Abstract
Extracellular lysophosphatidate and sphingosine 1-phosphate (S1P) are important bioactive lipids, which signal through G-protein-coupled receptors to stimulate cell growth and survival. The lysophosphatidate and S1P signals are terminated partly by degradation through three broad-specificity lipid phosphate phosphatases (LPPs) on the cell surface. Significantly, the expression of LPP1 and LPP3 is decreased in many cancers, and this increases the impact of lysophosphatidate and S1P signaling. However, relatively little is known about the physiological or pharmacological regulation of the expression of the different LPPs. We now show that treating several malignant and nonmalignant cell lines with 1 μg/ml tetracycline, doxycycline, or minocycline significantly increased the extracellular degradation of lysophosphatidate. S1P degradation was also increased in cells that expressed high LPP3 activity. These results depended on an increase in the stabilities of the three LPPs and increased expression on the plasma membrane. We tested the physiological significance of these results and showed that treating rats with doxycycline accelerated the clearance of lysophosphatidate, but not S1P, from the circulation. However, administering 100 mg/kg/day doxycycline to mice decreased plasma concentrations of lysophosphatidate and S1P. This study demonstrates a completely new property of tetracyclines in increasing the plasma membrane expression of the LPPs.
Keywords: autotaxin, matrix metalloproteinase, doxycycline, sphingosine 1-phosphate
Lysophosphatidate (LPA) has potent growth and migratory effects on cells through its activation of six G-protein-coupled receptors (1–3). Extracellular LPA is formed mainly through the hydrolysis of lysophosphatidylcholine by autotaxin (ATX), a secreted enzyme with lysophospholipase D activity (4). ATX activity and LPA signaling are essential for vasculogenesis and the formation of the neural crest in embryos (5, 6). A major function of increasing LPA availability in adult animals is in wound healing (7). ATX activity and LPA signaling is increased in response to inflammation, which normally results in tissue repair and resolution of the inflammation (4, 8). If the inflammation is not resolved, then LPA signaling remains increased and contributes to adverse pathologies seen in asthma, arthritis, fibrosis, Crohn’s disease and ulcerative colitis, atherosclerosis, hepatitis, and multiple sclerosis (4, 9). LPA signaling is also increased in many cancers (10–13), which have been described as “wounds that do not heal” (14). LPA provides part of the signal that leads to increased tumor growth, angiogenesis, metastasis, and the development of resistance to chemotherapy (1, 15). LPA signaling also produces a vicious inflammatory cycle because it increases the production of multiple inflammatory cytokines by cancer cells and in surrounding adipose tissue in the case of breast cancer (16). These cytokines further increase ATX expression. Consequently, considerable effort has been directed to decreasing LPA signaling by various strategies including blocking LPA formation (4, 9, 17) using antibodies against LPA (18) or using LPA receptor antagonists (19).
Another component in controlling the accumulation of extracellular LPA is its dephosphorylation by a family of three enzymes called lipid phosphate phosphatases (LPPs) (1, 20, 21). The LPPs are integral membrane enzymes whose catalytic domains face the extracellular space or the luminal space when the LPPs are located on the plasma membranes or internal membranes, respectively (22). LPPs dephosphorylate a wide variety of lipid phosphates and pyrophosphates (20, 23). One of their major functions is to dephosphorylate extracellular LPA and possibly sphingosine 1-phosphate (S1P). Significantly, the activities of LPP1 and LPP3 are decreased in many cancers (2, 20), which together with increased ATX activity (4), exposes tumors to higher extracellular LPA concentrations.
Therefore, an alternative way to reduce LPA signaling in various inflammatory conditions including cancers is by increasing the low expressions of LPP1 and LPP3, which are often observed in different malignancies (20, 24–26). So far, it has been established that gonadotropin-releasing hormone increases LPP activity and LPP3 expression in ovarian cancer cells (27). Overexpression of LPP1 in ovarian cancer cell lines increases LPA hydrolysis, which is associated with a marked inhibition of cell proliferation and colony-forming activity and increased apoptosis (28). In SKOV3 and OVCAR-3 ovarian cancer cells, LPP3 overexpression promotes extracellular LPA hydrolysis, decreases LPA-stimulated colony formation, and decreases tumor growth in mice (29). Similarly, increasing the low expression of LPP1 expression in breast and thyroid cancer cells increases the dephosphorylation of extracellular LPA and also attenuates signaling downstream of LPA and other G-protein-coupled receptors (23). These effects resulted in a dramatic suppression in breast and thyroid tumor growth in mouse models (23).
However, designing therapies that will increase the activity of an enzyme represents much more of a challenge compared with creating an enzyme inhibitor. During our previous work, we used a doxycycline-inducible promoter to express LPP1 in the cancer cells (23). We observed that treating cancer cells with increasing doxycycline concentrations enhanced the degradation of exogenous LPA, even in those cells that did not express recombinant LPP1. We, therefore, initiated the present work to elucidate this new effect of doxycycline.
Tetracyclines were discovered in 1940s, and they have developed into a family of broad-spectrum antibiotics with activities against bacteria, chlamydiae, mycoplasmas, rickettsiae, and protozoan parasites (30). The bacteriostatic activity is caused by preventing the binding of aminoacyl tRNA to ribosomes, which is necessary for protein synthesis (31). It is also well documented that tetracyclines inhibit matrix metalloproteinase (MMP) activities (32). In the present study, we demonstrate for the first time that doxycycline increases the degradation of extracellular LPA in multiple cell lines through enhancing the stabilities of LPP1, LPP2, and LPP3 and the expression of these enzymes on the plasma membrane. The physiological relevance of these findings was demonstrated because doxycycline also increased the rate of LPA clearance from the circulation of rats, and it decreased the steady-state concentrations of LPA and S1P in the plasma of mice. The present work, therefore, establishes a novel effect of tetracyclines in decreasing the circulating levels of LPA through increasing the degradation of extracellular LPA. This property could decrease excessive signaling by LPA through its receptors.
MATERIALS AND METHODS
Reagents
Oleoyl-LPA (233019) was from Avanti Polar Lipids (Alabaster, AL). Minocycline hydrochloride (475843), marimastat (444289), mouse anti myc-tag (05-724) antibody, mouse anti HA tag (human influenzahemagglutinin amino acids 98-106) antibody (05-904), rabbit anti-integrin α3 (AB1920), and β1 (04-1109) antibodies were from EMD Millipore (Etobicoke, Ontario, Canada). Doxycycline hyclate (0219895525) was from MP Biomedicals (Solon, OH). Rabbit anti EGFR (sc-03) and rabbit anti E-cadherin (H-108) antibodies were from Santa Cruz (Dallas, TX). Tetracycline hydrochloride (T7660), fatty acid-free albumin from bovine serum (A8806), mouse anti-tubulin (T6074) antibody, and protease inhibitor cocktail (P8340) were from Sigma (St. Louis, MO). The transfection reagent PolyJet (SL100688) was from SignaGen Laboratories (Gaithersburg, MD). Sulfo NHS-SS-biotin (PG82077), streptavidin agarose (20349), G418 sulfate (11811-031), pENTR/D-TOPO Cloning Kit (K2400-20), and LR clonase enzyme mix (11791-019) were from Life Technologies (Grand Island, NY). PfuUltra DNA polymerase (600385) was from Agilent Technologies (Santa Clara, CA). Anti-GFP antibody was kindly provided by Dr. L. Berthiaume (Department of Cell Biology, University of Alberta, Edmonton, Alberta, Canada), and [γ-32P]ATP (BLU002250UC) was from PerkinElmer (Woodbridge, Ontario, Canada). Human recombinant sphingosine kinase-1 (10348) was from Cayman Chemical (Burlington, Ontario, Canada).
Cell culture and lentivirus generation
Human mammary carcinoma cell lines MDA-MB-231 and MCF-7, mouse mammary carcinoma cell line 4T1, human mammary epithelial cell line MCF10A, and human embryonic kidney 293 (HEK293) and HEK293T cell lines were from ATCC (Manassas, VA). MCF10A cells were cultured in DMEM/F12 medium with 10% FBS, 20 ng/ml epidermal growth factor (EGF), 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, and 10 μg/ml insulin. Other cell lines were maintained in DMEM supplemented with 10% FBS. C-terminus myc-tagged human LPP1, LPP2, and LPP3 sequences (plpp1, plpp2, and plpp3 genes, respectively) were generated by PCR using cDNA from HEK293 cells. They were first cloned into the pENTR/D-TOPO vector and then the lentiviral destination vector pLenti-PGK-Neo-DEST (Addgene19067) carrying a neomycin selection marker through LR recombination using Gateway® Technology. Lentivirus was generated as described previously (20) by cotransfecting the lentiviral vector and packaging vectors into HEK293T cells. C-terminus green fluorescent protein (GFP)-tagged human LPP1, LPP2, and LPP3 were transiently expressed by using pEZY3 plasmid. Human LPP1, LPP2, and LPP3 sequences were first cloned into pENTR1A-GFP-N2 vector (Addgene 19364) between HindIII and SacII sites, and then recombinated into the pEZY3 vector (Addgene 18672) through LR reaction.
Preparation of 32P-labeled LPA and S1P and measurement of LPP activity
[32P]S1P was prepared by incubating 0.4 μmol sphingosine with 0.01 U recombinant sphingosine kinase-1 and 400 μCi [γ-32P]ATP for 12 h at 37°C in 200 µl of reaction buffer, which contained 20 mM Tris-HCl, pH 7.4; 20% glycerol; 1 mM 2-mercaptoethenol; 1 mM EDTA; 1 mM sodium orthovanadate; 10 mM MgCl2; and 1 mM ATP. [32P]S1P was extracted into butan-1-ol. [32P]LPA and was prepared as described previously (33, 34). [32P]LPA and [32P]S1P were then purified by thin-layer chromatography as described previously (33, 34), and they were then diluted with nonradioactive substrate to a specific radioactivity of ∼1 × 107 dpm/µmol. Ecto-LPP activity against [32P]LPA and [32P]S1P and total LPP activity against [3H]phosphatidate (PA) were measured as described by Jasinska et al. (33).
Real-time PCR and Western blotting
LPP1, LPP2, and LPP3 mRNA levels were determined by quantitative RT-PCR using GAPDH as a reference mRNA. Protein levels were measured by Western blotting as described previously (23). Immunoblots were analyzed by Odyssey infrared imaging system (LI-COR Biosciences).
Expression of LPPs on the plasma membrane
The expression of plasma membrane proteins was determined by tagging the exposed proteins with a noncell permeable biotinylation reagent (35). Briefly, cells were cultured in 10 cm dishes and pretreated with or without doxycycline (0.5–2 μg/ml) for 24 h. Cells were placed on ice for 20 min and washed with ice-cold PBS. Cells were incubated with 0.4 mg/ml Sulfo-NHS-SS-biotin in PBS for 30 min on ice and then washed three times with quenching buffer (50 mM glycine in PBS, pH 7.4) and incubated on ice in quenching buffer for 15 min. Cells were washed with PBS and lysed in RIPA buffer. After adjusting the protein concentrations of cell lysates, biotinylated proteins were precipitated using 30 μl streptavidin agarose. The LPPs were resolved by SDS-PAGE and revealed by immunoblotting.
LPA and S1P clearance in the circulation and whole blood of rats
Sprague Dawley rats (300 ± 20 g) were housed with free access to water and food (7001 Teklad 4% fat diet), and they were then pretreated with 50 mg/kg/day doxycycline through ip injection for 3 days. For measuring LPA clearance, rats were anesthetized with ip injection of 50 mg/kg sodium pentobarbital. A straight incision was made at the middle of the abdomen, and the peritoneal cavity was opened. The portal vein and hepatic artery were exposed by using two cotton swabs to gently lift the liver. After separating the adjacent connective tissues, the portal vein and hepatic artery were ligated using 2.0 silk sutures, and the peritoneal cavity was closed. A straight incision was then made at the middle of neck. The right carotid artery was separated by blunt dissection, and catheterization was performed with a 22 GA catheter. [32P]LPA (∼500,000 dpm) was added in 0.5 ml saline containing 0.5 μM nonradioactive LPA, 500 μM Evans blue, and 0.1% BSA and injected into the circulation through the catheter. About 300 μl of blood was collected into an EDTA-coated insulin syringe through the catheter at 30, 60, 90, 120, 180, 240, and 300 s after the injection. Blood samples were centrifuged at 14,000 rpm for 1 min, and 100 μl plasma was collected. HCl (1 M, 50 μl) was added into the plasma and mixed well. Plasma LPA was extracted twice with 100 μl butan-1-ol before measuring the radioactivity. Concentrations of Evans blue were calculated with a standard curve plotted at A620nm, and this value was used to estimate the volume of circulation and the amount of [32P]LPA in the circulation. Clearance of [32P]S1P in rat circulation was measured as for [32P]LPA, with the exception that animals were not subject to portal vein and hepatic artery ligation. Blood was collected at 1, 2, 3, 5, and 10 min after injection of [32P]S1P.
For measuring LPA and S1P degradation in whole blood in vitro, rats were injected ip with vehicle or 50 mg/kg/day of doxycycline for 3 days. They were anesthetized with ip injection of 50 mg/kg sodium pentobarbital. Blood was collected into an EDTA-coated syringe by right carotid artery catheterization. [32P]LPA or [32P]S1P were injected into the whole blood at ∼50,000 dpm/ml and incubated at 37°C for 1, 5, 10, 20, and 30 min. After adding 1 M HCl, LPA and S1P in the whole blood were extracted with butanol and radioactivity determined.
Measurement of plasma LPA and S1P concentrations
Female BALB/c mice (22 ± 2 g) were housed with free access to water and food (7001 Teklad 4% fat diet) and pretreated with 100 mg/kg/day doxycycline for 3 days through ip injection. Mice were euthanized, and blood was collected through the heart using an EDTA-coated syringe. Plasma LPA and S1P concentrations were measured as described previously (17). Blood samples were centrifuged at 14,000 rpm for 1 min, and plasma was collected. Plasma was treated with internal standards (isotope labeled C17:0-LPA and [13C2D2]S1P), and lipid phosphates were extracted into butan-1-ol. Lysophospholipids were measured by LC/MS using electrospray ionization in the negative mode using an Agilent 1200 series LC system coupled to a 3200 QTRAP mass spectrometer (AB Sciex, Concord, Ontario, Canada). The concentrations of different LPA species (C16:0-LPA, C20:4-LPA, C18:2-LPA, C18:0-LPA, and C18:1-LPA) were summed as total LPA concentration in plasma.
Statistical analysis
Results were analyzed by Student’s t-test or by ANOVA for multiple comparisons followed by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
Tetracyclines increased LPP activity in multiple cell lines
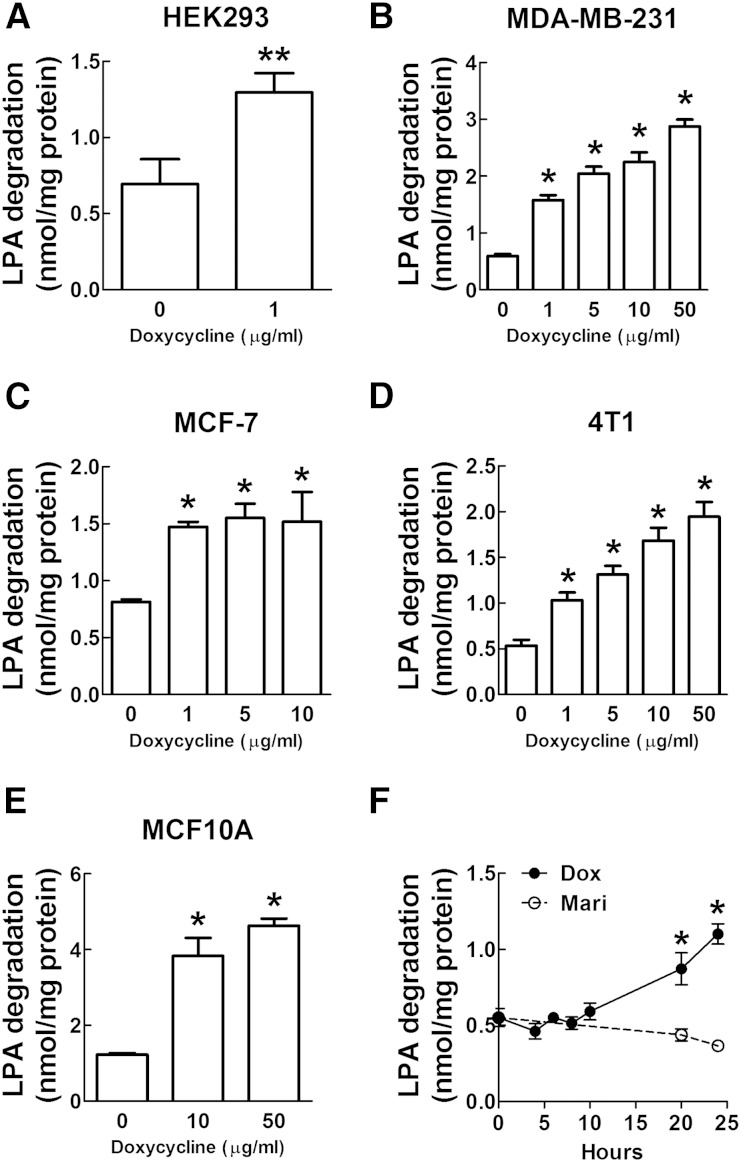
During our previous work (23), we used a doxycycline-inducible promoter to express LPP1 in lentivirus-transduced cells. We observed that doxycycline treatment for 24 h increased the dephosphorylation of exogenous [32P]LPA (ecto-activity) in HEK293 cells by ∼2-fold (Fig. 1A). Because LPP1 and LPP3 activities are decreased in many types of cancer cells, we next determined if doxycycline increased LPP activity in breast cancer cell lines. Treatment with 1 to 50 μg/ml (2 to 100 μM) doxycycline for 24 h increased dephosphorylation of extracellular LPA in human MDA-MB-231 and MCF-7 and mouse 4T1 breast cancer cell lines (Fig. 1B–D). In nontransformed human mammary epithelial MCF10A cell line, a 48 h treatment with doxycycline was required to increase LPP activity (Fig. 1E). We then treated MDA-MB-231 and 4T1 cells with 1 μg/ml tetracycline (2.1 μM) or 1 μg/ml minocycline (2.0 μM) and found that ecto-LPP activity was also increased by these two tetracyclines (supplementary Fig. 1A, B). These combined results demonstrate that tetraycyclines increased ecto-LPP activity in multiple cell types. To determine whether the effect of doxycycline resulted from the direct activation of LPPs, we measured the dephosphorylation of exogenous LPA at different times after incubation with 1 μg/ml (2 μM) doxycycline. LPP activity did not increase until after ∼20 h of doxycycline treatment (Fig. 1F). These results establish that doxycycline exerts a long-term effect rather than a direct activation of LPP activity.
Fig. 1.
Doxycycline increased the dephosphorylation of exogenous [32P]LPA (ecto-activity) in various cell lines. A: Treatment with doxycycline at 1 μg/ml (2 μM) for 24 h increased ecto-LPP activity in HEK293 cells. B: Treatment with doxycycline for 24 h increased ecto-LPP activity in human MDA-MB-231 breast cancer cell line. C: Treatment with doxycycline for 24 h increased ecto-LPP activity in human MCF-7 breast cancer cell line. D: Treatment with doxycycline for 24 h increased ecto-LPP activity in mouse 4T1 breast cancer cell line. E: Treatment with doxycycline for 48 h increased ecto-LPP activity in nontransformed human mammary epithelial MCF10A cell line. F: Ecto-LPP activity of MDA-MB-231 cells at different time points after treatment with doxycycline (Dox) at 1 μg/ml (2 μM) and pan MMP inhibitor marimastat (Mari) at 5 μM. Results are means ± SD from three independent experiments. Significant differences between drug-treated and nontreated cells are shown as * P < 0.05 and ** P < 0.01.
Because tetracyclines inhibit MMP activity, we determined whether MMP activity controls LPP activity and thus the degradation of LPA. Treatment with 5 μM marimastat, a pan MMP inhibitor, for 24 h did not change ecto-LPP activity in MDA-MB-231 cells (Fig. 1F). The IC50 of marimastat for MMPs are at nanomolar levels (36), and 5 μM marimastat is more than sufficient to inhibit different MMP isoforms. Tetracycline had a much higher IC50 for MMP-2 compared with marimastat: 160 μM versus 2.3 nM (supplementary Fig. 2A, B). Tetracyclines at 10 μg/ml (21 μM) did not change the amounts of secretion of MMP-2 and MMP-9 (supplementary Fig. 2C). These results indicated that the tetracycline-induced changes in LPP activity do not result from MMP inhibition.
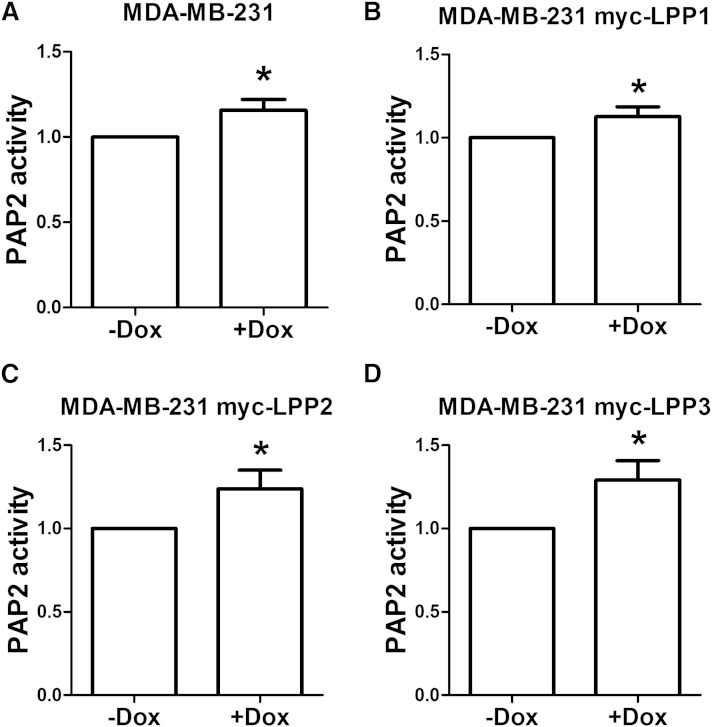
Doxycycline also increased the total LPP activity in cell lysates. In normal MDA-MB-231 cells, treatment with 5 μg/ml (10 μM) doxycycline for 48 h caused a small (∼16%) but consistent increase in total LPP activity (Fig. 2A). The same effect of doxycycline was also observed in MDA-MB-231 cells, which stably express myc-LPP1, myc-LPP2, and myc-LPP3 (Fig. 2B–D).
Fig. 2.
Doxycycline increased total LPP activity against [3H]PA. Treatment with doxycycline at 5 μg/ml (10 μM) for 48 h increased total LPP-activity in normal MDA-MB-231 cells (A), MDA-MB-231 cells expressing myc-LPP1 (B), MDA-MB-231 cells expressing myc-LPP2 (C), and MDA-MB-231 cells expressing myc-LPP3 (D). Results are means ± SD from four independent experiments with normal MDA-MB-231 cells or three MDA-MB-231 cells expressing myc-tagged LPPs. Significant differences between drug-treated and nontreated cells are shown as * P < 0.05.
Doxycycline increased the protein levels of LPP1, LPP2, and LPP3 and expression on the plasma membrane
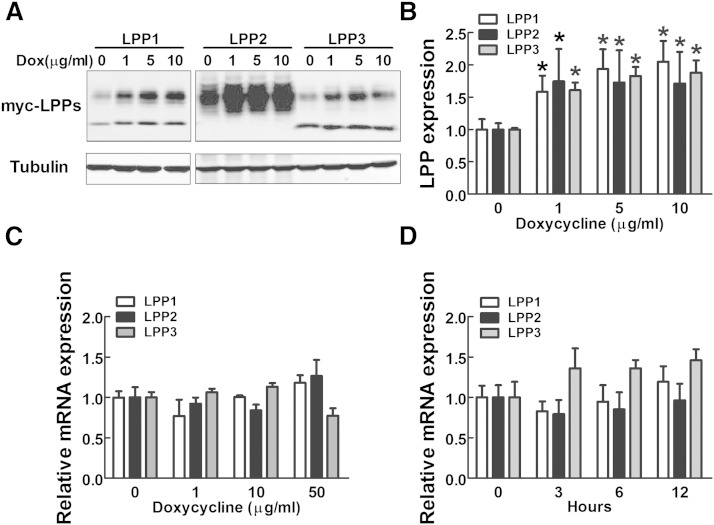
Because existing antibodies are not very effective in detecting endogenous levels of LPPs in cell lysates, we established three stable MDA-MB-231 cell lines expressing myc-tagged human LPP1, LPP2, and LPP3 by using lentivirus. Treatment with 1 μg/ml doxycycline for 24 h increased the expressions of myc-tagged LPP1, LPP2, and LPP3 (Fig. 3A, B). However, treatment with 1–50 μg/ml doxycycline for 24 h did not significantly change mRNA levels of LPPs in both normal (Fig. 3C) and virus-transduced MDA-MB-231 cells expressing myc-LPP1, LPP2, and LPP3 (supplementary Fig. 3). There was no significant change in mRNA levels of LPPs after 3–12 h of treatment with 1 μg/ml doxycycline (Fig. 3D). Therefore, doxycycline appears to increase the protein levels of LPPs through a posttranscriptional mechanism.
Fig. 3.
Doxycycline increases the expression of LPPs. A: Protein expressions of myc-tagged human LPP1, LPP2, and LPP3 in MDA-MB-231 cells with or without doxycycline (Dox) treatment at 1, 5, and 10 μg/ml (2, 10, and 20 μM) for 24 h. B: Quantification of expression levels of myc-LPP1, LPP2, and LPP3 in A. C: mRNA levels of LPP1, LPP2, and LPP3 relative to GAPDH mRNA in MDA-MB-231 cells after 24 h treatment with doxycycline. D: mRNA levels of LPP1, LPP2, and LPP3 relative to GAPDH mRNA in MDA-MB-231 cells after 1 μg/ml (2 μM) doxycycline treatment for different times. Results are means ± SD from three independent experiments. Significant differences between drug-treated and nontreated cells are shown as * P < 0.05.
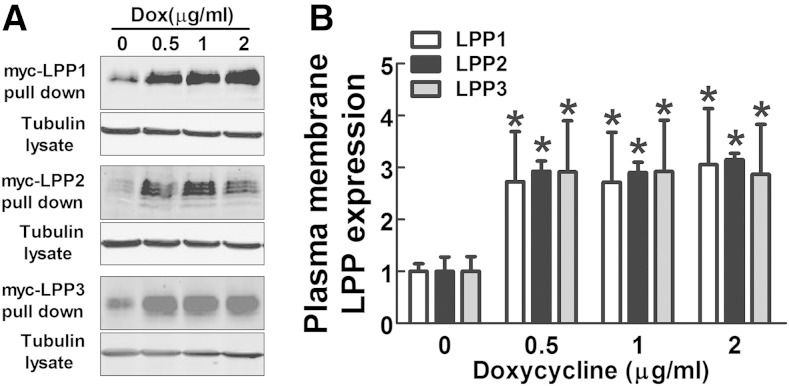
LPPs that degrade extracellular LPA are located on plasma membrane, and so we determined whether the amount of LPPs on the cell surface was changed by doxycycline. Plasma membrane proteins were labeled by using a noncell permeable biotinylation reagent and concentrated with streptavidin beads. Doxycycline treatment for 24 h significantly increased the amounts of myc-LPPs on plasma membrane (Fig. 4A, B). To determine whether these effects of doxycycline were specific to LPPs, we measured the levels of other plasma membrane proteins including integrin-α3, integrin-β1, EGF receptor, and E-cadherin. No significant changes in the expressions of these proteins were found after doxycycline treatment (supplementary Fig. 4A–C).
Fig. 4.
Doxycycline increases the expression of LPPs on the plasma membrane. A: Plasma membrane myc-LPP1, myc-LPP2, and myc-LPP3 in stable MDA-MB-231 cells after 24 h treatment with doxycycline. B: Quantification of plasma membrane myc-tagged LPP1, LPP2, and LPP3 in A. Results are means ± SD from three independent experiments. Significant differences between drug-treated and nontreated cells are shown as * P < 0.05.
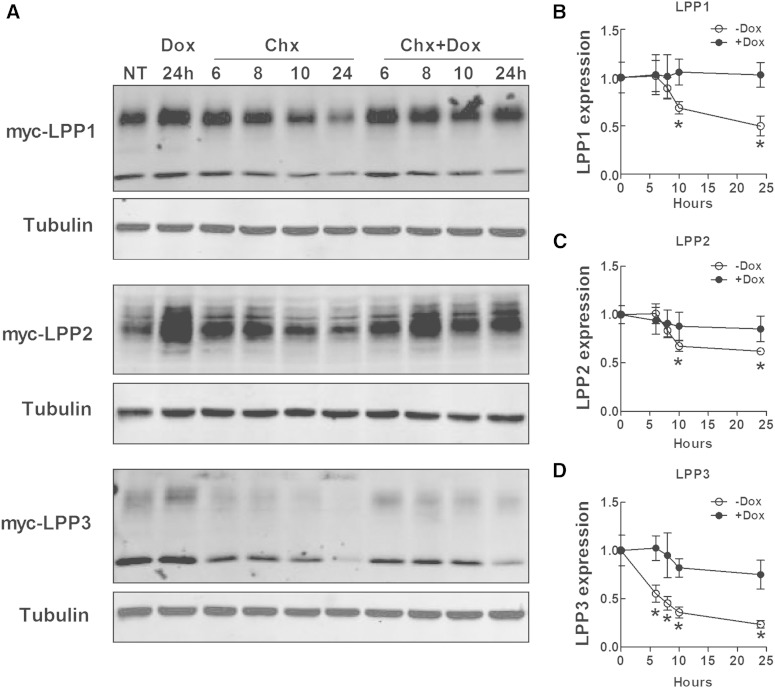
Doxycycline enhanced LPP1, LPP2, and LPP3 stability
Because doxycycline needs at least 20 h to increase LPP activity in cells and it did not affect mRNA levels for the LPPs, it seemed likely that doxycycline suppressed LPP degradation. To test this, we treated MDA-MB-231 cells that stably expressed myc-tagged LPP1, LPP2, and LPP3 with 50 μg/ml cycloheximide to inhibit protein synthesis. LPP1 and LPP2 levels decreased significantly after 10 h of cycloheximide treatment by ∼35% and 45%, respectively. Degradation of LPP3 was faster than LPP1 and LPP2. LPP3 levels were decreased by ∼45% after treatment for 6 h with cycloheximide. Adding doxycycline together with cycloheximide significantly slowed the degradation of the three LPPs (Fig. 5A–D). Doxycycline also increased the stability of GFP-LPP2 upon cycloheximide treatment in HEK293 cells, which transiently expressed GFP-LPP2 or HA-LPA receptor type 1 (LPAR1). However, doxycycline did not increase the stability of HA-tagged LPAR1 (supplementary Fig. 5A–D).
Fig. 5.
Doxycycline decreases the degradation of LPPs. A: Protein levels of myc-LPP1, myc-LPP2, and myc-LPP3 in stable MDA-MB-231 cells treated with 50 μg/ml cycloheximide (Chx) for 6, 8, 10, and 24 h in the presence or absence of 1 μg/ml doxycycline (Dox). The first two lanes on the left side are cells without treatment (NT) and cells treated with 1 μg/ml doxycycline for 24 h. B–D: Quantification of protein levels of myc-LPP1 (B), myc-LPP2 (C), and myc-LPP3 (D) in A. Results are means ± SD from three independent experiments. Significant differences between cells with or without doxycycline treatment are shown as * P < 0.05.
Protein levels of LPP1, LPP2, and LPP3 were also elevated by treatment with the lysosome inhibitor NH4Cl (50 mM), but not the proteasome inhibitor MG132 (10 μM) (supplementary Fig. 6A), indicating that LPPs are degraded in lysosomes. We then determine whether doxycycline decreased the degradation of the LPPs by increasing lysosomal pH using a ratiometric probe, LysoSensor™ Yellow/Blue DND-160 (Life Technologies). Doxycycline at concentrations of 2 μM did not change lysosmal pH, and this contrasted with the effects of 50 mM NH4Cl or 20 µM chloroquine, which increased lysosomal pH from about 4.2 to 10.8 and 6.8, respectively (supplementary Fig. 6B). We also tested whether doxycycline changes the cycling of LPPs between cytoplasm and plasma membrane by using a biotinylation method (37). We were unable to detect significant effects of doxycycline on the transportation of LPPs to cell surface or the recycling of LPPs (results not shown).
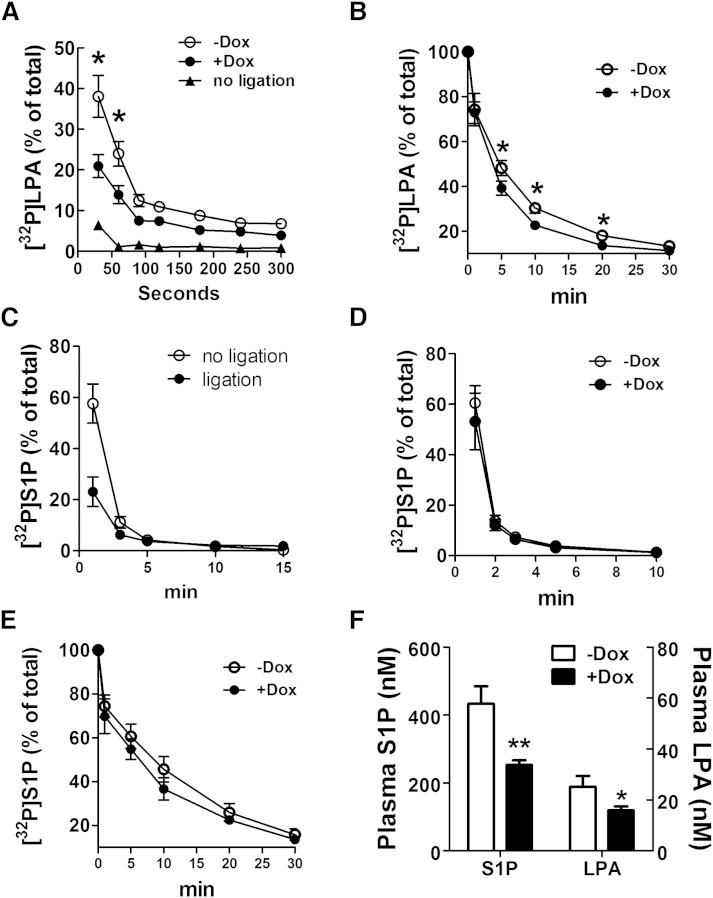
Doxycycline increased LPA clearance from the circulation of rats and in whole blood and decreased plasma LPA and S1P concentrations in mice
The importance of LPP1 in removing LPA from the circulation was demonstrated in LPP1 hypomorph mice (38). We, therefore, tested whether doxycycline could increase LPA clearance from the circulation by increasing LPP activity. Salous et al. (39) reported that the majority of intravenously injected LPA in mice is taken up by the liver, resulting in a rapid clearance of plasma LPA with the half-life of <30 s. Ligation of the dual hepatic blood supply significantly attenuated the rate of removal of intravenously administered LPA (39). We observed similar results in rats because 90% of [32P]LPA injected into the rat circulation was cleared within 30 s (Fig. 6A). The [32P]LPA that accumulated in liver accounted for ∼77% of that distributed among major organs (heart, liver, spleen, lung, kidney, and brain). Ligation of portal vein and hepatic artery completely eliminated the accumulation of [32P]LPA in liver (results not shown), and the time for clearing 90% of plasma [32P]LPA increased to ∼90 s (Fig. 6A). Consequently, we chose this strategy for measuring the effects of doxycycline on the LPP-dependent component of LPA clearance from the circulation. The clearance of [32P]LPA from the plasma was increased from 61% to 79% at 30 s and from 75% to 85% at 60 s after injection of [32P]LPA, in the doxycycline-treated rats compared with the controls (Fig. 6A).
Fig. 6.
Doxycycline increases the turnover of LPA in blood but not S1P. A: Effect of doxycycline on the turnover of [32P]LPA in circulation of rats. Rats with or without pretreatment of 50 mg/kg/day doxycycline (Dox) for 3 days were subjected to portal vein and hepatic artery ligation before experiment. Results from rats without ligation are also shown, n = 6. B: Effect of doxycycline on [32P]LPA degradation in whole blood. Blood was collected from rats with or without pretreatment with 50 mg/kg/day doxycycline (Dox) for 3 days, and incubated with [32P]LPA for the indicated time, n = 5. C: Removal of [32P]S1P from the circulation of rats with or without portal vein and hepatic artery ligation. D: Removal of [32P]S1P from circulation of rats with or without 50 mg/kg/day doxycycline treatment. E: Degradation of [32P]S1P in whole blood from rats with or without 50 mg/kg/day doxycycline treatment. F: Effect of doxycycline on plasma S1P and LPA concentrations in mice with or without 100 mg/kg/day doxycycline treatment for 3 days, n = 6. Results are means ± SD. Significant differences between animals with or without doxycycline treatment are shown as * P < 0.05 and ** P < 0.01.
We also measured LPA dephosphorylation in whole blood collected from control and doxycycline-treated rats because LPPs are expressed on blood cells. Doxycycline increased LPA dephosphorylation in the blood relative to control rats (Fig. 6B). [32P]LPA remaining in the whole blood was 48% versus 39% at 5 min, 30% versus 23% at 10 min, and 18% versus 14% at 20 min in control and doxycycline groups, respectively.
The dephosphorylation of S1P in the circulation differed significantly from that of LPA. First, S1P was not removed rapidly by the liver. In fact, ligation and exclusion of the liver from the circulation increased the disappearance of [32P]S1P (Fig. 6C). The half-life of injected [32P]S1P in whole blood was about 1 min, and doxycycline treatment of rats did not increase the removal of S1P from the circulation or the dephosphorylation of S1P in whole blood (Fig. 6D, E).
Dephosphorylation of exogenous [32P]S1P by native MDA-MB-231 cells was also not changed significantly by doxycycline (supplementary Fig. 7A). To explain this, we transiently expressed GFP-tagged human LPP1, LPP2, and LPP3 in HEK293 cells using the equal amount (0.75 μg/sample) of plasmids to achieve comparable expression levels of LPPs (supplementary Fig. 7B) and compared their respective activities against LPA and S1P. All three LPPs significantly increased LPA degradation, with the order of potency LPP3 > LPP1 > LPP2 (supplementary Fig. 7C). However, among the LPPs, LPP3 showed the greatest effect on S1P degradation, whereas overexpression of LPP1 and LPP2 produced limited increases of S1P dephosphorylation (supplementary Fig. 7D). Our result showed that LPP3 mRNA levels in MDA-MB-231 breast cancer cell line were ∼5-fold lower than the nontransformed MCF10A cell line (supplementary Fig. 7E), indicating that MDA-MB-231 may have a very low endogenous LPP3 level, which was not able to significantly increase S1P degradation even after doxycycline treatment. HEK293 cells overexpressing GFP-LPP3 showed a small but significant increase of S1P dephosphorylation by doxycycline (supplementary Fig. 7F).
To further support these results, we treated mice with 100 mg/kg/day doxycycline, and this significantly decreased the total concentration of LPA species in plasma by ∼38% (Fig. 6F). Doxycycline also decreased plasma SIP concentration by ∼42% (Fig. 6F).
DISCUSSION
In the present study, we report for the first time doxycycline increases the degradation of exogenous LPA by increasing the expressions of LPP1, LPP2, and LPP3 on the surface of cells. This increases the “ecto-activity” of the LPPs in degrading extracellular LPA. The mechanism for this effect involved the action of doxycycline in increasing the protein levels of LPP1, LPP2, and LPP3 by delaying their degradations. These results do not depend on the use of an myc-tag to identify the LPPs because doxycycline also increased the stability of GFP-tagged LPP2. The effect was specific because there was no effect of doxycycline on other plasma membrane proteins including integrin-α3, integrin-β1, EGF receptor, E-cadherin, and also HA-tagged LPAR1.
LPPs are plasma membrane proteins, which are likely to be degraded in lysosomes when they are internalized. This is compatible with the lack of effect of the proteasome inhibitor, MG132, on LPP levels and their increased expression in the presence of NH4Cl, which increases lysosomal pH to inhibit lysosomal proteases. However, doxycycline did not increase lysosomal pH. We were also not able to detect an effect of doxycycline on the cycling of the LPPs to and from the plasma membrane. The effects of doxycycline on LPP stability did not depend on the inhibition of MMP activity. The IC50 of tetracycline was ∼160 μM for inhibiting MMP2 activity, which is much higher than that of marimastat, ∼2.3 nM. For other MMP isoforms, the IC50 of marimastat are within nanomolar range (36). We showed that marimastat as high as 5 μM did not affect LPP activities.
As a consequence of the doxycycline-induced increase in localization of the LPPs on the plasma membrane, there is an increase in the dephosphorylation of extracellular LPA in multiple cell lines including cancer cells and in whole blood. Significantly, all three LPPs were effective in dephosphorylating extracellular LPA. We established the physiological significance of these findings in vivo by showing that doxycycline increased the clearance of LPA from the circulation in rats and decreased the steady-state concentrations of LPA in the plasma of mice.
The kinetics of the turnover of S1P in the circulation differed from that in LPA. S1P was not cleared rapidly by the liver. Doxycycline treatment did not increase S1P dephosphorylation significantly in the circulation or in whole blood. This is probably explained because dephosphorylation of extracellular S1P depends on the expression of LPP3, with LPP1 and LPP2 having little effect. These results indicate that although the LPPs show relatively little specificity for different lipid phosphates when these are in detergent micelles (40), there does appear to be selectivity for the ecto-activities in intact cells. Our conclusion that LPP3 acts preferentially on extracellular S1P is compatible with studies on the dephosphorylation of FTY720-P, which is an analog of S1P. Lysates from cells that overexpressed LPP1, LPP2, and LPP3 showed that only LPP3 dephosphorylated FTY720-P (41). LPP3 also acted as an ecto-phosphatase in intact cells in controlling the equilibrium between FTY720 and FTY720-P that was observed in vivo (41).
Doxycycline did decrease the steady-state level of circulating S1P in treated mice, but this reflects a balance of S1P production and removal. The effect could have resulted from increased LPP3 expression on plasma membranes throughout the body. It could also have been caused secondary to the decrease in LPA concentrations because LPA can increase S1P release from cells (42). However, we think that this is unlikely because decreasing plasma LPA concentrations with an ATX inhibitor did not lower the concentration of circulating S1P (13). A further possibility is that tetracyclines exert an anti-inflammatory effect (43, 44), which might indirectly decrease plasma S1P concentrations (45). Consequently, doxycycline can decrease the extracellular concentrations of two potent lysophospholipid mediators, LPA and S1P, in part by increasing the combined ecto-activities of LPP1, LPP2, and LPP3. This situation contrasts with our previous study in which we increased LPP1 expression only in the breast cancer cells that were injected into the mice. In this case, there were no significant changes in plasma LPA or S1P in the plasma or breast tumors of the mice (23). Also, doxycycline in the present work did not change S1P degradation significantly in MDA-MB-231 cells. This is probably because LPP3 is the major isoform that dephosphorylates exogenous S1P, and the levels of LPP1 and LPP3 are very low in several cancer cells including MDA-MB-231 cells. In fact, the level of LPP3 is approximately five times lower in MDA-MB-231 cells compared with nontransformed mammary epithelial cell line MCF10A. When HEK293 cells overexpressing GFP-LPP3 were treated with doxycycline, the degradation of exogenous S1P significantly increased.
We initiated this study because we observed that 1 μg/ml doxycycline or tetracycline, which is the dose commonly used for inducing the Tet-On/Off expression system, increased the dephosphorylation of LPA and S1P in intact cells. Therefore, this additional side effect of tetracyclines should be considered when using the Tet-On/Off system. LPA and S1P are very potent signaling molecules, and their signaling effects contribute to the progression of several inflammatory conditions such as asthma, arthritis, fibrosis, Crohn’s disease, ulcerative colitis, atherosclerosis, hepatitis, multiple sclerosis, and cancers. Increasing LPA degradation and decreasing its signaling actions have positive effects as cancer treatments. One strategy for achieving this is to increase the low expression of LPP1 and LPP3 in cancer cells (20, 23). However, increasing the activity of enzymes is relatively difficult compared with designing enzyme inhibitors. Our present work shows for the first time that doxycycline has the capacity to increase the degradation of extracellular LPA and S1P by increasing the expressions of the LPPs on plasma membranes of cells. This makes it reasonable to consider the application of tetracyclines for such diseases, which involve excessive S1P, and particularly LPA signaling.
Supplementary Material
Footnotes
Abbreviations:
- ATX
- autotaxin
- HA
- human influenzahemagglutinin (amino acids 98-106, HA-tag)
- LPA
- lysophosphatidic acid or lysophosphatidate (salt form at physiological pH)
- LPAR1
- LPA receptor type 1
- LPP
- lipid phosphate phosphatase
- MMP
- matrix metalloproteinase
- S1P
- sphingosine 1-phosphate
X.T. held a research fellowship from the Canadian Breast Cancer Foundation (CBCF), and D.N.B. was supported by grants from CBCF and the Women and Children’s Health Research Institute of the University of Alberta.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Brindley D. N., Lin F. T., and Tigyi G. J.. 2013. Role of the autotaxin-lysophosphatidate axis in cancer resistance to chemotherapy and radiotherapy. Biochim. Biophys. Acta. 1831: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samadi N., Bekele R., Capatos D., Venkatraman G., Sariahmetoglu M., and Brindley D. N.. 2011. Regulation of lysophosphatidate signaling by autotaxin and lipid phosphate phosphatases with respect to tumor progression, angiogenesis, metastasis and chemo-resistance. Biochimie. 93: 61–70. [DOI] [PubMed] [Google Scholar]
- 3.Tigyi G., and Parrill A. L.. 2003. Molecular mechanisms of lysophosphatidic acid action. Prog. Lipid Res. 42: 498–526. [DOI] [PubMed] [Google Scholar]
- 4.Benesch M. G., Ko Y. M., McMullen T. P., and Brindley D. N.. 2014. Autotaxin in the crosshairs: taking aim at cancer and other inflammatory conditions. FEBS Lett. 588: 2712–2727. [DOI] [PubMed] [Google Scholar]
- 5.van Meeteren L. A., Ruurs P., Stortelers C., Bouwman P., van Rooijen M. A., Pradere J. P., Pettit T. R., Wakelam M. J., Saulnier-Blache J. S., Mummery C. L., et al. 2006. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol. Cell. Biol. 26: 5015–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka M., Okudaira S., Kishi Y., Ohkawa R., Iseki S., Ota M., Noji S., Yatomi Y., Aoki J., and Arai H.. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem. 281: 25822–25830. [DOI] [PubMed] [Google Scholar]
- 7.Pilquil C., Ling Z. C., Singh I., Buri K., Zhang Q. X., and Brindley D. N.. 2001. Co-ordinate regulation of growth factor receptors and lipid phosphate phosphatase-1 controls cell activation by exogenous lysophosphatidate. Biochem. Soc. Trans. 29: 825–830. [DOI] [PubMed] [Google Scholar]
- 8.Panchatcharam M., Salous A. K., Brandon J., Miriyala S., Wheeler J., Patil P., Sunkara M., Morris A. J., Escalante-Alcalde D., and Smyth S. S.. 2014. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol. 34: 837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Federico L., Pamuklar Z., Smyth S. S., and Morris A. J.. 2008. Therapeutic potential of autotaxin/lysophospholipase d inhibitors. Curr. Drug Targets. 9: 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y., Shen Z., Wiper D. W., Wu M., Morton R. E., Elson P., Kennedy A. W., Belinson J., Markman M., and Casey G.. 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. J. Am. Med. Assoc. 280: 719–723. [DOI] [PubMed] [Google Scholar]
- 11.Schulte K. M., Beyer A., Kohrer K., Oberhauser S., and Roher H. D.. 2001. Lysophosphatidic acid, a novel lipid growth factor for human thyroid cells: over-expression of the high-affinity receptor edg4 in differentiated thyroid cancer. Int. J. Cancer. 92: 249–256. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Umezu-Goto M., Murph M., Lu Y., Liu W., Zhang F., Yu S., Stephens L. C., Cui X., Murrow G., et al. 2009. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 15: 539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benesch M. G., Ko Y. M., Tang X., Dewald J., Lopez-Campistrous A., Zhao Y. Y., Lai R., Curtis J. M., Brindley D. N., and McMullen T. P.. 2015. Autotaxin is an inflammatory mediator and therapeutic target in thyroid cancer. Endocr. Relat. Cancer. 22: 593–607. [DOI] [PubMed] [Google Scholar]
- 14.Dvorak H. F. 2015. Tumors: wounds that do not heal-redux. Cancer Immunol. Res. 3: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venkatraman G., Benesch M. G., Tang X., Dewald J., McMullen T. P., and Brindley D. N.. 2015. Lysophosphatidate signaling stabilizes Nrf2 and increases the expression of genes involved in drug resistance and oxidative stress responses: implications for cancer treatment. FASEB J. 29: 772–785. [DOI] [PubMed] [Google Scholar]
- 16.Benesch M. G., Tang X., Dewald J., Dong W. F., Mackey J. R., Hemmings D. G., McMullen T. P., and Brindley D. N.. 2015. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J. 29: 3990–4000. [DOI] [PubMed] [Google Scholar]
- 17.Benesch M. G., Tang X., Maeda T., Ohhata A., Zhao Y. Y., Kok B. P., Dewald J., Hitt M., Curtis J. M., McMullen T. P., et al. 2014. Inhibition of autotaxin delays breast tumor growth and lung metastasis in mice. FASEB J. 28: 2655–2666. [DOI] [PubMed] [Google Scholar]
- 18.Crack P. J., Zhang M., Morganti-Kossmann M. C., Morris A. J., Wojciak J. M., Fleming J. K., Karve I., Wright D., Sashindranath M., Goldshmit Y., et al. 2014. Anti-lysophosphatidic acid antibodies improve traumatic brain injury outcomes. J. Neuroinflammation. 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komachi M., Sato K., Tobo M., Mogi C., Yamada T., Ohta H., Tomura H., Kimura T., Im D. S., Yanagida K., et al. 2012. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 103: 1099–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang X., Benesch M. G., and Brindley D. N.. 2015. Lipid phosphate phosphatases and their roles in mammalian physiology and pathology. J. Lipid Res. 56: 2048–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brindley D. N., and Pilquil C.. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res. 50 (Suppl.): S225–S230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kok B. P., Venkatraman G., Capatos D., and Brindley D. N.. 2012. Unlike two peas in a pod: lipid phosphate phosphatases and phosphatidate phosphatases. Chem. Rev. 112: 5121–5146. [DOI] [PubMed] [Google Scholar]
- 23.Tang X., Benesch M. G., Dewald J., Zhao Y. Y., Patwardhan N., Santos W. L., Curtis J. M., McMullen T. P., and Brindley D. N.. 2014. Lipid phosphate phosphatase-1 expression in cancer cells attenuates tumor growth and metastasis in mice. J. Lipid Res. 55: 2389–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhattacharjee A., Richards W. G., Staunton J., Li C., Monti S., Vasa P., Ladd C., Beheshti J., Bueno R., Gillette M., et al. 2001. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl. Acad. Sci. USA. 98: 13790–13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis C., Shah S. P., Chin S. F., Turashvili G., Rueda O. M., Dunning M. J., Speed D., Lynch A. G., Samarajiwa S., Yuan Y., et al. 2012. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 486: 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshihara K., Tajima A., Komata D., Yamamoto T., Kodama S., Fujiwara H., Suzuki M., Onishi Y., Hatae M., Sueyoshi K., et al. 2009. Gene expression profiling of advanced-stage serous ovarian cancers distinguishes novel subclasses and implicates ZEB2 in tumor progression and prognosis. Cancer Sci. 100: 1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imai A., Furui T., Tamaya T., and Mills G. B.. 2000. A gonadotropin-releasing hormone-responsive phosphatase hydrolyses lysophosphatidic acid within the plasma membrane of ovarian cancer cells. J. Clin. Endocrinol. Metab. 85: 3370–3375. [DOI] [PubMed] [Google Scholar]
- 28.Tanyi J. L., Hasegawa Y., Lapushin R., Morris A. J., Wolf J. K., Berchuck A., Lu K., Smith D. I., Kalli K., Hartmann L. C., et al. 2003. Role of decreased levels of lipid phosphate phosphatase-1 in accumulation of lysophosphatidic acid in ovarian cancer. Clin. Cancer Res. 9: 3534–3545. [PubMed] [Google Scholar]
- 29.Tanyi J. L., Morris A. J., Wolf J. K., Fang X., Hasegawa Y., Lapushin R., Auersperg N., Sigal Y. J., Newman R. A., Felix E. A., et al. 2003. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 63: 1073–1082. [PubMed] [Google Scholar]
- 30.Rasmussen B., Noller H. F., Daubresse G., Oliva B., Misulovin Z., Rothstein D. M., Ellestad G. A., Gluzman Y., Tally F. P., and Chopra I.. 1991. Molecular basis of tetracycline action: identification of analogs whose primary target is not the bacterial ribosome. Antimicrob. Agents Chemother. 35: 2306–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onoda T., Ono T., Dhar D. K., Yamanoi A., and Nagasue N.. 2006. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int. J. Cancer. 118: 1309–1315. [DOI] [PubMed] [Google Scholar]
- 32.Myers S. A., and Wolowacz R. G.. 1998. Tetracycline-based MMP inhibitors can prevent fibroblast-mediated collagen gel contraction in vitro. Adv. Dent. Res. 12: 86–93. [DOI] [PubMed] [Google Scholar]
- 33.Jasinska R., Zhang Q. X., Pilquil C., Singh I., Xu J., Dewald J., Dillon D. A., Berthiaume L. G., Carman G. M., Waggoner D. W., et al. 1999. Lipid phosphate phosphohydrolase-1 degrades exogenous glycerolipid and sphingolipid phosphate esters. Biochem. J. 340: 677–686. [PMC free article] [PubMed] [Google Scholar]
- 34.Brindley D. N., Xu J., Jasinska R., and Waggoner D. W.. 2000. Analysis of ceramide 1-phosphate and sphingosine-1-phosphate phosphatase activities. Methods Enzymol. 311: 233–244. [DOI] [PubMed] [Google Scholar]
- 35.Chanoux R. A., Robay A., Shubin C. B., Kebler C., Suaud L., and Rubenstein R. C.. 2012. Hsp70 promotes epithelial sodium channel functional expression by increasing its association with coat complex II and its exit from endoplasmic reticulum. J. Biol. Chem. 287: 19255–19265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasmussen H. S., and McCann P. P.. 1997. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol. Ther. 75: 69–75. [DOI] [PubMed] [Google Scholar]
- 37.Bobulescu I. A., Dwarakanath V., Zou L., Zhang J., Baum M., and Moe O. W.. 2005. Glucocorticoids acutely increase cell surface Na+/H+ exchanger-3 (NHE3) by activation of NHE3 exocytosis. Am. J. Physiol. Renal Physiol. 289: F685–F691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomsig J. L., Snyder A. H., Berdyshev E. V., Skobeleva A., Mataya C., Natarajan V., Brindley D. N., and Lynch K. R.. 2009. Lipid phosphate phosphohydrolase type 1 (LPP1) degrades extracellular lysophosphatidic acid in vivo. Biochem. J. 419: 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salous A. K., Panchatcharam M., Sunkara M., Mueller P., Dong A., Wang Y., Graf G. A., Smyth S. S., and Morris A. J.. 2013. Mechanism of rapid elimination of lysophosphatidic acid and related lipids from the circulation of mice. J. Lipid Res. 54: 2775–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brindley D. N., Pilquil C., Sariahmetoglu M., and Reue K.. 2009. Phosphatidate degradation: Phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim. Biophys. Acta. 1791: 956–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mechtcheriakova D., Wlachos A., Sobanov J., Bornancin F., Zlabinger G., Baumruker T., and Billich A.. 2007. FTY720-phosphate is dephosphorylated by lipid phosphate phosphatase 3. FEBS Lett. 581: 3063–3068. [DOI] [PubMed] [Google Scholar]
- 42.Shida D., Fang X., Kordula T., Takabe K., Lepine S., Alvarez S. E., Milstien S., and Spiegel S.. 2008. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 68: 6569–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Caprio R., Lembo S., Di Costanzo L., Balato A., and Monfrecola G.. 2015. Anti-inflammatory properties of low and high doxycycline doses: an in vitro study. Mediators Inflamm. 2015: 329418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang J., Su W., Chen X., Cheng X., Dai Y., Han L., and Liang D.. 2015. Doxycycline attenuates endotoxin-induced uveitis by prostaglandin E2–EP4 signaling. Invest. Ophthalmol. Vis. Sci. 56: 6686–6693. [DOI] [PubMed] [Google Scholar]
- 45.Gomez-Muñoz A., Presa N., Gomez-Larrauri A., Rivera I. G., Trueba M., and Ordoñez M.. 2016. Control of inflammatory responses by ceramide, sphingosine 1-phosphate and ceramide 1-phosphate. Prog. Lipid Res. 61: 51–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.