Abstract
The SLP-76 family of immune cell-specific adaptors is composed of three distinct members named SLP-76, Blnk, and Clnk. They have been implicated in the signaling pathways coupled to immunoreceptors such as the antigen receptors and Fc receptors. Previous studies using gene-targeted mice and deficient cell lines showed that SLP-76 plays a central role in T-cell development and activation. Moreover, it is essential for normal mast cell and platelet activation. In contrast, Blnk is necessary for B-cell development and activation. While the precise function of Clnk is not known, it was reported that Clnk is selectively expressed in mast cells, natural killer (NK) cells, and previously activated T-cells. Moreover, ectopic expression of Clnk was shown to rescue T-cell receptor-mediated signal transduction in an SLP-76-deficient T-cell line, suggesting that, like its relatives, Clnk is involved in the positive regulation of immunoreceptor signaling. Stimulatory effects of Clnk on immunoreceptor signaling were also reported to occur in transfected B-cell and basophil leukemia cell lines. Herein, we attempted to address the physiological role of Clnk in immune cells by the generation of Clnk-deficient mice. The results of our studies demonstrated that Clnk is dispensable for normal differentiation and function of T cells, mast cells, and NK cells. Hence, unlike its relatives, Clnk is not essential for normal immune functions.
The SLP-76 family of adaptors includes three related polypeptides named SLP-76, Blnk (also termed SLP-65 or BASH), and Clnk (also named MIST) (14, 18, 28). These intracytoplasmic molecules share a common organization, which includes an amino-terminal acidic domain carrying sites of tyrosine phosphorylation, a central region with proline-rich motifs and arginine-rich sequences implicated in protein-protein interactions, and a carboxyl-terminal Src homology 2 (SH2) domain. Accumulating evidence indicates that SLP-76 and Blnk orchestrate the formation of multimolecular complexes leading to activation of phospholipase Cγ, as well as of the guanine nucleotide exchange factors Vav-1 and Sos, in response to stimulation of immunoreceptors including the antigen receptors and Fc receptors.
SLP-76 is expressed in T cells, mast cells, natural killer (NK) cells, macrophages, and neutrophils (14, 18, 28). Genetic studies using an SLP-76-deficient variant of the T-cell Jurkat line or genetically engineered SLP-76-deficient mice showed that SLP-76 is essential for T-cell differentiation and activation, due to its involvement in T-cell antigen receptor (TCR) signaling (8, 25, 30). It is also necessary for mast cell activation, platelet activation, vascular development, and neutrophil activation (1, 7, 21, 26). In contrast, SLP-76 is dispensable for NK cell and macrophage activation (4, 24). Unlike SLP-76, Blnk is found in B cells and macrophages. Experiments performed with a Blnk-deficient variant of the DT-40 B-cell line or Blnk-deficient mice revealed that Blnk is crucial for normal B-cell differentiation and activation, as a result of its implication in B-cell antigen receptor signaling (13, 15, 20, 23). Nevertheless, it is not required for macrophage activation (22).
Clnk (also known as MIST; hereafter named Clnk) is the most recently identified member of the SLP-76 family (6, 11). While it is not found in resting T cells, it is abundantly expressed in previously activated T cells grown in the presence of interleukin-2 (IL-2) (6). Clnk is also present in IL-2-activated NK cells and IL-3-propagated mast cells. Moreover, immunohistochemical studies showed that it is detectable in cutaneous mast cells (11). The physiological function of Clnk is not known. Nonetheless, overexpression studies with Jurkat T cells revealed that, like SLP-76, Clnk can promote TCR-induced activation of the IL-2 promoter and rescue TCR signaling in SLP-76-deficient Jurkat T cells (6, 31). Likewise, it was reported that Clnk can partially restore B-cell antigen receptor signaling in Blnk-deficient DT-40 B cells (12) and that it can enhance responses triggered by the high-affinity Fc receptor for immunoglobulin E (IgE) (FcɛRI) in RBL 2H3 cells (11).
While the precise biochemical mechanism(s) involved in these effects were not elucidated, Clnk was shown to undergo tyrosine phosphorylation in response to immunoreceptor, but not cytokine, stimulation (6). Furthermore, following immunoreceptor engagement, it associates by way of its SH2 domain with hemopoietic protein kinase 1 (HPK-1), a serine/threonine-specific protein kinase involved in other systems in the activation of NFκB and Jun N-terminal kinase (JNK) (31). Physical interactions with ADAP (also named Fyb or SLAP-130) and Grb2 were also reported (10, 12, 31). Hence, Clnk may be required to orchestrate signals involved in the positive regulation of immunoreceptor signaling in a subset of immune cells.
In order to elucidate the physiological function of Clnk, we have generated Clnk-deficient mice. Our detailed studies of T-cell, mast cell, and NK cell functions from these animals indicate that Clnk is dispensable for normal functioning of the immune system.
MATERIALS AND METHODS
Knockout constructs.
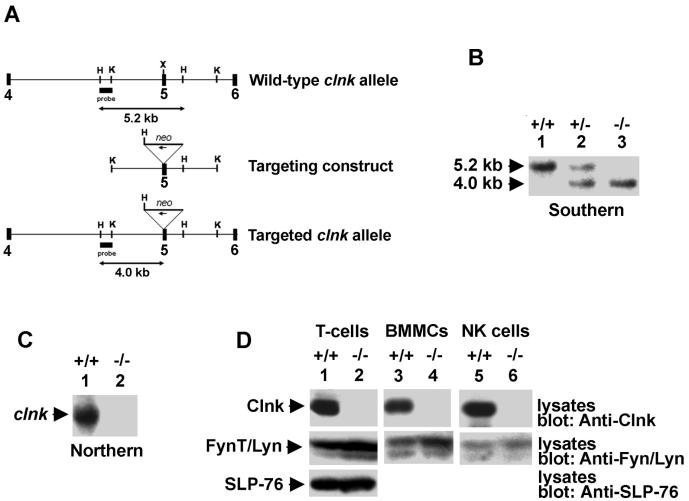
The clnk gene was cloned from a 129/Sv genomic DNA library by screening with a full-length clnk cDNA as a probe (6). For homologous recombination, we created a construct allowing disruption of exon 5 of clnk by insertion of the neo gene in the antisense orientation (Fig. 1A). Exon 5 was chosen for targeting because it is the first coding exon that cannot be alternatively spliced. R1 embryonic stem (ES) cells were transfected according to standard protocols, and G418-resistant colonies were tested for homologous recombination by Southern blotting. Two clones (C3 and G12) were injected into blastocysts and produced progeny having germ line transmission of the mutation. Mice were backcrossed to C57BL/6 (three to seven generations, depending on the experiments) and bred to homozygosity for our studies. clnk+/+ littermates were used as controls. Similar results were obtained with mice derived from either ES clone.
FIG. 1.
Molecular and biochemical characterization of Clnk-deficient mice. (A) clnk gene organization and targeting construct. A partial structure of the mouse clnk gene (exons 4 to 6) and a depiction of the targeting construct are shown. The position of the DNA fragment used as a probe for Southern blotting is shown. This probe hybridizes to a 5.2-kb HindIII fragment in clnk+/+ mice and to a 4.2-kb HindIII fragment in clnk−/− animals. (B) Southern blot depicting wild-type, heterozygous, and homozygous targeted alleles. Genomic DNA from mouse tails was digested with HindIII and tested by Southern blotting by using the probe depicted in panel A. (C) Northern blot. RNA was isolated from BMMCs derived from clnk+/+ and clnk−/− mice and probed by Northern blotting by using a full-length clnk cDNA as a probe. (D) Immunoblots. Lysates were prepared from anti-CD3 MAb-stimulated T cells (day 5 of IL-2 cultures), BMMCs, and NK cells. The presence of Clnk and that of SLP-76 were detected by immunoblotting by using polyclonal rabbit anti-Clnk (top panels) and anti-SLP-76 (bottom panel) sera, respectively. Equal loading was confirmed by reprobing the immunoblot membranes (middle panels) with anti-FynT (lanes 1 and 2) or anti-Lyn (lanes 3 to 6) antibodies.
Mice.
C57BL/6 mice were obtained from Harlan Sprague Dawley, Inc., Chicago, Ill. Anti-lymphocyte choriomeningitis virus gp33 major histocompatibility class I (MHC-I)-restricted TCR transgenic mice P14 (5) were obtained from Taconics, Germantown, N.Y.
Cells.
All cells were grown in RPMI medium supplemented with 10% fetal bovine serum, glutamine, and antibiotics. Primary mouse cell cultures were propagated in the same growth medium containing β-mercaptoethanol. NK cells were purified from spleens by negative selection with magnetic columns (StemCell Technologies, Vancouver, British Columbia, Canada) and were expanded in the presence of high concentrations of IL-2 (1,000 U per ml). Cells were used for experimentation after 7 to 9 days in culture, at which time >95% of cells were DX-5 positive and CD3 negative (data not shown). Bone marrow-derived mast cells (BMMCs) were established from mouse femurs by growth in medium containing 5% culture supernatant from the WEHI-3B cell line (a source of IL-3). Cells were used for experiments after 1 month in culture, at which time they were 100% positive for FcɛRI (data not shown). Naïve T cells (CD4+ and/or CD8+) were purified from spleens or lymph nodes by negative selection with magnetic columns (StemCell Technologies). Cell preparations were typically >90% CD3+ (data not shown). The cell lines P815 and EL-4 were obtained from American Type Culture Collection (Rockville, Md.). RMA and RMA-S cells were provided by Sylvain Latour, Hôpital Necker, Paris, France.
Antibodies and reagents.
Anti-Clnk and anti-SLP-76 polyclonal antibodies were described previously (6). Biotinylated anti-CD8, phycoerythrin (PE)-coupled anti-CD4 and anti-DX-5, fluorescein-isothiocyanate (FITC)-coupled anti-Vβ8.1/2, anti-Vβ6, anti-CD3, anti-CD8, and annexin V, as well as anti-2B4, anti-H-2Db, and anti-NK1.1, were purchased from BD PharMingen (Mississauga, Ontario, Canada). FITC-labeled anti-gamma interferon (IFN-γ) and anti-IL-2 used for intracellular staining were procured from R&D Systems (Minneapolis, Minn.). Streptavidin Quantum Red was obtained from Sigma (Oakville, Ontario, Canada) and used as a secondary reagent for biotinylated antibodies. Anti-CD3 monoclonal antibody (MAb) 145-2C11, anti-CD16 MAb 2.4G2, and anti-Thy MAb G7 were purified in our laboratory. 7-Aminoactinomycin D (7-AAD) and carboxyfluorescein succinimidyl ester (CFSE) were purchased from Molecular Probes (Eugene, Oreg.).
Northern blots.
Total RNA was extracted from mouse cells by use of the RNeasy RNA extraction kit (QIAGEN Inc., Mississauga, Ontario, Canada). RNA was then processed for Northern blotting by standard procedures.
Immunoblots.
Equal numbers of cells were directly lysed in boiling sodium dodecyl sulfate (SDS)-containing sample buffer, and lysates were resolved by gel electrophoresis. Immunoblots were done as described previously (29), with horseradish peroxidase-coupled protein A and enhanced chemiluminescence reagents (Amersham Biosciences, Baie d'Urfé, Québec, Canada).
In vitro T-cell assays.
To measure cytokine production, purified T cells were first stimulated in vitro with anti-CD3 MAb 145-2C11 coated on plastic. After 48 h, cells were propagated in the presence of IL-2 (100 units per ml). Following an additional 5 days in culture, cells were washed and stimulated, or not, in duplicate with the indicated concentrations of anti-CD3 MAb 145-2C11 and IL-2. Cytokine production was assessed by intracellular staining using FITC-labeled anti-IFN-γ and anti-IL-2, as outlined by the manufacturer (BD PharMingen). To measure proliferation, freshly isolated cells were incubated with CFSE (1 μM) for 10 min at 37°C. After stopping the incorporation of CFSE by addition of fetal bovine serum, cells were stimulated with anti-CD3 MAb 145-2C11 in the presence of recombinant IL-2 (100 units per ml). After 3 or 6 days, cells were analyzed by flow cytometry. To examine activation-induced cell death (AICD), IL-2-expanded cells were stimulated overnight with plate-bound MAb 145-2C11 and then stained with 7-AAD and FITC-labeled annexin V, according to the manufacturers' instructions.
T-cell memory.
Equal volumes of ovalbumin (1 mg per ml in phosphate-buffered saline [PBS]; Sigma, Mississauga, Ontario, Canada) and complete Freund's adjuvant (Sigma) were emulsified. Two hundred microliters of emulsion (i.e., 100 μg of ovalbumin) was then injected into the peritoneal cavity of 6- to 8-week-old mice. After 10 to 14 weeks, spleens were extracted and red blood cells were removed by Lympholyte (Cedarlane, Hornby, Ontario, Canada). Red blood cell-depleted spleen cells (2.5 × 105) were subsequently cultured for 3 days with the indicated concentrations of ovalbumin. Proliferation was measured by assaying for tritiated thymidine incorporation in triplicates, whereas cytokine secretion in the supernatant was assessed by enzyme-linked immunosorbent assay (ELISA) in duplicates, by use of kits purchased from R&D Systems.
T-cell anergy.
Mice (6 to 8 weeks old) were injected intraperitoneally with 100 μg of Staphylococcus aureus enterotoxin B (SEB) (Sigma)) in PBS. Control mice were injected with PBS alone. After 14 days, CD4+ T cells were purified from spleen and lymph nodes. Cells (5 × 105 per ml) were then cocultured with 3 × 106 irradiated (2,500 Rads) C57BL/6 splenocytes per ml in the presence of the indicated amounts of SEB. Proliferation assays (performed in triplicate) and cytokine secretion assays (performed in duplicate) were done after 3 days of culture, as detailed above.
Influenza virus infection assays.
Mice were infected intraperitoneally with 200 hemagglutinin units of influenza A virus strain HKx31 (H3N2) (2, 9). After 3 weeks, mice were challenged with the serologically distinct strain A/PR8/34 (PR8, H1N1), which shares the nucleoprotein (NP) gene with strain HKx31. Mice were sacrificed at days 5, 7, 14, and 21 after the primary infection and at days 7 and 28 after the secondary infection. For detection of antigen-specific CD8+ T cells by tetramer staining, splenocytes were labeled with phycoerythrin-conjugated anti-mouse CD8α, FITC-conjugated anti-mouse CD62L (eBioscience,San Diego, Calif.), and allophycocyanin-coupled tetramers consisting of murine MHC class I molecule H-2Db, β2-microglobulin, and influenza NP peptide NP366-374 (NIAID MHC Tetramer Core Facility, Atlanta, Ga.). For each experiment, appropriate isotype control MAbs were used. Cytotoxic activity of CD8+ T cells was measured at the peaks of the primary (day 7) and secondary (day 21 + 7) responses by an ex vivo cytotoxicity assay. Briefly, cells (at serial threefold dilutions) were assayed for anti-influenza NP-specific cytotoxic activity, by use of 51Cr-labeled EL-4 cells pulsed with 50 μM NP366-374 peptide. After 5 h, supernatants were harvested and counted in a Topcount scintillation counter (Canberra Packard Canada, Mississauga, Ontario, Canada). Maximum and spontaneous releases were determined in wells treated with 1% SDS and medium alone, respectively. Spontaneous release was consistently less than 10% (data not shown). Specific lysis was calculated with the following equation: (experimental 51Cr release − spontaneous 51Cr release) × 100/(maximum 51Cr release − spontaneous 51Cr release) = specific release (as a percentage). To measure IL-2-production by CD4+ T cells, spleen cells (5 × 106 cells) were incubated for 48 h with 250 hemagglutinin units of heat-killed influenza A virus strain HKx31 per ml. For IL-2 detection, serial dilutions of culture supernatants were prepared in duplicate and incubated for 24 h in 96-well plates with 104 IL-2-dependent indicator cells. Cells were labeled with tritiated thymidine (Amersham Biosciences) during the final 6 h of the incubation period.
Mast cell degranulation.
IL-3-propagated BMMCs were washed and resuspended in Tyrode's buffer (10 mM HEPES [pH 7.4], 130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% bovine serum albumin). They were preincubated with anti-2,4-dinitrophenol (DNP) mouse IgE MAb SPE-7 (Sigma) for 40 min on ice. After this incubation, cells were washed again and triggered or not for 40 min at 37°C by addition of human serum albumin (HSA)-coupled DNP (DNP-HSA) (Sigma). Supernatants were harvested, whereas cell pellets were lysed in Tyrode's buffer containing 0.5% Triton X-100. β-Hexosaminidase activity was measured in a colorimetric assay using p-nitrophenyl-N-acetyl-β-d-glucosaminide as a substrate (16). Control degranulation was measured by stimulating cells with ionomycin (3 μM). Degranulation was expressed as the percentage of maximal granule content, which was defined by lysing cells in SDS-containing buffer. Assays were done in duplicate.
Mast cell cytokine production.
Mast cells (2 × 105 per ml) were stimulated for 3 h at 37°C with the indicated concentrations of SPE-7. Supernatants were subsequently collected and assayed for IL-6 or TNF-α by ELISA. Assays were done in duplicate.
Mast cell proliferation.
BMMCs were rested for 8 h in medium lacking IL-3. After this incubation period, cells (4 × 105 per ml) were incubated for 24 h with the indicated concentrations of WEHI-3B supernatant (a source of IL-3). Proliferation was assayed in triplicates by measuring the incorporation of tritiated thymidine during the last 6 h of the incubation period.
NK cell-mediated cytotoxicity and IFN-γ secretion.
To test antibody-dependent cellular cytotoxicity (ADCC), IL-2-activated NK cells (at the specified raio of effector cell to target cell [E:T]) were incubated with EL-4 cells (5 × 104 cells per ml), preincubated or not with anti-Thy MAb G7, and prelabeled for 1 h at 37°C with 100 μCi of 51Cr. After 4 h, the supernatant was collected and radioactivity was measured. The percentage of maximum release was calculated as 100% × [cpm (experimental well) − cpm (spontaneous release)]/[cpm (maximum release) − cpm (spontaneous release)]. To assess natural cytotoxicity, NK cells were stimulated with MHC class I-positive RMA cells or MHC class I-negative RMA-S cells, and target cell killing was tested as detailed for ADCC. To assay IFN-γ secretion, NK cells (2 × 105 per ml) were stimulated overnight with the indicated antibodies, IL-12, or a combination of phorbol myristate acetate (PMA) plus ionomycin. Supernatants were then collected and tested for IFN-γ by ELISA. All assays were done in duplicate.
RESULTS AND DISCUSSION
Generation of Clnk-deficient mice.
To produce mice lacking Clnk, a targeting construct was created as depicted in Fig. 1A. This construct was aimed at disrupting exon 5, the first exon common to all clnk transcripts (data not shown). We wanted to avoid targeting the more upstream exons, as such an approach could yield alternatively spliced transcripts capable of encoding functional Clnk proteins. Properly targeted ES cells were identified by Southern blotting, and two independent targeted lines (G3 and C12) were used for production of chimeric mice and germ line transmission of the mutant allele. After breeding to homozygosity (Fig. 1B), RNA was isolated from IL-3-propagated BMMCs and probed by a Northern blotting assay using a full-length clnk cDNA as a probe (Fig. 1C). This analysis showed that no clnk transcripts were detectable in RNA samples from clnk−/− mice (lane 2). No truncated or aberrant transcript was observed. Likewise, immunoblotting studies showed that no Clnk protein was found in lysates prepared with IL-2-propagated splenic T cells (Fig. 1D, top panel, lane 2), BMMCs (lane 4), and NK cells (lane 6) from these animals. In addition, no truncated protein was seen. By contrast, the levels of SLP-76 (Fig. 1D, bottom panel) of IL-2-propagated T cells from clnk+/+ (lane 1) and clnk−/− (lane 2) mice were comparable. Thus, our targeting strategy produced a true clnk null allele. Clnk-deficient mice were born at a proper mendelian frequency, appeared normal, and were fertile (data not shown).
T-cell functions in Clnk-deficient mice.
Previous studies showed that Clnk is not expressed in freshly isolated thymocytes or splenic T cells (6). Moreover, it is not detectable in short-term cultures of TCR-activated T cells. However, Clnk is induced within 24 h of exposure of TCR-activated T cells to IL-2. In the light of the additional observation that Clnk expression enhanced TCR-mediated responses in Jurkat T cells, we propose that Clnk may be implicated in modulating TCR responses in previously activated T cells such as effector or memory T cells. These two types of T cells are characterized by an enhanced capacity to respond to TCR stimulation. Alternatively, Clnk may be involved in qualitatively changing the outcome of TCR stimulation in previously activated T cells. As a result, it may participate in phenomena like T-cell anergy or AICD (17).
As expected, the various thymocyte subsets were represented normally and freshly isolated peripheral T cells responded adequately to TCR stimulation in Clnk-deficient mice (data not shown). To assess whether Clnk regulates T-cell responsiveness when its expression is induced, purified splenic T cells from clnk+/+ and clnk−/− mice were activated for 48 h in vitro with antibodies against CD3 (anti-CD3). Cells were subsequently propagated for 2 to 4 days in the presence of IL-2 (100 units per ml), thereby producing high numbers of T cells with an effector phenotype (data not shown). Note that labeling with CFSE showed that the ability of T cells to grow in the presence of IL-2 was not appreciably influenced by Clnk deficiency (Fig. 2A). Moreover, an anti-Clnk immunoblot confirmed that Clnk was expressed in cells obtained from clnk+/+, but not from clnk−/−, mice (Fig. 1D; data not shown).
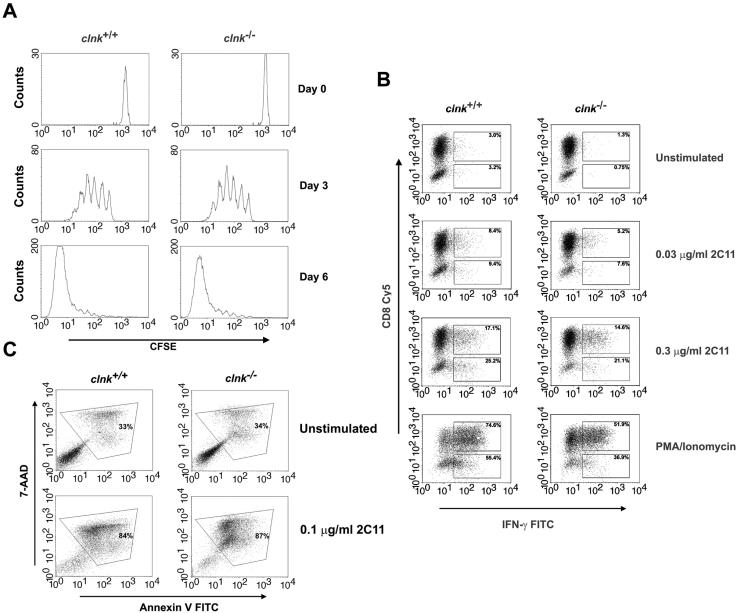
FIG. 2.
In vitro assays of T-cell functions. (A) T-cell proliferation. T cells were labeled with CFSE and stimulated by use of plastic coated with anti-CD3 MAb 145-2C11 (1 μg per ml) in the presence of 100 U of IL-2 per ml. T-cell proliferation was assessed by measuring CFSE labeling at the indicated times after the initial stimulation. Proliferation of T cells causes a progressive loss in CFSE label. (B) TCR-induced production of IFN-γ. T cells were stimulated for 48 h with anti-CD3 MAb 145-2C11 and then expanded for 5 days in IL-2-supplemented growth medium. After being washed, cells were stimulated for 4 h and analyzed for IFN-γ production by intracellular staining with FITC-coupled anti-IFN-γ antibodies. CD8+ T cells were identified by concomitant staining with Cy5-labeled anti-CD8. CD8− T cells presumably represent CD4+ cells. Percentages indicate the proportions of cells (CD8+ or CD8−) showing appreciable IFN-γ secretion. (C) Activation-induced T-cell death. T cells were stimulated for 48 h with anti-CD3 and expanded for 5 days with IL-2. Cells were then stimulated or not with plate-bound anti-CD3 MAb 145-2C11. This type of activation protocol is known to trigger AICD. After 18 h, AICD was assessed by staining cells with 7-AAD and FITC-labeled annexin V. Apoptotic cells (boxed) are positive for both 7-AAD and annexin V.
When these previously activated T cells were restimulated with anti-CD3, we found that there was no significant difference in the ability of T cells from clnk+/+ and clnk−/− animals to produce IFN-γ (Fig. 2B) and proliferate (data not shown). Analogous results were obtained when cells were tested for production of IL-2, IL-4, IL-10, tumor necrosis factor alpha (TNF-α), and granulocyte macrophage colony-stimulating factor (data not shown). Furthermore, labeling of cells with 7-AAD and annexin revealed that Clnk deficiency had no impact on the ability of anti-CD3 to trigger apoptosis, a phenomenon referred to as AICD (Fig. 2C). To study antigen-specific T-cell responses, we also crossed clnk−/− mice with an MHC class I-restricted TCR transgenic mouse model (P14). Experiments with previously activated T cells derived from these animals demonstrated that Clnk deficiency had no effect on antigen-triggered T-cell proliferation and cytotoxicity (data not shown).
Several in vivo assays of T-cell functions were also performed by using Clnk-deficient animals. To ascertain if Clnk may be involved in T-cell memory, mice were immunized with the antigen ovalbumin in the presence of complete Freund adjuvant (CFA). After 12 weeks, splenocytes were isolated and restimulated in vitro with various concentrations of ovalbumin. The persistence of memory-type T cells was determined by measuring ovalbumin-specific proliferation and cytokine production (Fig. 3A). These assays showed that Clnk deficiency did not interfere with the persistence of ovalbumin-specific T cells in immunized mice. Similar results were obtained when antigen-specific T cells were counted by enzyme-linked immunospot assay (data not shown).
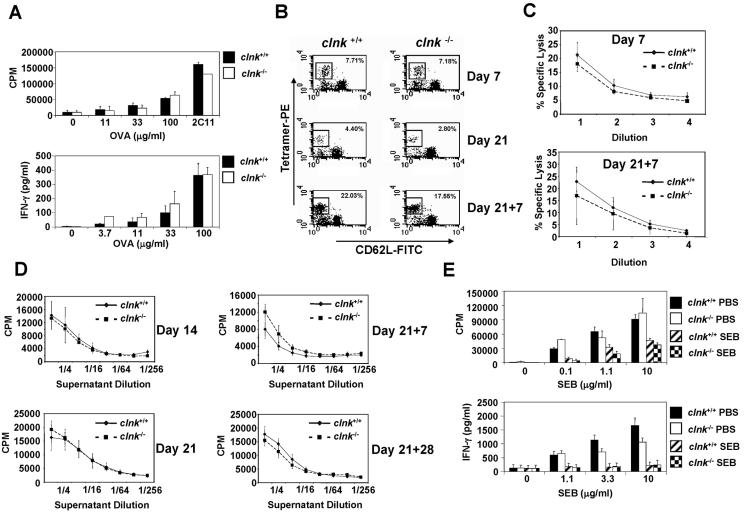
FIG. 3.
In vivo assays of T-cell functions. (A) T-cell memory. Mice were injected with ovalbumin (OVA) emulsified in CFA. Ten to fourteen weeks later, total spleen cells were stimulated with the indicated concentrations of OVA protein. Proliferation was assessed by measuring thymidine incorporation (top panel), whereas IFN-γ production was determined by ELISA (bottom panel). Control stimulation was performed by using soluble anti-CD3 MAb 145-2C11 (2C11) (3 μg per ml). Standard deviations are shown. (B) Antigen-specific anti-influenza virus T cells. Mice were infected with influenza virus as detailed in Materials and Methods. At the indicated times after primary (day 7 or day 21) or secondary (day 21 + 7) infection, MHC class I NP peptide tetramer-reactive CD8+ CD62Llo T cells (which represent antigen-specific effector T cells) were identified by flow cytometry. These results are representative of assays with three independent mice in each group. (C) Ex vivo cytotoxicity assay. Mice were infected as described in the legend for panel B. At the indicated times after primary (day 7) or secondary (day 21 + 7) infection, purified T cells were incubated in serial threefold dilutions (abscissa) with 51Cr-labeled EL-4 cells prepulsed with NP peptide. 51Cr release was measured and is presented as the percentage of maximal release. Standard deviations are shown. These results are representative of assays with three independent mice in each group. (D) CD4+ T-cell-mediated production of IL-2. Mice were infected as described for panel B. At the indicated times after primary (day 14 and day 21) or secondary (day 21 + 7 and day 21 + 28) infection, spleen cells were isolated and incubated for 48 h with heat-killed influenza virus. IL-2 production was then measured by monitoring CTLL-2 proliferation in the presence of serial dilutions (abscissa) of supernatant. Standard deviations are depicted. These results are representative of assays with three independent mice in each group. (E) T-cell anergy. Mice were injected intraperitoneally with SEB or PBS. After 14 days, CD4+ cells were purified and stimulated with the indicated concentrations of SEB in the presence of irradiated splenocytes. Proliferation (top panel) and IFN-γ production (bottom panel) were measured as detailed in the legend to Fig. 3A. In the experiment shown, 10 and 16% of purified CD4+ T cells were positive for Vβ8.1/2 in clnk+/+ and clnk−/− mice, respectively (data not shown). Standard deviations are shown.
The impact of Clnk was also tested by challenging mice with influenza A virus, a pathogen that elicits both CD8+ and CD4+ (Th1) T-cell responses (Fig. 3B through D). In this experiment, mice were first infected intraperitoneally with influenza A virus strain H3N2. After 3 weeks, they were challenged with a serologically distinct strain of influenza A virus, strain H1N1, which shares the NP gene with H3N2. Staining of CD8+ T cells with MHC class I peptide tetramers showed that, following either primary or secondary exposure to the virus, there was no difference in the ability of clnk+/+ and clnk−/− mice to produce NP-specific CD8+ effector T cells (Fig. 3B). Similar results were obtained when the proportion of IFN-γ-secreting CD8+ T cells was assessed (data not shown). Ex vivo cytotoxicity assays also showed that the ability of CD8+ T cells to kill EL-4 cells loaded with NP peptide was not significantly affected by Clnk deficiency (Fig. 3C). Likewise, primary and secondary CD4+ T-cell responses were not appreciably modified by the absence of Clnk, as judged by the assessment of IL-2-producing virus-specific T cells in the spleen (Fig. 3D) and the production of virus-specific IgG1 and IgG2a (data not shown). Combined, these results showed that Clnk is not required for primary and secondary anti-influenza virus responses involving CD8+ and CD4+ T-cells.
Finally, we tested the possibility that Clnk may be involved in the induction of T-cell anergy (Fig. 3E). In this experiment, mice were injected intraperitoneally with the superantigen SEB, which causes expansion followed by anergy of CD4+ T cells expressing Vβ8+ TCRs. After 2 weeks, CD4+ T cells were purified and restimulated in vitro with SEB in the presence of irradiated splenocytes. Proliferation was assayed by measuring thymidine incorporation, and IFN-γ secretion was determined by ELISA. This analysis showed that in vivo treatment with SEB caused similar degrees of anergy in clnk+/+ and clnk−/− mice, implying that Clnk expression is not involved in anergy induction.
Mast cell functions in Clnk-deficient mice.
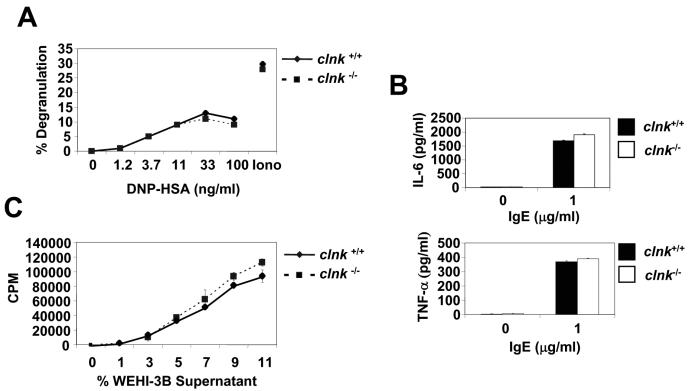
To examine the function of Clnk in mast cells, several assays using IL-3-propagated BMMCs from clnk+/+ and clnk−/− mice were performed (Fig. 4). First, cells were tested for their capacity to degranulate in response to FcɛRI stimulation (Fig. 4A). The result of this experiment showed that Clnk deficiency had no impact on the ability of BMMCs to release β-hexosaminidase in response to anti-DNP IgE and the antigen DNP-HSA. Second, we assessed the aptitude of BMMCs at secreting cytokines when stimulated with IgE alone (Fig. 4B). Once again, lack of Clnk had no effect on the capacity of BMMCs to secrete IL-6 and TNF-α in response to IgE. It should be pointed out, though, that a small enhancement of IL-3-induced proliferation was perhaps seen in Clnk-deficient BMMCs (Fig. 4C). The basis for this observation is not known.
FIG. 4.
Analyses of mast cell functions. (A) Antigen-induced degranulation. BMMCs were sensitized with anti-DNP IgE and triggered by addition of the indicated concentrations of the antigen DNP-HSA. Degranulation was assessed by measuring the release of β-hexosaminidase in the supernatant. Control degranulation was examined in the presence of ionomycin (Iono). Values are presented as percentages of maximal release. Degranulation was measured in response to DNP-HSA. (B) IgE-induced cytokine secretion. Cells were stimulated for 3 h with the indicated concentration of IgE, and secretions of IL-6 (top panel) and TNF-α (bottom panel) were determined by ELISA. Similar results were obtained over a range of different IgE concentrations (data not shown). Standard deviations are represented. (C) IL-3-induced proliferation. BMMCs were incubated in the absence of IL-3-containing medium for 8 h. Then, they were cultured for 18 h in the presence of the indicated concentrations (represented as a percentage of final volume) of WEHI-3B supernatant (a source of IL-3). Proliferation was determined by measuring thymidine incorporation. Standard deviations are represented.
NK cell-mediated responses in Clnk-deficient mice.
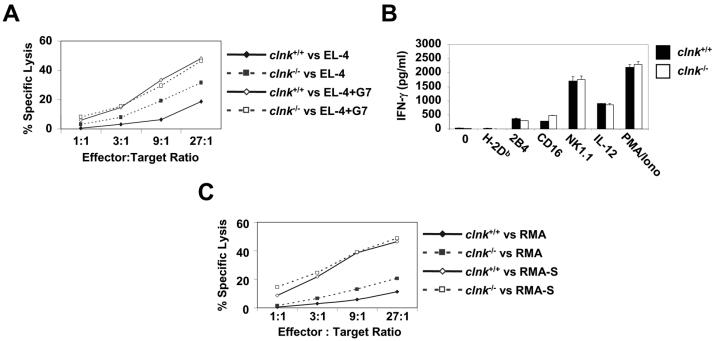
Next, we assessed the possible role of Clnk in NK cells (Fig. 5). First of all, we found that Clnk deficiency had no effect on the ability of spleen-derived NK cells to differentiate or proliferate in vitro in the presence of IL-2 (data not shown). Furthermore, it had no impact on the expression of NK cell markers CD16, NK1.1, DX-5, and 2B4 (data not shown). Several assays of NK cell functions were also performed. First, the ability of NK cells to mediate ADCC, a response mediated by an Fc receptor for IgG (CD16), was tested (Fig. 5A). This experiment showed that the ability of NK cells to kill anti-Thy MAb G7-coated EL-4 cells was not influenced by Clnk deficiency. It is noteworthy, though, that the ability of NK cells to kill EL-4 in the absence of anti-Thy MAb was greater for clnk−/− cells, suggesting that natural cytotoxicity was augmented in the absence of Clnk (see below). Second, we examined the ability of NK cells to release IFN-γ in response to antibody-mediated engagement of NK cell surface molecules such as 2B4, CD16, and NK1.1 (Fig. 5B). Once again, no difference between cells from clnk+/+ and clnk−/− mice was noted. Similar results were obtained when IFN-γ secretion in response to IL-12 or PMA plus ionomycin was examined (Fig. 5B).
FIG. 5.
Analyses of NK cell functions. (A) Antibody-mediated cellular cytotoxicity. NK cells were incubated at the indicated ratios of effector cells to target cells with 51Cr-labeled EL-4 target cells, in the presence or absence of anti-Thy MAb G7 (which binds Thy1 on EL-4 and triggers CD16 on NK cells). Target cell lysis was determined by measuring 51Cr release in the supernatant (values are percentages of maximal release). Standard deviations are shown. (B) IFN-γ secretion. Cells were stimulated with the indicated antibodies (1 μg per ml), IL-12 (5 ng per ml), or PMA (50 ng per ml) plus ionomycin (1 μg per ml). After 24 h, the production of IFN-γ was measured in the supernatant by ELISA. Standard deviations are shown. Similar results were obtained over a range of different antibody concentrations (data not shown). Standard deviations are represented. (C) Natural cytotoxicity. NK cells were stimulated at the indicated ratios of effector cells to target cells with 51Cr-labeled RMA (MHC class I-positive) or RMA-S (MHC class I-negative) cells. After 4 h, natural killing was assessed by measuring the release of 51Cr in the medium. Values are presented as percentages of maximal release. Standard deviations are shown.
Lastly, given the above-mentioned results with EL-4 cells (Fig. 5A), we extended our studies of the ability of NK cells to kill target cells through natural cytotoxicity (Fig. 5C). This process involves a multitude of known and, probably, some unknown NK cell receptors and is critical for NK cells to eliminate tumor cells or virus-infected cells (3). Intriguingly, we observed that the aptitude of Clnk-deficient NK cells to kill MHC class I-positive RMA cells was moderately enhanced in comparison to the aptitude of wild-type NK cells. A small enhancement in natural killing was also frequently seen when MHC class I-negative RMA-S cells were used as targets, especially at lower ratios of effector cells to target cells (Fig. 5C; data not shown). Combined, these findings showed that Clnk is not necessary for NK cell-mediated ADCC and IFN-γ secretion. However, they suggested that Clnk may play an inhibitory role in natural cytotoxicity.
CONCLUSIONS
Previous reports suggested that, like its relatives SLP-76 and Blnk, the Clnk protein is involved in the positive regulation of immunoreceptor signaling, albeit in a distinct subset of immune cells including previously activated T cells, mast cells, and NK cells (6, 11). However, the results presented herein showed that Clnk deficiency had no appreciable effect on immunoreceptor-mediated functions in the types of cells in which Clnk is normally found. Moreover, various in vivo studies revealed that Clnk is not necessary for induction of primary and secondary anti-influenza virus T-cell responses, T-cell memory, and T-cell anergy. Thus, based on the evidence obtained in this study, it seems unlikely that Clnk plays a crucial role during normal immune responses.
One likely explanation for this finding is that other members of the SLP-76 family, in particular SLP-76, provide a redundant function that can substitute for Clnk. The ability of Clnk and SLP-76 to associate with similar proteins (HPK-1, ADAP) and to mediate TCR signaling interchangeably in Jurkat T cells supports this idea (10, 27, 31). Alternatively, other molecules known to interact with Clnk, such as HPK-1 and ADAP, may be able to compensate for Clnk deficiency. Further insights into these possibilities will undoubtedly come from studies of crosses between Clnk-deficient mice and mice lacking SLP-76, HPK-1, or ADAP. It is also possible that Clnk is primarily implicated in pathways distinct from immunoreceptor signaling. While this seems unlikely based on the known functions of the other SLP-76-related adaptors, it is noteworthy that Clnk expression is induced by cytokines (6), suggesting that Clnk could participate in cytokine receptor signaling. Nevertheless, we found that tyrosine phosphorylation of Clnk and its association with other molecules was not induced by cytokines but rather was triggered by immunoreceptor stimulation (6). Additionally, Clnk deficiency had no effect on IL-2-induced proliferation by T cells and NK cells or on IL-12-triggered IFN-γ secretion by NK cells (this report). Furthermore, it did not reduce the ability of IL-3-containing medium to support the growth of BMMCs. Hence, it is improbable that Clnk is involved in cytokine receptor-induced responses.
The most intriguing result obtained during our experiments is the finding that natural cytotoxicity was moderately augmented in Clnk-deficient NK cells. In contrast, there was no change in other NK cell-mediated responses such as ADCC and receptor-mediated IFN-γ secretion. While the basis for this observation remains to be elucidated, it suggests that Clnk may have an inhibitory role in one or more signaling pathways mediating natural cytotoxicity. Unfortunately, as multiple types of NK cell receptors, including unknown receptors, are implicated in this function (3), we have been unable to identify the molecular defect responsible for the enhanced function. Nonetheless, it is worth mentioning that this inhibitory effect may not be restricted to natural killing, as we found that Clnk also inhibited TCR-mediated responses when constitutively expressed in T cells by transgenesis (O. Utting and A. Veillette, unpublished results). Moreover, one of the more prominent partners of Clnk, that is, HPK-1, was reported to inhibit TCR-induced activation of JNK when overexpressed in Jurkat T cells (19). This inhibitory impact may also not be limited to Clnk, since SLP-76-deficient NK cells were reported as well to exhibit increased natural killing (24). Clearly, future studies are warranted to address the physiological relevance and basis of these findings.
Acknowledgments
We thank Jie Yu for generating the targeting vector and Catherine Riou for screening the ES clones. We also thank Sylvain Latour for providing RMA and RMA-S cells and Andrew Makrigiannis for help with NK cell cultures.
This work was supported by grants from the National Cancer Institute of Canada (to A.V.) and the Canadian Institutes of Health Research (to A.V., T.H.W., and D.L.). O.U. holds a Fellowship from the Canadian Institutes of Health Research. A.V. is recipient of the Canada Research Chair in Immune Cell Signaling and is a Senior Scientist of the Canadian Institutes of Health Research.
REFERENCES
- 1.Abtahian, F., A. Guerriero, E. Sebzda, M. M. Lu, R. Zhou, A. Mocsai, E. E. Myers, B. Huang, D. G. Jackson, V. A. Ferrari, V. Tybulewicz, C. A. Lowell, J. J. Lepore, G. A. Koretzky, and M. L. Kahn. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299:247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertram, E. M., P. Lau, and T. H. Watts. 2002. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 168:3777-3785. [DOI] [PubMed] [Google Scholar]
- 3.Biassoni, R., C. Cantoni, D. Pende, S. Sivori, S. Parolini, M. Vitale, C. Bottino, and A. Moretta. 2001. Human natural killer cell receptors and co-receptors. Immunol. Rev. 181:203-214. [DOI] [PubMed] [Google Scholar]
- 4.Bonilla, F. A., R. M. Fujita, V. I. Pivniouk, A. C. Chan, and R. S. Geha. 2000. Adapter proteins SLP-76 and BLNK both are expressed by murine macrophages and are linked to signaling via Fcgamma receptors I and II/III. Proc. Natl. Acad. Sci. USA 97:1725-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandle, D., K. Burki, V. A. Wallace, U. H. Rohrer, T. W. Mak, B. Malissen, H. Hengartner, and H. Pircher. 1991. Involvement of both T cell receptor V alpha and V beta variable region domains and alpha chain junctional region in viral antigen recognition. Eur. J. Immunol. 21:2195-2202. [DOI] [PubMed] [Google Scholar]
- 6.Cao, M. Y., D. Davidson, J. Yu, S. Latour, and A. Veillette. 1999. Clnk, a novel SLP-76-related adaptor molecule expressed in cytokine-stimulated hemopoietic cells. J. Exp. Med. 190:1527-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements, J. L., J. R. Lee, B. Gross, B. Yang, J. D. Olson, A. Sandra, S. P. Watson, S. R. Lentz, and G. A. Koretzky. 1999. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J. Clin. Investig. 103:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clements, J. L., B. Yang, S. E. Ross-Barta, S. L. Eliason, R. F. Hrstka, R. A. Williamson, and G. A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281:416-419. [DOI] [PubMed] [Google Scholar]
- 9.DeBenedette, M. A., T. Wen, M. F. Bachmann, P. S. Ohashi, B. H. Barber, K. L. Stocking, J. J. Peschon, and T. H. Watts. 1999. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J. Immunol. 163:4833-4841. [PubMed] [Google Scholar]
- 10.Fujii, Y., S. Wakahara, T. Nakao, T. Hara, H. Ohtake, T. Komurasaki, K. Kitamura, A. Tatsuno, N. Fujiwara, N. Hozumi, C. Ra, D. Kitamura, and R. Goitsuka. 2003. Targeting of MIST to Src-family kinases via SKAP55-SLAP-130 adaptor complex in mast cells. FEBS Lett. 540:111-116. [DOI] [PubMed] [Google Scholar]
- 11.Goitsuka, R., H. Kanazashi, H. Sasanuma, Y. Fujimura, Y. Hidaka, A. Tatsuno, C. Ra, K. Hayashi, and D. Kitamura. 2000. A BASH/SLP-76-related adaptor protein MIST/Clnk involved in IgE receptor-mediated mast cell degranulation. Int. Immunol. 12:573-580. [DOI] [PubMed] [Google Scholar]
- 12.Goitsuka, R., A. Tatsuno, M. Ishiai, T. Kurosaki, and D. Kitamura. 2001. MIST functions through distinct domains in immunoreceptor signaling in the presence and absence of LAT. J. Biol. Chem. 276:36043-36050. [DOI] [PubMed] [Google Scholar]
- 13.Ishiai, M., M. Kurosaki, R. Pappu, K. Okawa, I. Ronko, C. Fu, M. Shibata, A. Iwamatsu, A. C. Chan, and T. Kurosaki. 1999. BLNK required for coupling Syk to PLC gamma 2 and Rac1-JNK in B cells. Immunity 10:117-125. [DOI] [PubMed] [Google Scholar]
- 14.Jordan, M. S., A. L. Singer, and G. A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110-116. [DOI] [PubMed] [Google Scholar]
- 15.Jumaa, H., B. Wollscheid, M. Mitterer, J. Wienands, M. Reth, and P. J. Nielsen. 1999. Abnormal development and function of B lymphocytes in mice deficient for the signaling adaptor protein SLP-65. Immunity 11:547-554. [DOI] [PubMed] [Google Scholar]
- 16.Latour, S., J. Zhang, R. P. Siraganian, and A. Veillette. 1998. A unique insert in the linker domain of Syk is necessary for its function in immunoreceptor signalling. EMBO J. 17:2584-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17:221-253. [DOI] [PubMed] [Google Scholar]
- 18.Leo, A., and B. Schraven. 2001. Adapters in lymphocyte signalling. Curr. Opin. Immunol. 13:307-316. [DOI] [PubMed] [Google Scholar]
- 19.Liou, J., F. Kiefer, A. Dang, A. Hashimoto, M. H. Cobb, T. Kurosaki, and A. Weiss. 2000. HPK1 is activated by lymphocyte antigen receptors and negatively regulates AP-1. Immunity 12:399-408. [DOI] [PubMed] [Google Scholar]
- 20.Minegishi, Y., J. Rohrer, E. Coustan-Smith, H. M. Lederman, R. Pappu, D. Campana, A. C. Chan, and M. E. Conley. 1999. An essential role for BLNK in human B cell development. Science 286:1954-1957. [DOI] [PubMed] [Google Scholar]
- 21.Newbrough, S. A., A. Mocsai, R. A. Clemens, J. N. Wu, M. A. Silverman, A. L. Singer, C. A. Lowell, and G. A. Koretzky. 2003. SLP-76 regulates Fcgamma receptor and integrin signaling in neutrophils. Immunity 19:761-769. [DOI] [PubMed] [Google Scholar]
- 22.Nichols, K. E., K. Haines, P. S. Myung, S. Newbrough, E. Myers, H. Jumaa, D. J. Shedlock, H. Shen, and G. A. Koretzky. 2004. Macrophage activation and Fcgamma receptor-mediated signaling do not require expression of the SLP-76 and SLP-65 adaptors. J. Leukoc. Biol. 75:541-552. [DOI] [PubMed] [Google Scholar]
- 23.Pappu, R., A. M. Cheng, B. Li, Q. Gong, C. Chiu, N. Griffin, M. White, B. P. Sleckman, and A. C. Chan. 1999. Requirement for B cell linker protein (BLNK) in B cell development. Science 286:1949-1954. [DOI] [PubMed] [Google Scholar]
- 24.Peterson, E. J., J. L. Clements, Z. K. Ballas, and G. A. Koretzky. 1999. NK cytokine secretion and cytotoxicity occur independently of the SLP-76 adaptor protein. Eur. J. Immunol. 29:2223-2232. [DOI] [PubMed] [Google Scholar]
- 25.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94:229-238. [DOI] [PubMed] [Google Scholar]
- 26.Pivniouk, V. I., T. R. Martin, J. M. Lu-Kuo, H. R. Katz, H. C. Oettgen, and R. S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Investig. 103:1737-1743. [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer, K., J. Liou, S. B. Singh, D. Yablonski, A. Weiss, and R. M. Perlmutter. 2001. The kinase HPK1 associates physically and functionally with the adaptor proteins BLNK and SLP-76 in lymphocytes. J. Biol. Chem. 276:45207-45216. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson, M. G., J. Lin, and A. Weiss. 2000. Lymphocytes with a complex: adapter proteins in antigen receptor signaling. Immunol. Today 21:584-591. [DOI] [PubMed] [Google Scholar]
- 29.Veillette, A., M. A. Bookman, E. M. Horak, and J. B. Bolen. 1988. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell 55:301-308. [DOI] [PubMed] [Google Scholar]
- 30.Yablonski, D., M. R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science 281:413-416. [DOI] [PubMed] [Google Scholar]
- 31.Yu, J., C. Riou, D. Davidson, R. Minhas, J. D. Robson, M. Julius, R. Arnold, F. Kiefer, and A. Veillette. 2001. Synergistic regulation of immunoreceptor signaling by slp-76-related adaptor clnk and serine/threonine protein kinase hpk-1. Mol. Cell. Biol. 21:6102-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]