Abstract
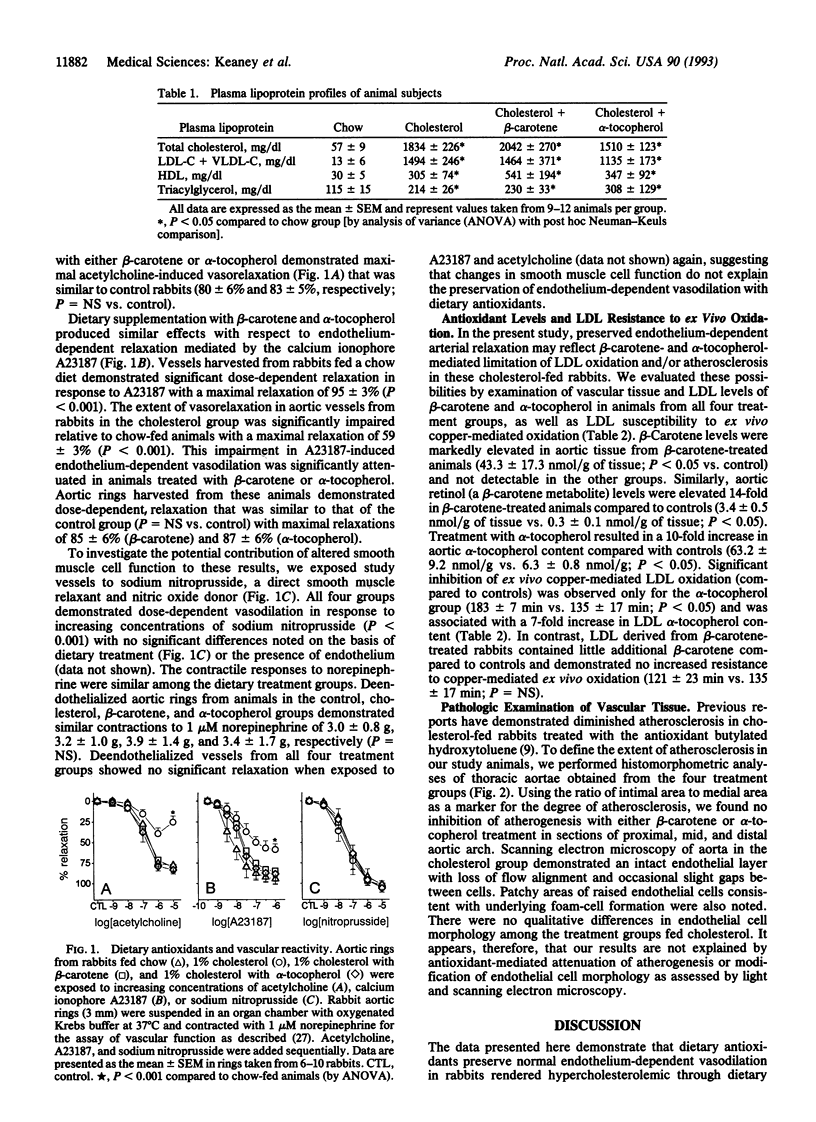
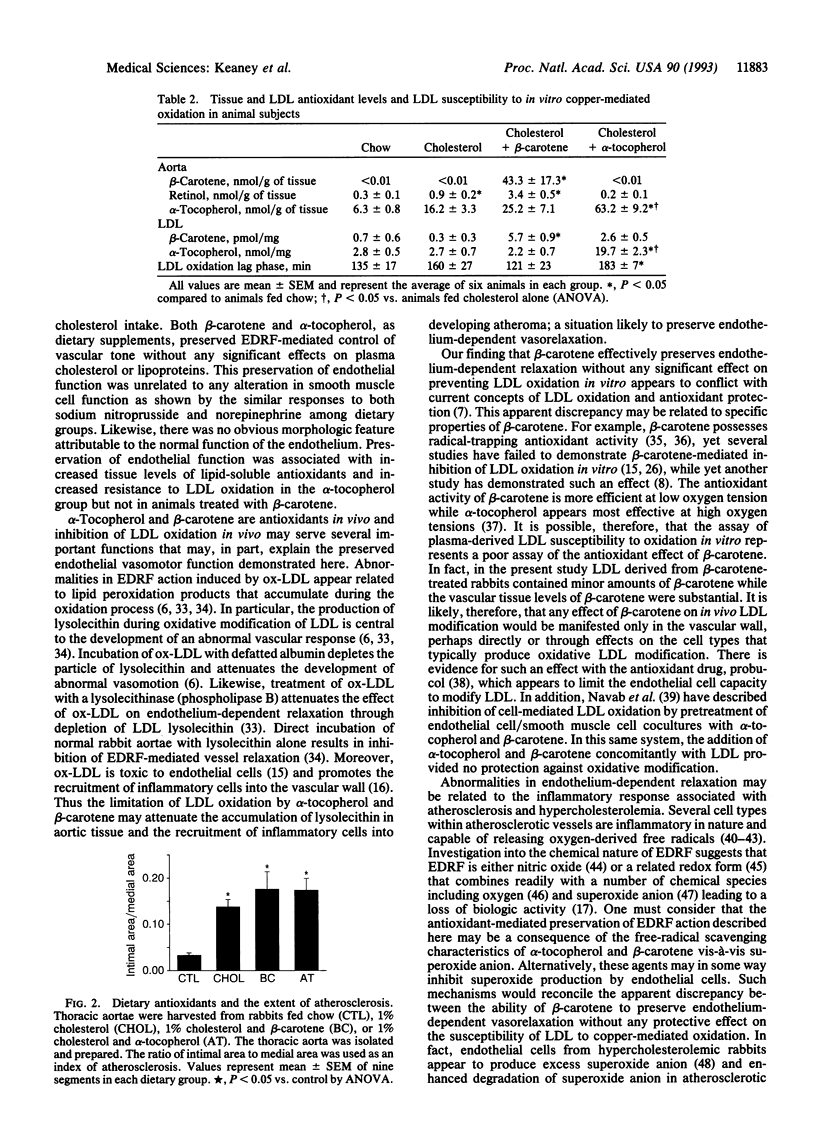
Recent evidence suggests that dietary therapy with lipid-soluble antioxidants may be beneficial for patients with atherosclerotic vascular disease but the potential mechanism(s) for these observations remain obscure. Abnormalities in endothelium-dependent control of vascular tone develop early in the course of atherosclerosis and may result from oxidative modification of low density lipoproteins. We examined the role of dietary antioxidants in preserving normal endothelial cell vasodilator function in cholesterol-fed rabbits with particular attention to possible effects on serum lipoproteins, low density lipoprotein oxidation, and atherogenesis. Male New Zealand White rabbits were fed diets containing no additive (controls), 1% cholesterol (cholesterol group), or 1% cholesterol chow supplemented with either beta-carotene (0.6 g/kg of chow) or alpha-tocopherol (1000 international units/kg of chow) for a 28-day period. After dietary therapy, thoracic aortae were harvested for assay of vascular function and for pathologic examination and tissue antioxidant levels. Compared to controls, acetylcholine- and A23187-mediated endothelium-dependent relaxations were significantly impaired in vessels from the cholesterol group (P < 0.001), whereas vessels from animals treated with beta-carotene or alpha-tocopherol demonstrated normal endothelium-dependent arterial relaxation. Preservation of endothelial function was associated with vascular incorporation of alpha-tocopherol and beta-carotene but was unrelated to plasma lipoprotein levels, smooth muscle cell function, or the extent of atherosclerosis. Increased low density lipoprotein resistance to ex vivo copper-mediated oxidation was observed only in the alpha-tocopherol group. Our results suggest that dietary antioxidants may benefit patients with atherosclerosis by preserving endothelial vasodilator function through a mechanism related to vascular tissue antioxidant content and not reflected by assay of low density lipoprotein resistance to ex vivo oxidation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Bossaller C., Habib G. B., Yamamoto H., Williams C., Wells S., Henry P. D. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5'-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. J Clin Invest. 1987 Jan;79(1):170–174. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton G. W., Joyce A., Ingold K. U. Is vitamin E the only lipid-soluble, chain-breaking antioxidant in human blood plasma and erythrocyte membranes? Arch Biochem Biophys. 1983 Feb 15;221(1):281–290. doi: 10.1016/0003-9861(83)90145-5. [DOI] [PubMed] [Google Scholar]
- Chin J. H., Azhar S., Hoffman B. B. Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest. 1992 Jan;89(1):10–18. doi: 10.1172/JCI115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B. H., Segrest J. P., Ray M. J., Brunzell J. D., Hokanson J. E., Krauss R. M., Beaudrie K., Cone J. T. Single vertical spin density gradient ultracentrifugation. Methods Enzymol. 1986;128:181–209. doi: 10.1016/0076-6879(86)28068-4. [DOI] [PubMed] [Google Scholar]
- Dieber-Rotheneder M., Puhl H., Waeg G., Striegl G., Esterbauer H. Effect of oral supplementation with D-alpha-tocopherol on the vitamin E content of human low density lipoproteins and resistance to oxidation. J Lipid Res. 1991 Aug;32(8):1325–1332. [PubMed] [Google Scholar]
- Esterbauer H., Dieber-Rotheneder M., Striegl G., Waeg G. Role of vitamin E in preventing the oxidation of low-density lipoprotein. Am J Clin Nutr. 1991 Jan;53(1 Suppl):314S–321S. doi: 10.1093/ajcn/53.1.314S. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992 Oct;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Frei B., England L., Ames B. N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B., Stocker R., Ames B. N. Antioxidant defenses and lipid peroxidation in human blood plasma. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9748–9752. doi: 10.1073/pnas.85.24.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Heinecke J. W., Baker L., Rosen H., Chait A. Superoxide-mediated modification of low density lipoprotein by arterial smooth muscle cells. J Clin Invest. 1986 Mar;77(3):757–761. doi: 10.1172/JCI112371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Hirata K., Yamada M., Hamamori Y., Matsuda Y., Akita H., Yokoyama M. Lysophosphatidylcholine inhibits bradykinin-induced phosphoinositide hydrolysis and calcium transients in cultured bovine aortic endothelial cells. Circ Res. 1992 Dec;71(6):1410–1421. doi: 10.1161/01.res.71.6.1410. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H., Zhu L., Beckman J. S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992 Nov 1;298(2):446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Ishii H., Horie S., Kizaki K., Kazama M. Retinoic acid counteracts both the downregulation of thrombomodulin and the induction of tissue factor in cultured human endothelial cells exposed to tumor necrosis factor. Blood. 1992 Nov 15;80(10):2556–2562. [PubMed] [Google Scholar]
- Jialal I., Norkus E. P., Cristol L., Grundy S. M. beta-Carotene inhibits the oxidative modification of low-density lipoprotein. Biochim Biophys Acta. 1991 Oct 15;1086(1):134–138. doi: 10.1016/0005-2760(91)90164-d. [DOI] [PubMed] [Google Scholar]
- Kugiyama K., Kerns S. A., Morrisett J. D., Roberts R., Henry P. D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990 Mar 8;344(6262):160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- Kunisaki M., Umeda F., Inoguchi T., Nawata H. Vitamin E restores reduced prostacyclin synthesis in aortic endothelial cells cultured with a high concentration of glucose. Metabolism. 1992 Jun;41(6):613–621. doi: 10.1016/0026-0495(92)90053-d. [DOI] [PubMed] [Google Scholar]
- Kuzuya M., Naito M., Funaki C., Hayashi T., Yamada K., Asai K., Kuzuya F. Antioxidants stimulate endothelial cell proliferation in culture. Artery. 1991;18(3):115–124. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mangin E. L., Jr, Kugiyama K., Nguy J. H., Kerns S. A., Henry P. D. Effects of lysolipids and oxidatively modified low density lipoprotein on endothelium-dependent relaxation of rabbit aorta. Circ Res. 1993 Jan;72(1):161–166. doi: 10.1161/01.res.72.1.161. [DOI] [PubMed] [Google Scholar]
- Melnykovych G., Clowes K. K. Growth stimulation of bovine endothelial cells by vitamin A. J Cell Physiol. 1981 Nov;109(2):265–270. doi: 10.1002/jcp.1041090209. [DOI] [PubMed] [Google Scholar]
- Minor R. L., Jr, Myers P. R., Guerra R., Jr, Bates J. N., Harrison D. G. Diet-induced atherosclerosis increases the release of nitrogen oxides from rabbit aorta. J Clin Invest. 1990 Dec;86(6):2109–2116. doi: 10.1172/JCI114949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr D., Bowry V. W., Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992 Jun 26;1126(3):247–254. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- Morel D. W., Hessler J. R., Chisolm G. M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983 Aug;24(8):1070–1076. [PubMed] [Google Scholar]
- Mügge A., Elwell J. H., Peterson T. E., Hofmeyer T. G., Heistad D. D., Harrison D. G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ Res. 1991 Nov;69(5):1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- Navab M., Imes S. S., Hama S. Y., Hough G. P., Ross L. A., Bork R. W., Valente A. J., Berliner J. A., Drinkwater D. C., Laks H. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88(6):2039–2046. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara Y., Peterson T. E., Harrison D. G. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest. 1993 Jun;91(6):2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne J. A., Lento P. H., Siegfried M. R., Stahl G. L., Fusman B., Lefer A. M. Cardiovascular effects of acute hypercholesterolemia in rabbits. Reversal with lovastatin treatment. J Clin Invest. 1989 Feb;83(2):465–473. doi: 10.1172/JCI113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M., van Poppel G., Vogelezang C., Buytenhek R., Kok F. J. Supplementation with vitamin E but not beta-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro. Effect of cigarette smoking. Arterioscler Thromb. 1992 May;12(5):554–562. doi: 10.1161/01.atv.12.5.554. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retsky K. L., Freeman M. W., Frei B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti- rather than prooxidant activity of vitamin C in the presence of transition metal ions. J Biol Chem. 1993 Jan 15;268(2):1304–1309. [PubMed] [Google Scholar]
- Rimm E. B., Stampfer M. J., Ascherio A., Giovannucci E., Colditz G. A., Willett W. C. Vitamin E consumption and the risk of coronary heart disease in men. N Engl J Med. 1993 May 20;328(20):1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubanyi G. M., Ho E. H., Cantor E. H., Lumma W. C., Botelho L. H. Cytoprotective function of nitric oxide: inactivation of superoxide radicals produced by human leukocytes. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1392–1397. doi: 10.1016/0006-291x(91)92093-y. [DOI] [PubMed] [Google Scholar]
- Saito H., Salmon J. A., Moncada S. Influence of cholesterol feeding on the production of eicosanoids, tissue plasminogen activator and superoxide anion (O2-) by rabbit blood monocytes. Atherosclerosis. 1986 Aug;61(2):141–148. doi: 10.1016/0021-9150(86)90074-2. [DOI] [PubMed] [Google Scholar]
- Saran M., Michel C., Bors W. Reaction of NO with O2-. implications for the action of endothelium-derived relaxing factor (EDRF). Free Radic Res Commun. 1990;10(4-5):221–226. doi: 10.3109/10715769009149890. [DOI] [PubMed] [Google Scholar]
- Shapiro S. S., Mott D. J., Machlin L. J. Kinetic characteristics of beta-carotene uptake and depletion in rat tissue. J Nutr. 1984 Oct;114(10):1924–1933. doi: 10.1093/jn/114.10.1924. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Stamler J. S., Singel D. J., Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992 Dec 18;258(5090):1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Stampfer M. J., Hennekens C. H., Manson J. E., Colditz G. A., Rosner B., Willett W. C. Vitamin E consumption and the risk of coronary disease in women. N Engl J Med. 1993 May 20;328(20):1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Stocker R., Bowry V. W., Frei B. Ubiquinol-10 protects human low density lipoprotein more efficiently against lipid peroxidation than does alpha-tocopherol. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1646–1650. doi: 10.1073/pnas.88.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K., Chan A. C. Effect of vitamin E enrichment on arachidonic acid release and cellular phospholipids in cultured human endothelial cells. Biochim Biophys Acta. 1988 Dec 16;963(3):468–475. doi: 10.1016/0005-2760(88)90315-3. [DOI] [PubMed] [Google Scholar]
- Verbeuren T. J., Jordaens F. H., Zonnekeyn L. L., Van Hove C. E., Coene M. C., Herman A. G. Effect of hypercholesterolemia on vascular reactivity in the rabbit. I. Endothelium-dependent and endothelium-independent contractions and relaxations in isolated arteries of control and hypercholesterolemic rabbits. Circ Res. 1986 Apr;58(4):552–564. doi: 10.1161/01.res.58.4.552. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Winterbourn C. C. Inhibition of adriamycin-promoted microsomal lipid peroxidation by beta-carotene, alpha-tocopherol and retinol at high and low oxygen partial pressures. FEBS Lett. 1988 Oct 10;238(2):353–356. doi: 10.1016/0014-5793(88)80511-8. [DOI] [PubMed] [Google Scholar]
- Vita J. A., Treasure C. B., Nabel E. G., McLenachan J. M., Fish R. D., Yeung A. C., Vekshtein V. I., Selwyn A. P., Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990 Feb;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Hirata K., Miyake R., Akita H., Ishikawa Y., Fukuzaki H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun. 1990 Apr 16;168(1):301–308. doi: 10.1016/0006-291x(90)91708-z. [DOI] [PubMed] [Google Scholar]



