Abstract
Objectives
Age-related decline of the five classical senses (vision, smell, hearing, touch, and taste) poses significant burdens on older adults. The co-occurrence of multiple sensory deficits in older adults is not well characterized and may reflect a common mechanism resulting in global sensory impairment.
Design, Setting, and Participants
The National Social Life, Health, and Aging Project, a representative, population-based study of community dwelling older US adults (57-85 years of age), collected biomarkers, social and health history, and other physiological measures, including sensory function.
Measurements
We estimated the frequency with which impairment co-occurred across the five senses as an integrated measure of sensory aging. We hypothesized that multisensory deficits would be common and reflect global sensory impairment which would largely explain the effects of age, gender, and race on sensory dysfunction.
Results
Two thirds (67%) of the older US population suffer from two or more sensory deficits, 27% from just one, and only 6% had none. Impairment of the sense of taste was the most common (74%); 70% had a poor sense of touch; 22% had poor sense of smell; 20% had impaired corrected vision; and 18% had poor corrected hearing. Older adults, men, African Americans, and Hispanics had greater multisensory impairment (all P<0.01). Global sensory impairment largely accounted for the effects of age, gender, and race on the likelihood of impairment of each of the five senses.
Conclusion
Multisensory impairment is prevalent in older US adults. These data support the concept of a common process that underlies sensory aging across the five senses. Clinicians assessing patients with a sensory deficit should consider further evaluation for additional cooccurring sensory deficits.
Keywords: sensory function, aging, hearing, vision, smell, taste, touch
INTRODUCTION
Aging has long been associated with decline in sensory function, a critical component of the health and quality of life of older people1. As an example, prior work has demonstrated that olfactory loss is associated with cognitive decline, highlighting its importance as an early warning sign of neurologic decline with its attendant morbidity and compromised physical function2–4, and that it strongly and independently predicts all-cause 5-year mortality2,5. These and other data are consistent with the idea that sensory function is a critical component of health and life itself.
Data from studies of single sensory deficits support this concept. For example, vision impairment is correlated with depression, poor quality of life, cognitive decline, and mortality6–8. Hearing loss is associated with slower gait speed9 (a marker of physical decline), poor cognition, and mortality10. Like smell, taste has been associated with nutritional compromise11 and in-patient mortality12, suggesting that chemosensory function is critical. Tactile discrimination declines with age13 due to the cumulative effects of decreased nerve conduction velocity14, decreased density of Meissner's and Pacinian corpuscles, and gray matter changes within the central nervous system, and is also associated with cognitive decline15.
Individual sensory impairments are common. The prevalence of hearing loss (33%) and vision impairment (18%) is high among older adults (age 70 and older)16,17. Similarly, deficits in smell (24%)18,19 and taste (up to ~61%) are widely prevalent in adults 70 and older20. Impairment of the sense of touch is noted in adults as young as 5521. These sensory losses have a major impact on how older adults live and function, often with profound consequences. As our population ages, these burdens will grow.
Despite these important consequences, little is known about the prevalence of deficits in multiple sensory systems, their combined impact, or common mechanisms that drive the underlying biology. While some studies have measured the prevalence of concurrent decreased vision and hearing22 (termed dual sensory impairment), to our knowledge none has measured other senses in a geriatric population. The importance of considering simultaneous impairments is clear from studies of vision and hearing, where dual losses interfere synergistically with independent function, presage cognitive decline and signal increased mortality23–25.
The close connection between these various sensory deficits, cognitive decline and even death itself suggest the possibility that global sensory decline, which we define as a common physiological process underlying deterioration of the classical senses, is an early indicator of neurodegeneration2,12,20,24, with attendant poor social and health outcomes26. Additionally, frequent associations with health outcomes across different senses may reflect common mechanisms underlying the effects of aging on these systems. These could include peripheral nerve dysfunction, changes in sensory integration at the central level27, decreased regenerative capacity28, or secondary metabolic effects (e.g., consequences of atherosclerosis or lipidemia)29. Finally, as dual sensory impairment has been shown to have worse effects on function compared to single deficits24, one would expect multisensory impairment (defined as impairment of more than two senses) to cause even more detrimental health effects. Despite this, to our knowledge, no study has examined multiple sensory impairments in a national population sample.
To address multisensory impairment, we analyzed data from the National Social Life, Health, and Aging Project (NSHAP), a longitudinal population-based study of adults ages 57-85, that collected extensive health and social measures through in-home interviews and respondent-administered questionnaires30. Sensory function was assessed in all five classical senses31. In addition, respondents were asked about their physical and mental health, medication use, cognition, and health behaviors. The NSHAP project and secondary analyses of these data have provided insights into a number of aspects of aging32–34. NSHAP offers a unique opportunity to examine sensory function broadly.
We estimated the prevalence of multisensory impairment and developed a model of global sensory impairment based on the interrelationships among measures of all five senses. We hypothesized that multisensory deficits are common among older adults, more prevalent in men and minorities, and occur more often with increasing age. Furthermore, we introduce the concept of global sensory impairment, a process that we hypothesize largely accounts for the effects of age, gender, and race on the likelihood of impairment in each of the five senses.
METHODS
NSHAP Study Design
Respondents
In 2005-6, interviewers from the National Opinion Research Center (NORC) conducted in-home interviews with 3,005 community-dwelling older adults (1,454 men and 1,551 women), identified using a probability sample of the population of community-dwelling adults born between 1920–47 (aged 57–85 years) in the United States (see 35,36). The Institutional Review Boards of NORC and The University of Chicago approved this study and all respondents provided written, informed consent.
NSHAP used a modular study design. All respondents were administered a core interview and provided a standard set of biomeasures in their homes, including olfaction and gustation testing and hearing assessment. In addition, half the respondents were randomized to receive both vision and touch testing31. The analytic sample includes the 2,968 respondents who had data on two or more of the five senses (Table 1). Of the 1,506 respondents eligible to receive all sensory modules, 1,301 (86%) individuals had complete data31. Race and Hispanic ethnicity were measured using the standard National Institutes of Health items as reported previously37, and respondents were coded as white (non-Hispanic), black or African-American, Hispanic (excluding those who self-identified as black or African-American) and other. Respondents were asked whether they had received a physician's diagnosis of diabetes, stroke, heart failure, hypertension or myocardial infarction, and were also asked to rate their overall physical health using the standard categories “Excellent,” “Very good,” “Good,” “Fair” or “Poor.” Data missing for one or more sensory measures due to the study design is, by definition, Missing Completely At Random (MCAR), and therefore does not introduce any bias into our analysis (only a loss of precision, relative to a design in which all respondents were administered all items). Although there was some item non-response due to respondent refusal (or responses of “Don't know”) for each sensory dysfunction item, this non-response was in general low, thereby limiting the magnitude of any potential bias.
Table 1.
Demographic and health characteristics of the US population of home-dwelling older adults based on the National Social Life, Health and Aging Project (2005-6)
| Percent1 |
|
|---|---|
| Age (yrs) (n=2,968) | |
| 57-64 | 41.4 |
| 65-74 | 34.9 |
| 75-85 | 23.7 |
| Gender (% men) (n=2,968) | 48.6 |
| Race/ethnicity (n=2,956) | |
| White | 80.7 |
| African-American (AA) | 9.9 |
| Hispanic (non-AA) | 6.9 |
| Other | 2.5 |
| Self-rated physical health (n=2,957) | |
| Poor | 6.8 |
| Fair | 17.9 |
| Good | 29.6 |
| Very good | 32.6 |
| Excellent | 13.1 |
| Comorbid diseases (n=2,968) | |
| Hypertension | 53.9 |
| Diabetes | 19.9 |
| Heart attack | 11.7 |
| Heart failure | 8.3 |
| Stroke | 8.2 |
| Sensory function (good/fair/poor) | |
| Hearing (n=2,968) | 82.0/12.8/5.3 |
| Vision2 (n=1,417) | 80.3/13.6/6.1 |
| Smell (n=2,939) | 77.8/18.8/3.5 |
| Touch2 (n=1,464) | 30.4/37.7/31.8 |
| Taste (n=2,735) | 26.0/25.8/48.2 |
| Number of impairments3 (n=1,301) | |
| 0 | 5.9 |
| 1 | 27.6 |
| 2 | 38.1 |
| 3 | 20.3 |
| 4 | 6.8 |
| 5 | 1.3 |
Estimates are weighted using the sample weights distributed with the dataset to yield population estimates of prevalence.
Measures were only administered to a randomly selected 50% of respondents.
An impairment was defined as fair or poor function; 1,301 respondents had data on all five senses.
Sensory Function
Vision
Participants wore their usual glasses or contact lenses, and corrected distance visual acuity was assessed under home lighting conditions via a Snellen chart test utilizing a standardized protocol31. Corrected vision was chosen to determine the actual functional level experienced by the respondent in daily life, consistent with prior benchmark studies38. Categories for visual acuity corresponded to those required for a driver's license: ‘good’ defined as 20/40 or better, ‘fair’ defined as between 20/50 or 20/63, and ‘poor’ defined as worse than 20/63.
Touch
Tactile sensitivity was assessed using a 2-point discrimination test on the index finger of the dominant hand while eyes were closed31. Three 2-point tests were conducted at inter-point distances of 12 mm, 8 mm, 4 mm as well as a single point following the 12 mm test. A 4 mm threshold (‘good’) was defined as correctly identifying two points at all three distances plus the single point test; an 8 mm threshold (‘fair’) was correctly identifying two points at 12 mm and 8 mm but not 4 mm and the single point test and a 12 mm threshold (‘poor’) was correctly identifying 2-points only at 12 mm and the single point test. All other response patterns were considered non-discriminating and also categorized as ‘poor’.
Smell
Olfaction was evaluated through a validated 5-item odor identification test using felt tip pens39 as previously described37. A single odorant was presented and respondents were instructed to select one of four word/picture choices with refusals coded as incorrect. Four or five errors were considered ‘poor’ (anosmic), two or three errors ‘fair’ (hyposmic) and one or no errors ‘good’ (normosmic).
Taste
Gustation was evaluated using 4 filter paper strips31, which were applied to the tongue in the following order: sour, bitter, sweet and salty with a sip of water between each application31. Respondents were asked to describe the taste using the same four descriptors. Responses of ‘tried unable to do,’ ‘refused’ or ‘don't know’ were counted as incorrect. Two to four errors were categorized as ‘poor’ (ageusic), one error ‘fair’ (hypogeusic), and zero errors ‘good’ (normogeusic).
Hearing
Respondents’ conversational hearing during the interview was assessed afterward by the field interviewer using a 5-point scale (1=practically deaf, 5=normal hearing) 31. Scores of 4 or 5 were categorized as ‘good’, 3 as ‘fair’, and 1 or 2 as ‘poor’. Respondents who chose to wear hearing aids during the interview were permitted to do so, but not required. Additional psychophysical measures of hearing (e.g., audiometry) were precluded by the time and resource constraints of the omnibus survey.
Statistical Analysis
Estimates of the prevalence of impairment (defined as having fair or poor function) for each sense and of the distribution of the total number of impairments among the U.S. national population of home-dwelling older adults (ages 57-85) were obtained by using the sampling weights provided with the dataset to account for differential probabilities of selection and non-response as previously described36. In addition, estimates of the population prevalence of several comorbid diseases and of the distribution of self-rated physical health and the demographic variables age, gender and race/ethnicity are also presented.
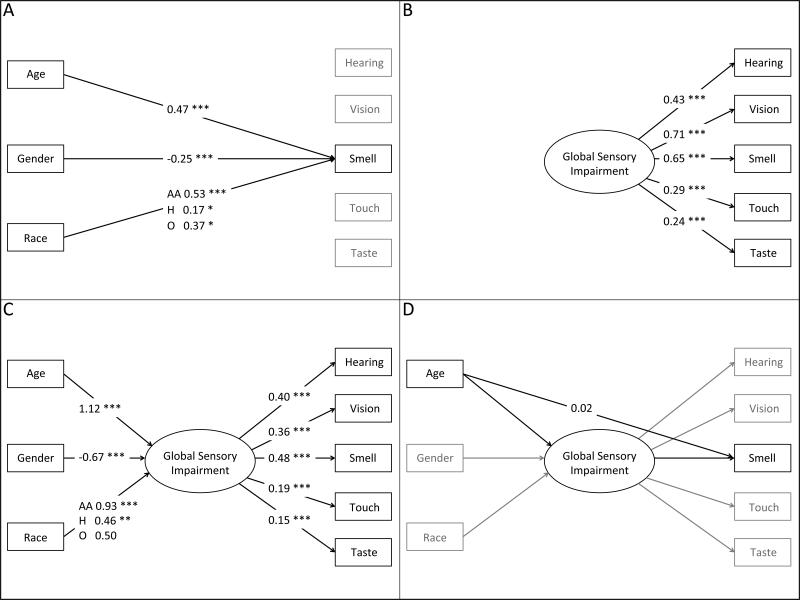
For each of the five senses individually, ordinal probit regression was used to model the relationship between sensory dysfunction (good/fair/poor) and age, gender and race/ethnicity (Figure 1A). Ordinal probit regression is a straightforward extension of the probit regression model, with the (standard) Normal distribution presumed to underlie the response being split according to multiple cutpoints (one fewer than the number of observed categories of the outcome) instead of just one. Thus, the coefficients for the covariates have an identical interpretation to those from probit regression—namely, as the change in standard units of the underlying Normal variable associated with a one unit change in the covariate. A generalized single factor measurement model (Figure 2B) was then fit to the five observed sensory dysfunction measures, assuming a single latent variable (with variance equal to one) capturing global sensory impairment which predicts each of the five sensory dysfunction measures via an ordinal probit regression40. For each dysfunction measure, the proportion of variance in its underlying distribution (as specified by the ordinal probit regression model) explained by global sensory impairment was calculated. This model was then expanded by specifying global sensory impairment to be a function of age (in decades), gender and race/ethnicity (white, black or African-American, Hispanic (non-black) and other) (Figure 2C). Finally, this Structural Equation Model (SEM) was augmented by adding direct effects of the demographic covariates on each of the sensory dysfunction measures, one at a time (Figure 2D). Indirect effects of age on each dysfunction (through its effect on global sensory impairment) were calculated and compared to the direct effects of age41. For all analyses, a two-sided p ≤ 0.05 was considered statistically significant. All analyses were performed with Stata (Release 13.1, StataCorp LP, College Station, Texas, USA).
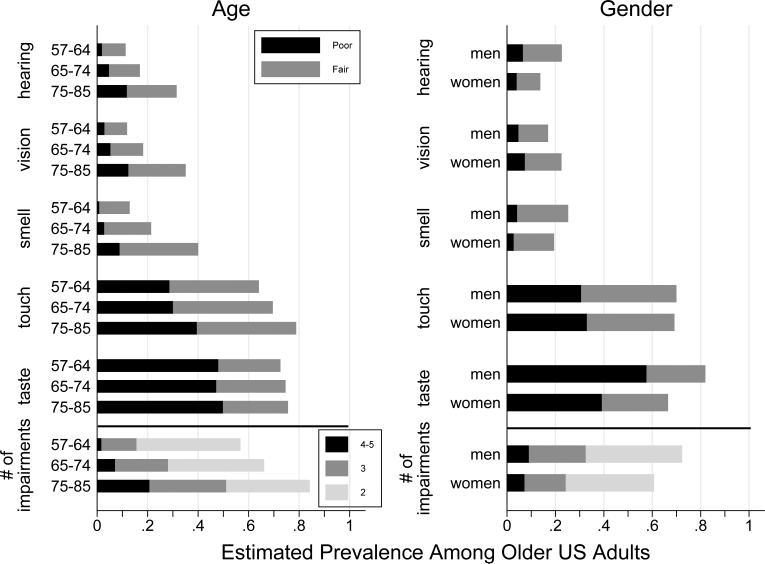
Figure 1.
Prevalence of sensory impairments for each of the five senses among community-dwelling older adults in the US, by age group and gender.
Figure 2.
Visual representation of the analytic models: (A) Overall effects of age, gender and race/ethnicity on each sensory dysfunction without global sensory impairment, using smell as an example (Table 2A); (B) Effects of global sensory impairment on each of the five sensory dysfunction measures (Table 3, Single Factor Model); (C) Effects of age, gender and race/ethnicity on global sensory impairment, and through global sensory impairment on each sensory dysfunction measure (Table 3, Structural Equation Model); (D) Direct effects of demographic variables on sensory dysfunction controlling for global sensory impairment, using the effect of age on smell as an example (Table 2B).
RESULTS
Prevalence of Individual Sensory Impairments
Overall
Taste impairment was the most prevalent sensory deficit, with 74% of respondents having an impaired sense of taste (26% fair/48% poor) (Table 1). Also prevalent was touch impairment, estimated to be fair in 38% of older adults and poor in 32%. Fourteen percent had fair corrected distance vision (20/50 or 20/63) while another 6% experienced poor corrected distance vision (20/80 or worse). The prevalence of fair and poor sense of smell was 19% and 3% respectively. Thirteen percent had fair corrected hearing and 5% had poor corrected hearing.
Association with age, gender, and race/ethnicity
Older people had worse function for all five senses, with the largest differences occurring for hearing, vision and smell (Table 2A; Figure 1). Men also had worse function for hearing, smell and taste, though demonstrated better corrected vision than women. African Americans and Hispanics had worse sensory function than whites on all measures except for hearing where there was no evidence of racial/ethnic differences and for taste where although African Americans still had worse function than whites, Hispanics had better function (Supplementary Figure 1).
Table 2.
Estimated effects of age, gender and race/ethnicity on individual sensory dysfunctions, both unadjusted and adjusting for global sensory impairment
| Sensory dysfunction | |||||
|---|---|---|---|---|---|
| Hearing | Vision | Smell | Touch | Taste | |
| A. Separate regression models showing the associations between demographics and sensory dysfunction1 | |||||
| Age (per decade) | 0.48*** | 0.41*** | 0.47*** | 0.21*** | 0.09** |
| Women (vs. men) | −0.39*** | 0.25** | −0.25*** | 0.04 | −0.44*** |
| Race/ethnicity (vs. white) | |||||
| African-American (AA) | −0.01 | 0.47*** | 0.53*** | 0.36*** | 0.16** |
| Hispanic (non-AA) | 0.06 | 0.39** | 0.17* | 0.65*** | −0.16* |
| Other | 0.08 | 0.01 | 0.37* | −0.03 | 0.01 |
| B. Associations between demographics and sensory dysfunction, holding constant global sensory impairment2 | |||||
| Age (per decade) | 0.28*** | −0.48 | 0.02 | 0.02 | −0.21* |
| Women (vs. men) | −0.30*** | 1.08** | 0.06 | 0.15 | −0.32*** |
| Race/ethnicity (vs. white) | |||||
| African-American (AA) | −0.28* | −0.18 | 0.33** | 0.22* | −0.09 |
| Hispanic (non-AA) | −0.07 | 0.13 | −0.02 | 0.60*** | −0.33** |
| Other | −0.04 | −0.42 | −0.04 | −0.11 | −0.13 |
Ordinal probit regression models, fit individually to each of the 5 sensory dysfunctions. Coefficients for each covariate indicate the change in the likelihood (on the probit scale) of being above a given cut point associated with a one unit increase in the covariate.
Direct effects of age, gender and race/ethnicity on each of the 5 sensory dysfunctions, adjusting for global sensory impairment. Obtained by adding direct effects to the Structural Equation Model in Table 3, separately for each dysfunction.
P<0.05,
P<0.01,
P<0.001
Prevalence of Co-occurring Sensory Impairments
Sensory deficits were widely prevalent in older US adults, with 94% demonstrating at least one sensory deficit (Table 1). Two-thirds (67%) of older adults had two or more sensory deficits, with two impairments being the most common (38%) (Figure 1). These deficits were correlated; for example, 34% had 0–1 and 8% had 4–5, as compared to the 28% and 6%, respectively, that would be expected under the null hypothesis of independence among the senses. Nearly two-thirds of adults (65%) experienced substantial impairment (i.e., poor functioning) in at least one sense, and 22% suffered from substantial impairment of two or more senses.
Global Sensory Impairment
Each of the sensory outcomes was associated with a single common factor (Table 3, Single Factor Model; Figure 2B), with the strongest associations for vision and smell, followed by hearing. This factor explains a significant proportion of the variation in the underlying distributions of the individual sensory deficits: 0.15 (hearing), 0.33 (vision), 0.30 (smell), 0.08 (touch) and 0.05 (taste). The effects of a +/− one SD change in this factor on the actual probability of each deficit is illustrated in Supplementary Figure 2.
Table 3.
Impact of global sensory impairment on the likelihood of individual sensory dysfunctions, and its association with age, gender and race/ethnicity.
| Single Factor Model | Structural Equation Model | |||
|---|---|---|---|---|
| Coefficient | P value | Coefficient | P value | |
| Sensory Dysfunction1 | ||||
| Hearing dysfunction | 0.43 | <0.001 | 0.40 | <0.001 |
| Vision dysfunction | 0.71 | <0.001 | 0.36 | <0.001 |
| Smell dysfunction | 0.65 | <0.001 | 0.48 | <0.001 |
| Touch dysfunction | 0.29 | <0.001 | 0.19 | <0.001 |
| Taste dysfunction | 0.24 | <0.001 | 0.15 | <0.001 |
| Demographics2 | ||||
| Age (per decade) | 1.12 | <0.001 | ||
| Women (vs. men) | −0.67 | <0.001 | ||
| Race/ethnicity (vs. white) | ||||
| African-American (AA) | 0.93 | <0.001 | ||
| Hispanic (non-AA) | 0.46 | 0.002 | ||
| Other | 0.50 | 0.066 | ||
Coefficients from ordinal probit regressions of each three-category sensory dysfunction measure on the underlying factor (global sensory impairment), each indicating the change in the likelihood (on the probit scale) of being above a given cut point associated with a one standard deviation increase in the underlying factor.
Coefficients indicate the change in the underlying factor associated with a one-unit change in the demographic covariate (residual variance of the underlying factor is constrained to equal one).
Global sensory impairment (i.e., the common factor) was strongly associated with age, gender and race/ethnicity (Table 3, Structural Equation Model; Figure 2C). Consistent with the individual results for each sense reported above, older age was associated with an increase in global sensory impairment, which was also higher for men than for women and among African Americans and Hispanics relative to whites.
To test the hypothesis that global sensory impairment accounts for much of the association between age and individual sensory deficits, as well as to examine the fit of the model, we added direct effects of the demographic covariates on each of the sensory deficits one at a time to the structural equation model (Table 2B; Figure 2D). For vision, smell and touch, most (if not all) of the association with age is explained by the effect of age on global sensory impairment, as judged by the fact that the direct effects of age on these senses were not statistically significant. Only for hearing and taste were the direct effects of age significant. For hearing, the direct effect of age was 0.28 (slightly larger than the indirect effect through global sensory impairment of 0.22), while for taste, the estimated negative direct effect of −0.21 reflects the fact that the association between age and taste dysfunction is the weakest of all the senses (as noted above). Thus, we find that global sensory impairment explains most of the association between age and the individual sensory dysfunction outcomes.
DISCUSSION
Multisensory loss is remarkably common among older US adults and seems to be driven by a common underlying process. To our knowledge, this population-based study is the first to examine the full spectrum of sensory loss across the five classical senses in a representative sample of older adults, and emphasizes the broad and prevalent sensory burden faced by this growing segment of the population.
Prior studies have established that 6% of older adults have impaired vision and hearing22. Our results suggest that these same adults may also have additional sensory impairments. Across all five senses, we find that 38% of older adults have two impairments and 28% have three or more. Twenty-two percent have a substantial impairment (i.e., poor function) in two or more sensory modalities, representing a significant burden. Other recent studies of multiple sensory impairments to date support our findings here, and suggest important associations with function and quality of life with carefully measured sensory measures42–44. We note that these studies did not focus exclusively on older adults, address representative populations, include touch, or, in one case44, utilize objective measures. These and other factors may explain variability in findings among studies.
A significant amount of the variation in each of the sensory dysfunctions may be explained by a single underlying factor, which we interpret as global sensory impairment. This single factor accounts for much of the association between age and each of the sensory impairments, suggesting a common process of sensory aging. There are several possible mechanisms, shared across the senses, which could link their deficits during aging: neurodegeneration20,45, secondary effects of common environmental insults29,46, underlying genetics such as variation in genes involved in nerve maintenance or innate immunity47, coordinate cellular senescence, or combinations thereof.
The concept of global sensory impairment also leads to new ways of thinking about how other factors such as gender and race/ethnicity may affect sensory function through this common mechanism. Many studies have found associations between gender and race/ethnicity and individual sensory impairment22,37 and have proposed mechanisms to explain these. Our results here differ in that they investigate the relationship between these factors across all five senses. For example, other than for corrected vision, women seem to be protected from sensory loss compared to men, highlighting the prospect of a biologic mechanism. The higher prevalence and severity of multisensory impairment among African Americans is especially troubling given the well-documented disparities in access, treatment, and outcomes faced by African Americans.
There are important clinical implications to these data. Clinicians who see patients with single or dual sensory deficits (e.g., with hearing or vision loss or both) should consider evaluation of the other senses, as it is highly likely that such patients will have these undiagnosed conditions. Patients with multisensory impairment may be at higher risk for important sequelae such as neurodegeneration, complications from falls, burns, food poisoning, smoke inhalation, and others. If these other conditions are identified, even in the face of limited treatment options, mitigation through awareness, social intervention via family or caregiver support, or other means may be instituted. This burden of multisensory impairment may impact patients’ ability to cope with social, cognitive, and physical stresses, so attention to these issues is critical.
There are several limitations to our findings. We found high rates of multisensory impairment in the general population of older adults living at home, however individuals in a clinic or institutionalized setting may be at even higher risk. Conversely, one recent study that included objective measures of sensory function showed minimal multisensory impairment in adults younger than 45 years of age43. Although we measured corrected vision and hearing, we still found deficits in the home environment, which should prompt clinicians to be sensitive to the discrepancy between clinic and home-based assessment of sensory function and the consequences for patient care. For example, clinic-based estimates of sensory function, under optimal controlled conditions, may minimize the impact on daily life since they do not account for “real world” experience of the patients in their own home environments.
Additionally, this model does not explain everything we observe in the data and there are some important deviations, which are consistent with prior findings in the literature. For example, gender reliably predicted sensory dysfunction, with men being worse than women in all senses except for corrected vision where women were worse. This difference may reflect a gender disparity in obtaining or using adequate corrective lenses. Women may also have more rapid rates of decline in vision than men, perhaps indicating an underlying susceptibility to age-related vision loss. This is troubling given previous work demonstrating that in older women, vision impairment is a risk factor for cognitive decline23. Medicare fails to cover eyeglasses or contact lenses, so this issue may also reflect lack of financial resources among women.
Finally, our integrated measure of hearing was based on the interviewer's assessment during social conversation in the home. Subsequent work could expand on this study by including audiometry as an objective measure of hearing (e.g., at speech and other frequencies), although its inclusion is challenging (but not impossible, for screening at least26) in large field studies without specialized personnel and equipment (audiometer, sound booth, etc.). We view our measure of the ability to hear conversational speech in the home as a major strength of NSHAP in that the focus is on their typical environment and social context as experienced by older adults in their everyday lives, in contrast to clinic or hospital based assessments. From this perspective, we are likely underestimating the burden of hearing loss in this population. Similarly, our measures of sensory function could be enriched (e.g. the addition of near vision, olfactory sensitivity, more precise measures, etc.). However, we do not believe such enriched measures would alter our main results.
What are the implications of these findings? Because of the critical nature of these sensory modalities in daily living, our results of multisensory loss may explain, in part, why older adults report decreased quality of life and challenges in interacting with the environment and other people16. Our data also prompt further questions about the relationship between global sensory impairment and physical frailty, the concept of decline across multiple domains of physical performance, potentially including shared physiological mechanisms38. Are they independent processes, or does global sensory loss develop simultaneously with physical frailty, worsening its impact (e.g., weight loss, falls, and decreased physical activity) and even increasing the risk of mortality? Given that older adults face major changes in sensory perception, creating deleterious effects on both health and function, future work should characterize global sensory impairment and examine the trajectory of its decline in longitudinal studies. Current measures of physical frailty have proven important in the management of geriatric health care needs and an analogous consideration of multisensory impairment may prove useful.
In summary, further examination of multisensory loss in older adults will provide answers to these and other gaps in our knowledge and allow us to begin to design preventative measures or identify therapeutic targets in the underlying biology that is common to these five sensory modalities, with great promise for alleviating the suffering that they cause.
Supplementary Material
ACKNOWLEDGMENTS
Some of these data were presented at the Association of Chemoreception Sciences annual meeting in 2014.
We thank Linda J. Waite, Ph.D., Elbert Huang, M.D., M.P.H., Robert M. Naclerio, M.D., Fuad M. Baroody, M.D., and members of the Geriatric Assessment Group and Olfactory Research Group within NSHAP for useful comments provided freely. We gratefully acknowledge the participation of the NSHAP respondents.
Funding: This work was supported by the National Institute on Aging (R37 AG030481; AG036762; AG029795; K23 AG036762), the National Institute of Allergy and Infectious Disease (Chronic Rhinosinusitis Integrative Studies Program [CRISP], U01AI106683), the Institute for Translational Medicine at The University of Chicago (KL2RR025000), the McHugh Otolaryngology Research Fund, and the American Geriatrics Society. DWK was supported by a Mellon Foundation Social Sciences Dissertation-Year Fellowship.
Sponsor's Role
The funders had no role in the design, methods, subject recruitment, data collections, analysis and preparation of this paper.
Footnotes
Author Contributions
Camil Correia analyzed the data, made important intellectual contributions, and drafted the manuscript. Kevin J. Lopez collected and analyzed the data, and made important intellectual contributions. Kristen E. Wroblewski performed all statistical analyses, edited the paper, provided important intellectual content including data interpretation, and drafted the figures and tables. Megan Huisingh-Scheetz contribute to the study design and made important intellectual contributions, and edited the paper. David W. Kern made important intellectual contributions, provided data interpretation, and edited the paper. Rachel C. Chen made important intellectual contributions. L. Philip Schumm assisted with analyses and provided intellectual input, including editing of the manuscript. William Dale provided important intellectual input and edited the manuscript. Martha K. McClintock designed and oversaw collection of the NSHAP biomeasures and acquired the data; she provided important intellectual contributions including study design, data analysis, and edited the manuscript. Jayant M. Pinto conceived of and supervised the study, developed the analysis strategy, interpreted the data, and edited the manuscript. JMP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors declare no conflicts of interest.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
REFERENCES
- 1.Fischer ME, Cruickshanks KJ, Klein BEK, et al. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009;16:346–353. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto JM, Wroblewski KE, Kern DW, et al. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS One. 2014;9:e107541. doi: 10.1371/journal.pone.0107541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Michaels-Marston KS, Liu X, et al. Olfactory deficits in patients with mild cognitive impairment predict Alzheimer's disease at follow-up. Am J Psychiatry. 2000;157:1399–1405. doi: 10.1176/appi.ajp.157.9.1399. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Schneider JA, Arnold SE, et al. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RS, Yu L, Bennett DA. Odor identification and mortality in old age. Chem Senses. 2011;36:63–67. doi: 10.1093/chemse/bjq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JJ, Mitchell P, Simpson JM, et al. Visual impairment, age-related cataract, and mortality. Arch Ophthalmol. 2001;119:1186–1190. doi: 10.1001/archopht.119.8.1186. [DOI] [PubMed] [Google Scholar]
- 7.Rovner BW, Ganguli M. Depression and disability associated with impaired vision: The MoVies Project. J Am Geriatr Soc. 1998;46:617–619. doi: 10.1111/j.1532-5415.1998.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 8.Harrabi H, Kergoat M-J, Rousseau J, et al. Age-related eye disease and cognitive function. Invest Ophthalmol Vis Sci. 2015;56:1217–1221. doi: 10.1167/iovs.14-15370. [DOI] [PubMed] [Google Scholar]
- 9.Li L, Simonsick EM, Ferrucci L, et al. Hearing loss and gait speed among older adults in the United States. Gait Posture. 2013;38:25–29. doi: 10.1016/j.gaitpost.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genther DJ, Betz J, Pratt S, et al. Association of hearing impairment and mortality in older adults. J Gerontol Ser A Biol Sci Med Sci. 2014;70:85–90. doi: 10.1093/gerona/glu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schiffman SS, Graham BG. Taste and smell perception affect appetite and immunity in the elderly. Eur J Clin Nutr. 2000;54:S54–S63. doi: 10.1038/sj.ejcn.1601026. [DOI] [PubMed] [Google Scholar]
- 12.Solemdal K, Møinichen-Berstad C, Mowe M, et al. Impaired taste and increased mortality in acutely hospitalized older people. Chem Senses. 2014;39:263–269. doi: 10.1093/chemse/bjt116. [DOI] [PubMed] [Google Scholar]
- 13.Decorps J, Saumet JL, Sommer P, et al. Effect of ageing on tactile transduction processes. Ageing Res Rev. 2014;13:90–99. doi: 10.1016/j.arr.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Besné I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol. 2002;138:1445–1450. doi: 10.1001/archderm.138.11.1445. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Ogasa T, Ohta Y, et al. Decline of human tactile angle discrimination in patients with mild cognitive impairment and Alzheimer's disease. J Alzheimer's Dis. 2010;22:225–234. doi: 10.3233/JAD-2010-100723. [DOI] [PubMed] [Google Scholar]
- 16.Campbell VA, Crews JE, Moriarty DG, et al. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults in the United States, 1993-1997. MMWR CDC Surveill Summ. 1999;48:131–156. [PubMed] [Google Scholar]
- 17.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. Am J Public Health. 2004;94:823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern DW, Wroblewski KE, Schumm LP, et al. Field survey measures of olfaction: The Olfactory Function Field Exam (OFFE). Field Methods. 2014;26:421–434. doi: 10.1177/1525822X14547499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy C, Schubert CR, Cruickshanks KJ, et al. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- 20.Welge-Lüssen A. Ageing, neurodegeneration, and olfactory and gustatory loss. B-ENT. 2009;5(Suppl 13):129–132. [PubMed] [Google Scholar]
- 21.Wickremaratchi MM, Llewelyn JG. Effects of ageing on touch. Postgrad Med J. 2006;82:301–304. doi: 10.1136/pgmj.2005.039651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider J, Gopinath B, McMahon C, et al. Prevalence and 5-year incidence of dual sensory impairment in an older Australian population. Ann Epidemiol. 2012;22:295–301. doi: 10.1016/j.annepidem.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 24.Gopinath B, Schneider J, McMahon CM, et al. Dual sensory impairment in older adults increases the risk of mortality: A population-based study. PLoS One. 2013;8:e55054. doi: 10.1371/journal.pone.0055054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loprinzi P, Smit E, Lin F. Accelerometer-assessed physical activity and objectively determined dual sensory impairment in US adults. Mayo Clin. 2013;88:690–696. doi: 10.1016/j.mayocp.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Fischer ME, Cruickshanks KJ, Klein BEK, et al. Multiple sensory impairment and quality of life. Ophthalmic Epidemiol. 2009;16:346–353. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertolini G, Wicki A, Baumann CR, et al. Impaired tilt perception in Parkinson's disease: A central vestibular integration failure. PLoS One. 2015;10:e0124253. doi: 10.1371/journal.pone.0124253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerezoudi E, Thomas PK. Influence of age on regeneration in the peripheral nervous system. Gerontology. 1999;45:301–306. doi: 10.1159/000022109. [DOI] [PubMed] [Google Scholar]
- 29.Schubert CR, Cruickshanks KJ, Fischer ME, et al. Carotid intima media thickness, atherosclerosis, and 5-year decline in odor identification: The Beaver Dam Offspring Study. J Gerontol Ser A Biol Sci Med Sci. 2015;70:879–884. doi: 10.1093/gerona/glu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith S, Jaszczak A. Instrument development, study design implementation, and survey conduct for the National Social Life, Health, and Aging Project Soc. 2009;64(Suppl 1):i20–i29. doi: 10.1093/geronb/gbn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schumm LP, McClintock M, Williams S, et al. Assessment of sensory function in the National Social Life, Health, and Aging Project. J Gerontol Ser B Psychol Sci Soc Sci. 2009;64(Suppl 1):i76–i85. doi: 10.1093/geronb/gbp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Waite LJ. Relationship quality and shared activity in marital and cohabiting dyads in the National Social Life, Health, and Aging Project, Wave 2. J Gerontol B Psychol Sci Soc Sci. 2014:1–11. doi: 10.1093/geronb/gbu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qato DM, Alexander GC, Conti RM, et al. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–2878. doi: 10.1001/jama.2008.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzman R. The National Social Life, Health, and Aging Project: An introduction. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i5–i11. doi: 10.1093/geronb/gbp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the national social life, health, and aging project. J Gerontol B Psychol Sci Soc Sci. 2009;64(Suppl 1):i12–i19. doi: 10.1093/geronb/gbp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinto JM, Schumm LP, Wroblewski KE, et al. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69:323–329. doi: 10.1093/gerona/glt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs JM, Hammerman-Rozenberg R, Maaravi Y, et al. The impact of visual impairment on health, function and mortality. Aging Clin Exp Res. 2013;17:281–286. doi: 10.1007/BF03324611. [DOI] [PubMed] [Google Scholar]
- 39.Mueller C, Renner B. A new procedure for the short screening of olfactory function using five items from the “Sniffin' Sticks” identification test kit. Am J Rhinol. 2006;20:113–116. [PubMed] [Google Scholar]
- 40.Skrondal A, Rabe-Hesketh S. Generalized latent variable modeling: Multilevel, longitudinal, and structural equation models. CRC Press; 2004. [May 24, 2015]. Available at: https://books.google.com/books?hl=en&lr=&id=YUpDqCzb-WMC&pgis=1. [Google Scholar]
- 41.MacKinnon DP, Lockwood CM, Brown CH, et al. The intermediate endpoint effect in logistic and probit regression. Clin Trials. 2007;4:499–513. doi: 10.1177/1740774507083434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khil L, Wellmann J, Berger K. Impact of combined sensory impairments on health-related quality of life. Qual Life Res. 2015:15–19. doi: 10.1007/s11136-015-0941-7. [DOI] [PubMed] [Google Scholar]
- 43.Khil L, Wellmann J, Berger K. Determinants of single and multiple sensory impairments in an urban population. Otolaryngol Head Neck Surg. 2015;153:364–371. doi: 10.1177/0194599815588913. [DOI] [PubMed] [Google Scholar]
- 44.Michikawa T, Nishiwaki Y, Takebayashi T. Are you conscious of any age-related taste impairment? Prevalence of and factors associated with taste impairment in Japan. J Am Geriatr Soc. 2010;58:981–983. doi: 10.1111/j.1532-5415.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 45.Steinbach S, Hundt W, Vaitl A, et al. Taste in mild cognitive impairment and Alzheimer's disease. J Neurol. 2010;257:238–246. doi: 10.1007/s00415-009-5300-6. [DOI] [PubMed] [Google Scholar]
- 46.Chinta SJ, Lieu CA, Demaria M, et al. Environmental stress, ageing and glial cell senescence: A novel mechanistic link to Parkinson's disease? J Intern Med. 2013;273:429–436. doi: 10.1111/joim.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaum E, Winborn CS, Bhattacharya S. Genomic regulation of senescence and innate immunity signaling in the retinal pigment epithelium. Mamm Genome. 2015;26:210–221. doi: 10.1007/s00335-015-9568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.