Abstract
The protective effect of aspirin during exposure to heat stress in broiler chickens was investigated. We assayed pathological damage, expression and distribution of Hsp90 protein and hsp90 mRNA expression in chicken heart tissues after oral administration of aspirin following exposure to high temperature for varying times. Heat stress induced increases in plasma aspartate aminotransferase, creatine kinase and lactate dehydrogenase activities while causing severe heart damage, which was characterized by granular and vacuolar degeneration, nuclear shrinkage and even myocardium fragmentation in cardiac muscle fibers. After aspirin administration, myocardial cells showed fewer pathological lesions than broilers treated with heat alone. A high positive Hsp90 signal was always detected in the nuclei of myocardial cells from broilers treated with aspirin, while in myocardial cells treated with heat alone, Hsp90 in the nuclei decreased, as did that in the cytoplasm. Aspirin induced rapid and significant synthesis of Hsp90 before and at the initial phase of heat stress, and significant expression of hsp90 mRNA was stimulated throughout the experiment when compared with cells exposed to heat stress alone. Thus, specific pre-induction of Hsp90 in cardiovascular tissue was useful for resisting heat stress damage because it produced stable damage-related enzymes and fewer pathologic changes.
Keywords: Hsp90, aspirin, chicken, heart, heat stress damage
Introduction
Stress is the most common and serious condition affecting the intensive livestock breeding industry. For example, when poultry are exposed to high temperatures, many indicators such as food consumption, growth rate, feeding efficiency, eggshell quality and survival ability decline [9,35]. Biochemical and structural changes occur widely in the heart, liver, kidney and other organs of post-stressed animals [4,5,37]. Stress can induce specific pathological damage in the myocardium of heat-stressed broilers, as well as in transported pigs, and may even cause death [38,39]. Acute heart failure may be correlated with these stress phenomena. Although several factors and physiological reactions have been identified to date, the mechanisms underlying heat-induced heart failure are not completely understood [17].
All living organisms respond to environmental stresses by synthesizing a variety of proteins, originally known as heat shock proteins (Hsps). Hsps can be divided into six families of sequence-related proteins based on their molecular weights, small Hsps, Hsp40, Hsp60, Hsp70, Hsp90, and Hsp110 [22]. Among various forms of Hsps, the Hsp90 family, which is found in all kingdoms except archaea, is the most frequently expressed stress protein. Hsp90 is highly conserved in unstressed cells and inducible in response to cellular stress factors [7,30]. In addition to cell signaling and tumor repression, the functions of Hsp90 include enfolding synthesized proteins and stabilizing and refolding denatured proteins after stress, thus maintaining normal cell activity [31]. Under heat stress (HS), the expression of Hsp90 is necessary for viability. Yeast cells showed a temperature-sensitive phenotype under conditions in which the levels of cytosolic Hsp90 were reduced to 5% to 10% [29].
Nonsteroidal anti-inflammatory drugs (NSAIDs), originally identified as cyclooxygenase activity inhibitors that mediate inflammatory reaction, have been reported to modulate the HS response in yeast, drosophila and mammals [10,20]. Aspirin is an NSAID with antipyretic properties that is used for the reduction of fever, as well as to prevent coronary artery disease and stroke. Aspirin treatment has also been shown to lower the temperature threshold needed to induce Hsps gene transcription and promote thermotolerance [2]. Long-term administration of aspirin results in development of resistance to aspirin-induced mucosal damage, as well as increased Hsp expression correlated with mucosal resistance to aspirin [14]. In the fish cell line, CHSE-214, co-exposure to aspirin and heat shock (24℃) enhanced and prolonged heat shock-induced Hsp70 expression, presumably through activation of heat shock factors (HSFs). In contrast, heat exposure-induced Hsp90 expression is inhibited by these NSAIDs [19].
The above-mentioned studies primarily focused on the expression and mechanism of Hsps in various organisms or cells. Aspirin has been investigated in many studies of HS response, especially in resistance and adaptation studies. When compared with other kinds of domestic animals, poultry are more sensitive to higher ambient temperatures [8]. However, few studies have investigated the induction of Hsp90 and influence of aspirin on chickens exposed to heat treatment. In the present study, changes in myocardial damage-related enzymes and histopathological lesions, as well as changes in expression of Hsp90 induced by aspirin in chicken heart cells, and the possible protection offered by aspirin to myocardial cells subjected to HS were studied.
Materials and Methods
Experimental design
A total of 270 one-day-old specific pathogen free chicks were obtained from Qian Yuan Hao Biotechnology Company, Nanjing, China, and raised in an artificial climate chamber at 25 ± 1℃ and 75% humidity. The broilers were vaccinated against Newcastle disease and infectious bursal disease on day 7 and 14, respectively. After 21 days of adaptation feeding, the chickens were divided into three groups of 90 at random. Chickens in the HS group were heat-stressed without aspirin administration, while those in the aspirin administered before HS (ASA+HS) group were heat-stressed after oral administration of aspirin at 1 mg/kg body two hours before exposure to heat and those in the aspirin administration (ASA) group were only administered oral aspirin two hours in advance without HS. After aspirin administration, all chickens from the HS and ASA+HS groups were exposed to HS by rapidly increasing the air chamber temperature from (25 ± 1)℃ to (40 ± 1)℃ while maintaining the humidity at 80% for 0, 1, 2, 3, 5, 7, 10, 15 and 24 h, respectively. The chickens in ASA were maintained under normal conditions after oral administration of aspirin for the designated time. Birds were allowed free access to a commercial broiler feed and water ad libitum during HS. At each designed time point of HS, 10 experimental chickens were humanely sacrificed by decapitation after collecting blood samples. Heart samples were collected, and specimens for morphological studies were fixed in 10% formalin, while those for biochemical analysis were frozen in liquid nitrogen. Clinical symptoms such as breathing, water intake and mental state were also recorded at the same time.
The experiment was undertaken following the guidelines of the regional Animal Ethics Committee and approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University.
Detection of heart damage-related enzymes in the serum of chickens
A total of 1.5 mL of serum from each chicken was collected after exposure to different periods of HS. The enzyme activities of aspartate aminotransferase (AST), creatine kinase (CK) and lactate dehydrogenase (LDH) in the samples were measured using commercial kits according to the manufacturer's instructions (Nanjing Jiancheng Biochemical Reagent, China) and using a clinical biochemical indicator auto-analyzer (Vital Scientific NV, The Netherlands). Each sample was analyzed three times, after which the enzyme activities were determined as unit of enzyme activity/L (U/L).
Histopathological examination of heart tissues in experimental broilers
The fixed specimens were embedded in paraffin, after which serial sections (5 µm thick) were cut from the paraffin-embedded blocks. Following dewaxing with xylene and hydration with different concentrations of alcohol, sections were stained with hematoxylin and eosin (H&E) for 5 min and 1 min, respectively, and sealed with neutral balsam. The slices were examined by light microscopy (CX41; Olympus, Japan).
Immunohistochemical detection
Serial sections of the heart tissue were immunostained using the standard avidin-biotin complex (ABC) immunoperoxidase detection system. After the sections were deparaffinized in xylene, hydrated with ethanol, and rinsed with distilled water, endogenous peroxidase was blocked using 3% H2O2 for 15 min at room temperature (RT). Following further rinsing with PBS, heart tissue sections were boiled in citrate buffer (pH = 6) for 20 min to retrieve the antigen. The slides were then blocked by incubation with 5% BSA for approximately 30 min at 37℃. Next, sections were incubated with rat primary antibodies raised against Hsp90(α) (ADI-SPA-840; Enzo, USA) in a 1: 50 working solution of 1% BSA at 37℃ for 1 h in a humidified chamber. After washing with PBS, the slides were incubated with HRP-labeled Goat Anti-Rat IgG (diluted 1:250 in PBS Tween-20; HD0005-1; Dingguo, China) for 1 h. Following treatment with two drops of readymade diaminobenzidine (DAB) substrate chromogen solution (Ar1022; Boster, China) for 10 min, the reaction was stopped by adding water. Next, the sections were counterstained with hematoxylin and immunohistochemical images were obtained under light microscopy. The corresponding negative controls were prepared by omitting the antibody.
Detection of hsp90 mRNA by fluorescent quantitative real-time PCR
Total RNA was isolated from heart tissues in all of treatment groups using TRIZOL reagent according to the manufacturer's instructions (Invitrogen, USA). The concentration of RNA was measured at 260 nm using a spectrophotometer (Stratagene Mx3000P; Thermo Fisher Scientific, USA). Serial dilutions of RNA were prepared with ribonuclease-free water, after which 2 µg of each sample was reverse-transcribed using the Transcript M-MLV kit (Invitrogen) following the manufacturer's protocols and stored at −80℃. Moloney murine leukemia virus (M-MLV) reverse transcriptase has higher stability and lower intrinsic RNase H activity than AMV reverse transcriptase. Random decamers and oligo (dT) were provided in the RETROscript Kit (AM1710; Ambion, USA). The sequences of hsp90 and GAPDH were obtained from the National Center for Biotechnology Information (NCBI) GenBank database (accession Nos. X07265, and K01458, respectively). These sequences were used to design primers with the Primer Premier 5.0 software for conventional and RT-PCR amplification. The designed primer sequences were as follows: 5'-AGTCCCAGTTCATTGGC TAC-3' and 5'-TCCAGTCATTGGTGAGGCT-3' for hsp90; and 5'-TGAAAGTCGGAGTCAACGGAT-3' and 5'-ACGC TCCTGGAAGATAGTGAT-3' for GAPDH. Using the extracted total mRNA as a template in a 25 µL reaction system, GAPDH and hsp90 fragments were amplified by qPCR. The qPCR products were analyzed by 1.5% agarose gel electrophoresis. Quantitative real-time PCR amplification was performed using an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories, USA). Test samples were assayed in a 25 µL reaction mixture system. The thermal profile for real-time RT-PCR consisted of 45 min reverse transcription at 42℃ and 1 min initial denaturation at 95℃, followed by 40 cycles of PCR at 94℃ for 30 sec (denaturation), 56℃ for 30 sec (annealing), and 72℃ for 30 sec (extension). The reaction system and procedure for GAPDH was the same. The quantity of hsp90 mRNA was normalized to that of GAPDH using the following algorithm:
| Relative quantity of hsp90 mRNA = 2−ΔΔCt −ΔΔCt = −[(Cthsp90mRNA−CtGAPDH)control group − (Cthsp90mRNA−CtGAPDH)test group], |
where Ct is the cycle threshold.
Hsp90 expression levels detected by enzyme-linked immunosorbent assay
After sufficient washing in ice-cold phosphate-buffered saline (PBS), the heart samples were homogenized on ice using an Ultra-Turrax homogenizer, then centrifuged at 12,000 × g for 20 min at 4℃. The supernatant was collected and stored at −20℃ for protein quantification. The levels of Hsp90 in the hearts of chickens were measured using commercially available ELISA kits (P17142998; CUSABIO, USA). Immunological detection of the proteins was performed according to the manufacturer's instructions. Quantification of samples was conducted using a standard concentration curve constructed from the detection results of a serial dilution standard sample provided by manufacturer.
Statistical analysis
Differences between HS groups and the control group were analyzed by one-way analysis of variance (ANOVA), followed by the least significant difference (LSD) multiple comparison test using the Statistical Package for Social Sciences (SPSS ver. 20.0 for Windows; IBM, USA). Results were expressed as the mean ± standard deviation (SD). P values < 0.05 (*) or < 0.01 (**) were considered statistically significant. All experiments were performed in triplicate (n = 3).
Results
Clinical symptoms
Experimental chickens were raised adaptively to be in good physical condition and without any abnormity of their mental state before HS. The broilers in the HS group started to show mouth breathing, increased respiratory rate, and uneasiness immediately upon exposure to HS. The mouth breathing became more rapid as the HS continued. Additionally, water consumption increased and feed intake decreased correspondingly. The body temperature of the heat stressed chickens increased to 43℃. During the later period of HS, experimental chickens laid down and had difficulty opening their eyes. Birds in the ASA+HS group began to flap their wings, and increased their water consumption and respiratory rate significantly after 2 h of HS. Chickens in the ASA group behaved normally for the duration of the test.
Variations in damage-related serum enzyme activities after HS
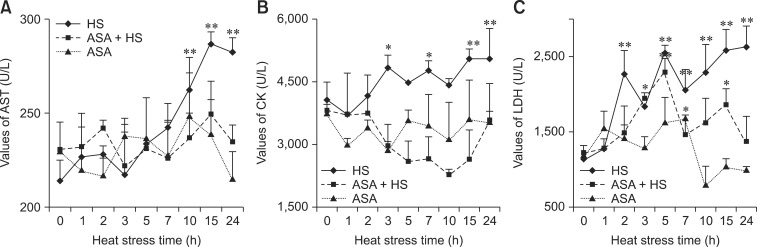
The levels of the damage-related serum enzymes AST, CK and LDH from different groups are shown in Fig. 1. The activity of AST in the serum of chickens from the HS group increased continuously after heat exposure. Significant induction started from 10 h of HS (p < 0.01) and was maintained at a high level after 15 h of HS (p < 0.01) relative to normal chickens without HS. The AST levels of chickens from the ASA+HS and ASA groups showed no significant variations. The level of CK in the serum of the chickens from the HS group displayed an increasing tendency, with obvious induction from 3 h of HS, with a higher level maintained from 15 h of HS (p < 0.01) until the end of the experiment (p < 0.01). There was no significant change in the CK of the serum of chickens from the ASA+HS and ASA groups until the end of the experiment, although the CK in the ASA+HS group showed a decreasing tendency (p > 0.05) from 3 h to 10 h of HS. Compared with the higher level in the HS group, the LDH activity in the serum of chickens from the ASA+HS group was much lower, except for a temporary induction at 3 h (p < 0.05) to 5 h (p < 0.01) of HS. There was no change in LDH induction in the chicken serum from the ASA group except for a transient increase at 7 h.
Fig. 1. Temporal changes in enzyme activities in the serum of experimental chickens. (A) The levels of aspartate aminotransferase (AST) showed an obvious increase from 10 h of heat exposure in the heat stress (HS) group to the end of the test, while the AST in the aspirin administered before heat stress (ASA+HS) and aspirin administration (ASA) groups stayed relatively stable. (B) The levels of creatine kinase (CK) showed an increasing tendency after 3 h of HS in the HS group, while no difference was detected in the CK of the ASA+HS and ASA group (except for a decline at 10 h). (C) The levels of lactate dehydrogenase (LDH) showed a significant increasing tendency in the HS group, with that of the ASA-HS group showing a marked rise after 5 h of HS, then returning to the basal level. No difference was detected for LDH in the ASA group, except for an increase at 7 h. Differences between levels of enzymes in every group at different times and that at 0 h in the HS group are indicated by *p < 0.05, and by **p < 0.01. U/L, unit of enzyme activity/L.
Histopathological examination
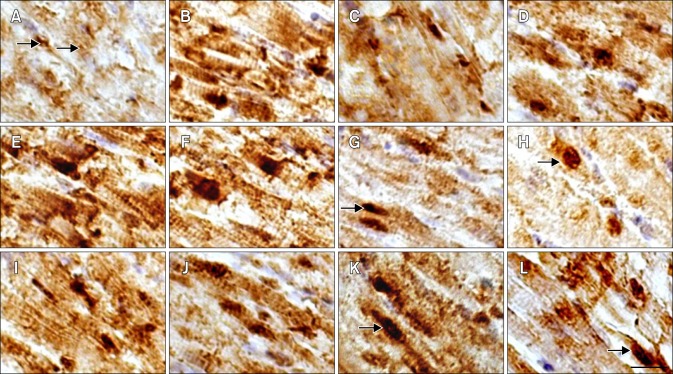
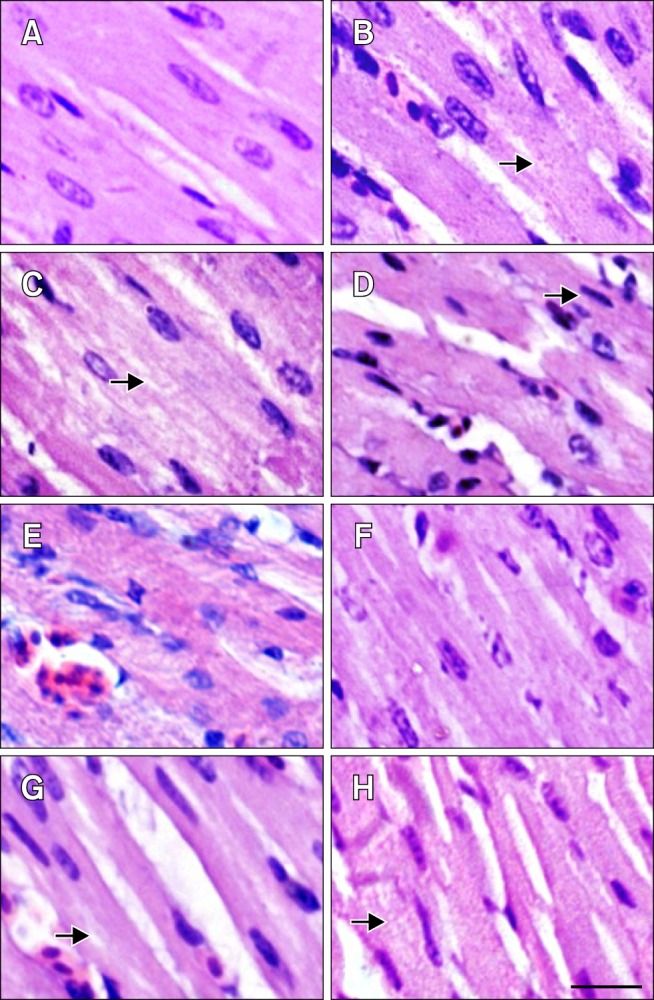
The histopathological lesions of chicken myocardial cells in the HS, ASA+HS and ASA groups after heat treatment in vivo are shown in Fig. 2. The myocardial cells from chicken heart tissues in the HS group were swollen and enlarged. Additionally, they showed granular degeneration characterized by numerous fine and tiny granules in the cytoplasm, as well as vacuolar degeneration characterized by tiny water droplets in the cytoplasm soon after exposure to HS. Necrosis, which is characterized by pyknosis in the myocardium cells and partly dissolved muscle fibrils in myofibers, was occasionally observed in some areas of the heart. The acute degeneration was continuous until the end of HS, and the damage to myocardial cells was more serious. In the ASA+HS group, hemangiectasis and hyperemia were obvious in heart tissues at 0 h of HS. Although the severity of lesions in the ASA+HS group was obviously weaker than that of the HS group, signs of acute degeneration, such as enlarged cells, granular degeneration and vacuolar degeneration in the myocardial cells, were also observed early in HS (from 0 to 5 h of heat exposure) in the ASA+HS group. After 10 h of stress treatment, the swollen myocardial cells were characterized by numerous granular particles in the cytoplasm. However, in the ASA+HS group, few myocardium nuclei with pyknosis were observed during the later period of HS. No obvious histopathological lesions were observed in the ASA groups from the beginning to the end of the experiment, except for hemangiectasis and congestion.
Fig. 2. Pathological changes in the myocardial fibers of tested chickens. (A–D) HS group. (E–H) ASA+HS group. (A) Heart tissue at 0 h of HS. (B) After 2 h of HS, the myocardial fibres showed the decreasing in size and granular degeneration characterised by numerous tiny granules in the cytoplasm (arrow). (C) After 5 h, vacuolar degeneration characterized by tiny water droplets in the cytoplasm, and partly dissolved muscle fibrils (arrow) was observed occasionally. (D) After 10 h, necrosis characterized by pyknosis (arrow) was evident. (E) At 0 h of HS, hemangiectasis and hyperemia were observed. (F) After 2 h, myocardial fibers sizes enlarged. (G) After 5 h, granular degeneration and vacuolar degeneration were observed occasionally (arrow). (H) After 10 h, swollen myocardial cells characterized by numerous cytoplasmic particles (arrow) were observed. H&E stain. Scale bar = 10 µm.
Immunohistochemical assays
The location and distribution of Hsp90 positive signals in the myocardial cells and the heart vessels of the heat-treated chickens are shown in Figs. 3 and 4. Immunohistochemically, the positive signals of Hsp90 were located in the nucleus and the cytoplasm of myocardial cells of the chickens in the HS, ASA+HS and ASA groups in the form of intensely immunoreactive granules. In addition, intense immunoreactivity was primarily located in the nuclei and perinuclear cytoplasm of normal chicken myocardial cells in vivo. However, the density of Hsp90 positive signals changed with HS. In the HS group, the density of Hsp90 positive signals in the nucleus and the cytoplasm of myocardial cells increased immediately upon exposure to heat (panel B in Fig. 3), and obviously stronger signals were observed in the nucleus (panel B in Fig. 3). After a lower density at 5 h and 10 h of HS (panel C in Fig. 3), the Hsp90 signal density became stronger again at 24 h of HS (panel D in Fig. 3).
Fig. 3. Distribution Distribution of Hsp90 in the myocardial cells of tested chickens. (A–D) HS group. (I–L) ASA group. (E–H) ASA+HS group. (A) At 0 h of HS, Hsp90 was constitutively expressed in the nucleus and cytoplasm (arrow). (B) After 2 h, Hsp90 expression increased obviously. (C) After 5 h, the maximum intensity of Hsp90 signal weakened. (D) After 24 h, Hsp90 signal showed the second peak. (E) Before HS (0 h), cellular Hsp90 signal was extremely strong. (F) After 2 h of HS, extremely high Hsp90 expression was still observed. (G) After 5 h, cytoplasmic Hsp90 signal decreased while the signal in the nucleus did not (arrow). (H) After 24 h, Hsp90 signal was still present in the nucleus (arrow). (I) At 0 h of test, strong Hsp90 signal was detected in both the nucleus and cytoplasm. (J) The Hsp90 density decreased after 2 h. (K) Hsp90 level rebounded after 5 h, especially in the nucleus (arrow). (L) After 24 h, Hsp90 expression persisted in the cytoplasm and nucleus (arrow). Immunohistochemical staining with hematoxylin counterstain. Scale bar =10 µm.
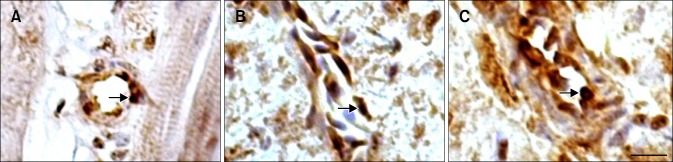
Fig. 4. The location of Hsp90 in the blood vessels of the tested chickens. (A) Immunoreactive Hsp90 was observed in the endotheliocyte as well as in the vascular wall of heart artery in the HS group after 1 h of heat stress. (B) Hsp90 was identified in the vein wall cells of the HS group after 1 h of heat stress. (C) The strong, positive signal of the heart aortic endotheliocyte in the ASA+HS group was maintained at 24 h of heat stress. Immunohistochemical staining with hematoxylin counterstain. Scale bar =10 µm.
Following oral administration of aspirin to the ASA+HS group, similar and much stronger distributions of Hsp90 positive signals in the nucleus and the cytoplasm of chicken myocardial cells were observed. Relatively higher levels of Hsp90 proteins were detected at 0 h in the myocardial cells of the chickens in the ASA+HS group, which had been pretreated with aspirin for 2 h before heat treatment compared with that of chickens in the HS group. The Hsp90 signal density in the cytoplasm became lower at later times of HS. However, the nuclei of cardiocytes still showed a relatively distinct reaction. The Hsp90 signal in the myocardial cells of chickens in the ASA group also showed high density at the beginning of the test, followed by a decline at 2 h and a moderate increase during the later period of the test. Strong immunoreactivity of Hsp90 was always detected in cellular nuclei during the experiment.
Interestingly, Hsp90 positive signals were also detected in the arteries and veins of the heart tissue (Fig. 4). The fine Hsp90 signals were scattered along the walls of the blood vessels, especially in the nuclei and cytoplasm of endothelial cells of both the heat stressed and the heat stressed plus aspirin chickens. Stronger and more persistent immunoreactive staining was observed in the artery, especially in the endothelial cells of ASA+HS chickens (panel C in Fig. 4). Similar results were detected in ASA chickens.
Variations in hsp90 mRNA transcription in chicken heart tissues after stress
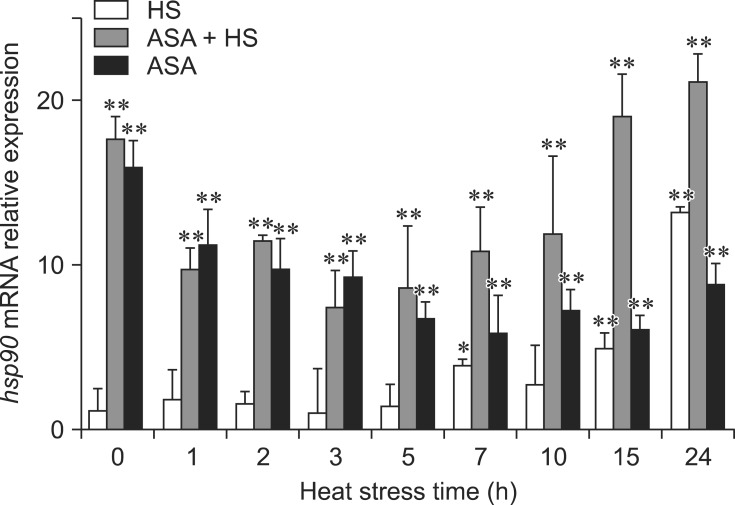
The transcription levels of hsp90 mRNA are shown in Fig. 5. After exposure to HS, the transcription level of hsp90 mRNA in chicken heart tissues from the HS group remained steady and changed only slightly (p > 0.05) for 5 h. The induction of hsp90 mRNA transcription started at 7 h (p < 0.05) and obvious induction of hsp90 mRNA transcription was displayed at 15 h, and especially at 24 h, of HS (p < 0.01). However, significant induction of hsp90 mRNA transcription (p < 0.01) was observed in the heart tissues from chickens in the ASA+HS group at 0 h of HS (after 2 h of aspirin administration). The transcription level of hsp90 mRNA decreased significantly and remained at lower levels until 15 h and 24 h of HS. Significant induction of hsp90 mRNA transcription (p < 0.01) started again after 15 h of HS. During heat exposure, while there were fluctuations in the transcript levels, hsp90 mRNA in the heart tissues of chickens subjected to aspirin in the ASA+HS group was maintained at much higher levels (p < 0.01) compared to every heat treatment time for chickens from the HS group. Although it was always significantly higher (p < 0.01) than that in normal chicken hearts tissues, hsp90 mRNA in ASA decreased gradually to a low level during the experiment.
Fig. 5. Relative expression of hsp90 mRNA in different groups. *p < 0.05, **p < 0.01 for the expression of hsp90 mRNA in the HS, ASA+HS and ASA groups at different times compared with that at 0 h in the HS group. Values indicated are the mean ± SD.
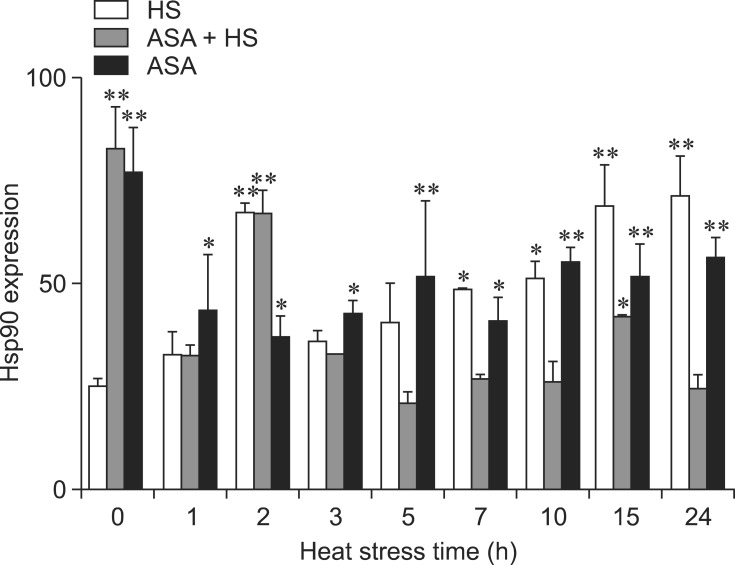
Variations in Hsp90 expression in heart tissues after HS
Variations in the amount of Hsp90 in chicken heart tissues after exposure to heat are shown in Fig. 6. As soon as HS started, the expression of Hsp90 in the chicken heart tissues from the HS group gradually increased and reached higher levels (2-fold higher) at 2 h of HS (p < 0.01) compared with that at 0 h (control). After an obvious reduction at 3 h, the expression levels of Hsp90 increased gradually from 5 h, reaching 2-fold higher levels again at 15 h and 24 h of HS (p < 0.01, respectively) compared with the level at 0 h. In the ASA+HS chickens, the level of Hsp90 protein detected in the heart tissues was dramatically and significantly elevated by 3-fold before heat exposure after 2 h of oral aspirin administration. At 2 h after exposure to HS, the levels of Hsp90 increased (p < 0.01) after undergoing a brief drop at 1 h of HS. With increased exposure to heat, the expression levels of Hsp90 in the heart tissues of the ASA+HS chickens remained steady, except for an obvious increase (p < 0.01) at 15 h of HS. Hsp90 expression in the ASA group showed a similar increase (p < 0.01) at the beginning of the test. After a reduction between 1 h to 2 h of stress, Hsp90 was maintained at a significantly higher level than in normal chicken tissues, but was lower than that of chicken heart in the HS group during the later period of the test.
Fig. 6. Levels of Hsp90 in the heart tissues of different groups of broilers. The difference between the expression level of Hsp90 in the HS, ASA+HS and ASA groups at different times of HS and that of 0 h in the HS group. *p < 0.05, **p < 0.01.
Discussion
Stress responses can be studied using indicators such as clinical behavior, blood constituents and histological diagnoses, which can indicate tissue damage [39]. Increased levels of CK, CK-MB AST, ALT and LDH are consistently correlated with cardiac infarcts. Therefore, these indicators are used to investigate heart damage in livestock [34]. CK is usually detected in muscles and cardiac muscle fibers after intensive physical suffering of animals. In response to myocardial injury from external stress, CK is released from myocardial cells into the blood, increasing the serum CK activity [37]. Heart and liver diseases or damage are always accompanied by elevated blood levels of AST [36]. In vitro tests showed that the LDH content is a sensitive parameter of myocardial damage [6]. In the present study, heart damage was monitored in real-time during HS based on fluctuations in AST, CK and LDH. Exposure to HS induced a significant elevation of plasma AST, CK and LDH activity in experimental chickens, which implied severe, acute heart damage during prolonged HS. However, the levels of these enzymes in ASA+HS chickens that had been treated with aspirin showed no significant changes, which was indicative of normal cardiac function. Histopathological examination of heart tissues of the ASA+HS birds revealed more mild pathological lesions, which were characterized by enlarged myocardial fiber size and granular degeneration compared with the features of vacuolar degeneration, nuclear shrinkage, and myocardium fragmentation observed in the cardiac muscle fibers of HS birds. Taken together, these results indicated that aspirin plays an important role in preserving heart structure and function. However, the hemangiectasis and hyperemia induced by aspirin to improve blood flow to important organs (heart tissue), which was partly responsible for remission of myocardial ischemia and then myocardial damage from HS, was also observed in the ASA+HS group and in the ASA group after administration of aspirin.
Hsps can mediate stress protection, although HS is thought to be among the most common and worst stressors for chicken [5,37,38,39]. Aspirin has analgesic, anti-inflammatory, anti-pyretic and anti-thrombotic activities, and it has become accepted that it can induce HS proteins to enhance thermotolerance [2]. The different expression levels and location patterns of Hsp90 observed in myocardial cells from different treatment groups may have several functions. Immunohistochemistry and ELISA demonstrated that, before HS (0 h) the distribution density of Hsp90 in the cytoplasm and nuclei of chicken myocardial cells of the ASA+HS group was significantly higher than the expression of Hsp90 in the HS group. Environmental stress often stimulates demand for ATP in stressed cells, which induces damage to cytoskeletal structures. As a molecular chaperone, Hsp90 makes a significant contribution to stabilization of the intracellular protein structure and preservation of the integrity of the cytoskeleton by binding to actin and tubulin, the major constituents of the cell cytoskeleton, especially after stress [16]. Hsp90 is also a key player in the refolding of denatured proteins into their proper conformation, which maintains cellular homeostasis during the cell's response to stress [27,31]. Therefore, the specific pre-induction of Hsp90 by aspirin treatment in the myocardium may be sufficient preparation to respond to HS, in which Hsp90 functions to clear denatured proteins, activate related kinases and transcription factors, and guarantee correct protein maturation. The present study revealed that, in the HS group, the amount of Hsp90 in the nuclei decreased during HS, and this decrease was accompanied by a reduction in the cytoplasm. However, the Hsp90 signal in the nuclei was maintained at relatively high levels in the ASA+HS group, regardless of its decline in the cytoplasm. It has been reported that the selective expression of Hsp90 and its cellular redistribution are involved in transcription of genes that encode a protein or proteins connected with stress resistance [5]. In HeLa cells transfected with heat shock transcription factor (HSF4), less Hsp90 was synthesized, while Hsp72 expression was maintained [26]. Therefore, the intracellular Hsp90 expression in myocardial cells after treatment with aspirin might be related to the mechanism by which myocardial cells synthesize other HS proteins that are more sensitive to HS to protect them from myocardial ischemia. For example, Hsp70 is induced by aspirin's effect on certain HSFs [24]. Intracellular Hsp90 is reorganized from the cytoplasm to the nucleus upon stress, which results in protection of genetic material and its regulation following stress events. These findings agreed with those of a previous study [33]. Aspirin also promoted the self-protective translocation of Hsp90.
The protection offered by Hsps in the blood capillaries has been studied [21,38]. The results of the present study verify those of a previous study, in which Hsp90 was reported to be located in endothelial cells and vessel walls of heart arteries [5,38]. Notably, the positive signal of Hsp90 was also detected in the endothelial cells of vein walls of chicken heart tissues, with or without HS. These are crucial for vasoconstriction, which influences blood reallocation for the whole body and backflow to the heart. It has been reported that the protection of pig hearts via Hsps was weakened during transportation, resulting in hypoxia from blood vessel constriction based on reductions in Hsp70, Hsp86, Hsp90 and Hsp27 levels [5]. The present study revealed that, as the duration of HS increased, the amount of Hsp90 in arterial endothelial cells and vessel wall cells in the ASA+HS group remained at a high level, while it gradually declined in the heart arteries of the HS group. Although an animal's stress response decreases over time when exposed to the same stressors [18], aspirin can reverse this weakening trend, at least in the cardiovascular system.
The semi-quantitative results in the present study showed a gradual and transient increase to a peak value in the expression of Hsp90 in the HS group during 2 h of HS. In contrast, after 0 h to 2 h of HS, administration of aspirin in the ASA+HS group induced a rapid increase in Hsp90 synthesis, except for a consumptive decline in cellular stress-protection at 1 h. Elevation of Hsps in the heart contributes to protection against ischemia and reperfusion-associated damage [32]. For example, reports showed that high levels of Hsp90 resulted in significantly greater survival of myocytes during HS, while heat exposure induced irreversible damage and death of certain myocardial cells owing to a decline in Hsp90α levels [11,12]. Hsp90 regulates the activity of specific enzymes and hormone receptors, as well as the transcription of other Hsps by an association with transcription factors [25]. The earlier high expression of Hsp90 in ASA+HS may have produced a significant preexisting protection from acute HS, which was correlated with milder pathological changes in the ASA+HS group compared to the HS group over the same time period. This protective effect of Hsp90 also correlated with stable LDH and CK activity relative to their significant increases (p < 0.01) in the HS group. Interestingly, Hsp90 expression in the ASA+HS group remained at a relatively low level after 5 h of heat treatment, while it remained high in the ASA group. It has been reported that Hsp90 has three structural domains, the N-terminal domain, the middle domain and the C-terminal domain, through which two Hsp90 proteins are connected to form a dimer to function [13]. According to the results of the present study, the transformation of Hsp90 to functional dimers may be reinforced by co-treatment of aspirin and heat for better cell protection during HS, which could result in the relatively low level of Hsp90 that was observed in the ASA+HS group during the late experimental period. However, further study is needed to elucidate the mechanism in detail. In addition, Hsp90 overexpression in the ASA group was lower than that in the HS group in the later stages of the experiment, indicating that the induction of Hsp90 by aspirin at the tested dose was weaker than that of HS at 42℃ in the adaptive cells.
Changes in hsp90 mRNA transcription in chickens with and without treatment by aspirin exposed to HS were detected in the present study. The difference between transcription and translation during the course of heat exposure alone was observed, similar to previous reports [38], which may be a consequence of complicated post-transcriptional mechanisms depending on a number of factors in addition to mRNA levels [3,28]. The expression of other HSP families could also influence the synthesis of Hsp90, as validated in the rat stomach [1]. As expected, aspirin induced the expression of hsp90 mRNA throughout exposure to HS and in non-stress chickens. A previous study indicated that aspirin induces heat shock factor-1 (HSF-1) and facilitates its binding with DNA, but did not stimulate Hsp90 gene expression in vitro [15], which is not consistent with the present results. Aspirin caused HSF-1 to be phosphorylated at threonine and form an HSF-1-HSE complex, which is necessary but insufficient to activate transcription, while HS could induce the HSF-1-HSE complex fully [15]. According to this theory, aspirin pretreatment alone seemed to be insufficient to cause heat shock gene transcription, which was not consistent with the results of the ASA+HS and ASA groups. However, HSPs transcription and expression involves four heat shock factors (1–4) and is regulated in a complex manner. For instance, HSF-2 also plays important roles in heat shock genes transcription [23]. It is possible that hsp90 mRNA expression may only be partly HSF-1-dependent, and that another HSPs regulation pathway possibly involving HSF-2 or other factors also contributes to the expression of hsp90 mRNA. When combined with the results observed in the ASA+HS group after exposure to HS, subsequent HS may be able to convert the aspirin-induced inert HSF-1-HSE complex into a fully functional complex, causing co-stimulation with aspirin and leading to higher hsp90 mRNA transcription. However, the detailed mechanisms responsible for this remain to be elucidated.
Acknowledgments
This work was supported by grants from the National Key Basic Research Program of China (973 Program) (2014CB138502), the National Natural Science Foundation of China (31372403), the National Department Public Benefit Research Foundation (Agriculture) (201003060-11), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), and the Sino-German Agricultural Cooperation Project of the Federal Ministry of Food, the Agriculture and Consumer Production, Berlin, Germany.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Alderman BM, Cook GA, Familari M, Yeomans ND, Giraud AS. Resistance to apoptosis is a mechanism of adaptation of rat stomach to aspirin. Am J Physiol Gastrointest Liver Physiol. 2000;278:G839–G846. doi: 10.1152/ajpgi.2000.278.6.G839. [DOI] [PubMed] [Google Scholar]
- 2.Amici C, Rossi A, Santoro MG. Aspirin enhances thermotolerance in human erythroleukemic cells: an effect associated with the modulation of the heat response. Cancer Res. 1995;55:4452–4457. [PubMed] [Google Scholar]
- 3.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 4.Bao E, Sultan KR, Nowak B, Hartung J. Expression and distribution of heat shock proteins in the heart of transported pigs. Cell Stress Chaperones. 2008;13:459–466. doi: 10.1007/s12192-008-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao ED, Sultan KR, Nowak B, Hartung J. Localization and expression of heat shock proteins in liver of transport stressed pigs. Zhongguo nong ye ke xue. 2002;35:1130–1133. [Google Scholar]
- 6.Buriro R, Lv YJ, Ali I, Tang S, Liu ZJ, Zhang M, Adem A, Hartung J, Bao ED. Temporal variations of Hsp60 and HSF-1 in primary rat myocardial cells in vitro under heat stress. Genet Mol Res. 2013;12:3003–3016. doi: 10.4238/2013.August.20.2. [DOI] [PubMed] [Google Scholar]
- 7.Csermely P, Schnaider T, Soti C, Prohászka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 8.Geraert PA, Guillaumin S, Leclercq B. Are genetically lean broilers more resistant to hot climate? Br Poult Sci. 1993;34:643–653. doi: 10.1080/00071669308417623. [DOI] [PubMed] [Google Scholar]
- 9.Geraert PA, Padilha JCF, Guillaumin S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: growth performance, body composition and energy retention. Br J Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- 10.Giardina C, Lis JT. Sodium salicylate and yeast heat shock gene transcription. J Biol Chem. 1995;270:10369–10372. doi: 10.1074/jbc.270.18.10369. [DOI] [PubMed] [Google Scholar]
- 11.Heads RJ, Yellon DM, Latchman DS. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol. 1995;27:1669–1678. doi: 10.1016/s0022-2828(95)90722-x. [DOI] [PubMed] [Google Scholar]
- 12.Islam A, Lv YJ, Abdelnasir A, Rehana B, Liu ZJ, Zhang M, Tang S, Cheng YF, Chen HB, Hartung J, Bao ED. The role of Hsp90XMLLink_XYZ in heat-induced apoptosis and cell damage in primary myocardial cell cultures of neonatal rats. Genet Mol Res. 2013;12:6080–6091. doi: 10.4238/2013.December.2.6. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SE. Hsp90: structure and function. Top Curr Chem. 2013;328:155–240. doi: 10.1007/128_2012_356. [DOI] [PubMed] [Google Scholar]
- 14.Jin M, Otaka M, Okuyama A, Itoh S, Otani S, Odashima M, Iwabuchi A, Konishi N, Wada I, Pacheco I, Itoh H, Tashima Y, Masamune O, Watanabe S. Association of 72-kDa heat shock protein expression with adaptation to aspirin in rat gastric mucosa. Dig Dis Sci. 1999;44:1401–1407. doi: 10.1023/a:1026603919224. [DOI] [PubMed] [Google Scholar]
- 15.Jurivich DA, Sistonen L, Kroes RA, Morimoto RI. Effect of sodium salicylate on the human heat shock response. Science. 1992;255:1243–1245. doi: 10.1126/science.1546322. [DOI] [PubMed] [Google Scholar]
- 16.Kabakov AE, Gabai VL. Heat-shock proteins maintain the viability of ATP-deprived cells: what is the mechanism? Trends Cell Biol. 1994;4:193–196. doi: 10.1016/0962-8924(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 17.Kamarck T, Jennings JR. Biobehavioral factors in sudden cardiac death. Psychol Bull. 1991;109:42–75. doi: 10.1037/0033-2909.109.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Klemcke HG. Responses of the porcine pituitary-adrenal axis to chronic intermittent stressor. Domest Anim Endocrinol. 1994;11:133–149. doi: 10.1016/0739-7240(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 19.Koo HN, Oh SY, Kang KI, Moon DY, Kim HD, Kang HS. Modulation of HSP70 and HSP90 expression by sodium salicylate and aspirin in fish cell line CHSE-214. Zoolog Sci. 2000;17:1275–1282. [Google Scholar]
- 20.Lee BS, Chen J, Angelidis C, Jurivich DA, Morimoto RI. Pharmacological modulation of heat shock factor 1 by anti-inflammatory drugs results in protection against stress-induced cellular damage. Proc Natl Acad Sci U S A. 1995;92:7207–7211. doi: 10.1073/pnas.92.16.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei L, Yu J, Bao E. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br Poult Sci. 2009;50:504–511. doi: 10.1080/00071660903110851. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 23.Mathew A, Mathur SK, Morimoto RI. Heat shock response and protein degradation: regulation of HSF2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–5098. doi: 10.1128/mcb.18.9.5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM. Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action. J Thorac Cardiovasc Surg. 2006;132:72–79. doi: 10.1016/j.jtcvs.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 25.Nadeau K, Das A, Walsh CT. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem. 1993;268:1479–1487. [PubMed] [Google Scholar]
- 26.Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A. 1997;94:12949–12956. doi: 10.1073/pnas.94.24.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostlund G, Sonnhammer EL. Quality criteria for finding genes with high mRNA-protein expression correlation and coexpression correlation. Gene. 2012;497:228–236. doi: 10.1016/j.gene.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348:166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 30.Prohászka Z, Füst G. Immunological aspects of heat-shock proteins-the optimum stress of life. Mol Immunol. 2004;41:29–44. doi: 10.1016/j.molimm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- 32.Seok SH, Baek MW, Lee HY, Kim DJ, Na YR, Noh KJ, Park SH, Lee HK, Lee BH, Ryu DY, Park JH. Arsenite-induced apoptosis is prevented by antioxidants in zebrafish liver cell line. Toxicol In Vitro. 2007;21:870–877. doi: 10.1016/j.tiv.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular pathophysiology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461. [DOI] [PubMed] [Google Scholar]
- 34.Tang S, Buriro R, Liu Z, Zhang M, Ali I, Adam A, Hartung J, Bao E. Localization and expression of Hsp27 and XMLLink_XYZB-crystallin in rat primary myocardial cells during heat stress in vitro. PLoS One. 2013;8:e69066. doi: 10.1371/journal.pone.0069066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Hel der, Versteqen MW, Pijls L, van Kampen M. Effect of two-day temperature exposure of neonatal broiler chicks on growth performance and body composition during two weeks at normal conditions. Poult Sci. 1992;71:2014–2021. doi: 10.3382/ps.0712014. [DOI] [PubMed] [Google Scholar]
- 36.Wada H, Kamiike W. Aspartate aminotransferase isozymes and their clinical significance. Prog Clin Biol Res. 1990;344:853–875. [PubMed] [Google Scholar]
- 37.Yu H, Bao ED, Zhao RQ, Lv QX. Effect of transportation stress on heat shock protein 70 concentration and mRNA expression in heart and kidney tissues and serum enzyme activities and hormone concentrations of pigs. Am J Vet Res. 2007;68:1145–1150. doi: 10.2460/ajvr.68.11.1145. [DOI] [PubMed] [Google Scholar]
- 38.Yu J, Bao E, Yan J, Lei L. Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones. 2008;13:327–335. doi: 10.1007/s12192-008-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Xin L, Bao E, Hartung J, Yue Z. Variation in the expression of Hsp27, XMLLink_XYZB-crystallin mRNA and protein in heart and liver of pigs exposed to different transport times. Res Vet Sci. 2011;90:432–438. doi: 10.1016/j.rvsc.2010.06.028. [DOI] [PubMed] [Google Scholar]