Abstract
Impulsivity is a multi-faceted personality construct that plays a prominent role in drug abuse vulnerability. Dysregulation of 5-hydroxytryptamine (serotonin, 5-HT) systems in subregions of the prefrontal cortex has been implicated in impulsivity. Extracellular 5-HT concentrations are regulated by 5-HT transporters (SERTs), indicating that these transporters may be important molecular targets underlying individual differences in impulsivity and drug abuse vulnerability. The present study evaluated the role of SERT in mediating individual differences in impulsivity. Rats were tested for both impulsive action using the cued go/no-go task and for impulsive choice using a delay discounting task in a counterbalanced design. Following behavioral evaluation, Km and Vmax were obtained from kinetic analysis of [3H]5-HT uptake by SERT using synaptosomes prepared from both orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC) obtained from each individual rat. Vmax for SERT in OFC, but not mPFC, was negatively correlated with mean adjusted delay scores in the delay discounting task. In contrast, Vmax for SERT in OFC and mPFC was not correlated with performance in the cued go/no-go task. To further evaluate the relationship between SERT function and impulsive choice, a selective SERT inhibitor, fluoxetine (0, 15, 50 and 150 pmol/side) was microinjected bilaterally into OFC and effects on the delay discounting task determined. Following stabilization of behavior, fluoxetine increased mean adjusted delay scores (decreased impulsivity) in high impulsive rats compared to saline microinjection, but had no effect in low impulsive rats. These ex vivo and in vivo results suggest that enhanced SERT function in OFC underlies high impulsive choice behavior.
Keywords: Cued go/no-go, Delay discounting, Impulsivity, Serotonin transporter
1. Introduction
The majority of individuals who experiment with drugs do not develop a substance abuse disorder [1]. An important goal is to identify factors underlying individual differences in drug abuse vulnerability. One factor known to play a prominent role is impulsivity. Increased levels of impulsivity have been implicated in alcohol, cocaine, methamphetamine, opioid and nicotine abuse [2]. Personality and behavioral tests have been developed to measure different components of impulsivity, including the Five Factor Model of personality, which identifies different facets of impulsivity [3]. Two commonly used behavioral tasks employing human subjects and measuring distinct components of impulsivity include the cued go/no-go task, a measure of impulsive action, and the delay discounting task, a measure of impulsive choice [4]. In the cued go/no-go task, responding during a go cue is reinforced, but not reinforced during a no-go cue [5]. Individuals who fail to extinguish responding during the no-go cue are considered to have greater levels of impulsive action. In the delay discounting task, individuals choose between a small, immediate reward and a larger, delayed reward [6]. Individuals who consistently choose the small immediate reward over the large delayed reward are considered to exhibit greater impulsive choice. As indicated by personality traits and behavioral measurements in the cued go/no-go and delay-discounting tasks, individuals with high impulsivity have been reported to consume greater amounts of alcohol, tobacco and marijuana [7,8], and have a high liability for substance abuse.
Although a positive relationship between impulsivity and drug abuse has been reported, whether impulsivity is an antecedent condition or an outcome of drug use is not known and difficult to evaluate in human subjects [2]. Preclinical models are well-suited to address this question due to the ability to evaluate pre-existing individual differences in impulsivity in drug naive subjects followed by subsequent evaluation of drug abuse liability using established behavioral models. In this respect, rats exhibiting high impulsivity in the delay discounting task self-administer greater amounts of cocaine, methylphenidate, and nicotine relative to low impulsive rats [9], consistent with findings from human studies.
Importantly, neurobehavioral mechanisms underlying vulnerability to drug abuse also may be evaluated in animal models to establish causal links. Several studies have shown that the serotonin (5-HT) system in the prefrontal cortex (PFC) plays a major role in the neurochemical effects of drugs of abuse, specifically psychostimulants which increase extracellular 5-HT in a number of brain regions and contribute to the development and maintenance of addiction [10]. In addition to the role of 5-HT in addiction, 5-HT in orbitofrontal cortex (OFC) and medial prefrontal cortex (mPFC) is involved in modulating different aspects of impulsivity [10]. For instance, lesions in OFC and mPFC alter delay discounting behavior [11–14, but see 15,16]. Further, 5-HT in OFC and mPFC mediate behavior in the delay discounting task, as indicated by results from a number of experimental approaches, e.g., microinjection, lesion and microdialysis [17]. In addition, both acute dietary tryptophan depletion in humans and forebrain 5-HT depletion induced by infusion of 5,7-dihydroxytryptamine into rat dorsal raphe increase impulsive choice in the delay discounting task [18–20]. With respect to impulsive action, 5-HT in the mPFC has been linked to the rate of acquisition of behavior in the cued go/no-go task [21]. In addition, injection of 5,7-dihydroxytryptamine into median raphe increases impulsive action [16,22–24]. However, neurochemical mechanisms in mPFC and OFC that underlie impulsive behavior in the cued go/no-go task have not been evaluated in depth using preclinical models, which is surprising considering the established relationship between impulsivity and drug abuse vulnerability in humans when employing this task [7].
Extracellular 5-HT concentrations are regulated primarily by plasma membrane transporters, i.e., the 5-HT transporter (SERT; [25]). Polymorphisms in genes encoding SERT are associated with impulsivity in both normal individuals and in neuropsychiatric conditions associated with high impulsivity (e.g., attention deficit hyperactivity disorder) [26,27]. A role for SERT in impulsivity is supported also by pharmacological evidence in which citalopram, a SERT inhibitor, decreased impulsive choice in the delay discounting task in rats [28]. However, relationships between basal SERT function specifically in prefrontal cortical subregions and impulsive behavior have not been investigated.
The present study determined whether individual differences in SERT function in the OFC and/or mPFC have a role in the expression of individual differences in impulsive action and choice. Kinetic parameters, affinity (Km) and maximal transport velocity (Vmax), for [3H]5-HT uptake were determined in OFC and mPFC synaptosomes obtained from individual rats that were trained in both cued go/no-go and delay discounting tasks using a counterbalanced design. In addition to these ex vivo studies, effects of fluoxetine microinjection, a selective SERT inhibitor, on impulsive choice were determined to directly evaluate the relationship between basal SERT function and impulsivity.
2. Materials and methods
2.1. Materials
5-[1,2-3H(N)]-Hydroxytryptamine creatinine sulfate ([3H]5-HT; specific activity, 27.1 Ci/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). 5-HT, desipramine HCl, 1-(2-bis(4-fluorphenyl)-methoxy)-ethyl-4-(3-phenyl-propyl) piperazine HCl (GBR 12909), fluoxetine HCl, pargyline HCl, catechol, and L-ascorbic acid were purchased from Sigma–Aldrich (St. Louis, MO). D-Glucose was purchased from Aldrich Chemical Co. (Milwaukee, WI). Xylazine was purchased from Lloyd Laboratories Inc. (Metro Manila, Philippines). Ketamine was purchased from Putney Inc. (Portland, ME). Acepromazine was purchased from Vedco Inc. (St. Joseph, MO). Carprofen was purchased from Pfizer Animal Health (New York, NY). All other chemicals were purchased from Fisher Scientific (Pittsburgh, PA).
2.2. Subjects
Thirty two male Sprague–Dawley rats (250–275 g, 59–63 days old; Harlan Laboratories, Indianapolis, IN) were housed individually in a temperature- and humidity-controlled colony with a 12/12h light/dark cycle. Rats were food restricted (85% of free feeding body weight), and had free access to water in their home cages. Experiments were conducted during the light phase. Rats were cared for in accordance with the 2011 edition of the “Guide for the Care and Use of Laboratory Animals” and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.3. Behavioral apparatus
Operant chambers (28 × 21 × 21 cm; ENV-008; MED Associates, St. Albans, VT) with aluminum front and back walls and Plexiglas sides were located inside sound-attenuating chambers (ENV-018M; MED Associates). A recessed food tray (5 × 4.2 cm) was 2 cm above the floor in the bottom-center of the front wall. Retractable levers (4.5 cm) were 6 cm above the floor on each side of the food tray. A 28-V white cue light was 6 cm above each lever. A white house light was mounted in the center of the back wall. All responses and scheduled consequences were recorded and controlled by a computer interface using Med-IV software.
2.4. Experimental design
Rats (n = 20) were tested in both the cued go/no-go and delay discounting tasks, with order of testing counterbalanced. Following the last behavioral test day (rats were ∼88 days old), synaptosomes were prepared from OFC and mPFC obtained from each rat to determine kinetic parameters of SERT function. In a separate experiment, rats (n = 12) were implanted bilaterally with intracranial guide cannulae into OFC and then tested only in the delay discounting task for 21 days. Following stabilization of mean adjusted delay scores, fluoxetine was microinjected into OFC and effects on delay discounting determined.
2.5. Cued go/no-go task
The cued go/no-go task was conducted using previously described procedures and data analyses [29,30], with minor modifications. Training began with 3 days of auto-shaping, in which both levers were extended and the house light was illuminated. During 60-min sessions, rats received sucrose pellets (45 mg; F0021 dust-less precision pellet, Bio-Serve, Frenchtown, NJ) non-contingently on a variable time (VT) 100 s schedule of reinforcement, and responding on the active lever resulted in contingent delivery of a sucrose pellet on a continuous schedule of reinforcement. Position of the active lever was counterbalanced across sessions for each rat. Responses on the inactive lever were recorded, but had no programmed consequence. Following either contingent or non-contingent delivery of a sucrose pellet, both levers were retracted for 2 s. Auto-shaping sessions ended after either 60 min elapsed or delivery of 60 reinforcers. Following auto-shaping, training continued for 4 consecutive daily 20-min sessions employing a variable interval (VI) schedule (VI-4, VI-8, VI-14, and VI-20 s) of sucrose pellet reinforcement.
The cued go/no-go task was employed for 14 consecutive daily 40-min sessions. Sessions consisted of 2-min go components in which reinforcers were available, alternated with 2-min no-go components in which reinforcers were not available (extinction). Go components were signaled by illumination of the cue light above the lever for the entire 2-min period, and active lever responses on a VI-20 s schedule resulted in sucrose pellet reinforcement. No-go components were signaled by the absence of the cue light illumination; responses on the active lever were recorded, but had no programmed consequence. During both go and no-go components, responses on the inactive lever were recorded, but had no programmed consequence. The primary dependent measure from the cued go/no-go task was the ratio of responses during go trials to responses during no-go trials (VI/EXT ratio) averaged across the last 7 sessions [29].
2.6. Delay discounting task
The delay discounting task was conducted for 21 days using previously described procedures [31]. Sessions began with house light illumination and ended following completion of 60 trials or when 2 h elapsed. Each session included 15 blocks of 4 trials. For each block, the first 2 trials were forced-choice trials and the last 2 trials were free-choice trials. During forced-choice trials, only one lever (left or right; counterbalanced across trials) was extended, and the cue light above the extended lever was illuminated. During free-choice trials, both levers were extended, and cue lights above both levers were illuminated. A response on one lever [fixed ratio (FR) 1 schedule of reinforcement] resulted in immediate delivery of one sucrose pellet, and a response on the other lever (FR 1) resulted in delivery of 3 sucrose pellets after a delay. The location of the levers delivering 1 or 3 pellets alternated across sessions. The delay to the larger reinforcer (initially set at 0 s) adjusted according to responses during the free-choice trials in each block. Responding on the lever delivering 3 pellets increased the delay to the larger, delayed rein-forcer by 1 s. Responding on the lever associated with the small, immediate reinforcer decreased the delay to the larger reinforcer by 1 s. A minimum delay of 0 s and a maximum delay of 45 s for the 3-pellet reinforcer were imposed. The delay on the final free-choice trial during each session was used as the initial delay for the first free-choice trial of the next session. During the delay, cue lights were turned off, although the house light remained illuminated until delivery of the 3 pellets. Following a response on either lever, an adjusting inter-trial interval (ITI) was imposed, such that the ITI lasted 60 s. During the ITI, both cue lights and house light were extinguished, and lever presses had no programmed consequence. The main outcome measure, mean adjusted delay (MAD) score, was calculated by averaging all the adjusting delays on the free-choice trials during the session. MAD scores for the last 7 sessions were averaged as previously described [29].
2.7. [3H]5-HT uptake assay
SERT function was assessed using synaptosomes obtained from both mPFC and OFC following the conclusion of the cued go/no-go and delay discounting tasks. Kinetic parameters of transporter function, i.e., Vmax (pmol/min/mg) and Km (μM), for [3H]5-HT uptake were determined using a previously published method [32,33]. Rat brains were dissected on an ice-cold plate to obtain the mPFC and OFC. Regions were identified according to the atlas of Paxinos and Watson [34] and obtained by making a coronal cut in the frontal cortex just anterior to the olfactory tubercles. Olfactory bulbs were removed. mPFC was obtained following two sagittal cuts 1.2 mm on either side of the midline. OFC was obtained as the ventral segment following a transverse cut in the remaining cortical tissue.
Each brain region was homogenized in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer (clearance, ∼0.003 inch). Homogenates were centrifuged at 2000 × g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000 × g for 17 min at 4°C. Resulting pellets were resuspended in 2.2 ml of ice-cold assay buffer (125 mM NaCl, 5 mM KCl,1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM L-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4) to obtain synaptosomal suspensions. Nonspecific [3H]5-HT uptake was determined in the presence of 10 μM fluoxetine. [3H]5-HT uptake was determined using buffer containing desipramine (1 μM) and GBR 12909 (50 nM) to inhibit uptake by the norepinephrine transporter and the dopamine transporter, respectively [32,33,35,36].
mPFC and OFC synaptosomes (40 and 50 μg protein/100 μl, respectively) were incubated in buffer containing desipramine and GBR 12909 (125 μl) in a metabolic shaker for 5 min at 34 °C. Then, 1 of 7 [3H]5-HT concentrations (1–300 nM, in 25 μl) was added for a total incubation volume of 250 μl. Incubations continued for 5 min at 34 °C and were terminated by addition of 3 ml of ice-cold assay buffer. Samples were filtered immediately through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h) using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Filters were washed 3 times with 3 ml of ice-cold buffer containing 1 mM pyrocatechol. Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences, Boston, MA). Protein concentrations were determined using bovine serum albumin as the standard [37]. Vmax and Km values were determined using the commercially available Graph-Pad Prism 4.0 program (Graph-Pad Software Inc., San Diego, CA).
2.8. Microinfusion of fluoxetine into OFC
In a separate experiment, rats (n = 12) were anesthetized with a ketamine (75 mg/kg)/xylazine (7.5 mg/kg)/acepromazine (0.75 mg/kg) cocktail (i.p.) and implanted with bilateral intracranial guide cannulae (26 gauge, Plastics ONE, Inc., Roanoke, VA) into OFC (AP: +3.7, ML: ±2.4, DV: −3.4; from skull) [38]. Each guide cannula was fitted with a dummy cannula (Plastics ONE) and embedded in a cap of dental acrylic affixed to the skull with stainless-steel jeweler screws. Rats were allowed 4 days to recover and then they were trained on the delay discounting task described previously until responding stabilized (21 sessions). On test days (rats were ∼93 days old), dummy cannulae were removed from the guide cannulae and 33 gauge injector cannulae (Plastic ONE) were inserted into the guide cannulae. The injector cannula protruded 1 mm below guide cannula and was connected to a 10 μl syringe (Hamilton Company, Reno, NV) via PE50 tubing (Small Parts, Inc, Miramar, FL). Fluoxetine (0, 15, 50 and 150 pmol/side; dissolved in saline) was infused bilaterally at a volume of 0.5 μl/side over 2 min. Fluoxetine doses were chosen based on a previous study [39]. Injector cannulae were left in place for 1 min after the infusion, at which time the injector cannulae were removed, the dummy cannulae replaced and the rat placed immediately into the operant chamber. A Latin-square design was used to randomize the order of drug doses across individuals. A three-day washout occurred between each test day, where rats received no infusions. Cannula placements were verified histologically using the rat brain atlas of Paxinos and Watson [34].
2.9. Data analyses
Data analyses were conducted using Graph-Pad Prism 4.0 and SAS (version 9.3; SAS, Cary, NC). To determine stability in the cued go/no-go and delay discounting tasks, linear trend analyses were performed on VI responses/EXT responses (VI/EXT) and MAD scores, respectively, across the last 7 sessions, with a nonsignificant slope from both measures defining stability [40]. That is, a slope not significantly different from zero indicated stability of performance. Further, linear trend analysis increases power while reducing Type I and Type II errors [41]. For the [3H]5-HT uptake assay, kinetic parameters, Vmax (pmol/min/mg) and Km (μM), indicate maximal transport velocity and affinity of 5-HT for SERT, respectively. Log-transformed Km values were used for statistical analyses. Pearson's correlation analyses determined relationships of VI/EXT ratios with either Vmax or Km for [3H]5-HT uptake in OFC and mPFC. Separate Pearson's correlation analyses also determined relationships of MAD scores with either Vmax or Km for [3H]5-HT uptake in OFC and mPFC. Further, to determine whether a narrow spread in the data weakened the Pearson's correlation, a Spearman correlation, which evaluates the ranked data and is insensitive to the spacing of VI/EXT values along the horizontal axis, also was employed. Microinjection data were analyzed using linear mixed models [38] to relate log-transformed saline-normalized scores to group (high vs. low impulsivity based on median split) and fluoxetine dose (15, 50, 100 pmol/side), both treated as categorical predictor variables as described previously [38]. Random effects were included in the models to account for the lack of independence of within-subject repeated measurements. Statistical significance was declared at p < 0.05. For OFC and mPFC, 2 and 3 rats, respectively, were excluded from the data analysis due to the lack of saturation in the [3H]5-HT uptake assay, which did not allow determination of Vmax.
3. Results
3.1. Cued go/no-go and delay discounting tasks
Linear trend analyses revealed that rats evaluated for SERT function ex vivo showed no significant change in VI/EXT responses across the final 7 sessions of the cued go/no-go task (Fig. 1, left panel; t(138) = 1.73, p = 0.1) or in MAD scores across the final 7 sessions of the delay discounting task (Fig. 1, right panel; t(138) = −0.30, p = 0.76). Furthermore, performance in the cued go/no-go task was not correlated with performance in the delay discounting (data not shown; Pearson r = 0.14, p = 0.55).
Fig 1.
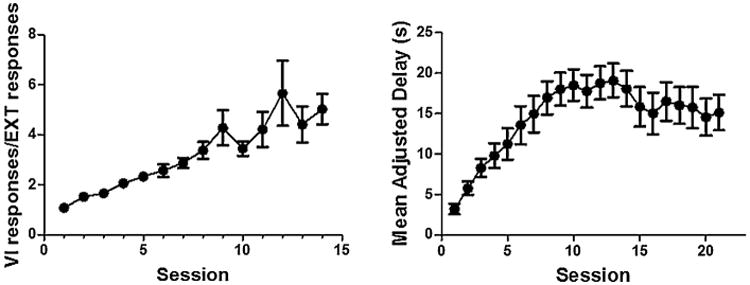
Acquisition of the cued go/no-go and delay discounting tasks in rats evaluated for SERT function ex vivo (n = 20). Left panel: mean (±SEM) ratio of variable interval responses and extinction responses (VI/EXT responses) are plotted as a function of session for 14 days of cued go/no-go. Right panel: mean (±SEM) adjusted delay (MAD) is plotted as a function of session for 21 days of delay discounting.
3.2. Correlation analyses of mPFC and OFC kinetic parameters for [3H]5-HT uptake with behavioral outcome measures
Fig. 2 illustrates that specific uptake was saturable for [3H]5-HT uptake in both OFC and mPFC obtained from individual rats. Kinetic parameters (Vmax and Km) for [3H]5-HT uptake were obtained from saturation curves, and these values were used in correlational analyses with behavioral outcome measures. Pearson's correlation analyses were conducted to determine whether SERT kinetic parameters from OFC and mPFC were associated with behaviors in the cued go/no-go or delay discounting tasks. For the cued go/no-go task, Vmax for [3H]5-HT uptake in OFC and mPFC were not correlated with VI/EXT responses (Pearson r for mPFC, −0.06 and for OFC, −0.153; Fig. 3a and b). Further, Spearman r values were −0.124 for mPFC and −0.153 for OFC, suggesting that Pearson's correlations between SERT function and VI/EXT were not masked by the small range over which 90% of the data fell. Further, a mediation analysis was performed as previously described [42] to determine whether VI/EXT ratios were primarily influenced by responses during the go or no-go component. Results show that responding during the go component (VI) has no effect on VI/EXT ratios and that VI/EXT responses were completely mediated by EXT responses.
Fig 2.
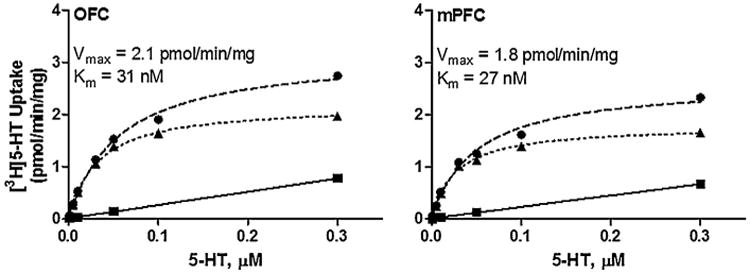
Saturation curves for [3H]5-HT uptake at SERT in OFC and mPFC synaptosomes from a representative individual rat. Nonspecific [3H]5-HT uptake was determined in the presence of 10 μM fluoxetine. Specific uptake (▲) was obtained by subtracting nonspecific uptake (■) from total uptake (●). Nonlinear curve fits of data for uptake used the Michaelis–Menten equation to obtain Vmax and Km values. Vmax represents the maximal velocity of [3H]5-HT uptake and Km represents affinity of 5-HT at SERT.
Fig 3.
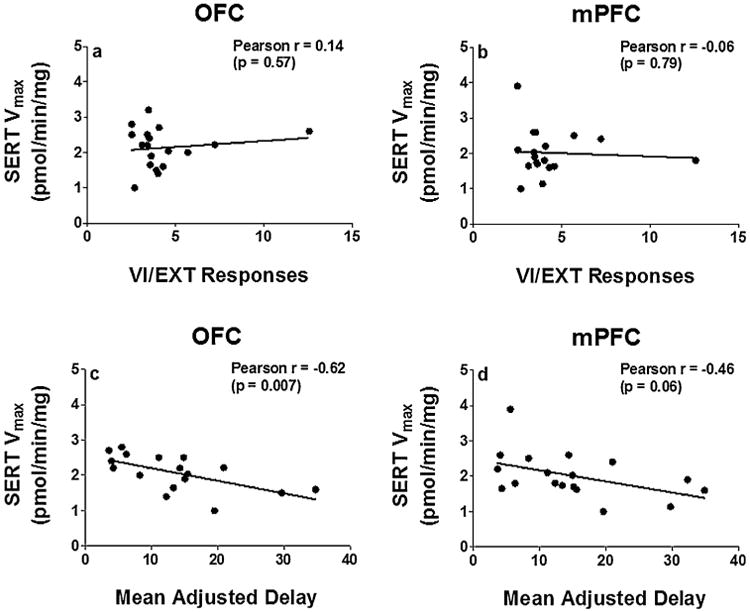
Pearson's correlation of Vmax for [3H]5-HT uptake in OFC and mPFC with VI/EXT responses (panels a and b) and mean adjusted delay (panels c and d). Note that lower VI/EXT responses indicate increased impulsive action and lower MAD scores indicate increased impulsive choice. Data points represent individual rats (n = 17–18).
With respect to impulsive choice, Vmax for [3H]5-HT uptake in OFC from individual rats was negatively correlated with MAD scores from the delay discounting task (Pearson r = −0.62, p < 0.05, Fig. 3c), but not in mPFC (Pearson r = −0.46, p = 0.06, Fig. 3d). In addition, since there was a trend (p = 0.06) in the relationship between mPFC SERT function and impulsive choice, the data were analyzed further using a quadratic trend analysis. The quadratic trend analysis evaluated the possibility that the lack of a linear relationship between mPFC SERT function and MAD scores weakened the correlation and masked the relationship. Results showed that there were no significant quadratic terms corresponding to the correlations, providing no evidence of nonlinear relationships between MAD behavioral measures and SERT function in mPFC and OFC. Furthermore, no significant correlations were obtained between Km for [3H]5-HT uptake in OFC and mPFC with either behavioral task (data not shown).
3.3. Effect of microinjection of fluoxetine into OFC on impulsive choice
To directly evaluate the association between OFC SERT function ex vivo and impulsive choice shown in Fig. 3c, the selective SERT inhibitor, fluoxetine (0, 15, 50 and 150 pmol/side) was microinjected into OFC and effects on impulsive choice determined. Results from microinjection experiment using raw MAD scores revealed a main effect of group (F(1,10) = 5.01, p < 0.05) and a significant group × fluoxetine dose interaction (F(4,40) = 3.43, p < 0.05). To further analyze the effects of fluoxetine microinjected into OFC, percent change from baseline scores revealed increased MAD scores in high impulsive rats compared to saline (Fig. 4a, t(18) = 2.44, p = 0.025, n = 6), but no effect in low impulsive rats (t(18) = −0.15, p = 0.883, n = 5).
Fig 4.
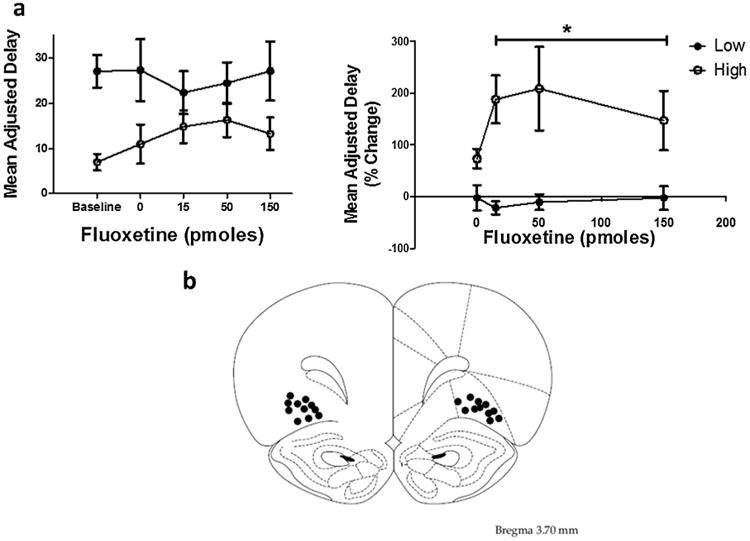
Panel a. Mean adjusted delay raw scores following OFC microinfusions of fluoxetine in high (n = 6) and low (n = 5) impulsive rats based on a median split (left). Mean (±SEM) adjusted delay scores following OFC microinfusions of fluoxetine expressed as a % change from baseline in high and low impulsive rats based on a median split (right). Note that high MAD scores indicate decreased impulsive choice.
*Represents significant overall drug effect in high impulsive rats (averaged across the three doses) compared to saline, p < 0.05.
Panel b. Diagram shows locations for bilateral intra-OFC cannula injection sites for rats that were included in the statistical analysis. One rat was excluded from the data analysis due to the probe placement being outside of OFC.
3.4. Histology
Fig. 4b illustrates the site of drug injection in the OFC plotted on a representative section from the atlas of Paxinos and Watson [34]. Cannulae were implanted into OFC in 12 rats; only 11 rats had correct cannulae placements, and data from these rats were included in statistical analyses.
4. Discussion
While previous lesion and inactivation studies have implicated prefrontal cortical subregions in impulsivity [11–14], the present study elucidates a pharmacologically-relevant neurochemical mechanism associated with individual differences in impulsive action and impulsive choice, as measured by a cued go/no-go task and delay discounting task, respectively. Functional assays to determine [3H]5-HT uptake kinetics were optimized to employ cortical subregions (OFC and mPFC) from individual rats to allow for correlational analysis between the kinetic parameters of neurotransmitter transporter function and behavioral measures of impulsivity. Performance on the delay discounting task correlated with OFC SERT function. Consistent with the ex vivo results, infusion of fluoxetine into OFC decreased impulsive choice in high impulsive rats. The current results, in conjunction with previous findings showing that extracellular 5-HT concentrations are regulated by pre-synaptic uptake processes, support the contention that SERT is an important molecular target linked to individual differences in impulsivity. Thus, the present study provides the first direct evidence that OFC SERT function is specifically implicated in impulsive choice.
4.1. Delay discounting
In the delay discounting task, choosing immediate small reinforcers (lower MAD scores) indicates high impulsive choice. Current results showed that maximal velocity of 5-HT uptake at SERT in OFC, but not in mPFC, negatively correlated with this measure of impulsive choice, suggesting that greater impulsive choice is associated with increased 5-HT uptake in OFC. These results are consistent with our recent observations that increased negative urgency, which is also a facet of impulsivity, is associated with increased OFC SERT function [43]. Though there is abundant evidence that SERT regulates extracellular 5-HT concentrations [25], increased SERT function does not always reflect lower extracellular 5-HT concentrations, because 5-HT release also contributes to extracellular 5-HT concentrations. Further, 5-HT release is regulated, at least in part, by activation of pre- and post-synaptic 5-HT receptors [44]. Specifically, 5-HT1A and 5-HT1B autoreceptors, which are highly expressed in PFC, inhibit 5-HT neuronal firing and 5-HT release from terminals [44–47]. Also, postsynaptic receptor subtypes are located on non-5-HT neurons, which modulate 5-HT neuronal activity and release through neural inputs to 5-HT neurons [48]. Thus, since the current study did not directly measure extracellular 5-HT in OFC, the relationship between increased transporter function and extracellular 5-HT concentrations are only speculative.
Consistent with the current ex vivo results, fluoxetine (0, 15, 50 and 150 pmol/side) infusion into the OFC decreased impulsivity in high impulsive rats compared to saline, but had no effect in low impulsive rats. Although baseline MAD scores were different between high and low impulsive individuals treated with saline, the overall effect of fluoxetine (averaged over the three doses) in high impulsive rats was significantly different compared to high impulsive rats treated with saline. The current results showing that microinjection of fluoxetine into OFC decreases impulsive choice are in agreement with reports that impulsive choice is decreased following systemic administration of SERT inhibitors, citalopram and fluoxetine [19,28]. Another report from our laboratory showed that intra-OFC microinjection of 8-OH-DPAT, a 5-HT1A autoreceptor agonist that decreases extracellular 5-HT [49], also decreased impulsive choice using the same adjusting delay discounting task [38]. Interestingly, in addition to acting as a 5-HT1A autoreceptor agonist, 8-OH-DPAT acts nonselectively as a SERT inhibitor [50,51]. To the extent that 8-OH-DPAT acts as a SERT inhibitor, the behavioral outcome of the current study is consistent with our previous findings. That is, decreased impulsive choice is associated with reduced OFC SERT function. Thus, one neurochemical model to explain the decrease in impulsivity could be that inhibition of SERT increases extracellular 5-HT concentration, which increases 5-HT1A and 5-HT1B autoreceptor activation, and thereby inhibits 5-HT neuronal firing and decreases 5-HT release from its nerve terminals.
The present study supports the suggestion that OFC plays a critical role in impulsivity [52–54]. Winstanley et al. [55] previously demonstrated that 5-HT levels in mPFC increased during a fixed, ascending order, increasing delay discounting procedure; whereas herein, a relationship was found between OFC SERT function and delay discounting performance within an adjusting delay procedure. The discrepancy in results between the present study and that of Winstanley et al. [55] may be explained by differences in procedures. Delay order (ascending vs. descending) is well known to have a substantial effect on the magnitude of discounting [56]. The mean adjusting delay procedure used herein, where the experienced delay to the larger reinforcer is contingent on the choice between the small and large options, creates a unique varied delay order for each subject within each session. In other words, each choice either increased or decreased the delay trial by trial for each participant within each session. Therefore, the titrating schedule within the present mean adjusting delay procedure functionally results in a variable order in which each animal experiences the delay to the larger reinforcer in each session. Previous studies have shown that OFC plays a large role in environments where response outcomes are variable. For example, discrimination processes regarding responses that yield different rewards, and updating the value of these response outcomes as they change, have been demonstrated to be specifically OFC-dependent [57]. Thus, because the current mean adjusting delay procedure produced a varying order of experienced delayed consequences across the session, this continuous change in response outcomes may have produced an increase in OFC activity. Furthermore, because the response outcomes were associated with experienced delays, one might expect changes in 5-HT function within OFC, rather than the mPFC. Overall, the current data are consistent with the role of 5-HT signaling in delay-associated tasks, as indicated by Winstanley et al. [55], and suggests that when the experienced delays for each choice are continuously varying, the delay-associated 5-HT signaling is governed by OFC.
4.2. Cued go/no-go task
In the current study, we used a cued go/no-go procedure that has been published previously [29,30], and VI/EXT performance from this procedure has been interpreted as a measure of impulsive action [29]. One caveat to the current study is that the cue that signaled each component was not counterbalanced (i.e., the stimulus lights were used only to signal “go” components). The low impulsive animals in our experiment may have merely responded more during the “go” component, thus displaying increased sign–tracking behavior [58]. However, this explanation seems unlikely given that mediational analyses showed that VI/EXT ratios resulted from differences in responding during the no-go component. Furthermore, it is important to note that Hellemans et al. [30] counterbalanced the stimuli used to signal each component and found no differences in task performance.
In contrast to the relation between impulsive choice and OFC SERT function, individual differences in impulsive action measured by the cued go/no-go task were not correlated with SERT function in the OFC or mPFC. These results were surprising considering that the 5-HT system has been implicated previously in impulsive action and perseverative responding. For example, systemic administration of the selective 5-HT reuptake inhibitor, citalopram, increased neuronal activity (measured by fMRI) in subregions of PFC in humans during no-go trials of a go/no-go task [59]; however, these latter BOLD signal results may be due to nonselective effects of citalopram at other monoaminergic transporters [59]. In monkeys, 5-HT depletion in OFC increases perseverative responding in a reversal task measuring behavioral flexibility [60]. In rats, 5-HT depletion impairs acquisition rate [21] and increases impulsive action in a go/no-go task [61]. Conversely, greater 5-HT levels in PFC also are associated with increased impulsive responding during the 5-choice serial reaction time (CSRT) task [62,63]. Most importantly, using the 5-CSRT task, 5-HT1A, 5-HT2A, 5-HT2C receptor subtypes also have been shown to mediate impulsive action [64]. Thus, different tasks (5-CSRT and cued go/no-go) employed to measure impulsive action in the previous studies show that the cortical 5-HT systems play a role in inhibitory control of prepotent responses.
Contrary to this previous literature, however, OFC SERT function in the current study did not appear to contribute to the observed differences in 5-HT system function between high and low impulsive rats as measured by the cue no/no-go task. As explained above, in addition to transporter function, 5-HT levels are also regulated by variations in 5-HT release and/or activation of 5-HT receptors [44], expressed in the PFC. The serotonin system is complex, with over 14 different types of 5-HT receptors currently identified that can have both excitatory and inhibitory effects on serotonergic neurons and the cells they target (e.g., dopaminergic and glutamatergic neurons [44]). Thus, this diversity of the 5-HT system may provide an explanation for the inconsistencies in the literature regarding the role of this neurotransmitter and its transporter function in both impulsive action and choice. Although the current results are promising, more work will be required to elucidate the underlying neural mechanisms of impulsive choice and action. Specifically, future studies are needed to determine the potential role of the mPFC involvement in impulsivity and also the role of different 5-HT receptors in impulsive behavior.
5. Conclusion
The present study demonstrates that individual differences in SERT function in OFC are associated with individual differences in impulsive choice. Consistent with correlations from the ex vivo studies, microinfusion of fluoxetine into OFC decreased impulsive choice. Thus, these results indicate that enhanced SERT function in OFC underlies high impulsive choice and suggest that therapeutics specifically targeting SERT in OFC may be beneficial for individuals with disorders associated with high impulsive choice.
Highlights.
OFC SERT function is negatively correlated with delay discounting behavior.
Fluoxetine microinjection into OFC decreased impulsivity in high impulsive rats.
Enhanced SERT function in OFC underlies high impulsive choice.
Acknowledgments
This work was supported by National Institutes of Health grant P50 DA05312 (Center for Drug Abuse Research Translation) and UL1 TR000117.
Abbreviations
- mPFC
medial prefrontal cortex
- OFC
orbitofrontal cortex
- 5-HT
5-hydroxytryptamine (serotonin)
- SERT
serotonin transporter
- 5-CSRTT
five-choice serial reaction time task
- Km
affinity
- Vmax
maximal transport velocity
Footnotes
The authors declare no conflict of interest.
References
- 1.Ellenbroek BA, van der Kam EL, van der Elst MC, Cools AR. Individual differences in drug dependence in rats: the role of genetic factors and life events. Eur J Pharmacol. 2005;526:251–258. doi: 10.1016/j.ejphar.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 2.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Diff. 2001;30:669–689. [Google Scholar]
- 4.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostling EW, Fillmore MT. Tolerance to the impairing effects of alcohol on the inhibition and activation of behavior. Psychopharmacology (Berl) 2010;212:465–473. doi: 10.1007/s00213-010-1972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weafer J, Milich R, Fillmore MT. Behavioral components of impulsivity predict alcohol consumption in adults with ADHD and healthy controls. Drug Alcohol Depend. 2011;113:139–146. doi: 10.1016/j.drugalcdep.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollins SH, McClernon FJ, Fuemmeler BF. Association between smoking and attention-deficit/hyperactivity disorder symptoms in a population-based sample of young adults. Arch Gen Psychiatry. 2005;62:1142–1147. doi: 10.1001/archpsyc.62.10.1142. [DOI] [PubMed] [Google Scholar]
- 9.Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, et al. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2002;160:290–298. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 12.Kheramin S, Body S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, et al. Effects of orbital prefrontal cortex dopamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2004;175:206–214. doi: 10.1007/s00213-004-1813-y. [DOI] [PubMed] [Google Scholar]
- 13.Rudebeck PH, Walton ME, Smyth AN, Bannerman DM, Rushworth MF. Separate neural pathways process different decision costs. Nat Neurosci. 2006;9:1161–1168. doi: 10.1038/nn1756. [DOI] [PubMed] [Google Scholar]
- 14.Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–1196. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- 16.Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- 17.Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34:50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, Doya K. Low-serotonin levels increase delayed reward discounting in humans. J Neurosci. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bizot J, Le Bihan C, Puech AJ, Hamon M, Thiebot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology (Berl) 1999;146:400–412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- 20.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- 21.Masaki D, Yokoyama C, Kinoshita S, Tsuchida H, Nakatomi Y, Yoshimoto K, et al. Relationship between limbic and cortical 5-HT neurotransmission and acquisition and reversal learning in a go/no-go task in rats. Psychopharmacology (Berl) 2006;189:249–258. doi: 10.1007/s00213-006-0559-0. [DOI] [PubMed] [Google Scholar]
- 22.Fletcher PJ. Effects of combined or separate 5,7-dihydroxytryptamine lesions of the dorsal and median raphe nuclei on responding maintained by a DRL 20s schedule of food reinforcement. Brain Res. 1995;675:45–54. doi: 10.1016/0006-8993(95)00037-q. [DOI] [PubMed] [Google Scholar]
- 23.Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berl) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- 24.Mobini S, Chiang TJ, Al-Ruwaitea AS, Ho MY, Bradshaw CM, Szabadi E. Effect of central 5-hydroxytryptamine depletion on inter-temporal choice: a quantitative analysis. Psychopharmacology (Berl) 2000;149:313–318. doi: 10.1007/s002130000385. [DOI] [PubMed] [Google Scholar]
- 25.O'Reilly CA, Reith ME. Uptake of [3H]serotonin into plasma membrane vesicles from mouse cerebral cortex. J Biol Chem. 1988;263:6115–6121. [PubMed] [Google Scholar]
- 26.Carver CS, Johnson SL, Joormann J, Kim Y, Nam JY. Serotonin transporter polymorphism interacts with childhood adversity to predict aspects of impulsivity. Psychol Sci. 2011;22:589–595. doi: 10.1177/0956797611404085. [DOI] [PubMed] [Google Scholar]
- 27.Sonuga-Barke EJ, Kumsta R, Schlotz W, Lasky-Su J, Marco R, Miranda A, et al. A functional variant of the serotonin transporter gene (SLC6A4) moderates impulsive choice in attention-deficit/hyperactivity disorder boys and siblings. Biol Psychiatry. 2011;70:230–236. doi: 10.1016/j.biopsych.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bizot JC, Thiebot MH, Le Bihan C, Soubrie P, Simon P. Effects of imipramine-like drugs and serotonin uptake blockers on delay of reward in rats. Possible implication in the behavioral mechanism of action of antidepressants. J Pharm Exp Ther. 1988;246:1144–1151. [PubMed] [Google Scholar]
- 29.Marusich JA, Darna M, Charnigo RJ, Dwoskin LP, Bardo MT. A multivariate assessment of individual differences in sensation seeking and impulsivity as predictors of amphetamine self-administration and prefrontal dopamine function in rats. Exp Clin Psychopharmacol. 2011;19:275–284. doi: 10.1037/a0023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellemans KGC, Nobrega JN, Olmstead MC. Early environmental experience alters baseline and ethanol-induced cognitive impulsivity: relationship to forebrain 5-HT1A receptor binding. Behav Brain Res. 2005;159:207–220. doi: 10.1016/j.bbr.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- 32.Darna M, Beckmann JS, Gipson CD, Bardo MT, Dwoskin LP. Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 2015;1599:115–125. doi: 10.1016/j.brainres.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrholm SD, Horton DB, Dwoskin LP. The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology. 2007;53:982–989. doi: 10.1016/j.neuropharm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th. Academic Press; London: 1998. [Google Scholar]
- 35.Bymaster FP, Katner JS, Nelson DL, Hemrick-Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J, Zhang A, Kläss T, Johnson KM, Wang CZ, Ye YP, Kozikowski AP. Biaryl analogues of conformationally constrained tricyclic tropanes as potent and selective norepinephrine reuptake inhibitors: synthesis and evaluation of their uptake inhibition at monoamine transporter sites. J Med Chem. 2003;46:1997–2007. doi: 10.1021/jm020596w. [DOI] [PubMed] [Google Scholar]
- 37.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 38.Yates JR, Perry JL, Meyer AC, Gipson CD, Charnigo R, Bardo MT. Role of medial prefrontal and orbitofrontal monoamine transporters and receptors in performance in an adjusting delay discounting procedure. Brain Res. 2014;1574:26–36. doi: 10.1016/j.brainres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bubar MJ, McMahon LR, De Deurwaerdère P, Spampinato U, Cunningham KA. Selective serotonin reuptake inhibitors enhance cocaine-induced locomotor activity and dopamine release in the nucleus accumbens. Neuropharmacology. 2003;44:342–353. doi: 10.1016/s0028-3908(02)00381-7. [DOI] [PubMed] [Google Scholar]
- 40.Marusich JA, Beckmann JS, Gipson CD, Bardo MT. Methylphenidate as a reinforcer for rats: contingent delivery and intake escalation. Exp Clin Psychopharmacol. 2011;3:257–266. doi: 10.1037/a0019814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. 1st. Cambridge University Press; New York: 2006. [Google Scholar]
- 42.Barron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 43.Yates JR, Darna M, Gipson CD, Dwoskin LP, Bardo MT. Dissociable roles of dopamine and serotonin transporter function in a rat model of negative urgency. Behav Brain Res. 2015;291:201–208. doi: 10.1016/j.bbr.2015.05.023. Epub May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;8:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 45.Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of the dorsal raphe: reciprocal changes in firing induced by peripheral nerve stimulation. Brain Res. 1978;153:169–175. doi: 10.1016/0006-8993(78)91140-x. [DOI] [PubMed] [Google Scholar]
- 46.Farnebo LO, Hamberger B. Drug-induced changes in the release of 3H-monoamines from field stimulated rat brain slices. Acta Physiol Scand. 1971;371(Suppl):35–44. doi: 10.1111/j.1748-1716.1971.tb05213.x. [DOI] [PubMed] [Google Scholar]
- 47.Göthert M, Weinheimer G. Extracellular 5-hydroxytryptamine inhibits 5-hydroxytryptamine release from rat brain cortex slices. Naunyn Schmiedebergs Arch Pharmacol. 1979;310:93–96. doi: 10.1007/BF00499879. [DOI] [PubMed] [Google Scholar]
- 48.Sharp T, Boothman L, Raley J, Quérée P. Important messages in the ‘post’: recent discoveries in 5-HT neurone feedback control. Trends Pharmacol Sci. 2007;28:629–636. doi: 10.1016/j.tips.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 49.Iskra-Jopa J, Gołembiowska K, Dziubina A, Cybulski M, Duszyńska B, Chilmonczyk Z. In-vivo effects of the 1,2,4-piperazine derivatives MM5 and MC1, putative 5-HT agonists, on dopamine and serotonin release in rat prefrontal cortex. J Pharm Pharmacol. 2005;57:205–211. doi: 10.1211/0022357055425. [DOI] [PubMed] [Google Scholar]
- 50.Assié MB, Koek W. [(3)H]-8-OH-DPAT binding in the rat brain raphe area: involvement of 5HT(1A) and non-5-HT(1A) receptors. Br J Pharmacol. 2000;130:1348–1352. doi: 10.1038/sj.bjp.0703426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Assié MB, Koek W. Possible in vivo 5-HT reuptake blocking properties of 8-OH-DPAT assessed by measuring hippocampal extracellular 5-HT using microdialysis in rats. Br J Pharmacol. 1996;119:845–850. doi: 10.1111/j.1476-5381.1996.tb15749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eagle DM, Bari A, Robbins a TW. The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 2008;199:439–456. doi: 10.1007/s00213-008-1127-6. [DOI] [PubMed] [Google Scholar]
- 53.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 54.Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW. Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex. 2006;16:106–114. doi: 10.1093/cercor/bhi088. [DOI] [PubMed] [Google Scholar]
- 56.Slezak JM, Anderson KG. Effects of variable training, signaled and unsignaled delays, and D-amphetamine on delay-discounting functions. Behav Pharmacol. 2009;20:424–436. doi: 10.1097/FBP.0b013e3283305ef9. [DOI] [PubMed] [Google Scholar]
- 57.Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomie A, Grimes KL, Pohorecky LA. Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Rev. 2008;58:121–135. doi: 10.1016/j.brainresrev.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Del-Ben CM, Deakin JF, McKie S, Delvai NA, Williams SR, Elliott R, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 60.Clarke HF, Dalley JW, Crofts HS, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–880. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 61.Harrison AA, Everitt BJ, Robbins TW. Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res. 1999;100:99–112. doi: 10.1016/s0166-4328(98)00117-x. [DOI] [PubMed] [Google Scholar]
- 62.Puumala T, Sirvio J. Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience. 1998;83:489–499. doi: 10.1016/s0306-4522(97)00392-8. [DOI] [PubMed] [Google Scholar]
- 63.Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- 64.Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol. 2011;164:1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


