Introduction
The Nocardia genus, belonging to the order Actinomycetales, is a group of gram-positive saprophytic bacteria that are found ubiquitously in the environment. In 2005, Nocardia takedensis was isolated from moat sediment and sludge around the Takeda shrine in Japan and reported as a novel species.1, 2 This species shows highest sequence similarity to Nocardia beijingensis (98.1%-98.3%), Nocardia brasiliensis (97.9%-98.0%), and Nocardia tenerifensis (97.9%-97.9%) based on 16S rRNA sequencing.1 We describe a case of cutaneous nocardiosis caused by N takedensis, which, to our knowledge, is the first report of an infection caused by this species in the United States.
Case report
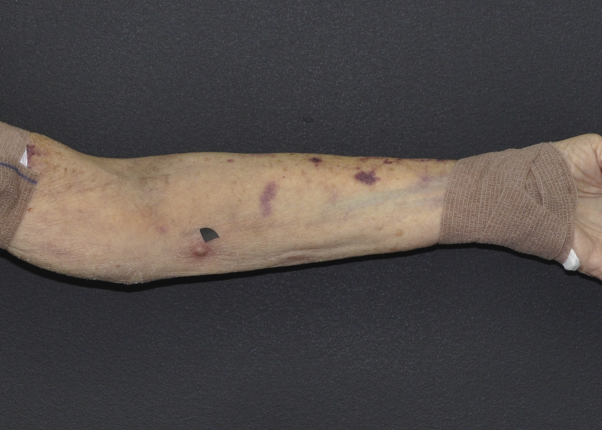
A 68-year-old-woman with a history of follicular lymphoma and myelodysplastic syndrome after allogeneic peripheral blood stem cell transplantation presented with new lesions on the left arm. The patient, a resident of suburban New York, reported a fall on her outdoor deck 3 months prior, which resulted in an abrasion on the left dorsal hand. Over the next several weeks, she had noted the development of painful lesions ascending the left forearm. Her oral medications were budesonide, 9 mg, sirolimus, 0.5 mg, and prednisone, 10 mg by mouth each daily. She denied any systemic symptoms, including shortness of breath. On examination, the patient's vital signs were within normal limits and 3 skin-colored to red, tender papules in a lymphangitic distribution were identified on the left forearm (Fig 1). A biopsy was performed for histopathologic examination (Fig 2).
Fig 1.
Cutaneous nocardiosis from N takedensis infection. Clinical image of the left upper extremity shows skin-colored to red papules in a lymphangitic distribution (black arrowhead corresponds to site of skin biopsy).
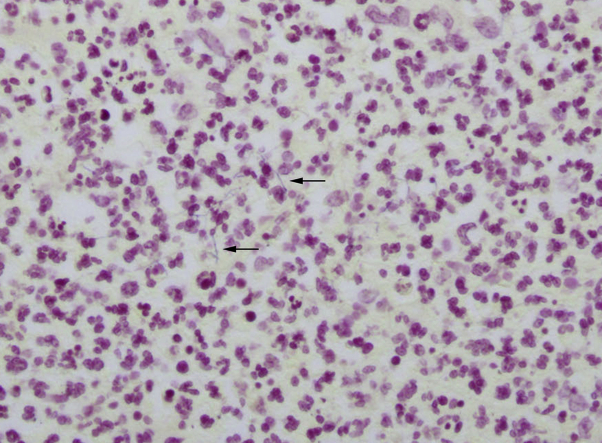
Fig 2.
Histopathologic image of the tissue specimen highlights thin filamentous branching gram-positive rods (black arrows) and abundant neutrophils. (Gram stain; original magnification: ×400.)
Histopathologic examination of the tissue specimen found dense multinodular pandermal and subcutaneous neutrophilic infiltrates. Gram and periodic acid–Schiff with diastase stains identified thin, filamentous rods suspicious for Actinomycetes species (Fig 2), and Grocott-Gomori's methenamine silver stain and repeated Fite stains were negative. After 6 days, a white, dry colony grew on chocolate agar and Columbia nalidixic agar media. Gram stain and modified Kinyoun stain of the colony showed thin, filamentous, gram-positive, acid-fast negative, beaded branching bacilli. The isolate was subcultured on a blood agar slant and sent to Mayo Medical Laboratories for definitive species identification. Middlebrook 7H10 agar and blood agar plates were used to grow the bacterium, which was then analyzed by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. The obtained mass spectrum was compared with an internal Mayo database but resulted in no matches. DNA was subsequently extracted for 16S rRNA gene sequencing. The obtained isolate sequence of 460 nucleotides was analyzed using the National Center for Biotechnology Information Basic Local Alignment Search Tool, which found 99.1% sequence similarity (4 total mismatches) to N takedensis.
At the time of the skin biopsy, the patient received cefuroxime, 500 mg by mouth twice daily (7 days), followed by amoxicillin/clavulanate, 875 mg by mouth twice daily (14 days), without improvement. After N takedensis was identified and systemic disease excluded, she completed 7 days of sulfamethoxazole/trimethoprim, 800/160 mg by mouth twice daily, which was discontinued because of worsening renal insufficiency after starting the drug. Despite the short therapeutic course, the skin lesions gradually resolved over the ensuing 4 weeks.
Discussion
Nocardiosis typically occurs in immunosuppressed patients, although as many as one-third of patients with nocardiosis are immunocompetent.3 Predisposing risk factors to Nocardia infections include depressed cell-mediated immunity, including lymphoma and HIV, hematopoietic stem cell or solid-organ transplants, and long-term immunosuppression.3, 4 Cutaneous manifestations of nocardiosis can be classified into primary cutaneous nocardiosis and disseminated disease. Primary cutaneous nocardiosis results from direct inoculation of pathogenic Nocardia present in soil into the skin. Disseminated disease is more common and usually occurs in the setting of primary pulmonary infection and underlying immunosuppression.3 Primary cutaneous nocardiosis most frequently presents as lymphangitic papules or draining abscesses. It typically arises in immunocompetent hosts and follows an indolent course.3 In the patient presented herein, we suspect that direct cutaneous inoculation from her fall was the etiology of her infection.
To identify any published cases of cutaneous nocardiosis caused by N takedensis, we performed a PubMed search for articles using the search term “nocardia takedensis” without date, language, publication, or article type restrictions. This search yielded 10 articles, which were independently reviewed by 2 authors (EC and MM). Two cases of primary cutaneous nocardiosis caused by N takedensis were identified. The first case was from a patient in Taiwan who had underlying chronic kidney disease and diabetes mellitus and presented with foot cellulitis. The second case was from a healthy male in Mexico who presented with actinomycetoma.4, 5 Both patients were successfully treated with oral trimethoprim-sulfamethoxazole (TMP-SMX). In addition, N takedensis has been isolated from the lungs in a patient from Spain with pulmonary eosinophilic granuloma and was reported to cause an unspecified infection in a patient in Japan with T-cell lymphoma.6, 7 No reports of patients infected with N takedensis in the United States were found.
Clinicians and pathologists should be aware that the Nocardia genus continues to undergo the addition of new species; more than 50 species have been identified to date. The species variability in general morphologic characteristics and acid fastness, which is caused by differences in cell wall mycolic acid composition and the type of stain used, make their identification and species differentiation challenging.3, 6, 7 As in the case presented herein, identification using molecular techniques is often required for a definitive diagnosis and is important when considering the optimal antimicrobial treatment. TMP-SMX is active against most species; however, clinicians should note that Nocardia otitidiscaviarum, Nocardia nova, and Nocardia farcinica have shown notable resistance and that up to 42% of Nocardia isolates submitted to the Centers for Disease Control and Prevention for antimicrobial susceptibility testing between 1995 and 2004 were resistant to TMP-SMX.3, 8, 9 Other antimicrobial agents that have been used to treat Nocardia infections include amikacin, impinem, meropenem, ceftriaxone, cefotaxime, minocycline, moxifloxacin, levofloxacin, linezolid, tigecycline, and amoxicillin-clavulanic acid.3
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Yamamura H., Hayakawa M., Nakagawa Y. Nocardia takedensis sp. nov., isolated from moat sediment and scumming activated sludge. Int J Syst Evol Microbiol. 2005;55(Pt 1):433–436. doi: 10.1099/ijs.0.63189-0. [DOI] [PubMed] [Google Scholar]
- 2.Japan MLJVTGo. Takeda Shrine. 2007-2012; http://www.mustlovejapan.com/subject/takeda_jinja/. Accessed October 29, 2014
- 3.Wilson J.W. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W.L., Lai C.C., Ko W.C. Clinical and microbiological characteristics of infections caused by various Nocardia species in Taiwan: a multicenter study from 1998 to 2010. Eur J Clin Microbiol Infect Dis. 2011;30(11):1341–1347. doi: 10.1007/s10096-011-1227-9. [DOI] [PubMed] [Google Scholar]
- 5.Kresch-Tronik N.S., Carrillo-Casas E.M., Arenas R. First case of mycetoma associated with Nocardia takedensis. J Dermatol. 2013;40(2):135–136. doi: 10.1111/1346-8138.12009. [DOI] [PubMed] [Google Scholar]
- 6.Betran A., Rezusta A., Lezcano M.A. First Spanish case of nocardiosis caused by Nocardia takedensis. J Clin Microbiol. 2009;47(6):1918–1919. doi: 10.1128/JCM.00090-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan C.K., Lai C.C., Lin S.H. Clinical and microbiological characteristics of Nocardiosis including those caused by emerging Nocardia species in Taiwan, 1998-2008. Clin Microbiol Infect. 2010;16(7):966–972. doi: 10.1111/j.1469-0691.2009.02950.x. [DOI] [PubMed] [Google Scholar]
- 8.Lerner P.I. Nocardiosis. Clin Infect Dis. 1996;22(6):891–903. doi: 10.1093/clinids/22.6.891. quiz 904-895. [DOI] [PubMed] [Google Scholar]
- 9.Uhde K.B., Pathak S., McCullum I., Jr. Antimicrobial-resistant nocardia isolates, United States, 1995-2004. Clin Infect Dis. 2010;51(12):1445–1448. doi: 10.1086/657399. [DOI] [PubMed] [Google Scholar]