scholar]
- 26.Ueda D., Yoto Y. The ten-day mark as a practical diagnostic approach for acute paranasal sinusitis in children. Pediatr Infect Dis J. 1996;15:576–579. doi: 10.1097/00006454-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Kakish K.S., Mahafza T., Batieha A., Ekteish F., Daoud A. Clinical sinusitis in children attending primary care centers. Pediatr Infect Dis J. 2000;19:1071–1074. doi: 10.1097/00006454-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Aitken M., Taylor J.A. Prevalence of clinical sinusitis in young children followed up by primary care pediatricians. Arch Pediatr Adolesc Med. 1998;152:244–248. doi: 10.1001/archpedi.152.3.244. [DOI] [PubMed] [Google Scholar]
- 29.Revai K., Dobbs L.A., Nair S., Patel J.A., Grady J.J., Chonmaitree T. Incidence of acute otitis media and sinusitis complicating upper respiratory tract infection: the effect of age. Pediatrics. 2007;119:e1408–e1412. doi: 10.1542/peds.2006-2881. [DOI] [PubMed] [Google Scholar]
- 30.Advani S., Sengupta A., Forman M., Valsamakis A., Milstone A.M. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. 2012;31:1221–1226. doi: 10.1097/INF.0b013e318265a804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–650. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 32.Loeffelholz M.J., Trujillo R., Pyles R.B., Miller A.L., Alvarez-Fernandez P., Pong D.L. Duration of rhinovirus shedding in the upper respiratory tract in the first year of life. Pediatrics. 2014;134:1144–1150. doi: 10.1542/peds.2014-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansen R.R., Wieringa J., Koekkoek S.M., Visser C.E., Pajkrt D., Molenkamp R. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–2636. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pappas D.E., Hendley J.O., Hayden F.G., Winther B. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J. 2008;27:8–11. doi: 10.1097/INF.0b013e31814847d9. [DOI] [PubMed] [Google Scholar]
- 35.Butler C.C., Hood K., Kinnersley P., Robling M., Prout H., Houston H. Predicting the clinical course of suspected acute viral upper respiratory tract infection in children. Fam Pract. 2005;22:92–95. doi: 10.1093/fampra/cmh713. [DOI] [PubMed] [Google Scholar]