Abstract
Objective
To determine the frequency of disease reclassification and to identify clinicopathologic variables associated with it in patients with favorable-risk prostate cancer undergoing active surveillance.
Patients and Methods
We assessed 191 men selected by what may be the most stringent criteria used in active surveillance studies yet conducted who enrolled in a prospective cohort active surveillance trial. Clinicopathologic characteristics were analyzed in a multivariate Cox proportional hazards regression model. Key features were an extended biopsy with a single core positive for Gleason score (GS) 3+3 (<3 mm) or 3+4 (<2 mm) and a prostate-specific antigen (PSA) level <4 ng/mL (adjusted for prostate volume). Biopsies were repeated every 1–2 years and clinical evaluations every 6 months. Disease was reclassified when PSA level increased 30% from baseline or when biopsy tumor length increased beyond the enrollment criteria, more than one positive core was detected, or any grade increased to a dominant 4 pattern or any 5 pattern.
Results
Disease was reclassified in 32 (16.8%) patients (median follow-up time among survivors, 3 years [interquartile range, 1.9–4.6 years]), including upgrading to GS 4+3 in 5 (2.6%). Overall, 13 of the 32 (40.6%) had incremental increases in GS. Tumor length (hazard ratio [HR], 2.95; 95% confidence interval [CI], 1.34–6.46; P=0.007) and older age (HR, 1.05; 95% CI, 1.00–1.09; P=0.05) were identified as, respectively, significant and marginally significant predictors of disease reclassification. Disease remained stable in 83.2% of patients.
Conclusion
Needs persist for improvements in risk stratification and predictive indicators of cancer progression.
Clinical trial information
Keywords: active surveillance, biopsy, prostate, prostate cancer, tumor, watchful waiting
Introduction
In the era of PSA screening in the United States, despite evidence supporting the use of active surveillance (AS), most men with low-risk prostate cancer receive curative treatment. As data emerge challenging this status quo interventional response [1, 2], the management paradigm is shifting away from immediate intervention toward active surveillance with curative treatment delayed until disease progression occurs [3]
The prospective AS study with the longest follow-up [4, 5], in general, including patients with mostly low-risk and some intermediate-risk prostate cancer, demonstrated the feasibility of AS. Application of AS to a wider cohort of men with early stage prostate cancer, however, is limited by currently available clinicopathologic tools (Gleason grading and prostate-specific antigen level). As shown by surgical series of low-risk prostate cancer cases selected for immediate surgical intervention, about a third of men are found to have radical prostatectomy specimen pathology characterized by increased disease volume, grade, and disease extension beyond the prostate [6, 7]. Additionally, prostate cancer’s widely variable heterogeneous biology and the absence of a clear definition of early progression remain unmet challenges in managing early stage prostate cancer by active surveillance. Aggregate studies [8, 9] have demonstrated three central and evolving concepts integral to AS, including selection criteria, surveillance interval and tools, and the trigger (or triggers) for treatment recommendation.
In 2006, to test the hypothesis that a systematic strategy of AS could shelter patients from unnecessary intervention yet detect tumor progression when disease remained curable, we initiated a multiyear prospective cohort study. Our aim was to determine the rate of disease reclassification of clinically organ-confined prostate cancer, including the most stringently selected favorable-risk cases, and compare it to rates in other AS series [8, 10].
PATIENTS AND METHODS
This single-institution prospective cohort study, conducted by a multidisciplinary team of urologic surgeons, radiation oncologists, and medical oncologists, was approved by The University of Texas MD Anderson Cancer Center (MDACC) Institutional Review Board. All patients signed an informed consent.
Patients with early stage prostate cancer were stratified to AS groups I (favorable risk), II (patient’s choice), or III (therapy prevented by comorbidities). For the favorable-risk group, inclusion criteria, based on previous work at MDACC that established clinical predictors of LV/LG disease [11], required that patients have a biopsy no more than 6 months before enrollment of ≥10 cores showing either a 3+3 Gleason score (GS) in one core (tumor focus, <3.0 mm) or a 3+4 GS in one core (tumor focus, <2.0 mm). The study entry PSA had to be <4 ng/mL or adjusted for volume [12]. Patients with a 3+3 GS or 3+4/4+3 GS, not meeting the criteria for group I were considered group II. Patients in group III had comorbidities precluding local therapy, as determined by the managing physician. In June 2007, the protocol was amended to include a confirmatory biopsy onsite at enrollment or within six months of diagnosis, unless MDACC performed the diagnostic biopsy.
Patients were evaluated at baseline and every 6 months by clinical examination (digital rectal examination) and laboratory studies (serum PSA, testosterone). Additionally, information on concomitant medications, including 5α-reductase inhibitors, was recorded as was body mass index (BMI) at each follow-up. All biopsies were performed with the transrectal ultrasound-guided technique using the 11-core multisite-directed biopsy scheme (sextant locations, one posterior midline, and two each laterally directed and designated as left and right anterior horn) [13]. Prostate biopsies were repeated every 1–2 years; if the biopsy was negative, then the following year’s biopsy was omitted, unless requested by the patient.
Disease was reclassified when change in biopsy findings or PSA level was significant. The threshold for a significant change was detecting an increase in tumor length in a positive core beyond study entry criteria, more than one positive core at biopsy, or an upgrade to a dominant Gleason grade 4 or any Gleason grade 5 component. If the location of disease on repeat biopsy (including contralateral location) otherwise met criteria, it was not reclassified. The threshold for a significant change in PSA level was finding a >30% increase, which had to be confirmed within a month of laboratory results or three months after biopsy.
Patients who declined repeat prostate biopsies were allowed to remain on protocol, provided their treating physician agreed, and were included in the analysis as not having had disease reclassified. Since patients’ compliance with long-term surveillance was also of interest, patients were allowed to remain on protocol unless they requested to go off, met the criteria for disease reclassification, or were diagnosed with a second malignancy.
PSA Metrics During AS
PSA velocity (PSAV) was computed by fitting a linear regression model on PSA values over time. The American Urological Association [14] suggests that a correct measurement of PSAV requires at least three PSA measurements after diagnosis over at least 18 months. PSA doubling time (PSADT) was calculated by regressing the ln(PSA) over time to obtain the slope (m), and the PSADT was defined as ln(2)/m.
Statistical Methods
Patient characteristics were summarized using median and range for continuous variables and frequency with percentage for categorical variables. Disease reclassification was defined by prespecified pathologic criteria and/or an increase in PSA value, and an unadjusted disease reclassification probability was estimated [15]. A log-rank test was performed to compare the time to disease reclassification between patients with and without delayed treatment. Cox proportional hazards models [16] were fit to assess the association between disease reclassification and baseline patient characteristics, including age, race, BMI, clinical stage, total PSA, testosterone level, total prostate volume by transrectal ultrasound, tumor length, and first-degree family history. PSA density (PSAD) was excluded from this model because in our unique cohort with stringent criterion for PSA, it was highly correlated with PSA and tumor length. However, in several other AS series, PSAD has been shown to be an important predictor of AS biopsy disease reclassification [17].
P values < 0.05 were deemed statistically significant. All analyses were performed in S-PLUS (TiBCO Spotfire S+ S.2 for Windows, Palo Alto, CA).
RESULTS
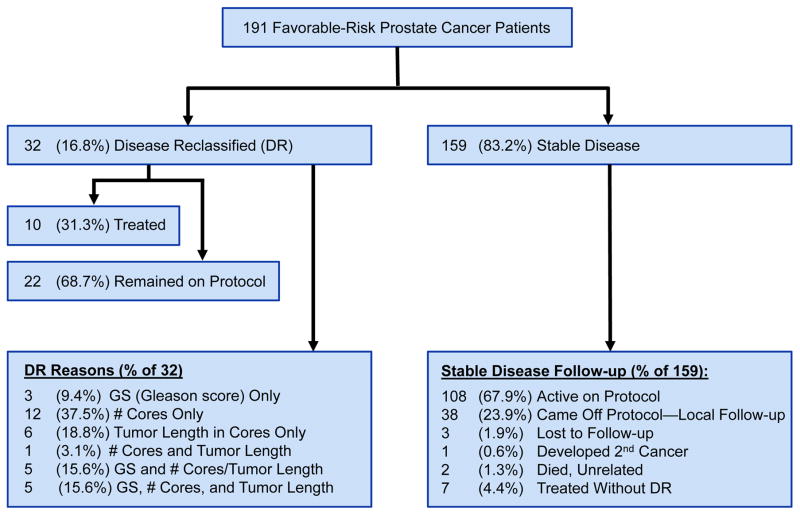
Between February 2006 and February 2012, 575 men were enrolled and one patient was deemed ineligible: 191 men entered group I; 369, group II; and 14, group III. We describe results for those in group I, all of whom had at least 6 months of follow-up. Among survivors, the median follow-up time was 3 years (interquartile range, 1.9–4.6 years), and the mean follow-up time was 3.2 years (Fig. 1 and Table 1). Of 191 patients, 150 enrolled after confirmatory biopsy onsite became mandatory at study entry or within 6 months of diagnosis, including 23 whose diagnostic extended biopsy was performed at MDACC within 6 months of study registration. Of the 41 enrolled beforehand, 15 underwent rebiopsy prior to study entry per treating physicians’ preference. Based on the stratification criteria for group I, patients were required to have no tumor on rebiopsy prior to study entry or confirmatory biopsy. There was no significant difference in the rate of disease reclassification between the patients who enrolled before and after confirmatory biopsy became mandatory (data not shown).
Fig. 1.
Disposition of cohort in active surveillance: patients with favorable-risk prostate cancer (Gleason score ≤6 [3 + 3] with no more than 1 positive biopsy core [tumor, <3 mm] or Gleason score 7 [3 + 4] with no more than 1 positive biopsy core [tumor, <2 mm] and PSA level <4 ng/mL).
Table 1.
Baseline demographic and clinical characteristics and surveillance PSA metrics of study participants in all patients and by reclassification status.
| Variable | All patients | No reclassification | Reclassification | P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| No. of patients | 191 | 100 | 159 | 100 | 32 | 100 | |
| Age (years) | 64 (57–69) | 63 (57–69) | 65.5 (59.5–70) | 0.18 | |||
| Race or ethnicity (n = 190) | 0.36 | ||||||
| White | 155 | 81.6 | 131 | 84.5 | 24 | 15.5 | |
| Black | 17 | 8.9 | 15 | 88.2 | 2 | 11.8 | |
| Hispanic | 15 | 7.9 | 11 | 73.3 | 4 | 26.7 | |
| Asian | 3 | 1.6 | 2 | 66.7 | 1 | 33.3 | |
| Body mass index | 29.1 (26.1–31.8) | 29.2 (26.2–31.8) | 27.9 (25.1–32.6) | 0.5 | |||
| PSA level (ng/mL) | |||||||
| PSA | 3.3 ( 2.2–4.3) | 3.3 (2.1–4.4) | 3 (2.4–4.2) | 0.68 | |||
| PSA density | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.1 (0.1–0.1) | 0.67 | |||
| PSA velocity (n = 124) | 0.1 (−0.1–0.6) | 0.1 (−0.2–0.5) | 0.4 (0–0.7) | 0.04 | |||
| PSA doubling time (n = 124) | 3.4 (−3.4–7.2) | 2.7 (−4–7.1) | 4.5 (1.4–7.7) | 0.34 | |||
| Testosterone (ng/dL) | 379 (295–479) | 379 (290–469) | 389 (323–494) | 0.44 | |||
| Transrectal ultrasound, total (mL) | 43.9 (29.7–56.3) | 44 (29.5–57.5) | 43.5 (33.6–53.2) | 0.81 | |||
| Gleason score | 1.00 | ||||||
| 5* | 1 | 0.5 | 1 | 100 | — | — | |
| 6 | 189 | 99 | 157 | 83.1 | 32 | 16.9 | |
| 7 | 1 | 0.5 | 1 | 100 | — | — | |
| Clinical stage | 0.86 | ||||||
| cT1a* | 5 | 2.6 | 4 | 80 | 1 | 20 | |
| cT1c | 164 | 85.9 | 137 | 83.5 | 27 | 16.5 | |
| cT2a | 20 | 10.5 | 16 | 80 | 4 | 20 | |
| cT2b | 1 | 0.5 | 1 | 100 | — | — | |
| cT2c | 1 | 0.5 | 1 | 100 | — | — | |
| Number of surveillance biopsies (n = 183) | <.0001 | ||||||
| 0 | 33 | 18 | 33 | 100 | — | — | |
| 1 | 72 | 39.3 | 62 | 86.1 | 10 | 13.9 | |
| 2 | 44 | 24 | 37 | 84.1 | 7 | 15.9 | |
| 3 | 25 | 13.7 | 17 | 68 | 8 | 32 | |
| 4 | 8 | 4.4 | 2 | 25 | 6 | 75 | |
| 5 | 1 | 0.5 | — | — | 1 | 100 | |
| Tumor length (mm) (n = 185) | 0.03 | ||||||
| < 0.5 | 10 | 5.4 | 8 | 80 | 2 | 20 | |
| 0.5–< 1 | 84 | 45.4 | 77 | 91.7 | 7 | 8.3 | |
| 1–< 2 | 68 | 36.8 | 52 | 76.5 | 16 | 23.5 | |
| 2–< 3 | 23 | 12.4 | 17 | 73.9 | 6 | 26.1 | |
| 5-alpha-reductase inhibitor: current or history of treatment | 0.3 | ||||||
| No | 159 | 83.2 | 130 | 81.8 | 29 | 18.2 | |
| Yes | 32 | 16.8 | 29 | 90.6 | 3 | 9.4 | |
| Family history, first degree (n = 190) | 0.66 | ||||||
| No | 139 | 73.2 | 114 | 82 | 25 | 18 | |
| Yes | 51 | 26.8 | 44 | 86.3 | 7 | 13.7 | |
| Family history, second degree (n = 190) | 0.29 | ||||||
| No | 161 | 84.3 | 136 | 84.5 | 25 | 15.5 | |
| Yes | 30 | 15.7 | 23 | 76.7 | 7 | 23.3 | |
Disease reclassification occurred in 32 (16.8%) patients, exclusively driven by repeat biopsy findings. The 3-year and 5-year freedom from the reclassification rate was 82.3% (95% CI: 76.2%–88.8%) and 69.8% (95% CI: 59.4%–82.2%), respectively. Disease reclassification was documented at first occurrence on repeat biopsy. Of the 32 patients with reclassified disease, 19 (59.4%) had increases in tumor volume, including 12 (37.5%) who had an increase in the number of cores with tumor, 6 (18.8%) who had increases in tumor length, and 1 (3.1%) who had both. Of the remaining 13 (40.6%), 3 (9.4%) had increases in tumor grade only, and 10 (31.3%) had increases in tumor grade plus increases in tumor volume (tumor length and/or tumor positive cores). All 13 increases in tumor grade were to GS 7: 3+4, n=8; 4+3, n=5. Therefore, although the overall rate of disease reclassification was 16.8%, the rate of biologically concerning disease reclassification occurred in 5 (2.6%), that is, in those cases in which GS was upgraded to 4+3.
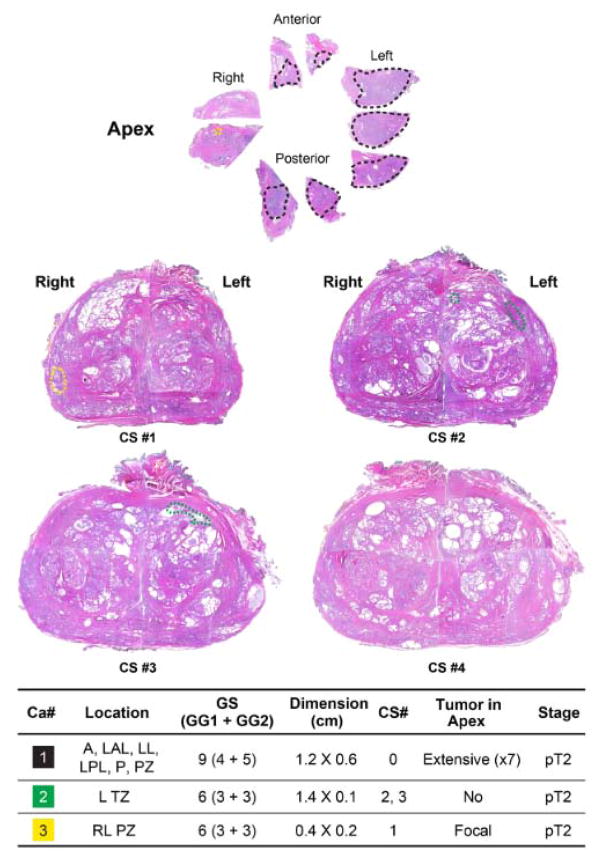
Four of 10 patients treated after disease reclassification underwent radical prostatectomy. One had pT3a GS 4+3 with a focal positive margin, two had organ-confined GS 7 (one 3+4 and the other 4+3), and one was further upgraded to organ-confined GS 4+5. The last of the four had a GS 6 tumor (≤1-mm focus in one core biopsy) at study entry, had reclassification of tumor on repeat biopsy at year 5 to GS 4+3 (4.5-mm focus in one core biopsy), and had further upgrading at prostatectomy to organ-confined GS 4+5. The tumor was at the apex (Fig. 2). All patients are disease free with limited follow-up. Two patients without disease reclassification who opted for radical prostatectomy had organ-confined GS 3+3 disease. Potential predictors of disease reclassification included higher PSA velocity (P=0.04), increasing number of repeat surveillance biopsies (P<0.0001), and increasing tumor length (P=0.03) (Table 2). In a multivariable Cox proportional hazards regression model in which PSA kinetics and PSAD were excluded, tumor length (hazard ratio [HR], 2.95; 95% confidence interval [CI], 1.34–6.46; P=0.007) and age at study enrollment (HR, 1.05; 95% CI: 1.00–1.09; P=0.05) remained significant or marginally significant (Table 3).
Fig. 2.
Cross-sectional analysis of the prostatectomy specimen of a patient who had Gleason 6 tumor at study entry, had reclassification of tumor on repeat biopsy at year 5 to Gleason 4 + 3, and had a further upgrade at prostatectomy to organ-confined Gleason 4 + 5 tumor in the apex. The apex and the four cross sections (CS#1-CS#4) are depicted. Three tumor foci (two in the peripheral zone and one in the transition zone) are indicated with black, yellow, and green dots). All tumor foci are confined to the prostate. The location and Gleason score/grades and dimensions for each tumor focus is listed at the bottom. (A = anterior; LAL = left anterior lateral; LL = left lateral; LPL = left posterolateral; P = posterior; PZ = peripheral zone; L = left; TZ = transition zone; RL = right lateral; GS = Gleason score; GG = Gleason grade; Ca#1 = Gleason 4 + 5 tumor at the apex.)
Table 2.
Univariate and multivariable Cox proportional hazards regression model for disease reclassification.
| Univariate Cox Models | Multivariable Cox Model | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% Confidence Interval | P | Hazard ratio | 95% Confidence Interval | P | |
| Age at registration | 1.04 | 0.99 –1.08 | 0.12 | 1.05 | 1.00–1.09 | 0.05 |
| Race | ||||||
| Black (vs. White) | 1.97 | 0.27 – 14.65 | 0.51 | |||
| Hispanic (vs. White) | 0.79 | 0.19 – 3.35 | 0.75 | |||
| Asian (vs. White) | 2.03 | 0.70 – 5.86 | 0.19 | |||
| BMI | 0.98 | 0.90 – 1.07 | 0.61 | |||
| Clinical stage (cT2 vs. cT1) | 0.84 | 0.12 – 6.18 | 0.87 | |||
| Tumor length of ≥1 mm (vs. < 1 mm) | 2.80 | 1.29 – 6.08 | 0.009 | 2.95 | 1.34–6.46 | 0.007 |
| PSA—total | 1.01 | 0.81 – 1.26 | 0.95 | 0.94 | 0.75–1.19 | 0.62 |
| Testosterone level/100 | 1.07 | 0.86 – 1.33 | 0.56 | |||
| Transrectal ultrasound(total) | 1.00 | 0.98 – 1.01 | 0.60 | |||
| First–degree family history—Yes (vs. No) | 0.86 | 0.37 – 1.99 | 0.72 | 0.75 | 0.32–1.76 | 0.5 |
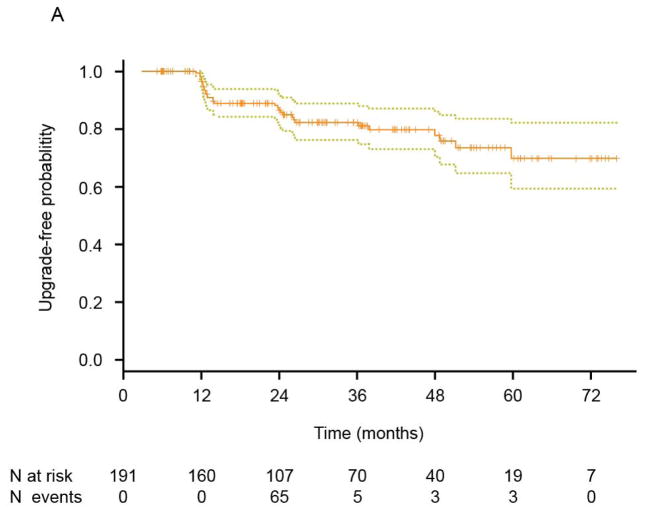
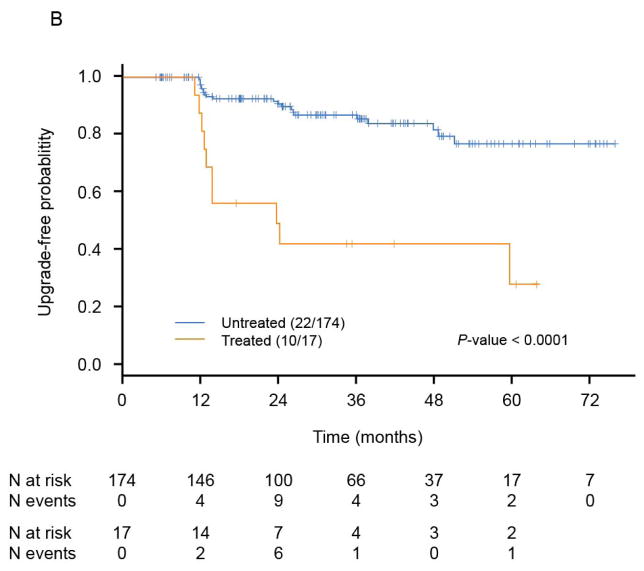
The time to disease reclassification was estimated by the Kaplan-Meier method and has been depicted for all patients (Fig. 3A) and by treatment status (Fig. 3B). Among all 191 patients, the median time to disease reclassification had not been reached after a median follow-up time of 3 years (Fig. 3A). The 3-year freedom from disease reclassification rate was 82.3% (95% CI: 76.2%–88.8%). Among the 17 treated patients, 10 (58.8%) experienced disease reclassification, and the median time to reclassification was 23.7 months (95% CI: 12.9 to an unestimable value). Among the 174 untreated patients, only 22 (12.6%) had disease that was reclassified, and the median time to reclassification had not been reached. There was a significant difference between these two groups in terms of the median time to disease reclassification (P<0.0001, log-rank test, Fig. 3B).
Fig. 3.
Kaplan-Meier estimates. Kaplan-Meier estimates for time to disease reclassification by the Kaplan-Meier method are illustrated in A and estimates for time to disease reclassification by treatment status are illustrated in B (patients untreated after reclassification = 22; patients treated after reclassification = 10).
Reclassification for patients was not based in any case on an increase in serum PSA level. PSAV and PSADT measurements using baseline and at least three PSA follow-up values over at least 18 months from baseline was feasible in 124 (64.9%) of 191 patients. The median PSAV was 0.1 (range, −2.0–8.4). The median PSADT was 3.4 years (PSA range, −67.9–351). As the negative values in the ranges indicate, some patients’ PSA levels decreased after starting AS.
DISCUSSION
Among 191 men with favorable-risk prostate cancer who met stringent criteria as compared to any other series for AS, 32 (16.8%) were reclassified at a median follow-up of 3 years, and 5 (2.6%) had biologically concerning upgrading to GS 4+3. Biopsy findings determined reclassification, most often by incremental changes in GS and/or length. Of 32 patients with reclassified disease, 10 (31.3%) chose treatment; the remainder continued surveillance. Three patients undergoing subsequent radical prostatectomy had findings of high-grade disease (GS, ≥7 [4+3]), two had confined disease, and the other had nonconfined disease.
PSA level, which was included in our protocol based on 2005 knowledge, had minimal impact on outcomes. To more specifically delineate each case, we adjusted PSA by prostate volume. In a previous study by our group, we had derived a nomogram predicting low-volume/low-grade (LV/LG) prostate cancer based on findings from 258 men with untreated early stage prostate cancer who underwent radical prostatectomy. We found age, PSA density, and tumor length in a biopsy core to be predictive of LV/LG cancer [18]. We adjusted PSA by simply multiplying the total prostate volume on transrectal ultrasound by a factor of 0.12 [12]. Among the total 191 patients, 63 had PSA ≥ 4 ng/mL, and among the 32 patients who had disease reclassification, 9 had a PSA value ≥ 4 ng/mL. Given that none of the cases reclassified were reclassified because of the preset PSA criterion, this attempt to refine PSA’s value had no effect on the conclusion of the study. Other authors have found similar limitations in correlating PSA with adverse pathology and/or triggers for intervention [5, 19, 20].
Our observations suggest that even with stringent AS selection criteria and frequent monitoring, inherent limitations of the tools available may allow biologically significant cases to go undetected. Our study did not demonstrate a zero risk of biologically concerning disease reclassification. With biopsy, that rate was 2.6%; with prostatectomy, it was 1%—a rate confounded by exclusion of treatments other than prostatectomy at disease reclassification. These rates are certainly low, but the exact interpretation for key decision points will vary by patients and physicians (a young patient may view such odds as too risky; an older patient, as very favorable).
The observed low incidence of higher-grade disease in our cohort suggests two interesting areas for future research and discussion. First, our cohort should have less occult high-grade disease than any similar population that is unbiopsied. For example, in the placebo arm of the Prostate Cancer Prevention Trial, 4,888 men with a PSA level ≤ 4 ng/mL who underwent biopsy were observed to have high-grade prostate cancer incidence of 3.2% [21]. Yet the biologic significance of upgrading in this multibiopsied cohort remains unclear, in part, because so many upgraded patients are ultimately treated. Recently Hussein et al. [22] reported on a cohort of active surveillance patients who were upgraded (3+4 or greater) on follow-up biopsy, and they found that 69% were treated with radical prostatectomy; however, among men who deferred treatment further, only 6% were later upgraded and 34% downgraded. Significant follow-up will be required to correlate the positive predictive value of upgrading to metastatic progression and cancer-specific mortality, and we should keep an open mind to the possibility that the biology of these patients is different (favorable) from that of men with higher grade disease initially undergoing biopsy.
The study design reflects knowledge about AS in 2005. During protocol design, it was well known that PSA screening led to increased prostate cancer detection; but no consensus existed, and no evidence linked it with a decline in mortality [23]. Therefore, the hypothesis that PSA screening led to overdetection of clinically insignificant tumors was widely accepted, but how to segregate tumors using clinically available testing (staging, biopsy, PSA) remained challenging. Albertsen et al. [24] demonstrated a very small risk of prostate cancer disease-specific mortality among men with low-grade disease, even after 20 years of follow-up.
Two prospective series were reported [4, 25] that specifically studied PSA-era patients selected for AS with predefined inclusion, monitoring, and reclassification (IMR). However, these studies demonstrate the difficulty in making advances when studies use different criteria. The Carter et al. [25] series emphasized yearly biopsies and minimal use of PSA metrics; the Klotz et al. [4] series emphasized PSA metrics but biopsies only every 3 to 4 years. Guidelines developed in this era [20] considered AS a standard option for all risk categories of clinically localized disease, but they did not specify parameters for IMR. Therefore, the stringent criteria used are both a strength and limitation in this study, as is the avoidance of protocol updates (other than entry repeat biopsy) during study follow-up. This protocol is meant to address a specific question in a small cohort of patients, whereas other series are meant to be for larger cohorts of patients, and they, therefore, evolve techniques over time [5]. The Klotz series [5], for example, contained multiple methodology updates after its 1995 inception: 2000, biopsy inclusion criteria changed; 2003, mandatory informed consent discontinued; 2009, PSA discontinued as sole trigger for intervention. A separate arm of our surveillance program will report on the outcomes of men with more heterogeneous entry criteria.
For our series to achieve the goal of defining a favorable-risk cohort, we utilized internal studies that sought to predict, using the criteria of Epstein et al. [26] levels of insignificant cancer from pretreatment factors [11]. Thus, patients with one core of GS 3+3 <3 mm or 3+4 <2 mm had the highest likelihood of harboring a total tumor volume of <0.5 mL3. In 2010, we reported a retrospective study of radical prostatectomies that met these same criteria, except PSA was <10 ng/mL [27]. Of 66 cases studied, 6% were upgraded to GS 4+3, and all were organ confined. Total tumor volume was <0.5 mL3 in 71% of cases, but in undersampled disease, the transition zone was a common location. We found that even subtle changes in inclusion criteria, such as those identified by Griffin et al. [28] and Chun et al. [29], were associated at radical prostatectomy with significant increases in extraprostatic extension and upgrading.
By contrast, the series of Carter et al. [25] showed 31% met criteria at a median follow-up of 1.2 years. In the Klotz et al. [4] series, inclusion criteria were much more liberal in comparison to those of the current study (cT2b or lower, PSA <15 ng/mL, and GS ≤7). Monitoring included a schedule of PSA and repeat biopsies, and thresholds for included PSA metrics such as a PSADT of less than 2 years, clinical progression, or histologic progression to a GS ≥8. At a median follow-up of 4.6 years, 60% remained on surveillance, and disease-specific survival was 97.2%. AS programs under way in North America, using variable IMR, have reported that 14%–41% of men move from surveillance to active treatment [30]. At least six additional series with defined IMR [10] [30–34] and two recent high-profile reviews [8], [9] compare differences in IMR and outcomes. Mandatory repeat biopsy onsite at study entry or within six months of diagnosis, in addition to stringent selection criteria, may be responsible for our study’s low rate of disease reclassification. This requirement often reveals higher volume or grade of disease that could significantly alter the appropriateness of surveillance. In screening after the protocol amendment requiring confirmatory biopsy, we observed approximately 15% disease reclassification (data not shown). Adamy et al. [34] demonstrated the importance of a confirmatory biopsy in 531 low-risk cases: 35% were excluded.
In the Klotz et al. [4] series, only five patients died of prostate cancer after initial surveillance and, in the authors’ opinion, had lethal disease at the outset [35]. Some did not pursue early curative therapy when disease was reclassified to high risk. Three other series confirmed this conclusion [30, 36, 37], indicating that errors in diagnosis are unlikely to lead to early mortality. In a 2015 report covering 15 years of follow-up, Klotz et al. [5] found that 2.8% of men in the study were diagnosed with metastatic disease and 1.5% had died of prostate cancer. In their study, men were 9.2 times more likely to die of other causes than they were of prostate cancer. Given such a low rate of mortality in patients undergoing AS, even when the most liberal criteria for selection are used [4], the number of men considered candidates for AS should be increased. Our study represents the other end of the spectrum in using the most stringent criteria to help define a framework for AS, but the important question of how liberal patient selection criteria for AS may be without compromising patient safety remains unanswered.
A significant burden rests on the patient in AS who undergoes tests and biopsies repeatedly. We found increasing tumor length to be predictive and older age to be marginally predictive of disease reclassification. Others also have shown older age to be predictive of pathological progression [38], and higher risk of upgrading and upstaging in men who met AS criteria undergoing prostatectomy [39]. Given that prostate carcinogenesis and progression represent a multistep and multiyear process, older men harboring small indolent tumors may find with time the tumors become clinically significant. It is of note that in our unique cohort in which the majority of patients (78.5% using the standard extended biopsy scheme) had negative confirmatory biopsy within six months of study entry, the extent of tumor on diagnostic biopsy was still predictive of disease reclassification. Biopsies themselves carry an infection risk, and some studies have raised the concern of a biopsy-related decline in sexual function [40, 41]. Urgently needed is a more rational approach to biopsy frequency and triggers for treatment, especially in patients with LV/LG disease. Researchers are hoping the Prostate Testing for Cancer Treatment (ProtecT) trial, a phase III study comparing active monitoring, radiotherapy, and radical prostatectomy, reveals answers about early diagnosis and active treatment in LV/LG disease [42]. Results are expected in 2016.
Upstream of the overdiagnosis and overtreatment dilemma, improved screening methods are needed to indicate when LV/LG disease could be safely ignored. We remain confident that improvement in accuracy of risk assessment and determination of biological potential of the disease based on integration of imaging and biomarkers will one day reduce or eliminate the uncertainty in prostate cancer AS that today prompts unnecessary therapy with adverse effects on quality of life. Finally, we can suggest from our study and from aggregate literature [43] that when patients approach ages 75–80 years and beyond with disease characteristics matching our stringent criteria that monitoring and reclassification thresholds be reduced to a plan more closely resembling traditional watchful waiting.
Acknowledgments
We thank all of the men who participated in this study.
Funding/Support and role of the sponsor: This study was supported in part by Cancer Center Core Grant (P30CA 16672) from The University of Texas MD Anderson Cancer Center.
Abbreviations
- PSA
prostate-specific antigen
Footnotes
Presented at the Forty-ninth Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 31 to June 4, 2013.
Author contributions: Jeri Kim had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kim, Davis, Logothetis.
Acquisition of data: Kim, Achim, Troncoso.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: All authors.
Critical revision of the manuscript for important intellectual content: All authors.
Final approval of the manuscript: All authors.
Statistical analysis: Wang.
Obtaining funding: Kim, Davis, Logothetis.
Administrative support: Kim, Davis, Troncoso, Logothetis.
Supervision: Kim.
Other (specify): None.
Financial disclosures: Jeri Kim certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (e.g., employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending) are the following: all authors report no financial disclosures or conflicts of interest.
References
- 1.Moyer VA U S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012 Jul 17;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012 Jul 19;367:203–13. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangma CH, Valdagni R, Carroll PR, van Poppel H, Klotz L, Hugosson J. Active Surveillance for Low-risk Prostate Cancer: Developments to Date. Eur Urol. 2015 Apr;67:646–8. doi: 10.1016/j.eururo.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010 Jan 1;28:126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 5.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015 Jan 20;33:272–7. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 6.Ploussard G, Salomon L, Xylinas E, et al. Pathological findings and prostate specific antigen outcomes after radical prostatectomy in men eligible for active surveillance--does the risk of misclassification vary according to biopsy criteria? J Urol. 2010 Feb;183:539–44. doi: 10.1016/j.juro.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro RH, Johnstone PA. Risk of Gleason grade inaccuracies in prostate cancer patients eligible for active surveillance. Urology. 2012 Sep;80:661–6. doi: 10.1016/j.urology.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: Progress and promise. J Clin Oncol. 2011 Sep 20;29:3669–76. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 9.Dall’Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: A systematic review of the literature. Eur Urol. 2012 Dec;62:976–83. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 10.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013 Apr;63:597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Ochiai A, Troncoso P, Chen ME, Lloreta J, Babaian RJ. The relationship between tumor volume and the number of positive cores in men undergoing multisite extended biopsy: Implication for expectant management. J Urol. 2005 Dec;174:2164–8. doi: 10.1097/01.ju.0000181211.49267.43. discussion 8. [DOI] [PubMed] [Google Scholar]
- 12.Babaian RJ, Miyashita H, Evans RB, Ramirez EI. The distribution of prostate specific antigen in men without clinical or pathological evidence of prostate cancer: relationship to gland volume and age. J Urol. 1992 Mar;147:837–40. doi: 10.1016/s0022-5347(17)37400-1. [DOI] [PubMed] [Google Scholar]
- 13.Babaian RJ, Toi A, Kamoi K, et al. A comparative analysis of sextant and an extended 11-core multisite directed biopsy strategy. J Urol. 2000 Jan;163:152–7. [PubMed] [Google Scholar]
- 14.American Urological Association. PSA Testing for the Pretreatment Staging and Posttreatment Management of Prostate Cancer: 2013 Revision of 2009 Best Practice Statement. Linthicum, MD: American Urological Association; [Accessed 25 November 2013]. 2013. at http://www.auanet.org/education/guidelines/prostate-specific-antigen.cfm. [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J Royal Stat Soc (Series B—Methodological) 1972;34:187–220. [Google Scholar]
- 17.Loeb S, Bruinsma SM, Nicholson J, et al. Active Surveillance for Prostate Cancer: A Systematic Review of Clinicopathologic Variables and Biomarkers for Risk Stratification. Eur Urol. 2015 Apr;67:619–26. doi: 10.1016/j.eururo.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi H, Wang X, Ochiai A, et al. A nomogram for predicting low-volume/low-grade prostate cancer: a tool in selecting patients for active surveillance. Cancer. 2007 Dec 1;110:2441–7. doi: 10.1002/cncr.23055. [DOI] [PubMed] [Google Scholar]
- 19.Vickers AJ, Savage C, O’Brien MF, Lilja H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009 Jan 20;27:398–403. doi: 10.1200/JCO.2008.18.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loblaw A, Zhang L, Lam A, et al. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J Urol. 2010 Nov;184:1942–6. doi: 10.1016/j.juro.2010.06.101. [DOI] [PubMed] [Google Scholar]
- 21.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004 May 27;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 22.Hussein AA, Welty CJ, Ameli N, et al. Untreated Gleason Grade Progression on Serial Biopsies during Prostate Cancer Active Surveillance: Clinical Course and Pathological Outcomes. J Urol. 2015 Jan 23; doi: 10.1016/j.juro.2015.01.077. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer—Part I: Evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999 Jun 16;91:1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 24.Albertsen PC, Hanley JA, Fine J. Twenty-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005 May 4;293:2095–101. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 25.Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: Preliminary results. J Urol. 2002 Mar;167:1231–4. [PubMed] [Google Scholar]
- 26.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994 Feb 2;271:368–74. [PubMed] [Google Scholar]
- 27.Davis JW, Kim J, Ward JF, et al. Radical prostatectomy findings in patients predicted to have low-volume/low-grade prostate cancer diagnosed by extended-core biopsies: An analysis of volume and zonal distribution of tumour foci. BJU Int. 2010 May;105:1386–91. doi: 10.1111/j.1464-410X.2009.08964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin CR, Yu X, Loeb S, et al. Pathological features after radical prostatectomy in potential candidates for active monitoring. J Urol. 2007 Sep;178:860–3. doi: 10.1016/j.juro.2007.05.016. discussion 3. [DOI] [PubMed] [Google Scholar]
- 29.Chun FK, Suardi N, Capitanio U, et al. Assessment of pathological prostate cancer characteristics in men with favorable biopsy features on predominantly sextant biopsy. Eur Urol. 2009 Mar;55:617–28. doi: 10.1016/j.eururo.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 30.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. 2011 Jan 10;29:228–34. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008 Dec;54:1297–305. doi: 10.1016/j.eururo.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 32.Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008 Jan;101:165–9. doi: 10.1111/j.1464-410X.2007.07190.x. [DOI] [PubMed] [Google Scholar]
- 33.van den Bergh RC, Roemeling S, Roobol MJ, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2009 Jan;55:1–8. doi: 10.1016/j.eururo.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011 Feb;185:477–82. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010 Jul;184:131–5. doi: 10.1016/j.juro.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 36.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: An update of the Johns Hopkins experience. J Clin Oncol. 2011 Jun 1;29:2185–90. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 37.Dall’Era MA, Cowan JE, Simko J, et al. Surgical management after active surveillance for low-risk prostate cancer: Pathological outcomes compared with men undergoing immediate treatment. BJU Int. 2011 Apr;107:1232–7. doi: 10.1111/j.1464-410X.2010.09589.x. [DOI] [PubMed] [Google Scholar]
- 38.Margel D, Nandy I, Wilson TH, Castro R, Fleshner N. Predictors of pathological progression among men with localized prostate cancer undergoing active surveillance: a sub-analysis of the REDEEM study. J Urol. 2013 Dec;190:2039–45. doi: 10.1016/j.juro.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 39.Busch J, Magheli A, Leva N, et al. Higher rates of upgrading and upstaging in older patients undergoing radical prostatectomy and qualifying for active surveillance. BJU Int. 2014 Oct;114:517–21. doi: 10.1111/bju.12466. [DOI] [PubMed] [Google Scholar]
- 40.Ehdaie B, Vertosick E, Spaliviero M, et al. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol. 2014 Mar;191:660–4. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 41.Punnen S, Cowan JE, Dunn LB, Shumay DM, Carroll PR, Cooperberg MR. A longitudinal study of anxiety, depression and distress as predictors of sexual and urinary quality of life in men with prostate cancer. BJU Int. 2013 Jul;112:E67–75. doi: 10.1111/bju.12209. [DOI] [PubMed] [Google Scholar]
- 42.Lane JA, Donovan JL, Davis M, et al. Active monitoring, radical prostatectomy, or radiotherapy for localised prostate cancer: study design and diagnostic and baseline results of the ProtecT randomised phase 3 trial. Lancet Oncol. 2014 Sep;15:1109–18. doi: 10.1016/S1470-2045(14)70361-4. [DOI] [PubMed] [Google Scholar]
- 43.Klotz L. Active surveillance for low-risk prostate cancer. Current Urology Reports. 2015 Apr;16:24. doi: 10.1007/s11934-015-0492-z. [DOI] [PubMed] [Google Scholar]