Abstract
Dyrk1A, a mammalian homolog of the Drosophila minibrain gene, encodes a dual-specificity kinase, involved in neuronal development and in adult brain physiology. In humans, a third copy of DYRK1A is present in Down syndrome (trisomy 21) and has been implicated in the etiology of mental retardation. To further understand this pathology, we searched for Dyrk1A-interacting proteins and identified Arip4 (androgen receptor-interacting protein 4), a SNF2-like steroid hormone receptor cofactor. Mouse hippocampal and cerebellar neurons coexpress Dyrk1A and Arip4. In HEK293 cells and hippocampal neurons, both proteins are colocalized in a speckle-like nuclear subcompartment. The functional interaction of Dyrk1A with Arip4 was analyzed in a series of transactivation assays. Either Dyrk1A or Arip4 alone displays an activating effect on androgen receptor- and glucocorticoid receptor-mediated transactivation, and Dyrk1A and Arip4 together act synergistically. These effects are independent of the kinase activity of Dyrk1A. Inhibition of endogenous Dyrk1A and Arip4 expression by RNA interference showed that both proteins are necessary for the efficient activation of androgen receptor- and glucocorticoid receptor-dependent transcription. As Dyrk1A is an activator of steroid hormone-regulated transcription, the overexpression of DYRK1A in persons with Down syndrome may cause rather broad changes in the homeostasis of steroid hormone-controlled cellular events.
Down syndrome (DS) is the most common genetic cause of mental retardation in humans (reviewed in reference 14). Besides a number of overt physical features and defects that affect, e.g., the heart and the immune system, mental retardation is the condition of overriding clinical importance in DS, being present in virtually all cases. A number of abnormalities of the central nervous system are associated with DS; these include (i) smaller brains with disproportionately smaller cerebellum and brain stem, (ii) reduced neuron numbers and densities in distinct brain regions, such as the hippocampus, cerebellum, and granular layers of the cerebral cortex, and (iii) abnormalities in neuron morphology, especially with regard to dendritic spines (reviewed in references 14 and 51).
The molecular and cellular mechanisms underlying these changes are not understood yet. In most cases, DS is caused by complete trisomy 21. In rare cases, however, only a part of chromosome 21 is present in three copies. Studies on individuals with partial trisomy 21 have suggested that a region on chromosome 21, referred to as the DS critical region, is the cause of mental retardation (12, 56, 57). Consequently, genes located in this region and having possible important functions in the emergence of mental retardation have gained much attention during recent years.
The human homolog of the Drosophila minibrain gene, DYRK1A (dual-specificity tyrosine phosphorylation-regulated kinase or dual-specificity YAK1-related kinase), was found to be located on chromosome 21 in the DS critical region (24, 62, 66), and the mouse homolog was located in the corresponding region on chromosome 16 (65). The Drosophila minibrain gene was originally identified in a screen for phenotypes affecting brain morphology (32). minibrain mutant flies are characterized by a markedly reduced brain volume caused by impaired postlarval neurogenesis due to decreased levels of the nuclear protein kinase MNB. They also exhibit some behavioral abnormalities, for example, reduced performance in odor discrimination learning (69).
Subsequently, the expression of Dyrk1A was shown to be increased 1.5-fold in the brains of DS fetuses (46) and 2.1-fold in the brains of Ts65Dn mice, which serve as an animal model for DS and which are trisomic for a region of chromosome 16 (23).
Dyrk1A codes for a kinase (Fig. 1A) which is able to autophosphorylate on tyrosine and which phosphorylates substrates on serine/threonine residues (reviewed in reference 4). Several potential substrates of Dyrk1A have been identified (reviewed in reference 18); these include Tau, eIF2Bɛ (72), dynamin (8), CREB (75), STAT3 (49), FKHR (73), Gli1 (45) and, very recently, cyclin L2 (10). The physiological relevance of all of these interactions remains to be clarified. Dyrk1A contains a nuclear localization signal (NLS), and transfected Dyrk1A-green fluorescent protein (GFP) fusions have been detected in the nuclei of various cell lines after transfection (1, 5). However, for the endogenous Dyrk1A protein, nuclear and cytoplasmic localizations in the brain and in primary cerebellar neurons have been described (26, 48). Dyrk1A is expressed in several brain regions in humans and in mice both during embryonic development and in adulthood (23, 48, 58). In the adult mouse brain, Dyrk1A expression in the hippocampal formation, the olfactory bulb, the cerebral and cerebellar cortices, and motor nuclei of the brain stem as well as in other regions has been described. This widespread but not ubiquitous expression pattern suggests diverse functions of Dyrk1A during development and in adult brain physiology.
FIG. 1.
Schematic representation of Dyrk1A and Arip4 proteins. (A) Dyrk1A. The kinase domain, the NLS, the PEST region, and histidine and serine/threonine repeats are indicated. (B) Arip4. The domain encoded by the yeast clone from the yeast two-hybrid screen, the putative Dyrk1A substrate consensus site, the NLSs, and LXXLL motifs typical of interacting proteins of steroid hormone receptors are indicated.
The notion that Dyrk1A plays a role in the developing brain and in the adult brain depending on the Dyrk1A gene dosage is supported by studies of genetically modified mice. Whereas homozygous Dyrk1A knockout mice die in utero, the deletion of one allele of Dyrk1A causes the malformation of several brain regions (16). The size of the brain is reduced in a region-specific manner, and neuronal densities are altered in some regions. Evidence for a role of Dyrk1A in cognitive processes came from the study of transgenic mice containing a yeast artificial chromosome carrying a copy of a 180-kb genomic region surrounding the human DYRK1A gene on chromosome 21. These mice exhibited, in analogy to DS, deficits in explicit learning and memory (63, 64, 70).
To elucidate the function of Dyrk1A in the adult mammalian brain, we searched for signaling mechanisms involving Dyrk1A. To find Dyrk1A-interacting proteins, we performed a yeast two-hybrid screen with the kinase domain of Dyrk1A as bait. We found that Dyrk1A interacts with the SNF2-like chromatin remodeling ATPase Arip4 (androgen receptor [AR]-interacting protein 4), which was originally described as a cofactor in AR-mediated transcription (61). In addition to the physical interaction of Dyrk1A with Arip4 in yeast and mammalian cells, we show the coexpression of the respective genes in the mouse brain and the colocalization of Dyrk1A and Arip4 proteins in hippocampal neurons. Finally, a functional interaction of these proteins is evidenced, as Dyrk1A and Arip4 are able to synergistically activate AR- and glucocorticoid receptor (GR)-mediated transcriptional activation.
MATERIALS AND METHODS
Materials.
Plasmids pSVL-HA-Dyrk1AWT and pSVL-HA-Dyrk1AK188R (37) were kindly provided by W. Becker (Rheinisch-Westfälische Technische Hochschule Aachen, Germany); pSHAG-1 (54) was provided by G. Hannon (Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.); and pSTC-AR, pSTC-GR (42), and pMTV-Luc were provided by S. Rusconi (University of Fribourg, Fribourg, Switzerland). pDaRed2-C1 was purchased from Clontech (Palo Alto, Calif.).
Plasmid construction.
The cDNA of mouse Dyrk1A (GenBank accession no. U58497) was cloned by reverse transcription (RT)-PCR. Nucleotides 1 to 1465 were cloned into vector pCRII (Invitrogen, Karlsruhe, Germany), and nucleotides 1360 to 2442 were cloned into pBluescript II KS (Stratagene, Amsterdam, The Netherlands). The construct obtained from the latter cloning, clone pBS-Dyrk1Ant1360-2442, was also used as a template for in vitro transcription to generate probes for in situ hybridization. The cDNA fragments of both clones were fused, and the product was subcloned into the EcoRI/XbaI sites of pBluescript II KS after reamplification by PCR with primers JS.56 (5′-ATTCCGGAATTCCCCCATCAGGATGATATGAGA-3′; the EcoRI site is shown in italic type, and the Dyrk1A sequence is underlined) and JS.59 (5′-CTAGCTAGTCTAGAGCGGTTCAGTGTGTTAATCTC-3′; the XbaI site is shown in italic type, and the Dyrk1A sequence is underlined) to generate clone pBS-Dyrk1A.
The bait plasmid used in the yeast two-hybrid screen, pGBKT7-Dyrk1A152-486, was cloned by PCR amplification of the part of the Dyrk1A cDNA encoding the kinase domain and insertion of a sequence coding for a linker of three glycine residues 5′ to the kinase domain-encoding region. The PCR amplification was done with primers JS.40 (5′-GGCCCGGAATTCGGTGGTGGTGAAAAGTGGATGGATCGGTATGAAATCGACT-3′; an EcoRI site is shown in italic type, the linker sequence is shown in bold type, and the Dyrk1A sequence is underlined) and JS.41 (5′-TTCAAGAAAACAGCTGATGAAGGTTGAGGATCCGCGCGC-3′; a stop codon is shown in bold type, a BamHI site is shown in italic type, and the Dyrk1A sequence is underlined). The PCR product was inserted into the EcoRI/BamHI sites of bait vector pGBKT7 (Clontech) in frame with the GAL4 DNA-binding domain encoded by the vector.
The entire coding region of the Dyrk1A cDNA was also amplified by using clone pBS-Dyrk1A as a template, and a sequence coding for a linker of three glycine residues was inserted 5′ to the coding region. Amplification was done with primers JS.60 (5′-GGATATCCATATGAAGATCTTCGGTGGTGGTATGCATACAGGAGGAGAGACT-3′; an NdeI site is shown in italic type, a BglII site is shown in bold italic type, the linker sequence in shown in bold nonitalic type, and the Dyrk1A sequence is underlined) and JS.61 (5′-CCGGAATTCCCGCTCGAGTCACGAGCTAGCTACAGGACT-3′; an EcoRI site is shown in italic type, and the Dyrk1A sequence is underlined). The PCR product, encoding (Gly)3-Dyrk1A, was inserted into the NdeI/EcoRI sites of bait vector pGBKT7 in frame with the GAL4 DNA-binding domain encoded by the vector to generate pGBKT7-Dyrk1A, which was used to test for the interaction of Arip4 with the entire Dyrk1A protein. From this construct, the cDNA was cut out at the BglII/EcoRI sites of the primers and transferred to pEGFP-C2 (Clontech) to generate pEGFP-Dyrk1A, which encodes a fusion of enhanced GFP (EGFP) with Dyrk1A, linked by a hinge of three glycine residues. pECFP-Dyrk1A and pEYFP-Dyrk1A, based on pECFP-C1 (Clontech) and pEYFP-C1 (Clontech), respectively, were generated in the same way. pCMV-FLAG-Dyrk1A, coding for FLAG-tagged Dyrk1A, used in coimmunoprecipitation experiments, was generated as follows. The Dyrk1A cDNA was cut out from pGBKT7-Dyrk1A at the BglII/XhoI sites, the BglII site was blunted, and the fragment was inserted into EcoRV/XhoI-cut pCMV-Tag2A (Stratagene).
To generate pBS-Arip4nt3420-4763 (containing nucleotides 3420 to 4763 of the mouse Arip4 cDNA; GenBank accession no. AJ132389), which was used as a template for in vitro transcription to generate probes for in situ hybridization, we subcloned into the XhoI/EcoRV sites of pBluescript II KS the Arip4 cDNA fragment that was present in the prey clone from the yeast two-hybrid screen and that was cut out from pACT2 (Clontech) with SfiI (blunted) and XhoI.
The entire cDNA of Arip4 was cloned as follows. The 5′ region of Arip4 (nucleotides 185 to 2717) was amplified from mouse brain RNA by RT-PCR with primers JS.106 (5′-GGAGCCATGTCAGACGAAT-3′) and JS.100 (5′-CCTTGTGGAGAGAAGGAACA-3′), cloned into pCRII-Topo, and then subcloned into pEGFP-C2 to generate pEGFP-Arip45′. We purchased human IMAGE clone 3138844 (GenBank accession no. BC001474; Deutsches Ressourcenzentrum für Genomforschong, Berlin, Germany), containing part of KIAA0809 (52) (GenBank accession no. NM015106), the human Arip4 ortholog. A fragment of this clone corresponding to the 3′ region of Arip4 (nucleotides 2519 to 4623 of the mouse Arip4 cDNA) was transferred to pEGFP-Arip45′ by using the BstBI site at position 2598 of the mouse Arip4 cDNA to clone the full-length coding region of Arip4 fused to EGFP (pEGFP-Arip4). From this construct, the Arip4 cDNA was cut out with EcoRI and HindIII and with XhoI and ApaI and subcloned into pCMV-Tag2B (Stratagene) to generate pCMV-FLAG-Arip4 or into pECFP-C1 and pEYFP-C1 to generate pECFP-Arip4 and pEYFP-Arip4, respectively.
To generate the deletion mutant of Arip4, Arip4Δ1165-1196, two fragments of the Arip4 cDNA were amplified by PCR with primers JS.161 (5′-GCCCCGGCATGTCCCATTGGGA-3′) and JS.167 (5′-GGCGGCATTGGTGGCCAGCCCCTCAGGGTCGG-3′), with primers JS.166 (5′-GAGGGGCTGGCCACCAATGCCGCCCTGCCTG-3′) and JS.165 (5′-CTTTCGCTTGTGGCCCCTTAA-3′), and with pCMV-FLAG-Arip4 as a template. These fragments were fused by PCR with primers JS.162 (5′-GCTCTGCCTCCAGCACAAAT-3′) and JS.165. The product of this reaction corresponded to nucleotides 3321 to 3958 of the Arip4 cDNA but lacked nucleotides 3683 to 3778 (coding for residues 1165 to 1196) and was inserted into pCMV-FLAG-Arip4 at the BstEII/AflII sites, yielding pCMV-FLAG-Arip4Δ1165-1196.
Short hairpin RNA (shRNA) expression constructs for RNA interference (RNAi) were cloned by inserting oligonucleotides into pSHAG-1. To design shRNAs to knock down Dyrk1A and Arip4, regions of the cDNAs that are identical in mice (Dyrk1A; GenBank accession no. U58497), rats (Dyrk1A; NM012791), and humans (Dyrk1A; AF108830) and that are identical in mice (Arip4; AJ132389) and humans (Arip4 [KIAA0809]; NM015106), respectively, were chosen. The oligonucleotides were designed by using the program RNAi OligoRetriever (G. Hannon, Cold Spring Harbor Laboratory; http://katahdin.cshl.org:9331/RNAi/html/rnai.html). The shRNA sequences were as follows (sense strand): 5′-GATCAAAAAACAGATATCCTTCAGTCACGTAATATGCAGAACAAGCTTCTCCTGCACATTACATGACTGAAGGACATCG-3′ (JS.138) and 5′-GATCAAAAAAATGTTAGATGTCCAGCTATGCCAATCCCTCGCAAGCTTCCAAGAGACTGGCATAGCTGGACATCCAACG-3′ (JS.140), targeting Dyrk1A; 5′-GATCAAAAAACTAGCCATAACAGTATACCCAACATACTACCCAAGCTTCGGCAGTATGTCGGGTATACCGTTATGGCCG-3′ (JS.154), 5′-GATCAAAAAAAGGGGCGCTACCATCTAACCAGAAGTAGCCGCAAGCTTCCAGCTACTTCCGGCTAGATGGTAGCACCCG-3′ (JS.156), and 5′-GATCAAAAAAGGACCCGCCGGCACCAGAGGATCAGTAGTCACAAGCTTCTGACTACTGACCCTCTGGTGCCAGCAGGCG-3′ (JS.158), targeting Arip4; and 5′-GATCAAAAAACTGCAAGGCGACTAAGTCGGATAACGCCAAGCAAGCTTCCCTGGCGTTACCCAACTTAATCGCCTTGCG-3′ (JS.174), 5′-GATCAAAAAATTAACACCAGACCAACTAGTAATAGTAGCAACAAGCTTCTCGCTACCATTACCAGTTGGTCTGGTGTCG-3′ (JS.176), and 5′-GATCAAAAAACAGACCGTTCACACAGAACTAGCAATCGTCCCAAGCTTCGAACGATCGCCAGTTCTGTATGAACGGTCG-3′ (JS.178), targeting lacZ. In all transactivation assays with RNAi, pools of equal amounts of the two (Dyrk1A) or three (Arip4 and lacZ) constructs targeting the respective mRNA were used.
All sequences amplified by PCR were completely sequenced, and constructs generated by subcloning were verified by sequencing of the regions adjacent to the cloning sites.
Yeast two-hybrid screen.
The yeast two-hybrid screen was carried out by using MATCHMAKER GAL4 two-hybrid system 3 (Clontech) and three independent reporter genes for the selection of interacting clones (ADE2, HIS3, and MEL1 [encoding α-galactosidase]). All procedures were performed according to the manufacturer's protocols. In brief, AH109 cells (Clontech) (35) were transformed with pGBKT7-Dyrk1A152-486 and selected for growth on medium lacking tryptophan. The transformed yeast was mated with yeast strain Y187 (29), pretransformed with an expression vector containing a cDNA library fused to the GAL4 activation domain. We used a pretransformed MATCHMAKER library from mouse brains pooled from 200 9- to 12-week-old BALB/c males; the average insert size was 2 kb (according to the manufacturer [Clontech]).
Positively interacting clones were selected and retested for expression of the reporter genes on synthetic defined medium lacking adenine, histidine, leucine, and tryptophan but supplemented with X-α-Gal (20 mg/ml; Clontech), a substrate for α-galactosidase which is converted into a blue product in colonies expressing the reporter genes. To eliminate clones with more than one library plasmid, clones were restreaked on plates lacking leucine and tryptophan to allow the loss of additional library plasmids but supplemented with X-α-Gal to identify colonies still containing positively interacting clones. To confirm the specificity of the interacting proteins, AH109 yeast cells were cotransformed (21) with one of the prey plasmids and either pGBKT7 or pGBKT7-lamin C (Clontech). In parallel, clones were tested for interactions with pGBKT7-Dyrk1A, coding for the complete Dyrk1A protein fused to the GAL4 DNA-binding domain. Approximately 3.5 × 106 individual library plasmids were screened based on the number of diploid colonies after the mating. Library plasmids from all six positively interacting clones were rescued from the yeast cells and sequenced.
Animals and tissue preparation.
Animals were housed in a temperature- and humidity-controlled room with a 12-h light-dark cycle and with access to food and water ad libitum. The experimental protocols were approved by the Ethical Committee on Animal Care and Use of the Government of Bavaria, Munich, Germany. Adult mice (3 to 5 months old; C57BL/6N) were killed by cervical dislocation. Brains were removed, snap-frozen on dry ice, and stored at −80°C prior to sectioning. Brains were mounted on Tissue Tek (Polysciences, Warrington, Pa.), and 16-μm-thick coronal sections in consecutive series were cut from the forebrain by using a cryostat Microtome HM560 (Microm, Walldorf, Germany). Sections were mounted on frozen SuperFrost/Plus slides (Fisher Scientific, Schwerte, Germany), dried on a 42°C warming plate, and stored at −20°C until used.
Synthesis of probes and in situ hybridization.
Radioactive 35S-labeled riboprobes for in situ hybridization were synthesized by in vitro transcription as previously described (47) with plasmids pBS-Dyrk1Ant1360-2442 and pBS-Arip4nt3420-4763 as templates. Restriction enzymes (New England Biolabs, Beverly, Mass.) used for linearization and RNA polymerases used for each probe were as follows: Dyrk1A sense, EcoRV and T7; Dyrk1A antisense, XbaI and T3; Arip4 sense, XhoI and T7; and Arip4 antisense, XbaI and T3. When these probes were used for in situ hybridization experiments, sense controls did not give any detectable signals (data not shown). It should be noted that pBS-Dyrk1Ant1360-2442 exhibits no significant sequence similarity to the Dyrk1A relatives Dyrk1B and Dyrk2, -3, and -4. The Arip4 probe derived from pBS-Arip4nt3420-4763 shows no match longer than 19 nucleotides to any other mouse mRNA.
Cell culture and transfection.
HEK293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Karlsruhe, Germany) with 10% heat-inactivated fetal calf serum (FCS) (Gibco), 2 mM glutamine (Gibco) and, to prevent bacterial and fungal contamination, 1% antibiotic-antimycotic mixture (penicillin-streptomycin-amphotericin) (Gibco) at 37°C in a humidified 5% CO2 incubator.
HEK293 cells for confocal laser scanning microscopy were transfected by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. In brief, 200,000 cells were used to seed poly-d-lysine-coated glass coverslips in the wells of a 24-well plate. After 2 days, the medium was changed to DMEM with 10% heat-inactivated FCS and 2 mM glutamine, and 1 μg of the expression vectors was transfected by using 1 μl of Lipofectamine 2000 per well. One day later, cells were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS; 10 mM sodium phosphate, 2.7 mM potassium chloride, 120 mM sodium chloride [pH 7.4]) and mounted on glass slides. For coimmunoprecipitation experiments, 3.8 × 106 HEK293 cells were transfected after cultivation for 12 h at 37°C in 5% CO2 in a 10-cm culture dish with 20 μg of DNA by the calcium phosphate method (7).
Primary hippocampal neurons were prepared from Sprague-Dawley rat embryos at embryonic day 16 (Charles River, Sulzfeld, Germany) as reported previously (60). A total of 2 × 105 cells in minimal essential medium (Gibco) supplemented with 10% heat-inactivated horse serum were used to seed poly-d-lysine-coated coverslips. Two days after preparation, after the medium was renewed or changed to Neurobasal (Gibco) supplemented with B27 (6), neurons were transfected by using Effectene (QIAGEN, Hilden, Germany). Transfection was performed according to the protocol recommended by the manufacturer for primary hippocampal neurons. A total of 0.5 μg of plasmid DNA was transfected per well with 1.6 μl of Enhancer (QIAGEN) and 2 μl of Effectene. At 12 h posttransfection, the medium was changed. After approximately 24 h, the neurons were fixed, stained with 5 μg of 4′,6′-diamidino-2-phenylindole (DAPI)/ml, mounted on glass slides, and analyzed by fluorescence microscopy.
Organotypic hippocampal slice cultures.
Hippocampal slice cultures from C57BL/6N mice were prepared by using the static interface culture method of Stoppini et al. (68) as described previously (40). Cultures were maintained on porous (0.4-μm-pore-size) transparent membrane inserts (30 mm in diameter; Millipore, Bedford, Mass.) in six-well culture plates with five or six slices on each insert for 2 to 3 weeks. Plates were kept in a humidified CO2 incubator (5% CO2, 95% atmospheric air) at 35.5°C. The medium (nutrient medium, composed of 25% heat-inactivated horse serum, 25% Hanks' balanced salt solution, and 50% Opti-MEM [all from Gibco] and supplemented with 25 mM d-glucose and 1 mM glutamine [both from Sigma, Munich, Germany]) was changed every 2 or 3 days. Neither antibiotics-antimycotics nor antimitotics were used. Transient transfection was performed by using Lipofectamine 2000 (Invitrogen) as follows. Ten microliters of Lipofectamine 2000 and of plasmid DNA (8 μg in total) were each diluted in 350 μl of Opti-MEM and, after incubation for 5 min, mixed with each other. After incubation for 20 min, the DNA-Lipofectamine 2000 complexes in Opti-MEM were pipetted onto the membrane harboring the slices. After 4 h, the solution covering the slices was removed; on the next day, the medium was changed. At 48 h posttransfection, cells were rinsed in PBS, fixed with 4% paraformaldehyde in PBS for 10 min, rinsed in PBS, stained with DAPI (1 μg/ml in PBS), washed again in PBS, and mounted in ProLong Antifade mounting medium (Molecular Probes, Leiden, The Netherlands).
Neuron selection.
Small numbers of principal neurons were transfected in each hippocampal slice culture, making it easy to distinguish the processes of individual neurons. Anatomical criteria were used to identify dentate gyrus granule cells, CA3 or CA1 pyramidal neurons, or interneurons as described by Danzer et al. (9). For CA1 pyramidal cells, (i) the soma was located in the CA1 pyramidal cell layer; (ii) apical and basal dendritic fields typical of a pyramidal cell were present; and (iii) the soma was smaller than that in CA3 pyramidal cells, and collateral dendrites branching from the proximal apical dendrite were present. Furthermore, neurons had to show no degenerative changes (e.g., dendritic bleb formation or nuclear fragmentation).
Imaging and analysis.
Light- and dark-field images of in situ hybridizations were acquired with a DMRB microscope (Leica, Solms, Germany).
Primary hippocampal neurons and neurons in slice cultures selected for analysis were analyzed by using an Axioplan 2 imaging system (Carl Zeiss, Göttingen, Germany) equipped with appropriate filters for the excitation and detection of enhanced cyan fluorescent protein (ECFP), EGFP, enhanced yellow fluorescent protein (EYFP), DAPI, and DsRed2.
Confocal images of HEK293 cells were taken by using an LSM 510 confocal microscope (Carl Zeiss, Jena, Germany) with a C-Apochromat 63×/1.2 water immersion objective and appropriate filters for the detection of ECFP and EYFP. ECFP was excited with the 458-nm line of an argon laser, and EYFP was excited with the 514-nm line. Zeiss AIM software (version 3.0) was used to acquire the images. The images obtained (see Fig. 4) were processed by using Adobe Photoshop to compensate for differences in brightness and contrast in the different colors.
FIG. 4.
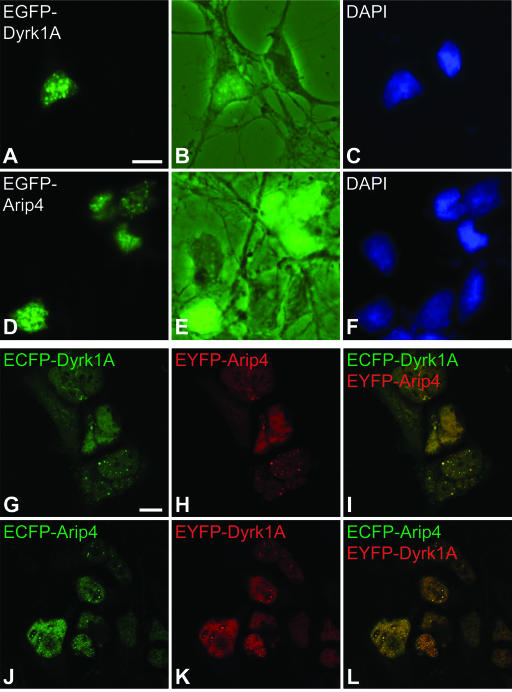
Intracellular localization of Dyrk1A and Arip4 in primary hippocampal neurons and in HEK293 cells. (A to F) Primary rat hippocampal neurons were transfected with EGFP-Dyrk1A (A to C) and EGFP-Arip4 (D to F). (A and D) Fusion proteins were detected by conventional fluorescence microscopy. (B and E) To visualize entire cells, corresponding images were taken with phase-contrast settings in combination with fluorescence. (C and F) Nuclei were counterstained with DAPI. Note that both EGFP-Dyrk1A and EGFP-Arip4 were localized in a speckle-like nuclear subcompartment. (G to L) Fusions of ECFP and EYFP with Dyrk1A and Arip4 were coexpressed in HEK293 cells and were analyzed by confocal laser scanning microscopy. ECFP signals (green; G and J) and EYFP signals (red; H and K) were merged (I and L) to visualize colocalization (yellow). Scale bars: A to F, 5 μm (bar shown only in panel A); G to L, 10 μm (bar shown only in panel G).
Series of light optical sections of nuclei from hippocampal slice cultures were recorded by using a TCS SP confocal laser scanning microscope equipped with a Plan Apo 100×/1.4 oil objective (Leica Lasertechnik, Heidelberg, Germany). Using the appropriate lines of an argon laser for the visualization of ECFP (457 nm), EYFP (514 nm), and DsRed2 (514 nm) and emission ranges of 460 to 500 nm (ECFP), 520 to 540 nm (EYFP), and 605 to 650 nm (DsRed2), stacks of equidistant (0.24-μm) 8-bit gray-scale images with a size of 512 × 512 pixels (40 × 40 μm) were obtained. Spreading of excited ECFP and EYFP into neighboring channels was minimized by recording the channels at each z position sequentially before moving to the next z position. In the analysis of ECFP-transfected cells with EYFP settings and vice versa, spreading was proven to be absent. To improve the signal-to-noise ratio, each image line was scanned eight times, and the values were averaged. Displayed confocal images were processed first in ImageJ (http://rsb.info.nih.gov/ij/) by using the Gaussian blur filter (radius, 2 pixels) and then in Adobe Photoshop to compensate for differences in brightness and contrast in the different colors.
Immunoprecipitation.
For coimmunoprecipitation experiments, HEK293 cells (3.8 × 106 cells) in a 10-cm culture dish were transfected 12 h after seeding with 10 μg of pCMV-FLAG-Dyrk1A or pCMV-FLAG-Arip4 and with 10 μg of pEGFP-Arip4 or pEGFP-Dyrk1A, respectively. One day after transfection, the cells were lysed for 30 min on ice in 200 μl of a lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10% glycerol, complete mini-EDTA-free protease inhibitor cocktail (Roche; one tablet per 7 ml of buffer), 1% phosphatase inhibitor cocktail II (Sigma), and 100 U of Benzonase (Sigma)/ml. The lysates were cleared by centrifugation at 4°C for 20 min at 16,000 × g. Twenty-microliter quantities of the supernatants were stored at −20°C, and the rest of the supernatants were subjected to precipitation with 40 μl of anti-FLAG M2 affinity matrix (Sigma) overnight at 4°C. The matrix was subsequently washed three times with 1 ml of 50 mM Tris-HCl-150 mM NaCl (pH 7.4). Bound proteins were eluted by boiling for 5 min in sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis loading buffer and, in parallel with 2.5% of the cleared lysates, were resolved by SDS-7.5% polyacrylamide gel electrophoresis. Proteins were blotted electrophoretically to Immobilon-P (Millipore). Blots were blocked in TBST (20 mM Tris-HCl, 138 mM NaCl, 0.1% Tween 20 [pH 7.6]) containing 5% fat-free milk powder for 1 h at room temperature. Blots were incubated overnight at 4°C with a monoclonal anti-GFP antibody (Roche) diluted 1:1,000 in TBST containing 5% fat-free milk powder. After being washed, the blots were incubated with a secondary antibody conjugated to horseradish peroxidase (1:2,000 in TBST containing 5% fat-free milk powder) (DAKO, Hamburg, Germany) and were developed by using the enhanced chemoluminescence method (ECL+; Amersham, Little Chalfont, United Kingdom). After being stripped (in 2% SDS-100 mM β-mercaptoethanol-50 mM Tris-HCl [pH 6.8] for 30 min at 70°C), the blots were incubated with a mouse monoclonal anti-FLAG antibody (1:4,000; M2; Sigma).
Transactivation assays.
A total of 120,000 CV-1 cells were cultured in DMEM (supplemented with 5% charcoal-stripped heat-inactivated FCS, 2 mM glutamine, and 1% antibiotic-antimycotic mixture) for approximately 16 h. The medium was changed to one without the antibiotic-antimycotic mixture, and the cells were transfected with the mouse mammary tumor virus (MMTV)-luciferase reporter gene plasmid pMTV-Luc and the appropriate plasmids for the expression of Dyrk1A, Arip4, and either AR or GR by using 1 μl of Lipofectamine 2000. For some experiments (see Fig. 6A), 480 ng of pMTV-Luc, 18 ng of pSTC-AR, 100 ng of pSVL-HA-Dyrk1AWT, and 40 ng of pCMV-FLAG-Arip4WT or pCMV-FLAG-Arip4Δ1165-1196 were transfected per well. In all wells, the total amount of cytomegalovirus (CMV) promoter-containing vectors was 140 ng; when necessary, this amount was adjusted with pCMV-Tag2. For other experiments (see Fig. 6B), 500 ng of pMTV-Luc, 15 ng of pSTC-AR, 50 ng of pCMV-FLAG-Arip4, and 100 ng of pSVL-HA-Dyrk1AWT or pSVL-HA-Dyrk1AK188R were transfected per well. The total amount of CMV promoter-containing vectors was adjusted to 150 ng per well with pCMV-Tag2. Experiments with GR (see Fig. 8) were performed with the same amounts as those used for the experiments just described (see Fig. 6B), but instead of pSTC-AR, pSTC-GR was used. In all experiments, the total amount of DNA per well was adjusted to 1 μg with pBluescript II KS. For RNAi experiments, 50 ng of pSVL-HA-Dyrk1A (see Fig. 7A), pCMV-FLAG-Arip4 (see Fig. 7B), or pCMV-Tag2 (see Fig. 7C) was cotransfected with 15 ng of pSTC-AR, 500 ng of pMTV-Luc, and 410 ng of the appropriate pSHAG-1-based RNAi construct. At 20 h posttransfection, the medium was changed to one containing 500 nM R1881 (an AR agonist) (for AR experiments), 10 nM dexamethasone (for GR experiments), or vehicle (0.1% ethanol). On the following day, the cells were washed once with PBS, lysed in 120 μl of a lysis buffer containing 100 mM potassium phosphate and 0.5 mM dithiothreitol per well, incubated for 15 min at 4°C, transferred to −80°C for 30 min, and thawed at 4°C. The lysates were cleared by centrifugation for 5 min at 16,000 × g, 50-μl quantities of the supernatants were used for the determination of luciferase activity with 50 μl of luciferase assay reagent (Promega), and protein concentrations in three 10-μl samples from each supernatant were determined by using a bicinchoninic acid (BCA) protein assay kit (Pierce). Luciferase activity was normalized to protein concentration.
FIG. 6.
The synergistic enhancement of AR-dependent transactivation by Dyrk1A and Arip4 expression is independent of the kinase activity of Dyrk1A. (A) Dyrk1A with Arip4WT or Arip4Δ1165-1196 was expressed in CV-1 cells together with the MMTV-luciferase reporter gene. Cells were stimulated with the synthetic AR agonist R1881. Note that Arip4Δ1165-1196, which lacks the Dyrk1A substrate consensus sequence, was also able to enhance the effect of Dyrk1A. WT, wild type. (B) Arip4 with Dyrk1AWT or Dyrk1AK188R was expressed in CV-1 cells together with the MMTV-luciferase reporter gene. Cells were stimulated with the synthetic AR agonist R1881. Note that kinase-deficient mutant Dyrk1AK188R was able to stimulate AR-mediated activation not different from that stimulated by Dyrk1AWT. Luciferase activities were normalized to protein content, and ratios were expressed relative to those obtained with AR in the presence of R1881 (value set at 1; second bar from left in panels A and B). Means and SEMs for three independent experiments are shown. For comparisons indicated by brackets, one asterisk indicates a P value of <0.05 and three asterisks indicate a P value of <0.0001.
FIG. 8.
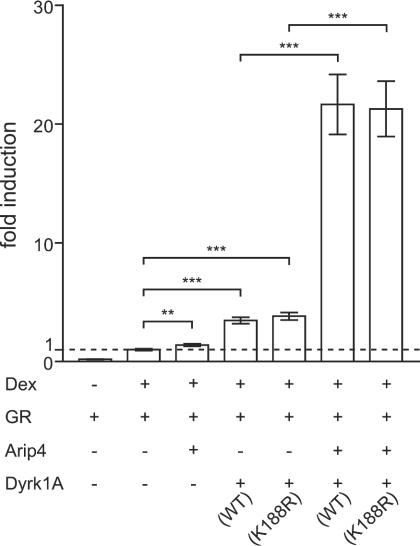
Dyrk1A and Arip4 are able to enhance glucocorticoid-induced transactivation. Arip4 with Dyrk1AWT or Dyrk1AK188R was expressed in CV-1 cells. WT, wild type. Luciferase activities were normalized to protein contents of the lysates, and ratios were expressed relative to those achieved with GR only in the presence of dexamethasone (Dex; value set at 1; second bar from left). Means and SEMs for three independent experiments are shown. For comparisons indicated by brackets, two asterisks indicate a P value of <0.01 and three asterisks indicate a P value of <0.0001.
FIG. 7.
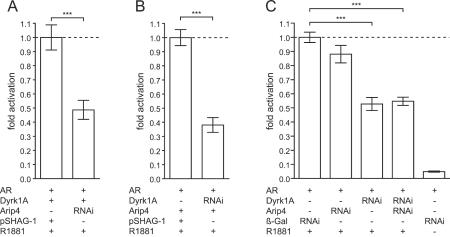
Effect of Dyrk1A and Arip4 knockdown by RNAi on AR-dependent transactivation. (A) Endogenous Arip4 is necessary for the full effect of Dyrk1A on AR-mediated transcription. Dyrk1A was ectopically expressed, and Arip4 expression was either unaltered (−) or inhibited by RNAi. RNAi targeting of Arip4 strongly reduced the effect of Dyrk1A. Values are expressed relative to that obtained by expression of Dyrk1A without knockdown of Arip4 (with pSHAG-1 as a control; value set at 1; left bar). (B) Endogenous Dyrk1A is necessary for the full effect of Arip4 on AR-mediated transcription. Arip4 was ectopically expressed, and Dyrk1A expression was either unaltered (−) or inhibited by RNAi. RNAi targeting of Dyrk1A strongly reduced the effect of Arip4. Values are expressed relative to that obtained by expression of Arip4 without knockdown of Dyrk1A (with pSHAG-1 as a control; value set at 1; left bar). (C) Endogenous Dyrk1A and Arip4 both contribute to AR-mediated transactivation. Knockdown of either Dyrk1A (third bar from left) or both Dyrk1A and Arip4 (fourth bar from left) significantly reduced the effect of the synthetic AR agonist R1881 on transcription, compared to the effect seen in controls expressing shRNA specific for the mRNA encoded by the lacZ gene (first bar from left). Values are expressed relative to those for the control (AR and lacZ RNAi in the presence of R1881; values set at 1; first bar from left). Luciferase activities were normalized to protein contents of the lysates. Means and SEMs for three independent experiments are shown. For comparisons indicated by brackets, three asterisks indicate a P value of <0.0001.
All transfections were done at least in triplicate, and the results are reported as the mean and standard error of the mean (SEM) for at least three experiments; thus, N is at least 9 for all transactivation assay data.
Statistical analysis of significant differences between two groups was performed by using the Student t test, and two-tailed P values were calculated. P values of <0.05 were considered statistically significant.
RESULTS
Identification of Arip4 as a Dyrk1A-interacting protein.
The kinase domain of Dyrk1A (residues 152 to 486) was used as bait in a yeast two-hybrid system to identify Dyrk1A-interacting proteins. Screening was performed with a pretransformed cDNA library generated from adult mouse brain in order to identify proteins which interact with Dyrk1A in the central nervous system. One of the six clones isolated encoded the C terminus (residues 1078 to 1466) of Arip4 (Fig. 1B), a recently identified cofactor of AR with ATP-dependent chromatin remodeling activity (61). This part of Arip4 contains a putative NLS, an LXXLL motif found in numerous proteins that interact with steroid hormone receptors (31), and a Dyrk1A substrate consensus sequence around serine 1168 (RPVSP) that perfectly matches recently described Dyrk1A phosphorylation sites (33).
To examine further the specificity of this interaction, cotransformation experiments with yeast cells were performed. The prey vector encoding GAL4 activation domain-Arip41078-1466 was transformed together with a vector encoding GAL4 DNA-binding domain-Dyrk1A. Reporter gene activation indicated an interaction of C-terminal Arip4 with the entire Dyrk1A protein. To exclude nonspecific interactions, the GAL4 activation domain-Arip41078-1466 construct was cotransformed with a GAL4 DNA-binding domain-lamin C or a GAL4 DNA-binding domain construct. No activation of reporter genes was detected in these controls. Subsequently, a cDNA encoding the entire Arip4 protein was cloned (see Materials and Methods).
Interaction of Dyrk1A with Arip4 in mammalian cells.
To check for the interaction of Dyrk1A with Arip4 in mammalian cells, HEK293 cells were transiently transfected with expression vectors encoding FLAG-tagged Dyrk1A and EGFP-tagged Arip4 or EGFP-tagged Dyrk1A and FLAG-tagged Arip4. Proteins from cell lysates were immunoprecipitated with anti-FLAG antibodies, and the binding of Dyrk1A to Arip4 was detected by Western blotting with anti-EGFP antibodies. Both combinations of transfections (FLAG-Dyrk1A with EGFP-Arip4 [Fig. 2 ] and EGFP-Dyrk1A with FLAG-Arip4 [data not shown]) revealed an interaction of Dyrk1A with Arip4. As a negative control, EGFP alone did not bind to FLAG-Dyrk1A (Fig. 2), and no EGFP-Dyrk1A was present in immunoprecipitates obtained from extracts of FLAG-transfected HEK293 cells (data not shown). These results indicate that Dyrk1A specifically interacts with Arip4 in mammalian cells.
FIG. 2.
Interaction of Dyrk1A with Arip4 in HEK293 cells. The indicated proteins were expressed in HEK293 cells. Whole-cell lysates were subjected to immunoprecipitation (IP) with anti-FLAG beads. Immunoprecipitated proteins (left panels) were analyzed by immunoblotting (Western blotting [WB]) with an anti-EGFP antibody for the detection of EGFP-Arip4 and EGFP (upper and lower panels). The membrane then was reprobed with an anti-FLAG antibody for the detection of FLAG-Dyrk1A (middle panel). Note that FLAG-Dyrk1A interacts with EGFP-Arip4 (upper panel, second lane; the lower band is a degradation product of the fusion protein) but not with EGFP alone (lower panel, first lane). Cell lysates were also immunoblotted to detect the expressed proteins (right panels; Input): EGFP-Arip4 (upper panel), FLAG-Dyrk1A (middle panel; arrowheads indicate the fusion protein), and EGFP (lower panel). Protein markers are indicated on the right.
Coexpression of Dyrk1A and Arip4 in the mouse brain.
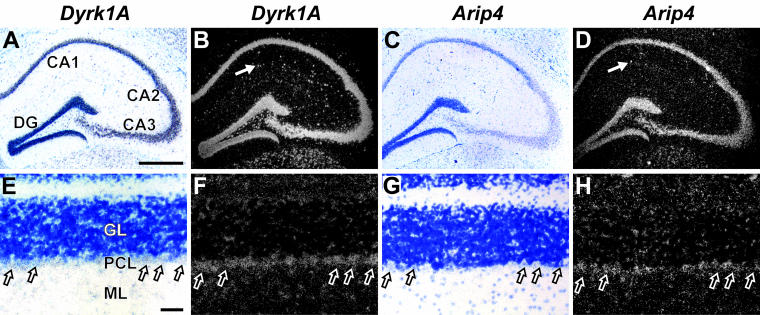
Dyrk1A is known to be widely expressed in the adult mouse brain (48). The pattern of expression of Arip4 has not yet been analyzed at the cellular level; the only data available show that Arip4 mRNA is present in whole-brain lysates from mice (61). A prerequisite for a physiologically relevant interaction of Dyrk1A with Arip4 is its coexistence within the same cell. Due to the low levels of expression of both Dyrk1A and Arip4, it was not possible to perform double in situ hybridization experiments. Therefore, single in situ hybridization experiments were performed with radiolabeled probes for Dyrk1A and Arip4 and with consecutive cryosections from various regions of the adult mouse brain. Special emphasis was placed on regions that are affected in persons with DS, such as the hippocampus and the cerebellum. Both Dyrk1A and Arip4 were found to be widely expressed in these regions (Fig. 3). Specifically, Dyrk1A and Arip4 were expressed in all principal neurons of the hippocampus and the dentate gyrus (Fig. 3A to D). Thus, the two transcripts were definitely coexpressed in these cell types. In addition, both genes were expressed in many interneurons in the hippocampal formation, although the exact subtype of the interneurons remains to be determined. In the cerebellum, Dyrk1A and Arip4 mRNAs were detected in all layers—granular, molecular, and Purkinje cell layers. Due to their large perikaryon, distinctly identifiable Purkinje cells were visualized in two consecutive sections, which were hybridized with probes for Dyrk1A and Arip4. In this way, it was possible to detect both mRNAs in identical cells (Fig. 3E to H). Taken together, the results of this in situ hybridization analysis showed the coexpression of Dyrk1A and Arip4 in principal neurons of the hippocampus and in cerebellar Purkinje cells.
FIG. 3.
Coexpression of Dyrk1A and Arip4 in selected brain regions of the adult mouse. In situ hybridization experiments were performed with 35S-labeled riboprobes and consecutive coronal sections to detect mRNAs of Dyrk1A and Arip4, respectively. Corresponding images of the same specimen were taken with bright-field (A, C, E, and G) and dark-field (B, D, F, and H) illumination. (A to D) Both genes are expressed in pyramidal cells of the CA1, CA2, and CA3 regions of the hippocampus, in interneurons of the hippocampal formation (arrows), and in granule cells of the dentate gyrus (DG). (E to H) In the cerebellum, both Dyrk1A and Arip4 are expressed in a large cell population of the granular layer (GL), in the molecular layer (ML), and in Purkinje cells (PCL; arrows). Scale bars: A to D, 500 μm (bar shown only in panel A); E to H, 50 μm (bar shown only in panel E).
Subcellular localization of Dyrk1A and Arip4.
Next, we determined the subcellular localization of the Dyrk1A and Arip4 proteins by transfection of expression constructs coding for fusions of EGFP with Dyrk1A and with Arip4, respectively, into primary hippocampal neurons. Transfection was performed after 2 days of culturing, and cells were analyzed by fluorescence microscopy on the following day to monitor the localization of the proteins (Fig. 4A to F). Both EGFP-Dyrk1A and EGFP-Arip4 were exclusively nuclear and concentrated in a speckle-like nuclear subcompartment in cells with neuronal morphology. In addition, they were also present in the nucleoplasm, although to a much lesser degree.
To address the question of whether Dyrk1A and Arip4 are localized in the same nuclear subcompartment, expression constructs coding for fusion proteins with the color variants of EGFP, i.e., ECFP and EYFP, were generated. These proteins were expressed in HEK293 cells. The localization of the fusion proteins was studied by confocal laser scanning microscopy. Again, both Dyrk1A and Arip4 were found to be localized in a speckle-like nuclear subcompartment and, to a lesser degree, diffusely throughout the nucleus, excluding the nucleolus (Fig. 4G, H, J, and K). Overlays of the images showed that Dyrk1A and Arip4 were colocalized throughout the nucleoplasm and, to a large extent, in a speckle-like nuclear subcompartment (Fig. 4I and L). However, the colocalization in the speckles was not complete, as some spots were found to be stained for either but not both of the proteins. Similar results were obtained after transfection of the color variants into hippocampal cell line HT22 (data not shown).
To study the subcellular localization of Dyrk1A and Arip4 in a context where the basic morphological and functional properties of the hippocampal neuronal network are preserved, we expressed ECFP-Dyrk1A and EYFP-Arip4 together with DsRed2 in organotypic hippocampal slice cultures and analyzed their localization by confocal laser scanning microscopy (Fig. 5). This strategy was chosen because no appropriate antibodies against Dyrk1A and Arip4 were available to detect the proteins by double-immunofluorescence methods. In these hippocampal slice cultures, transfected cells were visualized by DsRed2 fluorescence (Fig. 5B), and neurons were identified based on morphological criteria (9). Both in interneurons and in pyramidal neurons, Dyrk1A and Arip4 were found to be localized primarily in the nucleus and, to a much lesser extent, also in the cytoplasm of the perikaryon and in neurites. For most of the neurons analyzed, Dyrk1A and Arip4 were not dispersed throughout the entire nucleus but were concentrated in a speckle-like nuclear subcompartment (Fig. 5C to F). When examined by conventional fluorescence microscopy, ECFP-Dyrk1A- or EYFP-Arip4-positive regions in the nucleus appeared as one or several large aggregate(s), but when confocal analysis was used, these domains were resolved and were shown to consist of a large number of small speckles (Fig. 5C to F). By comparison of the ECFP-Dyrk1A- or EYFP-Arip4-positive regions with the distribution of chromatin, as visualized by DNA staining with DAPI and conventional fluorescence microscopy, we found evidence in several neurons that Dyrk1A- and Arip4-containing speckles occurred in nuclear regions where chromatin was less densely packed (Fig. 5F and G). In some neurons, where chromocenters were visible, Dyrk1A and Arip4 seemed to be excluded from these regions of densely packed chromatin (data not shown). In a few neurons, small aggregates containing Dyrk1A and/or Arip4 were also detected in the cytoplasm (Fig. 5A and data not shown).
FIG. 5.
Colocalization of Dyrk1A and Arip4 in hippocampal pyramidal neurons. Conventional fluorescence microscopy (A, B, and G) and confocal laser scanning microscopy (C to F) images show the same pyramidal neuron in an organotypic hippocampal explant transfected with plasmids encoding ECFP-Dyrk1A, EYFP-Arip4, and DsRed2 and stained with DAPI. Corresponding images of the same region were obtained to visualize ECFP-Dyrk1A (green) and EYFP-Arip4 (red), the cell body of transfected neurons (labeled with DsRed2; red), and the nuclei of all cells (DAPI; blue). Merged images of ECFP-Dyrk1A and EYFP-Arip4 were generated to show the colocalization of Dyrk1A and Arip4 (yellow; F). To visualize the nucleus and the distribution of the chromatin, the DAPI image (G) was superimposed on the confocal ECFP-Dyrk and EYFP-Arip4 image (F) to generate the image in panel C. Asterisks in panels F and G indicate corresponding locations. Open arrowheads (ECFP-Dyrk1A) and filled arrowheads (EYFP-Arip4) indicate speckles containing only Dyrk1A and only Arip4, respectively. Scale bars: A and B, 25 μm; C, 5 μm; D to G, 5 μm (bar shown only in panel D).
Dyrk1A and Arip4 regulate AR signaling.
Arip4 is known to interact physically and functionally with AR and was described as a coactivator of androgen-induced transcription (61). Therefore, we addressed the question of whether the interaction of Dyrk1A with Arip4 could lead to a modulation of androgen-induced transcription by performing luciferase reporter gene assays after transient transfections.
First, we cotransfected CV-1 cells with an MMTV-luciferase reporter gene which is regulated by androgen and an expression vector for AR. In addition, Dyrk1A, Arip4, both, or neither was expressed. As shown in Fig. 6A, the activation of AR with the synthetic androgen R1881 led to a robust induction of the reporter gene (7.1-fold). This level of luciferase activity induced by hormone-activated AR without cofactors was set at 1. The expression of Dyrk1A led to a 4.3-fold (SEM, ±0.35 [P < 0.0001]) further increase in reporter gene activity, whereas transfection with a plasmid encoding wild-type Arip4 caused an increase of 1.2-fold (±0.06 [P < 0.05]). The expression of Dyrk1A and Arip4 together strongly augmented the inducing effect on the reporter gene and led to an increase of 18-fold (±2.7 [P < 0.0001]) over that obtained with activated AR (Fig. 6A). This enhancement was much stronger than the sum of the effects of Dyrk1A and Arip4 transfected alone. Therefore, these findings indicate a functional interaction of Dyrk1A and Arip4 which has a synergistic effect on AR-mediated transcription.
Because Dyrk1A is a protein kinase and Arip4 contains a Dyrk1A substrate consensus sequence, it is reasonable to assume that Dyrk1A regulates the activity of Arip4 by phosphorylation. To test this hypothesis, we used a mutant of Arip4 (Arip4Δ1165-1196) which lacks the Dyrk1A substrate sequence and investigated the effect of the mutation on the activity of Arip4 in a transactivation assay with AR (Fig. 6A). Surprisingly, the mutation had no significant effect on the activity of Arip4 transfected alone (wild-type Arip4, 1.22-fold activation [±0.06]; Arip4Δ1165-1196, 1.27-fold activation [±0.11] [P = 0.69]) or on the synergistic effect on AR of the cotransfection of Arip4 with Dyrk1A (Dyrk1A plus wild-type Arip4, 18-fold activation [±2.7]; Dyrk1A plus Arip4Δ1165-1196, 16-fold activation [±2.0] [P = 0.56]). These results led us to conclude that the activation of Arip4 by Dyrk1A is not mediated by the phosphorylation of serine 1168. To further substantiate whether the phosphorylation of Arip4 by Dyrk1A plays an essential role in an activation mechanism, we cotransfected Arip4 together with a kinase-deficient mutant of Dyrk1A (Dyrk1AK188R) (Fig. 6B). In these experiments, Dyrk1AK188R was also able to activate AR-mediated transcription synergistically with Arip4, and the effect was not significantly different from that obtained with wild-type Dyrk1A (Arip4 plus wild-type Dyrk1A, 29.2-fold activation [±3.2]; Arip4 plus Dyrk1AK188R, 22.2-fold activation [±2.9] [P = 0.12]). Apparently, the effect of Dyrk1A on Arip4 and androgen-induced transactivation is not mediated by the phosphorylation of serine 1168 or phosphorylation at other sites.
Functions of endogenous Dyrk1A and Arip4 in AR-mediated transactivation.
To address the question of whether the inducing effect of Dyrk1A and Arip4 on steroid hormone-dependent transactivation can also be mediated by endogenous Dyrk1A and Arip4 without ectopic expression, we used RNAi to knock down the endogenously expressed Dyrk1A and Arip4 proteins. Both Dyrk1A and Arip4 are expressed in CV-1 cells, as detected by RT-PCR (data not shown). RNAi was performed by expressing shRNAs directed against either Dyrk1A or Arip4 after transfection of pSHAG-1-based constructs. Four constructs targeting Dyrk1A were tested by coexpression with EGFP-Dyrk1A in HEK293 cells. Two constructs were found to lead to a strong reduction in the level of expression of EGFP-Dyrk1A, whereas EGFP was not affected, as visualized by fluorescence microscopy (data not shown). In addition, lysates of these cells were immunoblotted, and EGFP-Dyrk1A was detected with an anti-EGFP antibody. The amount of Dyrk1A was reduced to undetectable or very low levels for the two most efficient shRNA-expressing constructs (data not shown). These two plasmids were used as a mixture to knock down Dyrk1A in CV-1 cells before transactivation assays. For RNAi-mediated knockdown of Arip4, three constructs were generated and used as a mixture. Knockout of endogenous Arip4 reduced the reporter gene-inducing effect of transfected Dyrk1A (Dyrk1A plus RNAi-targeting Arip4, 0.49-fold activity [±0.07]; Dyrk1A plus pSHAG-1, 1.0-fold activity [±0.09] [P < 0.001]) (Fig. 7A), and knockdown of endogenous Dyrk1A strongly reduced the effect of transfected Arip4 on androgen-induced transactivation (Arip4 plus RNAi targeting Dyrk1A, 0.38-fold activity [±0.05]; Arip4 plus pSHAG-1, 1.0-fold activity [±0.06] [P < 0.0001]) (Fig. 7B).
To monitor possible nonspecific effects of the pSHAG-1 vector and of the expression of double-stranded shRNAs, either the pSHAG-1 empty vector or a set of three plasmids expressing shRNAs targeting lacZ (pSHAG-1-lacZ) were included in the transfection experiments. In cotransfections with Dyrk1A or Arip4, the values obtained with pSHAG-1 and pSHAG-1-lacZ did not differ significantly (data not shown); thus, only one of these controls is shown for each experiment.
Without any ectopic expression by transfection of Dyrk1A or Arip4, knockdown of endogenous Dyrk1A or both Dyrk1A and Arip4 significantly reduced androgen-mediated transactivation (RNAi targeting Dyrk1A, 0.53-fold activity [±0.046]; RNAi targeting lacZ control, 1.0-fold activity [±0.036] [P < 0.0001]; and RNAi targeting Dyrk1A together with RNAi targeting Arip4, 0.55-fold activity [±0.029]; control, 1.0-fold activity [±0.036] [P < 0.0001]). However, the reduction of reporter gene activity after knockdown of endogenous Arip4 did not reach significance (0.88-fold activity [±0.062]; control, 1.0-fold activity [±0.036] [P = 0.11]) (Fig. 7C).
Dyrk1A and Arip4 regulate GR signaling.
In order to determine whether Dyrk1A and Arip4 were able to enhance transcription induced by other steroid hormone receptors, we expressed Dyrk1A and Arip4 in CV-1 cells together with a GR expression vector and with a GR-inducible luciferase reporter gene (MMTV-luciferase). As shown in Fig. 8, the expression of Dyrk1A or Arip4 resulted in an increase in GR-mediated transactivation to an extent similar to that observed for AR (Arip4, 1.4-fold activation [±0.10] [P < 0.01]; Dyrk1A, 3.5-fold activation [±0.27] [P < 0.0001]; both compared to control, 1.0-fold activity [±0.077]). When Dyrk1A and Arip4 were coexpressed, they synergistically increased the effect of dexamethasone on the expression of the reporter gene (21.7-fold activation [±2.5]; control, 1.0-fold activity [±0.077] [P < 0.0001]). In addition, we investigated for GR whether the phosphorylation of Arip4 by Dyrk1A plays a role as a mechanism for activating GR-mediated transcriptional effects. It was found that the kinase-deficient mutant Dyrk1AK188R exhibited the same activity as wild-type Dyrk1A (Arip4 plus wild-type Dyrk1A, 21.7-fold activation [±2.5]; Arip4 plus Dyrk1AK188R, 21.3-fold activation [±2.3] [P = 0.91]) (Fig. 8). Furthermore, we performed transactivation assays with GR and dexamethasone in combination with RNAi to knock down endogenous Dyrk1A and/or Arip4 and obtained results similar to those shown for AR and androgen in Fig. 7 (data not shown). In summary, not only AR-mediated transactivation but also GR-mediated induction of transcription is potentiated by Dyrk1A and Arip4, and these effects are not dependent on the kinase activity of Dyrk1A.
DISCUSSION
Arip4, a steroid hormone receptor cofactor, interacts with Dyrk1A.
In this study, we aimed to shed light on the function of Dyrk1A in the mammalian brain, in particular, on the cellular mechanisms by which a third copy of Dyrk1A could lead to cognitive impairments in mice and humans (27, 63, 64). During the last few years, several transcription factors that interact with Dyrk1A, including FKHR, CREB, and Gli1, were identified (45, 49, 73, 75). Despite these investigations, it is still unknown which signal transduction cascades are the key players modulated by Dyrk1A both under physiological circumstances and in pathophysiological situations, such as in DS.
In the present investigation, we identified Arip4 as a protein physically and functionally interacting with Dyrk1A. Arip4 was originally identified as an ATPase of the Rad54/ATRX subfamily of SNF2-like proteins, contains chromatin remodeling activity, interacts with AR, and modulates androgen-mediated transactivation (61). In general, SNF2-like proteins are thought to modify the structure of chromatin in a noncovalent manner through rearrangement of nucleosomes and are able to render condensed chromatin accessible to sequence-specific transcription factors (reviewed in references 3 and 44). The clone initially isolated in the yeast two-hybrid screen codes for the C terminus of Arip4. This region contains an NLS and an LXXLL motif, a potential binding site for ligand-activated nuclear receptors (31). Intriguingly, we identified in this C-terminal region of Arip4 a Dyrk1A substrate sequence perfectly matching the Dyrk1A substrate consensus sequence (33).
By coimmunoprecipitation, we confirmed the specific interaction of Dyrk1A and Arip4 in mammalian cells. It was shown by Rouleau et al. (61) that Arip4 interacts with the AR; therefore, it seems plausible to hypothesize that Dyrk1A, Arip4, and AR coexist in one complex in mammalian cells.
Colocalization of Dyrk1A with Arip4 in the mouse brain.
To elucidate the physiological relevance of this interaction, we performed coexpression and colocalization analyses. By in situ hybridization, we could show the coexpression of Dyrk1A and Arip4 in several brain regions. In our expression analysis, we placed special emphasis on those regions, which are affected in DS. Both for the cerebellum and for the hippocampal formation, disturbances in volume and neuronal numbers and/or densities were found, and the alterations in these regions were suggested to account, at least in part, for the behavioral phenotype in DS (reviewed in reference 51; see also reference 59). We found that both genes are broadly expressed in the adult mouse brain. In the cerebellum and in principal neurons of the hippocampus and the dentate gyrus, we found a high level of coexpression. Furthermore, we showed the colocalization of Dyrk1A and Arip4 in a speckle-like nuclear subcompartment in mammalian cell lines and hippocampal neurons.
From these data, we conclude that Dyrk1A and Arip4 are coexpressed and colocalized in neurons of the hippocampus, a brain region that plays a critical role in learning and memory and that is most clearly affected in the brains of persons with DS (51).
Dyrk1A and Arip4 colocalize in nuclear speckles.
To study the subcellular localization of Dyrk1A and Arip4, fusions of Dyrk1A and Arip4 with EGFP or its color variants ECFP and EYFP were expressed in HEK293 cells, primary hippocampal neurons, and organotypic hippocampal explants. Using confocal laser scanning microscopy, we found that Dyrk1A and Arip4 fusion proteins colocalize to a large extent in a speckle-like nuclear subcompartment. This localization appears to reflect the physiological situation of endogenously expressed Dyrk1A, as the presence of Dyrk1A in speckles is in agreement with the results of recent studies in which immunostaining detected endogenous Dyrk1A protein in primary cerebellar (48) and hippocampal (J. H. Sitz and B. Lutz, unpublished observations) neurons. The accumulation of Dyrk1A in speckles was shown to be dependent on a repeat of histidine residues located C-terminal to the kinase domain (1) (Fig. 1A). However, the overexpression of Dyrk1A was also reported to cause a diffuse distribution of Dyrk1A itself all over the nucleus and speckle disassembly in some cell types (1). In the present study, this feature, however, was not observed in the primary neurons analyzed. In hippocampal explants, few neurons were observed to contain Dyrk1A not present in a speckle-like pattern but more diffusely distributed in the nucleus. In HEK293 cells, we observed both speckle-like and more diffusely distributed localizations of Dyrk1A and Arip4. These distributions were not mutually exclusive but coexisted in many cells. In agreement with Alvarez et al. (1), we did not observe a correlation between expression level and subnuclear localization in any of the cell types studied. In contrast, Funakoshi et al. (17) reported that strongly overexpressed Dyrk1A is localized all over the nucleus in HeLa cells and induces multinucleation occurring after mitosis.
Dyrk1A colocalizes in speckles with the serine arginine-rich (SR) protein SC35 splicing factor (1) and is able to interact with and to phosphorylate several arginine/serine-rich (RS) domain proteins, including the recently identified Dyrk1A substrate cyclin L2, which binds to Dyrk1A via the RS domain (10). The RS domain is a typical feature of many proteins involved in pre-mRNA processing (22). These findings may be interpreted as evidence for a role of Dyrk1A as a splicing cofactor, but it should be noted that speckles are known to contain proteins that are part of the transcriptional machinery, e.g., hyperphosphorylated RNA polymerase II (50), transcription factors (43, 77), and the chromatin remodeling transcriptional cofactor HMG-17 (34). Moreover, the current view of the function of speckles is that they are not direct transcription and/or splicing centers but rather are storage, assembly, or modification compartments (reviewed in reference 41). It remains to be determined whether Dyrk1A is able to regulate splicing and in which nuclear subcompartment Dyrk1A primarily exerts its function(s). Dyrk1A accumulation in speckles is sensitive to the transcriptional state, as Dyrk1A localizes to larger speckles after inhibition of RNA polymerase II (1). This finding suggest that speckles may be storage compartments for superfluous Dyrk1A and that Dyrk1A is involved in transcription, splicing, or both.
Dyrk1A and Arip4 regulate steroid hormone-induced transactivation.
We analyzed the functional interaction of Dyrk1A and Arip4 by performing reporter gene assays after the expression of Dyrk1A and/or Arip4 in CV-1 cells. We were able to show that Dyrk1A and Arip4 activate androgen- and glucocorticoid-mediated transactivation synergistically. We were able to show not only that Dyrk1A and Arip4 participate in transcriptional regulation after induction by steroid hormones when they are ectopically expressed but also that endogenously expressed Dyrk1A and Arip4 have a stimulatory effect on androgen- and glucocorticoid-induced transcription. Whereas a physical and functional interaction of Arip4 with AR and a regulatory role for Arip4 in androgen-mediated transactivation had been described already by Rouleau et al. (61), the effect of Arip4 on glucocorticoid-induced transcription is shown here for the first time.
Dyrk1A is not a general transcriptional activator and functions without kinase activity.
Although Dyrk1A has been reported to interact with several transcription factors in other studies (reviewed in reference 18) and in this study, it should be noted that Dyrk1A is not a general transcriptional activator, as it does not enhance Elk-, c-Jun-, or LEF-1-mediated transactivation (45). It remains to be determined which of the signaling cascades leading to gene induction is most predominantly regulated by Dyrk1A or even requires Dyrk1A under physiological conditions in the mammalian brain.
Most of the transcription factors previously described as Dyrk1A substrates are phosphorylated by Dyrk1A. Remarkably, we found that the kinase activity of Dyrk1A is not necessary for the enhancing effect on steroid hormone-mediated transactivation, as neither mutation of the putative Dyrk1A phosphorylation site of Arip4 nor engagement of a kinase-negative mutant of Dyrk1A affected the synergistic activation of steroid hormone-mediated transactivation by Dyrk1A and Arip4. This finding is reminiscent of the interaction of Dyrk1A with FKHR. It was reported that FKHR-mediated transactivation of the glucose-6-phosphatase gene is stimulated by Dyrk1A and that the synergistic effect of FKHR and Dyrk1A on the glucose-6-phosphatase promoter is independent both of the kinase activity of Dyrk1A and of the presence of serine 329 of FKHR (71), which is phosphorylated by Dyrk1A (73).
Involvement of Dyrk1A in steroid hormone-regulated processes and in mental retardation.
Our results show a regulatory role of Dyrk1A in steroid hormone-induced transcription. In this process, the Dyrk1A-interacting protein Arip4 is able to potentiate the stimulatory effect of Dyrk1A in a synergistic manner. Notably, Dyrk1A together with Arip4 appears to be able to influence both AR- and GR-mediated transactivation in vivo in the mammalian brain, as both genes are coexpressed, e.g., in the hippocampus (Fig. 3), and as AR and GR are also present in this region (38, 67, 76). Thus, these functional interactions may play important roles both under physiological conditions in the mammalian brain and in pathophysiological states, such as in DS.
Dyrk1A appears to be involved in cognitive processes in the mammalian brain in a gene dosage-dependent manner. The overexpression of Dyrk1A in yeast artificial chromosome-transgenic mice causes an impairment in hippocampus-dependent spatial learning and memory (63, 64). Our work may suggest that these impairments could be explained, at least in part, by dysregulation of AR- and GR-mediated processes. Pubertal androgens have been reported to regulate synaptic plasticity in the hippocampal CA1 region and to reduce social memory (28, 30). Intriguingly, androgens modulate the behaviorally induced expression of c-fos (39), an immediate-early gene implicated in long-term memory (reviewed in reference 25), but whether androgens applied in adulthood affect performance in learning and memory tasks is still a matter of debate (reviewed in reference 13). An important role of corticosteroid receptors, in particular, GR, in hippocampus-dependent spatial learning and memory has been well established (reviewed in reference 36). There is evidence for an inverted U-shaped relationship between corticosteroid levels and the abilities to learn and to memorize (reviewed in reference 11). This effect is primarily mediated by transactivation through GR (53). This inverted U-shaped mode of action is in agreement with the current view of the gene dosage-dependent function of Dyrk1A and MNB in memory processing. Both overexpression in mice (64) and reduced expression in Drosophila (69) lead to impairments in learning and memory. Based on the results of the present work, it can be suggested that Dyrk1A functions in cognitive processes, such as hippocampus-dependent spatial memory, at least in part through its interaction with Arip4 and with steroid hormone receptors, in particular, GR.
Interestingly, mutations in the human ATRX gene, the closest relative of Arip4, cause the ATR-X syndrome, which is characterized by mental retardation and other defects (19, 20). Like Arip4, ATRX has been reported to form a complex with the transcriptional cofactor Daxx and to display chromatin remodeling activities (74). Another example of a human syndrome associated with mental retardation caused by mutations in a chromatin remodeling protein and leading to transcriptional dysregulation is the Rubinstein-Taybi syndrome, which is caused by mutations in the CREB-binding protein gene, encoding a transcriptional cofactor also involved in steroid hormone pathways (2, 55).
The finding that most (>95%) of the genes dysregulated by more than ±2 standard deviations in trisomy 21 human fetal cells do not map to chromosome 21 (15) may be explained by secondary effects caused by the overexpression of a multifunctional transcriptional coregulator(s) encoded on chromosome 21, among which Dyrk1A is a prototypical example.
Steroid hormone signaling may be altered under pathophysiological circumstances where Dyrk1A is overexpressed, such as in transgenic mice and in humans with DS, through the interaction of Dyrk1A with Arip4. However, it remains to be investigated whether disturbed chromatin remodeling and altered regulation of steroid hormone-regulated gene expression contribute to the phenotype of DS.
Acknowledgments
We thank Steffen Dietzel (University of Munich, Munich, Germany) and Peter Hutzler (GSF-National Research Center for Environment and Health, Neuherberg, Germany) for help with confocal laser scanning microscopy; Nadhim Bayatti for the preparation of primary hippocampal cultures; Maria Brenz Verca, Stefano Brenz Verca, and Theo Rein for valuable discussions and critically reading the manuscript; Walter Becker (Rheinisch-Westfälische Technische Hockschule Aachen, Aachen, Germany) for plasmids pSVL-HA-Dyrk1AWT and pSVL-HA-Dyrk1AK188R; Sandro Rusconi (University of Fribourg, Fribourg, Switzerland) for pSTC-AR, pSTC-GR, and pMTV-Luc; and Anika Daschner, Barbara Fackelmeier, and Barbara Wölfel for excellent technical assistance.
This work was supported by a Boehringer Ingelheim Fonds scholarship to J.H.S., by a Hertie Foundation scholarship to B.L., and by the Max Planck Society.
REFERENCES
- 1.Alvarez, M., X. Estivill, and S. de la Luna. 2003. DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J. Cell Sci. 116:3099-3107. [DOI] [PubMed] [Google Scholar]
- 2.Ausio, J., D. B. Levin, G. V. De Amorim, S. Bakker, and P. M. Macleod. 2003. Syndromes of disordered chromatin remodeling. Clin. Genet. 64:83-95. [DOI] [PubMed] [Google Scholar]
- 3.Becker, P. B., and W. Hörz. 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71:247-273. [DOI] [PubMed] [Google Scholar]
- 4.Becker, W., and H. G. Joost. 1999. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acids Res. Mol. Biol. 62:1-17. [DOI] [PubMed] [Google Scholar]
- 5.Becker, W., Y. Weber, K. Wetzel, K. Eirmbter, F. J. Tejedor, and H. G. Joost. 1998. Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J. Biol. Chem. 273:25893-25902. [DOI] [PubMed] [Google Scholar]
- 6.Brewer, G. J., J. R. Torricelli, E. K. Evege, and P. J. Price. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567-576. [DOI] [PubMed] [Google Scholar]
- 7.Chen, C., and H. Okayama. 1987. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 7:2745-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen-Hwang, M. C., H. R. Chen, M. Elzinga, and Y. W. Hwang. 2002. Dynamin is a minibrain kinase/dual specificity Yak1-related kinase 1A substrate. J. Biol. Chem. 277:17597-17604. [DOI] [PubMed] [Google Scholar]
- 9.Danzer, S. C., K. R. Crooks, D. C. Lo, and J. O. McNamara. 2002. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J. Neurosci. 22:9754-9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Graaf, K., P. Hekerman, O. Spelten, A. Herrmann, L. C. Packman, K. Bussow, G. Muller-Newen, and W. Becker. 2003. Characterization of cyclin L2, a novel cyclin with an arginine/serine-rich (RS) domain: phosphorylation by DYRK1A and colocalization with splicing factors. J. Biol. Chem. 279:4612-4624. [DOI] [PubMed] [Google Scholar]
- 11.De Kloet, E. R., M. S. Oitzl, and M. Joels. 1999. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 22:422-426. [DOI] [PubMed] [Google Scholar]
- 12.Delabar, J. M., D. Theophile, Z. Rahmani, Z. Chettouh, J. L. Blouin, M. Prieur, B. Noel, and P. M. Sinet. 1993. Molecular mapping of twenty-four features. of Down syndrome on chromosome 21. Eur. J. Hum. Genet. 1:114-124. [DOI] [PubMed] [Google Scholar]
- 13.Dohanich, G. 2002. Gonadal steroids, learning and memory, p. 265-327. In D. W. Pfaff, A. Arnold, A. M. Etgen, S. E. Fahrbach, and R. T. Rubin (ed.), Hormones, brain and behaviour, vol. 2. Academic Press, Inc., San Diego, Calif. [Google Scholar]
- 14.Epstein, C. J. 2000. Down syndrome (trisomy 21), p. 1223-1256. In C. R. Scriver and W. S. Sly (ed.), The metabolic and molecular bases of inherited disease. McGraw-Hill Book Co., New York, N.Y.
- 15.FitzPatrick, D. R., J. Ramsay, N. I. McGill, M. Shade, A. D. Carothers, and N. D. Hastie. 2002. Transcriptome analysis of human autosomal trisomy. Hum. Mol. Genet. 11:3249-3256. [DOI] [PubMed] [Google Scholar]
- 16.Fotaki, V., M. Dierssen, S. Alcantara, S. Martinez, E. Marti, C. Casas, J. Visa, E. Soriano, X. Estivill, and M. L. Arbones. 2002. Dyrk1A haploinsufficiency affects viability and causes developmental delay and abnormal brain morphology in mice. Mol. Cell. Biol. 22:6636-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Funakoshi, E., T. Hori, T. Haraguchi, Y. Hiraoka, J. Kudoh, N. Shimizu, and F. Ito. 2003. Overexpression of the human MNB/DYRK1A gene induces formation of multinucleate cells through overduplication of the centrosome. BMC Cell Biol. 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galceran, J., K. De Graaf, F. J. Tejedor, and W. Becker. 2003. The MNB/DYRK1A protein kinase: genetic and biochemical properties. J. Neural Transm. Suppl. 67:139-148. [DOI] [PubMed] [Google Scholar]
- 19.Gibbons, R. J., L. Brueton, V. J. Buckle, J. Burn, J. Clayton-Smith, B. C. Davison, R. J. Gardner, T. Homfray, L. Kearney, and H. M. Kingston. 1995. Clinical and hematologic aspects of the X-linked alpha-thalassemia/mental retardation syndrome (ATR-X). Am. J. Med. Genet. 55:288-299. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons, R. J., D. J. Picketts, L. Villard, and D. R. Higgs. 1995. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome). Cell 80:837-845. [DOI] [PubMed] [Google Scholar]
- 21.Gietz, D., A. St. Jean, R. A. Woods, and R. H. Schiestl. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20:1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graveley, B. R. 2000. Sorting out the complexity of SR protein functions. RNA 6:1197-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimera, J., C. Casas, X. Estivill, and M. Pritchard. 1999. Human minibrain homologue (MNBH/DYRK1): characterization, alternative splicing, differential tissue expression, and overexpression in Down syndrome. Genomics 57:407-418. [DOI] [PubMed] [Google Scholar]
- 24.Guimera, J., C. Casas, C. Pucharcos, A. Solans, A. Domenech, A. M. Planas, J. Ashley, M. Lovett, X. Estivill, and M. A. Pritchard. 1996. A human homologue of Drosophila minibrain (MNB) is expressed in the neuronal regions affected in Down syndrome and maps to the critical region. Hum. Mol. Genet. 5:1305-1310. [DOI] [PubMed] [Google Scholar]
- 25.Guzowski, J. F. 2002. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus 12:86-104. [DOI] [PubMed] [Google Scholar]
- 26.Hämmerle, B., A. Carnicero, C. Elizalde, J. Ceron, S. Martinez, and F. J. Tejedor. 2003. Expression patterns and subcellular localization of the Down syndrome candidate protein MNB/DYRK1A suggest a role in late neuronal differentiation. Eur. J. Neurosci. 17:2277-2286. [DOI] [PubMed] [Google Scholar]
- 27.Hämmerle, B., C. Elizalde, J. Galceran, W. Becker, and F. J. Tejedor. 2003. The MNB/DYRK1A protein kinase: neurobiological functions and Down syndrome implications. J. Neural Transm. Suppl. 67:129-137. [DOI] [PubMed] [Google Scholar]
- 28.Harley, C. W., C. W. Malsbury, A. Squires, and R. A. Brown. 2000. Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus 10:693-697. [DOI] [PubMed] [Google Scholar]
- 29.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 30.Hebbard, P. C., R. R. King, C. W. Malsbury, and C. W. Harley. 2003. Two organizational effects of pubertal testosterone in male rats: transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp. Neurol. 182:470-475. [DOI] [PubMed] [Google Scholar]
- 31.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 32.Heisenberg, M., and K. Böhl. 1979. Isolation of anatomical brain mutants of Drosophila by histological means. Z. Naturforsch. 34:143-147. [Google Scholar]
- 33.Himpel, S., W. Tegge, R. Frank, S. Leder, H. G. Joost, and W. Becker. 2000. Specificity determinants of substrate recognition by the protein kinase DYRK1A. J. Biol. Chem. 275:2431-2438. [DOI] [PubMed] [Google Scholar]
- 34.Hock, R., F. Wilde, U. Scheer, and M. Bustin. 1998. Dynamic relocation of chromosomal protein HMG-17 in the nucleus is dependent on transcriptional activity. EMBO J. 17:6992-7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James, P., J. Halladay, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kellendonk, C., P. Gass, O. Kretz, G. Schutz, and F. Tronche. 2002. Corticosteroid receptors in the brain: gene targeting studies. Brain Res. Bull. 57:73-83. [DOI] [PubMed] [Google Scholar]
- 37.Kentrup, H., W. Becker, J. Heukelbach, A. Wilmes, A. Schrmann, C. Huppertz, H. Kainulainen, and H. G. Joost. 1996. Dyrk, a dual specificity protein kinase with unique structural features. whose activity is dependent on tyrosine residues between subdomains VII and VIII. J. Biol. Chem. 271:3488-3495. [DOI] [PubMed] [Google Scholar]
- 38.Kerr, J. E., R. J. Allore, S. G. Beck, and R. J. Handa. 1995. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology 136:3213-3221. [DOI] [PubMed] [Google Scholar]
- 39.Kerr, J. E., S. G. Beck, and R. J. Handa. 1996. Androgens selectively modulate C-fos messenger RNA induction in the rat hippocampus following novelty. Neuroscience 74:757-766. [DOI] [PubMed] [Google Scholar]
- 40.Khaspekov, L. G., M. S. Brenz Verca, L. E. Frumkina, H. Hermann, G. Marsicano, and B. Lutz. 2004. Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity. Eur. J. Neurosci. 19:1691-1698. [DOI] [PubMed] [Google Scholar]
- 41.Lamond, A. I., and D. L. Spector. 2003. Nuclear speckles: a model for nuclear organelles. Nat. Rev. Mol. Cell. Biol. 4:605-612. [DOI] [PubMed] [Google Scholar]
- 42.Lanz, R. B., and S. Rusconi. 1994. A conserved carboxy-terminal subdomain is important for ligand interpretation and transactivation by nuclear receptors. Endocrinology 135:2183-2195. [DOI] [PubMed] [Google Scholar]
- 43.Larsson, S. H., J. P. Charlieu, K. Miyagawa, D. Engelkamp, M. Rassoulzadegan, A. Ross, F. Cuzin, H. van Heyningen, and N. D. Hastie. 1995. Subnuclear localization of WT1 in splicing or transcription factor domains is regulated by alternative splicing. Cell 81:391-401. [DOI] [PubMed] [Google Scholar]
- 44.Lusser, A., and J. T. Kadonaga. 2003. Chromatin remodeling by ATP-dependent molecular machines. Bioessays 25:1192-1200. [DOI] [PubMed] [Google Scholar]
- 45.Mao, J., P. Maye, P. Kogerman, F. J. Tejedor, R. Toftgard, W. Xie, G. Wu, and D. Wu. 2002. Regulation of Gli1 transcriptional activity in the nucleus by Dyrk1. J. Biol. Chem. 277:35156-35161. [DOI] [PubMed] [Google Scholar]
- 46.Mao, R., C. L. Zielke, H. R. Zielke, and J. Pevsner. 2003. Global up-regulation of chromosome 21 gene expression in the developing Down syndrome brain. Genomics 81:457-467. [DOI] [PubMed] [Google Scholar]
- 47.Marsicano, G., and B. Lutz. 1999. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11:4213-4225. [DOI] [PubMed] [Google Scholar]
- 48.Marti, E., X. Altafaj, M. Dierssen, L. De, V. Fotaki, M. Alvarez, R. Perez, I. Ferrer, and X. Estivill. 2003. Dyrk1A expression pattern supports specific roles of this kinase in the adult central nervous system. Brain Res. 964:250-263. [DOI] [PubMed] [Google Scholar]
- 49.Matsuo, R., W. Ochiai, K. Nakashima, and T. Taga. 2001. A new expression cloning strategy for isolation of substrate-specific kinases by using phosphorylation site-specific antibody. J. Immunol. Methods 247:141-151. [DOI] [PubMed] [Google Scholar]
- 50.Mortillaro, M. J., B. J. Blencowe, X. Wei, H. Nakayasu, L. Du, S. L. Warren, P. A. Sharp, and R. Berezney. 1996. A hyperphosphorylated form of the large subunit of RNA polymerase II is associated with splicing complexes and the nuclear matrix. Proc. Natl. Acad. Sci. USA 93:8253-8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadel, L. 2003. Down's syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2:156-166. [DOI] [PubMed] [Google Scholar]
- 52.Nagase, T., K. Ishikawa, M. Suyama, R. Kikuno, N. Miyajima, A. Tanaka, H. Kotani, N. Nomura, and O. Ohara. 1998. Prediction of the coding sequences of unidentified human genes. XI. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 5:277-286. [DOI] [PubMed] [Google Scholar]
- 53.Oitzl, M. S., H. M. Reichardt, M. Joels, and E. R. De Kloet. 2001. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. USA 98:12790-12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paddison, P. J., A. A. Caudy, E. Bernstein, G. J. Hannon, and D. S. Conklin. 2002. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16:948-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrij, F., R. H. Giles, H. G. Dauwerse, J. J. Saris, R. C. Hennekam, M. Masuno, N. Tommerup, G. J. van Ommen, R. H. Goodman, and D. J. Peters. 1995. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348-351. [DOI] [PubMed] [Google Scholar]
- 56.Rahmani, Z., J. L. Blouin, N. Creau-Goldberg, P. C. Watkins, J. F. Mattei, M. Poissonnier, M. Prieur, Z. Chettouh, A. Nicole, and A. Aurias. 1989. Critical role of the D21S55 region on chromosome 21 in the pathogenesis of Down syndrome. Proc. Natl. Acad. Sci. USA 86:5958-5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahmani, Z., J. L. Blouin, N. Creau-Goldberg, P. C. Watkins, J. F. Mattei, M. Poissonnier, M. Prieur, Z. Chettouh, A. Nicole, and A. Aurias. 1990. Down syndrome critical region around D21S55 on proximal 21q22.3. Am. J. Med. Gene Suppl. 7:98-103. [DOI] [PubMed] [Google Scholar]
- 58.Rahmani, Z., C. Lopes, M. Rachidi, and J. M. Delabar. 1998. Expression of the mnb (dyrk) protein in adult and embryonic mouse tissues. Biochem. Biophys. Res. Commun. 253:514-518. [DOI] [PubMed] [Google Scholar]
- 59.Raz, N., I. J. Torres, S. D. Briggs, W. D. Spencer, A. E. Thornton, W. J. Loken, F. M. Gunning, J. D. McQuain, N. R. Driesen, and J. D. Acker. 1995. Selective neuroanatomic abnormalities in Down's syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 45:356-366. [DOI] [PubMed] [Google Scholar]
- 60.Reisert, I., K. Lieb, C. Beyer, and C. Pilgrim. 1996. Sex differentiation of rat hippocampal GABAergic neurons. Eur. J. Neurosci. 8:1718-1724. [DOI] [PubMed] [Google Scholar]
- 61.Rouleau, N., A. Domans'kyi, M. Reeben, A. M. Moilanen, K. Havas, Z. Kang, T. Owen-Hughes, J. J. Palvimo, and O. A. J.änne. 2002. Novel ATPase of SNF2-like protein family interacts with androgen receptor and modulates androgen-dependent transcription. Mol. Biol. Cell 13:2106-2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shindoh, N., J. Kudoh, H. Maeda, A. Yamaki, S. Minoshima, Y. Shimizu, and N. Shimizu. 1996. Cloning of a human homolog of the Drosophila minibrain/rat Dyrk gene from the Down syndrome critical region of chromosome 21. Biochem. Biophys. Res. Commun. 225:92-99. [DOI] [PubMed] [Google Scholar]
- 63.Smith, D. J., and E. M. Rubin. 1997. Functional screening and complex traits: human 21q22.2 sequences affecting learning in mice. Hum. Mol. Genet. 6:1729-1733. [DOI] [PubMed] [Google Scholar]
- 64.Smith, D. J., M. E. Stevens, S. P. Sudanagunta, R. T. Bronson, M. Makhinson, A. M. Watabe, T. J. O'Dell, J. Fung, H. U. Weier, J. F. Cheng, and E. M. Rubin. 1997. Functional screening of 2 Mb of human chromosome 21q22.2 in transgenic mice implicates minibrain in learning defects associated with Down syndrome. Nat. Genet. 16:28-36. [DOI] [PubMed] [Google Scholar]
- 65.Song, W. J., S. H. Chung, and D. M. Kurnit. 1997. The murine Dyrk protein maps to chromosome 16, localizes to the nucleus, and can form multimers. Biochem. Biophys. Res. Commun. 231:640-644. [DOI] [PubMed] [Google Scholar]
- 66.Song, W. J., L. R. Sternberg, C. Kasten-Sportes, M. L. Keuren, S. H. Chung, A. C. Slack, D. E. Miller, T. W. Glover, P. W. Chiang, L. Lou, and D. M. Kurnit. 1996. Isolation of human and murine homologues of the Drosophila minibrain gene: human homologue maps to 21q22.2 in the Down syndrome “critical region.” Genomics 38:331-339. [DOI] [PubMed] [Google Scholar]
- 67.Sousa, R. J., N. H. Tannery, and E. M. Lafer. 1989. In situ hybridization mapping of glucocorticoid receptor messenger ribonucleic acid in rat brain. Mol. Endocrinol. 3:481-494. [DOI] [PubMed] [Google Scholar]
- 68.Stoppini, L., P. A. Buchs, and D. Muller. 1991. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods 37:173-182. [DOI] [PubMed] [Google Scholar]
- 69.Tejedor, F., X. R. Zhu, E. Kaltenbach, A. Ackermann, A. Baumann, I. Canal, M. Heisenberg, K. F. Fischbach, and O. Pongs. 1995. minibrain: a new protein kinase family involved in postembryonic neurogenesis in Drosophila. Neuron 14:287-301. [DOI] [PubMed] [Google Scholar]
- 70.Vicari, S., S. Bellucci, and G. A. Carlesimo. 2000. Implicit and explicit memory: a functional dissociation in persons with Down syndrome. Neuropsychologia 38:240-251. [DOI] [PubMed] [Google Scholar]
- 71.von Groote-Bidlingmaier, D. Schmoll, H. M. Orth, H. G. Joost, W. Becker, and A. Barthel. 2003. DYRK1 is a co-activator of FKHR (FOXO1a)-dependent glucose-6-phosphatase gene expression. Biochem. Biophys. Res. Commun. 300:764-769. [DOI] [PubMed] [Google Scholar]
- 72.Woods, Y. L., P. Cohen, W. Becker, R. Jakes, M. Goedert, X. Wang, and C. G. Proud. 2001. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem. J. 355:609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woods, Y. L., G. Rena, N. Morrice, A. Barthel, W. Becker, S. Guo, T. G. Unterman, and P. Cohen. 2001. The kinase DYRK1A phosphorylates the transcription factor FKHR at Ser329 in vitro, a novel in vivo phosphorylation site. Biochem. J. 355:597-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xue, Y., R. Gibbons, Z. Yan, D. Yang, T. L. McDowell, S. Sechi, J. Qin, S. Zhou, D. Higgs, and W. Wang. 2003. The ATRX syndrome protein forms a chromatin-remodeling complex with Daxx and localizes in promyelocytic leukemia nuclear bodies. Proc. Natl. Acad. Sci. USA 100:10635-10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang, E. J., Y. S. Ahn, and K. C. Chung. 2001. Protein kinase Dyrk1 activates cAMP response element-binding protein during neuronal differentiation in hippocampal progenitor cells. J. Biol. Chem. 276:39819-39824. [DOI] [PubMed] [Google Scholar]
- 76.Young, W. J., and C. Chang. 1998. Ontogeny and autoregulation of androgen receptor mRNA expression in the nervous system. Endocrine 9:79-88. [DOI] [PubMed] [Google Scholar]
- 77.Zeng, C., E. Kim, S. L. Warren, and S. M. Berget. 1997. Dynamic relocation of transcription and splicing factors dependent upon transcriptional activity. EMBO J. 16:1401-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]