Abstract
Elevated apoC-III levels predict increased cardiovascular risk when present on LDL and HDL particles. We developed novel high-throughput chemiluminescent ELISAs that capture apoB, lipoprotein (a) [Lp(a)], and apoA-I in plasma and then detect apoC-III on these individual lipoproteins as apoCIII-apoB, apoCIII-Lp(a), and apoCIII-apoAI complexes, respectively. We assessed the effects on these complexes of placebo or 100–300 mg volanesorsen, a generation 2.0+ antisense drug that targets apoC3 mRNA in patients with hypertriglyceridemia, including familial chylomicronemia syndrome (n = 3), volanesorsen monotherapy (n = 51), and as add-on to fibrate (n = 26), treated for 85 days and followed for 176 days. Compared with placebo, volanesorsen was associated with an 82.3 ± 11.7%, 81.3 ± 15.7%, and 80.8 ± 13.6% reduction in apoCIII-apoB, apoCIII-Lp(a), and apoCIII-apoA-I, respectively (300 mg dose; P < 0.001 for all), at day 92. Strong correlations in all assay measures were noted with total plasma apoC-III, chylomicron-apoC-III, and VLDL-apoC-III. In conclusion, novel high-throughput ELISAs were developed to detect lipoprotein-associated apoC-III, including for the first time on Lp(a). Volanesorsen uniformly lowers apoC-III on apoB-100, Lp(a), and apoA-I lipoproteins, and may be a potent agent to reduce triglycerides and cardiovascular risk mediated by apoC-III.
Keywords: hypertriglyceridemia, triglycerides, remnant lipoproteins, antisense oligonucleotides, cardiovascular disease, familial chylomicronemia syndrome, apolipoprotein C-III
ApoC-III is a 79 amino acid glycoprotein synthesized principally in the liver and associated with apoB-containing lipoproteins and HDL (1). apoC-III plays a key role in determining serum triglyceride levels and is a potent inhibitor of both the LPL-dependent and -independent pathways of triglyceride-rich lipoprotein (TRL) clearance. Thus, apoC-III inhibits the apoC-II-mediated activation of LPL and also inhibits hepatic lipase that plays an important role in the conversion of dense VLDL to IDL. Furthermore, apoC-III also inhibits TRL clearance by inhibiting receptor-mediated uptake of TRL by the liver (2).
Recent work has shown that the CVD risk of circulating plasma apoC-III is not uniform, but rather dependent on its association with specific lipoproteins (3–5). This suggests that the effect of both LDL and HDL on CVD risk is context-dependent on the presence or absence of apoC-III. However, current methods to detect apoC-III on LDL or HDL utilize labor-intensive immunoprecipitation of apoC-III and/or ultracentrifugation methods that can only be done in small numbers of samples and are not easily translatable to large outcome studies or clinical applications. Furthermore, lipoprotein (a) [Lp(a)] can contaminate both LDL and HDL fractions isolated with this technique. Therefore, we developed high-throughput sandwich chemiluminescent enzyme-linked immunoassays to detect apoC-III on individual lipoproteins in plasma.
Volanesorsen, a generation 2.0+ antisense oligonucleotide directed to mRNA of apoC3 has been shown to potently decrease plasma apoC-III and triglyceride levels by a mean of ∼70% in animal models, human volunteers, subjects with familial chylomicronemia syndrome (FCS), and individuals with primary hypertriglyceridemia (6–8). These assays were applied to a recent volanesorsen phase 2 randomized trial of apoC-III and triglyceride lowering (7, 8).
METHODS
Study subjects
The study subjects were derived from one phase 2 study of volanesorsen therapy (previously called ISIS 304801 and ISIS-APOC-IIIRx) (6–8), enrolling three groups of patients with hypertriglyceridemia: Group 1 included FCS subjects (n = 3) with triglyceride levels 1,406–2,083 mg/dl enrolled in an open-label study. These patients were administered volanesorsen, a generation 2.0+ antisense drug directed to APOC3 mRNA, at 300 mg once weekly for 13 weeks (no placebo arm) and then followed up to 176 days. Group 2 received volanesorsen as monotherapy [n = 51: placebo (n = 16), 100 mg (n = 11), 200 mg (n = 13), and 300 mg (n = 11)] in patients with hypertriglyceridemia of varying etiology with baseline fasting triglyceride levels 190–1,822 mg/dl; patients were randomized to volanesorsen at doses of 100, 200, and 300 mg or placebo once weekly for 13 weeks and then followed up to 176 days. Group 3 was comprised of subjects on stable fibrate therapy who received volanesorsen as an add-on [n = 26: placebo (n = 8), 200 mg (n = 8), and 300 mg (n = 10)] with fasting triglycerides between 204 and 932 mg/dl (7, 8). Patients were randomized to volanesorsen at doses of 200 and 300 mg or placebo once weekly for 13 weeks and then followed up to 176 days. Blood samples from this trial were available for the lipoprotein-associated apoC-III levels, as described below.
Laboratory measurements
Fasted blood samples were collected for measurement of lipid parameters at baseline and on days 57, 92, and 176 at the end of the posttreatment follow-up period, as previously described (8). Total plasma APOC-III, chylomicron/VLDL APOC-III (density gradient <1.006 g/ml), triglycerides, total cholesterol, HDL cholesterol (HDL-C) (precipitated), nonHDL-C (calculated; total cholesterol – HDL-C), LDL cholesterol (LDL-C) (isolated by ultracentrifugation), chylomicron/VLDL cholesterol (VLDL-C) (density gradient <1.006 g/ml), and apoB-48 levels were determined at MedPace Reference Labs (Cincinnati, OH). Triglycerides and cholesterol were measured by standard enzyme-based colorimetric assays. APOC-III was measured by rate nephelometry. NonHDL-C was calculated. VLDL and LDL fractions were isolated from samples by ultracentrifugation, and then assayed for apoB content (VLDL-apoB and LDL-apoB) at Ecogene-21 (Chicoutimi, QC, Canada). Lp(a) levels were performed as previously described (9).
Determination of lipoprotein-associated apoC-III complex levels: apoCIII-apoB, apoCIII-Lp(a), and apoCIII-apoAI
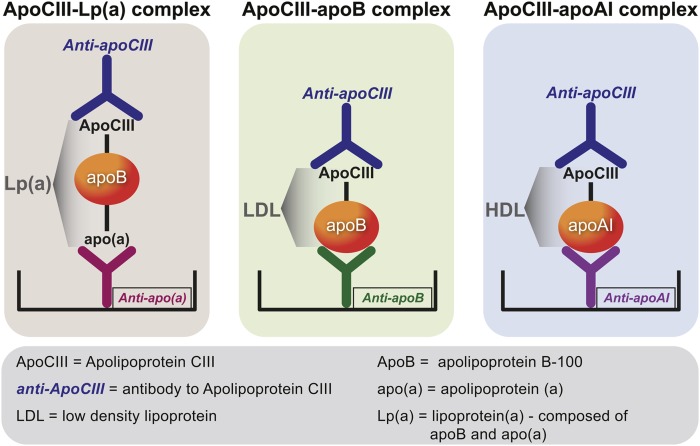
A sensitive and quantitative sandwich-based chemiluminescent ELISA was used to measure apoC-III associated with plasma lipoproteins containing apoB-100, Lp(a), and apoA-I (Fig. 1). Microtiter 96-well plates were coated overnight at 4°C with antibodies: MB47 to bind apoB-100 (10) (MB47 does not detect apoB-48); LPA4 (9) to bind Lp(a); and sheep anti-human apoA-I (The Binding Site, Birmingham, UK) (all at 5 μg/ml antigen of 40 μl/well). Conditions were established to ensure that the amount of plasma added was sufficient to provide a saturating and equal amount of each lipoprotein captured in each well. Excess material was washed off and the plates blocked with 1% TBS/BSA for 45 min. After the plates were washed, EDTA plasma was added at 1:50 dilution (40 μl/well) for 75 min to bind apoB-100, Lp(a), and HDL, respectively. After the plates were again washed, rabbit anti-apoC-III monoclonal antibody (Abgent, San Diego, CA) (at 1 μg/ml, 40 μ/well) was incubated with the plates for 60 min. After washing excess material off the plates, alkaline phosphatase-labeled goat anti-rabbit IgG (Sigma, St. Louis, MO) (40 μl/well) was added for 60 min. After a final washing, lumi-phos 530 (Lumigen, Inc., Southfield, MI) (25 l/well) was added for 75 min and luminescence read on a Dynex luminometer (Chantilly, VA). The results are reported as relative light units (RLU) in 100 milliseconds after the background (TBS/BSA) RLU were subtracted. High and low values were added to each 96-well plate as internal controls.
Fig. 1.
Methodology of lipoprotein-associated apoC-III assays. apoB-100, Lp(a), and apoA-I lipoproteins were captured from plasma with specific antibodies bound to microtiter well plates. Conditions were established so that the added plasma contains saturating amounts of lipoproteins to be captured. The content of apoC-III on each lipoprotein was then detected with a rabbit monoclonal anti-apoC-III antibody.
Although it would be ideal to provide absolute concentrations to lipoprotein-associated apoC-III in apoB, apoAI, and apo(a) fractions, technical limitations do not easily allow such a readout from this assay. If one used a known standard curve of apoC-III to provide a readout of apoC-III mass captured per well and presented this in milligrams per deciliter, it would be misleading, as the amount of apoC-III bound per given amount of apoB, apo(a), or apoAI cannot be easily determined. As the assays are currently performed, the amount of plasma is diluted to provide sufficient apoB, apo(a), and apoA-I to saturate the capacity of the given capture antibody such that each well has the same amount of lipoprotein as every other well, which allows one to compare the amount of bound apoC-III to each respective lipoprotein between subjects. We utilized an antibody approach to verify that each well contained equal amounts of the captured analyte to enable the measurement described above.
However, this assay does not allow quantifying the absolute amount of apoC-III per absolute amount of lipoprotein. It has been previously determined that the actual amounts of lipoproteins captured are in the nanogram range, and it would take a very sensitive method to verify the absolute amounts captured. This would then need to be separately verified for each captured analyte against a standard curve in each assay, which would greatly increase the workload for such an assay. Thus, as currently done, the relative amounts of apoB, apo(a), and apoA-I captured per well will always be equal in a given assay, but not necessarily the absolute amounts, and therefore one cannot express the absolute amount of apoC-III captured per absolute amount of lipoprotein.
Statistical analyses
Continuous variables are presented as mean ± SD or median [interquartile range (IQR)]. The percent change from baseline in lipoprotein-associated apoC-III levels between the volanesorsen-treated group and the placebo group are compared using the Wilcoxon Rank-sum test or t-test depending on the normality of the data. Spearman correlations were used to investigate the correlations between lipids, lipoproteins, and lipoprotein-associated apoC-III levels.
RESULTS
Baseline characteristics of the study group
The baseline characteristics of the combined groups used in this analysis are shown in Table 1. The patients tended to be primarily middle-aged males with increased BMI, and <50% were on statin therapy. They had markedly elevated triglycerides and low HDL-C. Baseline characteristics of the volanesorsen monotherapy and add-on fibrate had similar characteristics, as previously described (8).
TABLE 1.
Baseline characteristics of the combined study groups
| Volanesorsen Population (n = 80) | ||||
| Characteristic | Placebo (n = 24) | 100 mg (n = 11) | 200 mg (n = 21) | 300 mg (n = 24) |
| Gender, female:male | 7:17 | 4:7 | 3:18 | 9:15 |
| Age, years | 52.0 ± 14.1 | 53.0 ± 7.9 | 53.1 ± 12.0 | 52.1 ± 12.7 |
| BMI, kg/m2 | 31.9 ± 3.8 | 29.8 ± 4.4 | 30.3 ± 3.2 | 31.2 ± 4.6 |
| Statins, n (%) | 8 (33.3) | 5 (45.5) | 8 (38.1) | 8 (33.3) |
| Maximal statin dosea | 3 (12.5) | 1 (9.1) | 6 (28.6) | 2 (8.3) |
| Triglycerides, mg/dl | 500.8 ± 322.5 | 590.6 ± 260.3 | 504.5 ± 291.7 | 650.8 ± 522.7 |
| Median (IQR) | 464 (316, 582) | 558 (351, 825) | 448 (285, 687) | 451 (283, 810) |
| HDL-C, mg/dl | 33.6 ± 7.7 | 31.1 ± 4.2 | 34.0 ± 7.0 | 31.1 ± 12.3 |
| LDL-C, mg/dl | 101.4 ± 59.3 | 93.0 ± 32.6 | 95.2 ± 35.0 | 72.3 ± 36.8 |
| apoC-III, mg/dl | 21.1 ± 7.1 | 22.4 ± 7.7 | 20.2 ± 6.0 | 21.1 ± 6.6 |
Maximal statin (total daily dose): 40 mg and above of rosuvastatin, atorvastatin, pravastatin, or simvastatin.
Absolute changes in apoCIII-apoB, apoCIII-apoAI, and apoCIII-Lp(a) complex levels
As previously reported, plasma apoC-III and triglycerides were reduced by 71–90% and triglyceride levels by 56–86% in the FCS subjects who received the 300 mg dose (7). In the FCS group, plasma apoC-III levels were reduced by 71–90% and triglyceride levels by 56–86%. In the volanesorsen monotherapy group, apoC-III was decreased 40.0% in the 100 mg group, 63.8% in the 200 mg group, and 79.6% in the 300 mg group versus an increase of 4.2% in the placebo group. In the add-on to fibrate group, decreases of 60.2% in the 200 mg group and 70.9% in the 300-mg group versus a decrease of 2.2% in the placebo group were noted (8).
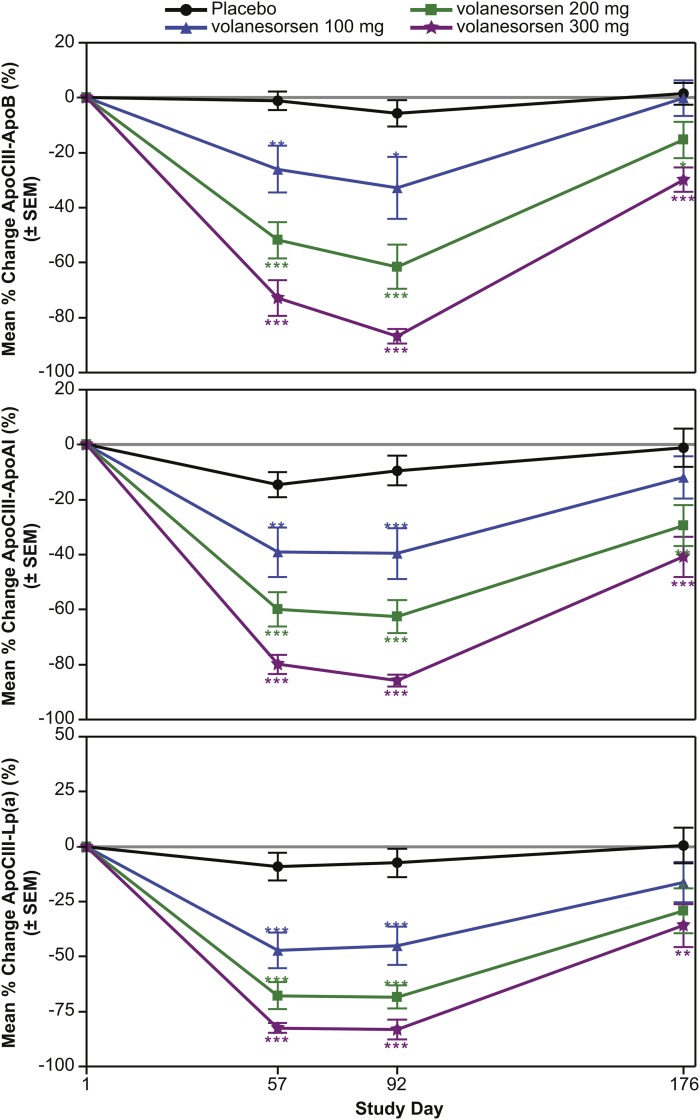
Table 2 displays the changes in levels of apoCIII-apoB, apoCIII-apoAI, and apoCIII-Lp(a) in the three groups of patients combined. Compared with the placebo group, significant decreases were present in apoCIII-apoB, apoCIII-apoAI, and apoCIII-Lp(a). The levels of all apoC-III-lipoprotein complexes reached a nadir at day 92 with 82.3 ± 11.7%, 81.3 ± 15.7%, and 80.8 ± 13.6% reductions in apoCIII-apoB, apoCIII-Lp(a), and apoCIII-apoA-I, respectively (300 mg dose; P < 0.001 for all) and a return toward baseline by day 176.
TABLE 2.
Baseline and follow-up mean absolute levels (IQR) of apoC-III-apoB, apoCIII-apoAI, and apoCIII-Lp(a)
| ISIS 304801 Monotherapy + Add-on to Fibrate + FCS | ||||
| Parameter | Placebo (n = 24) | 100 mg (n = 11) | 200 mg (n = 21) | 300 mg (n = 24) |
| apoCIII-apoB, RLU | ||||
| Baseline | 103,217 (17,063) | 107,604 (19,865) | 103,608 (14,876) | 97,936 (21,738) |
| Day 57 | 101,068 (20,549) | 76,027a (24,265) | 46,484c (27,242) | 28,342c (19,611) |
| Day 92 | 98,057 (23,356) | 68,531c (33,407) | 34,546c (25,071) | 17,483c (12,543) |
| Day 176 | 104,644 (21,042) | 104,675 (16,507) | 83,618c (21,362) | 731,447c (19,783) |
| apoCIII-apoAI, RLU | ||||
| Baseline | 100,606 (21,757) | 107,607 (22,186) | 106,888 (17,280) | 89,586 (24,604) |
| Day 57 | 87,092 (28,957) | 65,065a (33,715) | 39,949c (25,638) | 21,959c (13,813) |
| Day 92 | 91,511 (26,012) | 63,726b (32,981) | 36,571c (22,485) | 17,418c (12,976) |
| Day 176 | 95,938 (27,924) | 94,627 (31,242) | 69,900b (23,985) | 60,733c (26,865) |
| apoCIII-Lp(a), RLU | ||||
| Baseline | 79,731 (21,287) | 92,244 (24,821) | 81,260 (17,280) | 79,566 (29,656) |
| Day 57 | 72,705 (30,017) | 48,405b (29,098) | 25,099c (18,048) | 16,766c (12,285) |
| Day 92 | 73,712 (26,955) | 47,933c (26,511) | 23,050c (13,152) | 14,198c (10,559) |
| Day 176 | 78,076 (29,466) | 75,680 (29,471) | 51,614b (21,429) | 51,676c (24,673) |
Values are expressed as mean (SD).
P < 0.05 compared with placebo.
P < 0.01 compared with placebo.
P < 0.001 compared with placebo.
Mean percent changes in apoCIII-apoB, apoCIII-apoAI, and apoCIII-Lp(a) complex levels
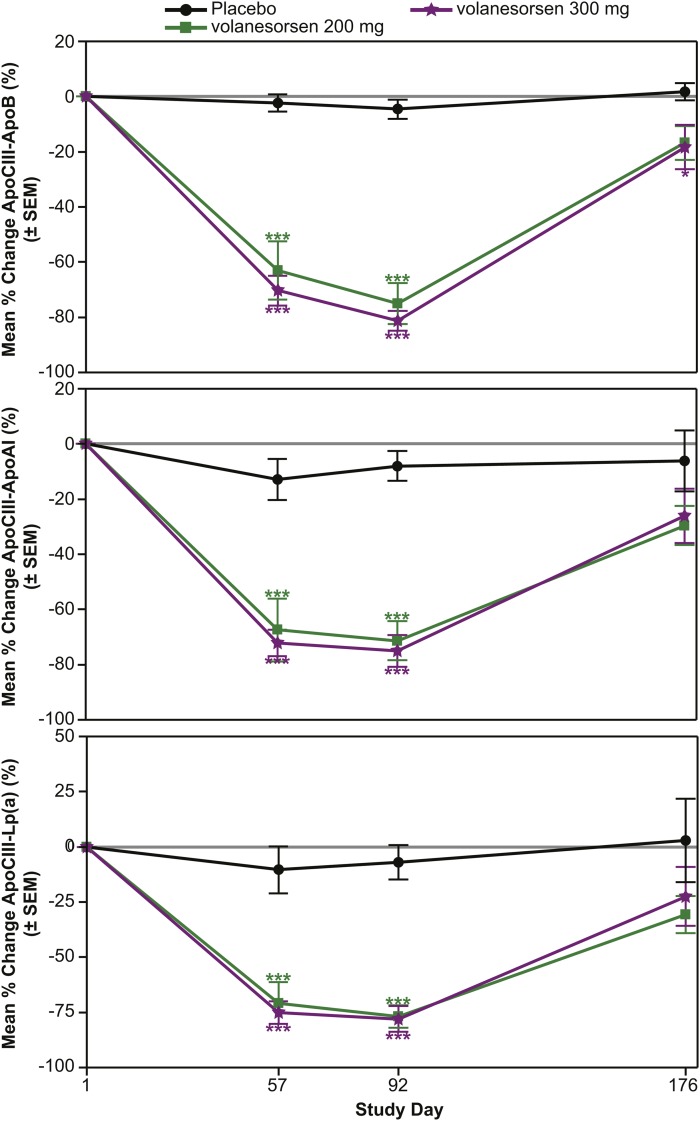
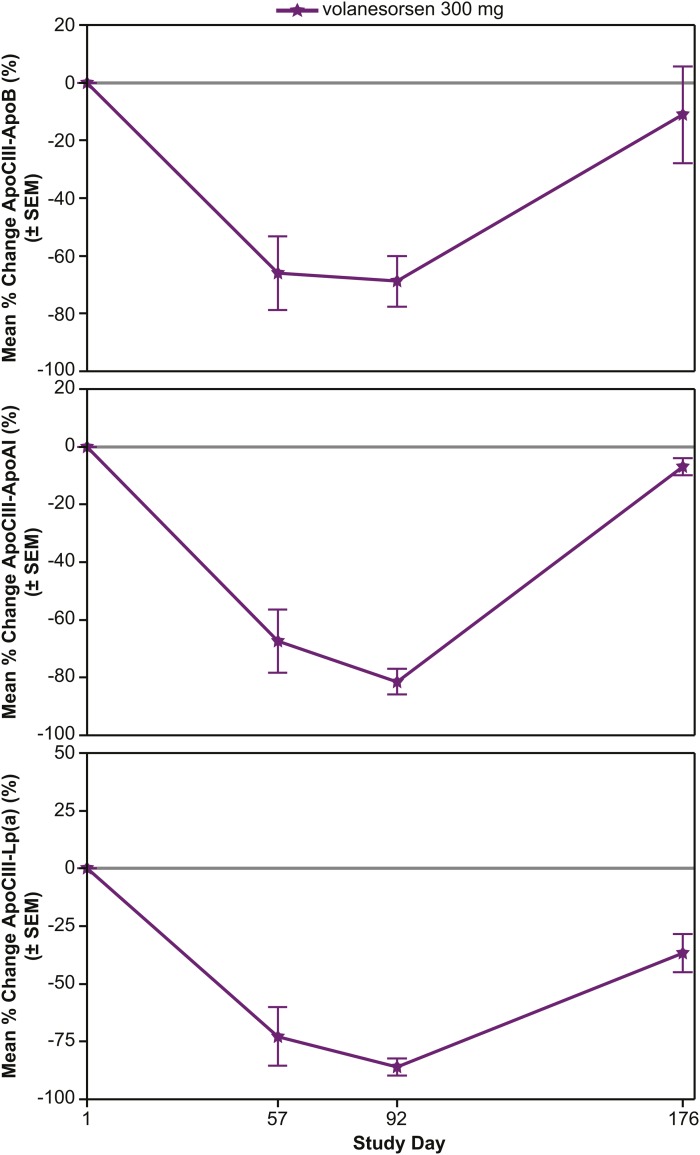
The mean percent changes were evaluated in the volanesorsen monotherapy (Fig. 2), add-on fibrate (Fig. 3), and familial chylomicronemia (Fig. 4) groups alone. In the volanesorsen monotherapy group compared with the placebo group, all three doses of volanesorsen resulted in time- and dose-dependent mean percent decreases in apoCIII-apoB, apoCIII-AI, and apoCIII-Lp(a) of approximately 80%. The levels of all three complexes reached a nadir at day 92 and tended to return to baseline, but not completely, by day 176. In the add-on to fibrate group, decreases in apoCIII-apoB, apoCIII-AI, and apoCIII-Lp(a) in the 200 and 300 mg doses were fairly similar, with approximately 75% reduction with less of a dose effect compared with placebo. Similar observations were noted in the FCS group with the 300 mg dose.
Fig. 2.
Mean percent reduction (± SEM) in apoCIII-apoB, apoCIII-apoA-I, and apoCIII-Lp(a) in the volanesorsen monotherapy group with 100, 200, and 300 mg/dose volanesorsen treatment.
Fig. 3.
Mean percent reduction (± SEM) in apoCIII-apoB, apoCIII-apoA-I, and apoCIII-Lp(a) in the add-on fibrate group with 100 and 300 mg/dose volanesorsen treatment.
Fig. 4.
Mean percent reduction (± SEM) in apoCIII-apoB, apoCIII-apoA-I, and apoCIII-Lp(a) in the familial chylomicronemia group following 300 mg/dose of volanesorsen treatment.
Spearman correlations between apoCIII-apoB, apoCIII-apoAI, and apoCIII-Lp(a) complex levels and lipid and lipoprotein levels
In the three groups combined, apoCIII-apoB levels correlated well with plasma total apoC-III levels, triglycerides, VLDL-C, VLDL-apoC-III, chylomicron-apoC-III, chylomicron-triglycerides, chylomicron-cholesterol, and LDL-C (Table 3). apoCIII-apoAI levels correlated well with plasma total apoC-III levels, VLDL-C, VLDL-apoC-III, chylomicron-apoC-III, total cholesterol, and nonHDL-C, and inversely with HDL-C. apoCIII-Lp(a) levels correlated well with plasma total apoC-III levels, triglycerides, VLDL-C, VLDL-apoC-III, chylomicron-apoC-III, chylomicron-triglycerides, chylomicron-cholesterol, total cholesterol, nonHDL-C, and inversely with LDL-C and HDL-C (Table 3). In individual groups, Spearman correlations between baseline apoC-III and triglyceride levels were r = 0.78 (P < 0.001) for the volanesorsen monotherapy group, r = 0.84 (P < 0.001) for the add-on fibrate group, and r = 1.00 (P < 0.001) for the FCS group.
TABLE 3.
Spearman correlations of baseline lipoprotein-associated apoC-III levels and lipids and lipoproteins
| apoC-III | TG | VLDL-C | apoB-48 | VLDL apoC-III | Chylomicron-apoC-III | Chylomicron TG | Chylomicron Cholesterol | TC | LDL-C | HDL-C | nonHDL-C | Lp(a) | |
| apoCIII-apoB | 0.60c | 0.48c | 0.43c | 0.40b | 0.38b | 0.58c | 0.64c | 0.52c | 0.15 | −0.35b | −0.28a | 0.17 | −0.08 |
| apoCIII-apoAI | 0.61c | 0.47c | 0.43b | 0.16 | 0.37b | 0.42b | 0.50c | 0.40b | 0.37b | 0.07 | −0.33a | 0.39b | −0.09 |
| apoCIII-apo(a) | 0.66c | 0.62c | 0.56c | 0.38b | 0.47c | 0.57c | 0.66c | 0.54c | 0.36b | −0.24 | −0.49c | 0.40b | −0.15 |
TC, total cholesterol; apoC-III, total plasma apoC-III.
P < 0.05.
P < 0.01.
P < 0.001.
DISCUSSION
This study demonstrates two novel findings: 1) that apoC-III can be detected directly on apoB-100, Lp(a), and apoA-I lipoproteins by immunocapture from plasma using novel ELISAs; and 2) that volanesorsen (7, 8), an antisense oligonucleotide targeting hepatic APOC3 mRNA significantly reduced the plasma content of apoC-III on apoB, Lp(a), and HDL lipoproteins in hypertriglyceridemic patients.
Elevated apoC-III levels and triglycerides are independent risk factors for CVD (5, 11). Recent genetic data has strongly suggested that apoC-III is a causal mediator of CVD (12, 13). Patients with elevated levels of triglycerides have concomitantly elevated levels of apoC-III and there is generally a high positive correlation between triglycerides and apoC-III (7, 8, 14). ApoC-III is a target of drug therapy with existing drugs, as well as drugs in development. However, all currently available drugs have modest effects on apoC-III levels. For example, fibrates, tesaglitazar, pioglitazone, fish-oils, niacin, statins, and ezetimibe reduce apoC-III levels by 10–30% (15–24).
Volanesorsen has been extensively evaluated in several animal models on different diets (6) in phase 1 (6) and phase 2 clinical trials (7, 8) and is currently being evaluated for triglyceride reduction in two phase 3 randomized placebo-controlled trials in patients with FCS and familial partial lipodystrophy. FCS patients, in particular, have the highest triglyceride levels among patients with hypertriglyceridemia and have frequent episodes of acute pancreatitis, which can be fatal, as well as long-term complications such as pancreatic insufficiency and diabetes mellitus (25). Ongoing studies with volanesorsen include the APPROACH [The APPROACH Study: A Study of Volanesorsen (Formerly ISIS-APOCIIIRx) in Patients with Familial Chylomicronemia Syndrome, NCT02211209] trial, the COMPASS [The COMPASS Study: A Study of Volanesorsen (Formally ISIS-APOCIIIRx) in Patients with Hypertriglyceridemia, NCT02300233] trial, and the BROADEN [The BROADEN Study: A Study of Volanesorsen (Formerly ISIS-APOCIIIRx) in Patients with Partial Lipodystrophy, NCT02527343] trial.
The clinical relevance of apoC-III measures on individual lipoprotein fractions isolated by ultracentifugation was shown by the Sacks’ group in the Nurses’ Health Study and Health Professionals Follow-Up Study using a nested case-control study design in patients with coronary heart disease (CHD). It was demonstrated that the risk of circulating plasma apoC-III is highly dependent on its association with specific lipoproteins (3, 4). For example, in detailed studies that evaluated the risk of lipoproteins on the basis of their content of apoC-III following full multivariable adjustment, it was shown that LDL or HDL containing apoC-III was associated with high risk of CHD events even after adjustment for triglycerides. In contrast, LDL or HDL lacking apoC-III did not predict CHD.
In view of the above, it is now shown that the levels of apoC-III on such atherogenic lipoproteins can be reduced by >80% with volanesorsen, and furthermore that such analyses can be performed with high-throughput assays on intact plasma. Even when apoC-III concentrations are high, it appears to distribute onto the surface of individual lipoproteins in a selective manner, depending on a number of properties of the lipoproteins. apoC-III can be present in multiple copies on some lipoproteins, is not present uniformly on all lipoproteins of the same type, and only a small proportion of lipoproteins contain apoC-III (3). The presence of apoC-III is thought to confer important metabolic and possibly pro-inflammatory and pro-atherogenic properties. Thus, apoC-III may impact metabolic behavior by inhibiting LPL activity and TRL lipolysis by mediating metabolic channeling of VLDL to certain LDL subclasses and by inhibiting receptor-mediated uptake of TRLs by the liver (11).
Using these rapid and relatively nonperturbing immunocapture techniques, it was also documented for the first time in this study that apoC-III travels on Lp(a). Lp(a) is now accepted as a causal independent genetic risk factor for CVD (26, 27) and for aortic stenosis (28–31). Lp(a) has multiple mechanisms leading to atherogenicity and inflammation, including its content of pro-inflammatory oxidized phospholipids (32–35). Therefore, Lp(a) is now a target of therapy with potent antisense oligonucleotides that lower Lp(a) levels and accompanying oxidized phospholipids ∼80% (36). It is possible that apoC-III, when present on Lp(a), may make Lp(a) even more pro-atherogenic by one or more of the apoC-III-specific mediated mechanisms discussed above. Finally, the clearance mechanisms of Lp(a) are not well-known, and whether apoC-III slows clearance of Lp(a) waits to be assessed experimentally. These immunocapture assays should facilitate such studies.
These assays are not necessarily a replacement for techniques that isolate specific lipoprotein fractions, but can be considered complimentary measures. For example, the apoCIII-apoB measure encompasses apoC-III on all apoB-100-containing lipoproteins, including VLDL, IDL, LDL, and Lp(a), and might be combined with ultracentrifugation techniques to study individual apoB-100 lipoproteins separately, such as VLDL or LDL. Similarly, the apoCIII-apoAI measure reflects those HDL particles with apoA-I on them, but would not allow study of apoC-III distribution on the multitude of different HDL subspecies. In turn, the apoCIII-Lp(a) measure reflects particles that have both apo(a) and apoB on them. Because Lp(a) distributes in the density range between LDL and HDL, ultracentrifugally isolated LDL and HDL lipoprotein fractions contain varying amounts of “contaminating” Lp(a), and because we now show that Lp(a) contains apoC-III, there may be a need to reassess the content of apoC on LDL and HDL isolated by ultracentrifugation.
In summary, we demonstrate high-throughput immunocapture ELISAs to detect apoC-III on apoB-100 and apoA-I lipoproteins and, also for the first time, on Lp(a). These techniques use plasma and are adaptable to high-throughput methodology where hundreds of samples can be measured per day. They may be used in future studies to understand whether baseline levels and changes in response to therapy of these lipoprotein-apoCIII complexes predict cardiovascular events.
Acknowledgments
The authors thank Tracy Reigle of Ionis Pharmaceuticals for preparation of the figures.
Footnotes
Abbreviations:
- CHD
- coronary heart disease
- FCS
- familial chylomicronemia syndrome
- HDL-C
- HDL cholesterol
- IQR
- interquartile range
- LDL-C
- LDL cholesterol
- Lp(a)
- lipoprotein (a)
- nonHDL-C
- total cholesterol – HDL cholesterol
- RLU
- relative light units
- TRL
- triglyceride-rich lipoprotein
- VLDL-C
- VLDL cholesterol
This work was supported by National Institutes of Health Grants R01-HL119828, P01-HL088093, P01-HL055798, R01-HL124174, and R01-HL086599 (Y.I.M., J.L.W., S.T.). Ionis Pharmaceuticals provided funding via a lab service contract to University of California San Diego to perform the apoC-III-lipoprotein assay measurements. S.T. and J.L.W. are co-inventors of and receive royalties from patents or patent applications owned by the University of California San Diego on oxidation-specific and other antibodies and apoC-III-lipoprotein assays. S.T. has a dual appointment at University of California San Diego and Ionis Pharmaceuticals, Inc. A.D. is a full-time employee of Akcea Therapeutics. J.L.W. has received honoraria for consulting for Ionis, CymaBay, Intercept, and Prometheus Pharmaceuticals. V.J.A. and Q.Y. are employees of Ionis Pharmaceuticals. The other authors report no conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Norata G. D., Tsimikas S., Pirillo A., and Catapano A. L.. 2015. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol. Sci. 36: 675–687. [DOI] [PubMed] [Google Scholar]
- 2.Huff M. W., and Hegele R. A.. 2013. Apolipoprotein C-III: going back to the future for a lipid drug target. Circ. Res. 112: 1405–1408. [DOI] [PubMed] [Google Scholar]
- 3.Mendivil C. O., Rimm E. B., Furtado J., Chiuve S. E., and Sacks F. M.. 2011. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 124: 2065–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen M. K., Rimm E. B., Furtado J. D., and Sacks F. M.. 2012. Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J. Am. Heart Assoc. 1: e000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wyler von Ballmoos M. C., Haring B., and Sacks F. M.. 2015. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J. Clin. Lipidol. 9: 498–510. [DOI] [PubMed] [Google Scholar]
- 6.Graham M. J., Lee R. G., Bell T. A. 3rd, Fu W., Mullick A. E., Alexander V. J., Singleton W., Viney N., Geary R., Su J., et al. 2013. Antisense oligonucleotide inhibition of apolipoprotein C-III reduces plasma triglycerides in rodents, nonhuman primates, and humans. Circ. Res. 112: 1479–1490. [DOI] [PubMed] [Google Scholar]
- 7.Gaudet D., Brisson D., Tremblay K., Alexander V. J., Singleton W., Hughes S. G., Geary R. S., Baker B. F., Graham M. J., Crooke R. M., et al. 2014. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 371: 2200–2206. [DOI] [PubMed] [Google Scholar]
- 8.Gaudet D., Alexander V. J., Baker B. F., Brisson D., Tremblay K., Singleton W., Geary R. S., Hughes S. G., Viney N. J., Graham M. J., et al. 2015. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N. Engl. J. Med. 373: 438–447. [DOI] [PubMed] [Google Scholar]
- 9.Tsimikas S., Lau H. K., Han K. R., Shortal B., Miller E. R., Segev A., Curtiss L. K., Witztum J. L., and Strauss B. H.. 2004. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 109: 3164–3170. [DOI] [PubMed] [Google Scholar]
- 10.Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., and Bernstein S.. 1986. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 6: 178–188. [DOI] [PubMed] [Google Scholar]
- 11.Sacks F. M. 2015. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr. Opin. Lipidol. 26: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosby J., Peloso G. M., Auer P. L., Crosslin D. R., Stitziel N. O., Lange L. A., Lu Y., Tang Z. Z., Zhang H., Hindy G., et al. ; TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute. 2014. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N. Engl. J. Med. 371: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jørgensen A. B., Frikke-Schmidt R., Nordestgaard B. G., and Tybjaerg-Hansen A.. 2014. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N. Engl. J. Med. 37: 32–41. [DOI] [PubMed] [Google Scholar]
- 14.Schonfeld G., George P. K., Miller J., Reilly P., and Witztum J.. 1979. Apolipoprotein C-II and C-III levels in hyperlipoproteinemia. Metabolism. 28: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 15.Chan D. C., Watts G. F., Ooi E. M., Ji J., Johnson A. G., and Barrett P. H.. 2008. Atorvastatin and fenofibrate have comparable effects on VLDL-apolipoprotein C-III kinetics in men with the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 28: 1831–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dallinga-Thie G. M., Berk-Planken I. I., Bootsma A. H., and Jansen H.; Diabetes Atorvastatin Lipid Intervention (DALI) Study Group. 2004. Atorvastatin decreases apolipoprotein C–III in apolipoprotein B-containing lipoprotein and HDL in type 2 diabetes: a potential mechanism to lower plasma triglycerides. Diabetes Care. 27: 1358–1364. [DOI] [PubMed] [Google Scholar]
- 17.Kastelein J. J., Maki K. C., Susekov A., Ezhov M., Nordestgaard B. G., Machielse B. N., Kling D., and Davidson M. H.. 2014. Omega-3 free fatty acids for the treatment of severe hypertriglyceridemia: the Epanova for Lowering Very High Triglycerides (EVOLVE) trial. J. Clin. Lipidol. 8: 94–106. [DOI] [PubMed] [Google Scholar]
- 18.Davidson M. H., Johnson J., Rooney M. W., Kyle M. L., and Kling D. F.. 2012. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: the ECLIPSE (Epanova(®) compared to Lovaza(®) in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 6: 573–584. [DOI] [PubMed] [Google Scholar]
- 19.Qamar A., Khetarpal S. A., Khera A. V., Qasim A., Rader D. J., and Reilly M. P.. 2015. Plasma apolipoprotein C-III levels, triglycerides, and coronary artery calcification in type 2 diabetics. Arterioscler. Thromb. Vasc. Biol. 35: 1880–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maki K. C., Bays H. E., Dicklin M. R., Johnson S. L., and Shabbout M.. 2011. Effects of prescription omega-3-acid ethyl esters, coadministered with atorvastatin, on circulating levels of lipoprotein particles, apolipoprotein CIII, and lipoprotein-associated phospholipase A2 mass in men and women with mixed dyslipidemia. J. Clin. Lipidol. 5: 483–492. [DOI] [PubMed] [Google Scholar]
- 21.Swahn E., von Schenck H., and Olsson A. G.. 1998. Omega-3 ethyl ester concentrate decreases total apolipoprotein CIII and increases antithrombin III in postmyocardial infarction patients. Clin. Drug Investig. 15: 473–482. [DOI] [PubMed] [Google Scholar]
- 22.Schuster H., Fagerberg B., Edwards S., Halmos T., Lopatynski J., Stender S., Birketvedt G. S., Tonstad S., Gause-Nilsson I., Halldorsdottir S., et al. 2008. Tesaglitazar, a dual peroxisome proliferator-activated receptor alpha/gamma agonist, improves apolipoprotein levels in non-diabetic subjects with insulin resistance. Atherosclerosis. 197: 355–362. [DOI] [PubMed] [Google Scholar]
- 23.Fagerberg B., Edwards S., Halmos T., Lopatynski J., Schuster H., Stender S., Stoa-Birketvedt G., Tonstad S., Halldorsdottir S., and Gause-Nilsson I.. 2005. Tesaglitazar, a novel dual peroxisome proliferator-activated receptor alpha/gamma agonist, dose-dependently improves the metabolic abnormalities associated with insulin resistance in a non-diabetic population. Diabetologia. 48: 1716–1725. [DOI] [PubMed] [Google Scholar]
- 24.Nagashima K., Lopez C., Donovan D., Ngai C., Fontanez N., Bensadoun A., Fruchart-Najib J., Holleran S., Cohn J. S., Ramakrishnan R., et al. 2005. Effects of the PPARgamma agonist pioglitazone on lipoprotein metabolism in patients with type 2 diabetes mellitus. J. Clin. Invest. 115: 1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brahm A. J., and Hegele R. A.. 2015. Chylomicronaemia–current diagnosis and future therapies. Nat. Rev. Endocrinol. 11: 352–362. [DOI] [PubMed] [Google Scholar]
- 26.Willeit P., Kiechl S., Kronenberg F., Witztum J. L., Santer P., Mayr M., Xu Q., Mayr A., Willeit J., and Tsimikas S.. 2014. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): prospective 15-year outcomes in the Bruneck study. J. Am. Coll. Cardiol. 64: 851–860. [DOI] [PubMed] [Google Scholar]
- 27.Kronenberg F., and Utermann G.. 2013. Lipoprotein(a): resurrected by genetics. J. Intern. Med. 273: 6–30. [DOI] [PubMed] [Google Scholar]
- 28.Capoulade R., Chan K. L., Yeang C., Mathieu P., Bosse Y., Dumesnil J. G., Tam J. W., Teo K. K., Mahmut A., Yang X., et al. 2015. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 66: 1236–1246. [DOI] [PubMed] [Google Scholar]
- 29.Bouchareb R., Mahmut A., Nsaibia M. J., Boulanger M. C., Dahou A., Lepine J. L., Laflamme M. H., Hadji F., Couture C., Trahan S., et al. 2015. Autotaxin derived from lipoprotein(a) and valve interstitial cells promotes inflammation and mineralization of the aortic valve. Circulation. 132: 677–690. [DOI] [PubMed] [Google Scholar]
- 30.Kamstrup P. R., Tybjaerg-Hansen A., and Nordestgaard B. G.. 2014. Elevated lipoprotein(a) and risk of aortic valve stenosis in the general population. J. Am. Coll. Cardiol. 63: 470–477. [DOI] [PubMed] [Google Scholar]
- 31.Thanassoulis G., Campbell C. Y., Owens D. S., Smith J. G., Smith A. V., Peloso G. M., Kerr K. F., Pechlivanis S., Budoff M. J., Harris T. B., et al. 2013. Genetic associations with valvular calcification and aortic stenosis. N. Engl. J. Med. 368: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byun Y. S., Lee J. H., Arsenault B. J., Yang X., Bao W., DeMicco D., Laskey R., Witztum J. L., Tsimikas S., and Investigators T. N. T. T.. 2015. Relationship of oxidized phospholipids on apolipoprotein B-100 to cardiovascular outcomes in patients treated with intensive versus moderate atorvastatin therapy: the TNT trial. J. Am. Coll. Cardiol. 65: 1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsimikas S., Duff G. W., Berger P. B., Rogus J., Huttner K., Clopton P., Brilakis E., Kornman K. S., and Witztum J. L.. 2014. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J. Am. Coll. Cardiol. 63: 1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsimikas S., Willeit P., Willeit J., Santer P., Mayr M., Xu Q., Mayr A., Witztum J. L., and Kiechl S.. 2012. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J. Am. Coll. Cardiol. 60: 2218–2229. [DOI] [PubMed] [Google Scholar]
- 35.Scipione C. A., Sayegh S. E., Romagnuolo R., Tsimikas S., Marcovina S. M., Boffa M. B., and Koschinsky M. L.. 2015. Mechanistic insights into lipoprotein(a)-induced interleukin-8 expression: a role for oxidized phospholipid modification of apolipoprotein(a). J. Lipid Res. 56: 2273–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsimikas S., Viney N. J., Hughes S. G., Singleton W., Graham M. J., Baker B. F., Burkey J. L., Yang Q., Marcovina S. M., Geary R. S., et al. 2015. Antisense therapy targeting apolipoprotein(a): a randomised, double-blind, placebo-controlled phase 1 study. Lancet. 386: 1472–1483. [DOI] [PubMed] [Google Scholar]