Surface-grafted polymer brushes impart chemical functionality to surfaces and interfaces, and control properties such as colloidal stability, adhesion, wettability, and friction.[1,2] The ability to introduce and modulate cross-links within surface-grafted polymer brushes is expected to influence their mechanical and chemical stability, permeability, and swelling characteristics.[3] Here we report that reversibly cross-linked polymer brushes provide a previously untapped resource for modulating the mechanical properties of surfaces. Bimetallic pincer-PdII complexes (Figure 1) provide reversible, exogenous cross-linking of end-grafted poly(4-vinylpyridine) (PVP) brushes. Atomic force microscopy (AFM) experiments show that structurally similar cross-linkers with different kinetic reactivities have different effects on the lateral mechanical properties of the brush layer. Surprisingly, not only the magnitude but also the direction of the change in lateral force upon cross-linking depends on the dissociation kinetics of the cross-linkers, which therefore provide a molecular handle by which to control the mechanical properties of surface-grafted polymer brushes.
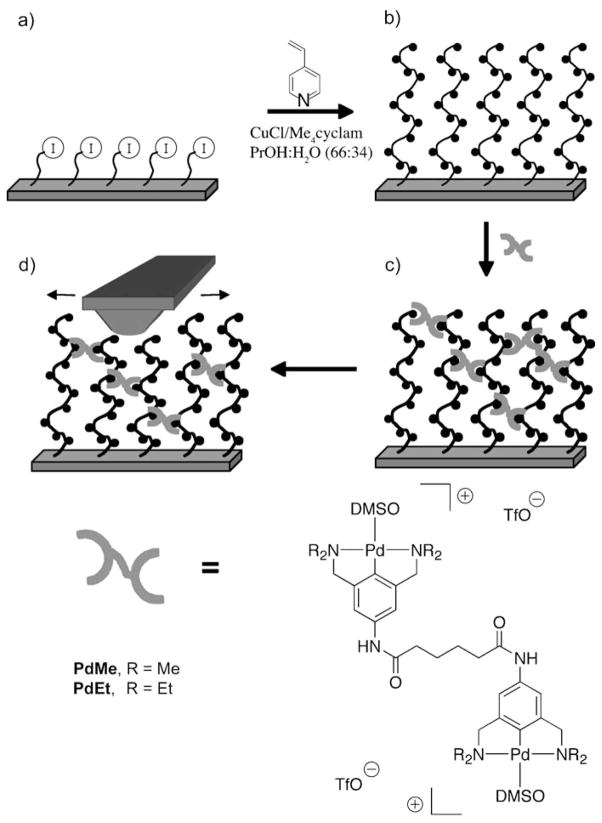
Figure 1.
Schematic description of the synthesis of cross-linked brushes and characterization. On a monolayer of mercaptoundecyl bromoisobutyrate on gold (a), (4-vinylpyridine) is polymerized to generate a surface-grafted polymer brush (b). Addition of the cross-linker PdMe or PdEt in DMSO yields a reversibly cross-linked polymer brush (c), whose lateral mechanics are probed by dragging an AFM tip across the surface (d). The figure is not to scale and does not imply a specific loading or distribution of the cross-links.
Reversibly cross-linkable polymer brushes were synthesized by atom-transfer radical polymerization (ATRP)[4] from an ω-mercaptoundecyl bromoisobutyrate initiator anchored to a gold surface (Figure 1). A low concentration[4,5] (0.020 mM) of the CuI catalyst was used to decrease the steady-state concentration of radicals and to minimize bimolecular termination reactions. Under these conditions, PVP brushes with a dry thickness of 33.5 nm (50 nm in DMSO) could be polymerized in two hours. Brush heights were inferred from cross-sectional profiles obtained by AFM imaging.[6]
The PVP brush substrate was divided in half, and the PVP brush was then cross-linked by the addition of DMSO solutions that contained the bis(PdII-pincer) compounds PdMe or PdEt (Figure 1), which reversibly coordinate to PVP. The compound abbreviations reflect the alkyl substituents on the amino groups of the PdII-pincer complexes. The steric bulk of these substituents has a minimal effect on the thermodynamics of pyridine (Pyr) coordination (Keq(PdMe–Pyr) = 33 M−1, Keq(PdEt–Pyr) = 29 M−1),[7] and so the uptake of PdMe and PdEt from equimolar solutions into the identical PVP brushes should be effectively equivalent, producing samples with comparable structure (number and placement of cross-links). A high concentration of the pincer complex ( ≈9 mM) was used initially to ensure significant cross-linking ( ≈30% of Pyr is bound to PdII, if Keq is unchanged in the brush). The mechanical response of the cross-links, however, is determined by the ligand dissociation rates, koff, and the bulk of the alkyl group has a dramatic influence on those rates (koff(PdMe–Pyr) ≈ 1100 s−1, koff(PdEt–Pyr) = 17 s−1).[7] The difference in the rates thus provides a probe of “mechanochemistry” that is reminiscent of the kinetic isotope effect in reaction chemistry.[8]
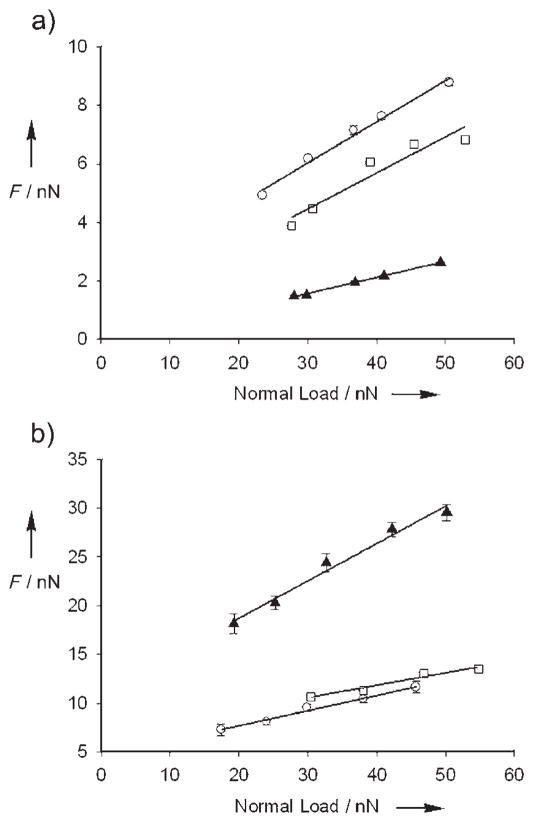
To study the effect of cross-linking on brush mechanics, we measured the lateral force exerted onto an AFM cantilever as the cantilever tip was dragged across the brush surface (scan size 1 μm, frequency 20 Hz). The lateral force, which is phenomenologically similar to friction, was measured as a function of the applied normal force for a range of solution conditions. Figure 2 shows the relationship between lateral force and normal force for six samples. The slope of the linear regression through each data set reports the dependence of lateral force on normal force (hereafter, the coefficient of lateral resistance, or Clat).[9] The addition of cross-linker causes a dramatic change in lateral force. When the faster PdMe cross-linker is added, both the absolute lateral force values and Clat drop to ≈ 30% of those of the noncross-linked PVP control (Figure 2a). That this change is due to PdMe–PVP coordination is verified by the addition of 100 mM dimethylaminopyridine (DMAP) inhibitor, which competes for the PdMe and restores the lateral forces to their original values. The slower cross-linker PdEt also changes the mechanics, but in this case the absolute lateral force values and Clat increase by more than twofold (Figure 2b). As with PdMe, the effect of cross-linking can be chemically reversed to close to that of the noncross-linked state by the addition of DMAP inhibitor.
Figure 2.
a) Lateral force as a function of applied normal load, measured between a silicon nitride cantilever and PVP brushes in DMSO (○), 10 mg PdMe + 1 mL DMSO (▲), and 10 mg PdMe + 1 mL of 0.1M DMAP (□) (Clat equals 0.14, 0.05, and 0.12 for the three solution conditions, respectively). b) Lateral force as a function of applied normal load, measured between a silicon nitride cantilever and PVP brushes in DMSO (○), 10 mg PdEt + 1 mL DMSO (▲), and 10 mg PdEt + 1 mL 0.1M DMAP (□) (Clat equals 0.16, 0.38, and 0.13 for the three solution conditions, respectively). The slope of each solid regression line through the data is Clat. Error bars represent the standard error of the mean. Note the different scale for the y axis in (a) and (b).
Although the quantitative study of the mechanical properties of thin polymer brushes poses still a significant technical challenge, lateral force measurements by AFM provide a sensitive, qualitative measure of changes in the surface mechanical properties of polymer thin films. To facilitate interpretation, the PVP brushes were fabricated and treated in parallel, and AFM conditions were kept constant for all samples. Furthermore, the two cross-linkers are structurally similar, and the thermodynamics of the cross-linking equilibria are effectively equal. Clat values are relatively independent of variations between samples (0.18 ± 0.05) for noncross-linked PVP brushes of different heights (40–130 nm) and roughnesses (2.5–6 nm rms over 1 μm2) in DMSO. The changes induced by cross-linking fall well outside of this experimental uncertainty. The magnitude of the lateral forces should depend also on the scan rate, and it is possible that the differences in the measured lateral forces might disappear altogether under certain experimental conditions. To test the effect of scan rate, we measured lateral force on our brush surfaces over a lateral scan rate range from 2 to 40 μms−1. Within this range, the rate dependencies in the measured lateral forces are small compared to the differences measured between the PdMe and PdEt samples. This demonstrates that the different mechanics are not limited to an isolated set of experimental conditions (see the Supporting Information).
Cross-linking could affect the measured lateral forces by changing the presentation of “dangling polymer ends”, heterogeneity, and roughness of the surface.[11,12] The structural effects should be in principle, and are in practice, indistinguishable for the two cross-linkers. The surfaces have minimal roughness (2–5 nm over 1 μm2) that varies little with or without cross-linker (Table 1). Normal force measurements show that the decay length of the steric repulsion provided by the brush surfaces of cross-linked brushes is significantly less than that for the noncross-linked brushes (data not shown). Importantly, however, the extent of the steric repulsion is independent of cross-linker type. The divergent effects of the two cross-linkers on lateral force thus suggest that the kinetics of the interaction, in addition to structural effects of the cross-linkers in the brush, make important contributions to brush mechanics.
Table 1.
A summary of the PVP brush heights and roughnesses measured on two samples fabricated in parallel, each in DMSO and then with 9 mM cross-linker. Initiator density in the monolayer is ca. 5 nm−2 (complete monolayer).
| Solution | Height [nm][a] | Roughness [nm][b] |
|---|---|---|
| in DMSO | 48.6 ± 4.4 | 5.1 ± 1.8 |
| + 9 mM PdMe | 53.5 ± 2.3 | 4.0 ± 0.2 |
| in DMSO | 54.7 ± 3.9 | 2.2 ± 0.1 |
| + 9 mM PdEt | 65.6 ± 4.7 | 2.8 ± 0.2 |
Estimated from dry height of 33.5 nm, assuming an active initiator density of ≈0.6 nm−2.[18]
Measured on an area of 1 × 1 μm2.
Kinetics contributions could originate from adhesion and adhesion hysteresis, and from molecular relaxations in the brush.[10–12] Non-zero intercepts in the Clat regression lines suggest a contribution to the lateral force from adhesion,[13] and the intercept is greater for PdEt–PVP than for PdMe–PVP. The greater adhesion in PdEt–PVP that is implied by the intercepts is also observed directly; under identical experimental conditions for both surfaces, the force at which the AFM tip dissociates from the PVP–PdEt surface is greater than that observed for the PVP–PdMe surface (see the Supporting Information). In addition, the differences in Clat could arise from cross-links that bear lateral shear stress exerted by the AFM tip, in that the kinetic stability of the cross-links influences the magnitude of their resistance. Such behavior is observed in bulk PVP–PdMe and PVP–PdEt[7,8] and the rupture of single molecules.[14]
The data thus suggest that cross-links reduce the lateral forces by limiting dangling-end contacts and/or penetration of the tip into the brush surface, but they increase the lateral force through mechanical resistance which depends on cross-link dissociation kinetics. The molecular origin of friction on “soft/wet” surfaces in general is an important and open area of research.[15] The use of well-defined kinetic probes, in combination with more sophisticated AFM techniques (e.g., microrheology[16]), offers potential for further mechanistic insight, for example, through frequency scaling.[7]
Polymer brush layers with controlled and stimuli-responsive properties are of significant current interest,[17] with potential applications in biomedical surface engineering and nanofabrication.[1] The modulation of cross-linking interactions is shown here to be one method by which to exert control. The cross-linking is reversed here by chemical competition, but responsiveness to other stimuli, such as temperature, could be engineered. Both the magnitude of the cross-linking effect and the importance of the cross-linking kinetics must depend on the specific context, including among other factors grafting density, polymer molecular weight, the number and distribution of cross-links, and solvent quality. Ongoing work in our laboratories is addressing these relationships.
Supplementary Material
Footnotes
This work was supported by the NSF (0503907) and by the NIH (EB-001037). D.M.L. acknowledges an NSF IGERT Fellowship (DGE-0221632) and Burroughs Wellcome Fellowship (Duke Department of Chemistry). S.Z. acknowledges an NSF CAREER award (NSF DMR-0239769). D.M.L. and N.I.A. contributed equally to this work.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
David M. Loveless,
Dr. Nehal I. Abu-Lail,
Dr. Marian Kaholek,
Prof. Stefan Zauscher, Email: zauscher@duke.edu,
Prof. Stephen L. Craig, Email: stephen.craig@duke.edu,
References
- 1.a) Rühe J. In: Polymer Brushes: Synthesis, Characterization, Applications. Advincula RC, Brittain WJ, Caster KC, Rühe J, editors. Wiley-VCH; Weinheim: 2004. pp. 1–32. [Google Scholar]; b) Caster KC. In: Polymer Brushes: Synthesis, Characterization, Applications. Advincula RC, Brittain WJ, Caster KC, Rühe J, editors. Wiley-VCH; Weinheim: 2004. pp. 331–352. [Google Scholar]
- 2.Fleer GJ, Cohen Stuart MA, Scheutjens JMHM, Cosgrove T, Vincent B. Polymers at Interfaces. Chapman and Hall; London: 1993. [Google Scholar]
- 3.a) Hyang W, Baker GL, Bruening ML. Angew Chem. 2005;117:1558–1560. [Google Scholar]; Angew Chem Int Ed. 2001;40:1510–1512. [Google Scholar]; b) Edmondson S, Huck WTS. Adv Mater. 2004;16:1327–1331. [Google Scholar]; c) Ionov L, Stamm M, Minko S, Hoffmann F, Wolff T. Macromol Symp. 2004;210:229–235. [Google Scholar]; d) Ulbricht M, Yang H. Chem Mater. 2005;17:2622–2631. [Google Scholar]; e) von Werne TA, Germack DS, Hagberg EC, Sheares VV, Hawker CJ, Carter KR. J Am Chem Soc. 2003;125:3831–3838. doi: 10.1021/ja028866n. [DOI] [PubMed] [Google Scholar]
- 4.Xia J, Zhang KW, Matyjaszewski K. Macromolecules. 1999;32:3531–3533. [Google Scholar]
- 5.Kim JB, Huang WX, Miller MD, Baker GL, Bruening ML. J Polym Sci Part A. 2003;41:386–394. [Google Scholar]
- 6.Kaholek M, Lee WK, Ahn SJ, Ma HW, Caster KC, LaMattina B, Zauscher S. Chem Mater. 2004;16:3688–3696. [Google Scholar]
- 7.Yount WC, Loveless DM, Craig SL. J Am Chem Soc. 2005;127:14488–14496. doi: 10.1021/ja054298a. [DOI] [PubMed] [Google Scholar]
- 8.Yount WC, Loveless DM, Craig SL. Angew Chem. 2005;117:2806–2808. doi: 10.1002/anie.200500026. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:2746–2748. doi: 10.1002/anie.200500026. [DOI] [PubMed] [Google Scholar]
- 9.The regression lines need not be linear (see reference [10]), and Clat is used here only phenomenologically.
- 10.Sills S, Overney RM. Phys Rev Lett. 2003;91:95501. doi: 10.1103/PhysRevLett.91.095501. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Maeda N, Tirrell M, Israelachvili J. Macromolecules. 2005;38:3491–3503. [Google Scholar]
- 12.Szoszkiewicz R, Bhushan B, Huey BD, Kulik AJ, Gremaud G. J Chem Phys. 2005;122:144708(6). doi: 10.1063/1.1886751. [DOI] [PubMed] [Google Scholar]
- 13.LaTorre C, Bhusan B. Ultramicroscopy. 2006;106:720–734. doi: 10.1016/j.ultramic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Kersey F, Yount WC, Craig SL. J Am Chem Soc. 2006;128:3886–3887. doi: 10.1021/ja058516b. [DOI] [PubMed] [Google Scholar]
- 15.Gong JP. Soft Matter. 2006;2:544–552. doi: 10.1039/b603209p. [DOI] [PubMed] [Google Scholar]
- 16.Alcaraz J, Buscemi L, Puig-de-Morales M, Colchero J, Baro A, Navajas D. Langmuir. 2002;18:716–721. [Google Scholar]
- 17.a) LeMieux MC, Minko S, Usov D, Shulha H, Stamm M, Tsukruk VV. Polym Mater Sci Eng. 2004;90:372–373. [Google Scholar]; b) Kaholek M, Lee WK, LaMattina B, Caster KC, Zauscher S. Polym Mater Sci Eng. 2004;90:226–227. [Google Scholar]; c) Granville AM, Boyes SG, Akgun B, Foster MD, Brittain WJ. Macromolecules. 2004;37:2790–2796. [Google Scholar]; d) Kaholek M, Lee WK, LaMattina B, Caster KC, Zauscher S. Nano Lett. 2004;4:373–376. [Google Scholar]; e) Kizhakkedathu JN, Norris-Jones R, Brooks DE. Macromolecules. 2004;37:734–743. [Google Scholar]; f) Motornov M, Minko S, Eichhorn KJ, Nitschke M, Simon F, Stamm M. Langmuir. 2003;19:8077–8085. [Google Scholar]; g) Minko S, Stamm M, Goreshnik E, Usov D, Sidorenko A. Polym Mater Sci Eng. 2000;83:533–534. [Google Scholar]; h) Moya S, Azzaroni O, Farhan T, Osborne VL, Huck WTS. Angew Chem. 2005;117:4654–4657. doi: 10.1002/anie.200500228. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:4578–4581. doi: 10.1002/anie.200500228. [DOI] [PubMed] [Google Scholar]
- 18.Jones DM, Brown AA, Huck WTS. Langmuir. 2002;18:9394–9395. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.