Abstract
NIPP1 (nuclear inhibitor of protein phosphatase 1) is a ubiquitously expressed nuclear scaffold protein that has been implicated in both transcription and RNA processing. Among its protein ligands are a protein kinase, a protein phosphatase, two splicing factors, and a transcriptional regulator, and the binding of these proteins to NIPP1 is tightly regulated by phosphorylation. To study the function of NIPP1 in vivo, we have used homologous recombination to generate mice that are deficient in NIPP1. NIPP1−/+ mice developed normally. However, NIPP1−/− embryos showed severely retarded growth at embryonic day 6.5 (E6.5) and were resorbed by E8.5. This early embryonic lethality was not associated with increased apoptosis but correlated with impaired cell proliferation. Blastocyst outgrowth experiments and the RNA interference-mediated knockdown of NIPP1 in cultured cells also revealed an essential role for NIPP1 in cell proliferation. In further agreement with this function, no viable NIPP1−/− cell lines were obtained by derivation of embryonic stem (ES) cells from blastocysts of NIPP1−/+ intercrosses or by forced homogenotization of heterozygous ES cells at high concentrations of Geneticin. We conclude that NIPP1 is indispensable for early embryonic development and cell proliferation.
NIPP1 is a nuclear protein of 39 kDa that is expressed in both plants and animals (11, 17, 29). It was originally purified as a potent and specific inhibitor of protein phosphatase 1 (PP1); hence its name, nuclear inhibitor of PP1 (3). NIPP1 contains at least two binding sites for PP1 and is complexed to about one-third of the nuclear pool of PP1 (2, 5, 19). The NIPP1-PP1 complex is inactive but can be activated by phosphorylation of NIPP1 with protein kinase A (1, 31), protein kinase CK2 (28, 31), and protein tyrosine kinases of the Src family (5). Overexpression of NIPP1 in Drosophila melanogaster is lethal in a range of tissues and developmental stages, probably as a result of the inhibition of PP1 (25).
NIPP1 also binds to the cell cycle-regulated maternal-embryonic leucine zipper kinase (MELK) (30). During mitosis, MELK interacts via a phosphorylated threonine with the Forkhead-associated (FHA) domain of NIPP1. Interestingly, MELK is a potent inhibitor of spliceosome assembly, and this inhibition requires a functional NIPP1-binding site. Thus, the MELK-NIPP1 interaction may contribute to splicing arrest during mitosis. Additional evidence for a key role of NIPP1 in pre-mRNA splicing comes from competition experiments with NIPP1 fragments (4) and from observations that NIPP1 is enriched both in the splicing factor storage sites, or “speckles,” and in spliceosomes (4, 17, 26). The targeting of NIPP1 to these subnuclear compartments is mediated by its FHA domain (4, 17) and is likely to be accounted for by the interaction of the FHA domain with phosphorylated forms of the splicing factors CDC5L (8) and SAP155 (7).
Further insights into the functional complexity of NIPP1 have come from recent observations that NIPP1 also interacts with the Polycomb protein EED (embryonic ectoderm development), a component of the Polycomb repressive complex 2 (PRC2) that is implicated in the maintenance of genes in their repressed state (20). Both EED and NIPP1 function as transcriptional repressors of targeted genes in transient transfection experiments. Moreover, a macromolecular complex that contains NIPP1, EED, PP1, and the histone deacetylase HDAC2 has been identified, suggesting a role for histone deacetylation in transcriptional repression by NIPP1.
The human NIPP1-encoding gene, PPP1R8, can give rise to multiple splice variants (27). The NIPP1 isoform that is ubiquitously expressed is referred to as NIPP1α (or just NIPP1). Another isoform is NIPP1γ/Ard1 (12, 27), which corresponds to the C-terminal one-third of NIPP1α. Interestingly, NIPP1γ displays an endoribonuclease activity with a specificity similar to that of bacterial RNase E, a key regulator of the decay and processing of various RNAs (21, 32). However, the widely expressed NIPP1α isoform shows no endoribonuclease activity (18), suggesting that the expression of this enzymatic activity is under tight control.
In conclusion, NIPP1 emerges from the available data as a multifunctional protein and as a candidate “integrator” of transcription, RNA processing, and cell cycle progression. To further study the complex function of NIPP1, we have disrupted the gene encoding NIPP1 (Ppp1r8) in mice by homologous recombination. NIPP1−/+ mice did not display an overt phenotype, while NIPP1−/− embryos died at around 6.5 days postconception. This early embryonic lethality was associated with a generally decreased ability of the cells to proliferate. Blastocyst outgrowth experiments and the RNA interference (RNAi)-mediated knockdown of NIPP1 in cultured cells confirmed the essential role of NIPP1 in cell proliferation.
MATERIALS AND METHODS
Construction of a targeting vector for homologous recombination.
Two overlapping 12.5-kb genomic phage clones, comprising exons 1 through 5 of Ppp1r8, were isolated from the 129SvJ mouse lambda library from Stratagene (La Jolla, Calif.) (Fig. 1A). Phage DNA was isolated, and all EcoRI and XbaI fragments were subcloned into pBluescript SK. The subclones were further characterized by restriction map analysis and sequencing and were used for the construction of the targeting vector (Fig. 1A). The targeting vector, with the pPNT plasmid as a backbone, contained 3.2 kb of Ppp1r8 sequence on the 5′ arm (flank 1) and 4.3 kb of Ppp1r8 on the 3′ arm (flank 2). A loxP-flanked neomycin resistance cassette (Neor) for positive selection was inserted between flanks 1 and 2, and a thymidine kinase-expressing cassette for negative selection was cloned outside flank 2. The neomycin cassette was introduced in a reverse orientation relative to the NIPP1 gene fragments. The integrity of the targeting vector was verified by restriction digestion and nucleotide sequencing.
FIG. 1.
Targeted disruption of the NIPP1 gene. (A) Partial restriction maps of the Ppp1r8 locus (mouse NIPP1 gene), the targeting vector, and the targeted locus. Two 12.5-kb phage genomic clones, isolated from the 129SvJ mouse lambda genomic library, are shown on top. In the wild-type (wt) locus, exons 1 to 5 (E1 to E5) are shown as solid boxes and “ATG” represents the first coding exon. Restriction sites: B, BamHI (not all indicated); E, EcoRI (all indicated); P, PstI (not all indicated). Dashed lines between the wt locus and the targeting vector delineate the regions that are common to the genomic locus and the replacement vector. The neomycin resistance cassette (Neor) in the replacement vector is flanked by loxP (not indicated), and a thymidine kinase cassette (TK) lies downstream of flank 2 in the replacement vector. The targeted locus represents the predicted structure of the NIPP1 locus after homologous recombination with the replacement vector. A 6.6-kb fragment comprising part of the promoter region, as well as exons 1 to 2, has been replaced by the Neo cassette. (B) Southern blot genotyping of neonates derived from intercrosses of NIPP1 heterozygotes. Genomic DNA was digested with EcoRI and hybridized with probe A, yielding a 9-kb band for the wt allele and a 5.8-kb band for the mutant allele. (C) PCR genotyping of E7.5 embryos obtained from crosses of NIPP1 heterozygotes. A wt-specific nested PCR, as explained in Materials and Methods, amplifies a band of 402 bp, and a mutant-specific PCR results in a band of 696 bp. M, marker; C, control. (D) RT-PCR genotyping of blastocysts (E3.5) isolated from NIPP1 heterozygous intercrosses. The presence of the wt NIPP1 allele results in a 350-bp fragment, while the mutated NIPP1 allele leads to amplification of a 696-bp product. (E) Detection of NIPP1 by immunostaining in E6.5 embryos from intercrosses of NIPP1 heterozygotes. Serial transverse sections of paraffin-embedded embryos were made from E6.5 deciduae. Sections were stained with anti-NIPP1 antibodies (a and c) or anti-HDAC2 antibodies (b and d). The figure shows an embryo that expresses NIPP1 (a and b) and an embryo that lacks NIPP1 (c and d). The control condition in panels c and d did not contain DNA template. Bar in panel Ea, 50 μm.
ES cell transfection and generation of NIPP1−/− mice.
The NotI-linearized targeting vector was electroporated into R1 embryonic stem (ES) cells (13). Following negative selection with ganciclovir (2 μM) and positive selection with Geneticin (200 μg/ml), resistant ES clones were obtained and analyzed by Southern blotting. NIPP1−/+ ES cells were aggregated with Swiss Webster morula-stage embryos to generate chimeric animals, as described previously (13). The resulting chimeric males were crossed with Swiss females, and NIPP1−/+ transgenic offspring were identified by Southern blot analysis of genomic DNA obtained from tail biopsies. Heterozygous mice were intercrossed to obtain NIPP1−/− mice with an overall 50:50 R1 129:Swiss genetic background.
Genotype analysis.
DNA for genotype analysis by Southern blotting or by PCR was isolated from ES clones, mouse tail tips, or embryos. The correct homologous recombination of NIPP1−/+ clones at both flanks was confirmed by Southern blot analysis using a 0.8-kb EcoRI-PstI fragment (probe A) located immediately upstream of flank 1 and a 1.7-kb HindIII-BamHI fragment (probe B) located downstream of flank 2 (Fig. 1A). To exclude additional random integration of the targeting vector, an internal 1.1-kb XbaI fragment (probe C) located in flank 2 was used to verify the absence of additional bands in SpeI-digested DNA. Hybridization of EcoRI-digested DNA with probe A detects a 9-kb band for the wild-type allele and a 5.8-kb band for the targeted allele (Fig. 1B). Hybridization of SpeI-digested DNA with probe B identifies a 13-kb band for the wild-type allele and an 8-kb band for the targeted allele (data not shown).
PCR genotyping of mouse tail tips and embryonic day 8.5 (E8.5) or E10.5 embryos (Table 1) was performed with a three-primer strategy: a common sense primer located in flank 1 (5′-CCTCAGCAGATAGCCCACGG-3′) can pair with a wild-type allele-specific antisense primer located in the deleted region (5′-GATGCTTGGCACTGGAAGAGGC C-3′) or with a mutant allele-specific antisense primer located in the neomycin gene (5′-CGCATCGCCTTCTATCGCCTTCTTGAC-3′). Amplification of the wild-type allele results in a 402-bp band, while amplification of the mutant allele yields a product of 623 bp. PCR genotyping of E7.5 embryos (Table 1; Fig. 1C) was performed by a wild-type-specific nested PCR and a mutant-specific PCR. The sense primer 5′-CCTCAGCAGATAGCCCACGG-3′ and the antisense primer 5′-GTACTTGGTAATCTGTAAGAGTCACCC-3′ were used to amplify a 475-bp fragment from the wild-type allele. The primary products were used as templates in a secondary PCR with the same sense primer and the nested antisense primer 5′-GATGCTTGGCACTGGAAGAGGCC-3′. This amplification results in a 402-bp wild-type-specific PCR product. The mutant-allele product was amplified by two neomycin-specific primers, 5′-CAACAGACAATCGGCTGCTCTGATGC-3′ and 5′-GATAGAAGGCGATGCGCTGCGAATCG-3′, and is 696 bp long.
TABLE 1.
Genotypes of offspring from NIPP1−/+ intercrosses
| Age | No. (%) with the following genotype:
|
No. (%) of resorp- tions | Total | Detection method | ||
|---|---|---|---|---|---|---|
| Wild type | Hetero- zygote | Homo- zygote | ||||
| E3.5 | 12 (23) | 29 (56) | 11 (21) | 52 | RT-PCR | |
| E6.5 | NDa | ND | 30 (19) | 4 (3) | 155 | Immunostaining |
| E7.5 | 15 (25) | 32 (54) | 1 (2) | 11 (19) | 59 | Nested PCR |
| E8.5 | 19 (44) | 12 (28) | 0 (0) | 12 (28) | 43 | PCR |
| E10.5 | 20 (33) | 22 (37) | 0 | 18 (30) | 60 | PCR + Southern blotting |
| Neonates | 95 (35) | 176 (65) | 0 | 271 | PCR + Southern blotting | |
ND, not determined. The total number of wild-type and heterozygote embryos at E6.5 was 121 (78%).
Reverse transcription-PCR (RT-PCR) was used to genotype E3.5 blastocysts. The flushed blastocysts were immediately frozen on dry ice and stored at −80°C until use. Blastocysts were lysed by three cycles of freezing and thawing. The first-strand cDNA was generated with an oligo(dT) primer according to the manufacturer's instructions (RevertAid First strand cDNA synthesis kit; Fermentas, St. Leon-Rot, Germany). For detection of wild-type cDNA, we performed the following nested PCR. The primary PCR was carried out with one-fifth of the RT product with the sense primer 5′-GAACTCCGGCTCCAGCCTCCCG-3′ and the antisense primer 5′-GATCTCGTCATCCTCACTGAAGG-3′ and resulted in a 612-bp PCR product. A 5-μl aliquot of this PCR mixture was used in a second PCR, which amplified a product of 350 bp by combination of the sense primer 5′-GATCTGTGTGACTTCACTATCGACC-3′ and the antisense primer 5′-GGCAGTGTTGAACTCTGTCAGG-3′. The mutant-allele product was amplified by a combination of the two neomycin-specific primers given in the preceding paragraph.
Histological analysis of embryos.
Deciduae were dissected free of uterine tissues, fixed overnight in freshly prepared 1% paraformaldehyde-phosphate-buffered saline (PBS), washed in PBS, dehydrated with increasing concentrations of ethanol, cleared in xylene, and embedded in paraffin. The paraffin-embedded blocks were then sectioned serially in a transverse or sagittal plane at a thickness of 7 μm. The first series of the equally spaced sections was stained with Harris hematoxylin and eosin (VWR, Leuven, Belgium).
Immunohistochemistry.
The TSA Biotin system of Perkin-Elmer (Wellesley, Mass.) was used for immunohistological staining. Paraffin sections were rehydrated (twice, for 5 min each time, in xylol; twice, for 3 min each time, in 100% ethanol; 3 min in 70% ethanol; 3 min in 50% ethanol; and 5 min in water), boiled in citric acid buffer (1.5 mM citric acid, 8.5 mM sodium citrate, 0.05% Dreft detergent) on a hot plate for 20 min, and cooled to room temperature. Sections were washed in TBST (20 mM Tris-HCl [pH 7.4], 0.15 M NaCl, 0.1% Triton X-100) for 5 min. Endogenous peroxidase was removed with 0.03% H2O2 in methanol for 20 min. Subsequently, the sections were washed in TBST three times, for 5 min each time, and then blocked in TNB blocking buffer (Perkin Elmer) with either 20% goat serum or 20% rabbit serum (DAKO, Glostrup, Denmark), according to whether a horseradish peroxidase (HRP)-conjugated goat anti-rabbit or rabbit anti-mouse antibody was used as the secondary antibody, respectively. The sections were incubated overnight in the primary antibody (1/50 in TNB), washed with TNT (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.05% Triton X-100) three times for 5 min each time, incubated at room temperature with the secondary antibody (1/100 in TNB) for 45 min, and washed with TNT three times for 5 min each time. The signal was amplified by incubating the sections for 8 min in biotinyl tyramide that had been diluted 50-fold in amplification diluent (Perkin Elmer), washed in TNT three times for 5 min each time, incubated with streptavidin-HRP (1/100) in TNB for 30 min, and finally washed in 50 mM Tris (pH 7.5) three times for 5 min each time. The signal was visualized by an HRP reaction using diaminobenzamidine (DAB; 1 mg/ml in 50 mM Tris-HCl [pH 7.5]) as a chromogen and hydrogen peroxide (0.01%) as a substrate. Sections were counterstained with Harris hematoxylin. Anti-Gata4 antibodies (sc9053), anti-Oct4 antibodies (sc5279), anti-HDAC2 antibodies (sc7899), and anti-Brachyury antibodies (sc17745) were obtained from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-Cdx2 antibodies were purchased from BioGenex (San Ramon, Calif.). Antibodies (M0744) against bromodeoxyuridine (BrdU) and HRP-conjugated goat anti-rabbit or rabbit anti-mouse secondary antibodies were obtained from DAKO. Anti-NIPP1 antibodies were raised against a synthetic peptide comprising residues 341 to 351 of NIPP1 (29).
In vitro culture of blastocysts.
Heterozygous NIPP1 males were intercrossed with superovulating heterozygous females. Blastocysts were flushed out of the uterus at day 3.5 of pregnancy in M2 medium (Sigma, St. Louis, Mo.) and cultured at 37°C and 5% CO2 on gelatin-coated chambered cover glasses (SanBio, Uden, The Netherlands) in TX-WES medium (Thromb-X, NV). Outgrowth morphology was recorded daily with a digital camera. NIPP1−/− blastocysts were identified by immunofluorescence after 5 days in culture by using NIPP1 antibodies.
BrdU labeling of embryos and blastocyst outgrowths.
BrdU (100 μg/g of body weight) was injected intraperitoneally into pregnant females at E6.5. The females were sacrificed 1 h after injection, uteri were removed, and decidual swellings were fixed overnight in freshly prepared 1% paraformaldehyde-PBS and processed for immunohistochemistry. BrdU (50 μM) was added to the culture medium of blastocysts that had been grown on gelatin-coated chambered cover glass for 4 days. Sixteen hours later, the cells were fixed in 2% paraformaldehyde-PBS, and immunofluorescence was performed.
RNAi.
Small interfering RNA molecules (siRNAs) for the knockdown of NIPP1 were made with the Dicer siRNA generation kit from Gene Therapy Systems, Inc. (San Diego, Calif.). To make double-stranded RNA (dsRNA) of NIPP1, the following PCR primers, with the first 20 bp derived from the T7 promoter, were used: 5′-GCGTAATACGACTCACTATAGGGAGAGGGTTCATGTTACTGCTGA-3′ and 5′-GCGTAATACGACTCACTATAGGGAGACCATGCTGCACTTGTCTACC-3′. The PCR product was transcribed in vitro with T7 RNA polymerase to form dsRNA by using the MEGAscript T7 kit (Ambion, Huntingdon, Cambridgeshire, United Kingdom). For transfection of one 24-well culture of HEK293 or U2OS cells, 1 μg of this dsRNA was cut in vitro with 1 U of recombinant Dicer enzyme. The lamin A/C siRNA duplex (5′-AACUGGACUUCCAGAAGAACA-3′) was bought from Dharmacon, Inc. (Lafayette, Colo.). The lamin dsRNA or the diced and purified NIPP1 dsRNA was transfected with the GeneSilencer siRNA transfection reagent according to the manufacturer's protocol (Gene Therapy Systems). For Western blot analysis, all cells were collected, washed twice with PBS, and subsequently lysed in a solution containing 50 mM Tris (pH 7.5), 0.3 M NaCl, 0.5% Triton X-100, 0.5 mM dithiothreitol, 0.5 mM phenylmethanesulfonyl fluoride, 0.5 mM benzamidine, and 5 μM leupeptin. After sonication, the lysates were cleared by centrifugation (for 10 min at 16,000 × g), and the protein concentration was measured to enable equal loading during sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Cell culture and immunofluorescence.
HEK293 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 2 mM l-glutamine. U2OS cells were grown in McCoy's 5A medium with l-glutamine, supplemented with 10% fetal calf serum, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. For immunofluorescence experiments, cells and blastocyst outgrowths were washed in PBS for 5 min, fixed in PBS-2% formaldehyde for 10 min, permeabilized in PBS-0.5% Triton X-100 for 10 min, and finally blocked in PBS containing 3% bovine serum albumin for 20 min. For BrdU staining, blastocyst outgrowths were permeabilized in PBS-0.5% Triton-0.1 M HCl. Primary and secondary antibodies (tetramethyl rhodamine isothiocyanate [TRITC]-conjugated anti-mouse immunoglobulin G; fluorescein isothiocyanate- and TRITC-conjugated anti-rabbit immunoglobulin G [DAKO]) were used at a final dilution of 1/200 and were left on the cells for 1 h. Each step was followed by three washes with PBS for 5 min. Microscopy was performed with a confocal microscope (Zeiss LSM 510).
TUNEL assay.
Terminal deoxynucleotidyltransferase (TDT)-mediated incorporation of dUTP into DNA ends was carried out on sectioned embryos by using the DeadEnd colorimetric apoptosis detection system (Promega, Leiden, The Netherlands). Briefly, deparaffinized and rehydrated sections adhering to glass slides were digested with proteinase K and postfixed with 4% paraformaldehyde in PBS. Incorporation of fluorescently labeled nucleotides by the TDT enzyme was carried out on slides at 37°C for 60 min. In each staining experiment, known TDT-mediated dUTP-biotin nick end labeling (TUNEL)-positive samples (DNase treated) were used as a positive control. As a negative control, the reaction was performed without TDT.
RESULTS
Targeted disruption of Ppp1r8.
To generate NIPP1−/+ ES cells by homologous recombination, we constructed a replacement-type targeting vector aimed at the swapping of a 6.6-kb fragment of Ppp1r8 for a neomycin cassette (Fig. 1A). The deleted Ppp1r8 fragment included a 3.2-kb fragment of the promoter region as well as exons 1 and 2. Following electroporation of the linearized targeting vector into R1 ES cells, eight NIPP1−/+ ES cell clones were obtained. The NIPP1−/+ ES cells were aggregated into Swiss mouse morulae, which were implanted into foster mothers. The resulting chimeric males were crossed with Swiss females, and the heterozygous offspring was identified by Southern blot analysis.
NIPP1−/+ mice did not show an overt phenotype for as long as 1 year (data not shown). Thus, the heterozygotes showed normal fertility and did not develop tumors in this period. Interestingly, immunoblot analysis did not show differences between NIPP1 expression levels in organs from wild-type versus NIPP1−/+ mice (data not shown), indicating that the loss of one NIPP1 allele does not affect the cellular NIPP1 concentration.
A deficiency of NIPP1 results in early embryonic lethality.
To examine the phenotype of NIPP1-null mice, NIPP1−/+ mice were intercrossed and their progeny was genotyped. PCR and Southern blot analysis of the neonates yielded no evidence of viable NIPP1−/− mice, while wild-type and heterozygous animals were obtained at the expected 1:2 ratio (Table 1; Fig. 1B). To examine at which stage during embryonic development the NIPP1−/− embryos died, we performed RT-PCR and immunohistochemical analyses of embryos from timed matings. Only 1 out of the 59 PCR-genotyped E7.5 embryos was homozygous, and no viable NIPP1−/− embryos were detected beyond 7.5 days (Table 1; Fig. 1C). However, we noted that 20 to 30% of the E7.5 to E10.5 embryos were resorbed (Table 1), which corresponds roughly to the number of expected NIPP1−/− embryos (25%). E3.5 blastocysts still showed a normal Mendelian distribution among NIPP1+/+ (23%), NIPP1−/+ (56%), and NIPP1−/− (21%) genotypes, as determined by RT-PCR analysis (Table 1; Fig. 1D). Also, immunostaining of sections from paraffin-embedded E6.5 embryos revealed that 19% of the embryos from the NIPP1−/+ intercrosses did not express NIPP1, while they showed a normal level of the unrelated nuclear histone deacetylase HDAC2 (Table 1; Fig. 1E). It should be noted that genotyping by immunofluorescence does not distinguish between NIPP1−/+ and NIPP1+/+ genotypes; therefore, embryos genotyped in this manner will be collectively referred to as wild type (NIPP1wt).
The data reported above indicated that NIPP1−/− embryos die between days 6.5 and 7.5 of development. Hematoxylin and eosin staining showed that E6.5 NIPP1−/− embryos were only about half the size of their wild-type E6.5 littermates (Fig. 2). The differences were apparent on both transverse and sagittal sections. In the wild-type embryos at E6.5, an embryonic axis could be observed (Fig. 2A), and the extraembryonic and embryonic ectoderms were organized into epithelia surrounding the proamniotic cavity (Fig. 2A to C). In the NIPP1-null embryos, however, the embryonic axis was hardly visible and the cells from the embryonic and extraembryonic ectoderms did not appear to be clearly organized into epithelial layers, accounting for the disorganized appearance of the NIPP1−/− embryos. In addition, the proamniotic cavity in the NIPP1-null embryos was reduced in size and sometimes was not even visible at all. Furthermore, an ectoplacental cone, which originates from the polar trophectoderm, could not be distinguished in NIPP1-null embryos (Fig. 2D to F).
FIG. 2.
Histological analysis of E6.5 NIPP1wt and NIPP1−/− littermates. The figure shows hematoxylin-and-eosin-stained sections of paraffin-embedded NIPP1wt (A to C) and NIPP1−/− (D to F) embryos at E6.5. NIPP1wt refers to either the NIPP1+/+ or the NIPP1−/+ genotype. Transverse sections at the extraembryonic (B and E) and embryonic (C and F) poles as well as sagittal sections (A and D) are shown. Arrowheads in panel A indicate the junction between the embryonic and extraembryonic regions. NIPP1−/− embryos are about half the size of their wild-type littermates. No ectoplacental cone can be distinguished. Ec, ectoplacental cone; ExEc, extraembryonic ectoderm, ExVE, extraembryonic visceral endoderm; EmEc, embryonic ectoderm; EmVE, embryonic visceral endoderm; R, Reichert membrane; PrC, proamniotic cavity. Bar in panel A, 50 μm.
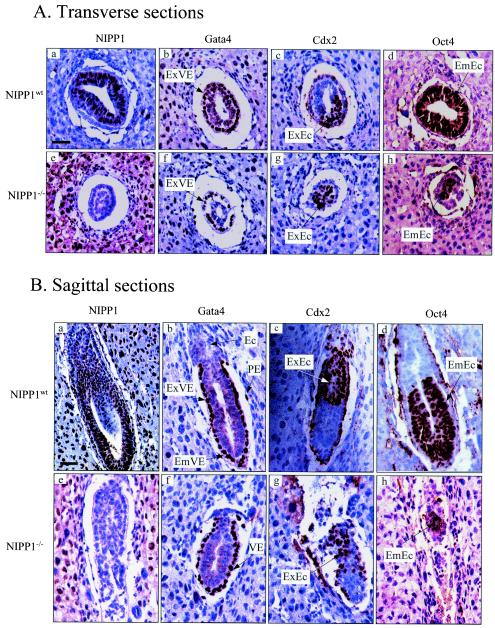
To examine in further detail the differentiation into distinct cell lineages, we performed immunostaining for specific markers of the parietal and visceral endoderm (Gata4), the extraembryonic ectoderm (Cdx2), and the embryonic ectoderm (Oct4). All three cell lineages were clearly present in the NIPP1−/− embryos (Fig. 3). While the visceral endoderm was well organized into an epithelial layer (Fig. 3Af and Bf), the cells from the extraembryonic (Fig. 3Ag and Bg) and embryonic (Fig. 3Ah and Bh) ectoderms appeared to be more scattered in the NIPP1-null embryos. Staining for the early-mesoderm marker Brachyury was positive in wild-type embryos but negative in NIPP1−/− embryos (data not shown).
FIG. 3.
Cell lineages in NIPP1wt and NIPP1−/− embryos. Transverse (A) and sagittal (B) sections of paraffin-embedded NIPP1wt and NIPP1−/− embryos (E6.5) were immunostained for NIPP1, Gata4, Cdx2, and Oct4 as indicated. Gata4 stains specifically the visceral and parietal endoderm, Cdx2 is a marker of the extraembryonic ectoderm, and Oct4 is specifically expressed in the embryonic ectoderm. All three cell lineages can be distinguished in the NIPP1−/− embryos, but the extraembryonic and the embryonic ectoderms are not organized into a clear epithelial layer. Abbreviations are as explained in the legend to Fig. 2. VE, visceral endoderm. Bars in panels Aa and Ba, 50 μm.
NIPP1-null embryos show a reduced rate of proliferation.
The data reported above demonstrated that NIPP1−/− blastocysts implanted normally and developed into a bilaminar egg cylinder, which, however, was severely reduced in size and had a disorganized ectoderm. NIPP1−/− embryos also failed to form an ectoplacental cone as well as a mesoderm. To further explore the cause of the early embryonic lethality of the NIPP1 knockouts, we investigated whether the NIPP1−/− embryos showed an increased level of apoptosis. A TUNEL assay on sections from E6.5 embryos showed similar levels of apoptosis in wild-type and knockout embryos (Fig. 4A). We then examined the effect of a lack of NIPP1 on cellular proliferation in vivo by measuring the incorporation of BrdU into DNA in the E6.5 embryos. A count of labeled and unlabeled nuclei revealed a labeling of only 45% of the cells in the NIPP1−/− embryos, compared to 63% for the NIPP1wt embryos. These data suggest a decreased rate of cell proliferation in NIPP1−/− embryos (Fig. 4B).
FIG. 4.
Apoptosis and proliferation rates in NIPP1wt and NIPP1−/− embryos. (A) Paraffin-embedded transverse sections of NIPP1wt (a and b) and NIPP1−/− (c and d) E6.5 embryos were stained with hematoxylin and eosin (a and c) and by the TUNEL assay (b and d) for the visualization of apoptosis. No difference in apoptosis between wild-type and knockout embryos was detected. Bar in panel Aa, 50 μm. (B) Pregnant mice were injected with BrdU 1 h before sacrifice. Sections of NIPP1wt and NIPP1−/− embryos (E6.5) were analyzed for BrdU incorporation by immunostaining with anti-BrdU antibodies. Stained nuclei of five NIPP1wt and six NIPP1−/− embryos were counted and expressed as a percentage of the total number of nuclei in the section. Nearly 20% lower BrdU incorporation was found in the mutant embryos, indicating a decreased rate of cell proliferation. Results are means ± standard errors (P = 0.0074 by an unpaired t test).
Delayed outgrowth of NIPP1-deficient blastocysts.
To acquire better insight into the nature of the retarded growth of NIPP1−/− embryos, we examined the growth capabilities of preimplantation embryos in vitro. Blastocysts were cultured in TX-WES medium to allow hatching from the zona pellucida, adherence to the gelatin-coated cover glasses, and expansion of the inner cell mass into a multilayer and of the trophectoderm into a trophoblast monolayer. Of the 42 blastocysts that were isolated from heterozygous intercrosses, 29 could be successfully cultured. Eight of these (27%) were identified by immunostaining as NIPP1−/− blastocysts. After 2 to 3 days of culture, all 29 embryos hatched from the zona pellucida and attached to the surface of the cover glass (Fig. 5A). After 3 days, however, the NIPPlwt blastocysts already showed clear outgrowths, while no outgrowth had been formed from the NIPP1−/− blastocysts (Fig. 5Ab and Ae). An outgrowth from the NIPP1−/− blastocysts was seen after a 5-day culture period (Fig. 5Af), but the number of cells in the inner cell mass, as detected by Hoechst staining, was clearly much lower than those in outgrowths from NIPP1wt blastocysts (Fig. 5Bc and Bf). In addition, the nuclei of trophoblast giant cells derived from NIPP1−/− blastocysts were smaller than those derived from wild-type blastocysts. Since trophoblast giant cells grow by repeated replication without intervening cell divisions (16), these data could indicate that NIPP1 is required for the G1/S transition or for replication (see Discussion).
FIG. 5.
Retarded outgrowth of NIPP1-deficient blastocysts. Blastocysts from intercrosses of heterozygotes were isolated, cultured, and genotyped as described in Materials and Methods. (A) Photographs of outgrowths of NIPP1wt (a to c) and NIPP1−/− (d to f) blastocysts following their culture in vitro for the indicated times. The inner cell mass (ICM) is surrounded by trophoblast giant cells (TG) after 3 to 5 days. After 3 days, the NIPP1−/− blastocysts had not yet formed an outgrowth. ZP, zona pellucida. (B) Following culture for 4 days, NIPP1wt and NIPP1−/− blastocyst outgrowths were incubated for 16 h with BrdU. BrdU incorporation was visualized by BrdU immunofluorescence (b and e). The figure also shows staining of blastocyst outgrowths for NIPP1 by immunofluorescence (a and d) and for DNA by Hoechst staining (e and f). NIPP1−/− blastocysts showed delayed outgrowth and severely reduced incorporation of BrdU. Ab, antibody. Bars, 20 μm.
We also incubated the 4-day-cultured blastocysts for 16 h in the presence of BrdU and subsequently immunostained the outgrowths with anti-BrdU antibodies to identify cells that had undergone DNA synthesis during the labeling period (Fig. 5Bb and Be). Compared to NIPP1wt blastocyst outgrowths, very few cells were labeled in NIPP1−/− blastocyt outgrowths, pointing to a considerably prolonged cell cycle for the latter condition.
Cell lines that lack NIPP1 are not viable.
To examine whether NIPP1 is also required for the proliferation of cultured cells, we first attempted to generate NIPP1−/− ES cells. A single clone was subjected to selection with an increased Geneticin concentration (2 mg/ml [23]), but among the 169 surviving ES subclones, not a single viable NIPP1−/− line was identified. As an alternative, we tried to derive ES cell lines from blastocysts of NIPP1−/+ intercrosses. Out of the 102 blastocysts that were processed for ES cell derivation, 42 cell lines were established. Genotyping by Southern blot analysis yielded 16 NIPP1+/+ (38%) and 26 NIPP1−/+ (62%) cell lines, but not a single cell line with a NIPP1−/− genotype (data not shown). Our failure to obtain NIPP1−/− ES clones suggests that NIPP1 is also essential for the viability of ES cells.
To further explore the requirement of NIPP1 for the proliferation of cultured cells, we examined the effect of the RNAi-mediated knockdown of NIPP1 in HEK293 cells (Fig. 6) and U2OS cells (data not shown). For that purpose, a 700-kb fragment of dsRNA derived from the NIPP1 transcript was fragmented in vitro with the Dicer enzyme and transfected into the cells. This resulted in the nearly complete disappearance of NIPP1 within 72 h, as detected by both immunoblotting (Fig. 6A) and immunofluorescence analysis (Fig. 6B). In contrast, this transfection did not affect the level of SIPP1 (Fig. 6A), an unrelated nuclear scaffold protein (22). Also, transfection of the cells with siRNAs for the knockdown of lamin A/C did not affect the level of NIPP1 (Fig. 6A). The RNAi-induced knockdown of NIPP1 was associated with a complete failure of the cells to proliferate, as evidenced by the low number of surviving cells (Fig. 6C).
FIG. 6.
Effects of the RNAi-mediated knockdown of NIPP1 on the proliferation of mammalian cells. (A) At 72 h after transfection of HEK293 cells with NIPP1-specific or lamin A/C-specific siRNAs or mock transfection (without siRNAs), the cells were lysed and processed for immunoblotting with anti-NIPP1 antibodies (upper panel) and with antibodies against SIPP1, a structurally unrelated nuclear scaffold protein (lower panel). (B) Levels of NIPP1 protein in mock-transfected cells (a) and in cells transfected with NIPP1-specific siRNAs (b), as determined by immunofluorescence analysis. Bar, 10 μm. (C) Number and morphology of HEK293 cells 72 h after mock transfection (a) or after transfection with NIPP1-specific siRNAs (b). The RNAi-induced knockdown of NIPP1 results in a lower number of cells, indicating slower growth. Bar, 30 μm.
DISCUSSION
NIPP1 is required for early embryonic development in the mouse.
To study the functional diversity of NIPP1 in vivo, we have targeted the NIPP1-encoding genes by homologous recombination. Surprisingly, the disruption of only a single NIPP1 allele did not affect the cellular NIPP1 concentration, which explains the lack of an overt phenotype in NIPP1−/+ mice. These findings fit in nicely with our previous observations that the cellular NIPP1 concentration is strictly controlled and may be subject to negative-feedback regulation. For example, transfection of mammalian cells with an expression vector for NIPP1 resulted in a huge accumulation of NIPP1 transcripts without a corresponding change in the concentration of NIPP1 protein; this could be accounted for by a translational repression mechanism involving the coding region of the NIPP1 transcript (17, 33). It is therefore possible that the concentrations of NIPP1 protein in heterozygote tissues are normal because of a lesser translational repression due to a lower basal level of NIPP1 transcripts. However, additional control mechanisms, e.g., at the level of transcription, cannot be excluded.
Disruption of both NIPP1 alleles was associated with early embryonic lethality. At E6.5, NIPP1−/− embryos were only about half the normal size (Fig. 2), and they showed a generally lower proliferation rate, which was not, however, associated with apoptosis (Fig. 4). Also, NIPP1−/− embryos failed to form an ectoplacental cone as well as a mesoderm, and their ectoderm cells did not organize into an epithelial layer (Fig. 2 and 3). By E7.5, nearly all NIPP1−/− embryos were resorbed (Table 1). Collectively, these data strongly indicate that NIPP1 is required for the rapid growth and differentiation phase of embryos around gastrulation. An intriguing question is why NIPP1−/− embryos survive until E6.5 (Table 1) while the knockdown of NIPP1 in cultured cells is associated with a complete block of proliferation within 3 days (Fig. 6). One possible explanation is that the need for NIPP1 increases with the speed of cell cycle progression. This could explain why NIPP1−/− embryos die in a period associated with an enhanced proliferation rate and why a loss of NIPP1 results in an immediate block of cell proliferation in the very rapidly dividing HEK293 or U2OS cells. In this respect, it is also striking that a lack of NIPP1 hampers the association of the rapidly dividing ectoderm cells into an epithelial layer but does not appear to affect the epithelial organization of the more slowly dividing endoderm cells (Fig. 3). Another possible explanation for the relatively late effect of a loss of NIPP1 on embryonic development is that the first days of embryonic development are maintained by maternally supplied NIPP1. In addition, it is possible that this pool of NIPP1 has a slower turnover than that in cultured cells. It is also interesting that the visceral endoderm, in contrast to the ectoderm, still forms an epithelial layer in NIPP1−/− embryos, which is in accordance with an apparently lower requirement for NIPP1 in the endoderm, as judged from the relatively low concentration of NIPP1 in the endoderms of wild-type embryos (Fig. 3Aa). Thus, because of their lower requirement for NIPP1, endodermal cells in NIPP1−/− embryos may thrive for a longer time than other cell lineages.
Role of NIPP1 in cell proliferation.
We have obtained various independent lines of evidence that link NIPP1 to cell proliferation. First, the lack of NIPP1 was associated with a decreased proliferation rate of cultured cells (Fig. 6) and of the inner cell mass of blastocyst outgrowths (Fig. 5B). Second, incorporation of BrdU in embryos (Fig. 4B) and blastocyst outgrowths (Fig. 5B) was severely impaired in the NIPP1−/− condition. Third, the nuclei of trophoblast giant cells in outgrowths of NIPP1−/− blastocysts remained relatively small (Fig. 5B). Since these cells endoreplicate (16), i.e., their nuclei increase in size by repeated cycles of replication without an intervening mitosis, these data suggest that a lack of NIPP1 is associated with a deficient G1/S transition and/or a hampered DNA replication.
We hypothesize that the contribution of NIPP1 to cell proliferation is somehow mediated by its protein ligands. For example, NIPP1 is a very potent inhibitor of PP1 (31), and it can be envisaged that the inhibition of PP1 may be required to enable a net phosphorylation of the retinoblastoma protein (pRb) by cyclin-dependent protein kinases, needed for the G1/S transition. In the absence of NIPP1, PP1 would not be restrained from dephosphorylating its established substrate, pRb (6, 10), thereby hampering entry into S phase. The NIPP1-mediated inhibition of the pool of PP1 that dephosphorylates pRb would add to the already established cell cycle-regulated mechanism of inhibition of PP1 by the phosphorylation of its C terminus (6, 10).
NIPP1 possibly also contributes to the G1/S transition by its ability to interfere with transcription and/or (alternative) splicing. The Polycomb protein EED has recently been identified as a novel ligand of NIPP1, and it has been found that NIPP1, like EED, functions as a transcriptional repressor in cultured cells (20). The transcriptional effects of EED have been largely explained by its ability to recruit the histone methyltransferase EZH2. Interestingly, the RNAi-mediated knockdown of either EED or EZH2 in cultured cells (9) results in the same phenotype that is associated with the knockdown of NIPP1 (Fig. 6). In addition, targeted disruption of the genes encoding EED (14, 15) and EZH2 (24) in mice is also associated with early embryonic lethality, albeit somewhat later than that seen with NIPP1−/− embryos. Finally, the expression of EED and EZH2 is controlled by the transcription factors E2F1 to E2F3 (9), which are negatively regulated by pRb, and the promoter of the NIPP1 gene also harbors E2F consensus binding sites (our unpublished observations). Collectively, these data suggest that NIPP1, EED, and EZH2 are all part of a regulatory pathway that promotes the G1/S transition in rapidly dividing cells.
Acknowledgments
This work was financially supported by the Fund for Scientific Research—Flanders (grant G.0374.01), by a Flemish Concerted Research Action, and by the Prime Minister's office.
Katleen Knaepen and Suzy Hermans provided expert technical assistance. We thank A. Zwijsen for medical advice on the dissection of embryos.
REFERENCES
- 1.Beullens, M., A. Van Eynde, M. Bollen, and W. Stalmans. 1993. Inactivation of nuclear inhibitory polypeptides of protein phosphatase-1 (NIPP-1) by protein kinase A. J. Biol. Chem. 268:13172-13177. [PubMed] [Google Scholar]
- 2.Beullens, M., A. Van Eynde, V. Vulsteke, J. Connor, S. Shenolikar, W. Stalmans, and M. Bollen. 1999. Molecular determinants of nuclear protein phosphatase-1 regulation by NIPP-1. J. Biol. Chem. 274:14053-14061. [DOI] [PubMed] [Google Scholar]
- 3.Beullens, M., A. Van Eynde, W. Stalmans, and M. Bollen. 1992. The isolation of novel inhibitory polypeptides of protein phosphatase 1 from bovine thymus nuclei. J. Biol. Chem. 267:16538-16544. [PubMed] [Google Scholar]
- 4.Beullens, M., and M. Bollen. 2002. The protein phosphatase-1 regulator NIPP1 is also a splicing factor involved in a late step of spliceosome assembly. J. Biol. Chem. 277:19855-19860. [DOI] [PubMed] [Google Scholar]
- 5.Beullens, M., V. Vulsteke, A. Van Eynde, I. Jagiello, W. Stalmans, and M. Bollen. 2000. The C-terminus of NIPP1 (nuclear inhibitor of protein phosphatase-1) contains a novel binding site for protein phosphatase-1 that is controlled by tyrosine phosphorylation and RNA binding. Biochem. J. 352:651-658. [PMC free article] [PubMed] [Google Scholar]
- 6.Bollen, M., and M. Beullens. 2002. Signaling by protein phosphatases in the nucleus. Trends Cell Biol. 12:138-145. [DOI] [PubMed] [Google Scholar]
- 7.Boudrez, A., M. Beullens, E. Waelkens, W. Stalmans, and M. Bollen. 2002. Phosphorylation-dependent interaction between the splicing factors SAP155 and NIPP1. J. Biol. Chem. 277:31834-31841. [DOI] [PubMed] [Google Scholar]
- 8.Boudrez, A., M. Beullens, P. Groenen, A. Van Eynde, V. Vulsteke, I. Jagiello, M. Murray, A. R. Krainer, W. Stalmans, and M. Bollen. 2000. NIPP1-mediated interaction of protein phosphatase-1 with CDC5L, a regulator of pre-mRNA splicing and mitotic entry. J. Biol. Chem. 275:25411-25417. [DOI] [PubMed] [Google Scholar]
- 9.Bracken, A. P., D. Pasini, M. Capra, E. Prosperini, E. Colli, and K. Helin. 2003. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 22:5323-5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ceulemans, H., and M. Bollen. 2004. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 84:1-39. [DOI] [PubMed] [Google Scholar]
- 11.Ceulemans, H., W. Stalmans, and M. Bollen. 2002. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays 24:371-381. [DOI] [PubMed] [Google Scholar]
- 12.Chang, A. C. Y., B. Sohlberg, L. Trinkle-Mulcahy, F. Claverie-Martin, P. Cohen, and S. N. Cohen. 1999. Alternative splicing regulates the production of ARD-1 endoribonuclease and NIPP-1, an inhibitor of protein phosphatase-1, as isoforms encoded by the same gene. Gene 240:45-55. [DOI] [PubMed] [Google Scholar]
- 13.Dewerchin, M., Z. Liang, L. Moons, P. Carmeliet, F. J. Castellino, D. Collen, and E. Rosen. 2000. Blood coagulation factor X deficiency causes partial embryonic lethality and fatal neonatal bleeding in mice. Thromb. Haemostas. 83:185-190. [PubMed] [Google Scholar]
- 14.Faust, C., A. Schumacher, B. Holdener, and T. Magnuson. 1995. The eed mutation disrupts anterior mesoderm production in mice. Development 121:273-285. [DOI] [PubMed] [Google Scholar]
- 15.Faust, C., K. A. Lawson, N. J. Schork, B. Thiel, and T. Magnuson. 1998. The Polycomb-group gene eed is required for normal morphogenetic movements during gastrulation in the mouse embryo. Development 125:4495-4506. [DOI] [PubMed] [Google Scholar]
- 16.Hattori, N., T. C. Davies, L. Anson-Cartwright, and J. C. Cross. 2000. Periodic expression of the cyclin-dependent kinase inhibitor p57Kip2 in trophoblast giant cells defines a G2-like gap phase of the endocycle. Mol. Biol. Cell 11:1037-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jagiello, I., A. Van Eynde, V. Vulsteke, M. Beullens, A. Boudrez, S. Keppens, W. Stalmans, and M. Bollen. 2000. Nuclear and subnuclear targeting sequences of the protein phosphatase-1 regulator NIPP1. J. Cell Sci. 113:3761-3764. [DOI] [PubMed] [Google Scholar]
- 18.Jagiello, I., M. Beullens, V. Vulsteke, S. Wera, B. Sohlberg, W. Stalmans, A. von Gabain, and M. Bollen. 1997. NIPP-1, a nuclear inhibitory subunit of protein phosphatase-1, has RNA-binding properties. J. Biol. Chem. 272:22067-22071. [DOI] [PubMed] [Google Scholar]
- 19.Jagiello, I., M. Beullens, W. Stalmans, and M. Bollen. 1995. Subunit structure and regulation of protein phosphatase-1 in rat liver nuclei. J. Biol. Chem. 270:17257-17263. [DOI] [PubMed] [Google Scholar]
- 20.Jin, Q., A. Van Eynde, M. Beullens, N. Roy, G. Thiel, W. Stalmans, and M. Bollen. 2003. The protein phosphatase-1 (PP1) regulator, nuclear inhibitor of PP1 (NIPP1), interacts with the polycomb group protein, embryonic ectoderm development (EED), and functions as a transcriptional repressor. J. Biol. Chem. 278:30677-30685. [DOI] [PubMed] [Google Scholar]
- 21.Jin, Q., M. Beullens, I. Jagiello, A. Van Eynde, V. Vulsteke, W. Stalmans, and M. Bollen. 1999. Mapping of the RNA-binding and endoribonuclease domains of NIPP1, a nuclear targeting subunit of protein phosphatase 1. Biochem. J. 342:13-19. [PMC free article] [PubMed] [Google Scholar]
- 22.Llorian, M., M. Beullens, I. Andrés, J.-M. Ortiz, and M. Bollen. 2004. SIPP1, a novel pre-mRNA splicing factor and interactor of protein phosphatase-1. Biochem. J. 378:229-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortensen, R. M., D. A. Conner, S. Chao, A. A. Geisterfer-Lowrance, and J. G. Seidman. 1992. Production of homozygous mutant ES cells with a single targeting construct. Mol. Cell. Biol. 12:2391-2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Carroll, D., S. Erhardt, M. Pagani, S. C. Barton, M. A. Surani, and T. Jenuwein. 2001. The Polycomb-group gene Ezh2 is required for early mouse development. Mol. Cell. Biol. 21:4330-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parker, L., S. Gross, M. Beullens, M. Bollen, D. Bennett, and L. Alphey. 2002. Functional interaction between nuclear inhibitor of protein phosphatase type 1 (NIPP1) and protein phosphatase type 1 (PP1) in Drosophila: consequences of over-expression of NIPP1 in flies and suppression by co-expression of PP1. Biochem. J. 368:789-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinkle-Mulcahy, L., P. Ajuh, A. Prescott, F. Claverie-Martin, S. Cohen, A. I. Lamond, and P. Cohen. 1999. Nuclear organisation of NIPP1, a regulatory subunit of protein phosphatase 1 that associates with pre-mRNA splicing factors. J. Cell Sci. 112:157-168. [DOI] [PubMed] [Google Scholar]
- 27.Van Eynde, A., E. Perez-Callejon, E. Schoenmakers, M. Jacquemin, W. Stalmans, and M. Bollen. 1999. Organization and alternate splice products of the gene encoding nuclear inhibitor of protein phosphatase-1 (NIPP-1). Eur. J. Biochem. 261:291-300. [DOI] [PubMed] [Google Scholar]
- 28.Van Eynde, A., M. Beullens, W. Stalmans, and M. Bollen. 1994. Full activation of a nuclear species of protein phosphatase-1 by phosphorylation with protein kinase A and casein kinase-2. Biochem. J. 297:447-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Eynde, A., S. Wera, M. Beullens, S. Torrekens, F. Van Leuven, W. Stalmans, and M. Bollen. 1995. Molecular cloning of NIPP-1, a nuclear inhibitor of protein phosphatase-1, reveals homology with polypeptides involved in RNA processing. J. Biol. Chem. 270:28068-28074. [DOI] [PubMed] [Google Scholar]
- 30.Vulsteke, V., M. Beullens, A. Boudrez, S. Keppens, A. Van Eynde, M. H. Rider, W. Stalmans, and M. Bollen. 2004. Inhibition of spliceosome assembly by the cell-cycle regulated protein kinase MELK and involvement of the splicing factor NIPP1. J. Biol. Chem. 279:8642-8647. [DOI] [PubMed] [Google Scholar]
- 31.Vulsteke, V., M. Beullens, E. Waelkens, W. Stalmans, and M. Bollen. 1997. Properties and phosphorylation sites of baculovirus-expressed nuclear inhibitor of protein phosphatase-1 (NIPP-1). J. Biol. Chem. 272:32972-32978. [DOI] [PubMed] [Google Scholar]
- 32.Wang, M., and S. N. Cohen. 1994. ard-1: a human gene that reverses the effects of temperature-sensitive and deletion mutations in the Escherichia coli rne gene and encodes an activity producing RNase E-like cleavages. Proc. Natl. Acad. Sci. USA 91:10591-10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wera, S., A. Van Eynde, W. Stalmans, and M. Bollen. 1997. Inhibition of translation by mRNA encoding NIPP-1, a nuclear inhibitor of protein phosphatase-1. Eur. J. Biochem. 247:411-415. [DOI] [PubMed] [Google Scholar]