Abstract
The influence of the hypercholesterolemia associated with atherosclerosis on monocytes is poorly understood. Monocytes are exposed to high concentrations of lipids, particularly cholesterol and lysophosphatidylcholine (lyso-PC). Indeed, in line with recent reports, we found that monocytes accumulate cholesteryl esters (CEs) in hypercholesterolemic mice, demonstrating the need for studies that analyze the effects of lipid accumulation on monocytes. Here we analyze the effects of cholesterol and lyso-PC loading in human monocytes and macrophages. We found that cholesterol acyltransferase and CE hydrolase activities are lower in monocytes. Monocytes also showed a different expression profile of cholesterol influx and efflux genes in response to lipid loading and a different pattern of lyso-PC metabolism. In monocytes, increased levels of CE slowed the conversion of lyso-PC into PC. Interestingly, although macrophages accumulated glycerophosphocholine, phosphocholine was the main water-soluble choline metabolite being generated in monocytes, suggesting a role for mono- and diacylglycerol in the chemoattractability of these cells. In summary, monocytes and macrophages show significant differences in lipid metabolism and gene expression profiles in response to lipid loading. These findings provide new insights into the mechanisms of atherosclerosis and suggest potentials for targeting monocyte chemotactic properties not only in atherosclerosis but also in other diseases.
Keywords: atherosclerosis, dyslipidemias, foam cells, glycerophosphocholine, lysophosphatidylcholine, lysophospholipid, phosphocholine
The hallmark of atherosclerosis is the accumulation of lipid-loaded macrophages in the arterial wall (1, 2). These lipid-loaded macrophages, or macrophage foam cells, differentiate from peripheral blood monocytes that migrate into areas of “damage” in the arterial wall as a result of chemotactic stimuli (3, 4). Monocyte adherence, their differentiation into macrophages, and other phenotypic modulations, including polarization into anti- and proinflammatory subsets, are involved in normal and abnormal pathophysiology. These aspects are well studied in the context of atherosclerosis. However, monocytes could also be contributing to the pathogenesis of atherosclerosis by preaccumulating lipids and transporting them into the atherosclerotic lesions. Monocytes do express scavenger receptors (SRs) to some degree (5–7) and are exposed to hypercholesterolemic conditions during disease state. Recent studies in mice and in hypercholesterolemic patients have shown lipid accumulations in blood monocytes (8–10), but the consequences of cellular lipid accumulation, which have been well characterized in macrophages, are still unclear in monocytes.
Macrophages ingest cholesterol by endocytosis of aggregated and native LDL via the LDL receptor (LDLR), and by uptake of modified lipoproteins through SRs such as scavenger receptor class A type 1 (SR-A1) and cluster of differentiation 36 (CD36) (11, 12). Cholesteryl esters (CEs) derived from the lipoproteins are hydrolyzed in late endosomes/lysosomes to release free cholesterol, which is then distributed to the plasma membrane and peripheral organelles (13). Excess free cholesterol is reesterified on the endoplasmic reticulum by ACAT1 (13, 14), and stored in cytoplasmic lipid droplets. This lipid-scavenging function of macrophages is initially beneficial, but under conditions of unregulated or increased lipid uptake, it leads to excessive accumulation of CE in macrophages that results in foam cell formation (14, 15). In this way, acceptor-mediated cholesterol efflux plays a key role in protecting against atherosclerosis. By removing excess cellular cholesterol, cholesterol efflux is critical in preventing foam cell formation. CE hydrolysis to release free cholesterol from the CE stored in lipid droplets is the initial step in cholesterol efflux (16). The generated free cholesterol is then transported to extracellular acceptors such as lipid-poor ApoA1 or HDL by the ATP-transporters ABCA1 and ABCG1, respectively (17).
In addition to cholesterol, plasma components such as lysophosphatidylcholine (lyso-PC), which is increased in dyslipidemic serum (18–20), and which has a great affinity for cholesterol (21), might have a role in the cholesterol enrichment of monocytes. More importantly, lyso-PC levels are increased during the oxidation of LDL (22). Oxidized LDL (OxLDL) is important in the generation of foam cells and is involved in many aspects of the pathogenesis of atherosclerosis, and as lyso-PC is the major bioactive lipid component of OxLDL (11, 23), it has been suggested that lyso-PC is the major factor contributing to the proatherogenic potential of OxLDL (24–26).
In the present study, we demonstrate that lipid loading, specifically cholesterol, CE, and lyso-PC, had significantly different effects on monocytes compared with macrophages. Using the human monocytic cell line THP-1, we found that monocytes showed differences in the metabolism of cholesterol, CE, and lyso-PC, and in the expression of genes involved in cholesterol influx and efflux compared with THP-1-derived macrophages. Furthermore, our results show that monocytes are able to efficiently efflux cholesterol to HDL, even at higher rates than macrophages.
MATERIALS AND METHODS
Reagents
RPMI-1640, FBS, penicillin-streptomycin, 1× PBS, NBD Cholesterol (22-(N-(7-Nitrobenz-2-Oxa-1,3-Diazol-4-yl)Amino)-23,24-Bisnor-5-Cholen-3β-Ol), TRIzol™, HCS LipidTOX™ Red Neutral Lipid Stain, ProLong® Gold Antifade Reagent, Amplex Red Cholesterol Assay Kit, and primers are from Life Technologies (Carlsbad, CA). Cholesterol, cholesteryl oleate, lyso-PC from egg yolk, oleic acid, sphingomyelin, choline, phosphocholine, glycerophosphocholine, phosphatidylcholine (PC), cytidine diphosphate-choline (CDP-choline), acetylcholine, snake venom phospholipase A2, phorbol 12-myristate 13-acetate (PMA), acetonitrile, ammonium formate, formic acid, Histopaque 1083, filipin complex from Streptomyces filipinensis, 1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), and TLC plates are from Sigma-Aldrich (St. Louis, MO). Chloroform, methyl alcohol, hexane, diethyl ether, acetic acid, isopropyl alcohol, and ethyl alcohol are from VWR international (Randor, PA). [1, 2-3H (N)]cholesterol, [9, 10-3H]cholesteryl oleate, and [methyl-14C]choline chloride are from American Radiolabeled Chemicals (St. Louis, MO). Oil Red O was purchased from Fisher Scientific (Hampton, NH).
Animals
Twenty 4-week-old LDLR−/− female mice (B6.129S7-(Ldlrtm1Her/J) weighing 18–20 g were purchased from Jackson Laboratory (Bar Harbor, ME) and fed a high-fat diet (17% anhydrous milk fat, 0.2% cholesterol; TD.04287; Harlan Teklad, Madison, WI) for 45 days. All procedures were performed according to protocol approved by the Institutional Animal Care and Use Committee. At the end of the feeding, mice were anesthetized and peripheral blood was collected via cardiac puncture into anticoagulant tubes and centrifuged at 3,000 rpm for 20 min. Plasma was collected and lipid profiles measured using a Cholestech LDX analyzer according to the manufacturer’s instructions (Cholestech Corp., Hayward, CA). Mononuclear cells were isolated from the cell fraction by centrifugation on a sucrose density gradient (Histopaque 1083) according to the manufacturer’s instructions. Monocytes were purified from the rest of monocytic cells by 1 h plastic adherence followed by extensive washing with 1× PBS (pH 7.4) to remove nonadherent cells (27). Macrophages from the peritoneal cavity were isolated by peritoneal lavage using 3 ml of cold saline, centrifuged, and plated for 1 h in serum-free RPMI-1640 at 37°C in a 5% CO2 incubator followed by washes with 1× PBS to remove nonadherent cells (28).
Cell culture
The human monocytic cell line THP-1 was used as a model for monocytes. For macrophages, THP-1 cells differentiated with 50 ng/ml PMA for 72 h and RAW264.7 macrophages were used. Cells were maintained in RPMI-1640 supplemented with 10% FBS, 1% penicillin-streptomycin.
Lyso-PC micelles and cell treatments
Micelles were prepared as described previously (29). Micelle ratios were chosen on the basis of results from previous studies from our laboratory, which showed lower lyso-PC-induced lysis when increasing concentrations of cholesterol were present (29), and results from the cell viability assays (supplementary Fig. 3). A final concentration of 120 µM cholesterol/60 µM lyso-PC was chosen to ensure minimal cytotoxicity for both monocytes and macrophages. A lower concentration of CE was used for the micelles (80 µM CE/60 µM lyso-PC), as CE/lyso-PC micelles induced more cell lysis, and increasing CE did not reduce the cytotoxicity of the micelles [(29) and supplementary Fig. 3]. When added alone, 60 µM lyso-PC was cytotoxic. In order to avoid any cytotoxicity in the treatments with lyso-PC alone, we used a lower concentration (12 µM) than that in the micelles. Monocytes/macrophages (1 × 106 cells/well in 2 ml culture medium) were starved in serum-free RPMI-1640 for 3 h prior to all treatments, and then incubated for the indicated times with the micelles in serum-free RPMI-1640. In all treatments, 40 µM oleic acid was added. Fluorescent NBD-cholesterol was used to monitor micelle uptake by the cells using fluorescence microscopy (Axio Observer.A1; Carl Zeiss AG, Jena, Germany).
Cell viability
Cell viability after treatment with the micelles was determined using the WST-1 assay kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Cells were seeded into 96-well plates (5 × 104 cells/well in 100 µl culture medium) in triplicate. Measurements were taken after 24 h treatment.
Oil Red O staining
THP-1-derived macrophages were washed with 1× PBS and fixed directly in cell culture chamber slides with 10% formalin for 20 min and washed twice with deionized water. THP-1 monocytes were fixed with 10% formalin for 20 min, centrifuged, and resuspended in deionized water, and the cell suspension was applied onto gelatin-coated slides and placed on a hot plate to dry. Monocytes and macrophages were then incubated 5 min with 60% isopropanol followed by staining with Oil Red O for 30 min. The slides were washed and counterstained with hematoxylin. Cells were imaged using phase contrast microscope (Leica, Solms, Germany).
Immunofluorescence staining
Monocytes and macrophages were stained for the presence of free cholesterol using filipin dye (0.5 mg/ml in 1× PBS for 1 h) as previously described (30), and the presence of CE using HCS LipidTOX (Red Neutral Lipid Stain; Life Technologies) following the manufacturer’s instructions. The samples were mounted in ProLong® Gold Antifade Reagent and visualized using fluorescence microscopy (Axio Observer.A1).
Quantitative real-time PCR
Total RNA from the cells was isolated using TRIzol™ reagent according to the manufacturer’s instructions; 1 µg of total RNA was used to synthesize cDNA, and quantitative real-time PCR was performed using CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA) with SYBR Green as detection dye. mRNA levels of target genes were normalized to GAPDH. Primers are detailed in supplementary Table 1.
Quantification of cellular cholesterol
Cellular cholesterol content was measured using the Amplex Red Cholesterol Assay Kit as described previously (31). Briefly, cells were lysed in 1× reaction buffer supplemented with protease inhibitor cocktail. An aliquot of the lysate was used for protein quantification using the Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories). The rest of the lysate was used for cell cholesterol measurement. Samples were heated at 60°C for 30 min to inactivate enzymes that could compete with the assay. With this assay both total cholesterol and CE can be quantified by including cholesterol esterase into the reaction. Cholesterol content was normalized to the amount of total cellular protein.
Isolation and modifications of lipoproteins
Following Institutional Review Board approval, blood was collected in heparinized tubes from consenting healthy donors. Plasma was separated by centrifugation at 3,000 rpm for 20 min, and lipoproteins were isolated by sequential ultracentrifugation using a Beckman TL-100 tabletop ultracentrifuge (Beckman, Palo Alto, CA) (29). Isolated lipoproteins were dialyzed against 0.3 mM EDTA in 1× PBS (pH 7.4) overnight and sterilized using 0.22 μm syringe filter. Protein concentration was estimated using the Bio-Rad DC Protein Assay Kit. LDL was acetylated using acetic anhydride (32), and acetylated LDL (AcLDL) was dialyzed overnight and sterilized using 0.22 μm syringe filter. Tagging AcLDL with 3H-cholesterol was done as previously described (33). Briefly, AcLDL (10 µg protein/ml) and 3H-cholesterol (0.25 µCi/ml) were coincubated in serum-free RPMI-1640 for 24 h at 37°C.
Cholesterol efflux assay
Cells were incubated with 3H-cholesterol/lyso-PC micelles, 3H-CE/lyso-PC micelles, and 3H-cholesterol-AcLDL for 24 h and then washed twice with 1× PBS and incubated with 25 µg/ml of native HDL for 4 h. Cell lipids were extracted using the Bligh and Dyer method (24), and 3H-cholesterol/3H-CE in the medium and in the cells was measured by liquid scintillation counter (MicroBeta2 Plate Counter; PerkinElmer, Waltham, MA). Efflux of free-3H-cholesterol from the cells into the medium was calculated as percent of the total 3H-cholesterol/3H-CE (sum of 3H counts in cells and in medium).
Cholesterol, CE, and lyso-PC metabolism
To analyze cellular cholesterol and CE, cells were incubated with 3H-cholesterol/lyso-PC and 3H-CE /lyso-PC micelles for the indicated times to a final specific radioactivity of 5,000 cpm/ml. Cellular lipids were extracted using the Bligh and Dyer method (34), run on TLC using hexane-diethyl ether-acetic acid (30:6:0.5, v/v/v) solvent system, and visualized with iodine vapors (35). Bands were identified by comparing with authentic standards, and TLC spots corresponding to cholesterol and CE were analyzed with liquid scintillation counter (MicroBeta2 Plate Counter; PerkinElmer).
Lyso-PC metabolism was analyzed using 14C-choline-lyso-PC, synthesized from 14C-choline chloride as previously described (29). Cells were incubated for the indicated times with 14C-choline-lyso-PC/cholesterol micelles, 14C-choline-lyso-PC/CE micelles, and 14C-choline-lyso-PC micelles to a final specific activity of 5,000 cpm/ml. Cell lipids were extracted using the Bligh and Dyer method (34). The aqueous and chloroform phases were separated and dried under a stream of nitrogen. The chloroform phase was analyzed by TLC using chloroform-methanol-water (65:35:6, v/v/v) solvent system and visualized with iodine vapors to identify the lipids by comparing with authentic standards of PC, sphingomyelin, and lyso-PC. The appropriate TLC spots were then analyzed using autoradiography phosphor screen/Cyclone Plus and OptiQuant software (PerkinElmer). The aqueous phase was analyzed by TLC using a solvent system of 0.5% NaCI/methanol/acetic acid (25:75:0.5, v/v/v) (36), visualized by exposure to iodine vapors and compared with authentic standards of choline, acetylcholine, phosphocholine, glycerophosphocholine, and CDP-choline. The plate was analyzed as described above. Radioactivity data were expressed as percent of total radioactivity in the cell lipid fraction for cholesterol, CE, and lipid-soluble choline metabolites, and as percent of total radioactivity in the cell aqueous fraction for water-soluble choline metabolites.
Identification of metabolites by LC/MS
TLC bands of interest were cut according to authentic standards and compounds extracted using water-methanol (30:70, v/v). Ammonium acetate in acetonitrile-water (50:50, v/v) was added to the samples to 100 mM final concentration. The samples were analyzed using the Flexar FX-15 ultra-high-performance liquid chromatograph coupled to Axion2ToF MS (Perkin-Elmer) as previously described (37), with some modifications. MS and chromatographic conditions are detailed in supplementary Methods. From the acquired data, the extracted ion chromatograms (EICs) corresponding to choline (m/z 104.1075), acetylcholine (m/z 146.1181), CDP-choline (m/z 489.1146), phosphocholine (m/z 184.0738), and glycerophosphocholine (m/z 258.1101) ions were extracted with 100 ppm extraction window. EIC areas with retention times corresponding to the authentic standards were calculated. The EICs of acetycholine and CDP-choline were absent in all lanes, and only trace amounts were detected.
Statistics
Data are represented as mean ± SD from at least three independent experiments. Statistical significance was determined using one-way ANOVA with Dunnett’s posttest or two-way ANOVA with Bonferroni’s or Tukey’s posttest, at levels of P < 0.05, P < 0.01, and P < 0.001. All data were analyzed using GraphPad Prism 6 software (GraphPad Software, San Diego, CA).
RESULTS
Monocytes and macrophages from hypercholesterolemic mice accumulate CE
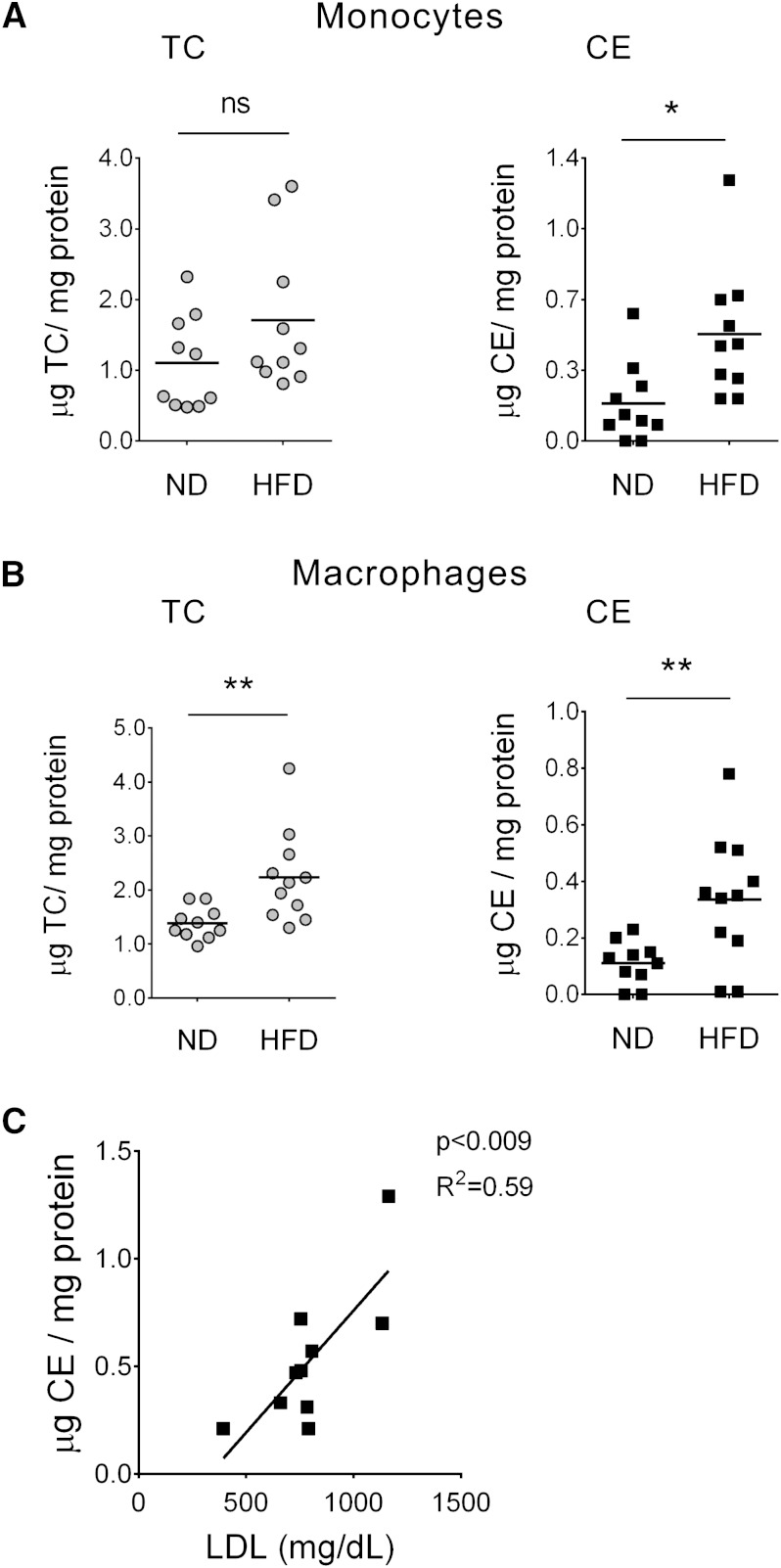
First, we analyzed if high cholesterol levels in blood would induce CE accumulation in peripheral blood monocytes. LDLR−/− mice fed a high-fat diet for 45 days were used as a model of hypercholesterolemia. As shown in Table 1, total cholesterol, LDL-cholesterol, and triglyceride concentrations were significantly increased in the high-fat diet fed mice compared with mice on a normal chow. The ratio of total cholesterol to HDL and LDL to HDL was also higher in the high-fat diet group. Peripheral blood monocytes and peritoneal macrophages from these mice were isolated and compared with monocytes and macrophages from mice fed a normal chow diet. In mice on a high-fat diet both monocytes and macrophages showed significantly higher levels of CE compared with cells from mice fed a normal diet (Fig. 1A, B, respectively). Moreover, there was a positive correlation between plasma LDL and monocyte CE content in the high-fat diet group (Fig. 1C). In vitro studies showed that THP-1 monocytes were able to take up AcLDL, although at lower levels than THP-1-derived macrophages (supplementary Fig. 1). Taken together, these data show that monocytes, similar to macrophages, can accumulate CE when exposed to high levels of cholesterol.
TABLE 1.
Mouse plasma lipid profiles after 45 days of high-fat or normal chow diet
| Groups | ||
| Parameter (mg/dl) | ND | HFD |
| Triglycerides | 81.7 ± 23.4 | 144.1 ± 75.7a |
| TC | 170.8 ± 24.1 | 864.6 ± 224.7b |
| HDL-cholesterol | 36.9 ± 6.8 | 40.5 ± 16.6 |
| LDL-cholesterol | 117.8 ± 27.2 | 797.2 ± 220.1b |
| VLDL-cholesterol | 16.2 ± 4.5 | 27.1 ± 16.6 |
| TC/HDL ratio | 5.0 ± 1.7 | 23.8 ± 10.1b |
| LDL/HDL ratio | 3.4 ± 1.5 | 22.2 ± 9.8b |
Mean ± SD (n = 10/group).
P < 0.05.
P < 0.0001, ND versus HFD (t-test).
Fig. 1.
Peripheral blood monocytes and peritoneal macrophages from hypercholesterolemic mice accumulate CE. A, B: Total cholesterol (TC) and CE in peripheral blood monocytes (A) and peritoneal macrophages (B) isolated from LDLR−/− mice fed a high-fat diet (HFD) or a normal diet (ND). TC and CE levels were normalized to cellular protein content. Each point represents an individual; horizontal lines indicate mean, n = 10/group. ND versus HFD, * P < 0.05, ** P < 0.01, ns = nonsignificant (t-test). C: Positive correlation between CE content in circulating monocytes from HFD mice and the plasma LDL. Each point represents an individual, n = 10 (Pearson correlation, P < 0.009; R2 = 0.59).
Cholesterol acyltransferase and CE hydrolase activities increase with the differentiation from monocytes to macrophages
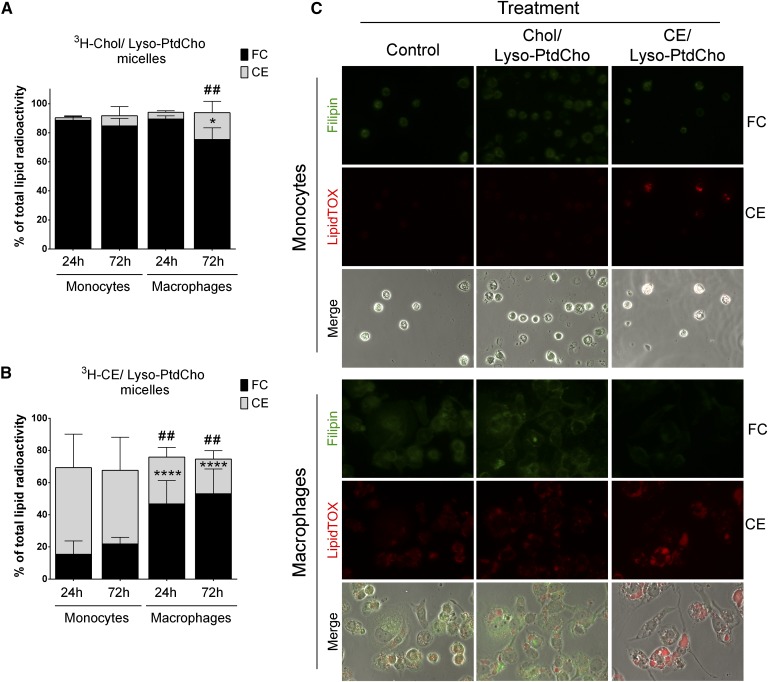
To study the differences in cholesterol and CE metabolism between monocytes and macrophages, we used a novel assay developed in our laboratory (29) that is based on the ability of lyso-PC to solubilize and deliver cholesterol into the cells. This method allowed us to study the differences between loading with free cholesterol or with CE avoiding the use of any inhibitors. The specific cholesterol, CE, and lyso-PC ratios were chosen as described in Materials and Methods. Using this technique, we efficiently loaded THP-1 monocytes and THP-1-derived macrophages with free cholesterol using cholesterol/lyso-PC micelles (120 µM cholesterol/60 µM lyso-PC) or with CE using CE/lyso-PC micelles (80 µM CE/60 µM lyso-PC), as demonstrated by the presence of fluorescent NBD-cholesterol inside the cells (supplementary Fig. 2A) and by accumulation of lipid droplets after 72 h incubation with the micelles (supplementary Fig. 2B). The micelles had no significant cytotoxic effects at the concentrations used for the assays (supplementary Fig. 3).
Using this method, we determined the fate of cholesterol in undifferentiated and differentiated THP-1 cells after incubation with radioactively labeled micelles. When loaded with 3H-cholesterol/lyso-PC micelles, monocytes showed poor conversion of free cholesterol into CE compared with macrophages, even after 72 h incubation (Fig. 2A). Monocytes also showed significantly lower levels of CE hydrolysis into free cholesterol when loaded with 3H-CE/lyso-PC (Fig. 2B). In contrast to monocytes, macrophages efficiently incorporated free cholesterol into CE and efficiently hydrolyzed it into free cholesterol when loaded directly with CE micelles (Fig. 2A, B).
Fig. 2.
Analysis of cholesterol and CE metabolism in THP-1 monocytes and macrophages. A–B: Cholesterol and CE fractions after incubation with 3H-cholesterol (Chol)/lyso-PC (Lyso-PtdCho) (A) or 3H-CE/lyso-PC (B) micelles for the indicated times. Data are mean ± SD (n = 3, each condition in duplicate) and are expressed as percent of total radioactivity in the cell lipid fraction. Monocyte free cholesterol (FC) versus macrophage FC, * P < 0.05, **** P < 0.0001; monocyte CE versus macrophage CE, ## P < 0.01 (two-way ANOVA with Tukey’s posttest). C: FC and CE accumulation in THP-1 monocytes and macrophages after 72 h treatment with the indicated micelles. Filipin stain for unesterified cholesterol (FC, green) and LipidTOX for neutral lipid staining (CE, Red). Magnification ×40.
We confirmed these results using filipin complex to stain free cholesterol and LipidTOX reagent to stain for neutral lipids in monocytes and macrophages incubated with the different micelles for 72 h (Fig. 2C). Monocytes incubated with free cholesterol micelles showed lower levels of neutral lipid accumulation compared with macrophages, suggesting that monocytes can be more resistant to become foam cells compared with fully differentiated macrophages.
The differentiation process alters the expression of genes involved in cholesterol metabolism and transport
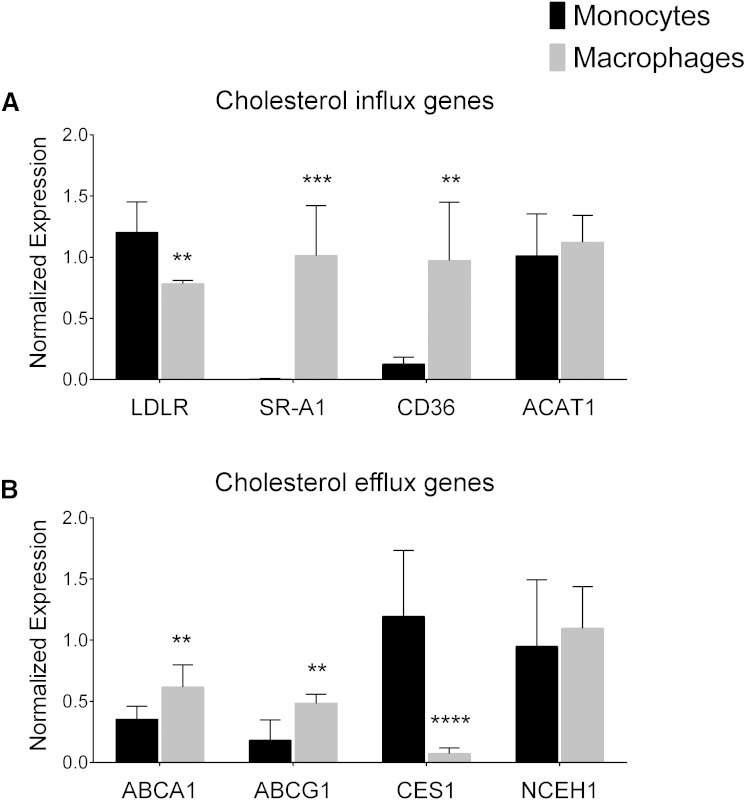
The analysis of genes associated with cholesterol metabolism and transport showed that monocytes and macrophages have a significantly different expression profile. First, we analyzed the expression of LDLR, involved in native LDL uptake, and the expression of two SRs involved in the uptake of modified LDL, CD36 and SR-A1 (11, 12). We found that LDLR was significantly downregulated during the differentiation from monocytes to macrophages, in contrast to CD36 and SR-A1 expression levels that were significantly higher in macrophages compared with monocytes (Fig. 3A).
Fig. 3.
Gene expression levels in untreated THP-1 monocytes and macrophages. A: Expression levels of genes involved in cholesterol influx: LDLR, SRs SR-A1 and CD36, and the cholesterol acyltransferase ACAT1. B: Expression levels of the genes involved in cholesterol efflux: ABCA1, ABCG1, and the CE hydrolases CES1 and NCEH1. Data are mean ± SD, n = 6. Monocytes versus macrophages, ** P < 0.01, *** P < 0.001, **** P < 0.0001 (t-test).
Analysis of genes involved in cholesterol efflux showed that the expression of ABCA1 and ABCG1 was upregulated during the differentiation process (Fig. 3B). The expression levels of the CE hydrolase CES1 were significantly higher in monocytes than in macrophages (Fig. 3B). There were no differences in the expression of the ACAT1 gene (Fig. 3A) or the neutral CE hydrolase (NCEH1) gene (Fig. 3B).
Lipid loading has a different effect on the gene expression profile in monocytes and macrophages
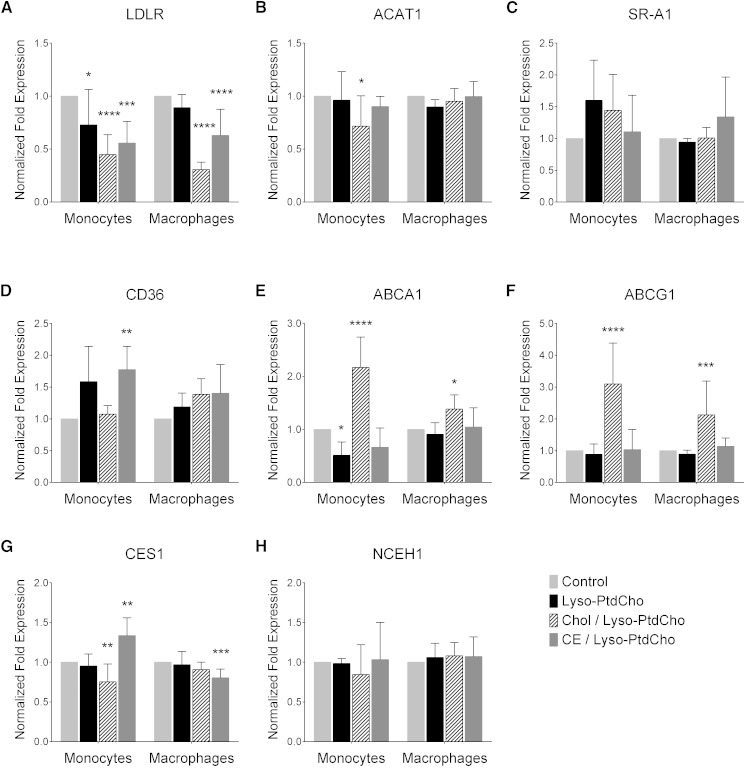
In macrophages, the effect of high cholesterol levels on gene expression has been well studied. However, in monocytes, the changes in the expression of key genes related to atherosclerosis are less understood. Therefore, we analyzed the expression of several genes in lipid-loaded monocytes and used lipid-loaded macrophages as reference. Cholesterol accumulation is known to suppress the expression of LDLR through the deactivation of sterol regulatory element-binding protein transcription factors (38). Indeed, we found that cholesterol/lyso-PC and CE/lyso-PC micelles induced a significant decrease in the expression of LDLR in monocytes and macrophages (Fig. 4A, dashed bars and dark gray bars, respectively). When we analyzed the expression of the SRs CD36 and SR-A1, we found that CE/lyso-PC micelles induced a significant increase in the expression of the CD36 only in monocytes (Fig. 4D, dark gray bars), but no changes were found for the SR-A1 gene expression in any of the conditions (Fig. 4C).
Fig. 4.
Effects of cholesterol, CE, and lyso-PC on the expression of the genes involved in cholesterol influx [LDLR (A), ACAT1 (B), SR-A1 (C), and CD36 (D)] and cholesterol efflux [ABCA1 (E), ABCG1 (F), CES1 (G), and NCEH1 (H)] in THP-1 monocytes and macrophages after 24 h treatment. Data are mean ± SD, n = 6. Control versus treatment, * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001 (one-way ANOVA, Dunnett’s posttest).
In line with previous studies showing an increase in ABCA1 and ABCG1 in lipid-loaded macrophages (39, 40), loading monocytes with free cholesterol also induced a significant increase in the expression of ABCA1 and ABCG1 (Fig. 4E, F, dashed bars). Surprisingly, loading with CE did not induce significant changes in the expression of these genes in either cell type (Fig. 4E, F, dark gray bars).
Only in monocytes did cholesterol/lyso-PC micelles decrease the expression of ACAT1 and CES1 (Fig. 4B, G, respectively). CES1 gene expression increased with CE/lyso-PC micelles incubation in monocytes but decreased in macrophages with the same treatment (Fig. 4G). NCEH1 gene expression levels remained unchanged with the treatments (Fig. 4H). Interestingly, loading with lyso-PC alone induced a significant decrease in the expression of LDLR and ABCA1 in monocytes (Fig. 4A, E). In summary, monocytes and macrophages showed a significantly different expression profile in response to cholesterol and CE loading.
The cholesterol efflux rate is higher in monocytes than in macrophages
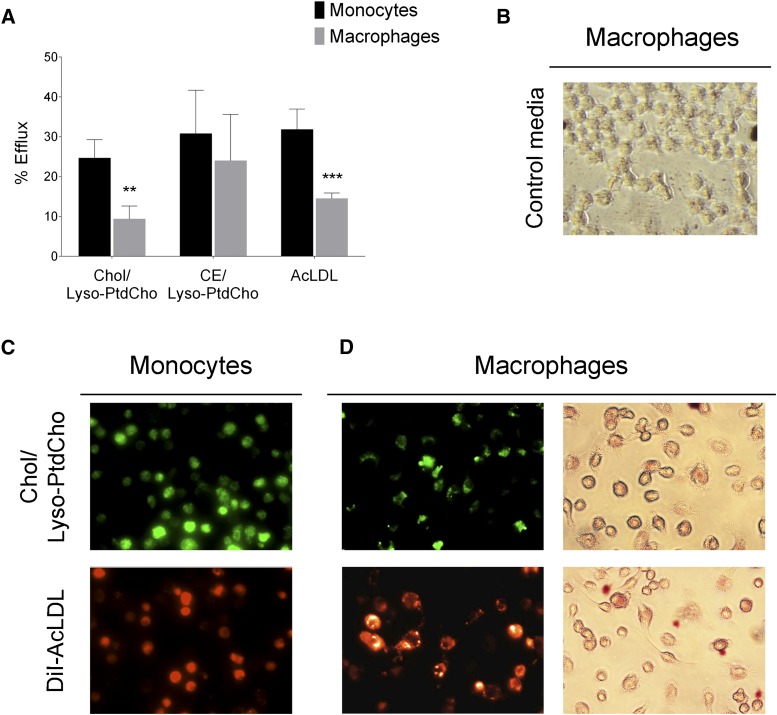
The increase in ABCA1 and ABCG1 expression with cholesterol loading suggests that cholesterol from monocytes in circulation could potentially be reverse transported. Given that cholesterol efflux from macrophage foam cells occurs via ABCA1 to preβ-HDL and via ABCG1/G4 to HDL (17), we decided to test the differences in the cholesterol efflux to HDL between monocytes and macrophages when cells were loaded with either free cholesterol or CE. Cells loaded with AcLDL were also analyzed. After incubation of THP-1 monocytes and macrophages with 3H-cholesterol/lyso-PC, 3H-CE/lyso-PC, and 3H-cholesterol-AcLDL for 24 h, cholesterol efflux to HDL was determined (Fig. 5A). We found that the rate of cholesterol efflux to HDL was significantly higher in monocytes compared with macrophages when cells were loaded with free cholesterol or AcLDL (Fig. 5A). In contrast, when cells were loaded with CE, the rate of cholesterol efflux was similar in both cell types (Fig. 5A).
Fig. 5.
Monocytes can efflux cholesterol. A: Cholesterol efflux to HDL in THP-1 monocytes and macrophages. THP-1 monocytes and macrophages were incubated for 24 h with 3H-cholesterol (Chol)/lyso-PC, 3H-CE/lyso-PC, and 3H-cholesterol-labeled AcLDL (25 μg/ml). Cholesterol efflux assay was performed as described in Materials and Methods in the presence of HDL (25 μg/ml) for 4 h. Data are mean ± SD (n = 3, each condition in duplicate) and are expressed as percentage of radioactivity in the medium of the total radioactivity in the cells plus medium. Monocytes versus macrophages, ** P < 0.01, *** P < 0.001 (two-way ANOVA with Bonferroni’s posttest). B: Oil Red O staining of RAW264.7 macrophages after 24 h incubation with the 24 h conditioned media from nonloaded monocytes (Control media). C: THP-1 monocytes after 24 h incubation with cholesterol/lyso-PC micelles (NBD-cholesterol; green) or DiI-AcLDL (50 µg/ml; orange). D: Lipid accumulation in RAW264.7 macrophages after 24 h incubation with the 24 h conditioned media from lipid-loaded monocytes from C, shown by fluorescence from NBD-cholesterol (upper left; green), DiI-AcLDL (lower left; orange), and neutral lipid accumulation by Oil Red O staining (right).
The monocyte capacity for cholesterol efflux suggests that lipid-loaded monocytes deliver lipids into the circulation (or into the lesions once they have migrated) and that these lipids might be uptaken by macrophages to form foam cells. To tests this hypothesis, THP-1 monocytes were incubated with cholesterol/lyso-PC micelles or DiI-AcLDL (50 µg/ml) for 24 h; cells were then imaged to monitor uptake by cells (Fig. 5C) and then washed and incubated with serum-free RPMI-1640 for 24 h. In the absence of acceptors, the amount of cholesterol efflux from lipid-loaded monocytes during the 24 h incubation reflects basal levels of efflux. Moreover, we felt that any conditions that would promote efflux should not be carried to the next step of the experiment, as it would be counterproductive for cholesterol loading. To demonstrate that the monocyte-derived cholesterol was taken up by macrophages, the conditioned medium was used on RAW264.7 macrophages to evaluate the transfer of the lipids from lipid-loaded monocytes to macrophages. Indeed, we found that macrophages are able to take up lipids released to the medium by loaded monocytes (Fig. 5D). The formation of neutral lipids was confirmed by Oil Red O staining (Fig. 5D), indicating that such lipids might be used for foam cell formation. No lipid droplets were observed when macrophages were incubated with conditioned media from nonloaded monocytes (Fig. 5B).
Lyso-PC metabolism in monocytes is affected by the presence of high levels of CE
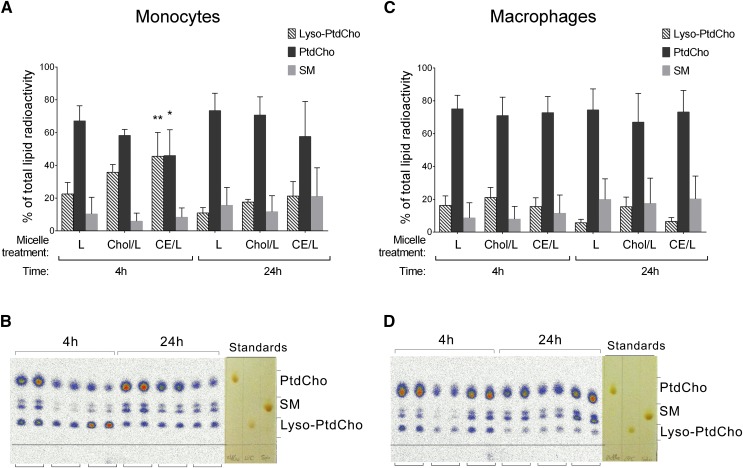
Considering that hypercholesterolemic conditions are characterized not only by high levels of cholesterol but also high levels of lyso-PC (18–20), and that lyso-PC is a major bioactive lipid component of OxLDL (24–26), we decided to test whether cholesterol or CE accumulation would have any effects on the metabolism of lyso-PC. To test this, we loaded THP-1 monocytes and macrophages with 14C-lyso-PC alone, cholesterol/14C-lyso-PC, and CE/14C-lyso-PC micelles and incubated for 4 h and 24 h. Lipid and aqueous fractions were extracted from the cells and analyzed by TLC and autoradiography analysis. The analysis of the lipid fraction showed that monocytes were less efficient than macrophages in metabolizing the incorporated lyso-PC, more significantly when added together with CE (Fig. 6A, B). In monocytes incubated with lyso-PC alone, or lyso-PC together with free cholesterol, by 4 h the incorporated lyso-PC was already metabolized, mainly into PC and a small percentage into sphingomyelin (Fig. 6A, B). However, in monocytes incubated with CE/lyso-PC micelles, at 4 h the radioactivity remained mainly in the lyso-PC fraction (Fig. 6A, B). In contrast, macrophages independently of loading with lyso-PC alone, or lyso-PC together with free cholesterol or CE, readily metabolized the incorporated lyso-PC (Fig. 6C, D). These results show that CE content influences the metabolism of lyso-PC only in monocytes.
Fig. 6.
Analysis of lyso-PC metabolism in the lipid fraction of monocytes (A, B) and macrophages (C, D) after 4 h and 24 h incubation with 14C-lyso-PC (L), cholesterol/14C-lyso-PC (Chol/L), and CE/14C-lyso-PC (CE/L) micelles. A, C: Radioactivity corresponding to lyso-PC (dashed bars), PC (black bars), and sphingomyelin (SM; gray bars) fractions, quantified as digital luminescence units from the TLC autoradiograph and represented as percent of total lipid radioactivity. Data are mean ± SD (n = 4, each condition in duplicate). Lyso-PC-treated cells versus other treatments, * P < 0.05, ** P < 0.01 (two-way ANOVA with Tukey’s posttest). B, D: Representative TLC autoradiograph with the corresponding I2-stained standards, each condition in duplicate.
Monocytes and macrophages show a different pattern of choline metabolites
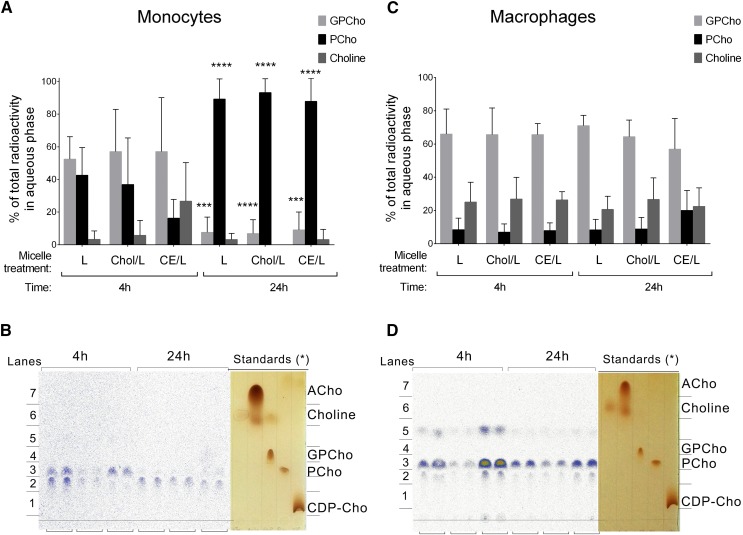
The analysis of the aqueous cell fraction showed that the water-soluble choline metabolites derived from the incorporated lyso-PC were different in monocytes and macrophages (Fig. 7). The data showed that while in macrophages the main percentage of radioactive counts in the aqueous phase were found in the glycerophosphocholine fraction, regardless of the treatment and the incubation time (Fig. 7C, D), in monocytes the 14C-choline was found mainly in the phosphocholine fraction after 24 h incubation (Fig. 7A, B). Macrophages also showed a higher percentage of choline being generated from the incorporated lyso-PC compared with monocytes. There were no differences between the different micelle treatments in the water-soluble choline metabolites derived from the lyso-PC metabolism. Because the standards of the water-soluble choline compounds showed variations in their retention time on the TLC compared with the sample spots found on the autoradiographs (Fig. 7C, D), the presence of phosphocholine, glycerophosphocholine, and choline on the corresponding TLC lanes was confirmed by LC/MS (supplementary Fig. 4). We found that there was an increased retention time on the test samples compared with the standards, probably because of the presence of ions on the sample solvent, and confirmed that the lanes corresponding to the autoradiograph spots were phosphocholine, glycerophosphocholine, and choline.
Fig. 7.
Analysis of lyso-PC metabolism in the aqueous fraction of monocytes (A, B) and macrophages (C, D) after 4 h and 24 h incubation with 14C-lyso-PC (L), cholesterol/14C-lyso-PC (Chol/L), and CE/14C-lyso-PC (CE/L) micelles. A, C: Radioactivity corresponding to glycerophosphocholine (GPCho; light gray bars), phosphocholine (PCho; black bars), and choline (dark gray bars) fractions, quantified as digital luminescence units from the TLC autoradiograph and represented as percent of total lipid radioactivity. Data are mean ± SD (n = 4, each condition in duplicate). The 4 h treatment versus 24 h treatment, *** P < 0.001, **** P < 0.0001 (two-way ANOVA with Tukey’s posttest). B, D: Representative TLC autoradiograph with the corresponding I2-stained standards, each condition in duplicate. The asterisk indicates that the increased retention time of the choline metabolites present in the sample compared with the authentic standards was confirmed by LC/MS.
DISCUSSION
In this study, we provide evidence showing that monocyte properties are altered by hypercholesterolemia in a different manner than in macrophages. Monocytes, as macrophages, play an important role in the pathogenesis of atherosclerosis. However, the majority of studies in this field have centered on the macrophages. In this study, we have focused on the effects that the hypercholesterolemia (a condition associated with atherosclerosis development and progression) has on monocytes and how they compare with macrophages. Monocytes like macrophages express LDLR and SRs and are exposed to cholesterol and other lipids during hypercholesterolemic conditions. In this context, we show in a mouse model of hypercholesterolemia that peripheral blood monocytes accumulate CE when exposed to high levels of cholesterol in circulation, in line with previous studies showing that both monocytes and macrophages are able to uptake modified LDL (8–10). These results indicate that foamlike monocytes could be contributing to lesion formation in other ways than as a precursors for lesional macrophages, for example, as a source of lipids that are transported from the bloodstream into the plaques, demonstrating the need for studies that analyze the effects that lipid accumulation has on the monocyte.
During hypercholesterolemia, in addition to cholesterol, the levels of other lipids such as lyso-PC are also increased in plasma (18–20). Lyso-PC is also known to be increased during the oxidation of LDL (22) and has been attributed many proatherogenic properties (24, 25). These, together with our findings showing that CE content in monocytes positively correlated with plasma LDL (Fig. 1C), would suggest that under hypercholesterolemic conditions not only is CE accumulated in monocytes, but lyso-PC levels would also be increased in these cells. This might also indicate that cholesterol and lyso-PC could influence each other’s metabolism and effects. Moreover, formation of monocyte and macrophage foam cells might be conductive to the generation and recruitment of more foam cells not only through protein factors like MCP-1, RANTES, and so forth, but also via lyso-PC degradation products through phospholipase C/D enzymes (41).
To study the effects of both these molecules, we took advantage of a method developed in our laboratory that uses lyso-PC micelles to incorporate cholesterol and CE into the cells (29). Cells do not just take up cholesterol, even under in vitro conditions. Past studies from our laboratory demonstrated that a highly nonpolar molecule, β-carotene, could be very efficiently solubilized using lyso-PC as a natural “detergent” (42). Using this method, we were able to enrich human THP-1 monocytes and macrophages with cholesterol and CE (supplementary Fig. 2).
The analysis of cholesterol metabolism showed that macrophages were significantly more efficient than monocytes in esterifying the incorporated cholesterol (Fig. 2A). In other words, the ACAT activity of macrophages appeared to be far greater compared with that of monocytes. In this regard, it has been shown that ACAT1 protein increases significantly during the monocyte-macrophage differentiation process (43, 44). Moreover, the fact that monocytes showed poor conversion of free cholesterol to CE, together with our in vivo experiments showing CE accumulation in peripheral blood monocytes from hypercholesterolemic mice (Fig. 1A), suggests that monocytes in circulation mainly uptake the cholesterol in the form of CE.
Monocytes also showed limited capacity to hydrolyze CE when loaded with CE micelles (Fig. 2B). Gene expression of the CE hydrolase CES1 was significantly higher in monocytes than in macrophages, but the expression levels of another CE hydrolase, NCEH1, were similar in both cell types (Fig. 3B). Recent findings show that NCEH1 is the enzyme primarily involved in the hydrolysis of CE in human macrophages, and the contribution of CES1 is lower (45). Future experiments should address the differences in the activity of the specific CE hydrolases between monocytes and macrophages.
Analysis of the effects of cholesterol and CE loading on gene expression also revealed significant differences between monocytes and macrophages (Fig. 4). Monocytes showed a different profile in the expression of the SR CD36 and the enzymes involved in cholesterol metabolism, ACAT1 and CES1, after cholesterol and CE loading (Fig. 4B, D, G). While SR-A1 gene expression was not affected by any treatment, CD36 gene expression was significantly upregulated only in monocytes treated with CE micelles (Fig. 4D). Indeed, it has been shown that CD36 is upregulated with LDL uptake (5, 46). CD36 is associated with the uptake of OxLDL (12). This would suggest that once monocytes uptake CE, they would be primed to load more lipids through increased SR expression.
Free cholesterol loading induced a similar response in the expression of the cholesterol responsive genes LDLR, ABCA1, and ABCG1 in monocytes and macrophages (Fig. 4A, E, F), in line with what has been previously described for macrophages (38–40). In contrast, although CE loading downregulated LDLR gene expression (Fig. 4A), CE loading failed to induce an upregulation of ABCA1 and ABCG1 (Fig. 4E, F). This would indicate that unesterified cholesterol induces more efficiently the expression of the ABCA1 and ABCG1 transporters compared with CE, whereas LDLR downregulation is induced by both free and esterified cholesterol at similar level. Indeed, it has been described that stimulation of CE hydrolysis in macrophages induces the expression of ABCA1 (47).
The results showing that monocytes upregulate the expression of ABC transporters suggested that monocytes are targets for cholesterol efflux in the presence of HDL. Our results show that monocytes loaded with free cholesterol or AcLDL had higher rates of cholesterol efflux to the HDL compared with macrophages, but not when cells were loaded with CE (Fig. 5A). Taking these results together with the cholesterol metabolism studies suggests that monocytes can be more resistant to become foam cells as compared with fully differentiated macrophages under normal physiological conditions. However, under conditions in which CE is increased in plasma, such as in hypercholesterolemia, monocytes would not efficiently hydrolyze the accumulated CE, and because HDL can only remove unesterified cholesterol from the cells, monocytes would be more prone to become foamlike cells. These foamlike monocytes would adhere and migrate into the lesions, where they would differentiate into macrophages that most probably will already have the characteristics of foam cells, contributing to the accumulation of lipids within the atherosclerotic lesion. Furthermore, our results show that macrophages can uptake the lipids released by loaded monocytes (Fig. 5D). During the performance of the experiment, no cholesterol acceptors such as ApoA1 or HDL were included; therefore, the cholesterol effluxed from the lipid-loaded monocytes reflects basal levels of efflux. Whether there is an intermediate carrier involved in this step is unknown. Based on the secretion of cholesterol-phospholipid-apoE complexes by lipid-laden macrophages (48, 49), it is possible that the secreted lipids are in the form of such complexes, and whether the uptake of such complexes by macrophages involves receptor(s) other than SRs is a topic for future study.
Therefore, lipids transported into the lesions by monocytes could contribute to the formation of foam cells. However, further experiments are needed to validate this theory. In order to explain our observation of similar efflux rates from CE-loaded monocytes and macrophages (Fig. 5A), and lower cholesterol efflux rates in macrophages when loaded with AcLDL, future experiments should analyze the differences of cholesterol acyltransferases and CE hydrolases at the protein level, and the differences at specific steps of the intracellular cholesterol transport between monocytes and macrophages. Also, it is important to point out that monocytes and macrophages showed different AcLDL uptake and this could influence the cholesterol efflux rate. In macrophages, we observed a higher uptake of AcLDL than in monocytes, as shown by the higher levels of DiI-AcLDL (supplementary Fig. 1), and by the higher levels of 3H-cholesterol after incubation with radioactively labeled AcLDL (data not shown), which correlates with the observation that macrophages had significantly higher gene expression levels of the SRs CD36 and SR-A1 (Fig. 3A).
Similar to cholesterol metabolism, the analysis of lyso-PC metabolism also revealed significant differences between monocytes and macrophages (Figs. 6 and 7). Lyso-PC is very efficiently utilized by cells in different ways: a) lysophosphatidylcholine acyltransferase (LPCAT) enzymes yield PC from lyso-PC, b) lysophospholipase cleaves the acyl-ester bond and removes the detergent nature of lyso-PC, and c) intermolecular trans-esterification reaction creates a molecule of PC and glycerophosphocholine.
The analysis of the cell lipid fraction revealed that CE loading influences the metabolism of lyso-PC in monocytes (Fig. 6A, B). Monocytes show a slower metabolism of lyso-PC compared with macrophages, which readily metabolize the incorporated lyso-PC independently of the cholesterol or CE content (Fig. 6). The effect of CE on lyso-PC metabolism could be due to the association of LPCAT enzymes to lipid droplets (50). It has been recently described that LPCAT1 knockdown induces an increase in lipid droplet size (50). Thus, it is possible that high intracellular CE levels influence the activity of LPCAT enzymes to adjust the properties of the lipid droplet surface monolayer.
The analysis of the aqueous fraction revealed that monocytes have a different pattern of choline metabolism compared with macrophages (Fig. 7). While in macrophages the radioactive counts in the aqueous fraction were found mainly in the form of glycerophosphocholine, with no significant changes over time (Fig. 7C, D), monocytes preferentially generated phosphocholine, more significantly after 24 h (Fig. 7A, B). Based on our results, the increased levels of phosphocholine we find at 24 h in monocytes could come from the generated PC, through the action of phospholipase C, producing also diacylglycerol (DAG) from PC. Sphingomyelin can also be a precursor for phosphocholine through the action of the sphingomyelinases.
Accumulation of glycerophosphocholine in macrophages not only suggests active acylation/transacylation and lysophospholipase-catalyzed deacylation mechanisms, but also a poor capacity to breakdown glycerophosphocholine by diesterases. Interestingly, it has been proposed that a glycerophosphocholine choline phosphodiesterase exists that catalyzes the hydrolysis of glycerophosphocholine generating glycerol and phosphocholine (51, 52). This will explain better our results in monocytes, where we see at 24 h the disappearance of the glycerophosphocholine found at 4 h, which perhaps is being hydrolyzed into the phosphocholine we find at 24 h (Fig. 7A, B). In this way, our results would suggest that the glycerophosphocholine choline phosphodiesterase is lower or dysfunctional in macrophages.
The synthesis and hydrolysis of choline metabolites generates mitogenic and chemotactic molecules such as phosphocholine (53), DAG (54), phosphatidic acid, lysophosphatidic acid, and arachidonic acid, which can be further metabolized to other signaling molecules (54). It would be interesting to analyze if, in the same way that monocytes and macrophages preferentially generate either phosphocholine or glycerophosphocholine, these signaling molecules are also differentially generated in these cell types. A difference in the generation of DAG, phosphatidic acid, or lysophosphatidic acid would mean that lyso-PC uptake might activate different pathways in monocytes and macrophages, thereby influencing different cellular functions.
Further studies characterizing the phenotypic and functional changes in lipid-loaded monocytes and how they compare with lipid-loaded macrophages can help in the development of better strategies for the treatment and prevention of atherosclerosis. For example, macrophages esterify cholesterol and accumulate CE efficiently, in contrast to monocytes. Thus, free cholesterol would accumulate more readily in monocytes and would influence cellular functions. Finally, identifying how the enzymes that control lyso-PC metabolism are differentially regulated during the differentiation process of monocytes to macrophages could help in developing targets for novel therapeutics or markers for atherosclerosis and other diseases where these lipids play a role.
Supplementary Material
Footnotes
Abbreviations:
- AcLDL
- acetylated LDL
- CD36
- cluster of differentiation 36
- CDP
- cytidine diphosphate
- CE
- cholesteryl ester
- DAG
- diacylglycerol
- EIC
- extracted ion chromatogram
- LDLR
- LDL receptor
- LPCAT
- lysophosphatidylcholine acyltransferase
- lyso-PC
- lysophosphatidylcholine
- OxLDL
- oxidized LDL
- PC
- phosphatidylcholine
- SR
- scavenger receptor
- SR-A1
- scavenger receptor class A type 1
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Libby P. 2002. Inflammation in atherosclerosis. Nature. 420: 868–874. [DOI] [PubMed] [Google Scholar]
- 2.Ross R. 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 362: 801–809. [DOI] [PubMed] [Google Scholar]
- 3.Gerrity R. G., Naito H. K., Richardson M., and Schwartz C. J.. 1979. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am. J. Pathol. 95: 775–792. [PMC free article] [PubMed] [Google Scholar]
- 4.Aqel N. M., Ball R. Y., Waldmann H., and Mitchinson M. J.. 1984. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 53: 265–271. [DOI] [PubMed] [Google Scholar]
- 5.Kapinsky M., Torzewski M., Buchler C., Duong C. Q., Rothe G., and Schmitz G.. 2001. Enzymatically degraded LDL preferentially binds to CD14(high) CD16(+) monocytes and induces foam cell formation mediated only in part by the class B scavenger-receptor CD36. Arterioscler. Thromb. Vasc. Biol. 21: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 6.Sampson M. J., Davies I. R., Braschi S., Ivory K., and Hughes D. A.. 2003. Increased expression of a scavenger receptor (CD36) in monocytes from subjects with Type 2 diabetes. Atherosclerosis. 167: 129–134. [DOI] [PubMed] [Google Scholar]
- 7.Draude G., von Hundelshausen P., Frankenberger M., Ziegler-Heitbrock H. W. L., and Weber C.. 1999. Distinct scavenger receptor expression and function in the human CD14+/CD16+ monocyte subset. Am. J. Physiol. 276: H1144–H1149. [DOI] [PubMed] [Google Scholar]
- 8.Mosig S., Rennert K., Buttner P., Krause S., Lutjohann D., Soufi M., Heller R., and Funke H.. 2008. Monocytes of patients with familial hypercholesterolemia show alterations in cholesterol metabolism. BMC Med. Genomics. 1: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosig S., Rennert K., Krause S., Kzhyshkowska J., Neunubel K., Heller R., and Funke H.. 2009. Different functions of monocyte subsets in familial hypercholesterolemia: potential function of CD14+ CD16+ monocytes in detoxification of oxidized LDL. FASEB J. 23: 866–874. [DOI] [PubMed] [Google Scholar]
- 10.Wu H., Gower R. M., Wang H., Perrard X-Y. D., Ma R., Bullard D. C., Burns A. R., Paul A., Smith C. W., Simon S. I., et al. 2009. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 119: 2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., and Witztum J. L.. 1989. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 320: 915–924. [DOI] [PubMed] [Google Scholar]
- 12.Greaves D. R., and Gordon S.. 2009. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res. 50: S282–S286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liscum L., and Munn N. J.. 1999. Intracellular cholesterol transport. Biochim. Biophys. Acta. 1438: 19–37. [DOI] [PubMed] [Google Scholar]
- 14.Chang T. Y., Chang C. C., and Cheng D.. 1997. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66: 613–638. [DOI] [PubMed] [Google Scholar]
- 15.Accad M., Smith S. J., Newland D. L., Sanan D. A., King L. E. Jr., Linton M. F., Fazio S., and Farese R. V. Jr. 2000. Massive xanthomatosis and altered composition of atherosclerotic lesions in hyperlipidemic mice lacking acyl CoA:cholesterol acyltransferase 1. J. Clin. Invest. 105: 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh S. 2012. Early steps in reverse cholesterol transport: cholesteryl ester hydrolase and other hydrolases. Curr. Opin. Endocrinol. Diabetes Obes. 19: 136–141. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy M. A., Barrera G. C., Nakamura K., Baldán Á., Tarr P., Fishbein M. C., Frank J., Francone O. L., and Edwards P. A.. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131. [DOI] [PubMed] [Google Scholar]
- 18.Eisenberg S., Stein Y., and Stein O.. 1969. Phospholipases in arterial tissue. 3. Phosphatide acyl-hydrolase, lysophosphatide acyl-hydrolase and sphingomyelin choline phosphohydrolase in rat and rabbit aorta in different age groups. Biochim. Biophys. Acta. 176: 557–569. [DOI] [PubMed] [Google Scholar]
- 19.Portman O. W., and Illingworth D. R.. 1974. Factors determining the concentrations of lysolecithin in plasma and tissues. Scand. J. Clin. Lab. Invest. Suppl. 137: 49–55. [PubMed] [Google Scholar]
- 20.Wells I. C., Peitzmeier G., and Vincent J. K.. 1986. Lecithin: cholesterol acyltransferase and lysolecithin in coronary atherosclerosis. Exp. Mol. Pathol. 45: 303–310. [DOI] [PubMed] [Google Scholar]
- 21.Rand R. P., Pangborn W. A., Purdon A. D., and Tinker D. O.. 1975. Lysolecithin and cholesterol interact stoichiometrically forming bimolecular lamellar structures in the presence of excess water, of lysolecithin or cholesterol. Can. J. Biochem. 53: 189–195. [DOI] [PubMed] [Google Scholar]
- 22.Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., and Steinberg D.. 1984. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc. Natl. Acad. Sci. USA. 81: 3883–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parthasarathy S., Steinbrecher U. P., Barnett J., Witztum J. L., and Steinberg D.. 1985. Essential role of phospholipase A2 activity in endothelial cell-induced modification of low density lipoprotein. Proc. Natl. Acad. Sci. USA. 82: 3000–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassa B. V., Roh D. D., Kirschenbaum M. A., and Kamanna V. S.. 1998. Atherogenic lipoproteins stimulate mesangial cell p42 mitogen-activated protein kinase. J. Am. Soc. Nephrol. 9: 488–496. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto T., Kobayashi T., and Kamata K.. 2007. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 14: 3209–3220. [DOI] [PubMed] [Google Scholar]
- 26.Aiyar N., Disa J., Ao Z., Ju H., Nerurkar S., Willette R. N., Macphee C. H., Johns D. G., and Douglas S. A.. 2007. Lysophosphatidylcholine induces inflammatory activation of human coronary artery smooth muscle cells. Mol. Cell. Biochem. 295: 113–120. [DOI] [PubMed] [Google Scholar]
- 27.Böyum A. 1968. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Invest. Suppl. 97: 77–89. [PubMed] [Google Scholar]
- 28.Selvarajan K., Moldovan L., Chandrakala A. N., Litvinov D., and Parthasarathy S.. 2011. Peritoneal macrophages are distinct from monocytes and adherent macrophages. Atherosclerosis. 219: 475–483. [DOI] [PubMed] [Google Scholar]
- 29.Sengupta B., Narasimhulu C. A., and Parthasarathy S.. 2013. Novel technique for generating macrophage foam cells for in vitro reverse cholesterol transport studies. J. Lipid Res. 54: 3358–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ginsbach C., and Fahimi H. D.. 1987. Labeling of cholesterol with filipin in cellular membranes of parenchymatous organs. Standardization of incubation conditions. Histochemistry. 86: 241–248. [DOI] [PubMed] [Google Scholar]
- 31.Corcoran M. P., Lichtenstein A. H., Meydani M., Dillard A., Schaefer E. J., and Lamon-Fava S.. 2011. The effect of 17beta-estradiol on cholesterol content in human macrophages is influenced by the lipoprotein milieu. J. Mol. Endocrinol. 47: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basu S. K., Goldstein J. L., Anderson G. W., and Brown M. S.. 1976. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc. Natl. Acad. Sci. USA. 73: 3178–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kritharides L., Christian A., Stoudt G., Morel D., and Rothblat G. H.. 1998. Cholesterol metabolism and efflux in human THP-1 macrophages. Arterioscler. Thromb. Vasc. Biol. 18: 1589–1599. [DOI] [PubMed] [Google Scholar]
- 34.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 35.Raghavamenon A., Garelnabi M., Babu S., Aldrich A., Litvinov D., and Parthasarathy S.. 2009. Alpha-tocopherol is ineffective in preventing the decomposition of preformed lipid peroxides and may promote the accumulation of toxic aldehydes: a potential explanation for the failure of antioxidants to affect human atherosclerosis. Antioxid. Redox Signal. 11: 1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundler R., and Akesson B.. 1975. Regulation of phospholipid biosynthesis in isolated rat hepatocytes. Effect of different substrates. J. Biol. Chem. 250: 3359–3367. [PubMed] [Google Scholar]
- 37.Xiong Y., Zhao Y. Y., Goruk S., Oilund K., Field C. J., Jacobs R. L., and Curtis J. M.. 2012. Validation of an LC-MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 911: 170–179. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein J. L., and Brown M. S.. 2009. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawn R. M., Wade D. P., Garvin M. R., Wang X., Schwartz K., Porter J. G., Seilhamer J. J., Vaughan A. M., and Oram J. F.. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 104: R25–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klucken J., Buchler C., Orso E., Kaminski W. E., Porsch-Ozcurumez M., Liebisch G., Kapinsky M., Diederich W., Drobnik W., Dean M., et al. 2000. ABCG1 (ABC8), the human homolog of the Drosophila white gene, is a regulator of macrophage cholesterol and phospholipid transport. Proc. Natl. Acad. Sci. USA. 97: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn M. T., Parthasarathy S., and Steinberg D.. 1988. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc. Natl. Acad. Sci. USA. 85: 2805–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Augé N., Santanam N., and Parthasarathy S.. 1998. An efficient method for solubilizing β-carotene in aqueous solutions. J. Med. Food. 1: 39–43. [Google Scholar]
- 43.Miyazaki A., Sakashita N., Lee O., Takahashi K., Horiuchi S., Hakamata H., Morganelli P. M., Chang C. C., and Chang T. Y.. 1998. Expression of ACAT-1 protein in human atherosclerotic lesions and cultured human monocytes-macrophages. Arterioscler. Thromb. Vasc. Biol. 18: 1568–1574. [DOI] [PubMed] [Google Scholar]
- 44.Wang H., Germain S. J., Benfield P. P., and Gillies P. J.. 1996. Gene expression of acyl-coenzyme-A:cholesterol-acyltransferase is upregulated in human monocytes during differentiation and foam cell formation. Arterioscler. Thromb. Vasc. Biol. 16: 809–814. [DOI] [PubMed] [Google Scholar]
- 45.Igarashi M., Osuga J., Uozaki H., Sekiya M., Nagashima S., Takahashi M., Takase S., Takanashi M., Li Y., Ohta K., et al. 2010. The critical role of neutral cholesterol ester hydrolase 1 in cholesterol removal from human macrophages. Circ. Res. 107: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 46.Tuomisto T. T., Riekkinen M. S., Viita H., Levonen A. L., and Yla-Herttuala S.. 2005. Analysis of gene and protein expression during monocyte-macrophage differentiation and cholesterol loading–cDNA and protein array study. Atherosclerosis. 180: 283–291. [DOI] [PubMed] [Google Scholar]
- 47.Tazoe F., Yagyu H., Okazaki H., Igarashi M., Eto K., Nagashima S., Inaba T., Shimano H., Osuga J., and Ishibashi S.. 2008. Induction of ABCA1 by overexpression of hormone-sensitive lipase in macrophages. Biochem. Biophys. Res. Commun. 376: 111–115. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W. Y., Gaynor P. M., and Kruth H. S.. 1996. Apolipoprotein E produced by human monocyte-derived macrophages mediates cholesterol efflux that occurs in the absence of added cholesterol acceptors. J. Biol. Chem. 271: 28641–28646. [DOI] [PubMed] [Google Scholar]
- 49.Braesch-Andersen S., Paulie S., Smedman C., Mia S., and Kumagai-Braesch M.. 2013. ApoE production in human monocytes and its regulation by inflammatory cytokines. PLoS One. 8: e79908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moessinger C., Kuerschner L., Spandl J., Shevchenko A., and Thiele C.. 2011. Human lysophosphatidylcholine acyltransferases 1 and 2 are located in lipid droplets where they catalyze the formation of phosphatidylcholine. J. Biol. Chem. 286: 21330–21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kanfer J. N., and McCartney D. G.. 1989. Glycerophosphorylcholine phosphocholine phosphodiesterase activity of rat brain myelin. J. Neurosci. Res. 24: 231–240. [DOI] [PubMed] [Google Scholar]
- 52.Yuan J., and Kanfer J. N.. 1994. Purification and properties of a glycerophosphocholine phosphodiesterase from bovine brain myelin. Neurochem. Res. 19: 43–48. [DOI] [PubMed] [Google Scholar]
- 53.Cuadrado A., Carnero A., Dolfi F., Jimenez B., and Lacal J. C.. 1993. Phosphorylcholine: a novel second messenger essential for mitogenic activity of growth factors. Oncogene. 8: 2959–2968. [PubMed] [Google Scholar]
- 54.Price B. D., Morris J. D., Marshall C. J., and Hall A.. 1989. Stimulation of phosphatidylcholine hydrolysis, diacylglycerol release, and arachidonic acid production by oncogenic ras is a consequence of protein kinase C activation. J. Biol. Chem. 264: 16638–16643. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.