Abstract
Pregnancy is a period of considerable physiological adaption in neuroendocrine, cardiovascular, as well as immune function. Understanding of typical changes in inflammatory immune responses during healthy pregnancy is incomplete. In addition, despite considerable racial difference in adverse pregnancy outcomes, data are lacking on potential racial differences in such adaptation. This repeated measures prospective cohort study included 37 Black and 39 White women who provided blood samples during early, mid-, and late pregnancy and 8–10 weeks postpartum. Peripheral blood mononuclear cells were incubated with lipopolysaccharide (LPS) for 24 hours and supernatants assayed by electrochemiluminescence to quantify interleukin(IL)-6, tumor necrosis factor(TNF)-α, IL-1β, and IL-8 production. While no changes were observed in IL-8 production over time, significant increases in IL-6, TNF-α, and IL-1β production were observed from early to late pregnancy, with subsequent declines approaching early pregnancy values at postpartum (ps<.05). Overall, inflammatory response patterns were highly similar among Black versus White women. However, Black women had greater TNF-α production during mid-pregnancy (p=.002) and marginally lower IL-1β production at postpartum (p=.054). These data show a clear trajectory of change in the inflammatory immune response across pregnancy and postpartum. In this cohort of generally healthy women, Black and White women exhibited minimal differences in LPS-stimulated cytokine production across the perinatal period. Future prospective studies in Black and White women with healthy versus adverse outcomes (e.g., preeclampsia, preterm birth) would inform our understanding of the potential role of immune dysregulation in pregnant women and in relation to racial disparities in perinatal health.
Keywords: cytokines, immunology, lipopolysaccharide, postpartum, pregnancy, race, racial disparities, stimulated cytokine production, inflammation
1. INTRODUCTION
Pregnancy is a period of considerable physiological adaption in neuroendocrine, cardiovascular, as well as immune function. The innate arm of the immune system is critical in the context of an immune challenge such as an infection or wound. Major functions of this arm of the immune system include recruitment of immune cells to the site of infection or injury, activation of the complement cascade, and activation of the adaptive immune system through antigen presentation. Understanding of typical changes in inflammatory immune responses during healthy pregnancy is incomplete.
Studies using animal models have primarily reported the inflammatory and fever response to be diminished during late pregnancy versus non-pregnancy (Aguilar-Valles et al. 2007, Fofie, Fewell & Moore 2005, Ashdown et al. 2007, McClure et al. 2005), but also enhanced during midand late gestation versus non-pregnancy (Faas et al. 2003, Vizi et al. 2001). Repeated assessment of innate immune function using animal models is rare but available data suggest the possibility of progressive enhancement of inflammatory immune response across pregnancy followed by a drop near term (Faas et al. 2003, Kabaroff, Boermans & Karrow 2006).
For clear ethical reasons, studies in pregnant women have focused primarily on ex vivo stimulation methods. Most commonly, peripheral blood mononuclear cells (PBMCs) are incubated with lipopolysaccharide (LPS) and response quantified according to proinflammatory cytokine production. Results of such studies are mixed; some data show no change in LPS-stimulated proinflammatory cytokine production across the course of pregnancy (Denney et al. 2011), while others have reported reduced (Amoudruz et al. 2006, Faas et al. 2014) or heighted (Daher et al. 1999, Brewster et al. 2008) production of some proinflammatory cytokines in later pregnancy as compared to early pregnancy and non-pregnancy. Thus, available data are inconsistent. In addition, existing data are primarily cross-sectional, in relatively small samples of 16–45 total pregnant women, and almost exclusively in non-US samples (UK, Netherlands, Sweden, Brazil; Amoudruz et al. 2006, Faas et al. 2014, Brewster et al. 2008, Daher et al. 1999). No data are available on race as a potential predictor of differential immune adaptation.
The role of race is of interest given considerable racial disparities in risk for adverse birth outcomes; in the US, Black women show 1.3 to 1.5-fold higher risk than Whites for adverse outcomes including gestational hypertension and preterm birth (Hamilton et al. 2015, Ghosh et al. 2014, Cabacungan, Ngui & McGinley 2012).These outcomes have been linked with dysregulation of inflammatory processes (Kuklina, Ayala & Callaghan 2009, Reddy et al. 2015, Behrman, Stith Butler 2007, Mendola et al. 2015, Hamilton et al. 2015). Better understanding of typical changes in immune function during healthy pregnancy is need to recognize patterns of immune-dysregulation. In a similar vein, data on potential racial differences in immune adaptation would be informative.
Addressing gaps in the literature, this study examined innate immune function, as measured by ex vivo LPS-stimulated proinflammatory cytokine production, during early, mid-, and late pregnancy and postpartum among 76 US women, inclusive of 37 Blacks and 39 Whites who were recruited to be demographically similar. This study aimed to: 1) describe longitudinal changes in the inflammatory immune response across pregnancy and postpartum, and 2) determine if racial differences are present in observed patterns of adaptation.
2. MATERIALS AND METHODS
2.1 Study Design and Participants
This repeated measures prospective cohort study enrolled 84 women (41 non-Hispanic White, 41 non-Hispanic Black, and 2 Hispanic White) assessed during early, mid-, and late pregnancy (early pregnancy mean 12 weeks, range 9–17 weeks; mid-pregnancy mean 21 weeks, range 19–24 weeks; late pregnancy mean 29 weeks, range 26–35 weeks) and at 7–11 weeks postpartum (mean 9 weeks, range 7–11 weeks). In the current analyses, immune adaptation in healthy pregnancy was the focus. Thus, per medical record review, women who developed gestational hypertension or preeclampsia during pregnancy (n = 3) or delivered preterm (n = 5) were excluded, resulting in a final sample size of 76 (39 White including 2 Hispanics, and 37 Black).
Participants were recruited from The Ohio State University Wexner Medical Center (OSUWMC) and surrounding community of Columbus, Ohio. Exclusion criteria included multifetal gestation, diagnosed fetal anomaly, chronic conditions (e.g., cancer, systemic lupus erythematosus) or use of medications (e.g., progesterone) with implications for immune function, illicit drug use other than marijuana, and consumption of >2 alcoholic beverages per week per self-report or medical record at time of enrollment. Women reporting acute illness, such as cold- or flu-like symptoms, or antibiotic use within ten days of a study visit were rescheduled. Blood samples were obtained between 8:00AM and 4:00PM. The study was approved by The Ohio State University Biomedical Institutional Review Board. Informed consent and HIPAA authorizations were obtained from all participants and modest compensation provided.
2.2 Demographics, Health, and Pregnancy Outcomes
Race, ethnicity, age, education, annual household income, smoking status, gravidity, and parity were determined by self-report. Maternal pre-pregnancy BMI (kg/m2) was calculated using self-reported pre-pregnancy weight and height measured at a study visit. Development of gestational hypertension, preeclampsia, and preterm birth during the current pregnancy was determined by post-delivery medical record review.
2.3 Inflammatory Immune Response
PBMCs at a concentration of 1 × 106 cells/ml were stimulated with 1ug/ml LPS in RPMI-1640 supplemented with 10% human male serum for 24 hours. A non-LPS media control was incubated simultaneously. After 24 hours, samples were centrifuged and aliquots removed and frozen at −80°C until assayed. Media samples were assayed neat, while LPS samples were diluted 1:6. Samples were assayed in duplicate for IL-6, TNF-α, IL-1β, and IL-8 (pg/ml) using human ProInflammatory II multiplex tissue culture kits from Meso Scale Discovery (MSD; 1601 Research Blvd., Rockville, MD). Plates were read by an MSD Sector Imager 2400 measuring electrochemiluminescence. The inter-assay coefficients of variation were 8.28, 6.02, 8.59, and 9.23, for IL-6, TNF-α, IL-1β, and IL-8, respectively. The intraassay coefficients of variation were 3.2, 2.36, 1.91, and 2.93, for IL-6, TNF-α, IL-1β, and IL-8, respectively.
2.4 Statistical Analyses
Patient characteristics were examined by descriptive statistics as mean/standard deviation or count/frequency. For the primary analyses, following log transformation of stimulated cytokine values, a linear mixed model with a random subject effect was fit to each stimulated cytokine endpoint. The random subject effects accounted for the correlation in repeated measures from the same subject across time. Also, the quadratic trend across the four time points was tested. In order to test whether patterns in stimulated cytokine production differed between White and Black women, race by visit interactions were used. Parameter contrasts were estimated within each model to compare cytokine means at each pair of time points, to compare the races at each time point, and to test for differences in the quadratic trends. Two outliers (>3 SD below the mean) for stimulated production of IL-8 were excluded from analysis for early and mid-pregnancy. All tests were evaluated at the α = 0.05 level of significance. No adjustments were made for multiple comparisons. All analyses were performed in SAS/STAT software version 9.3 (Cary, NC).
3. RESULTS
3.1 Descriptive Statistics and Comparisons by Race
Demographics, health, and birth outcome descriptive statistics and comparisons between White and Black women are shown in Table 1. White and Black women did not significantly differ in age, education, annual household income, smoking status, gravidity, parity, or maternal pre-pregnancy BMI (ps ≥ 0.10). Black women were marginally more likely to be classified in the lower income category (p = 0.06).
Table 1.
Descriptive Statistics and Comparisons by Maternal Race
|
White (n=39) Mean(SD) or n(%) |
Black (n=37) Mean(SD) or n(%) |
t-test/χ2 p Value |
|
|---|---|---|---|
| Age | 25.5 (3.4) | 25.5 (4.8) | 0.98 |
|
Education < High School Graduate High School Graduate Some College ≥ College Degree |
3 (7.7%) 9 (23.1%) 14 (35.9%) 13 (33.3%) |
3 (8.1%) 7 (18.9%) 15 (40.5%) 12 (32.4%) |
0.97 |
|
Annual Household Income <$15,000 ≥$15,000 |
9 (23.1%) 30 (76.9%) |
16 (43.2%) 21 (56.8%) |
0.06 |
|
Smoking Status Current Smoker Current Non-Smoker |
5 (12.8%) 4 (87.2%) |
33 (89.2%) 4 (10.8%) |
0.79 |
|
Gravidity 1 2 3 ≥4 |
12 (30.8%) 12 (30.8%) 7 (18.0%) 8 (20.5%) |
6 (16.2%) 11 (29.7%) 12 (32.4%) 8 (21.6%) |
0.35 |
|
Parity 0 1 ≥2 |
17 (43.6%) 12 (30.8%) 10 (25.6%) |
8 (21.6%) 13 (35.1%) 16 (43.2%) |
0.10 |
| Maternal Pre-Pregnancy BMI | 28.4 (6.3) | 27.1 (5.2) | 0.34 |
| Gestational Age at Deliverya | 39.5 (1.0) | 39.2 (1.1) | 0.37 |
data available from n=37 White and n=36 Black participants
3.2 Inflammatory Immune Response across Pregnancy and Postpartum
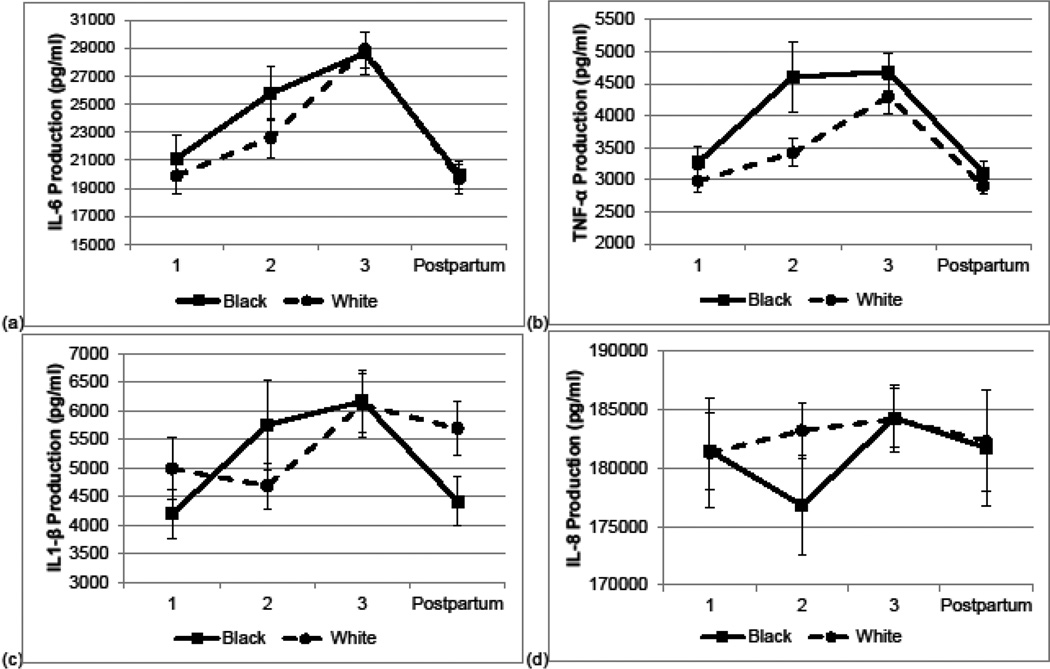
For the overall sample, quadratic patterns were evidenced for production of interleukin (IL)-6 (p < .001), tumor necrosis factor (TNF)-α (p < .001), and IL-1β (p = .01). For IL-6 and TNF-α production, values rose from early to mid-pregnancy (ps < .005) and mid- to late pregnancy (ps < .05), and declined postpartum relative to late pregnancy (ps < .001). For IL-1β production, values rose from early to late pregnancy (p < .001) and declined postpartum relative to late pregnancy (p = .03). For IL-8 production, there were no significant changes or differences in values over the course of pregnancy (ps > 0.14).
3.3 Effects of Race on Stimulated Cytokine Production
Overall, patterns of cytokine production were highly similar among White and Black women (Figure 1 a–d). Racial differences were observed in only two instances. Black women had greater TNF-α production during midpregnancy (p = .002) and marginally lower IL-1β production at postpartum (p = .054).
Fig. 1. Inflammatory Immune Response across Pregnancy and Postpartum according to Maternal Race.
Lipopolysacharide-stimulated production of (a) IL-6, (b) TNF-α, (c) IL-1β, and (d) IL-8 during early (1), mid- (2), and late (3) pregnancy and postpartum among Black and White women. Black women exhibited greater TNF-α production during mid-pregnancy (p = .002) and marginally lower IL-1β production at postpartum (p = .054). No other statistically significant differences were observed. Error bars = ±1 SE.
4. DISCUSSION
The current study demonstrates a clear trajectory of change in the inflammatory immune response across pregnancy. Specifically, while LPS-stimulated cytokine production of IL-8 remained relatively stable, production of IL-6, TNF-α, and IL-1β increased progressively across pregnancy. Our findings of overall increases in inflammatory responses are in line with the findings of two prior observational studies in non-US samples that utilized cross-sectional designs with 13 – 47 pregnant women at a given time point (Brewster et al. 2008, Daher et al. 1999). The current findings are also consistent with two studies using murine and ovine models (Vizi et al. 2001, Kabaroff, Boermans & Karrow 2006). In contrast, in the only longitudinal and US study, Denney et al. reported no change in production of IL-6, TNF-α, or IL-1β through pregnancy among 43 women (79% non-Hispanic Black; Denney et al. 2011). The basis for this difference in findings is unknown, but could be related to assay methodology.
In this sample, LPS-stimulated IL-6, TNF-α, and IL-1β production returned to levels comparable to early pregnancy values by 8–10 weeks postpartum. To our knowledge, this is the first study assessing rebound of these pregnancy-associated changes among the same participants at postpartum. Our findings are in line with the observation of similar TNF-α production among 28 pregnant women in their 1st trimester compared to 19 healthy non-pregnant controls in a Brazilian study (Daher et al. 1999).
This study also provides novel data on pregnancy-related immune adaptation in women of different races. We found that Black and White women displayed quite similar patterns of ex vivo LPS-stimulated proinflammatory cytokine production across pregnancy and postpartum. Black women exhibited higher TNF-α production at the mid-pregnancy assessment and lower IL-1β production at postpartum. However, given the lack of a consistent pattern of effects, these differences may be due to chance. Replication would clarify if this is the case. Overall, these data suggest that in healthy pregnant women, adaptation of the innate immune response, as measured by LPS-stimulated cytokine production, is similar among Black and White women.
Women with gestational hypertension, preeclampsia, and preterm birth (n = 8) were excluded from analyses in the current study and were not represented in large enough numbers to compare to healthy controls. This approach allowed conclusions regarding immune adaptation in healthy pregnancy. However, important empirical questions remain regarding the potential role of inflammatory immune dysregulation in predicting onset of these disorders. In addition, if racial differences in immune dysregulation do contribute to the racial disparities observed in these conditions, they may be observable only among those who ultimately exhibit the adverse outcome. This warrants examination in future studies.
A strength of the current study includes collection of immune data longitudinally across four time points in pregnancy and postpartum. This allowed for evaluation of patterns as opposed to cross-sectional comparisons between pregnant women assessed at different points in pregnancy. Further, ex vivo LPS-stimulated proinflammatory cytokine production has been minimally studied; however, the initial, non-specific response to challenges that mimic in vivo infection is likely of particular importance during pregnancy. Overly robust responses may lead to chronic inflammation and/or initiation of an inflammatory cascade while insufficient responses may increase vulnerability to infection.
Data from women is required to inform understanding of immune function and perinatal health. While animal models have applicability to understanding human reproductive biology, there are limitations to generalizability to humans. For example, in rats, increasing inflammatory responses from days 4–11 of pregnancy, followed by a drop to non-pregnant levels near term on day 20 have been reported (Faas et al. 2003). In contrast, there is no evidence to suggest that the inflammatory response declines among humans approaching term and considerable evidence supports enhanced inflammation during labor (Romero, Dey & Fisher 2014, Romero et al. 2007, Gomez-Lopez et al. 2014). There may be fundamental differences in the role of inflammation in labor initiation in humans versus other mammals. For example, declining progesterone levels appear to be critical in labor initiation among mice and sheep (Ratajczak, Fay & Muglia 2010). In women, progesterone levels remain elevated but changes in receptor profiles reduce signaling during labor (Mesiano, Wang & Norwitz 2011). An advancing inflammatory cascade is capable of, and may even be key in, inducing functional progesterone withdrawal among humans (Jiang et al. 2012, Condon et al. 2006).
In conclusion, these data demonstrate that ex vivo LPS-stimulated IL-6, TNF-α, and IL-1β production changes significantly through pregnancy and postpartum, with the greatest production of these cytokines observed in late pregnancy. In contrast, LPS-stimulated production of IL-8 appears to be unchanged throughout pregnancy and postpartum. Overall, observed patterns of cytokine production were highly similar among Black versus White women. Given the robust and consistent pattern observed in healthy pregnancy, it is possible that women deviating from this pattern of adaptation will exhibit risk for adverse pregnancy outcomes (e.g., gestational hypertension, preeclampsia, preterm birth). Further, prospective data in Black and White women with healthy versus adverse outcomes would inform our understanding of the potential role of immune-dysregulation in women in general and in relation to racial disparities in perinatal health.
Highlights.
We examined LPS-stimulated cytokine responses across pregnancy and postpartum.
IL-6, TNF-α, and IL-1β production increased from early to late pregnancy.
Subsequent declines approaching early pregnancy values were seen at postpartum.
Minimal differences were observed among Black versus White women.
Future data on adaptation in healthy versus disordered pregnancy would be informative.
Acknowledgments
We appreciate the contributions of our Clinical Research Assistants to data collection. We would like to thank our study participants and the staff at the OSU Clinical Research Center and Wexner Medical Center Prenatal Clinic.
ROLE OF FUNDING SOURCES
This study was supported by NICHD (R21HD067670, LMC) and NINR (R01NR013661, LMC). Manuscript preparation was also supported by NINR (F31NR014605, SLG). The project described was supported by the Ohio State University Clinical Research Center, funded by the National Center for Research Resources (UL1TR001070). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. NIH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Aguilar-Valles A, Poole S, Mistry Y, Williams S, Luheshi GN. Attenuated fever in rats during late pregnancy is linked to suppressed interleukin-6 production after localized inflammation with turpentine. The Journal of physiology. 2007;583(Pt 1):391–403. doi: 10.1113/jphysiol.2007.132829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoudruz P, Minang JT, Sundstrom Y, Nilsson C, Lilja G, Troye-Blomberg M, Sverremark-Ekstrom E. Pregnancy, but not the allergic status, influences spontaneous and induced interleukin-1beta (IL-1beta), IL-6, IL-10 and IL-12 responses. Immunology. 2006;119(1):18–26. doi: 10.1111/j.1365-2567.2006.02400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashdown H, Poole S, Boksa P, Luheshi GN. Interleukin-1 receptor antagonist as a modulator of gender differences in the febrile response to lipopolysaccharide in rats. American journal of physiology.Regulatory, integrative and comparative physiology. 2007;292(4):R1667–R1674. doi: 10.1152/ajpregu.00274.2006. [DOI] [PubMed] [Google Scholar]
- Behrman RE, Stith Butler A, editors. Preterm birth: Causes, consequences, and prevention. Washington, D.C.: The National Academies Press; 2007. [PubMed] [Google Scholar]
- Brewster JA, Orsi NM, Gopichandran N, McShane P, Ekbote UV, Walker JJ. Gestational effects on host inflammatory response in normal and pre-eclamptic pregnancies. European journal of obstetrics, gynecology, and reproductive biology. 2008;140(1):21–26. doi: 10.1016/j.ejogrb.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Cabacungan ET, Ngui EM, McGinley EL. Racial/ethnic disparities in maternal morbidities: a statewide study of labor and delivery hospitalizations in Wisconsin. Maternal and child health journal. 2012;16(7):1455–1467. doi: 10.1007/s10995-011-0914-6. [DOI] [PubMed] [Google Scholar]
- Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Molecular endocrinology (Baltimore, Md.) 2006;20(4):764–775. doi: 10.1210/me.2005-0242. [DOI] [PubMed] [Google Scholar]
- Daher S, Fonseca F, Ribeiro OG, Musatti CC, Gerbase-DeLima M. Tumor necrosis factor during pregnancy and at the onset of labor and spontaneous abortion. European journal of obstetrics, gynecology, and reproductive biology. 1999;83(1):77–79. doi: 10.1016/s0301-2115(98)00252-8. [DOI] [PubMed] [Google Scholar]
- Denney JM, Nelson EL, Wadhwa PD, Waters TP, Mathew L, Chung EK, Goldenberg RL, Culhane JF. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53(2):170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas MM, Kunnen A, Dekker DC, Harmsen HJ, Aarnoudse JG, Abbas F, De Vos P, Van Pampus MG. Porphyromonas Gingivalis and E-coli induce different cytokine production patterns in pregnant women. PloS one. 2014;9(1):e86355. doi: 10.1371/journal.pone.0086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas MM, Moes H, van der Schaaf G, de Leij LF, Heineman MJ. Total white blood cell counts and LPS-induced TNF alpha production by monocytes of pregnant, pseudopregnant and cyclic rats. Journal of reproductive immunology. 2003;59(1):39–52. doi: 10.1016/s0165-0378(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fofie AE, Fewell JE, Moore SL. Pregnancy influences the plasma cytokine response to intraperitoneal administration of bacterial endotoxin in rats. Experimental physiology. 2005;90(1):95–101. doi: 10.1113/expphysiol.2004.028613. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Grewal J, Mannisto T, Mendola P, Chen Z, Xie Y, Laughon SK. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethnicity & disease. 2014;24(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cellular & molecular immunology. 2014 doi: 10.1038/cmi.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton BE, PhD, Martin JA, Osterman MJ, MHS, Curtain SC., MA Births: Preliminary Data for 2014. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2015;64(6):1–19. [PubMed] [Google Scholar]
- Jiang ZY, Guo YY, Ren HB, Zou YF, Fan MS, Lv Y, Han P, De W, Sun LZ. Tumor necrosis factor (TNF)-alpha upregulates progesterone receptor-A by activating the NF-kappaB signaling pathway in human decidua after labor onset. Placenta. 2012;33(1):1–7. doi: 10.1016/j.placenta.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Kabaroff L, Boermans H, Karrow NA. Changes in ovine maternal temperature, and serum cortisol and interleukin-6 concentrations after challenge with Escherichia coli lipopolysaccharide during pregnancy and early lactation. Journal of animal science. 2006;84(8):2083–2088. doi: 10.2527/jas.2005-625. [DOI] [PubMed] [Google Scholar]
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstetrics and gynecology. 2009;113(6):1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- McClure L, O'Connor AE, Hayward S, Jenkin G, Walker DW, Phillips DJ. Effects of age and pregnancy on the circulatory activin response of sheep to acute inflammatory challenge by lipopolysaccharide. The Journal of endocrinology. 2005;185(1):139–149. doi: 10.1677/joe.1.06051. [DOI] [PubMed] [Google Scholar]
- Mendola P, Mumford SL, Mannisto TI, Holston A, Reddy UM, Laughon SK. Controlled direct effects of preeclampsia on neonatal health after accounting for mediation by preterm birth. Epidemiology (Cambridge, Mass.) 2015;26(1):17–26. doi: 10.1097/EDE.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reproductive sciences (Thousand Oaks, Calif.) 2011;18(1):6–19. doi: 10.1177/1933719110382922. [DOI] [PubMed] [Google Scholar]
- Ratajczak CK, Fay JC, Muglia LJ. Preventing preterm birth: the past limitations and new potential of animal models. Disease models & mechanisms. 2010;3(7–8):407–414. doi: 10.1242/dmm.001701. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Rice MM, Grobman WA, Bailit JL, Wapner RJ, Varner MW, Thorp JM, Jr, Leveno KJ, Caritis SN, Prasad M, Tita AT, Saade GR, Sorokin Y, Rouse DJ, Blackwell SC, Tolosa JE Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network. Serious maternal complications after early preterm delivery (24–33 weeks'gestation. American Journal of Obstetrics and Gynecology. 2015 doi: 10.1016/j.ajog.2015.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Goncalves LFMD, Kusanovic JP, Friel L, Hassan S. The Role of Inflammation and Infection in Preterm Birth. Seminars in reproductive medicine. 2007:21. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science (New York, NY) 2014;345(6198):760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Szelenyi J, Selmeczy ZS, Papp Z, Nemeth ZH, Hasko G. Enhanced tumor necrosis factor-alpha-specific and decreased interleukin-10-specific immune responses to LPS during the third trimester of pregnancy in mice. The Journal of endocrinology. 2001;171(2):355–361. doi: 10.1677/joe.0.1710355. [DOI] [PubMed] [Google Scholar]