Abstract
Background
Major depressive disorder (MDD, 10% over a person's lifetime) is common and costly to the health system. Unfortunately, many MDD cases are resistant to treatment with antidepressant drugs and require other treatment to reduce or eliminate depression. Electroconvulsive therapy (ECT) has long been used to treat persons with treatment-resistant depression (TRD). Despite its effectiveness, ECT has side effects that make patients intolerant to the treatment, or they refuse to use it. Repetitive transcranial magnetic stimulation (rTMS), which has fewer side effects than ECT and might be an alternative for TRD patients who are ineligible for or unwilling to undergo ECT, has been developed to treat TRD.
Objectives
This analysis evaluates the cost-effectiveness of rTMS for patients with TRD compared with ECT or sham rTMS and estimates the potential budgetary impact of various levels of implementation of rTMS in Ontario.
Review Methods
A cost-utility analysis compared the costs and health outcomes of two treatments for persons with TRD in Ontario: rTMS alone compared with ECT alone and rTMS alone compared with sham rTMS. We calculated the six-month incremental costs and quality-adjusted life-years (QALYs) for these treatments. One-way and probabilistic sensitivity analyses were performed to test the robustness of the model's results.
A 1-year budget impact analysis estimated the costs of providing funding for rTMS. The base-case analysis examined the additional costs for funding six centres, where rTMS infrastructure is in place. Sensitivity and scenario analyses explored the impact of increasing diffusion of rTMS to centres with existing ECT infrastructure.
All analyses were conducted from the Ontario health care payer perspective.
Results
ECT was cost effective compared to rTMS when the willingness to pay is greater than $37,640.66 per QALY. In the base-case analysis, which had a six-month time horizon, the cost and effectiveness for rTMS was $5,272 and 0.31 quality-adjusted life-years (QALYs). The cost and effectiveness for ECT were $5,960 and 0.32 QALYs. This translates in an incremental cost-effectiveness ratio of $37,640.66 per QALY gained for ECT compared to rTMS. When rTMS is compared with sham rTMS, an additional $2,154.33 would be spent to gain 0.02 QALY. This translates to an ICER of $98,242.37 per QALY gained. Probabilistic sensitivity analysis showed that the probability of rTMS being cost-effective compared to sham rTMS was 2% and 45% at the thresholds of $50,000 and $100,000 per QALY gained, respectively.
Conclusions
Repetitive transcranial magnetic stimulation may be cost-effective compared to sham treatment in patients with treatment-resistant depression, depending on the willingness-to-pay threshold.
BACKGROUND
The Programs for Assessment of Technology in Health (PATH) Research Institute was commissioned by Health Quality Ontario to evaluate the cost- effectiveness of repetitive transcranial magnetic stimulation (rTMS) in the treatment of resistant depression. Published economic evaluations are reviewed, and the structure and inputs of the economic model used to estimate cost-effectiveness are summarized. The results of the economic analyses are presented for rTMS alone versus electroconvulsive therapy (ECT) alone and for rTMS alone compared with sham rTMS, and the budget impact of implementing rTMS is estimated.
Health Quality Ontario conducts full evidence-based analyses, including economic analyses, of health technologies being considered for use in Ontario. These analyses are then presented to the Ontario Health Technology Advisory Committee, whose mandate it is to examine proposed health technologies in the context of available evidence and existing clinical practice, and to provide advice and recommendations to Ontario health care practitioners, the broader health care system, and the Ontario Ministry of Health and Long-Term Care.
DISCLAIMER: Health Quality Ontario uses a standardized costing method for its economic analyses. The main cost categories and associated methods of retrieval from the province's perspective are described below.
Hospital costs: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency department visit, and day procedure costs for the designated International Classification of Diseases diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in the estimated costs of the diagnoses and procedures under consideration. Due to difficulties in estimating indirect costs in hospitals associated with a particular diagnosis or procedure, Health Quality Ontario normally defaults to a consideration of direct treatment costs only.
Non-hospital costs: These include physician services costs obtained from the Ontario Schedule of Physician Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible, or from the device manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied (to both costs and effects/quality-adjusted life-years [QALYs]), as recommended by economic guidelines.
Downstream costs: All reported downstream costs are based on assumptions of population trends (i.e., incidence, prevalence, and mortality rates), time horizon, resource utilization, patient compliance, health care patterns, market trends (i.e., rates of intervention uptake or trends in current programs in place in the province), and estimates of funding and prices. These may or may not be realized by the Ontario health care system or individual institutions and are often based on evidence from the medical literature, standard listing references, and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach.
The economic analysis represents an estimate only, based on the assumptions and costing methods explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
NOTE: Numbers may be rounded to the nearest decimal point, as they may be reported from an Excel spreadsheet.
Objective of Analysis
This analysis aimed to evaluate the cost-effectiveness of rTMS for persons with treatment-resistant depression (TRD) compared with ECT or sham rTMS, as well as to estimate the potential budgetary impact of various levels of implementation of rTMS in Ontario from the perspective of the Ontario Ministry of Health and Long-Term Care.
Clinical Need and Target Population
Description of Disease/Condition
Major depressive disorder (MDD) is a common, disabling, and highly prevalent condition across the world and also within Canada.1 The Canadian Network for Mood and Anxiety Treatments (CANMAT) adopts the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV), general definition of a mental disorder as “a clinically significant behavioural or psychological syndrome or pattern that occurs in a patient and that is associated with present distress or disability or with a significantly increased risk of suffering, death, pain, disability, or an important loss of freedom.”1 The essential feature of MDD is the occurrence of one or more major depressive episodes. These episodes are defined as periods lasting at least two weeks characterized either by depressed mood (most of the day, nearly every day) or by markedly diminished interest or pleasure in all, or almost all activities (most of the day, nearly every day). In total, during the same two-week period, there must be at least one symptom of either depressed mood or loss of interest or pleasure and there must be five or more accompanying symptoms, as per the American Psychiatric Association.2
For a diagnosis of MDD, a patient must have experienced a major depressive episode as well as symptoms that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. The episode must not be attributable to the physiologic effects of a substance or to another medical condition.
An important subset of the MDD population is patients with TRD, which is characterized as MDD that persists even after a typically adequate course of antidepressant therapy. The European Union's Committee for Human Proprietary Medicinal Products defines cases as TRD if “consecutive treatment with two products of different pharmacological classes, used for a sufficient length of time at an adequate dose, fail to induce an acceptable effect.”3
Prevalence and Incidence
Worldwide, 1-year prevalence rates for MDD range between 0.64 per 100 to 22.5 per 100 persons.4 Lifetime prevalence values are higher, ranging from 0.88 per 100 to 29.6 per 100 persons.4 Approximately 4.7% of Canadians reported a major depressive episode in the last year, and 11.3% reported an episode in their lifetime. (5) It is more common for female patients to report a major depressive episode. The percentage reporting an episode also decreases with increasing age. The percentage of Ontarians reporting an episode in the last year ranges from 9% in female patients aged 15 to 24 years to 1.4% in male patients aged 65 years and older.5
Given the lack of consensus on the definition of TRD, disagreement on the prevalence is unsurprising. In Canada, one case series observed a prevalence of 21.7% for TRD, defined as failure of at least two antidepressants from different classes, among MDD patients in primary care with no observable differences in prevalence rates between sexes or ethnicities.6 Prevalence of TRD among MDD patients has been estimated to be as high as 30% to 60% in the literature.7,8
Burden of Illness
In an Institute of Clinical Evaluative Sciences (ICES) review of the Ontario burden of mental illness and addictions, MDD had the highest estimated number of health-adjusted life-years (HALYs) lost of all of the mental illnesses and addictions. In Ontario, more than 200,000 HALYs are lost per year.8 This total is more than double the second highest number estimated of HALY lost related to mental health (for bipolar disorder), primarily because of the greater prevalence of MDD. The HALY is a composite measure of lost years from premature mortality and year-equivalent lost from reduced functioning or disability. Almost all of the life-years lost owing to MDD were a result of reduced functioning and disability. Just as more Canadian women report a major depressive episode, Ontario women lost a much greater number of HALYs than men. In comparison with other diseases, such as cancer and infections, MDD still totals the highest number of HALYs lost per year. In 2004, the World Health Organization9 identified unipolar depressive disorders as having the third highest disease burden worldwide, calculated by total disability-adjusted life-years lost. For women aged 15 to 44, MDD was the leading cause of disease burden for high-income as well as low- and middle-income countries. It is projected that by 2030, this condition will cause the greatest disease burden worldwide.
Intervention Under Evaluation
Repetitive Transcranial Magnetic Stimulation
Repetitive transcranial magnetic stimulation involves a noninvasive, superficial magnetic stimulation of the brain. An electromagnetic coil is used to generate a magnetic field (1.5–2.5 T) that is delivered through the skull.10 The magnetic field is believed to affect neuronal function, but the mechanism of effect is still not completely known. Both rTMS and ECT are believed to work similarly by stimulating the prefrontal cortex to rebalance areas in the brain responsible for mood regulation.11
Repetitive transcranial magnetic stimulation is delivered in intervals, or “trains,” that last several seconds, followed by inter-train periods. Several trains can be delivered per session, and usually five sessions are delivered over a week for the acute phase of treatment. Intensity of the stimulus is based on the patient's motor threshold (the minimal intensity of field required to produce muscle twitches), typically using 90% to 120% of this threshold for treatment. The left or right dorsolateral prefrontal cortex is targeted (the left is most common).10
In general, rTMS is a safe and well-tolerated treatment with short-term side effects that include headaches and scalp pain. There is no evidence of cognitive impairment with rTMS.10 To date, there have been no systematic long-term safety evaluations of rTMS, although open-label reports on maintenance rTMS treatment suggest that it is safe in the long term.11,12
Electroconvulsive Therapy
Electroconvulsive therapy involves induction of a seizure by applying an electrical current to the brain. The current typically complies with the following parameters: current (500–800 mA); frequency (20–120 Hz); pulse width (0.25–2 ms) and duration (0.5 ≥ 8 seconds). The minimum charge to induce a seizure is referred to as the seizure threshold. The electrodes can be placed bilaterally (either bitemporal or bifrontal) or unilaterally (typically on the right side of the head).13 The need for rapid onset of antidepressant effects must be weighed against the deleterious effects of more frequent ECT treatment.
When ECT is prescribed as a first-line treatment or in individuals with a history of antidepressant medication trials of inadequate dose or duration, response rates as high as 80% to 90% have been reported.13 While ECT does have a high response rate, it is associated with some adverse events and side effects. Electroconvulsive therapy is a safe procedure with a very low mortality rate (0.2 per 100,000 treatments); however, several short-term side effects have been reported (including nausea, headache, muscle pain, oral lacerations, dental injuries, and persistent myalgia and the fear of having seizure induction).14
Ontario Context
Health Canada approved rTMS for TRD in 2002, but the treatment is currently funded only in Quebec and Saskatchewan.15,16 Private clinics have offered rTMS paid either through private insurance or out of pocket by patients. According to experts, rTMS is available in fewer than 10 centres in Ontario, but only two of these operate at high volumes (more than 200 patients yearly).17
ECONOMIC ANALYSIS
Research Questions
This report addresses the following research questions:
What is the cost-effectiveness of rTMS versus ECT for patients who have TRD and would be eligible for and willing to undergo either treatment?
What is the cost-effectiveness of rTMS versus standard of care (sham rTMS) for patients with TRD who are ineligible for or refuse ECT treatment?
What is the 1-year budgetary impact of implementing rTMS for patients with TRD from the perspective of the Ontario Ministry of Health and Long-term Care?
Economic Literature Review
Objective
This literature review aimed to identify existing economic evaluations that have addressed the cost-effectiveness of implementing rTMS in patients with TRD.
Research Methods
Literature Search Strategy
A literature search of the following databases was performed on November 20, 2014: Ovid MEDLINE, MEDLINE In-Process & Other Non-Indexed Citations, Embase, PsycINFO, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Health Technology Assessment and UK National Health Service (NHS) Economic Evaluation Database. A copy of the search strategy can be found in Appendix 11. The websites of Canadian Health Technology Assessment (HTA) agencies (Canadian Agency for Drugs and Technologies in Health [CADTH], Institute of Health Economics, University of Calgary Institute for Public Health HTA unit, Institut national d'excellence en sante et en services sociaux [INESSS], Centre for Evaluation of Medicines McMaster University, Centre for Health Services and Policy Research University of British Columbia, Institute for Clinical Evaluative Sciences [ICES] Ontario, Technology Assessment Unit at McGill University Health Centre) were further searched for reports relating to rTMS. Reports from Health Quality Ontario were excluded because this report updates a previous report by Health Quality Ontario on this topic.
Titles and abstracts were first assessed by a single reviewer on the basis of the inclusion criteria listed below, followed by full-text screening by two independent reviewers for all potentially relevant articles identified from the first screen. References to relevant publications included after full-text screening were hand-searched for additional citations.
Inclusion Criteria
English-language full-text publications and HTA reports
published between January 1, 2000, and July 2014
full economic evaluations, such as cost-utility analyses, cost-effectiveness analyses, and cost-benefit analyses
economic evaluations reporting incremental cost-effectiveness ratios (ICERs) (e.g., cost per quality-adjusted life-year [QALY], life-years gained, or cost per event avoided)
studies in patients with TRD
studies using rTMS as an intervention
adults (≥ 18 years of age)
Exclusion Criteria
narrative reviews, letters or editorials, abstracts, posters, unpublished studies
studies of pediatric populations
foreign-language publications
Data from relevant articles were extracted using a standardized data abstraction form. This form recorded relevant study information, such as study characteristics (e.g., authors, year of publication); description of setting; type of economic evaluation (e.g., model-based vs. trial-based, methods) and results (Table 1).
Table 1:
Economic Literature Review
| Results | |||||||
|---|---|---|---|---|---|---|---|
| Author Name, Year, Country | Study Design and Perspective | Population/Comparator | Interventions | Health Outcomes | Costs | Cost-Effectiveness | |
| Non-HTA publications | |||||||
| Kozel et al, 2004, United States18 |
|
|
|
|
|
|
|
| Simpson et al, 2009, United States19 |
|
|
|
Not reported |
|
|
|
| HTA reports | |||||||
| University of Calgary, 2014, Canada16 |
|
|
|
|
|
|
|
Abbreviations: CAD, Canadian dollar; ECT, electroconvulsive therapy; HTA, health technology assessment; ICER, incremental cost-effectiveness ratio; MDD, major depressive disorder; NHS, UK National Health Service; QALY, quality-adjusted life-year; RCT, randomized controlled trial; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Results of Economic Literature Review
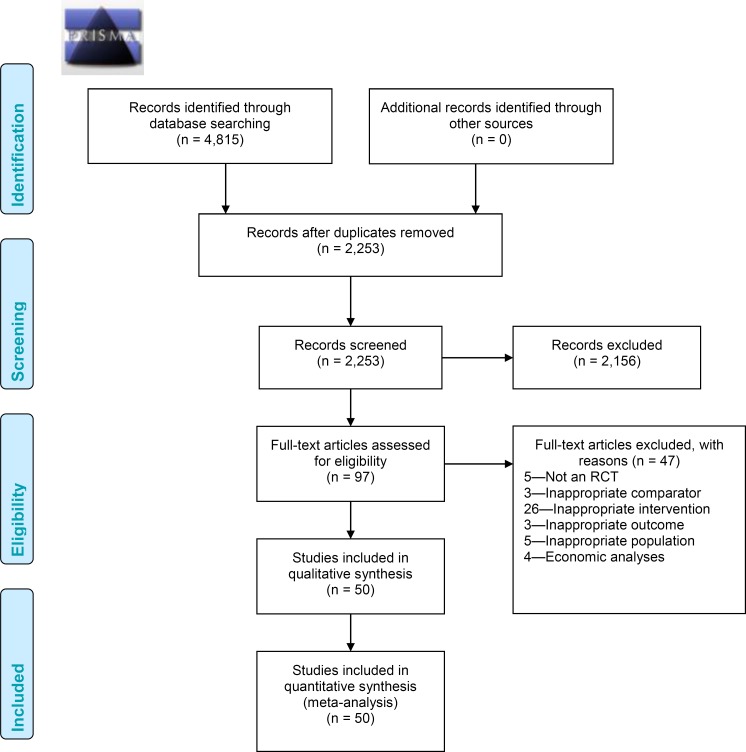
A total of 2,255 citations were identified in the database search, representing 2,253 unique studies once duplicates were removed. After the title and abstract review, 2,152 articles were excluded and the full texts of the remaining 103 articles were reviewed (Figure A1). One study met the inclusion criteria from the database search.18 In addition, two reports were identified from two Canadian agencies performing health technology assessments: Canadian Agency for Drugs and Technologies in Health and University of Calgary Institute for Public Health HTA unit.16,19 A review of the reference lists did not turn up any additional articles. In total, three unique reports were identified that addressed the pre-specified research question.
Studies Related to rTMS From Published Literature
Of the unique economic evaluations published, all were cost-utility analyses. The models lasted approximately 1 year. Two cost-utility analyses were conducted from the US societal perspective, while the final cost-utility analysis was from the Canadian healthcare perspective. Below we briefly summarize each study (Table 1).
A decision tree was constructed by Kozel et al18 comparing the cost utility of three treatment options: rTMS, ECT, or rTMS followed by ECT for nonresponders (rTMS-to-ECT). Both short-term (initial 3 weeks) and long-term (subsequent 49 weeks) regimens were modelled. Clinical outcomes and utility estimates were taken from the published literature, while costs were based on charges at the authors’ medical centre or by estimation. The number of treatments modelled was based on typical practice: rTMS consisted of 15 acute sessions and 49 treatments in the maintenance phase, while ECT consisted of eight acute sessions and 11 treatments in the maintenance phase. Given the US societal perspective, rTMS was found to be less efficacious but also less costly. The ICER of ECT compared with rTMS was $460,031 per QALY gained. However, when comparing ECT to rTMS-to-ECT, rTMS-to-ECT dominated because it offered greater gain in QALY at lower cost.
The last study, by Simpson and colleagues,19 compared rTMS against sham rTMS using decision analysis. Their hybrid model consisted of (a) a decision tree representing the initial acute treatment phase (9 weeks) and (b) a Markov cohort model representing the subsequent follow-up phase (54 weeks). Clinical progression inputs originated from a series of related studies: an initial 6-week multicentre RCT; followed by a 4-week open-label trial for participants who completed the initial study; and, last, a 24-week open-label follow-up study for participants who completed the first open-label study.20–22 Participants in these trials had unipolar, nonpsychotic MDD and were moderately-to-severely resistant to pharmaceutical antidepressants, as measured by the Antidepressant Treatment History Form. Resource use was collected from participants’ self-reports at the beginning of the initial study and at the end of the 24-week open-label follow-up. For the acute phase, rTMS therapy consisted of five sessions per week for four to 6 weeks. The follow-up phase consisted of tapering off rTMS over six sessions during a 33-week period. In both arms of the model, patients who had not responded to therapy by Week 9 of treatment were assumed to have switched to a new aggressive drug regimen consisting of an antidepressant, mood stabilizer, and an atypical antipsychotic. At an estimate of $300 per session, rTMS was associated with an ICER of $34,999 (USD)/QALY compared with sham rTMS. When productivity gains were included, the ICER associated with rTMS dropped to $6,667/QALY.
Reports Related to rTMS from Canadian HTA Agencies
Another HTA report, published in 2014 by the HTA Unit at the University of Calgary, addressed rTMS specifically for treatment-resistant MDD.16 Treatment-resistant MDD was defined (as in our model) as depression that does not subside despite adequate pharmaceutical and behaviour treatment. Included in this report was a decision analysis comparing rTMS against standard therapy or ECT; results of the latter comparison are reported here. The model was constructed with a 3- to 6-week horizon from the perspective of Alberta's publicly funded health care system. Differences between treatment arms were based on the response and remission rates identified from a clinical literature review completed as a part of the HTA. The number of sessions estimated for each treatment strategy was 12 for ECT and 20 for rTMS. When the health states were defined on the basis of response to therapy (i.e., at least 50% reduction in baseline scores), rTMS was the dominant strategy (incremental QALY: 0.02; incremental cost: –$2,373). If remission was the health state definition (i.e., less than 8 on the Ham-D or Montgomery-Asberg Depression Rating Scale), the cost difference between treatment strategies remained at –$2,373 although rTMS was associated with fewer QALYs (incremental QALY: −0.01). The ICER of $328,325/QALY was calculated and indicates that, at a willingness-to-pay threshold less than $328,325/QALY, rTMS would be considered cost-effective; at any higher willingness-to-pay thresholds, ECT would be cost-effective. The model was found to be sensitive to the cost of rTMS. The probabilistic sensitivity analysis demonstrated that, at a willingness-to-pay threshold of $50,000/QALY, the probability at which rTMS is cost-effective was 98.2% or 84.5% when the outcome was defined as response or remission, respectively. In addition to the economic model, this report included interviews focusing on the societal issues (burden of illness, patterns of care, and capacity) and patient experience with this technology; a systematic review and meta-analysis on its efficacy and safety; and a literature review on the economic evidence.16
Appraisal
The three unique economic evaluations were generally well conducted with an explicit research question and a clearly defined model perspective. In most of these analyses, however, it was unclear how TRD was defined.16,18,19 Study interventions and comparators were clearly identified. Between studies, however, the treatment protocol for both rTMS and ECT differed considerably in terms of the number of sessions. The models generally captured a range of costs including facility, labour, and equipment cost.
Each economic evaluation was assessed. For the first study by Kozel et al,18 given the few RCTs with sufficient follow-up, both the efficacy and cost parameters were built on several simplifying assumptions and the use of observational trials. Uncertainty was explored only by one-way sensitivity analyses that, in this case, selected parameters considered critical or that had fewer data supporting the conclusion.
The third study, by Simpson et al,19 lasted longest and used the most complex model to capture both the acute initiation stage and the chronic maintenance stage. The clinical efficacy parameters were informed from a multicentre RCT and its extension phases, with the efficacy parameters extrapolated beyond the trial duration (although this was not clearly described). Despite mention of a sensitivity analysis being conducted, only the methods and findings of the two scenario analyses were described: changing model perspective to incorporate indirect costs and varying the costs of a suicide attempt.
The last and most recent economic evaluation by the University of Calgary looked only at the acute cost-effectiveness between rTMS and ECT.16 The model was simple in considering only response and remission, although it is uncertain that these outcomes can be adequately captured in a model that lasted only 4 to 6 weeks. The data informing the clinical parameters were pooled from a systematic review. This study had the most extensive sensitivity analysis; scenario analysis incorporated a broader societal perspective, one-way sensitivity analysis on relative risk efficacy parameters, threshold analysis to identify the cost at which rTMS is no longer cost-effective, and probabilistic analysis on a limited set of parameters. Further, the way results were presented raises the question of whether the analysis was performed correctly. Given the model is limited to a maximum of 6 weeks, the maximum QALY in this model should be 0.11 QALYs (as maximum QALY in a year is 1.0). Yet the QALY within each treatment arm far exceeds this maximum (0.59 for rTMS and 0.57 for ECT).
Despite the fact that depression is a chronic condition, the longest time horizon in these models was 63 weeks19 (minimum time horizon: 6 weeks16). Yet none of the studies reported a discount factor. Only two analyses considered the acute and maintenance stages of therapy, as the remaining analyses were limited to comparing acute initiation. Given differences are known to exist in resource consumption between these two stages, existing evaluations might not have adequately captured the full costs and consequences associated with long-term rTMS and the impact of relapse.
Summary
Current studies have conflicting conclusions on the potential economic value of rTMS. This conflict could be caused by consideration of different comparators, health outcomes, settings/perspectives, and treatment regimens. As such, it was deemed important to conduct an economic evaluation specifically in the context of the province of Ontario.
Primary Economic Evaluation
Research Methods
Type of Analysis
A cost-utility analysis was conducted from the Ontario health care payer's perspective (Ministry of Health and Long-Term Care), estimating the 6-month costs and health outcomes (i.e., QALYs) for rTMS and its relevant comparator in two populations:
patients who are eligible for and willing to be given either treatment
patients who refuse ECT because of intolerance or medical reasons
The primary outcomes were QALYs, cost (in 2014 Canadian dollars), and the incremental cost-effectiveness ratio (ICER).
Intervention Evaluated
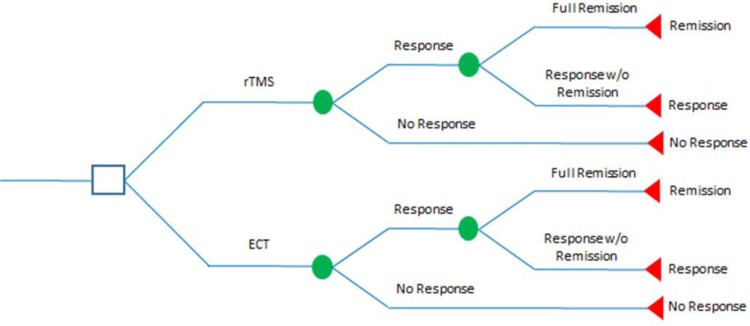
For TRD patients who are eligible for (and willing to use) either treatment, a decision-analysis model was developed to compare costs and health outcomes using rTMS alone versus ECT alone (Figure 1).
Figure 1: Decision Analysis Comparing rTMS to ECT in Treating Treatment-Resistant Depression.
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; w/o, without.
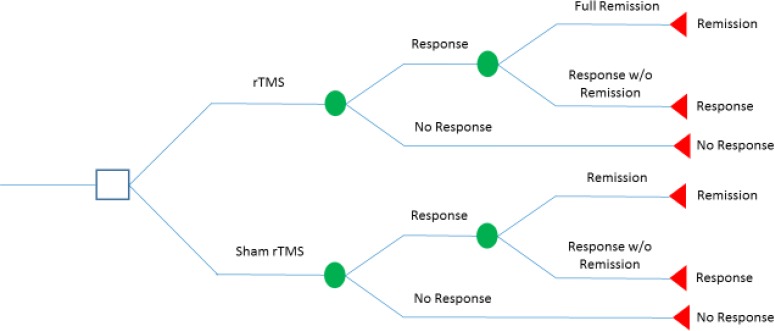
For TRD patients who are unsuitable for ECT treatment for either medical reasons or intolerance, a decision-analysis model was developed to compare costs and health outcomes using rTMS versus sham rTMS (Figure 2).
Figure 2: Decision Analysis Comparing rTMS to Sham rTMS in Treating Treatment-Resistant Depression.
Abbreviations: rTMS, repetitive transcranial magnetic stimulation; w/o, without.
Perspective
The analysis was conducted from the perspective of the Ontario Ministry of Health and Long-Term Care.
Discounting and Time Horizon
The time horizon of the model was 6 months after the initiation of rTMS therapy. Because the time horizon for this analysis is less than 1 year, neither costs nor QALYs were discounted.
Target Population
The hypothetical population of this study was patients diagnosed with MDD who have failed to benefit from two or more antidepressive treatments and whose disease is thus considered treatment resistant. For the economic evaluation, the hypothetical population was considered to be (a) eligible for both treatment options (rTMS and ECT) and none refuse either treatment or (b) refuse ECT treatment for medical reasons or intolerance.
Variability and Uncertainty
Variability and uncertainty were assessed using deterministic and probabilistic sensitivity analyses. One-way sensitivity analyses were conducted by varying each model's parameter separately over plausible ranges. The results were presented in tornado diagrams.
Given the level of uncertainty in the input parameters of the model, probabilistic sensitivity analyses were conducted using assigned distributions on point estimates where possible. With random sampling from each distribution, the models were run repeatedly to provide a range of possible costs and outcomes. The results would inform the probability of cost-effectiveness through a range of willingness-to-pay values and would be presented in the cost-effectiveness acceptability curve.
Generalizability
The generalizability of this study is limited to patients who are diagnosed with MDD and experience TRD.
Model Structure
We developed two decision-analysis models, comparing rTMS alone to ECT alone (Model 1) and rTMS alone to sham rTMS (Model 2). These models incorporate the probability of response, remission, and no response of various treatments. In general, all models followed a hypothetical Ontario cohort of TRD patients over 6 months. These models are composed of two phases: acute and maintenance. Symptoms were expected to be resolved during the acute phase; complete resolution of the index episode or developing a new episode were expected to occur during the maintenance phase. Figures 5 and 6 present the model structures of various treatments for TRD patients. Response rates (> 50% reduction) and remission rates for rTMS, ECT, and sham rTMS were taken from the systematic review and meta-analyses of the clinical evidence review (Appendix 4).
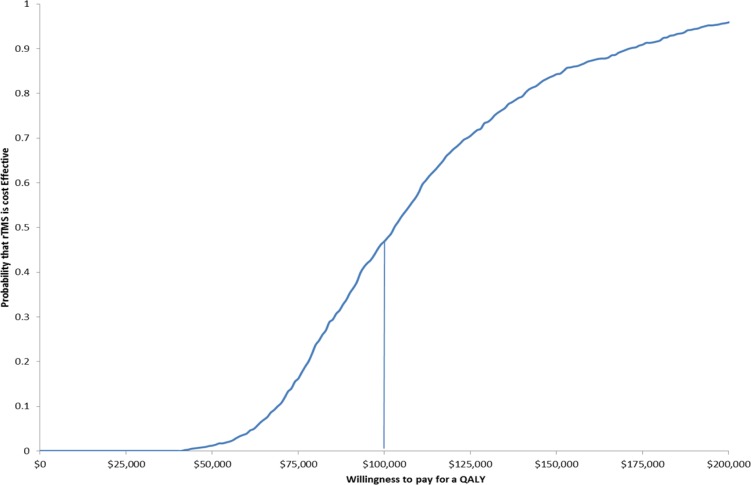
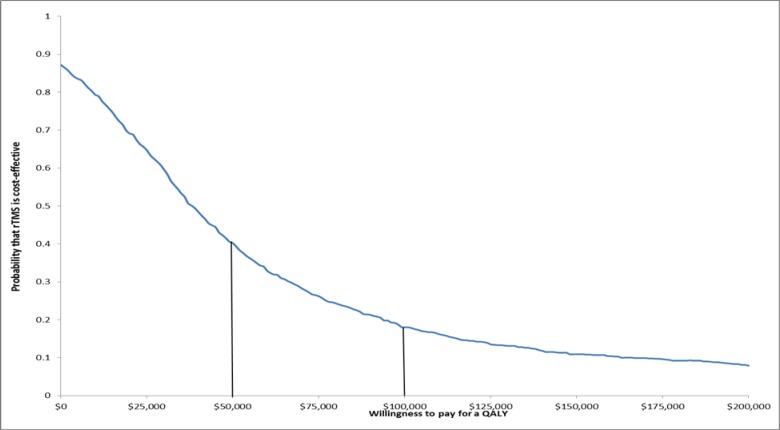
Figure 5: Cost-Effectiveness Acceptability Curve as a Function of Willingness to Pay Comparing rTMS to Sham rTMS.
Abbreviations: QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
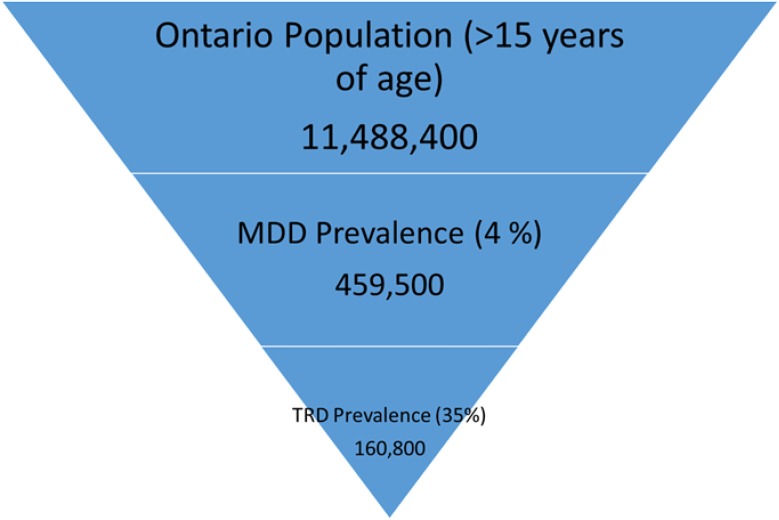
Figure 6: Estimated Treatment-Resistant Depression Population of Ontario in 2014.
Abbreviations; MDD, major depressive disorder; TRD, treatment-resistant depression.
We assumed that response rates were the same for both the acute and maintenance phases. At the acute phase, a TRD patient initially receiving one of the treatments (rTMS, ECT, or sham rTMS) either responded or did not respond to the assigned therapy. Upon achieving either response or no response to the treatment at the acute phase, TRD patients entered the maintenance phase. During the maintenance phase, patients who responded to therapy would subsequently either achieve full remission or continue to respond without full remission. Those who achieved full remission remained in remission and those who responded to the treatment without remission continued to respond until the end of the cycle. Those who did not respond at the acute phase would subsequently switch to pharmacotherapy during the maintenance phase.
Key assumptions in the base-case model were (a) TRD patients who did not respond to an initial treatment would continue with sham rTMS for the rest of the cycle and (b) patients who achieved remission or continued to respond after the initial treatment would remain in remission or as responders to the treatment for the rest of the treatment cycle.
Input Parameters
Effectiveness Measures
Primary outcome measures captured in the models are remission and response rates. The definitions are described below and the values are taken from a systematic review conducted internally by PATH (Appendix 4). We decided not to use the reported values of response and remission rates from the clinical report conducted by Health Quality Ontario because the clinical values in the meta-analysis would come from studies that might report remission and response rates separately. To ensure consistency and for the purpose of modelling, we aimed to look at studies that reported both remission and response rates. We also ensured that studies used were also included in the report by Health Quality Ontario. Details of the systematic review and meta-analyses are reported in Appendix 4. We converted the remission and response rates into probability using the formulae reported by Briggs et al.23
Remission
The primary outcome measure was the end-of-treatment score on the 17-item Hamilton Rating Scale for Depression (Ham-D)24 and the rate of remission; remission was defined as a Ham-D score of 8 or less.
Response
Response was defined as a 50% reduction in the baseline Ham-D score at the end of treatment.24
Quality of Life
Various utility weights were applied to patients according to their response status (Table 2). A utility weight of 0.58 was applied to patients who had no response to treatment. A utility weight of 0.72 was applied to patients who had a partial response to treatment, while a utility weight of 0.85 was applied to patients who had full remission after treatment. These utility weights were derived from Sapin et al.25
Table 2:
Model Parameters for Base-Case Analysis
| Variables | Base Case | Range | Comments |
|---|---|---|---|
| Model 1: rTMS vs. ECT | |||
| rTMS | |||
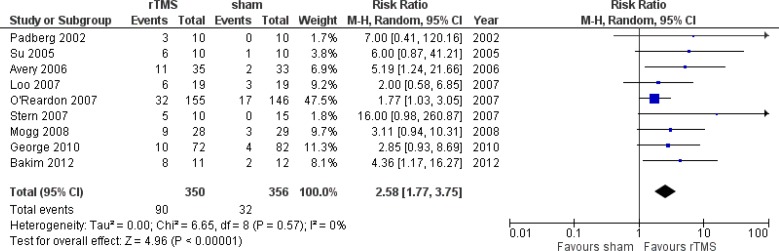
| Probability of any response | 0.418 | 0.296–0.552 | Meta-analysisa |
| Probability of remission given any response | 0.481 | 0.395–0.586 | Meta-analysisa |
| ECT | |||
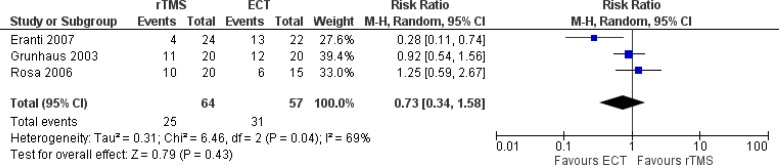
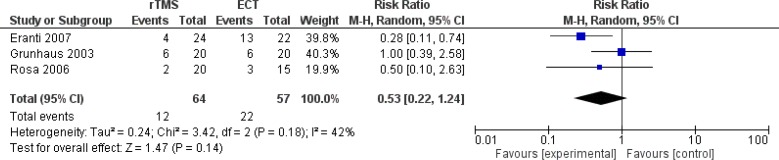
| Probability of any response | 0.544 | 0.413–0.670 | Meta-analysisa |
| Probability of remission given any response | 0.731 | 0.660–0.804 | Meta-analysisa |
| Model 2: rTMS vs. pharmacotherapy | |||
| rTMS | |||
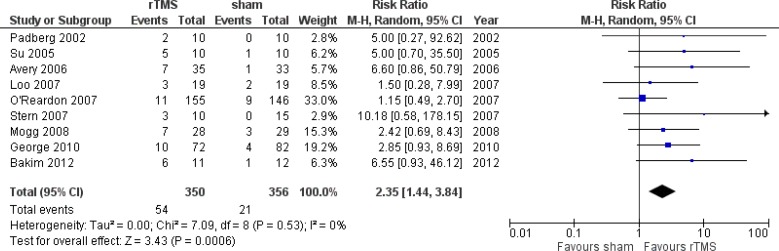
| Probability of any response | 0.331 | 0.229–0.452 | Meta-analysisa |
| Probability of remission given any response | 0.665 | 0.585–0.754 | Meta-analysisa |
| Pharmacotherapy | |||
| Probability of any response | 0.103 | 0.074–0.142 | Meta-analysisa |
| Probability of remission given any response | 0.650 | 0.629–0.714 | Meta-analysisa |
| Quality of life | |||
| Depression | 0.33 | 0.297–0.363 | Sapin, 200425 |
| Nonresponder | 0.58 | 0.517–0.643 | Sapin, 200425 |
| Responder without remission | 0.72 | 0.675–0.765 | Sapin, 200425 |
| Responder with remission | 0.85 | 0.821–0.879 | Sapin, 200425 |
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation.
Meta-analysis includes subgroup of studies completed in our report.
Resources and Costs
The costs for the acute treatment phase and maintenance treatment phase for both rTMS and ECT are presented in Table 3. (Appendix 3 provides a detailed breakdown of the unit costs). The number of sessions for each treatment phase for rTMS and ECT were determined through consultation with physicians and represent a basis for a standardized approach to be adopted with further diffusion of rTMS. The acute phase for rTMS consists of five weekly treatments for 4 weeks, followed by a maintenance phase that involves tapering to once a month; two sessions per week for 6 weeks followed by one session per week for 6 weeks, then down to one treatment per month. It is assumed that there are 20 rTMS sessions in total during the acute phase and 22 rTMS sessions during the maintenance phase. The acute phase for ECT consists of three weekly treatments for 4 weeks, followed by a maintenance phase that involves tapering to once a month; one treatment per week for 4 weeks followed by one session every 2 weeks for 8 weeks, then down to one treatment per month. It is assumed that there are 12 ECT sessions in total during the acute phase and six sessions during the maintenance phase.
Table 3:
Average Cost of Treatment Phases for rTMS and ECT
| Treatment Phase | rTMS, $ | ECT, $ |
|---|---|---|
| Acute phase | 2,190.40 | 3,325.32 |
| Maintenance phase | 2,409.44 | 1,662.66 |
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation.
Another important consideration regarding costs is the additional health care resource use avoided through treatment by rTMS or ECT. Additional physician visits, hospital admissions, and depression drug costs were estimated on the basis of literature and expert consultation (Table 4). An average number of physician visits was calculated from a Canadian study by Burdett et al26 for both rTMS and ECT (10.9 for treatment responsive and 17 for treatment unresponsive). Each visit was accorded a cost of $80.30 on the basis of expert consultation and the assumption that visits would be mental health–related (Ontario Schedule of Benefits [OSB] fee codes K197 for outpatient psychotherapy and K198 for outpatient psychiatric care). Hospital admission costs were also adopted from Burdett et al26 and inflated to 2014 Canadian dollars according to the health care portion of the Consumer Price Index. Finally, antidepressant costs were based upon assumptions and expert consultation. A drug-responsive patient was expected to incur costs for two prescriptions at an average price of $0.35 daily (average price of all antidepressant drugs listed on the Ontario Drug Benefit Formulary), while treatment of unresponsive patients would incur costs for three prescriptions.27 Further, an estimated 30% of patients would not have drug costs covered through private insurance plans and thus could be dependent upon the Ministry.
Table 4:
Annual Background Costs
| Resource Item | Cost, $ | Comments |
|---|---|---|
| Treatment-resistant (nonresponders) | ||
| Physician visits | 1,328.97 | Number of physician visits taken from Burdett et al26 multiplied by $80.30, which is OSB for psychiatric visits (expert consultation) |
| Hospital admission | 4,768.28 | 2004 costs from Burdett et al26 inflated to 2014 CAD |
| Antidepressant therapy | 114.98 | Assumes three drugs at $0.35 a day each, with 30% of patients covered by provincial drug plan (expert consultation) |
| Annual total | 6,212.23 | |
| Treatment-resistant (responders) | ||
| Physician visits | 875.27 | Number of physician visits taken from Burdett et al26 multiplied by $80.30, which is OSB for psychiatric visits (expert consultation) |
| Hospital admission | 2,134.61 | 2004 costs from Burdett et al26 inflated to 2014 CAD |
| Antidepressant therapy | 76.65 | Assumes three drugs at $0.35 a day each, with 30% of patients covered by provincial drug plan (expert consultation) |
| Annual total | 3,086.53 | |
Abbreviations: CAD, Canadian dollars; OSB, Ontario Schedule of Benefits.
Sensitivity Analyses
To explore the uncertainty surrounding the base-case analysis, several one-way sensitivity analyses were conducted to determine the effect of changing single-model inputs on the overall outcome. A probabilistic sensitivity analysis was also conducted to investigate the uncertainty of the point estimate of model inputs.
For the probabilistic sensitivity analysis, the uncertainty of model inputs reported through the confidence interval or standard deviation of the mean result were incorporated into the model. From this information, a distribution can be constructed for each of the inputs, with uncertainty allowing for repeated runs of the model. Given the wide level of uncertainty, 10,000 simulations of the model were conducted. Distributions used for the model inputs are presented in Table 5.
Table 5:
Variables Modified for Probabilistic Sensitivity Analyses
| Model Input | Base Case | Distribution |
|---|---|---|
| Model 1: rTMS vs. ECT | ||
| rTMS | ||
| Probability of any response | 0.418 | Beta (α = 23.65; β = 32.93) |
| Probability of remission given any response | 0.481 | Beta (α = 49.53; β = 53.44) |
| ECT | ||
| Probability of any response | 0.544 | Beta (α = 31.40; β = 26.32) |
| Probability of remission given any response | 0.731 | Beta (α = 104.27; β = 38.37) |
| Model 2: rTMS vs. Pharmacotherapy | ||
| rTMS | ||
| Probability of any response | 0.331 | Beta (α = 22.23; β = 44.93) |
| Probability of remission given any response | 0.665 | Beta (α = 79.46; β = 40.03) |
| Pharmacotherapy | ||
| Probability of any response | 0.103 | Beta (α = 29.27; β = 254.89) |
| Probability of remission given any response | 0.650 | Beta (α = 304.88; β = 164.16) |
| Quality of life | ||
| Depression | 0.33 | Beta (α = 263.50; β = 534.99) |
| Nonresponder | 0.58 | Beta (α = 135.18; β = 97.89) |
| Responder without remission | 0.72 | Beta (α = 272.65; β = 106.03) |
| Responder with remission | 0.85 | Beta (α = 482.24; β = 85.10) |
| Treatment cost ($) | ||
| Cost for responders to any therapy | 1,543 | Gamma (α = 25.01; λ = 0.02) |
| Cost for nonresponders to any therapy | 3,115 | Gamma (α = 24.99; λ = 0.01) |
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation.
Primary Results of Economic Evaluation
Base-Case Results
Table 6 presents projected health outcomes, costs, and incremental cost-effectiveness ratios for two scenarios: rTMS alone versus ECT alone and rTMS versus sham rTMS.
Table 6:
Base-Case Results for rTMS Therapy Compared With ECT or Sham rTMS for TRD Patients
| Treatment Options | Cost | QALYs | ICER ($/QALY) |
|---|---|---|---|
| rTMS | 5,272.27 | 0.31 | |
| ECT | 5,960.21 | 0.32 | |
| Incremental | (687.94) | (0.01) | 37,640.66 |
| rTMS | 5,132.44 | 0.30 | |
| Pharmacotherapy | 2,978.12 | 0.28 | |
| Incremental | 2,154.33 | 0.02 | 98,242.37 |
Abbreviations: ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Comparing ECT to rTMS for TRD would result in an increase of $687.94 for a gain of 0.01 QALY. Assessing the cost of this potential gain, the ICER for using ECT compared with rTMS was calculated to be $37,640.66 per QALY gained. Therefore, rTMS would be considered cost-effective only if the willingness-to-pay is less than $37,640.66 per QALY. Otherwise ECT would be considered cost-effective. When rTMS is compared with sham rTMS, an additional $2,154.33 would be spent to gain 0.02 QALY. This translates to an ICER of $98,242.37 per QALY gained. If the willingness to pay for a QALY is greater than $98,242.37, rTMS would be cost-effective. The ICER for rTMS versus sham rTMS revealed that rTMS would be cost-effective for treating TRD patients who either refuse or do not tolerate ECT if the willingness to pay is greater than $98,242.37.
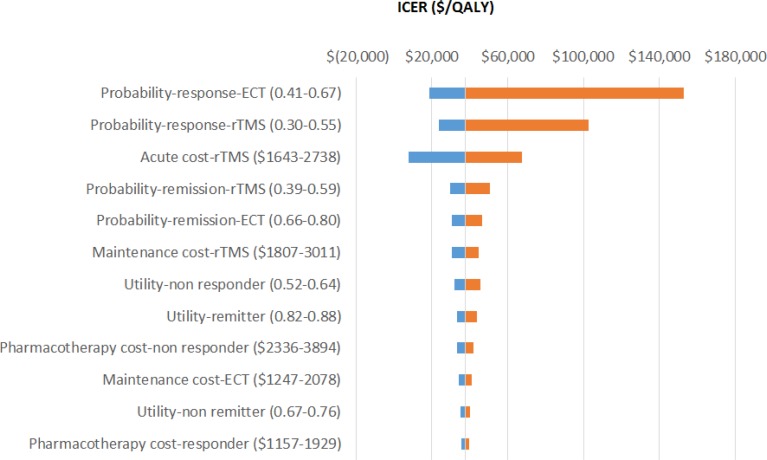
One-Way Sensitivity Analysis
The results for the one-way sensitivity analyses are presented in Table 7 and Figure 3, in which rTMS is compared with ECT. The sensitivity analyses revealed that these results are quite robust across a range of parameters for all comparisons. The results were most sensitive to the rate of ECT, the rate of rTMS, and the cost of rTMS.
Table 7:
Difference in Incremental Cost-Effectiveness Ratios as a Result of Changes to Model Parameters
| Variable | Base Case | Modified Value | Modified ICER |
|---|---|---|---|
| Probability of response to ECT | 0.544 | 0.431–0.670 | 18,738–153,053 |
| Probability of response to rTMS | 0.418 | 0.296–0.552 | 23,638–102,817 |
| Utility-remitter | 0.850 | 0.821–0.879 | 33,138–43,592 |
| Cost of acute phase for rTMS | 2,190.40 | 1,643–2,738 | 7,6738–67,591 |
| Cost of maintenance phase for rTMS | 2,409.44 | 1,807–3,012 | 30,438–44,791 |
| Cost of pharmacotherapy for responder | 1,543.27 | 1,157–1,929 | 35,338–39,891 |
| Cost of pharmacotherapy for nonresponder | 3,115.11 | 2,336–3,893 | 33,138–42,184 |
| Cost of maintenance phase for ECT | 1,662.66 | 1,246–2,078 | 34,338–40,965 |
| Probability of remission for rTMS | 0.481 | 0.395–0.586 | 29,638–50,444 |
| Probability of remission for ECT | 0.731 | 0.660–0.804 | 30,338–46,566 |
| Utility-nonresponder | 0.580 | 0.517–0.643 | 32,038–45,624 |
| Utility-responder but not remit | 0.720 | 0.675–0.765 | 35,338–40,273 |
Abbreviations: ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; rTMS, repetitive transcranial magnetic stimulation.
Figure 3: One-Way Sensitivity Analyses for Comparison of rTMS With ECT for TRD Nonresponders.
Abbreviations: ECT, electroconvulsive therapy; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Probabilistic Sensitivity Analysis
A total of 10,000 simulations of the decision-analysis model comparing rTMS to ECT and rTMS to sham rTMS were run with random draws of all model parameters within the assigned distributions (Table 5). A range of ICERs result, presented in cost-effectiveness acceptability curves (Figures 4 and 5). Given a willingness to pay of $50,000 per QALY, there is a 45% chance that rTMS would be cost-effective (Figure 4). Given a willingness to pay of $100,000 per QALY, there is a 20% chance that rTMS would be cost-effective. If willingness to pay is $50,000 per QALY, there is only 2% chance that rTMS would be more cost-effective than sham rTMS (Figure 1). However, if the willingness to pay increases to $100,000 per QALY, the probability that rTMS would become more cost-effective than sham rTMS rises to 45%.
Figure 4: Cost-Effectiveness Acceptability Curve as a Function of Willingness to Pay Comparing rTMS to ECT.
Abbreviations: ECT, electroconvulsive therapy; QALY, quality-adjusted life-year; rTMS, repetitive transcranial magnetic stimulation.
Budget-Impact Analysis
A budget-impact analysis was conducted from the perspective of the Ontario Ministry of Health and Long-Term Care to estimate the 1-year cost burden for implementing rTMS to treat TRD patients who would not otherwise have received ECT. All costs are reported in 2014 Canadian dollars. Costs include procedure costs only and do not take into account potential downstream costs or savings.
Research Methods
Target Population
To estimate the budgetary impact of funding the rTMS procedure, we first estimated the number of people in Ontario who would be eligible for the procedure given the prevalence of TRD, and then subtracted the number of patients currently receiving ECT (assuming these patients would still receive ECT because of the severity of their disease). We assumed that all TRD patients who did not receive ECT would be eligible and willing to undergo rTMS because it could be used as an alternative for less severe patients or as a method to avoid ECT in TRD patients who have previously refused (or would refuse) or have not been offered ECT.
rTMS-Eligible Patients
To estimate the number of patients eligible for rTMS in 2014, we determined the number of Ontarians who have TRD and are not treated with ECT. We know that 4% of the Ontario population over 15 years of age (11,488,400)5 could be classified as having MDD.28 Consequently an estimated 459,500 Ontarians older than the age of 15 years are believed to have MDD. The prevalence of TRD among those who suffer from MDD varies from approximately 30% to 60%. The lower bound (Figure 6) gives 160,800 TRD patients in Ontario.
Base-Case Analysis
Annual use data for ECT among TRD patients in Ontario aged 18 years or older was collected from the IntelliHealth database. Given the vast number of TRD patients in Ontario who do not receive ECT or other treatments to stabilize their depression, the potential demand exceeds what can be provided by the existing rTMS infrastructure. Thus, a base case was chosen to reflect current mental health facilities and professionals and technicians capable of delivering rTMS. Sensitivity analyses addressed the budgetary impact of expanding rTMS availability to the existing ECT infrastructure and of granting access to rTMS equivalent to current access to ECT in local health integration networks (LHINs). Analyses considered the lowest, median, and highest number of machines per capita, as well as maximal expansion under the current infrastructure.
The additional cost of implementing rTMS in the base case (six centres with rTMS currently available: Ottawa, Kingston, Sudbury, Hamilton, Centre for Addiction and Mental Health [five treatment suites], and the University Health Network [three treatment suites], representing a total of 12 treatment suites that are funded and operate at capacity) assuming 60 sessions per week, operating 48 weeks per year (1,500 annual treatments per suite) was estimated. Total annual costs for this scenario were then calculated, representing the budgetary impact; it represents an incremental cost that does not replace ECT. Neither was a reduction in health care resource use taken into account.
Scenario Analysis
Various scenarios based on current access to ECT throughout the LHINs and through expert consultation were developed (described in the Resource Utilization section). In total, we estimated the expected budgetary impact of implementing rTMS for four scenarios:
Funding rTMS across all LHINs, matching the lowest per-capita access to ECT
Funding rTMS across all LHINs, matching the median per-capita access to ECT
Funding rTMS across all LHINs, matching the highest per capita access to ECT
Funding rTMS to provide three treatment suites at each ECT centre across the province (expert recommendation)
Providing access to rTMS for all TRD patients is not feasible; thus several likely scenarios developed around the current ECT infrastructure in the province.
Current access to ECT by LHINs was determined through mapping the centres reported in Delva et al29 and by estimating per-capita access according to 2014 census data used to estimate the prevalence of TRD in Ontario, above.30 According to this study, 7.4% of Ontarians live farther than a 5-hour drive from an ECT centre; 86.6% of Ontarians live within a 1-hour drive. These estimates were used to create benchmarks for expanded rTMS access, including lowest, highest, and median access. The number of additional rTMS machines and sites was then estimated per LHIN, thus providing the same geographic access as ECT.29
Table 8 shows the population, current number of rTMS and ECT machines, and calculated per-capita access to both types of machines based on current population estimates for each LHIN. The number of ECT sites was taken from Burdett et al, with the assumption that there was one ECT machine per centre. This was chosen as a conservative estimate, because only approximately 1,600 patients are treated with ECT per year, indicating substantial barriers (likely other than geographic). These numbers are meant to be illustrative and may not be perfectly accurate.
Table 8:
Current Per-Capita Access to rTMS and ECT Across Local Health Integration Networks in Ontario
| No. of Machines | Per-Capita ECT Access for Patients | ||||
|---|---|---|---|---|---|
| Local Health Integration Network | Population | rTMS | ECT | rTMS | ECT |
| 1: Erie St Clair | 640,000 | 0 | 2 | – | 5,376 |
| 2: South West | 962,500 | 0 | 4 | – | 4,043 |
| 3: Waterloo Wellington | 775,000 | 0 | 2 | – | 6,510 |
| 4: Hamilton Niagara Haldimand Brant | 1,420,000 | 1 | 3 | 23,856 | 7,952 |
| 5: Central West | 850,000 | 0 | 1 | – | 14,280 |
| 6: Mississauga Halton | 1,200,000 | 0 | 3 | – | 6,720 |
| 7: Toronto Central | 1,200,000 | 8 | 9 | 2,520 | 2,240 |
| 8: Central | 1,800,000 | 0 | 4 | – | 7,560 |
| 9: Central East | 1,572,453 | 0 | 4 | – | 6,604 |
| 10: South East | 500,000 | 1 | 3 | 8,400 | 2,800 |
| 11: Champlain | 1,200,000 | 1 | 6 | 20,160 | 3,360 |
| 12: North Simcoe Muskoka | 461,700 | 0 | 3 | – | 2,586 |
| 13: North East | 565,000 | 1 | 4 | 9,492 | 2,373 |
| 14: North West | 231,120 | 0 | 2 | – | 1,941 |
| Total Ontario Population | 13,377,773 | 12 | 50 | ||
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive magnetic stimulation.
Based on the per-capita access to ECT, the four scenarios listed above were explored and the additional number of rTMS machines needed to match various benchmarks of ECT access were calculated. In more detail, the four scenarios explored were as follows:
Access to rTMS matches the lowest per-capita access to ECT (i.e., highest number of TRD patients per machine, which occurs in the Central West LHIN at 14,280).
Access to rTMS matches the median per-capita access to ECT (i.e., the average of the Erie St Clair LHIN and the South West LHIN at 4,709 TRD patients per machine).
Access to rTMS matches the highest per-capita access to ECT (i.e., the lowest number of TRD patients per machine, which occurs in the North West LHIN at 1,941 TRD patients per machine).
In a scenario of extreme diffusion suggested through expert consultation, rTMS was assumed to be available at all centres that have ECT facilities, with three treatment suites per site to provide the highest possible access given our existing infrastructure.
Table 9 shows the required number of rTMS suites and the resultant per-capita access to rTMS for each LHIN for the four scenarios. The number of rTMS suites required for the four scenarios are 27 for the current lowest ECT access, 59 for median ECT access, 122 for highest ECT access, and 150 for three treatment suites at each existing ECT centre.
Table 9:
rTMS Machines Needed Across Local Health Integration Networks to Match Per-Capita Access to ECT
| Lowest Per-Capita Access (∼15,000 Machines Per TRD Patient) | Median Per-Capita Access (∼4,700 Machines Per TRD Patient) | Highest Per-Capita Access (∼1,900 Machines Per TRD Patient) | 3 rTMS Treatment Suites Per ECT Centre | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Local Health Integration Network | Population | # rTMS Machi nes | Per-Capita rTMS Access | # rTMS Machines | Per-Capita rTMS Access | # rTMS Machines | Per-Capita rTMS Access | # rTMS Machines | Per-Capita rTMS Access |
| 1 | 640,000 | 1 | 10,752 | 3 | 3,584 | 6 | 1,792 | 6 | 1,792 |
| 2 | 962,500 | 2 | 8,085 | 4 | 4,043 | 9 | 1,797 | 12 | 1,348 |
| 3 | 775,000 | 1 | 13,020 | 3 | 4,340 | 7 | 1,860 | 6 | 2,170 |
| 4 | 1,420,000 | 2 | 11,928 | 6 | 3,976 | 13 | 1,835 | 9 | 2,651 |
| 5 | 850,000 | 1 | 14,280 | 4 | 3,570 | 8 | 1,785 | 3 | 4,760 |
| 6 | 1,200,000 | 2 | 10,080 | 5 | 4,032 | 11 | 1,833 | 9 | 2,240 |
| 7 | 1,200,000 | 8 | 2,520 | 8 | 2,520 | 11 | 1,833 | 27 | 747 |
| 8 | 1,800,000 | 2 | 15,120 | 7 | 4,320 | 16 | 1,890 | 12 | 2,520 |
| 9 | 1,572,453 | 2 | 13,209 | 6 | 4,403 | 14 | 1,887 | 12 | 2,201 |
| 10 | 500,000 | 1 | 8,400 | 2 | 4,200 | 5 | 1,680 | 9 | 933 |
| 11 | 1,200,000 | 2 | 10,080 | 5 | 4,032 | 11 | 1,833 | 18 | 1,120 |
| 12 | 461,700 | 1 | 7,757 | 2 | 3,878 | 4 | 1,939 | 9 | 862 |
| 13 | 565,000 | 1 | 9,492 | 3 | 3,164 | 5 | 1,898 | 12 | 791 |
| 14 | 231,120 | 1 | 3,883 | 1 | 3,883 | 2 | 1,941 | 6 | 647 |
| Total | 27 | 59 | 122 | 150 | |||||
Abbreviations ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation; TRD, treatment-resistant depression.
Draft—do not cite. Report is a work in progress and could change following public consultation
Resource Utilization
Because TRD is common in Ontario (approximately 160,800 persons affected), it would be difficult to treat all patients with rTMS. After patients have failed to respond to several lines of pharmacotherapy, ECT would be considered. However, ECT is used for only a few TRD patients, approximately 1,600 patients annually.17
Unit Costs
Table 10 presents the estimated session costs for both rTMS and ECT, including nurse/technician time, anesthesiologist fee for ECT, the fee for a psychiatric expert, and a related cost for the machine's purchase, maintenance, and disposables. All fees were confirmed through expert consultation and represent current practice for ECT and generally accepted protocols for rTMS. To further place the cost estimate of rTMS in context, the physician fee in Quebec is $175 (with a $350 fee that includes motor threshold testing at the first session).31 Further, the cost in private clinics across Canada is approximately $250, with US prices ranging from $300 to $400.17
Table 10:
Unit Costs for rTMS and ECT
| Resource Item | Cost, $ | Data Sources and Comments |
|---|---|---|
| ECT | ||
| Nurse/technician time | 41.70 | Based on hourly rate of $41.70 for nurse in Ontario32 |
| Anesthesiologist | 105.07 | Assumes 6 basic units, 1 time unit |
| Psychiatric expert | 85.92 | Based on weighted average of OHIP fee codes (54% outpatient/46% inpatient from IntelliHealth data): OSB G479 $92.60 for outpatient, OSB G478 $80.30 for inpatient |
| Cost of machine | 40.00 | Assumes $70,000 purchase, average 500 sessions yearly, amortized over 10 y, $13,000 in disposable airway tools16 |
| Per-session total | 276.82 | |
| rTMS | ||
| Nurse/technician time | 20.85 | Half hour of nurse/technician time |
| Psychiatric expert | 85.92 | Assumption of OHIP fee code for procedure equivalent to that of ECT |
| Cost of machine | 2.75 | Assumes Magstim machine ($80,000 cost, $5,000 import fee) divided by average 2,880 session yearly, amortized over 10 y16 |
| Per-session total | 109.52 | |
Abbreviations; ECT, electroconvulsive therapy; OHIP, Ontario Health Insurance Plan; OSB, Ontario Schedule of Benefits; rTMS, repetitive transcranial magnetic stimulation.
Sensitivity Analysis
Uncertainty in input parameter values because of model assumptions and reliance on expert opinion was addressed through several one-way sensitivity analyses over reasonably expected ranges. Variables examined for the base case and additional scenarios include the number of annual rTMS procedures per centre and the cost per session of rTMS. The number of procedures was decreased to a minimum of 100 procedures per year to represent an underuse of rTMS, increasing to 2,880 (12 sessions daily, 5 days a week, 48 weeks yearly) and 3,360 (14 sessions daily, 5 days a week, 48 weeks yearly). Costs were varied by 15% to account for variability in costs.
Results of Budget-Impact Analysis
Base-Case Results
Assuming that rTMS would be provided as incremental treatment for TRD patients not currently receiving ECT treatment, the expected 1-year costs associated with funding six centres with rTMS (representing 12 rTMS machines) in Ontario would be approximately $1.97 million.
To illustrate the budget impact, consider:
cost per procedure: $109.52
annual procedures per centre: 1,500
number of rTMS machines: 12
budgetary impact: $1,971,383
Scenario Analyses
Table 11 shows the budgetary impact if rTMS is expanded to provide varying levels of geographic access equivalent to current ECT access (Table 9). Table 9 also shows the estimated number of procedures and resultant costs if rTMS is expanded to match the maximum, minimum, and median access to ECT, as well as a scenario where every ECT suite is adapted to have three rTMS treatment suites as a maximal scenario. The budgetary impact is $4.4 million for the scenario in which rTMS access matches that of the LHIN with the lowest per-capita access to ECT, $9.6 million when it matches the median per-capita access to ECT, and $19.9 million when it matches the highest per-capita access to ECT. Finally, the expected budget impact when three rTMS treatment suites are opened at each currently existing ECT centre is approximately $24.5 million.
Table 11:
Estimated Budget Impact for rTMS Among TRD Patients Not Receiving ECT for Four Scenarios
| Scenario | ||||
|---|---|---|---|---|
| Parameter | Minimal ECT Access | Median ECT Access | Maximum ECT Access | Maximal Diffusion to all ECT Centres |
| Cost per procedure | $109.52 | $109.52 | $109.52 | $109.52 |
| Number of rTMS machines funded | 27 | 59 | 122 | 150 |
| Number of annual procedures | 40,500 | 88,500 | 183,000 | 225,000 |
| Budgetary impact | $4,435,611 | $9,692,631 | $20,042,389 | $24,642,282 |
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation.
Sensitivity Analyses
Table 12 shows the budgetary impact for rTMS for the base-case scenario. Results were sensitive to the annual number of procedures per suite and cost of rTMS session, as would be expected. The budget impact increases to a maximum of $4.3 million if the number of annual procedures per suite reaches 3,360.
Table 12:
Results of One-Way Sensitivity Analyses for Base Case
| Parameter | Base-Case Value | Sensitivity-Analysis Value | Budget Impact, $ |
|---|---|---|---|
| Base-Case Results | 1,971,383 | ||
| Annual number of rTMS procedures per suite | 1,500 | 100 | 177,580 |
| 500 | 690,082 | ||
| 1,000 | 1,330,710 | ||
| 2,880 | 3,739,469 | ||
| 3,360 | 4,354,472 | ||
| Cost of rTMS per session | $109.52 | $125.95 | 2,267,038 |
| $93.09 | 1,675,637 |
Abbreviations: rTMS, repetitive transcranial magnetic stimulation.
Table 13 shows the budgetary impact for rTMS for each of the expanded diffusion scenarios.
Table 13:
One-Way Sensitivity Analyses of Diffusion Scenarios
| Budgetary Impact | ||||||
|---|---|---|---|---|---|---|
| Parameter | Base-Case Value | Sensitivity-Analysis Value | Low-Access Scenario, $ | Median-Access Scenario, $ | High-Access Scenario, $ | Implementation at All ECT Centres, $ |
| Base-case results | 4,435,509 | 9,692,409 | 20,041,931 | 24,641,719 | ||
| Annual number of rTMS procedures per suite | 1,500 | 100 | 399,556 | 873,104 | 1,805,401 | 2,219,755 |
| 500 | 1,552,685 | 3,392,905 | 7,015,838 | 8,626,030 | ||
| 1,000 | 2,994,097 | 6,542,657 | 13,528,884 | 16,633,874 | ||
| 2,880 | 8,413,806 | 18,385,725 | 28,017,939 | 46,743,368 | ||
| 3,360 | 9,797,561 | 21,409,486 | 44,270,462 | 54,430,896 | ||
| Cost of rTMS per session | $109.52 | $125.95 | 4,110,836 | 11,146,271 | 23,048,221 | 28,337,977 |
| $93.09 | 3,770,182 | 8,238,548 | 17,035,642 | 20,945,461 | ||
Abbreviations: ECT, electroconvulsive therapy; rTMS, repetitive transcranial magnetic stimulation.
Discussion
Resources for health care are scarce relative to needs or wants, and an economic evaluation is intended to inform the choices decision-makers face in these circumstances. This study investigated the resource allocations and cost-effectiveness of rTMS compared with ECT and of rTMS compared with sham rTMS. Resulting information can help ascertain the best treatments for people with TRD. The implementation of rTMS for TRD would potentially bring clinical and economic benefits for patients who are unwilling to use or intolerant to ECT therapies for medical reasons. For TRD patients who are intolerant to ECT or refuse to use ECT, rTMS would be more effective than standard of care but be cost-effective only if the willingness to pay is greater than $98,242 per QALY.
Limitations
Our models encountered several limitations. The first is a simplification of clinical care. The models followed patients for only 6 months; therefore, the long-term effects of either treatment were not captured. Details were limited by the short remission and by models’ inability to capture deaths from suicide. If the models were followed for 1 year, the QALYs would be doubled but the costs would not double; therefore, the ICER would favour rTMS more. Several assumptions were also made, such as assuming full remission does not require maintenance costs for either ECT or rTMS. Disutility associated with either treatment was ignored. Had disutility been considered in the model, rTMS would be more cost-effective compared to ECT, as the therapy had fewer adverse events than the latter. Ideally, a Markov model should be developed to follow TRD patients for a lifetime.
One key limitation of the budget-impact analysis concerns the difficulty of developing an algorithm to capture the number of TRD patients within administrative databases to estimate the total number of patients potentially eligible for rTMS treatment. In the literature and in clinical practice, a general definition of patients having failed two courses of antidepressant drugs is commonly used; however, there is no accepted guideline on duration of treatment, on drugs used, and so forth. The budget-impact analysis assumed that expansion of funding for rTMS (i.e., funding at six existing centres and eventual expansion to 50 centres in Ontario with ECT facilities) would be controlled. Given the relatively high prevalence of MDD and TRD in Ontario, it is reasonable to assume that these rTMS centres would have an adequate pool of patients to treat.
The method used to estimate increased diffusion in the province addresses geographic barriers to ECT and rTMS, but might not take into account other factors that could prevent patients from receiving treatment.
ConclusionS
Relative to repetitive transcranial magnetic stimulation, electroconvulsive therapy would be cost-effective if the willingness to pay is greater than $37,640 per quality-adjusted life-year (QALY). In comparison to pharmacotherapy, rTMS would be cost-effective when the willingness to pay is greater than $98,242 per QALY.
The estimated 1-year budgetary impact of funding rTMS for treatment of treatment-resistant depression examined a base case in which rTMS was added at six centres with existing rTMS infrastructure (constituting 12 treatment suites). This addition was estimated to increase costs to the Ministry of Health and Long-Term Care by $1.97 million. Four other scenarios of increased diffusion were based on the current infrastructure for and access to ECT. The budget impact was $4.4 million, $9.7 million, and $20.0 million where expansion of rTMS was assumed to reach the minimum, median, and maximum per-capita ECT access across all local health integration networks. The final scenario where it was assumed rTMS would expand to three treatment suites at each of the 50 existing ECT sites would result in approximately $24.6 million in additional spending by the province.
Acknowledgments
The medical editor was Elizabeth Jean Betsch. Others involved in the development and production of this report were Irfan Dhalla, Nancy Sikich, Andree Mitchell, Claude Soulodre, Chris Pagano, and Jessica Verhey.
We are grateful to the following experts for their valuable advice:
Jeff Daskalakis, MD, PhD, FRCPC, Temerty Chair in Therapeutic Brain Intervention; Chief, Mood and Anxiety Division; and Director, Scientist Development, at the Centre for Addiction and Mental Health.
Amer M. Burhan, Associate Professor and Chair of Geriatric Psychiatry at Schulich School of Medicine (Western University), Clinical Physician Lead for Theraputic Brain Stimulation (ECT and TMS) at Parkwood Institute-Mental Health in London Ontario.
Glossary
LIST OF ABBREVIATIONS
- ECT
Electroconvulsive therapy
- HALY
Health-adjusted life-year
- Ham-D
Hamilton Depression Rating Scale
- ICER
Incremental cost-effectiveness ratio
- LHIN
Local health integration network
- MDD
Major depressive disorder
- NHS
UK National Health Service
- PATH
Programs for Assessment of Technology in Health
- QALY
Quality-adjusted life-year
- rTMS
Repetitive transcranial magnetic stimulation
- TRD
Treatment-resistant depression
APPENDICES
Appendix 1: Economic Literature Search Strategies
Search date: Nov 20, 2014
Databases searched: Ovid MEDLINE/In-Process, Embase, EBM Databases, PsycINFO Limits: 1994-current; English; conference abstracts removed
Databases: EBM Reviews – Cochrane Database of Systematic Reviews 2005 to October 2014, EBM Reviews – Database of Abstracts of Reviews of Effects 4th Quarter 2014, EBM Reviews – Cochrane Central Register of Controlled Trials October 2014, EBM Reviews – Cochrane Methodology Register 3rd Quarter 2012, EBM Reviews – Health Technology Assessment 4th Quarter 2014, EBM Reviews – NHS Economic Evaluation Database 4th Quarter 2014, Ovid MEDLINE(R) Daily Update November 19, 2014, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, Embase 1974 to 2014 November 19
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | Depression/ | 341444 |
| 2 | exp Depressive Disorder/ use prmz,acp,cctr,coch,clcmr,dare,clhta,cleed | 92220 |
| 3 | Major Depression/ use oemezd | 37851 |
| 4 | Treatment Resistant Depression/ use oemezd | 742 |
| 5 | (depressi* or dysthymic or melancholia or TRD or “involutional psychos*” or paraphrenia).ti,ab. | 642939 |
| 6 | or/1–5 | 783031 |
| 7 | Transcranial Magnetic Stimulation/ | 22067 |
| 8 | (((transcranial or trans-cranial) adj2 magnetic adj2 stimulation*) or rtms or tms).mp. | 36404 |
| 9 | or/7–8 | 36404 |
| 10 | 6 and 9 | 4766 |
| 11 | limit 10 to yr=“1994 -Current” [Limit not valid in DARE; records were retained] | 4743 |
| 12 | limit 11 to english language [Limit not valid in CDSR,ACP Journal Club,DARE,CLCMR; records were retained] | 4305 |
| 13 | remove duplicates from 12 | 2734 |
Database: PsycINFO <1987 to November Week 3 2014>
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Major Depression/ | 93059 |
| 2 | (depressi* or dysthymic or melancholia or TRD or “involutional psychos*” or paraphrenia).ti,ab. | 180727 |
| 3 | or/1–2 | 186314 |
| 4 | exp Transcranial Magnetic Stimulation/ | 4565 |
| 5 | (((transcranial or trans-cranial) adj2 magnetic adj2 stimulation*) or rtms or tms).mp. | 6312 |
| 6 | or/4–5 | 6312 |
| 7 | 3 and 6 | 1182 |
| 8 | limit 7 to (english language and yr=“1994 -Current”) | 1081 |
HEED
depressi* OR dysthymic OR melancholia OR TRD OR psychos* OR paraphrenia =all data
AND
transcranial OR trans-cranial OR rtms OR tms =all data
5 results
Figure A1: PRISMA Flow Diagram.
Appendix 2: Model Input Literature Search Results
Search date: Nov 20, 2014
Databases searched: Ovid MEDLINE/In-Process, Embase, EBM Databases, PsycINFO
Limits: 1994-current; English; conference abstracts removed
Databases: EBM Reviews – Cochrane Database of Systematic Reviews 2005 to October 2014, EBM Reviews – Database of Abstracts of Reviews of Effects 4th Quarter 2014, EBM Reviews – Cochrane Central Register of Controlled Trials October 2014, EBM Reviews – Cochrane Methodology Register 3rd Quarter 2012, EBM Reviews – Health Technology Assessment 4th Quarter 2014, EBM Reviews – NHS Economic Evaluation Database 4th Quarter 2014, Ovid MEDLINE(R) Daily Update November 19, 2014, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, Embase 1974 to 2014 November 19
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | Depression/ | 341444 |
| 2 | exp Depressive Disorder/ use prmz,acp,cctr,coch,clcmr,dare,clhta,cleed | 92220 |
| 3 | Major Depression/ use oemezd | 37851 |
| 4 | Treatment Resistant Depression/ use oemezd | 742 |
| 5 | (depressi* or dysthymic or melancholia or TRD or “involutional psychos*” or paraphrenia).ti,ab. | 642939 |
| 6 | or/1–5 | 783031 |
| 7 | Transcranial Magnetic Stimulation/ | 22067 |
| 8 | (((transcranial or trans-cranial) adj2 magnetic adj2 stimulation*) or rtms or tms).mp. | 36404 |
| 9 | or/7–8 | 36404 |
| 10 | 6 and 9 | 4766 |
| 11 | limit 10 to yr=“1994 -Current” [Limit not valid in DARE; records were retained] | 4743 |
| 12 | limit 11 to english language [Limit not valid in CDSR,ACP Journal Club,DARE,CLCMR; records were retained] | 4305 |
| 13 | remove duplicates from 12 | 2734 |
Database: PsycINFO <1987 to November Week 3 2014>
Search Strategy:
| # | Searches | Results |
|---|---|---|
| 1 | exp Major Depression/ | 93059 |
| 2 | (depressi* or dysthymic or melancholia or TRD or “involutional psychos*” or paraphrenia).ti,ab. | 180727 |
| 3 | or/1–2 | 186314 |
| 4 | exp Transcranial Magnetic Stimulation/ | 4565 |
| 5 | (((transcranial or trans-cranial) adj2 magnetic adj2 stimulation*) or rtms or tms).mp. | 6312 |
| 6 | or/4–5 | 6312 |
| 7 | 3 and 6 | 1182 |
| 8 | limit 7 to (english language and yr=“1994 -Current”) | 1081 |
Figure A2: PRISMA Flow Diagram for Model Input Parameters.
Appendix 3: Systematic Review of Clinical Evidence
Model Input Parameters
A systematic review33 was undertaken in part to derive model input parameters of effectiveness of rTMS versus sham and of rTMS versus ECT.
Literature Search Strategy
A literature search was performed on November 20, 2014 searching the following databases: EBM Reviews – Cochrane Database of Systematic Reviews 2005 to October 2014, EBM Reviews – Database of Abstracts of Reviews of Effects 4th Quarter 2014, EBM Reviews – Cochrane Central Register of Controlled Trials October 2014, EBM Reviews – Cochrane Methodology Register 3rd Quarter 2012, EBM Reviews – Health Technology Assessment 4th Quarter 2014, EBM Reviews – NHS Economic Evaluation Database 4th Quarter 2014, Ovid MEDLINE(R) Daily Update November 19, 2014, Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present, Embase 1974 to 2014 November 19. The search strategy is outlined in Appendix 2.
Titles and abstracts were first assessed by a single reviewer on the basis of the inclusion criteria listed below; followed by full-text screening by two independent reviewers for all potentially relevant articles identified from the first screen.
Inclusion Criteria
published between 1994 and 2014
English language
randomized controlled trial (RCT) (primary study)
intervention is rTMS, frequency of > 5 Hz
stimulating coil placed over the left dorsolateral prefrontal cortex (DLPEC)
patients received at least 2 weeks (or at least 10 days) of rTMS treatment.
adults (≥ 18 years) with treatment-resistant major depression
Studies that included unipolar patients only or that reported the proportion of bipolar patients as ≤ 20%
comparator is ECT, sham rTMS, or placebo
Exclusion Criteria
nonrandomized trials
studies on stimulation sites other than left dorsolateral prefrontal cortex
studies on bilateral rTMS or bilateral versus unilateral rTMS
studies on sequential combined low-frequency and high-frequency rTMS
studies on newer techniques (synchronized rTMS, pulsed rTMS, deep rTMS, rTMS with priming stimulation)
studies evaluating effects of rTMS on cognitive function
studies evaluating effectiveness of rTMS for depression in specific conditions (e.g., after stroke)
studies not reporting on response, remission, or relapse rates; that did not define these outcomes; or that reported the outcomes poorly
Results of Literature Review for Model Input Parameters
A total of 2,255 citations were identified in the database search, representing 2,253 unique studies once duplicates were removed. After the title and abstract review, 2,156 articles were excluded, and the full texts of the remaining 97 articles were reviewed. Fifty studies met the inclusion criteria (Figure A2).
A subgroup of studies were identified from the 50 studies that met the inclusion criteria.33 These 50 studies reported the outcomes of both response and remission rates at the end of treatment. Reporting both was necessary to ensure consistency in estimating the response and remission rates of each model's parameters. The end of treatment was chosen as our point of assessment because the number of treatment sessions subjects received varied between studies (e.g., 15 sessions of rTMS within a 4-week period in one study20 vs. 1 session of rTMS per weekday for 6 weeks in another study34).
Statistical Analysis: Direct Treatment Comparisons
The outcomes to be estimated (i.e., response and remission rates) were reported as rates of events. When necessary, missing data were derived from the papers according to the methods suggested in the Cochrane Handbook for Systematic Reviews of Interventions.35 For example, when not reported, the number of responders could be derived if the number of patients enrolled in the trial and the percentage of responders were reported in the text.
Pooled estimates of the relative risks (RR) of rTMS versus sham and rTMS versus ECT were analyzed using direct meta-analysis in Review Manager 5.3. A random effects model was used because we assumed that the true treatment effects had most likely varied between the included studies.36 Heterogeneity was assessed with the I2 statistic; more than 50% was considered moderate heterogeneity and more than 70% considered substantial heterogeneity as suggested by the Cochrane Handbook for Systematic Review of Interventions.
Results
From the systematic literature review, nine studies that compared rTMS versus sham were included,11,20,22,34,37–41 and three studies that compared rTMS versus ECT24,42,43 were included in the quantitative analysis (Tables A1 and A2) where both response and remission rates were reported within the same publication. Treatment-related parameters, end of treatment points (in weeks), response rates at end of treatment, and remission rates at end of treatment for these studies are presented in Table A1 (for rTMS vs. sham) and Table A2 (for rTMS vs. ECT). Response was defined as 50% or more reduction relative to pretreatment baseline on the Hamilton Rating Scale for Depression (Ham-D) or on the Montgomery-Asberg Depression Rating Scale (MADRS). Remission was also defined in these studies (e.g., 17- or 21-item Ham-D score ≤ 7 or ≤ 10, respectively34,40 or MADRS score ≤ 1037).
Table A1:
Included rTMS Versus Sham Studies: rTMS Parameters, End-of-Treatment Points, Response and Remission Rates
| Response | Remission | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rTMS parameters | rTMS Group | Sham Group | rTMS Group | Sham Group | |||||||||
| Study | Sessions, n | Frequency, Hz | Total Pulses | % rMT | End of Treatment Point (Weeks) | Events | Total | Events | Total | Events | Total | Events | Total |
| Bakim et al, 201234 | 30 | 20 | 24,000 | 110 | 6 | 8 | 11 | 2 | 12 | 6 | 11 | 1 | 12 |
| George, 201011 | 15 | 10 | 45,000 | 120 | 6 | 10 | 72 | 4 | 82 | 10 | 72 | 4 | 82 |
| Mogg, 200838 | 10 | 10 | 10,000 | 110 | 2 | 9 | 28 | 3 | 29 | 7 | 28 | 3 | 29 |
| Loo, 200737 O'Reardon, 2007 | 20 | 10 | 30,000 | 110 | 2 | 6 | 19 | 3 | 19 | 3 | 19 | 2 | 19 |
| 22 | 20 | 10 | 60,000 | 120 | 4 | 32 | 155 | 17 | 146 | 11 | 155 | 9 | 146 |
| Stern, 200740 | 10 | 10 | 16,000 | 110 | 2 | 5 | 10 | 0 | 15 | 3 | 10 | 0 | 15 |
| Avery, 200620 | 15 | 10 | 24,000 | 110 | 4 | 11 | 35 | 2 | 33 | 7 | 35 | 1 | 33 |
| Su, 200541 | 10 | 20 | 16,000 | 100 | 2 | 6 | 10 | 1 | 10 | 5 | 10 | 1 | 10 |
| Padberg, 200239 | 10 | 10 | 15,000 | 100 | 2 | 3 | 10 | 0 | 10 | 2 | 10 | 0 | 10 |
| Total | 90 | 350 | 32 | 356 | 54 | 350 | 21 | 356 | |||||
Abbreviations: rMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation.
Table A2:
Included rTMS Versus ECT Studies: rTMS/ECT Parameters, End-of-Treatment Points, Response, and Remission Rates
| Response | Remission | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rTMS Parameters | ECT Parameters | End-of-Treatment Point (Wk) | rTMS Group | ECT Group | rTMS Group | ECT Group | ||||||||
| Study | Sessions, n | Frequency, Hz | Total Pulses | % rMT | Sessions, n | Events | Total | Events | Total | Events | Total | Events | Total | |
| Eranti et al, 200724 | 15 | 10 | 15,000 | 110 | 6.3 ± 2.5 | 3 | 4 | 24 | 13 | 22 | 4 | 24 | 13 | 22 |
| Rosa et al, 200643 | 20 | 10 | 50,000 | 100 | 10 ± 1.5 | 4 | 10 | 20 | 6 | 15 | 2 | 20 | 3 | 15 |
| Grunhaus et al, 200342 | 20 | 10 | 24,000 | 90 | 10.25 ± 3.1 | 4 | 11 | 20 | 12 | 20 | 6 | 20 | 6 | 20 |
| Total | 25 | 64 | 31 | 57 | 12 | 64 | 22 | 57 | ||||||
Abbreviations: ECT, electroconvulsive therapy; rMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation.
Response and Remission Rates at End of Treatment
rTMS Versus Sham
Response
Overall, 90/350 (25.7%) subjects receiving rTMS and 32/356 (9.0%) subjects receiving sham were classified as responders. The pooled RR was 2.58 (95% confidence interval [CI] 1.77–3.75, z = 4.96, P < .00001), which indicates a significant difference in response rates favouring rTMS over sham rTMS. Heterogeneity between studies did not exceed that expected by chance (degrees of freedom [df] = 8, P = .57, I2 = 0%). The associated Forest plot is presented in Figure A3.
Figure A3: Meta-Analysis for Response Rates at End of Treatment for rTMS Versus Sham rTMS.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; rTMS, repetitive transcranial magnetic stimulation.
Remission
Overall, 54/350 (15.4%) subjects receiving rTMS and 21/356 (5.9%) subjects receiving sham rTMS were classified as remitters. The pooled RR was 2.35 (95% CI 1.44–3.84, z = 3.43, P = .0006), which indicates a significant difference in remission rates favouring rTMS over sham. Heterogeneity between studies did not exceed that expected by chance (df = 8, P = .53, I2 = 0%). The associated Forest plot is presented in Figure A4.
Figure A4: Meta-Analysis for Remission Rates at End of Treatment for rTMS Versus Sham rTMS.
Abbreviations: CI, confidence interval; df, degrees of freedom; M-H, Mantel-Haenszel; rTMS, repetitive transcranial magnetic stimulation.
rTMS Versus ECT
Response
Overall, 25/64 (39.1%) subjects receiving rTMS and 31/57 (54.4%) subjects receiving ECT were classified as responders. The pooled RR was 0.73 (95% CI 0.34–1.58, z = 0.79, P = .43), which indicates a nonsignificant difference in response rates between ECT and rTMS. Heterogeneity between studies exceeded that expected by chance (df = 2, P = .04, I2 = 69%). The associated Forest plot is presented in Figure A5.
Figure A5: Meta-Analysis for Response Rates at End of Treatment for rTMS Versus ECT.
Abbreviations: CI, confidence interval; df, degrees of freedom; ECT, electroconvulsive therapy; M-H, Mantel-Haenszel; rTMS, repetitive transcranial magnetic stimulation.
Remission
Overall, 12/64 (18.8%) subjects receiving rTMS and 22/57 (38.6%) subjects receiving ECT were classified as remitters. The pooled RR was 0.53 (95% CI 0.22–1.24, z = 1.47, P = .14), which indicates a nonsignificant difference in remission rates between ECT and rTMS. Heterogeneity between studies did not exceed that expected by chance (df = 2, P = .18, I2 = 42%). The associated Forest plot is presented in Figure A6.
Figure A6: Meta-Analysis for Remission Rates at End of Treatment for rTMS Versus ECT.
Abbreviations: CI, confidence interval; df, degrees of freedom; ECT, electroconvulsive therapy; M-H, Mantel-Haenszel; rTMS, repetitive transcranial magnetic stimulation.
Author contributions
This report was developed by a multi-disciplinary team composed of Hong Anh Tu, Stefan Palimaka, and Shayan Sehatzadeh from Health Quality Ontario and Gord Blackhouse, Belinda Yap, Bernice Tsoi, Jim Bowen, and Daria O'Reilly from the Programs for Assessment of Technology in Health (PATH). The medical librarians were Corinne Holubowich and Kellee Kaulback (Health Quality Ontario) and Kaitryn Campbell (PATH).
KEY MESSAGES
Major depressive disorder is common worldwide and within Canada. Many patients are treatment-resistant, meaning that they do not respond to several drugs meant to treat depression. These patients have more physician visits, more hospitalizations, and higher drug costs than patients without treatment resistance. Once a patient has tried at least two antidepressants without getting better, he or she might be offered electroconvulsive (shock) therapy to help with depression; however, use of this therapy is limited and has caused many side effects.
Health Quality Ontario investigated the use of repetitive transcranial magnetic stimulation and its costs for Ontario's health care system. An evidence-based clinical analysis that compared repetitive transcranial magnetic stimulation with drug therapy and with electroconvulsive therapy found that repetitive transcranial magnetic stimulation could be useful with few side effects.
We compared the costs and health outcomes of two treatments for patients with treatment-resistant depression in Ontario: repetitive magnetic transcranial stimulation alone compared with electroconvulsive therapy alone and repetitive magnetic transcranial stimulation alone compared with drug therapy. We calculated the changes in costs over 6 months and changes in patients’ quality of life for these treatments.
Repetitive transcranial magnetic stimulation has more value than electroconvulsive therapy if more than $37,640 will be spent to give 1 year of perfect health to patients. Compared with drug therapy, repetitive transcranial magnetic stimulation is worth the cost only when people are willing to pay more than $98,242 yearly for their improved quality of life.
The cost of running six centres that can provide repetitive transcranial magnetic stimulation for treatment-resistant depression for 1 year was estimated at $1.97 million. Costs increased by $4.4 million to provide lowest access in all local health integration networks, by $9.7 million for median access, and by $20.0 million for highest access.
Contributor Information
Health Quality Ontario:
Hong Anh Tu, Stefan Palimaka, Shayan Sehatzadeh, Gord Blackhouse, Belinda Yap, Bernice Tsoi, Jim Bowen, Daria O'Reilly, Corinne Holubowich, Kellee Kaulback, and Kaitryn Campbell
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches.
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Patten SB, Kennedy SH, Lam RW, O'Donovan C, Filteau MJ, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) clinical guidelines for the management of major depressive disorder in adults. I. Classification, burden and principles of management. J Affect Disord. 2009; 117 Suppl 1:S5–14. [DOI] [PubMed] [Google Scholar]
- (2).American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Association; 2013.
- (3).Committee for Medicinal Product for Human Use. Concept paper on the need for revision of note for guidance on clinical investigation of medicinal products in the treatment of depression with regard to treatment resistant depression [Internet]. London, UK: European Medicines Agency; 2009 [cited 2014 Feb 12]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/10/WC500004861.pdf
- (4).Waraich P, Goldner EM, Somers JM, Hsu L. Prevalence and incidence studies of mood disorders: a systematic review of the literature. Can J Psychiatry. 2004; 49(2): 124–38. [DOI] [PubMed] [Google Scholar]
- (5).Statistics Canada. Mental health profile, Canadian Community Health Survey – mental health (CCHS), by age group and sex, Canada and provinces [Internet]. Ottawa, ON: Government of Canada; 2013 [cited 2014 Nov 4]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1051101&paSer=&pattern=&stByVal=1&p1=1&p2=31&tabMode=dataTable&csid=
- (6).Rizvi SJ, Grima E, Tan M, Rotzinger S, Lin P, Mcintyre RS, et al. Treatment-resistant depression in primary care across Canada. Can J Psychiatry. 2014; 59(7): 349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003; 53(8): 649–59. [DOI] [PubMed] [Google Scholar]
- (8).Ratnasingham S CJ, Rehm J, Manson H, Kurdyak PA. Opening Eyes, Opening Minds: The Ontario Burden of Mental Illness and Addictions Report. An ICES/PHO Report. Toronto: Institute for Clinical Evaluative Sciences and Public Health Ontario; 2012. [Google Scholar]
- (9).World Health Organization. The global burden of disease, 2004 update [Internet]. Geneva, Switzerland: The Organization; 2015 [cited 2015 September 17]. Available from: http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf
- (10).Kennedy SH, Lam RW, Cohen NL, Ravindran AV. Clinical guidelines for the treatment of depressive disorders. IV. Medications and other biological treatments. Can J Psychiatry. 2001; 46 Suppl 1:38S–58S. [PubMed] [Google Scholar]
- (11).George MS, Lisanby SH, Avery D, McDonald WM, Durkalski V, Pavlicova M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010; 67(5): 507–16. [DOI] [PubMed] [Google Scholar]
- (12).O'Reardon JP, Blumner KH, Peshek AD, Pradilla RR, Pimiento PC. Long-term maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry. 2005; 66(12): 1524–8. [DOI] [PubMed] [Google Scholar]
- (13).Petrides G, Fink M, Husain MM, Knapp RG, Rush AJ, Mueller M, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001; 17(4): 244–53. [DOI] [PubMed] [Google Scholar]
- (14).Wijeratne C, Halliday GS, Lyndon RW. The present status of electroconvulsive therapy: a systematic review. Med J Aust. 1999; 171(5): 250–4. [DOI] [PubMed] [Google Scholar]
- (15).University Health Network. University Health Network rTMS Clinic [Internet]. 2015 [cited 2015 Jan 2]. Available from: http://rtmsclinic.ca/?pageid=11University
- (16).University of Calgary. Repetitive transcranial magnetic stimulation for treatment resistant depression [Internet]. Calgary (AB): University of Calgary; 2014 [cited 2014 Nov 1]. Available from: http://www.health.alberta.ca/documents/AHTDP-rTMS-Resistant-Depression-UofC.pdf
- (17).Donar J, Blumberger D, Daskalakis J. An analysis of the cost-effectiveness of repetitive transcranial magnetic stimulation in the treatment of medication-resistant major depression in Ontario. Forthcoming.
- (18).Kozel FA, George MS, Simpson KN. Decision analysis of the cost-effectiveness of repetitive transcranial magnetic stimulation versus electroconvulsive therapy for treatment of nonpsychotic severe depression. CNS Spectr. 2004; 9(6): 476–82. [PubMed] [Google Scholar]
- (19).Simpson KN, Welch MJ, Kozel FA, Demitrack MA, Nahas Z. Cost-effectiveness of transcranial magnetic stimulation in the treatment of major depression: a health economics analysis. Adv Ther. 2009; 26(3): 346–68. [DOI] [PubMed] [Google Scholar]
- (20).Avery DH, Holtzheimer PE, III, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006; 59(2): 187–94. [DOI] [PubMed] [Google Scholar]
- (21).Janicak PG, O'Reardon JP, Sampson SM, Husain MM, Lisanby SH, Rado JT, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. 2008; 69(2): 222–32. [DOI] [PubMed] [Google Scholar]
- (22).O'Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007; 62(11): 1208–16. [DOI] [PubMed] [Google Scholar]
- (23).Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- (24).Eranti S, Mogg A, Pluck G, Landau S, Purvis R, Brown RG, et al. A randomized, controlled trial with 6-month follow-up of repetitive transcranial magnetic stimulation and electroconvulsive therapy for severe depression. Am J Psychiatry. 2007; 164(1): 73–81. [DOI] [PubMed] [Google Scholar]
- (25).Sapin C, Fantino B, Nowicki ML, Kind P. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004; 2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Burdett A, Gard C, Longo C, Raskin J, Gilsenan A, Hopkins J, et al. Prevalence and Costs of Difficult-to-Treat Depression in a Canadian Claims Database. International Society for Pharmacoepidemiology 20th International Conference on Pharmacoepidemiology; Bordeaux, France2004.
- (27).Ministry of Health and Long-Term Care. Ontario drug benefit formulary [Internet]. Toronto (ON): Queen's Printer; 2015 [cited 2014 Nov 1]. Available from: https://www.healthinfo.moh.gov.on.ca/formulary
- (28).Nemeroff CB. Prevalence and management of treatment-resistant depression. J Clin Psychiatry. 2007; 68 Suppl 8:17–25. [PubMed] [Google Scholar]
- (29).Delva NJ, Graf P, Patry S, Gosselin C, Milev R, Gilron I, et al. Access to electroconvulsive therapy services in Canada. J ECT. 2011; 27(4): 300–9. [DOI] [PubMed] [Google Scholar]
- (30).Statistics Canada. Table 051-0001 – estimates of population, by age group and sex for July 1, Canada, provinces and territories, annual (persons unless otherwise noted) [Internet]. Ottawa (ON): Government of Canada; [cited 2015 Feb 2]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=0510001&paSer=&pattern=&stByVal=1&p1=1&p2=37&tabMode=dataTable&csid=
- (31).Regie de l'assurance maladie Quebec. Billing manual. Specialty. [Internet]. Quebec City (QC): Regie de l'assurance maladie Quebec; 2015 [cited 2015 Jan 10]. Available from: http://www.ramq.gouv.qc.ca/SiteCollectionDocuments/professionnels/manuels/150-facturation-specialistes/012_b_tarif_visites_acte_spec.pdf
- (32).Canadian Federation of Nurses Unions. Overview of key nursing contract provisions [Internet]. Ottawa (ON): Canadian Federation of Nurses Unions; 2015 [updated Oct 4 2012; cited 2014 Nov 1]. Available from: https://nursesunions.ca/sites/default/files/contract_comparison_english.pdf
- (33).Health Quality Ontario. High-frequency repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont Health Technol Assess Ser. 2015. [PMC free article] [PubMed]
- (34).Bakim B, Uzun E, Karamustafalioglu O, Ozcelik B, Alpak G, Tankaya O, et al. The combination of antidepressant drug therapy and high-frequency repetitive transcranial magnetic stimulation in medication-resistant depression. Bull Clin Psychopharmacol. 2012; 22(3): 244–53. [Google Scholar]
- (35).The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Internet]. [cited 2015 Feb 5]. Available from: www.cochrane-handbook.org
- (36).Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011; 342:d549. [DOI] [PubMed] [Google Scholar]
- (37).Loo CK, Mitchell PB, McFarquhar TF, Malhi GS, Sachdev PS. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007; 37(3): 341–9. [DOI] [PubMed] [Google Scholar]
- (38).Mogg A, Pluck G, Eranti SV, Landau S, Purvis R, Brown RG, et al. A randomized controlled trial with 4-month follow-up of adjunctive repetitive transcranial magnetic stimulation of the left prefrontal cortex for depression. Psychol Med. 2008; 38(3): 323–33. [DOI] [PubMed] [Google Scholar]
- (39).Padberg F, Schule C, Zwanzger P, Baghai T, Ella R, Mikhaiel P, et al. Relation between responses to repetitive transcranial magnetic stimulation and partial sleep deprivation in major depression. J Psychiatr Res. 2002; 36(3): 131–5. [DOI] [PubMed] [Google Scholar]
- (40).Stern WM, Tormos JM, Press DZ, Pearlman C, Pascual-Leone A. Antidepressant effects of high and low frequency repetitive transcranial magnetic stimulation to the dorsolateral prefrontal cortex: a double-blind, randomized, placebo-controlled trial. J Neuropsychiatry Clin Neurosci. 2007; 19(2): 179–86. [DOI] [PubMed] [Google Scholar]
- (41).Su TP, Huang CC, Wei IH. Add-on rTMS for medication-resistant depression: a randomized, double-blind, sham-controlled trial in Chinese patients. J Clin Psychiatry. 2005; 66(7): 930–7. [DOI] [PubMed] [Google Scholar]
- (42).Grunhaus L, Schreiber S, Dolberg OT, Polak D, Dannon PN. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003; 53(4): 324–31. [DOI] [PubMed] [Google Scholar]
- (43).Rosa MA, Gattaz WF, Pascual-Leone A, Fregni F, Rosa MO, Rumi DO, et al. Comparison of repetitive transcranial magnetic stimulation and electroconvulsive therapy in unipolar non-psychotic refractory depression: a randomized, single-blind study. Int J Neuropsychopharmacol. 2006; 9(6): 667–76. [DOI] [PubMed] [Google Scholar]