Abstract
Cardiovascular disease (CVD) is the leading cause of death worldwide. There is a consistent inverse relationship between fruit intake with CVD events and mortality in cross-sectional and prospective observational studies, but the relationship of fruit intake with measurements of atherosclerosis in humans is less clear. Nutritional effects on abdominal aortic calcification (AAC), a marker for subclinical intimal and medial atherosclerotic vascular disease, have not been studied previously. The aim of this study was to examine the cross-sectional relationship of total and individual fruit (apple, pear, orange and other citrus, and banana) intake with AAC, scored between 0 and 24. The current study assessed baseline data for a cohort of 1052 women over 70 years of age who completed both a food frequency questionnaire assessing fruit intake, and underwent AAC measurement using dual energy X-ray absorptiometry. AAC scores were significantly negatively correlated with total fruit and apple intakes (p < 0.05), but not with pear, orange or banana intakes (p > 0.25). In multivariable-adjusted logistic regression, each standard deviation (SD; 50 g/day) increase in apple intake was associated with a 24% lower odds of having severe AAC (AAC score >5) (odd ratio OR): 0.76 (0.62, 0.93), p = 0.009). Total and other individual fruit intake were not associated with increased odds of having severe AAC. Apple but not total or other fruit intake is independently negatively associated with AAC in older women.
Keywords: apples, fruit, abdominal aortic calcification, atherosclerosis, cardiovascular disease
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide. One of the most consistent relationships observed in cross-sectional and prospective observational lifestyle studies is that a diet rich in fruit is associated with a lower risk of CVD [1,2,3,4]. In a meta-analysis of 16 prospective cohort studies, the mean reduction in risk of CVD mortality was 5% for each additional serving of fruit per day [5]. A poor dietary pattern, including a low intake of fruit, is estimated to contribute to over 4% of the global burden of disease, around five million deaths worldwide. It is the third highest contributor to mortality after high blood pressure and smoking [6].
Several studies have explored the relationships of intakes of specific fruit, primarily apples and oranges, with CVD-related outcomes. Higher apple [7,8,9,10,11] and orange [10,12] intakes have been associated with lower risk of coronary heart disease and stroke. This may be due in part to their content of soluble fibre, vitamins (vitamin C), minerals (potassium and magnesium) and/or phytochemicals (flavonoids) [13]. In particular, apples and oranges are often major contributors to the intake of fibre [14] and specific flavonoids have been linked to benefits on CVD risk [15,16,17,18,19]. However, the type of fibre and the amount and structure of the flavonoids differs between these fruits [19,20], which could result in differential relationships with CVD risk.
While the association of fruit intake with CVD events and mortality is clear [2,21,22], the relationship of fruit intake with markers and measures of atherosclerosis in humans is less clear. Atherosclerosis, a thickening of the artery wall as a result of the accumulation of calcium and fat, is recognized as a fundamental contributor to CVD events and is major underlying contributor to CVD events in post-menopausal women. Abdominal aortic calcification (AAC), an emerging subclinical measure of atherosclerosis [23], corresponds to the degree of coronary calcification [24] and atherosclerosis in other arteries [25], and predicts CVD mortality [26,27]. The aim of this study was to examine the cross-sectional associations of total and individual fruit (apple, pear, orange and other citrus, and banana) intake with AAC in a cohort of women over 70 years of age.
2. Subjects and Methods
2.1. Participants
Subjects included in this study were originally recruited in 1998 to a 5-year, double-blind, randomized control trial of daily calcium supplementation to prevent osteoporotic fracture, the Calcium Intake Fracture Outcome Study (CAIFOS), which has been previously described [28]. Participants were recruited from the Western Australian general population of women aged over 70 years by mail, using the electoral roll which is a requirement of citizenship. Of the 5586 women approached, 1510 women were willing and eligible to participate in the study and of these 1500 women were recruited into the study. All participants were ambulant with an expected survival beyond 5 years and were not receiving any medication (including hormone replacement therapy) known to affect bone metabolism. Baseline disease burden and medications were comparable between these participants and the general population of similar age although the participants were more likely to be from higher socio-economic groups [29]. In the subsequent 5 years following inclusion in the study, participants received 1.2 g of elemental calcium as calcium carbonate daily or a matching placebo. The current study included participants from the CAIFOS study who completed a food frequency questionnaire at baseline (1998), which assessed apple and other fruit intake (n = 1456). In 1052 of these women, abdominal aortic calcification (AAC) was also measured at baseline (1998 or 1999). Informed consent was obtained and the study was approved by the Human Ethics Committee of the University of Western Australia.
2.2. Dietary Assessment
Baseline (1998) dietary intake was assessed using a validated semi-quantitative food frequency questionnaire (FFQ) developed by the Anti‑Cancer Council of Victoria [30]. The intake of energy and nutrients were estimated based on frequency of consumption and usual portion size [31]. Intakes of individual fruits were assessed using specific items on the FFQ and quantified in grams per day (g/day).
2.3. Assessment of Abdominal Aortic Calcification (AAC)
Measurements of AAC were collected during 1998 (baseline) and 1999 (Year 1). Following bone density measurement, an additional lateral spine image was captured to measure AAC. All AAC scores from 0 to 24 were derived from digitally enhanced lateral single-energy images of the thoraco-lumbar spine using a Hologic 4500A bone densitometry machine (Hologic, Bedford, MA, USA). A single experienced investigator read all images using the validated semi quantitative scoring system [32]. The AAC 24 scoring system scores aortic calcification relative to each vertebral height (L1–L4) and is scored as; 0 (no calcification), 1 (≤1/3 of the aortic wall), 2 (>1/3 to ≤2/3 of the aortic wall) or 3 (>2/3 of the aortic wall) for both the anterior and posterior aortic walls giving a maximum possible score of up to 24. Severity of AAC was then categorized using previously published groupings [33]: low (AAC score 0 or 1); moderate (AAC score 2–5); and severe (AAC score >5) by an experienced assessor.
2.4. Covariates
Baseline questionnaires were used to determine values for potential confounding variables including age, BMI, energy intake, energy expended in physical activity, alcohol consumption, previous type 2 diabetes, use of antihypertensive medication, use of cholesterol-lowering medication (statins), and current or previous smoking. Previous atherosclerotic vascular disease was determined from primary discharge diagnoses from hospital records (1980–1998) as described previously [34].
The participants provided their previous medical history and current medications verified by their General Practitioner. These data were coded using the International Classification of Primary Care-Plus (ICPC-Plus) method. This coding methodology allows aggregation of different terms for similar pathologic entities as defined by the International Classification of Disease coding system. These data were then used to determine the presence of pre-existing diabetes (T89001-90009). Smoking status was coded as non-smoker or ex-smoker/current smoker (there were only 3 current smokers) if a participant had ever consumed more than 1 cigarette per day for more than 3 months during the past.
Weight was assessed using digital scales with participants wearing light clothes and no shoes. Height was assessed using a stadiometer and the body mass index was calculated in kg/m2 at baseline.
For physical activity, the women filled in a validated questionnaire that allowed estimation of energy used during exercise in kJ/day with the use of published energy costs of specific activities [35,36]. The women were asked whether they participated in any sports, recreation, or regular physical activity. Women who answered “no” to this question scored zero, and women who answered “yes” were asked to list up to 4 sports, recreational activities, or forms of regular physical activity, including walking, which were undertaken in the past 3 months. Energy expenditure (in kJ/day) for these activities was calculated with the use of published energy costs. This measure of energy expenditure is associated with bone density [37]. Total energy and alcohol intake, nutrient intakes and flavonoid intakes were estimated from the FFQ.
2.5. Statistical Analysis
Analyses were undertaken using IBM SPSS Statistics version 21 (2012, IBM Corp, Armonk, NY, USA). Statistical significance was set at p < 0.05 for all tests. Descriptive data are presented as mean ± SD for continuous variables, as number (n) and percentage (%) for categorical variables and as median (x̃) (interquartile range (IQR)) for variables not normally distributed. As AAC and total fruit intake were not normally distributed, we used a non-parametric correlation test (Spearman’s correlation) to assess univariate associations between AAC, fruit intake (g/day) and demographic variables; the results are presented as (Spearman’s rho (ρ), p-value). To assess the differences in median values of AAC, and total fruit intake between groups based on binary clinical measures (e.g., atherosclerosis) we used a non-parametric t-test (Mann Whitney U test). We compared AAC scores across total fruit intake tertiles and apple intake tertiles using univariate ANCOVA, with Bonferroni adjustment for multiple comparisons. AAC was then split into two groups: not severe (0–5) and severe (>5) and logistic regression analysis was used to assess the OR (odds ratio) and 95% confidence interval for severe AAC for each standard deviation (SD) increase in fruit intake. Two models of adjustment were used for the univariate ANCOVA and logistic regression analyses: age-adjusted and multivariable-adjusted (age, BMI, energy intake, energy expended in physical activity, alcohol consumption, prevalent atherosclerotic vascular disease, previous type 2 diabetes, use of antihypertensive medication, use of cholesterol-lowering medication (statins), and current or previous smoking). We also assessed associations between AAC and fruit intake with additional adjustment for total vegetable intake and saturated fat intake, as a diet rich in fruit is associated with higher vegetable consumption [38] and lower consumption of saturated fat-rich food [39]. In addition, the potential mediating effects of dietary factors found in fruit that are linked to chronic disease protection was investigated using further adjustment for intakes of flavonoids, total dietary fibre, potassium, magnesium, and vitamin C. The OR and 95% confidence interval of severe abdominal aortic calcification for each SD increase in apple intake (50 g/day) was then stratified by BMI, health status and the use of medications. To assess if relationships of apple intake with AAC differed according to these parameters an interaction term was also included in the models.
3. Results
The baseline characteristics of the participants are shown in Table 1. At baseline, AAC score was positively correlated with age (ρ = 0.066, p = 0.032) and alcohol intake (ρ = 0.078, p = 0.011) and was significantly higher in women with prevalent atherosclerotic cardiovascular disease (2 (0–4) vs. 3 (1–6), p < 0.001)), taking antihypertensive medication (2 (0–4) vs. 2 (0–5), p = 0.011)), taking cholesterol-lowering medication (2 (0–4) vs. 3 (1–6), p < 0.001)) and in previous or current smokers (2 (0–4) vs. 3 (1–5), p < 0.001)). At baseline, total fruit intake (g/day) was positively correlated with energy intake (ρ = 0.23, p < 0.001) and physical activity (ρ = 0.087, p < 0.001), and negatively correlated with alcohol intake (ρ = −0.098, p < 0.001).
Table 1.
Baseline characteristics.
| Characteristic | Mean ± SD 1 |
|---|---|
| Age (year) | 75.1 ± 2.7 |
| BMI (kg/m2) | 27.2 ± 4.7 |
| Energy intake (kJ/day) | 7244 ± 2172 |
| Physical activity (kJ/day) | 595 ± 658 |
| Alcohol (g/day) | 6.8 ± 9.9 |
| Prevalent ASVD (n (%)) | 172 (11.8) |
| Previous type 2 diabetes (n (%)) | 87 (6) |
| BP lowering medication use (n (%)) | 630 (43.3) |
| Statin use (n (%)) | 270 (18.5) |
| Smoked ever (n (%)) | 538 (37.2) |
| AAC score ( x̃ (IQ range)) | 2 (4) |
| Fruit intake | |
| Total ( x̃ (IQ range) g/day) | 230.9 (205.5) |
| Apple ( x̃ (IQ range) g/day) | 31.8 (60.3) |
| Pear ( x̃ (IQ range) g/day) | 11.3 (33.1) |
| Orange ( x̃ (IQ range) g/day) | 35.8 (80.8) |
| Banana ( x̃ (IQ range) g/day) | 44.6 (53.7) |
ASVD, atherosclerotic vascular disease; AAC, abdominal aortic calcification; BP, blood pressure; IQ, interquartile. 1 Values presented as mean ± SD unless otherwise stated
The univariate analysis of AAC score with total fruit and individual fruits are presented in Table 2. The AAC score was significantly negatively correlated with apple intake, but not with total fruit intake or other specific fruits. Total fruit and apple intake were then divided into tertiles and the difference in AAC score across the groups was explored using ANCOVA. In multivariable adjusted models AAC score was 4.16 ± 0.27, 3.98 ± 0.28 and 3.54 ± 0.28, for apple intake tertiles 1 (0–15 g/day), 2 (16–54 g/day) and 3 (>55 g/day), respectively (p = 0.04). Additional adjustment for total flavonoids, fibre, potassium, magnesium, vitamin C, total vegetable and saturated fat intake only marginally changed the observed association between apple intake and AAC24 score (p = 0.05). The AAC score was not significantly different across tertiles of total fruit or other individual fruit intakes (p > 0.20).
Table 2.
Spearman’s Correlation of Abdominal Aortic Calcification (AAC) scores at baseline with total fruit and individual fruit (apples, pears, oranges and bananas) consumption at baseline (g/day).
| AAC Score | Correlations | ||||
|---|---|---|---|---|---|
| Total fruit | Apples | Pears | Oranges | Bananas | |
| Spearman’s rho (ρ) | −0.061 | −0.089 | −0.016 | −0.012 | −0.035 |
| p-Value | 0.05 | <0.01 | 0.60 | 0.70 | 0.25 |
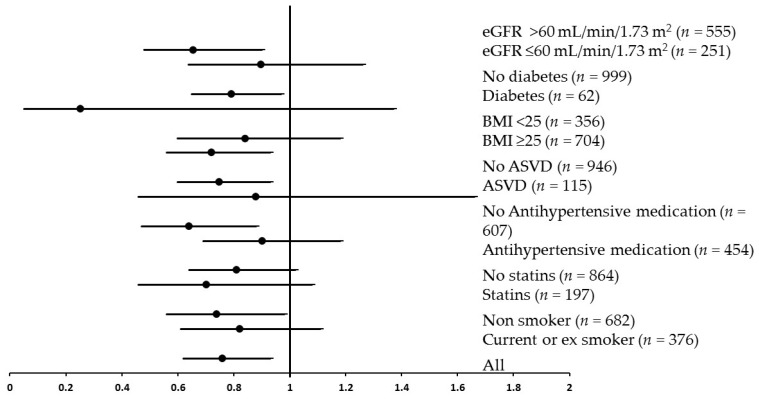
In order to further explore the relationship of fruit intake with the risk of severe calcification in the abdominal aorta, the women classified as having severe calcification (AAC > 5; n = 196) were compared to the women without severe calcification (AAC = 0–5; n = 865; Figure 1). In age-adjusted models, each SD increase in apple intake (50 g/day; approximately half of a small apple) was associated with a 26% lower odds of having severe AAC (OR: 0.74 (0.61, 0.90), p = 0.003). In multivariable-adjusted models, each SD increase in apple intake was associated with a 24% lower odds of having severe AAC (OR: 0.76 (0.62, 0.93), p = 0.009; Figure 1). There was no attenuation of this relationship after adjustment for total flavonoids, fibre, potassium, magnesium, vitamin C, total vegetable and saturated fat intake (OR: 0.70 (0.55, 0.91), p = 0.008). Total fruit intake and other individual fruits were not associated with increased odds of having severe AAC in age-adjusted or multivariable adjusted models (Figure 1). The OR for severe AAC in relation to apple intake was then stratified according to BMI, health status and the use of medications (Figure 2). There was no significant interaction of apple intake with any of the strata for these parameters in predicting severe AAC (p > 0.05).
Figure 1.
The odds ratio and 95% confidence interval of severe abdominal aortic calcification for each SD increase in total fruit, apple, pear, banana and orange intake. Multivariable adjusted for age, BMI, energy intake, energy expended in physical activity, alcohol consumption, prevalent atherosclerotic vascular disease, previous type 2 diabetes, use of antihypertensive medication, use of cholesterol-lowering medication (statins), and smoking status.
Figure 2.
The odds ratio and 95% confidence interval of severe abdominal aortic calcification for each SD increase in apple intake (50 g/day) stratified by estimated glomerular filtration rate (eGFR) diabetes, BMI, prevalent atherosclerotic vascular disease (ASVD), use of antihypertensive medication, use of statins and smoking status. Multivariable adjusted for age, BMI, energy intake, energy expended in physical activity, alcohol consumption, prevalent atherosclerotic vascular disease, previous type 2 diabetes, use of antihypertensive medication, use of cholesterol-lowering medication (statins), and smoking status. There was no significant interaction of apple intake with any of the strata for these parameters in predicting severe AAC (p > 0.05).
4. Discussion
The population included in this study were women over the age of 70. Although pre-menopausal women experience a lower rate of cardiovascular disease in comparison to men, after menopause the risk of CVD increases significantly [40]. For this reason it is important to investigate modifiable risk factors for CVD in this population, and their relationship with sub-clinical atherosclerosis, which is a major underlying contributor to CVD events. Higher fruit intake is consistently associated with lower risk for all-cause and disease-specific mortality in observational cohort studies [5]. It is also associated with a reduced risk of ischemic heart disease and stroke incidence [4].
We found that calcification of the abdominal aorta was negatively associated with apple intake, but not with the intake of pears, oranges and other citrus or bananas. A 1 SD (50 g/day) increase in apple intake was associated with 24% lower odds of having severe AAC. An apple usually weighs between 100 and 150 grams. These relationships were sufficiently robust to remain significant in multivariable-adjusted models that included factors known to be associated with a healthier diet, as well as nutrients in apples that may have potentially mediated the effects.
Abdominal aortic calcification is remarkably common, particularly in the elderly and those with chronic kidney disease [41,42]. Unlike coronary calcification, it can be made up of medial calcification, intimal calcification or a mix of both [43]. Using a simple scoring system for AAC, a relationship has been shown between the presence of calcific deposits in the abdominal aorta and increased CVD risk [44,45]. In our cohort, AAC was positively associated with age and alcohol intake and was higher in those with a history of atherosclerotic vascular disease, those taking antihypertensive medication or cholesterol lowering medication and in current and previous smokers. This was expected as elevated cholesterol, hypertension, aging and smoking are amongst the most widely accepted risk factors for atherosclerosis [46,47,48]. In addition, an increase in coronary artery calcification (CAC) has been observed with high dose and long-term statin therapy, but this CAC progression is likely to represent plaque repair rather than continuing plaque development [49].
Apple consumption has been related to benefits on lipid metabolism [50,51], blood pressure and vascular function [52], inflammation [50,53], oxidative stress [54,55] and type 2 diabetes [16,56]. These effects may be moderated by the microflora in the intestines [57]. It has been suggested that the beneficial effects of a diet rich in fruits can be attributed, in part, to flavonoids [16]. Apples, being one of the most ubiquitous fruits consumed worldwide, are often an important contributor to total flavonoid intake. In an acute, human intervention study, a flavonoid-rich apple blend improved measurements of endothelium-dependent vasodilation and decreased systolic blood pressure compared to a low flavonoid apple control [52], supporting the critical role of flavonoids in mediating the protective effects of apples. The main flavonoids found in apples are quercetin, phloridzin, chlorogenic acid and epicatechin [58]. Quercetin has been shown to reduce blood pressure in hypertensives [59] and there is evidence for its beneficial effects on atherosclerosis [60,61]. In the Finnish Mobile Clinic Health Examination Survey, it was found that high quercetin intake was associated with lower mortality from ischaemic heart disease, with much of the quercetin coming from apples [16]. In the current cohort of elderly women we have shown that quercetin intake was associated with a 40%–50% lower risk of atherosclerotic vascular disease mortality [62]. Indirect evidence also supports beneficial effects of other flavonoids found in apples in impeding the spread of inflammation [63]. Phloridzin prevents stimulated expression of endothelial adhesion molecules and reduces platelet aggregation [64], quercetin [59] and chlorogenic acid [65] prevent oxidation of LDL (low-density lipoprotein), and epicatechin can attenuate the development of atherosclerosis [66].
Other components of apple might also attenuate the development of atherosclerosis. In a recent study by Zhao et al., following the consumption of one apple/day for four weeks, plasma concentrations of oxidized LDL/beta2-glycoprotein I complex (oxLDL-β2GPI), a proposed contributor to atherosclerosis, were decreased considerably [67]. A smaller benefit was seen with capsules of apple polyphenol extract [67], indicating that the beneficial effects of apples are due to factors other than polyphenols. In a study by Ravn-Haren et al., decreases in serum total- and LDL-cholesterol were only observed after intervention with whole apple, apple pomace or cloudy apple juice compared to clear apple juice [68]. They conclude that the beneficial effects of apples as a whole could come from a synergistic effect between apple polyphenols and fibre, an interaction which may be mediated by the gut microflora [57]. Indeed higher fibre intake, particularly fruit fibre, has been associated with lower risk of cardiovascular disease [69,70,71]. Pectin, the most abundant fibre in apples, has been shown to reduce circulating cholesterol concentrations [72]. Additionally, there is evidence that magnesium [73], potassium [74] and vitamin C [75], all contribute to the beneficial effects of fruit against CVD. In the current study adjustment for these nutrients alongside fibre and flavonoids had little impact on multivariate adjusted effects indicating that a combination of components may be involved.
Limitations
The observational cross-sectional nature of this study is a limitation as it only allows for associations, rather than cause and effect, to be tested. In this study abdominal aortic calcification was assessed using a bone densitometer which is comparable to standard radiographs but is less sensitive than CT based assessment of AAC. Despite lower sensitivity, there is good agreement between the methods [76]. We were unable to distinguish between intimal and medial aortic calcification, with the latter occurring independently of atherosclerosis [43]. Although baseline cardiovascular and dietary confounders were adjusted for in the statistical models, residual confounding cannot be overlooked. Additionally, it is important to note that lifestyle behaviours tend to cluster; a higher intake of fruit is also associated with a better overall diet, not smoking, increased levels of physical activity, and being of a higher socioeconomic status. Furthermore, the use of a food frequency questionnaire can lead to an inaccurate representation of flavonoid intake due to recall bias and the fact that not all flavonoid sources are included in the questionnaire. Although the questionnaire approach has been vastly improved by the use of new databases of flavonoid composition, there is a large variation in flavonoid content between foods of the same type due to factors such as variety, storage and cooking. However, this method remains the best available for assessment of long-term habitual flavonoid intakes. The findings of this study are also limited to elderly women and therefore caution should be taken in generalizing the results to other populations.
5. Conclusions
In an analysis of a cohort of 1052 women over the age of 70 years, apple intake was dose-dependently associated with the severity of AAC. Furthermore, after multivariable adjustments for health and lifestyle factors, we found that for each 50-gram increase in apple intake per day, the odds of severe AAC decreased by 24%. It appears as though there is some truth behind the 19th century health promotion message “an apple a day keeps the doctor away”.
Acknowledgments
The authors wish to thank the staff at the Data Linkage Branch, Hospital Morbidity Data Collection and Registry of Births, Deaths and Marriages for their work on providing data for this study. The study was supported by Kidney Health Australia grant S07 10, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant and by project grants 254627, 303169 and 572604 from the National Health and Medical Research Council of Australia. Hologic Inc. provided the software for JTS for image review. The salary of JRL is supported by a National Health and Medical Research Council of Australia Career Development Fellowship. The salary of JMH is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship. The salary of NCW was supported by a MRF/UWA Postdoctoral Fellowship. DPK’s time was supported by a grant from the National Institute of Arthritis, Musculoskeletal and Skin Diseases (R01 AR 41398). None of the funding agencies had any role in the conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Contributions
N.P.B., J.R.L., R.L.P., W.H.L., G.W., C.P.B., N.C.W., J.M.H. were responsible for the project conception; J.R.L., R.L.P. collected the data; N.P.B., J.R.L., R.L.P., W.H.L., G.W., J.T.S., R.J.W., D.P.K., C.P.B., N.C.W., K.D.C., J.M.H. developed the research plan; N.P.B., J.R.L., R.J.W., J.M.H. analyzed the data; N.P.B., J.M.H. prepared the manuscript; J.R.L., R.L.P., W.H.L., G.W., J.T.S., R.J.W., D.P.K., C.P.B., N.C.W., K.D.C. critically reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Appel L.J., Moore T.J., Obarzanek E., Vollmer W.M., Svetkey L.P., Sacks F.M., Bray G.A., Vogt T.M., Cutler J.A., Windhauser M.M., et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 2.Joshipura K.J., Hu F.B., Manson J.E., Stampfer M.J., Rimm E.B., Speizer F.E., Colditz G., Ascherio A., Rosner B., Spiegelman D., et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 3.Hung H.-C., Joshipura K.J., Jiang R., Hu F.B., Hunter D., Smith-Warner S.A., Colditz G.A., Rosner B., Spiegelman D., Willett W.C. Fruit and vegetable intake and risk of major chronic disease. J. Natl. Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 4.Bazzano L.A., He J., Ogden L.G., Loria C.M., Vupputuri S., Myers L., Whelton P.K. Fruit and vegetable intake and risk of cardiovascular disease in US adults: The first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Am. J. Clin. Nutr. 2002;76:93–99. doi: 10.1093/ajcn/76.1.93. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Ouyang Y., Liu J., Zhu M., Zhao G., Bao W., Hu F.B. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ezzati M., Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N. Engl. J. Med. 2013;369:954–964. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 7.Knekt P., Jarvinen R., Reunanen A., Maatela J. Flavonoid intake and coronary mortality in Finland: A cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hertog M.G., Feskens E.J., Kromhout D., Hertog M., Hollman P., Katan M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- 9.Griep L.M.O., Verschuren W.M., Kromhout D., Ocké M.C., Geleijnse J.M. Colors of fruit and vegetables and 10-year incidence of stroke. Stroke. 2011;42:3190–3195. doi: 10.1161/STROKEAHA.110.611152. [DOI] [PubMed] [Google Scholar]
- 10.Takachi R., Inoue M., Ishihara J., Kurahashi N., Iwasaki M., Sasazuki S., Iso H., Tsubono Y., Tsugane S. Fruit and vegetable intake and risk of total cancer and cardiovascular disease Japan public health center-based prospective study. Am. J. Epidemiol. 2008;167:59–70. doi: 10.1093/aje/kwm263. [DOI] [PubMed] [Google Scholar]
- 11.Larsson S.C., Virtamo J., Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: A prospective study. Atherosclerosis. 2013;227:147–152. doi: 10.1016/j.atherosclerosis.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Johnsen S.P., Overvad K., Stripp C., Tjønneland A., Husted S.E., Sørensen H.T. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am. J. Clin. Nutr. 2003;78:57–64. doi: 10.1093/ajcn/78.1.57. [DOI] [PubMed] [Google Scholar]
- 13.Jensen E.N., Buch-Andersen T., Ravn-Haren G., Dragsted L.O. Mini-review: The effects of apples on plasma cholesterol levels and cardiovascular risk—A review of the evidence. J. Horticult. Sci. Biotechnol. 2009:34–41. [Google Scholar]
- 14.Figuerola F., Hurtado M.A.L., Estévez A.M., Chiffelle I., Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. doi: 10.1016/j.foodchem.2004.04.036. [DOI] [Google Scholar]
- 15.Mink P.J., Scrafford C.G., Barraj L.M., Harnack L., Hong C.-P., Nettleton J.A., Jacobs D.R. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 16.Knekt P., Kumpulainen J., Järvinen R., Rissanen H., Heliövaara M., Reunanen A., Hakulinen T., Aromaa A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 17.McCullough M.L., Peterson J.J., Patel R., Jacques P.F., Shah R., Dwyer J.T. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am. J. Clin. Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Justesen U., Knuthsen P., Leth T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A. 1998;799:101–110. doi: 10.1016/S0021-9673(97)01061-3. [DOI] [PubMed] [Google Scholar]
- 19.Harnly J.M., Doherty R.F., Beecher G.R., Holden J.M., Haytowitz D.B., Bhagwat S., Gebhardt S. Flavonoid content of US fruits, vegetables, and nuts. J. Agric. Food Chem. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- 20.Grigelmo-Miguel N., Martín-Belloso O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT Food Sci. Technol. 1999;32:503–508. doi: 10.1006/fstl.1999.0587. [DOI] [Google Scholar]
- 21.Dauchet L., Amouyel P., Hercberg S., Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006;136:2588–2593. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 22.He F., Nowson C., Lucas M., MacGregor G. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: Meta-analysis of cohort studies. J. Hum. Hypertens. 2007;21:717–728. doi: 10.1038/sj.jhh.1002212. [DOI] [PubMed] [Google Scholar]
- 23.Lewis J.R., Schousboe J.T., Lim W.H., Wong G., Zhu K., Lim E.M., Wilson K.E., Thompson P.L., Kiel D.P., Prince R.L. Abdominal Aortic Calcification Identified on Lateral Spine Images From Bone Densitometers Are a Marker of Generalized Atherosclerosis in Elderly Women. Arterioscler. Thromb. Vasc. Biol. 2016;36:166–173. doi: 10.1161/ATVBAHA.115.306383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oei H.-H.S., Vliegenthart R., Hak A.E., del Sol A.I., Hofman A., Oudkerk M., Witteman J.C. The association between coronary calcification assessed by electron beam computed tomography and measures of extracoronary atherosclerosis: The Rotterdam Coronary Calcification Study. J. Am. Coll. Cardiol. 2002;39:1745–1751. doi: 10.1016/S0735-1097(02)01853-3. [DOI] [PubMed] [Google Scholar]
- 25.Matsushita M., Nishikimi N., Sakurai T., Nimura Y. Relationship between aortic calcification and atherosclerotic disease in patients with abdominal aortic aneurysm. Int. Angiol. 2000;19:276–279. [PubMed] [Google Scholar]
- 26.Schousboe J.T., Taylor B.C., Kiel D.P., Ensrud K.E., Wilson K.E., McCloskey E.V. Abdominal aortic calcification detected on lateral spine images from a bone densitometer predicts incident myocardial infarction or stroke in older women. J. Bone Miner. Res. 2008;23:409–416. doi: 10.1359/jbmr.071024. [DOI] [PubMed] [Google Scholar]
- 27.Criqui M.H., Denenberg J.O., McClelland R.L., Allison M.A., Ix J.H., Guerci A., Cohoon K.P., Srikanthan P., Watson K.E., Wong N.D. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014;34:1574–1579. doi: 10.1161/ATVBAHA.114.303268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prince R.L., Devine A., Dhaliwal S.S., Dick I.M. Effects of calcium supplementation on clinical fracture and bone structure: Results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch. Intern. Med. 2006;166:869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 29.Bruce D.G., Devine A., Prince R.L. Recreational physical activity levels in healthy older women: The importance of fear of falling. J. Am. Geriatr. Soc. 2002;50:84–89. doi: 10.1046/j.1532-5415.2002.50012.x. [DOI] [PubMed] [Google Scholar]
- 30.Hodge A., Patterson A.J., Brown W.J., Ireland P., Giles G. The Anti Cancer Council of Victoria FFQ: Relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust. N. Z. J. Public Health. 2000;24:576–583. doi: 10.1111/j.1467-842X.2000.tb00520.x. [DOI] [PubMed] [Google Scholar]
- 31.Ireland P., Jolley D., Giles G., O’Dea K., Powles J., Rutishauser I., Wahlqvist M.L., Williams J. Development of the Melbourne FFQ: A food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac. J. Clin. Nutr. 1994;3:19–31. [PubMed] [Google Scholar]
- 32.Kauppila L.I., Polak J.F., Cupples L.A., Hannan M.T., Kiel D.P., Wilson P.W. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: A 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/S0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 33.Wilson P.W., Kauppila L.I., O’Donnell C.J., Kiel D.P., Hannan M., Polak J.M., Cupples L.A. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.CIR.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 34.Lewis J.R., Lim W., Dhaliwal S.S., Zhu K., Lim E.M., Thompson P.L., Prince R.L. Estimated glomerular filtration rate as an independent predictor of atherosclerotic vascular disease in older women. BMC Nephrol. 2012;13:58. doi: 10.1186/1471-2369-13-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McArdle W.D., Katch F.I., Katch V.L. Exercise Physiology: Nutrition, Energy, and Human Performance. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2010. [Google Scholar]
- 36.Pollock M.L. Health and Fitness through Physical Activity. John Wiley & Sons Canada, Limited; Mississauga, ON, Canada: 1978. [Google Scholar]
- 37.Devine A., Dhaliwal S.S., Dick I.M., Bollerslev J., Prince R.L. Physical activity and calcium consumption are important determinants of lower limb bone mass in older women. J. Bone Miner. Res. 2004;19:1634–1639. doi: 10.1359/JBMR.040804. [DOI] [PubMed] [Google Scholar]
- 38.Hu F.B., Rimm E.B., Stampfer M.J., Ascherio A., Spiegelman D., Willett W.C. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am. J. Clin. Nutr. 2000;72:912–921. doi: 10.1093/ajcn/72.4.912. [DOI] [PubMed] [Google Scholar]
- 39.Tucker K.L., Hallfrisch J., Qiao N., Muller D., Andres R., Fleg J.L. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: The Baltimore Longitudinal Study of Aging. J. Nutr. 2005;135:556–561. doi: 10.1093/jn/135.3.556. [DOI] [PubMed] [Google Scholar]
- 40.Kannel W.B., Hjortland M.C., Mcnamara P.M., Gordon T. Menopause and risk of cardiovascular disease: The Framingham study. Ann. Intern. Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- 41.Guérin A.P., London G.M., Marchais S.J., Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol. Dial. Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 42.Goodman W.G., London G., Group V.C.W., Amann K., Block G.A., Giachelli C., Hruska K.A., Ketteler M., Levin A., Massy Z. Vascular calcification in chronic kidney disease. Am. J. Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Jayalath R., Mangan S., Golledge J. Aortic calcification. Eur. J. Vasc. Endovasc. Surg. 2005;30:476–488. doi: 10.1016/j.ejvs.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Witteman J.M., Kok F., van Saase J.C., Valkenburg H. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;328:1120–1122. doi: 10.1016/S0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 45.Witteman J.C., Kannel W.B., Wolf P.A., Grobbee D.E., Hofman A., D’Agostino R.B., Cobb J.C. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am. J. Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-U. [DOI] [PubMed] [Google Scholar]
- 46.Mensah G.A., Ryan U.S., Hooper W.C., Engelgau M.M., Callow A.D., Kapuku G.K., Mantovani A. Vascular endothelium summary statement II: Cardiovascular disease prevention and control. Vasc. Pharmacol. 2007;46:318–320. doi: 10.1016/j.vph.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Kannel W.B., Cupples A. Epidemiology and risk profile of cardiac failure. Cardiovasc. Drugs Ther. 1988;2:387–395. doi: 10.1007/BF00633418. [DOI] [PubMed] [Google Scholar]
- 48.Bansal S., Buring J.E., Rifai N., Mora S., Sacks F.M., Ridker P.M. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 49.Henein M., Granåsen G., Wiklund U., Schmermund A., Guerci A., Erbel R., Raggi P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015;184:581–586. doi: 10.1016/j.ijcard.2015.02.072. [DOI] [PubMed] [Google Scholar]
- 50.Chai S.C., Hooshmand S., Saadat R.L., Payton M.E., Brummel-Smith K., Arjmandi B.H. Daily Apple versus Dried Plum: Impact on Cardiovascular Disease Risk Factors in Postmenopausal Women. J. Acad. Nutr. Diet. 2012;112:1158–1168. doi: 10.1016/j.jand.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Nagasako-Akazome Y., Kanda T., Ikeda M., Shimasaki H. Serum cholesterol-lowering effect of apple polyphenols in healthy subjects. J. Oleo Sci. 2005;54:143–151. doi: 10.5650/jos.54.143. [DOI] [Google Scholar]
- 52.Bondonno C.P., Yang X., Croft K.D., Considine M.J., Ward N.C., Rich L., Puddey I.B., Swinny E., Mubarak A., Hodgson J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012;52:95–102. doi: 10.1016/j.freeradbiomed.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 53.Chun O.K., Chung S.-.J., Claycombe K.J., Song W.O. Serum C-reactive protein concentrations are inversely associated with dietary flavonoid intake in US adults. J. Nutr. 2008;138:753–760. doi: 10.1093/jn/138.4.753. [DOI] [PubMed] [Google Scholar]
- 54.Wojdyło A., Oszmiański J., Laskowski P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. J. Agric. Food Chem. 2008;56:6520–6530. doi: 10.1021/jf800510j. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe K., Wu X., Liu R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 56.Song Y., Manson J.E., Buring J.E., Sesso H.D., Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: A prospective study and cross-sectional analysis. J. Am. Coll. Nutr. 2005;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 57.Koutsos A., Tuohy K.M., Lovegrove J.A. Apples and Cardiovascular Health—Is the Gut Microbiota a Core Consideration? Nutrients. 2015;7:3959–3998. doi: 10.3390/nu7063959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schieber A., Keller P., Carle R. Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J. Chromatogr. A. 2001;910:265–273. doi: 10.1016/S0021-9673(00)01217-6. [DOI] [PubMed] [Google Scholar]
- 59.Egert S., Bosy-Westphal A., Seiberl J., Kürbitz C., Settler U., Plachta-Danielzik S., Wagner A.E., Frank J., Schrezenmeir J., Rimbach G., et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009;102:1065–1074. doi: 10.1017/S0007114509359127. [DOI] [PubMed] [Google Scholar]
- 60.Ishisaka A., Kawabata K., Miki S., Shiba Y., Minekawa S., Nishikawa T., Mukai R., Terao J., Kawai Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS ONE. 2013;8:159. doi: 10.1371/journal.pone.0080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bondonno N.P., Bondonno C.P., Hodgson J.M., Ward N.C., Croft K.D. The Efficacy of Quercetin in Cardiovascular Health. Curr. Nutr. Rep. 2015;4:290–303. doi: 10.1007/s13668-015-0137-3. [DOI] [Google Scholar]
- 62.Ivey K.L., Lewis J.R., Prince R.L., Hodgson J.M. Tea and non-tea flavonol intakes in relation to atherosclerotic vascular disease mortality in older women. Br. J. Nutr. 2013;110:1648–1655. doi: 10.1017/S0007114513000780. [DOI] [PubMed] [Google Scholar]
- 63.Abedin M., Tintut Y., Demer L.L. Vascular calcification mechanisms and clinical ramifications. Arterioscler. Thromb. Vasc. Biol. 2004;24:1161–1170. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 64.Stangl V., Lorenz M., Ludwig A., Grimbo N., Guether C., Sanad W., Ziemer S., Martus P., Baumann G., Stangl K. The flavonoid phloretin suppresses stimulated expression of endothelial adhesion molecules and reduces activation of human platelets. J. Nutr. 2005;135:172–178. doi: 10.1093/jn/135.2.172. [DOI] [PubMed] [Google Scholar]
- 65.Bonita J.S., Mandarano M., Shuta D., Vinson J. Coffee and cardiovascular disease: In vitro, cellular, animal, and human studies. Pharmacol. Res. 2007;55:187–198. doi: 10.1016/j.phrs.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 66.Morrison M., van der Heijden R., Heeringa P., Kaijzel E., Verschuren L., Blomhoff R., Kooistra T., Kleemann R. Epicatechin attenuates atherosclerosis and exerts anti-inflammatory effects on diet-induced human-CRP and NFκB in vivo. Atherosclerosis. 2014;233:149–156. doi: 10.1016/j.atherosclerosis.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 67.Zhao S., Bomser J., Joseph E.L., DiSilvestro R.A. Intakes of apples or apple polyphenols decease plasma values for oxidized low-density lipoprotein/beta 2-glycoprotein I complex. J. Funct. Foods. 2013;5:493–497. doi: 10.1016/j.jff.2012.08.010. [DOI] [Google Scholar]
- 68.Ravn-Haren G., Dragsted L.O., Buch-Andersen T., Jensen E.N., Jensen R.I., Németh-Balogh M., Paulovicsová B., Bergström A., Wilcks A., Licht T.R., et al. Intake of whole apples or clear apple juice has contrasting effects on plasma lipids in healthy volunteers. Eur. J. Nutr. 2013;52:1875–1889. doi: 10.1007/s00394-012-0489-z. [DOI] [PubMed] [Google Scholar]
- 69.Threapleton D.E., Greenwood D.C., Evans C.E., Cleghorn C.L., Nykjaer C., Woodhead C., Cade J.E., Gale C.P., Burley V.J. Dietary fibre intake and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ. 2013;347:f6879. doi: 10.1136/bmj.f6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Streppel M.T., Ocké M.C., Boshuizen H.C., Kok F.J., Kromhout D. Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 year: The Zutphen Study. Am. J. Clin. Nutr. 2008;88:1119–1125. doi: 10.1093/ajcn/88.4.1119. [DOI] [PubMed] [Google Scholar]
- 71.Aune D., Chan D.S., Lau R., Vieira R., Greenwood D.C., Kampman E., Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theuwissen E., Mensink R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008;94:285–292. doi: 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 73.Leone N., Courbon D., Ducimetiere P., Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 74.Yang Q., Liu T., Kuklina E.V., Flanders W.D., Hong Y., Gillespie C., Chang M.-.H., Gwinn M., Dowling N., Khoury M.J., et al. Sodium and potassium intake and mortality among US adults: Prospective data from the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2011;171:1183–1191. doi: 10.1001/archinternmed.2011.257. [DOI] [PubMed] [Google Scholar]
- 75.Kobylecki C.J., Afzal S., Smith G.D., Nordestgaard B.G. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: A Mendelian randomization study. Am. J. Clin. Nutr. 2015;101:1135–1143. doi: 10.3945/ajcn.114.104497. [DOI] [PubMed] [Google Scholar]
- 76.Toussaint N.D., Lau K.K., Strauss B.J., Polkinghorne K.R., Kerr P.G. Determination and validation of aortic calcification measurement from lateral bone densitometry in dialysis patients. Clin. J. Am. Soc. Nephrol. 2009;4:119–127. doi: 10.2215/CJN.03410708. [DOI] [PMC free article] [PubMed] [Google Scholar]