Abstract
This study was conducted to identify the effectiveness of platelet-rich plasma (PRP) and efficacy of intralesional injection as a method of application to acute cutaneous wounds in dogs. Healthy adult beagles (n = 3) were used in this study. Autologous PRP was separated from anticoagulant treated whole blood in three dogs. Cutaneous wounds were created and then treated by intralesional injection of PRP in the experimental group, while they were treated with saline in the control group on days 0, 2 and 4. The healing process was evaluated by gross examination throughout the experimental period and histologic examination on day 7, 14 and 21. In PRP treated wounds, the mean diameter was smaller and the wound closure rate was higher than in the control. Histological study revealed that PRP treated wounds showed more granulation formation and angiogenesis on day 7, and faster epithelialization, more granulation formation and collagen deposition were observed on day 14 than in control wounds. On day 21, collagen deposition and epithelialization were enhanced in PRP treated groups. Overall, PRP application showed beneficial effects in wound healing, and intralesional injection was useful for application of PRP and could be a good therapeutic option for wound management in dogs.
Keywords: cutaneous wound, dog, intralesional injection, platelet-rich plasma, wound healing
Introduction
Wound healing is a complicated process mediated by physical, chemical, and cellular processes. The healing process is initiated by hemostasis and consists of inflammation, proliferation/repair, and remodeling, which are regulated by various cells, cytokines, and growth factors. The wound healing process can be slowed by host attributes, characteristics of the wound, external factors (e.g., concurrent disorders such as hyperadrenocorticism, diabetes mellitus, hepatic disorders and uremia), glucocorticoids and chemotherapy [15,17,25,28]. To enable repair of damaged tissue quickly and efficiently, many materials have been studied for delayed or chronic wounds, and platelet-rich plasma (PRP) has been shown to be effective in tissue healing [6,12,20,25,33].
Platelets play important roles in wound healing because of their hemostatic function and concentrated levels of naturally occurring cytokines and growth factors [5,9]. The α granules of platelets contain numerous growth factors including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF), and epidermal growth factor (EGF). Bioactive factors such as serotonin, histamine, dopamine, calcium, and adenosine are also stored in the dense granules [4,13].
PRP refers to the concentration of a large number of platelets in a small volume of plasma, at least 1,000,000 platelets/µL, resulting in a 3- to 5-fold increase in concentrations of growth factors. PRP works by secretion of growth factors following platelet activation, and released growth factors bind to the external surface of cell membranes in applied tissue. Studies have shown that adult stem cells, osteoblasts, fibroblasts, endothelial cells, and epidermal cells can express receptors to growth factors from PRP. Concentrated growth factors lead to accelerated endothelial and epithelial regeneration, and stimulate angiogenesis, collagen synthesis, soft-tissue healing, and hemostasis. Because PRP is known to be effective in various aspects of tissue regeneration, its clinical use has been reported for a variety of applications, such as in cosmetic, orthopedic, arthrologic, oral–maxillofacial, and dermatologic surgery [13,22,25,29].
Use of PRP in animals is steadily increasing, and its effects on tissue healing have been reported in many studies, particularly in bone regeneration and ligament or tendon reconstruction. Some studies have also described its healing effects in cutaneous wounds in dogs [2,18,27], goats [1], horses [7,23], and other species [10,19]. In both humans and animals, PRP is commonly processed for application to cutaneous wounds by mixing with calcium chloride and thrombin to form a platelet gel, which can be applied like an ointment [5,16,18]. A gel formulation is easy to use but has certain disadvantages, particularly in animals, such as uneven distribution at movable body parts, possible loss, limited accessibility to the wound surface, and limited duration of effect. Therefore, injection of PRP has been used in orthopedic procedures, cosmetic surgery, and cutaneous wounds [1,3,10,26]. The present study was conducted to investigate the efficacy of intralesional injection of PRP in cutaneous wounds in dogs by evaluating tissue healing parameters.
Materials and Methods
Animals
Three healthy adult beagle dogs (3–5 years of age; mean body weight, 12.47 kg; two intact female dogs and one intact male dog) were used for this study. The results of physical examination, complete blood cell count, and serum chemistry revealed no evidence of hypovolemia, dehydration, or hematologic abnormalities. All procedures were approved by the Institutional Animal Care and Use Committees of Gyeongsang National University.
Preparation of platelet-rich plasma
Whole blood was collected from the jugular vein of each dog. Briefly, 52 mL of blood was aspirated using a syringe fitted with a 19-gauge scalpel needle containing 8 mL of the anticoagulant citrate phosphate dextrose (CPDA-1; GreenCross Medical Science, Korea) to give 60 mL anticoagulant-treated blood, and the collection procedure was repeated to obtain 120 mL for each dog. Blood was immediately centrifuged and separated to prevent platelet activation and clot formation. Aliquots (18 mL) of anticoagulant-treated blood were injected into sterilized PRP-separation containers (PRO-PRP20; Goodmorning Bio, Korea) and centrifuged at 209 × g for 6 min. This resulted in separation of blood into three fractions: red blood cells (bottom layer), platelet-poor plasma (top layer), and buffy coat (middle layer, including white blood cells and platelets). Using a platelet separator (Goodmorning Bio), 1 mL PRP including the buffy coat was obtained. Obtained PRP was kept frozen at -76℃ for further use, except for 0.5 mL that was used for analysis.
Unseparated whole blood and PRP (0.5 mL samples of each) were used to obtain platelet and white blood cell (WBC) counts with an automatic blood cell counter (Celltacα MEK-6450K; Nihon Kohden, Japan).
Creation of cutaneous wounds
Dogs were premedicated with subcutaneous atropine sulfate (0.05 mg/kg; atropine sulfate injection; Jeil Pharmaceutical, Korea) and intravenous cefazolin sodium (20 mg/kg; cefazolin sodium injection; Chong Kun Dang Pharmaceutical, Korea), then sedated with intravenous medetomidine HCl (4 µg/kg; Domitor; Orion, Finland). Shortly before creating wounds, intramuscular tramadol HCl was administered (2 mg/kg; Tramadol HCl; Huons, Korea) for pain control. Dorsal hair was clipped and the skin was disinfected with 5% chlorhexidine and 70% isopropyl alcohol solution.
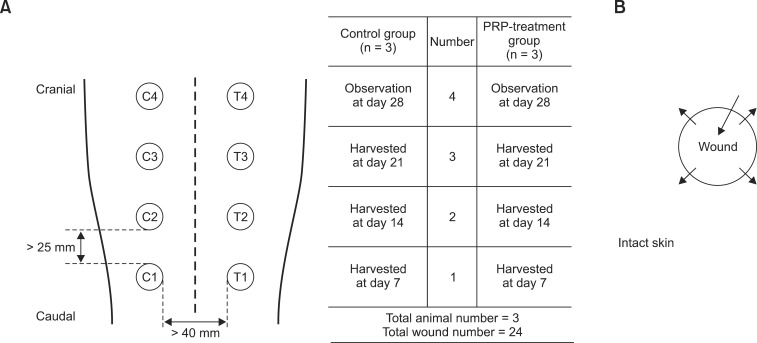
Full-thickness circular wounds were created in the dorsal skin of the thoracic region using a dermal biopsy punch 6 mm in diameter (Integra Miltex, USA) to harvest the epidermis and dermis, exposing subcutaneous fat. Wounds were covered with moist gauze to avoid desiccation until injection was performed. Eight wounds were created in each dog, resulting in 24 skin wounds in the three dogs. Wounds were at least 25 mm apart and divided into two groups (Fig. 1).
Fig. 1. Schematic illustration of location and experimental regimen for each wound, and injection protocol. (A) Representation of wounds creation and treatment regimen. Wounds are named C1–4 in the control group and T1–4 in the PRP-treated group. (B) 0.5 mL aliquot of saline (control group) or PRP (PRP-treated group) per wound was injected, divided into four intradermal injections and one injection into the wound bed. Each arrow represents injection of 0.1 mL solution. C, control group; T, PRP-treatment group.
Injection protocol
Overall, 0.5 mL PRP was injected into each PRP-treatment wound using a 26-G needle (T1, T2, T3, and T4; 12 wounds in three dogs). In addition, 0.1 mL was injected intradermally into the outward edge of the quadrant of the wound lesion and 0.1 mL was injected into the center of the wound bed (Fig. 1). Injections of saline into control wounds were performed using the same procedures. All injections were repeated on days 2 and 4.
Management
The dorsal wound area was covered with an occlusive dressing (Tegaderm; 3M Health Care, USA) and elastic bandage. On alternate days, wounds were cleaned with saline, taking care not to debride the wound, and the bandage was changed. Oral administration of tramadol HCL (2 mg/kg; Tridol; Yuhan, Korea) and cefadroxil (30 mg/kg; Cefadroxil; Ilyang Pharmaceutical, Korea) was conducted twice daily throughout the experimental period.
Wound assessment
Wounds were examined every two days during the experimental period. The diameters of wounds were measured with a ruler to the nearest millimeter (mm), after which the percentage of wound diameter to diameter at day 0 was calculated. The experimental day on which the wounds were closed and the wound closure rate were determined.
Microscopic evaluation
For histological evaluation, punch biopsies were conducted on days 7, 14, and 21. Full-thickness wound samples including surrounding non-wounded skin, were harvested with 8 mm circular biopsy punches. Tissues were fixed in 10% neutral buffer formalin solution, embedded in paraffin, sectioned at a thickness of 5 µm, and stained with hematoxylin-eosin reagent and Masson's trichrome method according to the manufacturer's instructions. For microscopic assessment, an upright microscope (Leica DM6000B; Leica, Germany), microscope camera (Leica DFC500; Leica), and imaging software (Leica Application Suite; Leica) were used. All measurements were performed by three investigators.
Epithelialization
HE-stained tissues were examined to evaluate epithelialization. Epidermal thickness was quantified in 10 different areas in each tissue. Epidermis overlying the wound edge on day 7 and the center of the wound on days 14 and 21 was evaluated, and re-epithelialization was compared between the control and PRP-treated wounds.
Granulation tissue
Granulation tissue was divided into two layers, upper granulation tissue, which is rich in vascularization, and lower granulation tissue rich in collagen. Total granulation tissue included both upper and lower granulation tissue. The amount of granulation tissue was evaluated using Masson's trichrome-stained slides and calculated as granulation indexes as previously described [30].
Angiogenesis
The degree of angiogenesis was determined by counting vascular structures on HE-stained sections. The number of vessels in the upper granulation tissue was measured in five random high-power fields by three investigators.
Collagen organization
The mid portion of the lower granulation tissue was observed in Masson's trichrome–stained sections and photographed to compare collagen deposition in control and PRP-treated wounds.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS ver. 12.0; IBM, USA). Data were expressed as the mean ± standard deviation. Statistical significance of the differences between the control and PRP-treated groups in the wound-closure time, epidermal thickness, granulation indexes, and number of blood vessels was analyzed using the nonparametric Mann–Whitney U-test. Wilcoxon signed-rank tests were used to compare mean wound diameters among control and treated wounds. For all analyses, values of p < 0.05 were considered statistically significant.
Results
Analysis of the PRP
Platelet counts in PRP were substantially increased compared with the baseline counts of unseparated blood. The average initial platelet concentration was 3.57 × 1011/L and the average platelet count in the PRP was 30.70 × 1011/L, resulting in significant increases of 8.60 times. The quantitative analysis of WBCs revealed an average increase of 2.90 fold in the PRP.
Macroscopic examination
Wound diameter
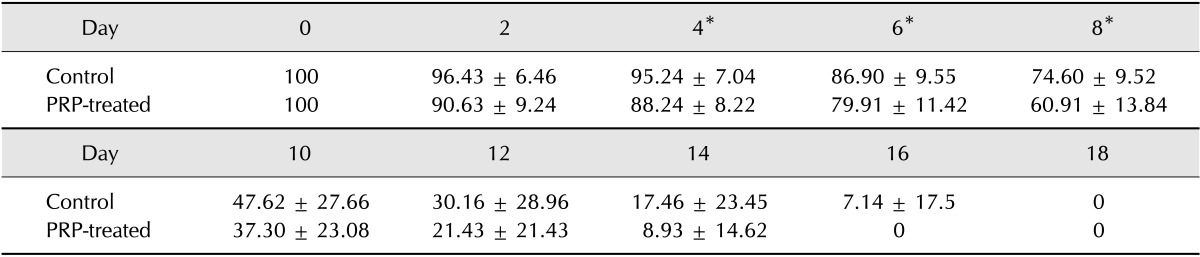
Statistical differences between control and PRP-treated wounds were identified on days 4 (p = 0.024), 6 (p = 0.040) and 8 (p = 0.027) (Table 1).
Table 1. Percentages of wound diameter to initial diameter on each examination day.
*Significant differences between control and PRP-treated wounds are observed (p < 0.05, Wilcoxon signed-rank test).
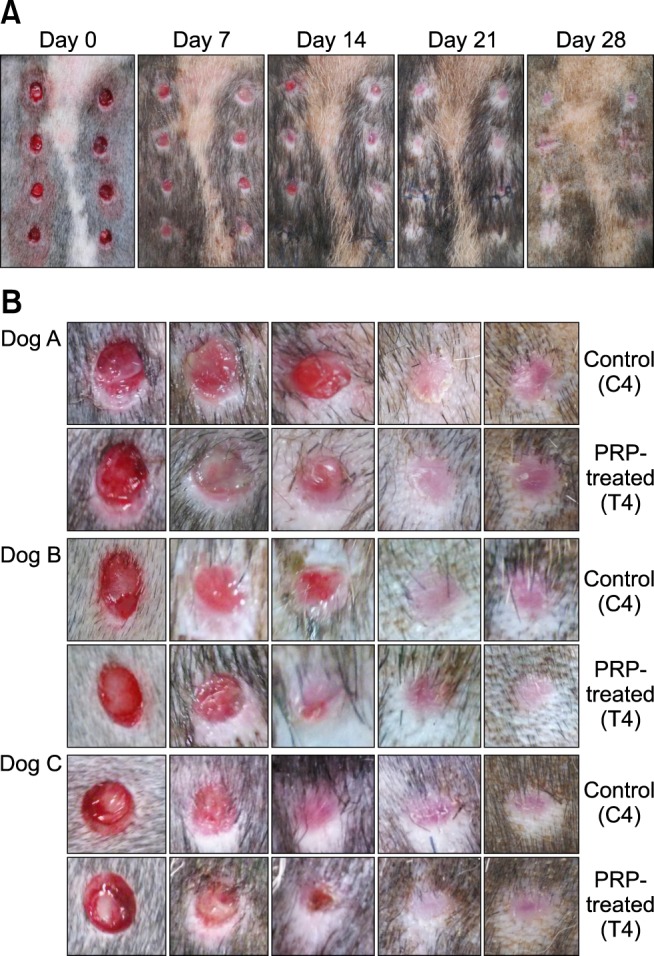
Time required for wound closure
Although there was no significant difference, mean wound closure time of the PRP-treated group (13.11 ± 2.47 days) was slightly faster than that of the control group (13.43 ± 2.76 days). Wound closure was observed between day 10 and day 18 in all dogs. During day 10 to day 18, the PRP-treated group showed a higher closure rate (Fig. 2).
Fig. 2. Steps of wound healing in control and PRP-treated wounds according to time. (A) Serial macroscopic examination of wounds in dog A. On day 14, a smaller wound diameter and higher wound closure rate was observed in PRP-treated wounds. (B) Serial examination of C4 and T4 during the experimental period in three dogs. In dogs A and B, wound diameters are smaller in T4 than C4 on day 14, and T4 are pale, small, and had less scar formation on days 14 and 28, compared with C4.
Microscopic examination
Epidermis
The average epidermal thickness decreased gradually in PRP-treated wounds throughout the experiment, but only from day 14 in control wounds. At day 14, the control tissue had an average thickness of 105.4 ± 29.79 µm, which was significantly thicker than that of the treated tissue at 82.7 ± 22.66 µm (p = 0.000), and more irregular layers appeared (Table 2).
Table 2. Mean epidermal thickness (µm).
*Significant differences between control and PRP-treated wounds are observed (p < 0.05, Mann-Whitney's U test).
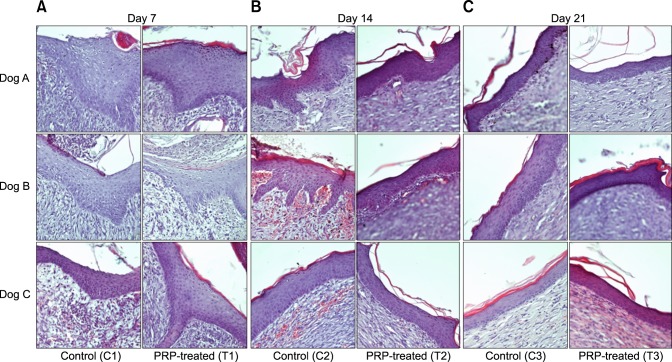
At day 7, epithelial layers had grown from the margins of the wounds and hypertrophied, but did not cover the granulation tissue in all wounds. All control wounds presented undifferentiated keratinocytes and had no or less keratohyaline granules in the stratum granulosum. Two of the three PRP-treated wounds exhibited hypertrophied epidermis with development of stratum spinosum and stratum granulosum, abundant keratohyaline granules, and differentiated keratinocytes.
At days 14 and 21, regenerated epidermal structures were compared in the center of the wound area. On day 14, hyperplastic epidermis with irregular thickness and no stratum corneum was observed in the control group, except in one dog. However, all PRP-treated wounds showed decreased thickness, and epithelial cells were flattened and keratinized with anuclear keratinocytes overlying the epidermis. On day 21, both control and PRP-treated wounds exhibited a decrease in cell layers, but keratohyaline granules persisted in the control group. Additionally, epithelialization appeared similar in PRP-treated wounds on day 14 and control wounds on day 21, indicating that the PRP-treated wounds had differentiated to a normal structure faster than the control wounds (Fig. 3).
Fig. 3. Photomicrographs of epidermis from control and PRP-treated groups on days 7, 14 and 21 in three dogs. (A) On day 7, epithelial layers of C1 are hypertrophied, but differentiation is limited with marked absence of keratinocytes, and dog B has no keratohyaline granules, although A and C have small amounts of keratohyaline granules. Epithelial layers of T1 exhibit hypertrophy and differentiated keratinocytes. More keratohyaline granules are observed in dogs A and C, with development of stratum spinosum and stratum granulosum, when compared with control wounds, respectively. Dog B shows no difference between the PRP-treated and control wound. (B) Epidermis at day 14. C2 still shows a hyperplastic epidermis, and dog A and B show irregular epidermis without stratum corneum. T2 shows decreased thickness, and epithelial cells are flattened and keratinized. (C) On day 21, the epidermis of C3 exhibits keratinization and decreased thickness with keratohyaline granules, although T3 exhibits more keratinization without keratohyaline granules. T2 and C3 appear to be at a similar stage of epithelialization. H&E stain. 200× (A–C).
Granulation tissue
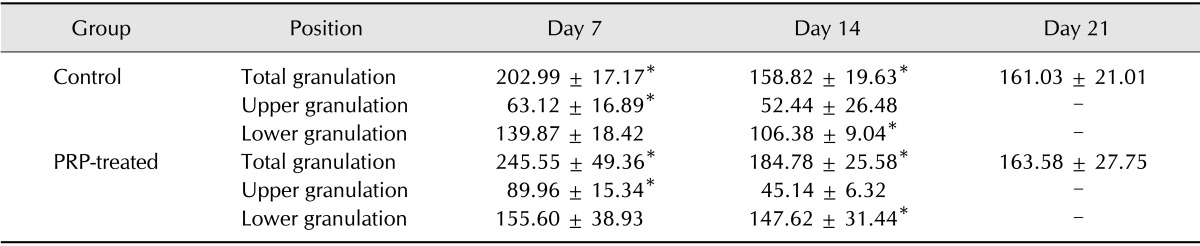
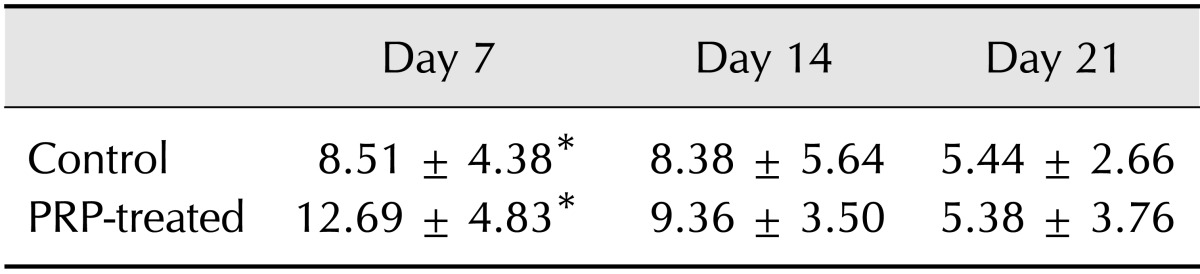
Total granulation, lower granulation, and upper granulation indexes are shown in Table 3. Lower and upper granulation indexes were not calculated on day 21 because they could not be distinguished. Compared with control wounds, total granulation indexes of PRP-treated wounds were increased on days 7 (p = 0.040) and 14 (p = 0.031). The upper granulation index of PRP-treated wounds on Day 7 was significantly higher (p = 0.006) than that of the control, while on day 14 the lower granulation index of the PRP-treated group was higher (p = 0.000).
Table 3. Granulation indexes.
*Significant differences between control and PRP-treated wounds were observed (p < 0.05, Mann-Whitney's U test).
Angiogenesis
The number of vessels was highest on day 7 in both the control and PRP-treated wounds and decreased gradually over the experimental period. More vessels were observed in the PRP-treated than control wounds on day 7 (p = 0.000), although no difference was found on Days 14 (p = 0.119) and 21 (p = 0.481) (Table 4).
Table 4. Mean number of vessels per high-power field (200×).
*Significant differences between control and PRP-treated wounds are observed (p < 0.05, Mann-Whitney's U test).
Collagen fibers
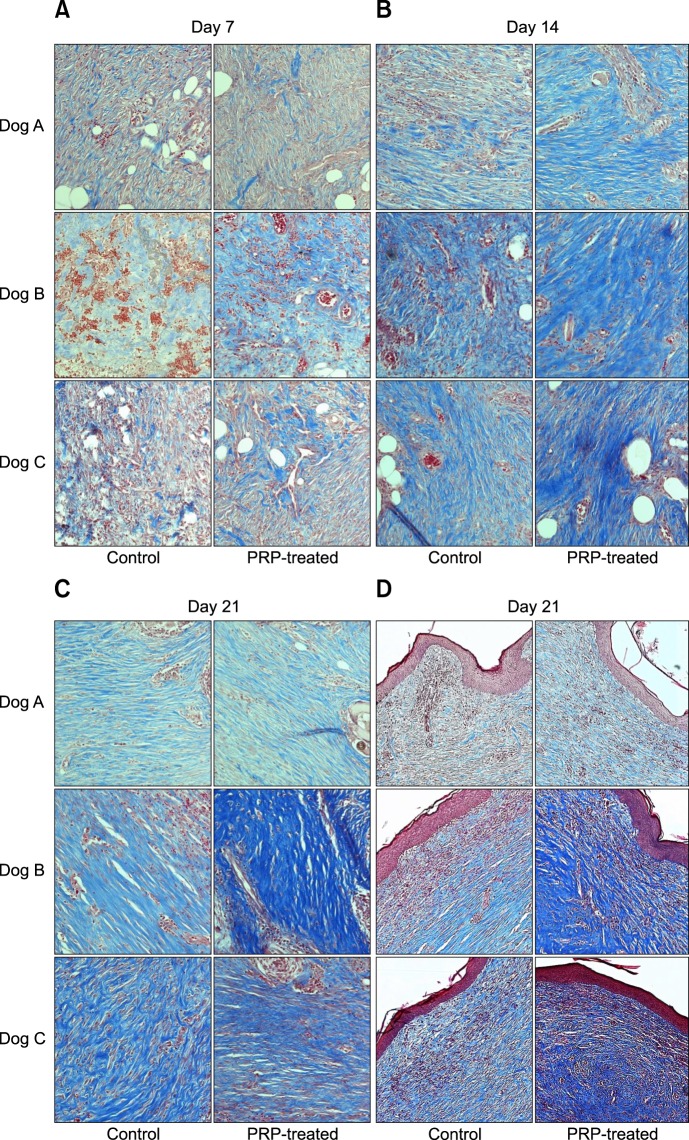
Evaluation of collagen fibers was performed in lower granulation tissue on days 7 and 14, but in the mid and upper portion of dermis on day 21. On day 7, PRP-treated wounds from two of three dogs displayed more collagen fibers than control wounds. The collagen fibers in all of the PRP-treated groups became denser and more abundant than those in the control on day 14. At day 21, two of the three mid-portions and all of the upper portions of the dermis in PRP-treated wounds showed tightly packed collagen fibers running parallel to each other and the epidermis relative to control wounds (Fig. 4).
Fig. 4. Photomicrographs of collagen deposition in granulation tissue and dermis. (A) On day 7, T1 from dogs B and C display more collagen deposition than C1. (B) On day 14, all T2 have dense and abundant collagen. (C). Tightly packed collagen fibers are observed running parallel to each other at the mid-portion of the dermis of T3 in dogs B and C on day 21. (D) All upper dermis sample of T3 exhibit more deposition of collagen than C3. Masson's trichrome stain. 200× (A–C), 100× (D).
Discussion
PRP application is an easily accessible cell therapy involving many bioactive factors, and therapeutic application may be achieved by simple techniques. In this study, PRP was injected intralesionally to identify the effects of PRP on experimental cutaneous wounds in dogs. Wounds were examined macroscopically and microscopically, and the results from control and PRP-treated groups were compared to evaluate the progression of healing.
According to the results of this study, PRP-treated wounds showed macroscopically and microscopically faster healing than control groups. These characteristics reflect accelerated tissue regeneration, which is one of the known effects of PRP application [29]. The proliferative phase is also known as repair or regeneration with granulation formation, angiogenesis, re-epithelialization, and collagen synthesis, and evaluation was performed 7, 14, and 21 days after wound creation in this study. To evaluate granulation in more detail, granulation tissue was subdivided into upper and lower granulation layers and granulation indexes, angiogenesis, and collagen deposition were then assessed in these areas [30]. PRP enhanced angiogenesis and upper granulation formation at day 7, in addition to collagen deposition and lower granulation formation at day 14. Accelerated re-epihelialization and epithelial differentiation were also observed in three sequential biopsies from PRP-treated wounds. Wound tension, glandular reformation, and hair regrowth can also be evaluated as healing parameters for longer periods, although this was not performed in this study [7,11,23].
Cutaneous wound healing indicates repair and regeneration of tissue, which is mediated by growth factors that regulate cell migration, proliferation, differentiation, production of proteins, enzymes, and extracellular matrix, and remodeling [15,28]. Important factors contributing to the healing effects of PRP include PDGF, TGF-β, VEGF, FGF, EGF, IGF, interleukin-8 (IL-8), and tumor necrosis factor α, as reported by several studies. Considering the functions of major growth factors, PDGF is a potent chemoattractant and mitogen for fibroblasts that stimulates collagen synthesis and angiogenesis. TGF-β stimulates mesenchymal, epithelial, and endothelial cell growth and proliferation. In addition, EGF enhances proliferation of fibroblasts and epidermal and epithelial cells, resulting in re-epithelialization and angiogenesis. VEGF and FGF also induce angiogenesis and collagen synthesis [3,21,25]. These mechanisms are complex and interrelated with each other over the healing periods. Because of the various mechanisms involved in the effects of PRP acting synergistically, further studies of usage determination and adverse effects are needed, although no remarkable complications have been reported to date [24,25,31].
To release the growth factors, platelets have to be activated by thrombin or excess calcium; therefore, platelet activation (for example, by adding autologous or bovine thrombin and calcium chloride) has commonly been performed before clinical application. However, one study suggested that no specific agent is needed for activation because platelets are activated by exposure to collagens in tissues, resulting in efficient release of growth factors [14]. Based on that report, no activation procedure was carried out in this study and appropriate effects of PRP were obtained, supporting the results of the previous study. As a result, this protocol provides an easier alternative to previously used protocols.
Certain conditions must be considered when determining PRP preparation and application. The potential candidate should be evaluated for general status and hematologic status to rule out hypovolemia, anemia, coagulopathy, and thrombocytopenia. Because a large volume of blood is required for preparation of PRP, it may not be possible to collect enough blood from candidates with hypovolemia and anemia. Moreover, thrombocytopenia and coagulopathy could influence PRP quality; therefore, candidates with these conditions should be excluded. Additionally, patients who have undergone profuse loss of red blood cells or who have underlying disorders place limitations on clinical usage [29].
Intralesional injection of PRP was performed for several reasons. Injection provides selective distribution in target regions of the wound that require more aid in the healing process and therefore a reduction in the required volume of PRP. In addition, injection techniques are simple and convenient, and regular dressing changes can be performed without loss of PRP from the wound [8]. Moreover, many fields in human medicine, including arthrology and cosmetic and orthopedic surgeries, make good use of PRP injection for various purposes [3,26,32]. In accordance with these advantages over the traditional gel form, injection is considered practical in wound management in veterinary medicine.
It should be noted that this study had several limitations. Because small and acute cutaneous wounds were created on normal skin in subjects without any disorders, clinical application in patients with delayed healing capacity or wide-ranging wounds may not correlate with the results of this study. Although wound remodeling continues for months to years to form a mature scar and normal structure, prolonged effects of PRP were not investigated and long-term observations were not made in this study. The platelet count in the PRP varied among the subjects and concentrations of growth factors were not identified. Regarding clinical usage, further studies for standardization, long-term evaluation, and application under various conditions will be required.
The present study reported that intralesional injection of autologous PRP was effective for cutaneous wound healing in dogs. Adequate enhancement of healing, including angiogenesis, granulation formation, organization of collagen fibers, and re-epithelialization, was obtained after PRP application. In accordance with this study, intralesional injection of PRP is considered a useful alternative in the management of cutaneous wounds by its healing effects and practicability. Further studies are required for usage determination and identification of complications to enable clinical application.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.AL-Bayati AH, Al-Asadi RN, Mahdi AK, Al-Falahi NH. Effects of autologous platelets rich plasma on full-thickness cutaneous wounds healing in goats. Int J Anim Vet Adv. 2013;5:233–239. [Google Scholar]
- 2.Alishahi MK, Mofidpoor H, Alishahi MAK. Histopathological evaluation of the effect of platelet-rich fibrin on canine cutaneous incisional wound healing. World Appl Sci J. 2014;31:676–680. [Google Scholar]
- 3.Alsousou J, Thompson M, Hulley P, Noble A, Willett K. The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery: a review of the literature. J Bone Joint Surg Br. 2009;91:987–996. doi: 10.1302/0301-620X.91B8.22546. [DOI] [PubMed] [Google Scholar]
- 4.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 5.Brissett AE, Hom DB. The effects of tissue sealants, platelet gels, and growth factors on wound healing. Curr Opin Otolaryngol Head Neck Surg. 2003;11:245–250. doi: 10.1097/00020840-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Carter K. Growth factors: the wound healing therapy of the future. Br J Community Nurs. 2003;8:S15–S16. S18–S19, S22–S23. doi: 10.12968/bjcn.2003.8.Sup4.11585. [DOI] [PubMed] [Google Scholar]
- 7.DeRossi R, Coelho ACAO, de Mello GS, Frazílio FO, Leal CRB, Facco GG, Brum KB. Effects of platelet-rich plasma gel on skin healing in surgical wound in horses. Acta Cir Bras. 2009;24:276–281. doi: 10.1590/s0102-86502009000400006. [DOI] [PubMed] [Google Scholar]
- 8.Dionyssiou D, Demiri E, Foroglou P, Cheva A, Saratzis N, Aivazidis C, Karkavelas G. The effectiveness of intralesional injection of platelet-rich plasma in accelerating the healing of chronic ulcers: an experimental and clinical study. Int Wound J. 2013;10:397–406. doi: 10.1111/j.1742-481X.2012.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 10.Fathi WK. The effect of hyaluronic acid and platelet-rich plasma on soft tissue wound healing: an experimental study on rabbits. Al–Rafidain Dent J. 2012;12:115–125. [Google Scholar]
- 11.Ferdousy RN, Rahman MM, Paul S, Khan MAHNA. Role of platelet rich plasma gel in the wound healing of black Bengal goat. IOSR J Agri Vet Sci. 2013;6:14–21. [Google Scholar]
- 12.Ferguson M, Byrnes C, Sun L, Marti G, Bonde P, Duncan M, Harmon JW. Wound healing enhancement: electroporation to address a classic problem of military medicine. World J Surg. 2005;29(Suppl 1):S55–S59. doi: 10.1007/s00268-004-2062-2. [DOI] [PubMed] [Google Scholar]
- 13.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 14.Fufa D, Shealy B, Jacobson M, Kevy S, Murray MM. Activation of platelet-rich plasma using soluble type I collagen. J Oral Maxillofac Surg. 2008;66:684–690. doi: 10.1016/j.joms.2007.06.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedlund CS. Surgery of the Integumentary System. In: Fossum TW, Hedlund CS, Johnson AL, Schulz KS, Seim HB, Willard MD, Bahr A, Carroll GL, editors. Small Animal Surgery. 3rd ed. St. Louis: Mosby Elsevier; 2007. pp. 159–170. [Google Scholar]
- 16.Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–183. doi: 10.1001/archfaci.9.3.174. [DOI] [PubMed] [Google Scholar]
- 17.Johnston DE. Wound healing in skin. Vet Clin North Am Small Anim Pract. 1990;20:1–25. doi: 10.1016/s0195-5616(90)50001-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Park C, Park HM. Curative effect of autologous platelet-rich plasma on a large cutaneous lesion in a dog. Vet Dermatol. 2009;20:123–126. doi: 10.1111/j.1365-3164.2008.00711.x. [DOI] [PubMed] [Google Scholar]
- 19.Kimura A, Ogata H, Yazawa M, Watanabe N, Mori T, Nakajima T. The effects of platelet-rich plasma on cutaneous incisional wound healing in rats. J Dermatol Sci. 2005;40:205–208. doi: 10.1016/j.jdermsci.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 21.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 23.Monteiro SO, Lepage OM, Theoret CL. Effects of platelet-rich plasma on the repair of wounds on the distal aspect of the forelimb in horses. Am J Vet Res. 2009;70:277–282. doi: 10.2460/ajvr.70.2.277. [DOI] [PubMed] [Google Scholar]
- 24.Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- 25.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:156–165. [PubMed] [Google Scholar]
- 26.Sampson S, Reed M, Silvers H, Meng M, Mandelbaum B. Injection of platelet-rich plasma in patients with primary and secondary knee osteoarthritis: a pilot study. Am J Phys Med Rehabil. 2010;89:961–969. doi: 10.1097/PHM.0b013e3181fc7edf. [DOI] [PubMed] [Google Scholar]
- 27.Sardari K, Emami MR, Kazemi H, Movasagi AR, Goli AA, Lotfi A, Malekzadeh S. Effects of platelet-rich plasma (PRP) on cutaneous regeneration and wound healing in dogs treated with dexamethasone. Comp Clin Pathol. 2011;20:155–162. [Google Scholar]
- 28.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 29.Smith RG, Gassmann CJ, Campbell MS. Platelet-rich plasma: properties and clinical applications. J Lancaster Gen Hosp. 2007;2:73–78. [Google Scholar]
- 30.Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, Kadosawa T, Fujinaga T. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–1414. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 31.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 32.Willemsen JC, van der Lei B, Vermeulen KM, Stevens HP. The effects of platelet-rich plasma on recovery time and aesthetic outcome in facial rejuvenation: preliminary retrospective observations. Aesthetic Plast Surg. 2014;38:1057–1063. doi: 10.1007/s00266-014-0361-z. [DOI] [PubMed] [Google Scholar]
- 33.Yang HS, Shin J, Bhang SH, Shin JY, Park J, Im GI, Kim CS, Kim BS. Enhanced skin wound healing by a sustained release of growth factors contained in platelet-rich plasma. Exp Mol Med. 2011;43:622–629. doi: 10.3858/emm.2011.43.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]