Abstract
Inflammation and haemostasis are part of the host's first line of defense to tick feeding. These systems are in part serine protease mediated and are tightly controlled by their endogenous inhibitors, in the serpin superfamily (serine protease inhibitors). From this perspective ticks are thought to use serpins to evade host defenses during feeding. The cattle tick Rhipicephalus microplus encodes at least 24 serpins, of which RmS-3, RmS-6, and RmS-17 were previously identified in saliva of this tick. In this study, we screened inhibitor functions of these three saliva serpins against a panel of 16 proteases across the mammalian defense pathway. Our data confirm that Pichia pastorisexpressed rRmS-3, rRmS-6, and rRmS-17 are likely inhibitors of pro-inflammatory and pro-coagulant proteases. We show that rRmS-3 inhibited chymotrypsin and cathepsin G with stoichiometry of inhibition (SI) indices of 1.8 and 2.0, and pancreatic elastase with SI higher than 10. Likewise, rRmS-6 inhibited trypsin with SI of 2.6, chymotrypsin, factor Xa, factor XIa, and plasmin with SI higher than 10, while rRmS-17 inhibited trypsin, cathepsin G, chymotrypsin, plasmin, and factor XIa with SI of 1.6, 2.6, 2.7, 3.4, and 9.0, respectively. Additionally, we observed the formation of irreversible complexes between rRmS-3 and chymotrypsin, rRmS-6/rRmS-17 and trypsin, and rRmS-3/rRmS-17 and cathepsin G, which is consistent with typical mechanism of inhibitory serpins. In blood clotting assays, rRmS-17 delayed plasma clotting by 60 s in recalcification time assay, while rRmS-3 and rRmS-6 did not have any effect. Consistent with inhibitor function profiling data, 2.0 µM rRmS-3 and rRmS-17 inhibited cathepsin G-activated platelet aggregation in a dose-responsive manner by up to 96% and 95% respectively. Of significant interest, polyclonal antibodies blocked inhibitory functions of the three serpins. Also notable, antibodies to Amblyomma americanum, Ixodes scapularis, and R. sanguineus tick saliva proteins cross-reacted with the three R. microplus saliva serpins, suggesting the potential of these proteins as candidates for universal anti-tick vaccines.
Keywords: immune response, tick saliva, platelet aggregation inhibitor, cathepsin G
1. Introduction
The cattle tick R. microplus is one the most harmful hematophagous ectoparasites of bovines, with significant impact on the cattle industry worldwide due to its spoliation action and its role as a vector of tick-borne pathogens such as Babesia spp and Anaplasma marginale, the agents of babesiosis and anaplasmosis, respectively (Grisi et al., 2014; Jongejan and Uilenberg, 2004). Current tick control strategies rely mostly on the use of chemical acaricides, even though selection of resistant tick populations to most used acaricides has been confirmed (Guerrero et al., 2002; Guerrero et al., 2012; Pohl et al., 2011). This is recognized as a worldwide drawback to successful tick control, not to mention environment and food chain contamination hazards. Immunization of cattle against R. microplus and other ticks has been recognized as an alternative against tick control strategy (de la Fuente et al., 2007; Willadsen et al., 1989). Thus, in the effort to find effective targets for tick vaccine development, our research group has endeavored to understand how ticks acquire blood meal.
Tick blood feeding occurs as two steps, namely the disruption of host tissue and the suction of blood that flows into the feeding lesion, triggering a host response that includes pain, itching, blood coagulation, inflammation, complement activation, tissue repair response, and adaptive immune response (Francischetti et al., 2009; Heinze et al., 2014). Serine proteases such as pro-coagulant (thrombin, factor Xa, factor XIa, and other blood coagulation factors), pro-inflammatory (neutrophil elastase, proteinase-3, chymase, tryptase, kallikrein, cathepsin G, trypsin-like, and chymotrypsin-like), and complement proteases (factors B, factor C, factor D, and component 2) have a role in these host defense responses to tick feeding (Cattaruzza et al., 2014; Davie et al., 1979; Korkmaz et al., 2008; Matsunaga et al., 1994). Ticks successfully acquire blood meals by inoculation of saliva proteins in order to counteract host defenses to tick feeding (Francischetti et al., 2009; Ribeiro, 1987; Ribeiro and Francischetti, 2003). Proteomic analysis of tick saliva revealed that it contains a great variety of proteins with antihemostatic, anti-inflammatory, and immunomodulatory roles, among which proteinase inhibitors that belong to different families such as serpin, Kunitz-type, Kazal-type, cystatin, alpha-2-macroglobulin, thyropin, and trypsin inhibitor-like (TIL) inhibitors (Carvalho-Costa et al., 2015; Diaz-Martin et al., 2013; Lewis et al., 2015; Mudenda et al., 2014; Oliveira et al., 2013; Radulovic et al., 2014; Tirloni et al., 2014a).
Members of the serpin (serine proteinase inhibitors) superfamily are irreversible inhibitors of serine protease mediators of host defense pathways to tick feeding (Gettins, 2002). In mammals serpins are known to regulate blood coagulation cascade, fibrinolysis, wound healing, angiogenesis, as well as inflammatory and immune responses (Rau et al., 2007; Silverman et al., 2001). This knowledge has led to the assumption that ticks inject serpins during feeding to disrupt the host homeostatic balance, as a way to prevent, slow down, and/or evade host defenses (Mulenga et al., 2001). Several tick serpin-encoding cDNAs have been cloned and characterized, including serpins from Amblyomma americanum (Kim et al., 2015; Mulenga et al., 2007; Mulenga et al., 2013; Porter et al., 2015), A. maculatum (Karim et al., 2011), Ixodes scapularis (Ibelli et al., 2014; Mulenga et al., 2009; Ribeiro et al., 2006), I. ricinus (Chmelar et al., 2011; Leboulle et al., 2002b; Prevot et al., 2006), R. microplus (Jittapalapong et al., 2010; Rodriguez et al., 2015; Rodriguez-Valle et al., 2012; Tirloni et al., 2014b), R. appendiculatus (Mulenga et al., 2003), R. haemaphysaloides (Yu et al., 2013), and Haemaphysalis longicornis (Imamura et al., 2005; Imamura et al., 2006; Sugino et al., 2003). Additionally, proteomic studies have identified serpins in saliva of blood-fed ticks, such as R. microplus (Tirloni et al., 2014a), A. americanum (Radulovic et al., 2014), Dermacentor andersoni (Mudenda et al., 2014), and H. longicornis (Tirloni et al., 2015), suggesting that the secretion of serpins is a common biologic strategy adopted by different tick species in order to counteract host’s defenses during tick feeding.
Recent evidence shows that some of the tick-encoded serpins are functional inhibitors that are likely associated with tick evasion of host defense. In A. americanum two salivary serpins were characterized: serpin 6 (Chalaire et al., 2011; Mulenga et al., 2007), an inhibitor of papain and trypsin-like proteinases with anti-blood clotting and anti-complement activation functions (Mulenga et al., 2013), and A. americanum serpin 19 (AAS19), a conserved serpin among ixodid ticks that acts as a broad spectrum inhibitor of trypsin-like proteases with anti-haemostatic functions (Kim et al., 2015). In I. scapularis, a blood meal induced serpin inhibited thrombin and reduced platelet aggregation (Ibelli et al., 2014). In I. ricinus the serpin IRIS is an inhibitor of pro-inflammatory protease elastase and exhibits immunomodulatory properties (Prevot et al., 2006; Prevot et al., 2009). Likewise, I. ricinus serpin IRS-2 inhibited pro-inflammatory proteases cathepsin G and chymase, in addition to Th17 differentiation inhibition (Chmelar et al., 2011; Palenikova et al., 2015). In R. haemaphysaloides two serpins with inhibitory activity against chymotrypsin were described (Yu et al., 2013).
In a previous study, three (RmS-3, RmS-6 and RmS-17) of the 24 R. microplus serpin (Tirloni et al., 2014a) were found in saliva of this tick. The objective of the present study was to characterize these the three R. microplus saliva serpins to gain insight into their role(s) in the tick-host relationship. The data obtained confirms and builds on previous studies that characterized inhibitor function profiles of RmS-3 and RmS-6 (Rodriguez-Valle et al., 2012).
2. Materials and Methods
2.1 Ethics statement
Animals used in these experiments were housed at Faculdade de Veterinária, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil. This study was conducted according to the ethic and methodological aspects preconized by the International and National Directives and Norms by the Animal Experimentation Ethics Committee of the UFRGS. The protocols were approved by the Comissão de Ética no Uso de Animais - CEUA – UFRGS.
2.2 Ticks and animals
R. microplus ticks (Porto Alegre strain), free of pathogens such as Babesia spp and Anaplasma spp were reared on Hereford calves (Bos taurus taurus), which were brought from a naturally tick-free area (Santa Vitória do Palmar, RS, Brazil; 33°32’2’’ S, 53°20’59’’ W) and maintained in individual sheds. Two naïve calves were infested with approximately 20,000 larvae that were 10 days old (from 1 g of R. microplus eggs). The larvae molt to nymphs and develop to females and males that mate. Female ticks feed to repletion and detaches from the host. The complete life cycle on the host takes roughly 21 days (Reck, Jr. et al., 2009). At 20th day, partially engorged adult females were manually detached from host.
2.3 Tick dissections, RNA and protein extractions, and cDNA synthesis
Partially engorged adult female ticks were manually and carefully removed from calves during tick feeding process. Ticks were collected, weighed, and divided in eight groups according to weight (as a measure of blood uptake index): group 1 (9.9 ± 1.6 mg); group 2 (16 ± 1.2 mg); group 3 (24.2 ± 1.9 mg); group 4 (34.8 ± 2.8 mg); group 5 (53.2 ± 2.3 mg); group 6 (84.2 ± 7.9 mg); group 7 (188.8 ± 15.4 mg); and group 8 (270.2 ± 14.9 mg). Ticks were rinsed with 70% ethanol, followed by dorsal surface dissecting with a scalpel blade to separate salivary glands (SG). Dissected SG from each group were washed with phosphate-buffered solution (PBS) in an RNase-free environment and placed in a tube containing TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). Total RNA and protein were extracted according to the manufacturer’s recommendations. The total RNA samples were resuspended in diethylpyrocarbonate (DEPC)-treated water and treated with DNase I (Invitrogen, Carlsbad, CA, USA). Total RNA concentration and purity were determined using a spectrophotometer. Protein samples were resuspended in 1% SDS solution and protein concentration was determined using the bicinchoninic acid method (BCA Protein Assay, Pierce, Rockford, USA) as previously described (Brown et al., 1989). Both total RNA and proteins were stored at −70 °C upon use.
2.4 Saliva collection
Tick saliva was collected as previously described (Clarke and Hewetson, 1971; Tirloni et al., 2014a). Partially engorged female ticks (varying from 20 to 200 mg) were manually and carefully removed from calves maintained in individual pens. Prior to saliva collection, any host contaminating tissue in tick mouthparts was removed using a scalpel blade and surgical forceps. Ticks were rinsed with sterile distilled water and induced to salivate by dorsal injection of 2–5 µL 2% pilocarpine (in PBS). Ticks were maintained at room temperature in a humid chamber for approximately 3 h, during which time saliva accumulated in the mouthparts was periodically collected using a pipette tip. Saliva protein concentration was determined by BCA and stored at −70 °C upon use.
2.5 Recombinant protein expression and purification of rRmS-3, rRmS-6 and rRmS-17
Serpin-encoding mature protein open reading frames of RmS-3, RmS-6, and RmS-17 (Tirloni et al., 2014b) were cloned into pPICZαC using a set of primers as described in Table S1. The pPICZαC/RmS expression plasmids were linearized with SacI and electroporated into Pichia pastoris X-33 strain according to the manufacturer’s recommendations (Life Technologies, Carlsbad, CA, USA). Transformed colonies were selected on yeast extract peptone dextrose medium (YPD) agar plates containing zeocin (100 µg/mL -Life Technologies, Carlsbad, CA, USA) and incubated at 28 °C. After five days, positive grown colonies were inoculated in buffered glycerol-complex medium (BMGY) and grown overnight at 28 °C with shaking (240 rpm). Subsequently, cells were used to inoculate buffered methanol-complex medium (BMMY) to OD600 of 1.0 and grown at 240 rpm at 28 °C during five days. Protein expression was induced daily during that period, by adding methanol to a 0.5 % final concentration. Recombinant proteins in spent culture media were precipitated by ammonium sulfate saturation (525 g/L of media) with stirring at 4 °C overnight. The precipitate was pelleted at 12,000 rpm for 1 h at 4 °C and resuspended in and dialyzed against buffer (20 mM Tris-HCl, 500 mM NaCl, pH 7.4). Expression of rRmS-3, rRmS-6, and rRmS-17 was confirmed resolving samples on a 12.5 % SDS-PAGE. Western blotting analysis was performed using an 1:5,000 dilution of antibody to the C-terminus hexa histidine tag (Life Technologies, Carlsbad, CA, USA), and positive signal was detected using a metal enhanced DAB chromogenic substrate kit (Thermo Scientific, Waltham, MA, USA). Recombinant proteins were affinity-purified under native conditions using Hi-Trap Chelating HP Columns (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). To evaluate purity, affinity-purified proteins were resolved on a 12.5% SDS-PAGE and stained with Coomassie brilliant blue. Affinity-purified proteins were dialyzed against 20 mM Tris-HCl, NaCl 150 mM buffer pH 7.4, protein concentration was determined by BCA and stored at −80 °C until use.
2.6 Antibody production and characterization
Rabbit anti-sera and murine monoclonal antibodies (MAbs) were raised for each serpin. To raise polyclonal antibodies against the three saliva serpins, rabbits were inoculated subcutaneously with 150 µg of either rRmS-3, rRmS-6 or rRmS-17 emulsified in oil adjuvant (Montanide 888 - Seppic and Marcol 52 – Exxon Mobil Corporation). Following this first administration, two 150-µg boosters of each recombinant serpin in the same adjuvant were applied at 15-day intervals. Antibodies from sera were purified by affinity chromatography on a protein G Sepharose resin according to the manufacturer’s instructions (GE Healthcare, Pittsburgh, PA, USA).
To raise monoclonal antibodies against the three saliva serpins, six-week-old BALB/c mice were injected three times intraperitoneally with 100 µg of either rRmS-6 or rRmS-17 (Freund´s adjuvant, Sigma-Aldrich, St. Louis, MO, USA) at 15-day intervals for monoclonal antibody production (Harlow and Lane, 1988). Indirect ELISA using recombinant protein was used to obtain the sera antibodies titer. Splenic lymphocytes were fused to murine Sp2/O-Ag14 myeloma cells in the presence of PEG 3350 (Sigma-Aldrich, St. Louis, MO, USA) and cultivated in DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 20% fetal calf serum (Cultilab, Campinas, SP, Brazil) and HAT (Sigma-Aldrich, St. Louis, MO, USA). Hybridomas growing in HAT medium were screened for specific antibodies by indirect ELISA, and those producing positive results were cloned twice using the limiting dilution technique. MAbs were purified by affinity chromatography on a protein G Sepharose resin according to the manufacturer’s instructions (GE Healthcare, Pittsburgh, PA, USA) and dialyzed against PBS. Concentrations of purified MAbs and polyclonal antibodies were determined at OD280nm, and the preparations were stored at −20 °C until use.
Anti-sera and MAbs specificity to native RmS targets in tick saliva was tested using western blotting and a sandwich ELISA assay. Purified rabbit IgG against rRmS-6 or rRmS-17 was used to coat 96-well polyestirene plates (310 ng/well) for 1 h at 37 °C. PBS-Tween (PBS-T) was used as a blocking solution for 1 h at 37 °C. Saliva from partially engorged R. microplus was added to the plate (800 ng of total saliva protein per well diluted in PBS-T) and incubated for 1 h at 37 °C, when MAbs were added to each well (100 ng/well diluted in PBS-T). After three washes in PBS-T, wells were incubated with a 1:2,000 dilution of horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (Sigma, St. Louis, MO, USA) for 1 h at 37 °C. After three washes in PBS-T, the reaction was visualized with 100 µL of enzyme substrate/chromogen solution (H2O2/ortophenylenediamine) in 100 mM citrate-phosphate buffer, pH 5.0, and the reaction was allowed to take place in the dark for 10 min. Optical density was read at 492 nm using the VersaMax tunable plate reader (Molecular Devices, Sunnyvale, CA, USA).
Purified rabbit IgG and purified MAbs were assayed in an indirect ELISA as above, with modifications to identify cross-reaction with different recombinant serpins. Briefly, 200 ng/well of either rRmS-3, rRmS-6 or rRmS-17 were used to coat 96-well polystyrene plates. After removal of unbound proteins and blocking unspecific binding sites, 1 µg/well of antibody was added to each well and incubated for 1 h at 37 °C. After three washes in PBS-T, wells were incubated with a 1:2,000 dilution of either horseradish peroxidase (HRP)-conjugated anti-mouse or anti-rabbit IgG secondary antibody (Sigma, St. Louis, MO, USA). The reaction was visualized as described above. Results were expressed as reaction percentage (the optical density from antibody raised to the antigen used as immunogen was taken as 100 %) from triplicates and were expressed as mean ± SD.
2.7 Expression analysis of salivary serpins by RT-PCR and western blotting
Expression profiles of the RmS-3, RmS-6 and RmS-17 genes in salivary glands from eight different adult female developmental stages were determined by RT-PCR and western blotting analysis. OligodT primed cDNA was synthesized from 5 µg of total RNA using the SuperScript III kit (Life Technologies, Carlsbad, CA, USA). Specific forward and reverse primers (Table S1) were used to determine transcription profile in pools of cDNA of SG from those eight groups of ticks (under item 2.3). For a constitutive gene control, forward and reverse primers targeting the 40S ribosomal protein S3a were used (Table S1). RT-PCR was carried out with 400 ng of cDNA using Taq DNA polymerase (Ludwig Biotecnologia, Porto Alegre, RS, Brazil). The reactions were performed according to the following steps: 5 min at 94 °C followed by 35 cycles of 30 s at 94 °C, 30 s at 50 °C, and 1 min and 30 s at 72 °C, with a final elongation at 72 °C for 5 min. PCR products were electrophoresed on a 1.0 % agarose gel and visualized by staining with GelRed™ (Uniscience, São Paulo, SP, Brazil). To analyze RmS-3, RmS-6, and RmS-17 protein expression profile in tick SG, total protein extracts were resolved on a 12 % SDS-PAGE (100 µg total protein per lane) and western blotting performed using rabbit pre-immune (1:500) or immune anti-sera raised for each recombinant serpin (1:500) as primary antibody. Subsequently, membranes were incubated with phosphatase-conjugated anti-rabbit IgG (Sigma–Aldrich) as secondary antibody for 1 h in a 1:5000 blocking buffer dilution, and BCIP (5-bromo-4-chloro-3-indolyl-phosphate) and NBT (nitro blue tetrazolium) were used for the colorimetric detection.
2.8 Deglycosylation assay
Amino acid sequence analyses predicted the existence of N-linked glycosylation sites in RmS-3, RmS-6, and RmS-17 (Tirloni et al., 2014b). To determine if yeast-expressed serpins and native salivary serpins were glycosylated, affinity-purified protein and total saliva proteins were treated with a protein deglycosylation enzyme mix according to the manufacturer’s instructions (New England Biolabs, Ipswich, MA, USA). Deglycosylation was confirmed by 12.5 % SDS-PAGE stained with Coomassie brilliant blue and western blotting using antibody to the C-terminus hexa histidine tag (as described in item 2.5) or rabbit anti-sera against serpins (1:200 dilution) and 1:2,500 dilution of horseradish peroxidase (HRP)-conjugated anti-rabbit IgG secondary antibody (Sigma, St. Louis, MO, USA).
2.9 Inter-species cross-reactivity assay
To analyze if recombinant R. microplus serpins react with serum from animals infested with R. microplus and if they cross-react with serum from animals infested with other ticks species, rRmS-3, rRmS-6, and rRmS-17 (2 µg both glycosylated as well as deglycosylated proteins) were subjected to western blotting analysis using: (i) cattle antibodies (1:50 dilution) generated to replete-fed (allowed to feed to completion) R. microplus (Reck, Jr. et al., 2009), and (ii) rabbit antibodies generated to replete-fed adult I. scapularis (1:25 dilution), A. americanum (1:50 dilution), and R. sanguineus (1:50 dilution) tick saliva proteins. After three washes in PBS, membranes were incubated with a 1:5,000 dilution of either horseradish peroxidase (HRP)-conjugated anti-bovine or a 1:2,000 dilution of anti-rabbit IgG secondary antibody (Sigma, St. Louis, MO, USA). After three washes with PBS, antibody binding was visualized using 3,3-diaminobenzidine tetrahydrochloride (DAB) substrate.
Antibodies to tick-saliva proteins from replete-fed I. scapularis and A. americanum were produced as previously published (Chalaire et al., 2011; Ibelli et al., 2014). To obtain antibodies to tick saliva proteins from replete-fed R. sanguineus, New Zealand White rabbits were repeatedly infested (three times) with 40 adult ticks (20 males and 20 females). Two weeks after the last infestation, immune serum was collected and stored at −70 °C.
2.10 Protease inhibition assay
To get insight into inhibitory activity of rRmS-3, rRmS-6, and rRmS-17, their protease inhibitory activities were evaluated against a panel of 16 mammalian serine proteases, most of which are related to host defense pathways against tick feeding (the amount of protease used is give in parenthesis): bovine thrombin (43 U), pancreatic porcine elastase (21.6 nM), pancreatic bovine trypsin (24.6 nM), pancreatic bovine α-chymotrypsin (96 nM), pancreatic porcine kallikrein (33 U), human chymase (10 U), human tryptase (10 U), human plasmin (10 nM) (Sigma-Aldrich, St. Louis, MO, USA), human neutrophil cathepsin G (166 nM) (Enzo Life Sciences Inc., Farmingdale, NY, USA), human factor XIa (3.68 nM), bovine factor IXa (306 nM), human factor XIIa (7.6 nM), human t-PA (32 nM), human u-PA (47.2 nM) (Molecular Innovations, Inc., Novi, MI, USA), bovine factor Xa (5.8 nM) (New England Biolabs, Ipswich, MA, USA), and human proteinase-3 (68 U) (EMD Millipore, Billerica, MA, USA). Substrates were used at 0.20 mM final concentration and purchased from Sigma-Aldrich: N α-benzoyl-DL-Arg-pNA for tryptase; N-succinyl-Ala-Ala-Pro-Phe-pNA for chymase, cathepsin G, and chymotrypsin; N-benzoyl-Phe-Val-Arg-pNA for thrombin and trypsin; and N-succinyl-Ala-Ala-Ala-p-nitroanilide for pancreatic elastase. The following substrates were purchased from Chromogenix, a daughter-company of Diapharma Inc. (Philadelphia, PA, USA): Bz-Ile-Glu(γ-OR)-Gly-Arg-pNA for factor Xa; H-D-Val-Leu-Lys-pNA for plasmin; and H-D-Pro-Phe-Arg-pNA for kallikrein, factor XIa, and factor XIIa. The substrate CH3SO2-D-CHG-Gly-Arg-pNA was purchased from Enzyme Research and used for factor IXa, u-PA and t-PA. The substrate N-methoxysuccinyl-Ala-Ala-Pro-Val-pNA was purchased from Enzo Life Sciences and used for proteinase-3. Reagents were mixed at room temperature in triplicate. Recombinant proteins (1 µM) were pre-incubated with indicated amounts of the protease for 15 min at 37 °C in 20 mM Tris-HCl, 150 mM NaCl, BSA 0.1%, pH 7.4. The corresponding substrate for each protease was added to a 100-µL final reaction volume. Substrate hydrolysis was measured at OD405nm every 11 s for 30 min at 30 °C using the Infinite M200 Pro plate reader (Tecan, Männedorf, Switzerland). Acquired OD405nm data were subjected to one phase decay analysis in Prism 6 software (GraphPad Software, La Jolla, CA, USA) to determine plateau values as proxy for initial velocity of substrate hydrolysis. The percent protease activity inhibition level was determined using the formula: 100 − (Vi/V0) × 100 where, Vi = activity in presence of, and V0 = activity in absence of recombinant serpins. Data are presented as mean percent of inhibition from triplicate readings and at least duplicate assays. The unit (U) definition used here is the amount of proteinase necessary for the increase of 0.001 OD405nm/min.
2.11 Stoichiometry of inhibition assay
Stoichiometry of inhibition (SI) indices were determined for each serpin displaying inhibitory activity. The kinetic of substrate hydrolysis in the absence and in the presence of recombinant serpins was evaluated. Preparations were pre-incubated for 1 h with constant concentration of: (i) chymotrypsin (10 nM), cathepsin G (200 nM), and pancreatic elastase (50 nM) for rRmS-3 (molar ratio ranging from 0 to 10); (ii) trypsin (10 nM), chymotrypsin (10 nM), factor Xa (5 nM), factor XIa (5 nM), and plasmin (50 nM) for rRmS-6 (molar ratio ranging from 0 to 10 for trypsin, chymotrypsin and plasmin, and from 0 to 20 for factor Xa and factor XIa); and (iii) trypsin (10 nM), chymotrypsin (10 nM), cathepsin G (200 nM), factor XIa (5 nM), and plasmin (50 nM) for rRmS-17 (molar ratio ranging from 0 to 10 for trypsin, chymotrypsin, cathepsin G and plasmin, and from 0 to 20 for factor XIa). Protease activity was measured using colorimetric substrates specific for each proteinase as described above. Data were plotted as the protease residual activity (Vi/V0) versus serpin:protease molar ratio. SI or the molar ratio serpin to protease (when protease activity is completely inhibited) was determined by fitting data onto the linear regression line and the SI index was estimated from the X-axis intercept (Kantyka and Potempa, 2011).
2.12 Serpin-protease complex formation
Formation of covalent complexes serpin-protease (Huntington et al., 2000) was evaluated as follows. Affinity-purified (i) rRmS-3 was incubated with chymotrypsin (0.5 µg) and cathepsin G (0.5 µg); (ii) rRmS-6 was incubated with trypsin (1.16 µg); and (iii) rRmS-17 was incubated with trypsin (1.16 µg) and cathepsin G (0.5 µg), all in varying molar ratios (from 0 to 10). These serpin-protease incubations were prepared in buffer (Tris-HCl 20 mM, NaCl 150 mM, pH 7.4) and incubated for 1 h at 37 °C. SDS-PAGE denaturing sample buffer was added to the reaction mix, and incubated for 5 min at 99 °C. Samples were subjected to 12.5 % SDS-PAGE and stained with Coomassie brilliant blue.
2.13 Recalcification time
The recalcification time (RT) assay was performed with 50 µL of universal coagulation reference human plasma Thermo Scientific, Waltham, MA, USA) pre-incubated in presence (10 µM) or absence of recombinant serpins in 90 µL of reaction buffer (20 mM Tris-HCl, 150 mM NaCl, pH 7.4) for 15 min at 37 °C. Adding 10 µL of pre-warmed 150 mM calcium chloride (CaCl2) triggered plasma clotting. Plasma clotting was monitored every 20 s for 30 min at OD605nm using the Infinite M200 Pro plate reader (Tecan, Männedorf, Switzerland) (Ciprandi et al., 2006).
2.14 Anti-platelet aggregation activity
Anti-platelet aggregation activity of rRmS-3 and rRmS-17 upon cathepsin G-induced platelet aggregation was determined using platelet-rich plasma (PRP) prepared from citrated (acid citrate dextrose) whole bovine blood as previously described (Berger et al., 2010; Kim et al., 2015). To prepare PRP, fresh citrated whole bovine blood was centrifuged at 200 g for 20 min at 18 °C. Subsequently, the PRP (top layer) was transferred into a new tube and centrifuged at 800 g for 20 min at 18 °C. The pellet containing platelets was washed and diluted with Tyrode solution pH 7.4 (137 mM NaCl, 2.7 mM KCl, 12 mM NaHCO3, 0.42 mM Na2HPO4, 1 mM MgCl2, 0.1 % glucose, 0.25 % BSA) until OD650 = 0.15. To determine anti-platelet aggregation activity, different amounts of rRmS-3 or rRmS-17 (varying from 3 µM to 0 µM) were pre-incubated for 15 min at 37 °C with cathepsin G (0.7 µM) in a 50-µL reaction. Adding 100 µL of pre-warmed PRP triggered platelet aggregation. Platelet aggregation was monitored every 20 s over 30 min at OD650nm using the Infinite M200 Pro plate reader (Tecan, Männedorf, Switzerland). In this assay, higher OD650nm was observed in our blank (platelet only), and increased platelet aggregation was correlated with reduction in OD650nm. Percentage of platelet aggregation inhibition was quantified by calculating the area under the curve and expressed as percent of the negative (only PRP and buffer) and positive control (absence of serpin). Data is presented as mean ± SEM of triplicate platelet aggregation assays.
2.15 Anti-rRmS antibodies effect upon serpin inhibitory activity
Purified rabbit IgG from anti-sera or MAbs raised against serpins were used to check if antibody-binding to serpins could block their protease inhibitory activity. rRmS-3 (55 nM), rRmS-6 (55 nM), and rRmS-17 (88 nM) were pre-incubated with purified rabbit IgG from anti-sera (varying from 0.8 to 3 µM) or purified mouse IgG from MAbs (varying from 0.3 to 1 µM) for 30 min at 37 °C before the addition of chymotrypsin (4.9 nM) for rRmS-3, or trypsin (1.6 nM) for rRmS-6 and rRmS-17, following a new 15-min incubation at 37 °C. Substrate (N-(p-Tosyl)-Gly-Pro-Lys-pNA for trypsin, and N-succinyl-Ala-Ala-Pro-Phe-pNA for chymotrypsin) was added to a 100-µL final reaction volume (final concentration 0.2 mM) and substrate hydrolysis was measured at OD405nm every 11 s for 15 min at 30 °C using the VersaMax tunable plate reader (Molecular Devices, Sunnyvale, CA, USA). Data are shown as mean of two assays done in duplicates.
3. Results
3.1 Salivary serpins are glycoproteins
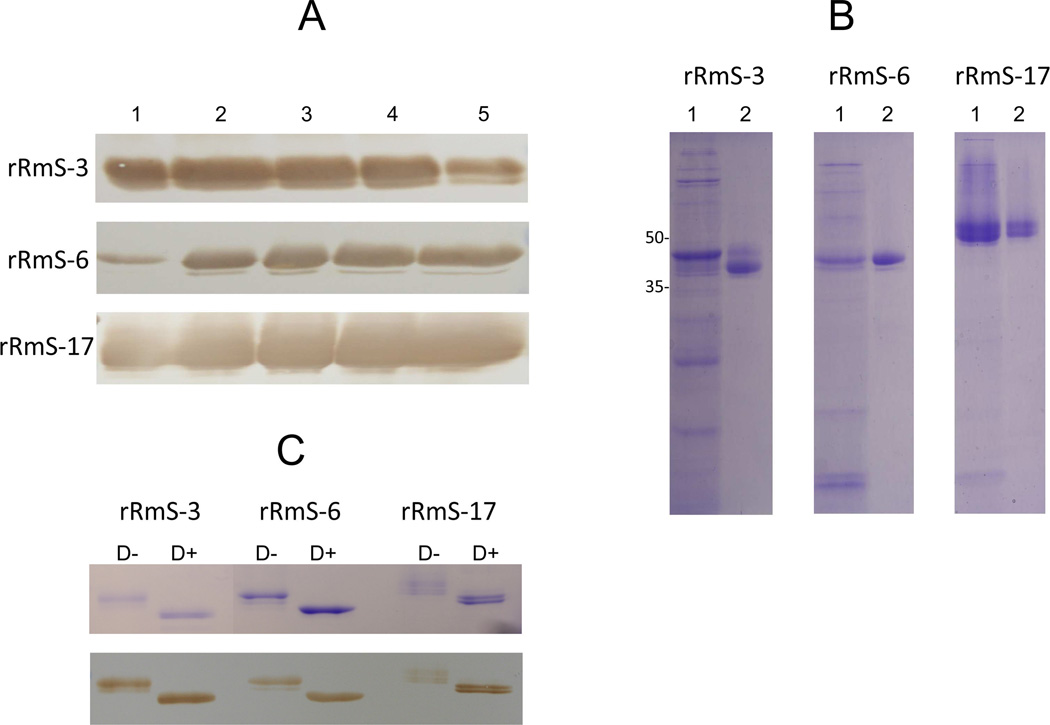
Data about expression and affinity purification of rRmS-3, rRmS-6, and rRmS-17 in P. pastoris are summarized in Fig.1A and 1B. Daily samples of yeast-expressed recombinant proteins were subjected to western blotting analysis using a specific antibody directed to the C-terminus histidine tag (Fig. 1A). Recombinant proteins were purified by affinity chromatography under native conditions and migrated according to molecular weight: ≈40 kDa for rRmS-3 and rRmS-6, and ≈50 kDa for rRmS-17 (Fig. 1B). When treated with deglycosylation enzymes, downward molecular weight shifts were observed (Fig. 1C), demonstrating that rRmS-3, rRmS-6, and rRmS-17 are glycosylated and consistent with sequence-based predictions (Tirloni et al., 2014b).
Figure 1. Recombinant expression of R. microplus salivary serpins in Pichia pastoris.
(A) Daily expression levels of rRmS-3, rRmS-6, and rRmS-17 throughout five days (1–5). Recombinant proteins in spent culture media were precipitated by ammonium sulfate and verification of protein expression was performed using an antibody to the C-terminus hexa histidine tag. (B) Total expression (1) and affinity purified-(2) rRmS-3, rRmS-6, and rRmS-17 were resolved on a 12.5 % SDS-PAGE following Coomassie brilliant blue staining. (C) Recombinant serpins treated with deglycosylation enzyme mix (D+) or without treatment (D−) were resolved on a 12.5 % SDS-PAGE following Coomassie brilliant blue staining and western blotting using anti-C-terminus hexa histidine tag antibody.
3.2 Serum from animals repeatedly infested with different tick species recognize R. microplus saliva serpins
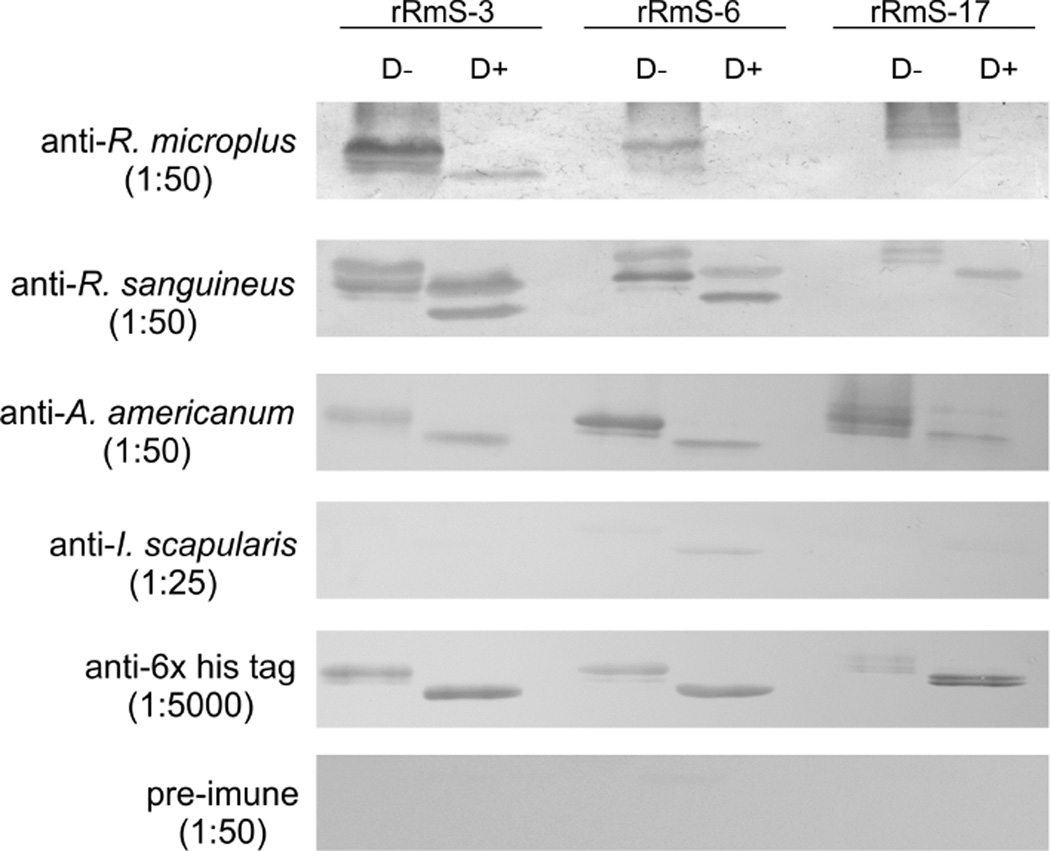
Immune serum from bovine repeatedly infested with R. microplus recognized all three recombinant salivary serpins (Fig. 2). Both glycosylated and deglycosylated forms of rRmS-3 bound R. microplus antibodies, while only glycosylated rRmS-6 and rRmS-17 were recognized by bovine anti-serum to R. microplus saliva proteins (Fig. 2). In addition to R. microplus-infested bovine serum, serum from R. sanguineus and A. americanum repeatedly infested rabbits bound both glycosylated and deglycosylated forms of the three serpins. Immune serum to I. scapularis weakly bound to rRmS-6 (Fig. 2).
Figure 2. Inter-species cross-reactivity assay.
Purified rRmS-3, rRmS-6 and rRmS-17 treated with deglycosylation enzyme mix (D+) or without treatment (D−) were resolved on a 12.5 % SDS-PAGE following western blotting analysis with: cattle serum generated by R. microplus tick infestation (dilution 1:50), rabbit serum generated by adult R. sanguineus infestations (dilution 1:50), A. americanum infestation (dilution 1:50), and adult I. scapularis infestation (dilution 1:25). Anti-C-terminus hexa histidine tag antibodies (dilution 1:5000) and rabbit pre-immune serum (dilution 1:50) were used as control.
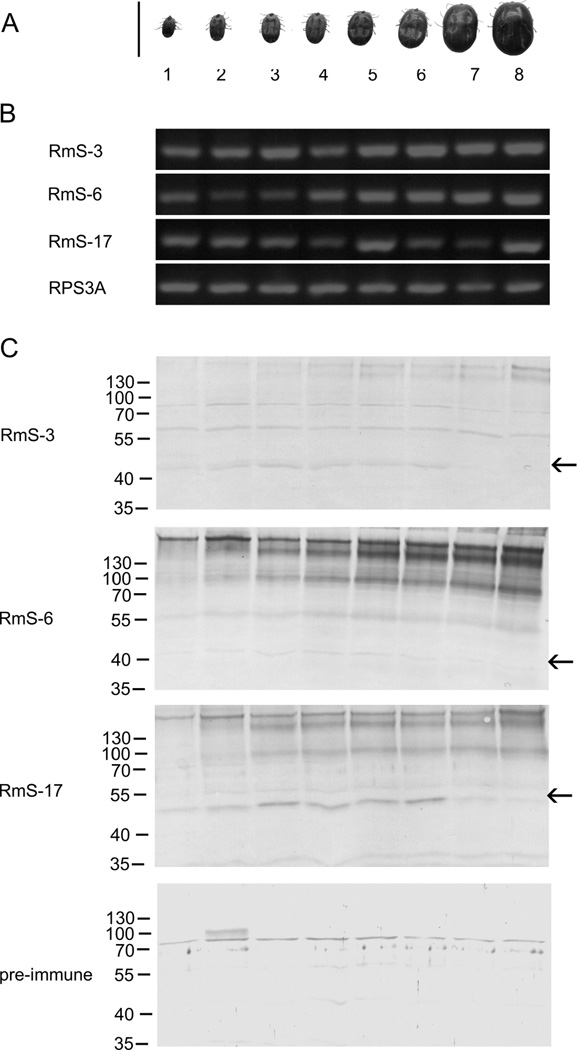
3.3 Feeding and salivary serpins expression profile
The temporal transcription analyses show that mRNA of serpins in tick salivary gland was present throughout all SG blood feeding stages investigated (Fig. 3A and Fig. 3B). In order to compare mRNA expression profiles with protein production, total tick salivary proteins were subjected to western blotting analysis (Fig. 3C). Polyclonal sera recognized all three serpins in the eight different groups of tick SG (Fig. 3C). Serpins appear as a band between 40 and 55 kDa (arrows in Fig. 3C). This molecular size is in accordance as previously published for identification of RmS-3, RmS-6, and RmS-17 in R. microplus saliva by GeLC-MS/MS (Tirloni et al., 2014a). RmS-3, RmS-6 and RmS-17 were expressed at some level throughout all groups, (Fig. 3C).
Figure 3. Transcription and expression profile of salivary serpins.
(A) Comparison of the feeding stage of ticks used for serpin expression profile.. (B) Total RNA was extracted from ticks and subjected to RT-PCR to amplify RmS-3, RmS-6, and RmS-17 fragments. Tick ribosomal protein S3a was used as reference. (C) Total protein extracts of SG from eight groups of ticks were subjected to western blotting analyses using antirRmS-3, anti-rRmS-6, rRmS-17 sera as well as pre-immune serum. Arrows indicate the position of the native protein.
3.4 R. microplus salivary serpins inhibit trypsin- and chymotrypsin-like proteases
The inhibitory profile of rRmS-3, rRmS-6, and rRmS-17 serpins against a panel of 16 mammalian proteases associated with host defense pathways showed a trypsinand chymotrypsin-like inhibitory pattern (Table 1). Incubation of serpins with the protease in a molar excess showed that rRmS-3 (1 µM) inhibited the activity of chymotrypsin (96 nM) by 96 %, the activity of cathepsin G (166 nM) by 78 %, the activity of pancreatic elastase (21.6 nM) by 92 %, and the activity of chymase (10 U) by 23 %. Comparatively, rRmS-6 (1 µM) inhibited the activity of trypsin (24.6 nM) by 73 %, the activity of plasmin (10 nM) by 24 %, the activity of factor Xa (5.8 nM) 32 %, the activity of factor XIa (3.68 nM) by 62 %, and the activity of chymotrypsin (96 nM) by 24 %. Also, rRmS-17 (1 µM) inhibited the activity of trypsin (24.6 nM), plasmin (10 nM), cathepsin G (166 nM), chymotrypsin (96 nM), and factor XIa (3.68 nM) by 87 %, 58 %, 78 %, 89 %, and 98 %, in that order (Table 1).
Table 1.
R. microplus salivary serpins inhibitory profile.
| Protease | Serpin | |||
|---|---|---|---|---|
| RmS-3 (1 µM) | RmS-6 (1 µM) | RmS-17 (1 µM) | ||
| Chymotrypsin (96 nM) | 96.94 ± 1.07 | 24.29 ± 7.39 | 89.76 ± 2.97 | |
| Factor IXa (300 nM) | n.i | n.i | n.i | |
| Cathepsin G (166 nM) | 78.74 ± 0.20 | n.i | 78.84 ± 0.29 | |
| Factor XIa (3.68 nM) | n.i | 62.86 ± 11.10 | 98.02 ± 1.24 | |
| Trypsin (24.6 nM) | n.i | 73.52 ± 11.91 | 87.03 ± 4.80 | |
| Factor Xa (5.8 nM) | n.i | 32.76 ± 10.68 | n.i | |
| Elastase (21.6 nM) | 92.62 ± 2.40 | n.i | n.i | |
| Plasmin (10 nM) | n.i | 24.13 ± 4.98 | 58.39 ± 13.52 | |
| Chymase (10 U) | 23.36 ± 4.64 | n.i | n.i | |
| Factor XIIa (7.6 nM) | n.i | n.i | n.i | |
| Thrombin (43 U) | n.i | n.i | n.i | |
| u-PA (47.2 nM) | n.i | n.i | n.i | |
| Tryptase (10 U) | n.i | n.i | n.i | |
| t-PA (32 nM) | n.i | n.i | n.i | |
| Proteinase-3 (68 U) | n.i | n.i | n.i | |
| Kallikrein (33 U) | n.i | n.i | n.i | |
n.i: not inhibited or inhibition rate lower than 20%.
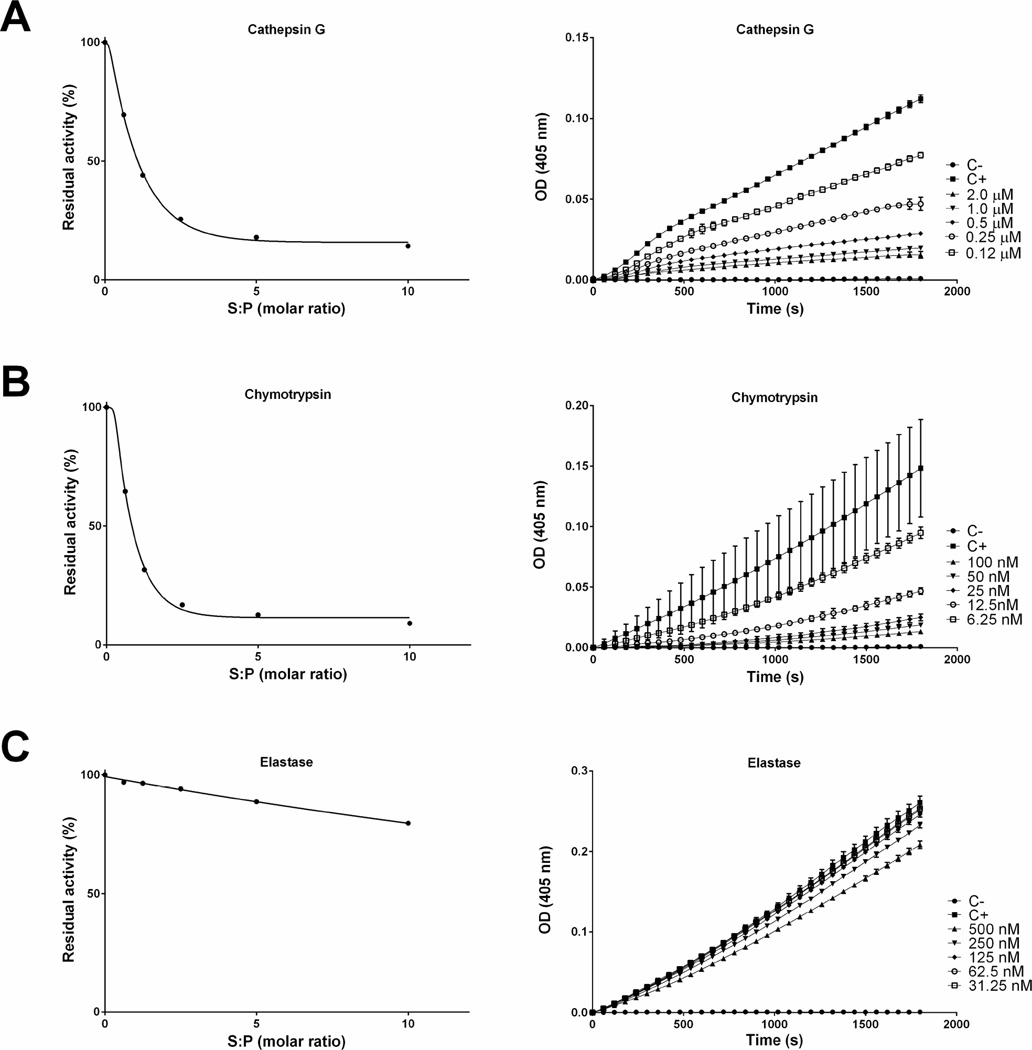
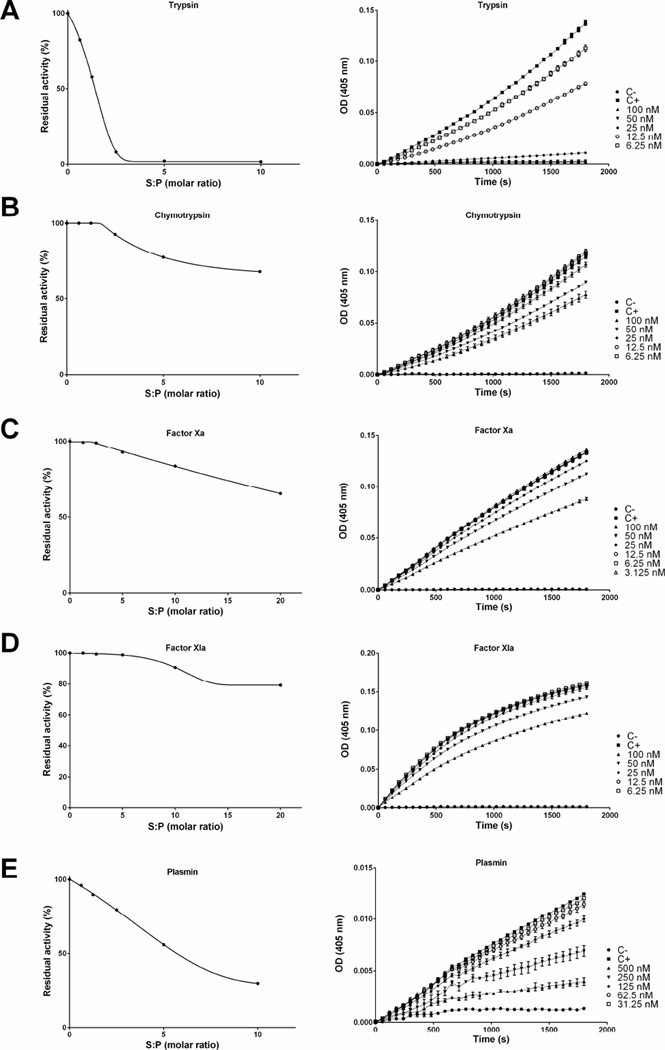
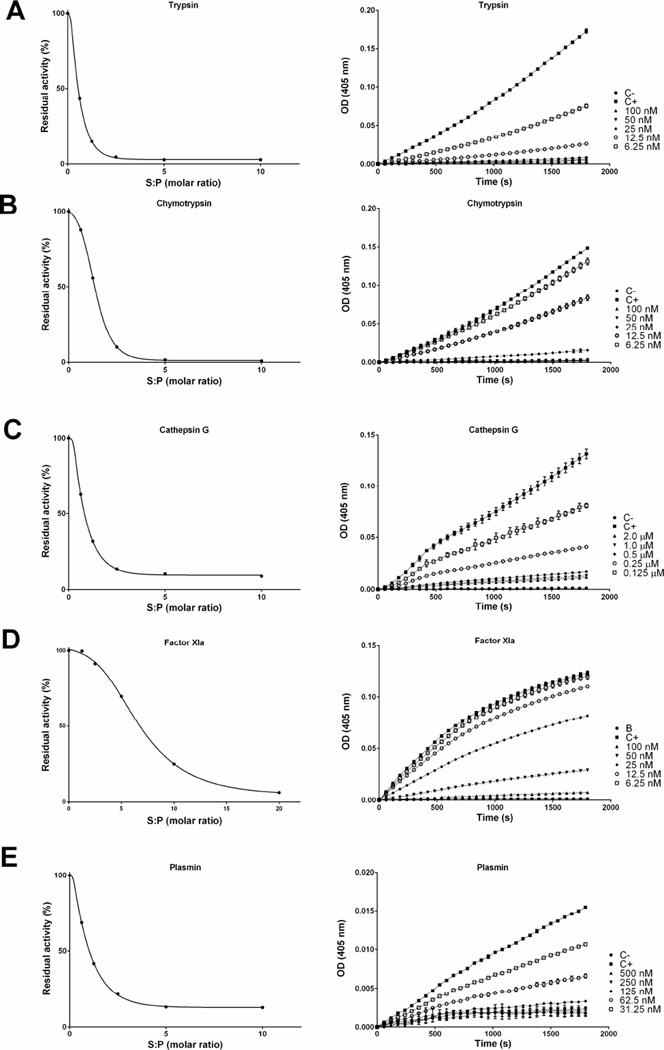
The inhibitory efficiency was evaluated using the SI (stoichiometry of inhibition) index where the kinetics of inhibition by tick serpins upon their sensible protease was tested at different molar ratios. Accordingly, the SI index for rRmS-3 was 2.0 for cathepsin G (Fig. 4A), 1.8 for chymotrypsin (Fig. 4B), and higher than 10 for pancreatic elastase (Fig. 4C). For rRmS-6, SI index was 2.6 for trypsin (Fig. 5A), near to 10 for plasmin (Fig. 5E) and higher than 10 for chymotrypsin (Fig. 5B), factor Xa (Fig. 5C) and factor XIa (Fig. 5D). For rRmS-17, SI index was 1.6 for trypsin (Fig. 6A), 2.7 for chymotrypsin (Fig. 6B), 2.6 for cathepsin G (Fig. 6C), 3.4 for plasmin (Fig. 6D), and higher than 10 for factor XIa (Fig. 6E).
Figure 4. rRmS-3 stoichiometry inhibition (SI) assay.
Residual protease activity in the presence and absence of rRmS-3 was evaluated pre-incubating serpin for 1 h at 37 °C with (A) cathepsin G (200 nM), (B) chymotrypsin (10 nM), and (C) pancreatic elastase (50 nM), resulting in molar ratios (serpin:protease) ranging from 0 to 10. Protease activity was measured using specific colorimetric substrate for each protease as described in Materials and Methods. The data were plotted as residual protease activity (Vi/V0) versus molar ratio (serpin:protease).
Figure 5. rRmS-6 stoichiometry inhibition (SI) assay.
Residual protease activity in the presence and absence of rRmS-6 was evaluated pre-incubating serpin for 1 h at 37 °C with (A) trypsin (10 nM), (B) chymotrypsin (10 nM), (C) factor Xa (5 nM), (D) factor XIa (5 nM), and (E) plasmin (50 nM), resulting in a molar ratio (serpin:protease) ranging from 0 to 10 for trypsin, chymotrypsin, and plasmin, and from 0 to 20 for factor Xa and factor XIa. Protease activity was measured using specific colorimetric substrate for each protease as described in Materials and Methods. The data were plotted as residual protease activity (Vi/V0) versus molar ratio (serpin:protease).
Figure 6. rRmS-17 stoichiometry inhibition (SI) assay.
Residual protease activity in the presence and absence of rRmS-17 was evaluated pre-incubating serpin for 1 h at 37 °C with (A) trypsin (10 nM), (B) chymotrypsin (10 nM), (C) cathepsin G (200 nM), (D) factor XIa (5 nM), and (E) plasmin (50 nM), resulting in a molar ratio (serpin:protease) ranging 0 to 10 (for factor XIa molar ratio ranges between 0 to 20). Protease activity was measured using specific colorimetric substrate for each protease as described in Materials and Methods. The data were plotted as residual protease activity (Vi/V0) versus molar ratio (serpin:protease).
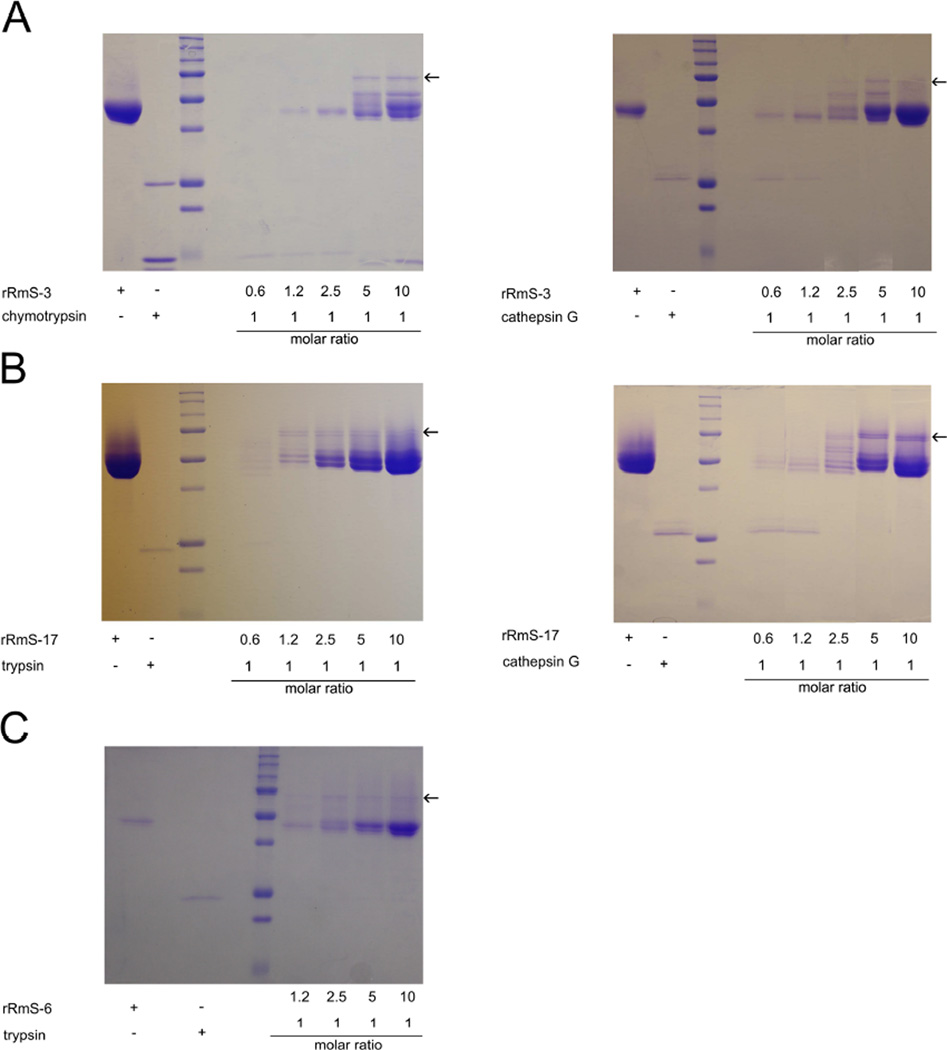
The mechanisms of action of rRmS-3, rRmS-6, and rRmS-17 confirmed that they behave like typical inhibitory serpins (Fig. 7). rRmS-3 is able to form covalent complex with chymotrypsin and cathepsin G (Fig. 7A), rRmS-6 is able to form a stable complex with trypsin (Fig. 7C), and rRmS-17 is able to form stable complexes with trypsin and cathepsin G (Fig. 7B). After incubation of a protease with a serpin, the resulting complex has molecular mass increased to a value corresponding to the sum of the masses of the target protease and the cleaved serpin (complexes indicated by arrows in Fig. 7). These irreversible complexes between serpins and the target proteases were observed at similar molar ratios shown in SI assays (Figs. 4 – 6).
Figure 7. Heat and SDS-stable complex formation assay.
Increasing amounts of recombinant serpins were pre-incubated for 1 h at 37 °C with a constant concentration of (A) chymotrypsin and cathepsin G for rRmS-3, (B) trypsin and cathepsin G for rRmS-17, and (C) trypsin for rRmS-6, resulting in molar ratios varying from 0.6:1 to 10:1 (serpin:protease). Samples were resolved on 12.5% SDS–PAGE and Coomassie blue-stained to identify SDS-stable complexes (indicated by arrows).
3.5 rRmS-3 and rRmS-17 inhibit cathepsin G-induced platelet aggregation
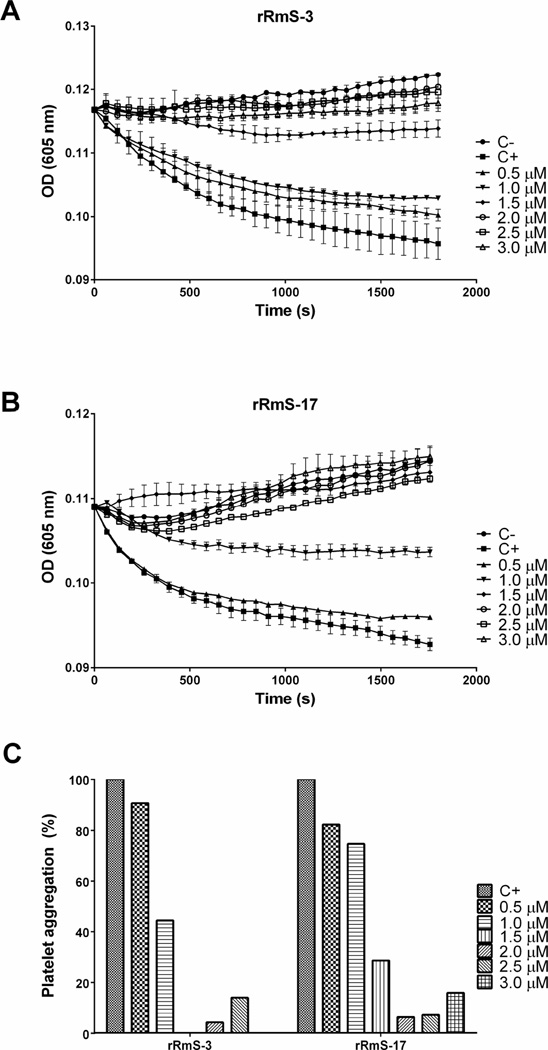
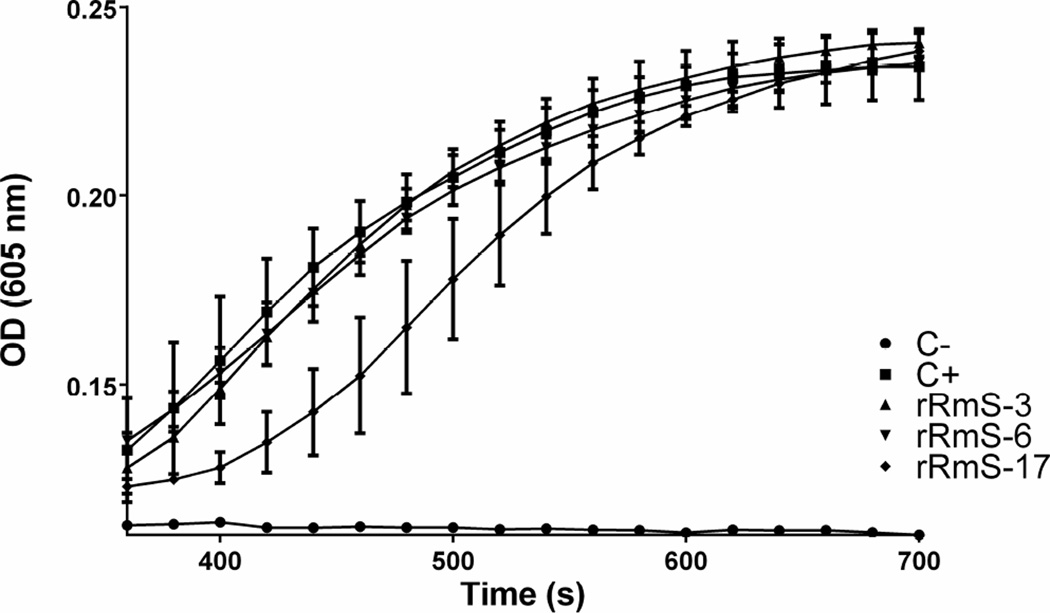
Cathepsin G released from activated neutrophils induces platelet aggregation via PAR4 activation (Sambrano et al., 2000; Selak et al., 1988). Since rRmS-3 and rRmS-17 were able to inhibit cathepsin G (Table 1, Fig. 4, and Fig. 6), we tested the ability of these serpins to inhibit cathepsin G-induced platelet aggregation. rRmS-3 inhibited cathepsin G-activated platelet aggregation in a dose-responsive manner by 10% at 0.5 µM to 96% at 2.5 µM (Fig. 8A and Fig. 8C). Similar result was observed for rRmS-17, which inhibited cathepsin G-activated platelet aggregation by 18% at 0.5 µM to 94% at 2.5 µM (Fig. 8B and Fig. 8C). R. microplus recombinant serpins did not have any effect on thrombin and ADP-induced platelet aggregation (data not shown). When subjected to blood clotting assays, rRmS-17 delayed recalcification time (RT) by 60 s (Fig. 9). However, none of these three serpins delayed plasma clotting time, aPTT, PT, and TT (data not shown).
Figure 8. rRmS-3 and rRmS-17 effect upon cathepsin G-induced platelet aggregation.
Platelet aggregation function induced by cathepsin G assay was done using bovine platelet rich plasma (PRP) as described in Materials and Methods. (A) Tyrode solution with varying amounts of rRmS-3 or rRmS-17 (3 µM, 2.5 µM, 2 µM, 1.5 µM, 1 µM, and 0.5 µM) were pre-incubated with cathepsin G (0.7 µM) in a 50-µL reaction for 15 min at 37 °C. Platelet aggregation was initiated with addition of 100 µL pre-warmed PRP and monitored at 20-s intervals over 30 min at OD650nm. (B) Percent of reduction of cathepsin G-induced platelet aggregation inhibited by rRmS-3 and rRmS-17.
Figure 9. Effect of R. microplus serpins upon plasma recalcification time.
Human reference plasma (50 µL) was incubated with rRmS-3 (10 µM), rRmS-6 (10 µM), and rRmS-17 (10 µM) in 90 µL of Tris–HCl reaction buffer for 15 min at 37 °C followed by the addition of 150 mM CaCl2 (10 µL). Clotting was measured every 20 s for 30 min.
3.6 Antibodies to RmS-3, -6 and -17 recognize native and recombinant serpins
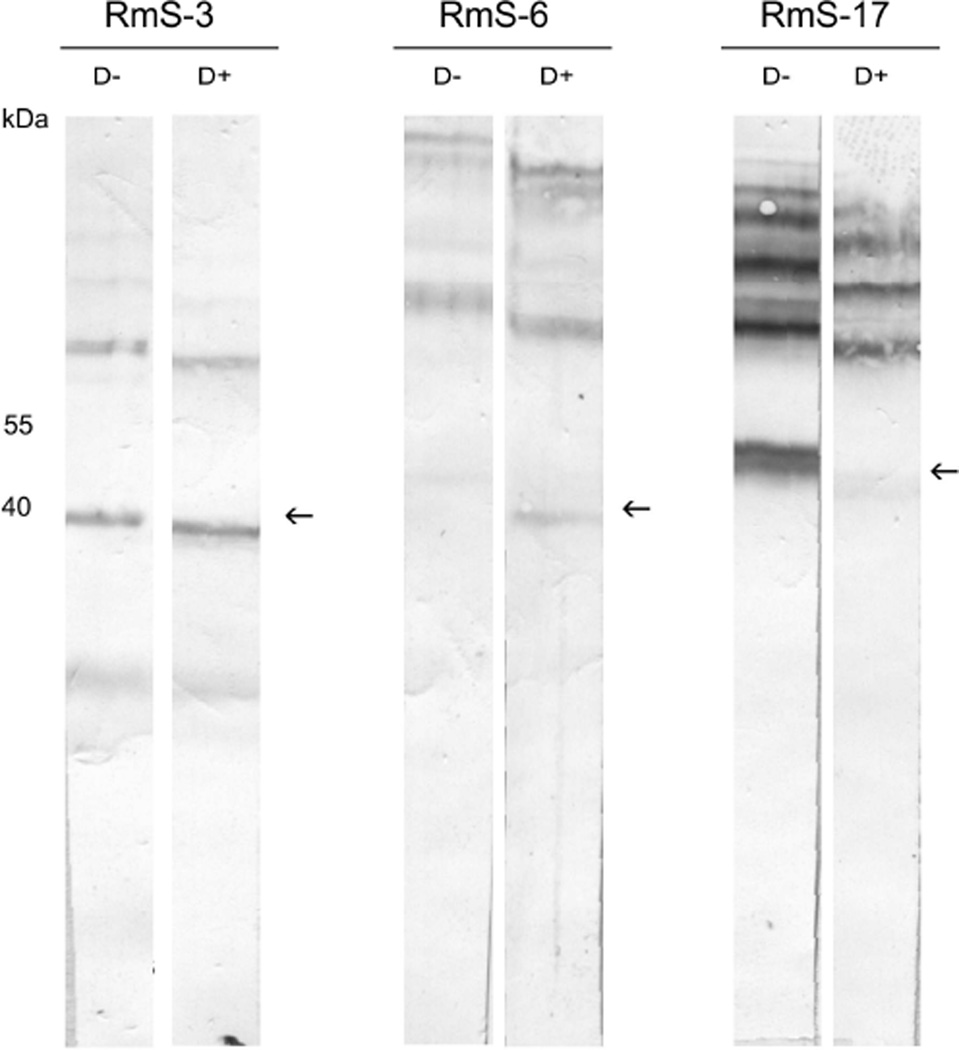
Five monoclonal antibodies (MAb) were generated from rRmS-6-immunized mice, and one MAb was obtained from an rRmS-17-immunized mouse. MAbs and purified rabbit IgG were tested in an indirect ELISA to detect cross-reactivity among the recombinant serpins (Table 2). MAbs BrBm41 and BrBm42 reacted only with rRmS-6, while MAbs BrBm39 and BrBm43 reacted with all three recombinant proteins. Although clone BrBm40 recognizes all three serpins, it reacts poorly with rRmS-17. Though MAb BrBm44 recognizes all three recombinant serpins, reactions were weak (Table 2). The three polyclonal sera reacted with all three recombinant proteins, showing cross-reactivity ranging from 30% to 55% with two other serpins (Table 2). Two of the MAbs (BrBm39 and BrBm40) were able to recognize native serpins in the saliva, as shown by a sandwich ELISA (Table 2), although no reaction was observed when MAbs were tested with denatured saliva proteins in a western blotting assay (data not shown). Additionally, all three polyclonal sera were able to recognize serpins in the saliva, whether they were glycosylated or deglycosylated (Fig. 10). The variation in molecular sizes of glycosylated and deglycosylated saliva proteins confirms that native RmS-3, RmS-6, and RmS-17 are secreted as glycosylated proteins (Fig. 10).
Table 2.
Antibodies reactivity with native salivary and recombinant serpins.
| Antibody | Immunogen | Cross-reactivity (%)* | |||
|---|---|---|---|---|---|
| Recombinant | Native | ||||
| Monoclonal | rRmS-3 | rRmS-6 | rRmS-17 | Saliva | |
| BrBm39 | rRmS-6 | 79 (±8) | 100 | 69 (±20) | 91 (±5.5) |
| BrBm40 | rRmS-6 | 51 | 100 | 30 | 106 (±19) |
| BrBm41 | rRmS-6 | NR** | 100 | NR** | NR** |
| BrBm42 | rRmS-6 | NR** | 100 | NR** | NR** |
| BrBm43 | rRmS-6 | 76 (±22) | 100 | 49 (±11) | NR** |
| BrBm44 | rRmS-17 | Weak** | Weak** | Weak** | NR** |
| Polyclonal | |||||
| Anti-RmS-3 | rRmS-3 | 100 | 47 (±10) | 40 (±9) | +*** |
| Anti-RmS-6 | rRmS-6 | 35 (±10) | 100 | 31 (±6) | +*** |
| Anti-RmS-17 | rRmS-17 | 46 (±10) | 56 (±14) | 100 | +*** |
Compared to primary reactivity. Results are shown as mean (±SD)
NR = No reaction; Weak = low reaction with all three serpins tested
+ : please refer to Figure 10 for western blotting reactions
Figure 10. Reactivity of anti-rRmS antibodies with RmS-3, RmS-6 and RmS-17 from tick saliva.
Pilocarpine-induced tick saliva were harvested as described in Material and Methods. Saliva total protein treated with deglycosylation enzyme mix (D+) or non-treated (D−) were resolved on a 12 % SDS-PAGE following western blotting using polyclonal sera (A) anti-rRmS-3, (B) anti-rRmS-6, and (C) anti-rRmS-17.
3.7 Antibodies abolish serpin inhibitory activity function
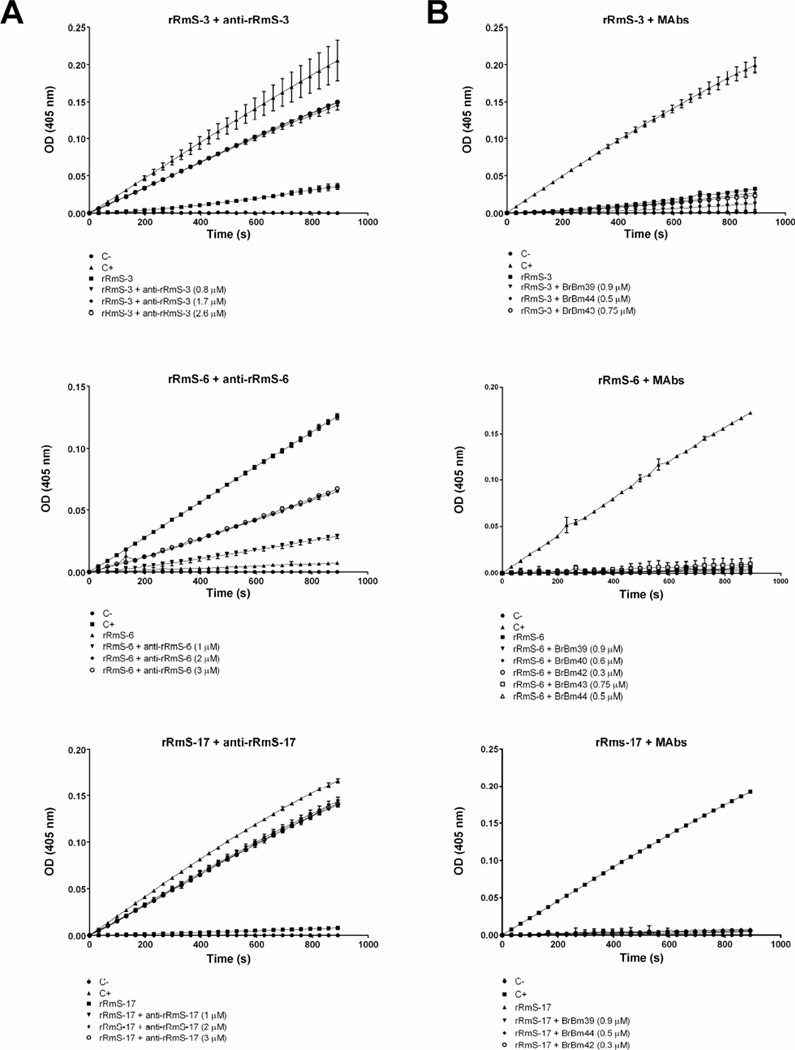
Purified IgG from polyclonal serum or MAbs were used for the serpin blocking activity assay. Each serpin was pre-incubated with IgG for 30 min followed by a second incubation with the test protease, when kinetics was monitored for 15 min. Blockage was observed exclusively when purified IgG from polyclonal serum was tested (Fig. 11 and Table 3), since IgG from MAbs was not able to block serpin inhibitory function (Fig. 11). This result shows that polyclonal antibodies were able to block the inhibitory activity of rRmS-3 by approximately 66%, of rRmS-6 by 50% and of rRmS-17 by 85% (Table 3). A non-related serpin anti-serum was used as control (anti-VTDCE) (Oldiges et al., 2012) and did not affect serpin inhibitory activity(Fig. S1).
Figure 11. Effect of antibodies upon serpin inhibitory activity.
Purified IgG from (A) rabbit polyclonal serum or (B) from mice MAbs (anti-rRmS-3, anti-rRmS-6, and rRmS-17) were incubated with rRmS-3 (55 nM), rRmS-6 (55 nM) or rRmS-17 (88 nM) at 37 °C for 30 min before the addition of chymotrypsin (4.9 nM) for rRmS-3, or trypsin (1.6 nM) for rRmS-6 and rRmS-17, following a new 15-min incubation. Protease kinetics was monitored for 15 min every 11 s.
Table 3.
Antibodies effect on serpin inhibitory activity.
| Antibody | Serpin activity inhibition (%)* |
|---|---|
| rRmS-3 | |
| rRmS-3 | 0 |
| rRmS-3 + anti-rRmS-3 (0.8 µM) | 66.7 |
| rRmS-3 + anti-rRmS-3 (1.7 µM) | 64.2 |
| rRmS-3 + anti-rRmS-3 (2.6 µM) | 66.8 |
| rRmS-6 | |
| rRmS-6 | 0 |
| rRmS-6 + anti-rRmS-6 (1 µM) | 18.9 |
| rRmS-6 + anti-rRmS-6 (2 µM) | 48.8 |
| rRmS-6 + anti-rRmS-6 (3 µM) | 50.4 |
| rRmS-17 | |
| rRmS-17 | 0 |
| rRmS-17 + anti-rRmS-17 (1 µM) | 85.6 |
| rRmS-17 + anti-rRmS-17 (2 µM) | 84.1 |
| rRmS-17 + anti-rRmS-17 (3 µM) | 84.3 |
4. Discussion
In this work we demonstrate the inhibitory properties of three salivary serpins from the cattle tick R. microplus. RmS-3, RmS-6, and RmS-17 are inhibitors of serine proteinases of the serpin superfamily (Tirloni et al., 2014a). In addition to the confirming that these serpins are clearly present in cattle tick saliva, as found using proteomic approaches in a previous study (Tirloni et al., 2014a), here it is shown that antibodies raised against these serpins produced in P. pastoris are able to recognize native serpins that are present in tick saliva. Additionally, immune serum from cattle repeatedly infested with R. microplus recognized all three recombinant salivary serpins, proving that these proteins are injected into animals during tick feeding. Consistent with earlier literature findings, according to which some serpins are indeed glycoproteins (Gettins, 2002), the present study shows that RmS-3, RmS-6 and, RmS-17 are secreted as glycoproteins (Fig. 10).
The protease inhibitory profile of R. microplus salivary serpins shows that rRmS-3 has anti-chymotrypsin activity, rRmS-6 has anti-trypsin activity, and rRmS-17 has both anti-trypsin and anti-chymotrypsin activity. Taking all data together, it becomes clear that rRmS-3 and rRmS-6 have a more strict inhibitory profile, while RmS-17 has a broad-spectrum of inhibition. rRmS-3 action upon chymotrypsin and pancreatic elastase is in accordance with previous work (Rodriguez et al., 2015). However, in that study, the authors showed that rRmS-6 has only anti-chymotrypsin activity (Rodriguez et al., 2015).
Aiming to compare the inhibitory efficiency of these serpins, stoichiometry of inhibition (SI) indices were determined. This index reflects the number of serpin molecules required to form a stable inhibitory complex with a given protease. Accordingly, rRmS-3 is an efficient inhibitor of chymotrypsin and cathepsin G. Although it also inhibits pancreatic elastase at a high excess molar ratio, SI data suggests rRmS-3 is not an efficient pancreatic elastase inhibitor. As pancreatic elastase (used in this study) and neutrophil elastase have different biochemical properties (Bode et al., 1989; Sinha et al., 1987), the inhibition of neutrophil elastase cannot be ruled out, and needs further characterization. Differences in elastase inhibition by some parasitic inhibitors was shown to be dependent on the source of elastase (Jin et al., 2011). rRmS-6 efficiently inhibits trypsin, while rRmS-17 has a broad spectrum of efficient inhibition, as demonstrated using trypsin, chymotrypsin, cathepsin G. However, it is less efficient upon plasmin and factor XIa. The high SI indices observed for some serpin-protease pairs point to the possibility that some serpins need a co-factor to enhance inhibition, as demonstrated for some mammalian serpins (Gettins, 2002; McCoy et al., 2003).
Tertiary serpin structure contains typically three β-sheets, eight or nine α-helices, and a reactive center loop (RCL). RCL is a solvent exposed flexible stretch of 21 amino acid residues positioned between β-sheets sA and sC and that acts as a suicide-substrate of the specific target protease (Gettins, 2002). RCL contains the peptide bond (between residues P1 and P1’) that is cleaved by the target protease, while the P1 residue in RCL is critical to define the specificity of a serpin for a particular protease (Hopkins et al., 1993; Hopkins and Stone, 1995). The predicted P1-P1’ residues of R. microplus salivary serpins are Leu-Ser for RmS-3, Arg-Ile for RmS-6, and Lys-Ser for RmS-17 (Tirloni et al., 2014b), which were predicted to inhibit chymotrypsin-like (RmS-3) and trypsin-like proteinases (RmS-6 and RmS-17) (Keil, 1992). This prediction is in accordance with the inhibitory profile described here. However, the finding that rRmS-17 was able to inhibit α-chymotrypsin is intriguing, since a serpin possessing a Lys at P1 has not been reported to inhibit chymotrypsin-like proteases, which have preference for large hydrophobic amino acids such as Phe, Tyr, or, to a lesser extent, Leu, Met, and His at the P1 position (Keil, 1992). It is possible that RmS-17 has two close reactive sites in RCL, allowing the interaction with both trypsin-like and chymotrypsin-like proteases, as yet demonstrated for another serpin (Liu et al., 2014). The P4-P3’ region of RmS-17 is comprised of Phe-Tyr-Thr-Lys-Ser-Ala-Val (Tirloni et al., 2014b), thus the Phe or Tyr at P4 and P3 position could be the real reactive site in RmS-17 for chymotrypsin inhibition.
rRmS-3 and rRmS-17 were able to inhibit cathepsin G. This protease has both trypsin- and chymotrypsin-like specificity, being able to accommodate both large, hydrophobic P1 residues (Phe, Leu, Met) as well as positive ones (Lys or Arg) (Hof et al., 1996). Modeling studies suggest that Ser40 in cathepsin G forms hydrogen bonds with P1’ Ser, providing a structural basis for the inhibition by natural cathepsin G inhibitors such as α1-antichymotrypsin, α1-antitrypsin, and squamous cell carcinoma antigen 2 (SCCA-2) (Korkmaz et al., 2008). This is in accordance with both RmS-3 and RmS-17 RCL, where the P1-P1’ residues for RmS-3 and RmS-17 are Leu-Ser and Lys-Ser, respectively. Interestingly, the RCL of RmS-3 resembles the chymotrypsin and cathepsin G cleavage site, as present in α1-antichymotrypsin and SCCA-2 serpins (Schick et al., 1997). Since serpins are natural inhibitors of proteases with a role in host defenses against tick feeding (Rau et al., 2007), we hypothesize that this finding is the result of a parasite convergent evolution process that, in ticks, selected a serpin with biologically equivalent recognition sites present in the host’s serpin, allowing tick saliva to target both cathepsin G and chymotrypsin-like proteases, mimicking proteins from the host itself.
After RCL P1-P1’ peptide bond cleavage, serpin covalently traps and distorts the target protease, resulting in a covalent complex serpin-protease, and therefore serpins are classified as suicide inhibitors (Huntington et al., 2000). Data presented here showed that R. microplus salivary serpins interact with proteases via this classical suicide inhibition, as demonstrated by the formation of heat- and SDS-stable complex between serpin and protease (Fig. 8). Altogether, these results demonstrate that RmS-3, RmS-6 and RmS-17 are classical serine protease inhibitors injected in the host during tick feeding, and that they target different host proteases. However, what could be the role of these protease inhibitory profiles regarding host defense modulation?
It was demonstrated that rRmS-6 and rRmS-17 are effective inhibitors of trypsin. Although they are produced predominantly by the pancreas as a means to degrade dietary proteins, trypsin-like proteases are also expressed in the nervous system and in epithelial tissues, where they are the most powerful protease-activated receptor 2 (PAR2) activator being important factors in neurogenic inflammation and pain in the skin (Cattaruzza et al., 2014; Steinhoff et al., 2000). Thus, injection of trypsin-like inhibitors such as RmS-6 and RmS-17 in the feeding site could interfere in pro-inflammatory and algesic responses during tick feeding.
Neutrophils play an essential part as triggers of an immune response against tick feeding (Heinze et al., 2012a; Heinze et al., 2012b). athepsin G, neutrophil elastase and proteinase 3 are the most abundant molecules present in neutrophil azurophil granules (Korkmaz et al., 2008; Pham, 2006; Pham, 2008). Accordingly, with results showed here, rRmS-3 and rRmS-17 effectively inhibited neutrophil cathepsin G (Table 1 and Fig. 5 and Fig. 7). So, these serpins can be involved in modulation of neutrophil function as was observed in I. scapularis saliva, pointing to its importance in the tick feeding process as well as in pathogen transmission (Guo et al., 2009; Ribeiro et al., 1990). Also, rRmS-3 and rRmS-17 were able to inhibit platelet aggregation induced by cathepsin G. The ability of neutrophils to enhance aggregation of human platelets in vitro is linked to cathepsin G activity (Faraday et al., 2013). Altogether, inhibition of cathepsin G could be potentially associated with blockage of several relevant pathways that may result in diminished neutrophil-mediated inflammation and thrombosis during tick feeding.
Another subset of cells with an important function in tick feeding are eosinophils. It was demonstrated that eosinophils have significant chymotrypsin-like activity, and that an inhibitor of the chymotrypsin-like protease markedly inhibited eosinophil peroxidase (EPO) release from eosinophils (Matsunaga et al., 1994). Since rRmS-3 and rRmS-17 have anti-chymotrypsin activity, these serpins could inhibit chymotrypsin-like proteases from cattle eosinophils. Interesting, the importance of eosinophils for tick feeding was highlighted in studies showing hosts upon repeated tick infestation present eosinophil accumulation in the attachment site, which has been linked to tick resistance (Brown et al., 1982; Carvalho et al., 2010; Schleger et al., 1981; Ushio et al., 1993).
Classically described as a protease related to fibrinolytic system, plasmin has been reported to participate also in pro-inflammatory processes (Burysek et al., 2002; Li et al., 2007; Li et al., 2010; Li et al., 2012; Syrovets et al., 1997). Plasmin inhibition by rRmS-6 and rRmS-17 could impair these plasmin immune functions. To investigate whether tick serpins could alter the classical coagulation parameters, standard coagulation tests in the presence of tick serpins were conducted. Given that rRmS-17 interferes with recalcification time, this feature seems to have a relationship with the inhibition of factor XIa. Serpins did not significantly alter plasma clotting time, TT, or aPTT (data not shown).
As serpins are secreted into host during tick feeding, and in order to investigate the specificity of host immune response against serpins during tick infestation, sera of repeated tick-infested hosts were used to probe glycosylated as well as deglycosylated serpins. Data showed that sera from tick-infested animals recognized either glycosylated or deglycosylated serpins. Interestingly, R. microplus serpins were also recognized by serum from hosts infested with A. americanum, R. sanguineus, and I. scapularis (Fig. 2), confirming that serpins are naturally secreted during tick feeding, and that hosts are able to produce a humoral immune response against these proteins after sequential tick infestations. These data are in accordance with a previous study in which RmS-3 was recognized by serum from tick-resistant cattle (Rodriguez-Valle et al., 2012). Furthermore, data showing cross-reactivity among sera from hosts infested with different tick species suggest secretion of similar serpins into saliva of other tick species, which highlight a potential use of salivary serpins as antigens in a universal anti-tick vaccine (Parizi et al., 2012). Serpins have been identified in saliva of other tick species, including D. andersoni, H. longicornis, and A. americanum (Chalaire et al., 2011; Kim et al., 2015; Mudenda et al., 2014; Tirloni et al., 2015), lending strength to the hypothesis that different tick species secrete these proteins in their tick saliva, and pointing to a role in the evasion from host defenses during tick feeding (Chmelar et al., 2011; Kim et al., 2015; Leboulle et al., 2002a; Mulenga et al., 2013; Prevot et al., 2006). Weak reactivity of sera from I. scapularis-infested animals against R. microplus serpins was observed (Fig. 2). Such an observation comes as no surprise, since I. scapularis is a prostriate tick while R. microplus, R. sanguineus, and A. americanum are metastriate ticks. The presence of specific either prostriate as well as metastriate specific-protein families in salivary glands has been clearly demonstrated (Francischetti et al., 2009), suggesting differences in salivary gland contents between these groups of ticks. According to western blotting results, the anti-R. microplus sera reactivity was lower with deglycosylated as compared with glycosylated serpins. These results suggest that the carbohydrate moieties of the serpin proteins participate in but are not essential for eliciting an immune response in the host, which could also be variable. Admittedly, the real importance of oligosaccharides concerning the immunogenicity of a particular glycoprotein cannot be determined without immunization experiments (Gavrilov et al., 2011). Many recombinant non-glycosylated proteins have been used as vaccine antigen, showing that glycosylation is not essential for the induction of a protective response (Imamura et al., 2005; Sugino et al., 2003).
MAbs and polyclonal sera raised against tick serpins crossreacted, though at different levels, with recombinant and native salivary serpins, suggesting that similar epitopes are present in different serpins. When poly- and monoclonal antibodies were used to assay the effect of antibodies against serpin activity, polyclonal antibodies abolished serpin inhibitory activity (Fig. 11). This is of major importance, when serpins are considered antigens in an anti-tick vaccine development. Host functional antibodies raised during immunization could block serpin inhibitory activity during tick feeding and interfere with modulatory functions of serpins, impairing blood meal acquisition and tick development. The different inhibition capacities of the MAbs and polyclonal antibodies can be explained based on the possibility that polyclonal antibodies recognize more than one epitope, while monoclonal antibodies can be recognized as a structure with unstable or non-functional epitopes (Kummer et al., 2004; Parra-Lopez et al., 2014; Saunders et al., 1998).
Analyzed together, our results and previous findings show that serpins are inoculated during tick infestation and play the role of immunogenic inductors of humoral responses. Indeed, serpins are involved in host-parasite interactions and help the parasite to evade the host immune system. In previous studies, proteins from I. ricinus (Prevot et al., 2007), H. longicornis (Sugino et al., 2003), and R. appendiculatus (Imamura et al., 2006; Imamura et al., 2008) were shown to induce the production of antibodies and to be associated with protection against tick infestation. Inducing an efficacious immune response against tick serpins could help hosts to limit and control parasite infections and, therefore, serpins may be included as suitable antigen candidates in an anti-tick vaccine.
Supplementary Material
Purified IgG from a non-related serpin rabbit polyclonal serum (anti-VTDCE; negative control) were incubated with rRmS-3 (55 nM), rRmS-6 (55 nM) or rRmS-17 (88 nM) at 37 °C for 30 min before the addition of chymotrypsin (4.9 nM) for rRmS-3, or trypsin (1.6 nM) for rRmS-6 and rRmS-17, following a new 15-min incubation. Protease kinetics was monitored for 15 min every 11 s.
Highlights.
The tick Rhipicephalus microplus secretes three types of salivary serpins.
R. microplus serpins inhibit pro-inflammatory and pro-coagulant proteases.
RmS-3 and RmS-17 inhibit cathepsin G-induced platelet aggregation.
Anti-serpin antibodies abolish this inhibitory activity.
Sera against other tick species recognize R. microplus serpins.
Acknowledgments
This research was supported by FAPERGS, FAPERJ, INCT-Entomologia Molecular, CNPq and CAPES from Brazil. LT is a receiver of the CNPq (Brazil) “Ciência sem Fronteiras” doctoral fellowship program (PVE 211273/2013-9). AM is a receiver of National Institutes of Health, USA grants (AI081093, AI093858, AI074789, AI074789-01A1S1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Berger M, Reck J, Jr, Terra RM, Pinto AF, Termignoni C, Guimaraes JA. Lonomia obliqua caterpillar envenomation causes platelet hypoaggregation and blood incoagulability in rats. Toxicon. 2010;55:33–44. doi: 10.1016/j.toxicon.2009.06.033. [DOI] [PubMed] [Google Scholar]
- Bode W, Meyer E, Jr, Powers JC. Human leukocyte and porcine pancreatic elastase: X-ray crystal structures, mechanism, substrate specificity, and mechanism-based inhibitors. Biochemistry. 1989;28:1951–1963. doi: 10.1021/bi00431a001. [DOI] [PubMed] [Google Scholar]
- Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal. Biochem. 1989;180:136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J. Immunol. 1982;129:790–796. [PubMed] [Google Scholar]
- Burysek L, Syrovets T, Simmet T. The serine protease plasmin triggers expression of MCP-1 and CD40 in human primary monocytes via activation of p38 MAPK and janus kinase (JAK)/STAT signaling pathways. J. Biol. Chem. 2002;277:33509–33517. doi: 10.1074/jbc.M201941200. [DOI] [PubMed] [Google Scholar]
- Carvalho WA, Maruyama SR, Franzin AM, Abatepaulo AR, Anderson JM, Ferreira BR, Ribeiro JM, More DD, Augusto Mendes MA, Valenzuela JG, Garcia GR, de Miranda Santos IK. Rhipicephalus (Boophilus) microplus: clotting time in tick-infested skin varies according to local inflammation and gene expression patterns in tick salivary glands. Exp. Parasitol. 2010;124:428–435. doi: 10.1016/j.exppara.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Costa T, Mendes M, da SM, da CT, Tiburcio M, Anhe A, Rodrigues V, Oliveira C. Immunosuppressive effects of Amblyomma cajennense tick saliva on murine bone marrow-derived dendritic cells. Parasit. Vectors. 2015;8:22. doi: 10.1186/s13071-015-0634-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaruzza F, Amadesi S, Carlsson JF, Murphy JE, Lyo V, Kirkwood K, Cottrell GS, Bogyo M, Knecht W, Bunnett NW. Serine proteases and protease-activated receptor 2 mediate the pro-inflammatory and algesic actions of diverse stimulants. Br. J. Pharmacol. 2014;171:3814–3826. doi: 10.1111/bph.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalaire KC, Kim TK, Garcia-Rodriguez H, Mulenga A. Amblyomma americanum (L.) (Acari: Ixodidae) tick salivary gland serine protease inhibitor (serpin) 6 is secreted into tick saliva during tick feeding. J. Exp. Biol. 2011;214:665–673. doi: 10.1242/jeb.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmelar J, Oliveira CJ, Rezacova P, Francischetti IM, Kovarova Z, Pejler G, Kopacek P, Ribeiro JM, Mares M, Kopecky J, Kotsyfakis M. A tick salivary protein targets cathepsin G and chymase and inhibits host inflammation and platelet aggregation. Blood. 2011;117:736–744. doi: 10.1182/blood-2010-06-293241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciprandi A, de Oliveira SK, Masuda A, Horn F, Termignoni C. Boophilus microplus: its saliva contains microphilin, a small thrombin inhibitor. Exp. Parasitol. 2006;114:40–46. doi: 10.1016/j.exppara.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Clarke RH, Hewetson RW. A modification to the collection of saliva from Boophilus microplus. J. Parasitol. 1971;57:194–195. [PubMed] [Google Scholar]
- Davie EW, Fujikawa K, Kurachi K, Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv. Enzymol. Relat Areas Mol. Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- de la Fuente J, Almazan C, Canales M, Perez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res. Rev. 2007;8:23–28. doi: 10.1017/S1466252307001193. [DOI] [PubMed] [Google Scholar]
- Diaz-Martin V, Manzano-Roman R, Valero L, Oleaga A, Encinas-Grandes A, Perez-Sanchez R. An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J. Proteomics. 2013;80C:216–235. doi: 10.1016/j.jprot.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Faraday N, Schunke K, Saleem S, Fu J, Wang B, Zhang J, Morrell C, Dore S. Cathepsin G-dependent modulation of platelet thrombus formation in vivo by blood neutrophils. PLoS. One. 2013;8:e71447. doi: 10.1371/journal.pone.0071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JM. The role of saliva in tick feeding. Front Biosci. (Landmark. Ed) 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilov BK, Rogers K, Fernandez-Sainz IJ, Holinka LG, Borca MV, Risatti GR. Effects of glycosylation on antigenicity and immunogenicity of classical swine fever virus envelope proteins. Virology. 2011;420:135–145. doi: 10.1016/j.virol.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Gettins PG. Serpin structure, mechanism, and function. Chem. Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- Grisi L, Leite RC, Martins JR, Barros AT, Andreotti R, Cancado PH, Leon AA, Pereira JB, Villela HS. Reassessment of the potential economic impact of cattle parasites in Brazil. Rev. Bras. Parasitol. Vet. 2014;23:150–156. doi: 10.1590/s1984-29612014042. [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Li AY, Hernandez R. Molecular diagnosis of pyrethroid resistance in Mexican strains of Boophilus microplus (Acari: Ixodidae) J. Med. Entomol. 2002;39:770–776. doi: 10.1603/0022-2585-39.5.770. [DOI] [PubMed] [Google Scholar]
- Guerrero FD, Lovis L, Martins JR. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Rev. Bras. Parasitol. Vet. 2012;21:1–6. doi: 10.1590/s1984-29612012000100002. [DOI] [PubMed] [Google Scholar]
- Guo X, Booth CJ, Paley MA, Wang X, DePonte K, Fikrig E, Narasimhan S, Montgomery RR. Inhibition of neutrophil function by two tick salivary proteins. Infect. Immun. 2009;77:2320–2329. doi: 10.1128/IAI.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies a laboratory manual. 1988 [Google Scholar]
- Heinze DM, Carmical JR, Aronson JF, Alarcon-Chaidez, F, Wikel S, Thangamani S. Murine cutaneous responses to the rocky mountain spotted fever vector, Dermacentor andersoni feeding. Front Microbiol. 2014;5:198. doi: 10.3389/fmicb.2014.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze DM, Carmical JR, Aronson JF, Thangamani S. Early immunologic events at the tick-host interface. PLoS. One. 2012a;7:e47301. doi: 10.1371/journal.pone.0047301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze DM, Wikel SK, Thangamani S, Alarcon-Chaidez FJ. Transcriptional profiling of the murine cutaneous response during initial and subsequent infestations with Ixodes scapularis nymphs. Parasit. Vectors. 2012b;5:26. doi: 10.1186/1756-3305-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof P, Mayr I, Huber R, Korzus E, Potempa J, Travis J, Powers JC, Bode W. The 1.8 A crystal structure of human cathepsin G in complex with Suc-Val-Pro-PheP-(OPh)2: a Janus-faced proteinase with two opposite specificities. EMBO J. 1996;15:5481–5491. [PMC free article] [PubMed] [Google Scholar]
- Hopkins PC, Carrell RW, Stone SR. Effects of mutations in the hinge region of serpins. Biochemistry. 1993;32:7650–7657. doi: 10.1021/bi00081a008. [DOI] [PubMed] [Google Scholar]
- Hopkins PC, Stone SR. The contribution of the conserved hinge region residues of alpha1-antitrypsin to its reaction with elastase. Biochemistry. 1995;34:15872–15879. doi: 10.1021/bi00048a033. [DOI] [PubMed] [Google Scholar]
- Huntington JA, Read RJ, Carrell RW. Structure of a serpin-protease complex shows inhibition by deformation. Nature. 2000;407:923–926. doi: 10.1038/35038119. [DOI] [PubMed] [Google Scholar]
- Ibelli AM, Kim TK, Hill CC, Lewis LA, Bakshi M, Miller S, Porter L, Mulenga A. A blood meal-induced Ixodes scapularis tick saliva serpin inhibits trypsin and thrombin, and interferes with platelet aggregation and blood clotting. Int. J. Parasitol. 2014;44:369–379. doi: 10.1016/j.ijpara.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura S, da Silva VJI, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine. 2005;23:1301–1311. doi: 10.1016/j.vaccine.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Imamura S, Namangala B, Tajima T, Tembo ME, Yasuda J, Ohashi K, Onuma M. Two serine protease inhibitors (serpins) that induce a bovine protective immune response against Rhipicephalus appendiculatus ticks. Vaccine. 2006;24:2230–2237. doi: 10.1016/j.vaccine.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Jin X, Deng L, Li H, Zhang Z, He Q, Yang C, Jiang H, Zhu XQ, Peng L. Identification and characterization of a serine protease inhibitor with two trypsin inhibitor-like domains from the human hookworm Ancylostoma duodenale. Parasitol. Res. 2011;108:287–295. doi: 10.1007/s00436-010-2055-z. [DOI] [PubMed] [Google Scholar]
- Jittapalapong S, Kaewhom P, Pumhom P, Canales M, de la Fuente J, Stich RW. Immunization of rabbits with recombinant serine protease inhibitor reduces the performance of adult female Rhipicephalus microplus. Transbound. Emerg. Dis. 2010;57:103–106. doi: 10.1111/j.1865-1682.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- Jongejan F, Uilenberg G. The global importance of ticks. Parasitology. 2004;129(Suppl):S3–S14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- Kantyka T, Potempa J. Human SCCA serpins inhibit staphylococcal cysteine proteases by forming classic "serpin-like" covalent complexes. Methods Enzymol. 2011;499:331–345. doi: 10.1016/B978-0-12-386471-0.00016-X. [DOI] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JM. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS. One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil B. Specificity of proteolysis. Berlin-Heidelberg-New York: Springer-Verlang; 1992. [Google Scholar]
- Kim TK, Tirloni L, Radulovic Z, Lewis L, Bakshi M, Hill C, Da Silva VI, Jr, Logullo C, Termignoni C, Mulenga A. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 2015 doi: 10.1016/j.ijpara.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz B, Moreau T, Gauthier F. Neutrophil elastase, proteinase 3 and cathepsin G: physicochemical properties, activity and physiopathological functions. Biochimie. 2008;90:227–242. doi: 10.1016/j.biochi.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Strik MC, Bladergroen BA, Hack CE. Production, characterization, and use of serpin antibodies. Methods. 2004;32:141–149. doi: 10.1016/s1046-2023(03)00205-6. [DOI] [PubMed] [Google Scholar]
- Leboulle G, Crippa M, Decrem Y, Mejri N, Brossard M, Bollen A, Godfroid E. Characterization of a novel salivary immunosuppressive protein from Ixodes ricinus ticks. J. Biol. Chem. 2002a;277:10083–10089. doi: 10.1074/jbc.M111391200. [DOI] [PubMed] [Google Scholar]
- Leboulle G, Rochez C, Louahed J, Ruti B, Brossard M, Bollen A, Godfroid E. Isolation of Ixodes ricinus salivary gland mRNA encoding factors induced during blood feeding. Am. J. Trop. Med. Hyg. 2002b;66:225–233. doi: 10.4269/ajtmh.2002.66.225. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Radulovic ZM, Kim TK, Porter LM, Mulenga A. Identification of 24h Ixodes scapularis immunogenic tick saliva proteins. Ticks. Tick. Borne. Dis. 2015 doi: 10.1016/j.ttbdis.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Laumonnier Y, Syrovets T, Simmet T. Plasmin triggers cytokine induction in human monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 2007;27:1383–1389. doi: 10.1161/ATVBAHA.107.142901. [DOI] [PubMed] [Google Scholar]
- Li X, Syrovets T, Genze F, Pitterle K, Oberhuber A, Orend KH, Simmet T. Plasmin triggers chemotaxis of monocyte-derived dendritic cells through an Akt2-dependent pathway and promotes a T-helper type-1 response. Arterioscler. Thromb. Vasc. Biol. 2010;30:582–590. doi: 10.1161/ATVBAHA.109.202044. [DOI] [PubMed] [Google Scholar]
- Li X, Syrovets T, Simmet T. The serine protease plasmin triggers expression of the CC-chemokine ligand 20 in dendritic cells via Akt/NF-kappaB-dependent pathways. J. Biomed. Biotechnol. 2012;2012:186710. doi: 10.1155/2012/186710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Han Y, Chen X, Zhang W. Structure-function relationship of SW-AT-1, a serpin-type protease inhibitor in silkworm. PLoS. One. 2014;9:e99013. doi: 10.1371/journal.pone.0099013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga Y, Kido H, Kawaji K, Kamoshita K, Katunuma N, Ogura T. Inhibitors of chymotrypsin-like proteases inhibit eosinophil peroxidase release from activated human eosinophils. Arch. Biochem. Biophys. 1994;312:67–74. doi: 10.1006/abbi.1994.1281. [DOI] [PubMed] [Google Scholar]
- McCoy AJ, Pei XY, Skinner R, Abrahams JP, Carrell RW. Structure of beta-antithrombin and the effect of glycosylation on antithrombin's heparin affinity and activity. J. Mol. Biol. 2003;326:823–833. doi: 10.1016/s0022-2836(02)01382-7. [DOI] [PubMed] [Google Scholar]
- Mudenda L, Pierle SA, Turse JE, Scoles GA, Purvine SO, Nicora CD, Clauss TR, Ueti MW, Brown WC, Brayton KA. Proteomics informed by transcriptomics identifies novel secreted proteins in Dermacentor andersoni saliva. Int. J. Parasitol. 2014;44:1029–1037. doi: 10.1016/j.ijpara.2014.07.003. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Blandon MA. Molecular and expression analysis of a family of the Amblyomma americanum tick Lospins. J. Exp. Biol. 2007;210:3188–3198. doi: 10.1242/jeb.006494. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Khumthong R, Chalaire KC. Ixodes scapularis tick serine proteinase inhibitor (serpin) gene family; annotation and transcriptional analysis. BMC. Genomics. 2009;10:217. doi: 10.1186/1471-2164-10-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Kim T, Ibelli AM. Amblyomma americanum tick saliva serine protease inhibitor 6 is a cross-class inhibitor of serine proteases and papain-like cysteine proteases that delays plasma clotting and inhibits platelet aggregation. Insect Mol. Biol. 2013;22:306–319. doi: 10.1111/imb.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Misao O, Sugimoto C. Three serine proteinases from midguts of the hard tick Rhipicephalus appendiculatus; cDNA cloning and preliminary characterization. Exp. Appl. Acarol. 2003;29:151–164. doi: 10.1023/a:1024278402288. [DOI] [PubMed] [Google Scholar]
- Mulenga A, Sugino M, Nakajim M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J. Vet. Med. Sci. 2001;63:1063–1069. doi: 10.1292/jvms.63.1063. [DOI] [PubMed] [Google Scholar]
- Oldiges DP, Parizi LF, Zimmer KR, Lorenzini DM, Seixas A, Masuda A, Da Silva VI, Jr, Termignoni C. A Rhipicephalus (Boophilus) microplus cathepsin with dual peptidase and antimicrobial activity. Int. J. Parasitol. 2012;42:635–645. doi: 10.1016/j.ijpara.2012.04.013. [DOI] [PubMed] [Google Scholar]
- Oliveira CJ, Anatriello E, de Miranda-Santos IK, Francischetti IM, Sa-Nunes A, Ferreira BR, Ribeiro JM. Proteome of Rhipicephalus sanguineus tick saliva induced by the secretagogues pilocarpine and dopamine. Ticks. Tick. Borne. Dis. 2013 doi: 10.1016/j.ttbdis.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palenikova J, Lieskovska J, Langhansova H, Kotsyfakis M, Chmelar J, Kopecky J. Ixodes ricinus Salivary Serpin IRS-2 Affects Th17 Differentiation via Inhibition of the Interleukin-6/STAT-3 Signaling Pathway. Infect. Immun. 2015;83:1949–1956. doi: 10.1128/IAI.03065-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parizi LF, Githaka NW, Logullo C, Konnai S, Masuda A, Ohashi K, Da Silva VI., Jr The quest for a universal vaccine against ticks: cross-immunity insights. Vet. J. 2012;194:158–165. doi: 10.1016/j.tvjl.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Parra-Lopez CA, Bernal-Estevez D, Yin L, Vargas LE, Pulido-Calixto C, Salazar LM, Calvo-Calle JM, Stern LJ. An unstable Th epitope of P. falciparum fosters central memory T cells and anti-CS antibody responses. PLoS. One. 2014;9:e100639. doi: 10.1371/journal.pone.0100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat. Rev. Immunol. 2006;6:541–550. doi: 10.1038/nri1841. [DOI] [PubMed] [Google Scholar]
- Pham CT. Neutrophil serine proteases fine-tune the inflammatory response. Int. J. Biochem. Cell Biol. 2008;40:1317–1333. doi: 10.1016/j.biocel.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl PC, Klafke GM, Carvalho DD, Martins JR, Daffre S, da Silva VI, Jr, Masuda A. ABC transporter efflux pumps: a defense mechanism against ivermectin in Rhipicephalus (Boophilus) microplus. Int. J. Parasitol. 2011;41:1323–1333. doi: 10.1016/j.ijpara.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Porter L, Radulovic Z, Kim T, Braz GR, Da Silva VI, Jr, Mulenga A. Bioinformatic analyses of male and female Amblyomma americanum tick expressed serine protease inhibitors (serpins) Ticks. Tick. Borne. Dis. 2015;6:16–30. doi: 10.1016/j.ttbdis.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot PP, Adam B, Boudjeltia KZ, Brossard M, Lins L, Cauchie P, Brasseur R, Vanhaeverbeek M, Vanhamme L, Godfroid E. Anti-hemostatic effects of a serpin from the saliva of the tick Ixodes ricinus. J. Biol. Chem. 2006;281:26361–26369. doi: 10.1074/jbc.M604197200. [DOI] [PubMed] [Google Scholar]
- Prevot PP, Beschin A, Lins L, Beaufays J, Grosjean A, Bruys L, Adam B, Brossard M, Brasseur R, Zouaoui BK, Vanhamme L, Godfroid E. Exosites mediate the anti-inflammatory effects of a multifunctional serpin from the saliva of the tick Ixodes ricinus. FEBS J. 2009;276:3235–3246. doi: 10.1111/j.1742-4658.2009.07038.x. [DOI] [PubMed] [Google Scholar]
- Radulovic ZM, Kim TK, Porter LM, Sze SH, Lewis L, Mulenga A. A 24–48 h fed Amblyomma americanum tick saliva immuno-proteome. BMC. Genomics. 2014;15:518. doi: 10.1186/1471-2164-15-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau JC, Beaulieu LM, Huntington JA, Church FC. Serpins in thrombosis, hemostasis and fibrinolysis. J. Thromb. Haemost. 2007;5(Suppl 1):102–115. doi: 10.1111/j.1538-7836.2007.02516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck J, Jr, Berger M, Marks FS, Zingali RB, Canal CW, Ferreira CA, Guimaraes JA, Termignoni C. Pharmacological action of tick saliva upon haemostasis and the neutralization ability of sera from repeatedly infested hosts. Parasitology. 2009;136:1339–1349. doi: 10.1017/S0031182009990618. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. Role of saliva in blood-feeding by arthropods. Annu. Rev. Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem. Mol. Biol. 2006;36:111–129. doi: 10.1016/j.ibmb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 2003;48:73–88. doi: 10.1146/annurev.ento.48.060402.102812. [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Weis JJ, Telford SR., III Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol. 1990;70:382–388. doi: 10.1016/0014-4894(90)90121-r. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Xu T, Kurscheid S, Lew-Tabor AE. Rhipicephalus microplus serine protease inhibitor family: annotation, expression and functional characterisation assessment. Parasit. Vectors. 2015;8:7. doi: 10.1186/s13071-014-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Valle M, Vance M, Moolhuijzen PM, Tao X, Lew-Tabor AE. Differential recognition by tick-resistant cattle of the recombinantly expressed Rhipicephalus microplus serine protease inhibitor-3 (RMS-3) Ticks. Tick. Borne. Dis. 2012;3:159–169. doi: 10.1016/j.ttbdis.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J. Biol. Chem. 2000;275:6819–6823. doi: 10.1074/jbc.275.10.6819. [DOI] [PubMed] [Google Scholar]
- Saunders DN, Buttigieg KM, Gould A, McPhun V, Baker MS. Immunological detection of conformational neoepitopes associated with the serpin activity of plasminogen activator inhibitor type-2. J. Biol. Chem. 1998;273:10965–10971. doi: 10.1074/jbc.273.18.10965. [DOI] [PubMed] [Google Scholar]
- Schick C, Kamachi Y, Bartuski AJ, Cataltepe S, Schechter NM, Pemberton PA, Silverman GA. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J. Biol. Chem. 1997;272:1849–1855. doi: 10.1074/jbc.272.3.1849. [DOI] [PubMed] [Google Scholar]
- Schleger AV, Lincoln DT, Kemp DH. A putative role for eosinophils in tick rejection. Experientia. 1981;37:49–50. doi: 10.1007/BF01965562. [DOI] [PubMed] [Google Scholar]
- Selak MA, Chignard M, Smith JB. Cathepsin G is a strong platelet agonist released by neutrophils. Biochem. J. 1988;251:293–299. doi: 10.1042/bj2510293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettins PG, Irving JA, Lomas DA, Luke CJ, Moyer RW, Pemberton PA, Remold-O'Donnell E, Salvesen GS, Travis J, Whisstock JC. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- Sinha S, Watorek W, Karr S, Giles J, Bode W, Travis J. Primary structure of human neutrophil elastase. Proc. Natl. Acad. Sci. U. S. A. 1987;84:2228–2232. doi: 10.1073/pnas.84.8.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, Trevisani M, Hollenberg MD, Wallace JL, Caughey GH, Mitchell SE, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Sugino M, Imamura S, Mulenga A, Nakajima M, Tsuda A, Ohashi K, Onuma M. A serine proteinase inhibitor (serpin) from ixodid tick Haemaphysalis longicornis; cloning and preliminary assessment of its suitability as a candidate for a tick vaccine. Vaccine. 2003;21:2844–2851. doi: 10.1016/s0264-410x(03)00167-1. [DOI] [PubMed] [Google Scholar]
- Syrovets T, Tippler B, Rieks M, Simmet T. Plasmin is a potent and specific chemoattractant for human peripheral monocytes acting via a cyclic guanosine monophosphate-dependent pathway. Blood. 1997;89:4574–4583. [PubMed] [Google Scholar]
- Tirloni L, Islam MS, Kim TK, Diedrich JK, Yates JR, III, Pinto AF, Mulenga A, You MJ, Da Silva VI., Jr Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasit. Vectors. 2015;8:338. doi: 10.1186/s13071-015-0918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates JR, III, Termignoni C, Pinto AF, Vaz Ida S., Jr Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS. One. 2014a;9:e94831. doi: 10.1371/journal.pone.0094831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirloni L, Seixas A, Mulenga A, Vaz Ida S, Jr, Termignoni C. A family of serine protease inhibitors (serpins) in the cattle tick Rhipicephalus (Boophilus) microplus. Exp. Parasitol. 2014b;137:25–34. doi: 10.1016/j.exppara.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Ushio H, Watanabe N, Kiso Y, Higuchi S, Matsuda H. Protective immunity and mast cell and eosinophil responses in mice infested with larval Haemaphysalis longicornis ticks. Parasite Immunol. 1993;15:209–214. doi: 10.1111/j.1365-3024.1993.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Willadsen P, Riding GA, McKenna RV, Kemp DH, Tellam RL, Nielsen JN, Lahnstein J, Cobon GS, Gough JM. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol. 1989;143:1346–1351. [PubMed] [Google Scholar]
- Yu Y, Cao J, Zhou Y, Zhang H, Zhou J. Isolation and characterization of two novel serpins from the tick Rhipicephalus haemaphysaloides. Ticks. Tick. Borne. Dis. 2013;4:297–303. doi: 10.1016/j.ttbdis.2013.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Purified IgG from a non-related serpin rabbit polyclonal serum (anti-VTDCE; negative control) were incubated with rRmS-3 (55 nM), rRmS-6 (55 nM) or rRmS-17 (88 nM) at 37 °C for 30 min before the addition of chymotrypsin (4.9 nM) for rRmS-3, or trypsin (1.6 nM) for rRmS-6 and rRmS-17, following a new 15-min incubation. Protease kinetics was monitored for 15 min every 11 s.