Abstract
The development and the function of central nervous system depend on thyroid hormones. In humans, the lack of thyroid hormones causes cretinism, a syndrome of severe mental deficiency. It is assumed that thyroid hormones affect the normal development and function of the brain by activating or suppressing target gene expression because several genes expressed in the brain have been shown to be under thyroid hormone control. Among these, the Rhes gene, encoding a small GTP-binding protein, is predominantly expressed in the striatal region of the brain. To clarify the role of Rhes in vivo, we disrupted the Rhes gene by homologous recombination in embryonic stem cells and generated mice homozygous for the Rhes null mutation (Rhes−/−). Rhes−/− mice were viable but weighed less than wild-type mice. Furthermore, they showed behavioral abnormalities, displaying a gender-dependent increase in anxiety levels and a clear motor coordination deficit but no learning or memory impairment. These results suggest that Rhes disruption affects selected behavioral competencies.
The thyroid hormones thyroxine (T4) and triiodothyronine (T3) have many physiological effects. They exert their actions in all tissues examined and affect many metabolic pathways. Some of the most prominent effects of thyroid hormones occur during fetal development and in early childhood. In humans, the lack of adequate levels of thyroid hormones in the first trimester of life, such as in iodine deficiency (endemic cretinism) (8, 9), or in developmental abnormalities of the thyroid gland (congenital hypothyroidism) (22, 28, 55) results in cretinism, a syndrome of severe mental deficiency, which may be accompanied by retarded growth and/or neurological deficits, such as spastic diplegia. Many of these developmental effects are not reversed by later treatment with hormone, indicating that thyroid hormone acts in a specific developmental window. Therefore, adequate levels of thyroid hormone are required for normal central nervous system development.
To date, several specific central nervous system genes whose expression is controlled by thyroid hormone have been identified. The expression of these genes may be decreased (2, 5) or increased (1, 18) in hypothyroidism. Furthermore, the total or partial absence of thyroid hormones may also affect either mRNA stability (43, 54) or the mRNA translational process (43, 57, 60). The identification of thyroid hormone target genes in the central nervous system and the understanding of their function in central nervous system development are important to understanding the pathogenesis of neurological cretinism at the molecular level.
In order to understand the molecular basis of neurological cretinism, we studied the Rhes (Ras homolog enriched in striatum) gene (24). Rhes is predominantly expressed in the striatum, and its expression is controlled by thyroid hormones (59). Interestingly, several lines of evidence indicate that in neurological cretinism, there is damage of striatum, which determines a striatopallidal syndrome with poor motor coordination and spastic diplegia (8, 9, 39).
Rhes, composed of 266 amino acids, belongs to the RASD subfamily of the Ras-related GTP-binding protein superfamily. Rhes has 95% identity with TEM2 (58) and 62% identity with Dexras1 (37), which are other members of the RASD subfamily. Ras family proteins are molecular switches that respond to extracellular signals and regulate intracellular signal pathways controlling cell growth (40, 41), gene transcription (20, 61), mRNA stability and translation (7, 15, 52), cytoskeleton organization (33, 38), peptide trafficking (23, 46, 50), and secretion (3, 45). In the central nervous system, Ras protein controls pathways that are involved in synaptic plasticity, learning, and memory (10).
To assess the role of Rhes in mature striatum and in the pathogenesis of neurological cretinism, we generated mice carrying null mutations in the Rhes gene by a gene-targeting method. In this paper, we show that mice homozygous for the Rhes mutation are viable and fertile but smaller than wild-type mice. Furthermore, they show a gender-dependent increase in anxiety levels and a motor coordination deficit but no learning or memory impairment.
MATERIALS AND METHODS
Tissue preservation and histological analysis.
Mice were killed by CO2 asphyxiation. Brains and embryos were isolated, fixed, and embedded in paraffin as described previously (19). Brains were cut in 10-μm sections; embryos were cut in 7-μm sections. In situ hybridization was carried out on paraffin-embedded sections as described previously (19). The Rhes riboprobe was transcribed from a 372-bp AflII-PvuII genomic fragment of the 3′ untranslated region located 42 bp downstream of the Rhes stop codon. The EGFP (enhanced green fluorescent protein) riboprobe was transcribed from a 747-bp NcoI-BamHI fragment, which contains all of the EGFP coding region.
RNA analysis.
Total RNA was isolated from adult mouse tissues, staged embryos brains, and cultured cells by the guanidine hydrochloride procedure as previously described (16); 5 μg of total RNA, previously treated with RNase-free DNase I (Roche), was used for reverse transcription (RT)-PCR analysis. Reverse transcription of mRNAs was carried out with the SuperScript preamplification system for first-strand cDNA synthesis (Life Technologies). Single-stranded cDNAs in 2 μl of a 25-μl reaction mixture were amplified by PCR with Taq DNA polymerase (Roche). Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) mRNA was amplified as an internal control for the reverse transcription reaction. The oligonucleotide primers used were Rhes (5′-ACTAGTTCAGGACAGAGCTCTGAC-3′ and 5′-CAGCAGGTGTCTTTATCCAGAGTC-3′) and G3PDH (5′-TCCACCACCCTGTTGCTGTA-3′ and 5′-ACCACAGTCCATGCCATCAC-3′). For Northern blot analysis, 15 μg of total RNA was separated on a 1% formaldehyde-agarose gel, blotted onto a Hybond N nylon membrane (Amersham), and hybridized with the 372-bp AflII-PvuII genomic fragment labeled with 32P.
Rhes antibody preparation.
The sequence encoding full-length rat Rhes, included between the NdeI and EcoRV sites, was cloned in the NdeI site and filled-in BamHI site of vector pET15b; the full-length protein fused with a 6His stretch at its NH2 terminus (6H-Rhes) was expressed in Escherichia coli BL21(DE3). The protein was solubilized in 4 M urea and injected into rabbits (30). The anti-Rhes antiserum was purified by affinity chromatography as previously described (19).
Western blot analysis.
Total protein extracts from wild-type and knockout striatum and from transfected cells were prepared as previously described (34), resolved by sodium dodecyl sulfate (SDS)-4 to 15% polyacrylamide gel electrophoresis (PAGE) on a precast gel (Bio-Rad), and transferred to a polyvinylidene difluoride membrane (Millipore). As a positive control, HeLa cells were transfected with the Pb-Rhes construct, encoding Rhes protein under the control of the PGK-1 promoter. The blot was probed with a 1:20,000 dilution of polyclonal anti-Rhes antibody and developed with the ECL Plus Western blotting detection reagent (Amersham Life Science).
Generation of knockout mice.
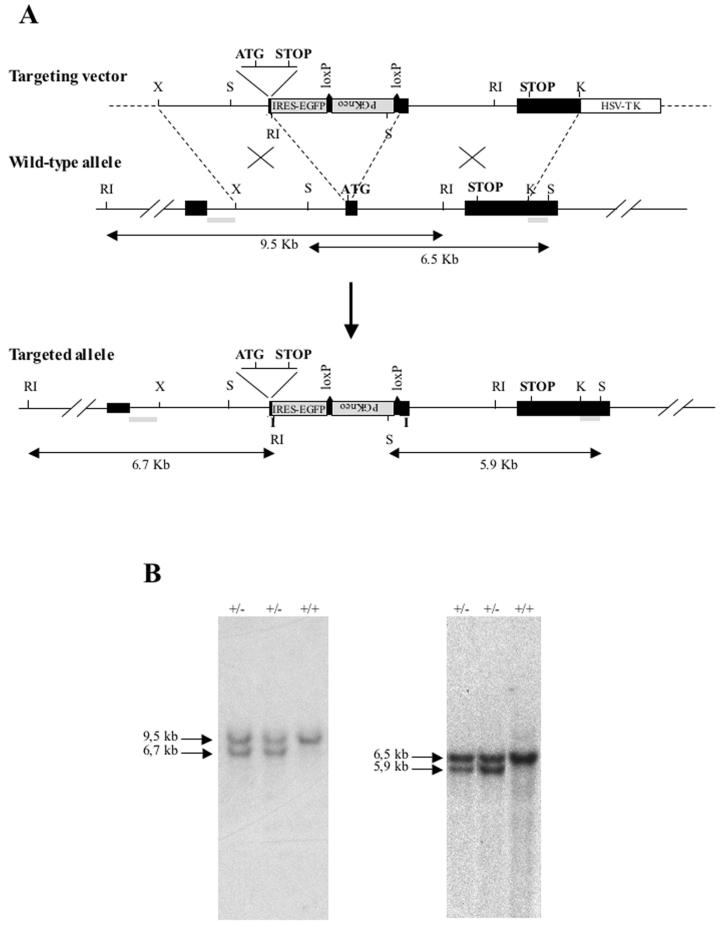
The Rhes genomic locus was isolated by PCR screening of the phage artificial chromosome library RPCI-21 (provided by the Yac Screening Center, DIBIT-HSR and IGBE-CNR, Milan, Italy). The internal ribosome entry site-EGFP cassette and the PGKneo cassette, flanked by loxP sequences, were flanked by two Rhes genomic DNA fragments: a 2.9-kb fragment including the Rhes translational start codon and a 5-kb fragment (see Fig. 3A). The 2.9-kb genomic fragment underwent a site-directed mutagenesis reaction which allowed insertion of a stop codon in each reading frame and EcoRI and XhoI restriction sites downstrea of the Rhes translational start codon. The site-directed mutagenesis reaction was performed with the QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions.
FIG. 3.
(A) Homologous recombination in ES cells. Targeting vector and wild-type and mutant Rhes allele maps are shown. Restriction enzyme sites and probes (represented as boxes located upstream and downstream of the homologous arms) are shown. The fragments obtained from wild-type and mutant allele digestions are also shown. K, KpnI; X, XbaI; S, StuI; RI, EcoRI. (B) Identification of ES cell recombinant clones by Southern blot analysis. Left panel: DNA isolated from ES cell clones was digested with EcoRI and probed with a 0.8-kb KpnI fragment (located 5′ to the genomic fragment for homologous recombination), yielding 9.5-kb and 6.7-kb bands for the wild-type and targeted alleles, respectively. Right panel: Homologous recombination was confirmed by digesting DNA from positive clones with StuI and probing it with a 0.55-kb KpnI-StuI fragment (located 3′ to the genomic fragment for homologous recombination), yielding 6.5- and 5.9-kb bands for the wild-type and targeted alleles, respectively. +/+, wild type; +/−, Rhes+/−.
The oligonucleotide primers used for the mutagenesis reaction were 5′-CTTAGCAGGCACCTCGAGTGTGGAATTCCTACTGGACTAGGTCTTCATCATG-3′ and 5′-CATGATGAAGACCTAGTCCAGTAGGAATTCCACACTCGAGGTGCCTGCTAAG-3′. The herpes simplex virus thymidine kinase cassette was positioned downstream of the 3′ homology arm. Transfection of the targeting vector and selection of the mutant embryonic stem (ES) cells (R1) were performed as described previously (21) except that 400 μg of G418 (Gibco) per ml was used. Screening of ES cell clones and genotyping of mice were carried out by Southern blot analysis. Genomic DNA samples were digested with EcoRI or StuI; the 0.8-kb KpnI and 0.55-kb KpnI-StuI fragments, located outside the homology arms, were used as probes (see Fig. 4A for probe positions and digestion product sizes). Embryo manipulations and aggregations of ES cell clones with mouse blastocysts of strain CD1 were carried out as described previously (36). Chimeric animals with a high contribution of the 129/Sv genetic background, as judged from coat color, were bred with CD1 mice. Offspring heterozygous for the disrupted Rhes gene were mated to each other to produce Rhes null mice.
FIG. 4.
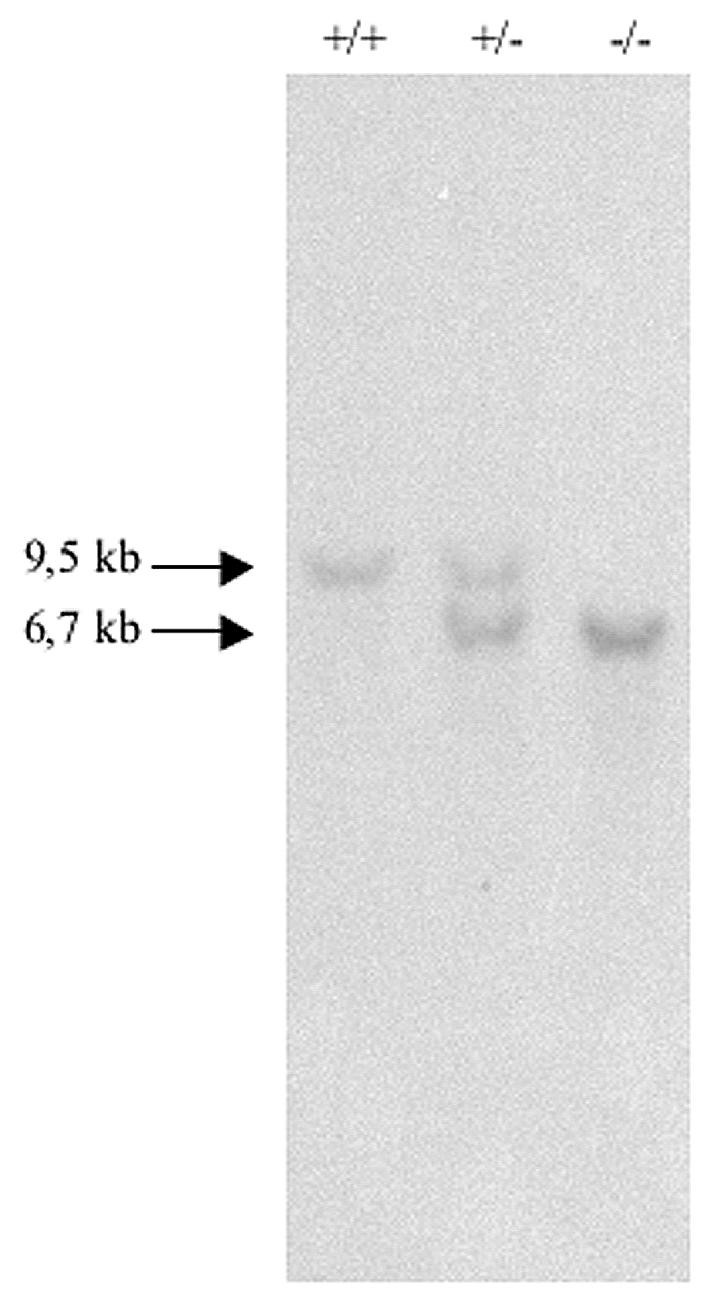
Identification of knockout mice. Southern blot analysis of offspring generated from mating of mice heterozygous for the Rhes null mutation. DNA isolated from tails was digested with EcoRI and probed with a 0.8-kb KpnI fragment, yielding 9.5- and 6.7-kb bands for the wild-type and targeted alleles, respectively. +/+, wild type; +/−, Rhes+/−; −/−, Rhes−/−.
Thyroid-stimulating hormone, glucose, and amylase measurements.
Thyroid-stimulating hormone levels in blood collected from wild-type and knockout mice on postnatal days 30 and 180 were analyzed with a rat thyroid-stimulating hormone radioimmunoassay kit (Amersham). The glucose levels in blood collected from wild-type and knockout mice on postnatal day 120 were analyzed as described previously (49). Amylase levels were analyzed as described previously (29).
Animals.
Ten adult (five males and five females) wild-type mice and 10 adult (five males and five females) knockout mice were used in all experiments. The wild-type mice were generated from crosses of the heterozygous mice to have the same genetic background as the Rhes−/− mice. The animals were housed in an air-conditioned room (temperature, 21 ± 1°C; relative humidity, 60 ± 10%) with the lights on from 2000 to 0800 h, in Plexiglas boxes (33 by 13 by 14 cm) with a metal top and sawdust as bedding. Pellet food (enriched standard diet, purchased from Mucedola, Settimo Milanese, Milan, Italy) and tap water were continuously available. Before each test, mice were individually weighed. For the Morris water maze test, they were weighed both the first day, before testing, and the last day, after testing.
Behavioral tests.
The tests below were carried out according to the references given in parentheses: open-field (14), passive avoidance (13, 17), elevated plus-maze (35, 53), rota-rod (11), and Morris water maze (12, 42).
Statistical analysis.
Analyses of variance were performed on body weight, duration, and frequency data in each behavioral category measured in the open-field and elevated plus-maze tests and on learning performance data for the Morris water maze. Post hoc comparisons were performed by Tukey's Honestly Significantly Different test. In the passive avoidance test, Mann-Whitney analysis was applied to evaluate the main effect of treatment and Wilcoxon analysis to evaluate the main effect of sex. In the rota-rod test, in order to assess the significance of the difference between wild-type and knockout mice, the Mann-Whitney U test was used. The main effect of speed was analyzed by the Friedman nonparametric analysis of variance, and Wilcoxon analysis was used to evaluate the main effect of sex. When no main effect of sex and/or genotype × sex interactions was found, the sex variable was not considered in the analysis.
RESULTS
Expression of Rhes during brain development and in adult tissues.
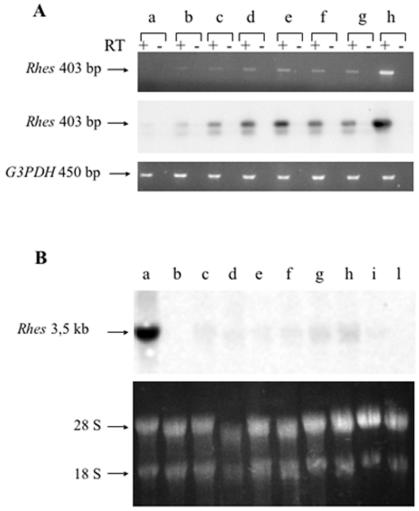
We analyzed the expression pattern of Rhes in the developing mouse brain by RT-PCR. Total RNA was extracted from the brains of CD1 staged embryos. RT-PCR analysis detected Rhes mRNA starting from embryonic day 13.5 (E13.5) (Fig. 1A). We analyzed the expression pattern of Rhes in CD1 adult mouse tissues by Northern blot analysis. Rhes was expressed at very high levels in brain and at low levels in kidney, thyroid, lung, heart, and testis (Fig. 1B). No Rhes mRNA was detected in liver. A low level of Rhes expression was also detected in the rat thyroid cell line FRTL-5 and in the mouse ES cell line R1 (44) (Fig. 1B). Since R1 cells were grown on mouse embryonic fibroblasts (MEFs), we also analyzed Rhes expression in MEF cells. The absence of the hybridization band in total RNA from MEF cells demonstrated bona fide Rhes expression in ES cells and not in the MEF layer.
FIG. 1.
Rhes expression profile during embryo development and in the adult mouse. (A) (Top panel) RT-PCR analysis performed with total RNAs prepared from E12.5 (a), E13.5 (b), E14.5 (c), E15.5 (d), E16.5 (e), E17.5 (f), and E18.5 (g) embryo brains. Total RNA from adult brain (h) was used as a positive control for PCR. The oligonucleotides were designed according to the sequence of the mouse Rhes gene and amplified a 403-bp fragment of the Rhes 3′ untranslated region. (Middle panel) Specificity of PCR products checked by hybridization with a 207-bp PstI-NcoI fragment derived from the Rhes sequence between the PCR primers. (Bottom panel) G3PDH gene amplification performed as an internal control for RT-PCR. (B) (Upper panel) Northern blot performed with total RNAs prepared from mouse adult tissues (brain [a], liver [b], kidney [c], thyroid [d], lung [e], heart [f], and testis [g]) and TL5 (h), R1 (i), and MEF (l) cells. The probe used was the 372-bp AflII-PvuII fragment of the mouse Rhes genomic locus, located 42 bp downstream of the translational stop codon. The lower panel shows the 28S and 18S rRNAs of each sample.
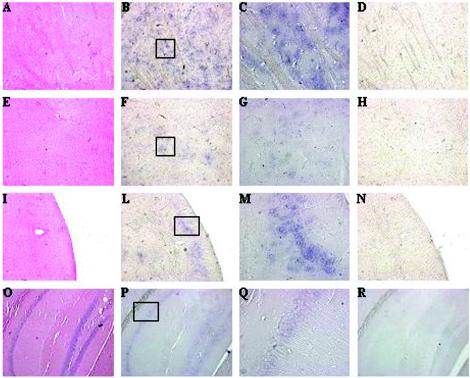
To obtain further insights into the distribution of Rhes mRNA during embryogenesis and in the adult brain, we performed in situ hybridization experiments (Fig. 2). Rhes mRNA was prominently expressed in the striatum, but it was also present in the accumbens nucleus (ventral part of striatum), in the olfactory tubercle, in the piriform cortex, and in the hippocampus dentate gyrus. No signal was detected with a Rhes sense riboprobe. Surprisingly, no signal was detected by in situ hybridization in the brain of E15.5 and E17.5 embryos, suggesting that, during embryogenesis, Rhes mRNA levels are below the limit of detection of our in situ hybridization technique.
FIG. 2.
Rhes expression in adult brain. Sagittal sections of CD1 adult mouse brain: striatum (A, B, C, and D), accumbens nucleus (E, F, G, and H), olfactory tubercle and piriform cortex (I, L, M, and N), and hippocampus (O, P, Q, and R). Sections A, E, I, and O were stained with hematoxylin and eosin; sections B, C, F, G, L, M, P, and Q were hybridized to the Rhes antisense riboprobe; sections D, H, N, and R were hybridized to the Rhes sense riboprobe. Sections C, G, M, and Q was higher magnifications of sections B, F, L, and P, respectively.
Targeting of the mouse Rhes locus.
In order to inactivate the Rhes locus, we constructed a targeting vector (Fig. 3A). The 5′ homology arm was mutagenized to insert a stop codon in each frame immediately downstream of the Rhes translational start codon. The targeting vector contained the internal ribosome entry site-EGFP cassette and PGKneo cassette flanked by loxP sequences between the homology arms (6). Furthermore, the construct was flanked by a cassette for the herpes simplex virus thymidine kinase gene for negative selection. The targeting vector was designed to abolish the synthesis of Rhes and to place the EGFP coding sequence downstream of the Rhes regulatory sequences (Fig. 3A). The targeting construct was transfected into RI ES cells, and the recombinant clones were identified by Southern blot analysis (Fig. 3B). Chimeric mice were generated with five independently targeted ES cell clones. In the progeny of one chimera, we obtained 48 ES cell-derived animals (agouti), of which 27 carried the wild-type Rhes allele and the remainder carried the targeted Rhes allele, consistent with a Mendelian pattern of transmission.
Rhes protein is not essential for normal embryo development.
To assess whether Rhes function is essential for normal development, we interbred Rhes+/− mice. The heterozygous mice were fertile; each mating was productive, and the litter sizes were indistinguishable from those obtained with wild-type mice. The genotypes of the newborns were assessed by Southern blot analysis (Fig. 4). Of 117 mice examined, 28 were wild type, 67 were heterozygous, and 22 were homozygous for the mutant allele. The ratio of the three classes of animals was not significantly different from the expected values for normal transmission of the wild-type and mutant alleles. The Rhes−/− mice were viable, showing that the Rhes gene product does not play a vital role, at least in the CD1 background. Furthermore, mice homozygous for the Rhes knockout allele mated and were fertile, and the litter sizes were indistinguishable from those of wild-type and heterozygous matings.
In situ hybridization and Western blot analysis on knockout mouse brain.
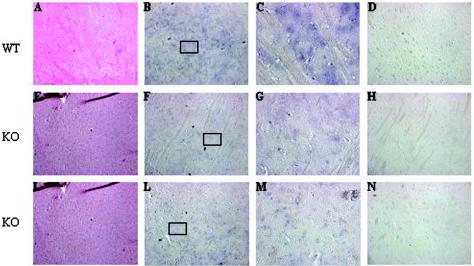
To determine Rhes expression in Rhes−/− mice, we performed in situ hybridization experiments and Western blot analyses. Since the main Rhes expression site was the striatum, we focused on this region of the brain. In situ hybridization showed that Rhes mRNA was barely detectable in the striatal region of the knockout mouse brain (Fig. 5), while it was clearly detected in wild-type animals. We also carried out an in situ hybridization experiments with an antisense riboprobe of EGFP because, in the targeting vector, the EGFP gene was controlled by the Rhes promoter. Figure 5 shows that EGFP mRNA was detected in the striatum of the Rhes−/− mouse. No signal was detected with the sense riboprobes for either Rhes or EGFP.
FIG. 5.
Rhes and EGFP expression in knockout mouse brain. The in situ hybridization experiments were carried out on sagittal sections of adult mouse brain from CD1 (wild type [WT]) (sections A, B, C, and D) and Rhes−/− (knockout [KO]) (sections E, F, G, H, I, L, M, and N) mice. Sections A, E, and I were stained with hematoxylin and eosin; sections B, C, F, and G were hybridized to the Rhes antisense riboprobe; sections D and H were hybridized to the Rhes sense riboprobe; sections L and M were hybridized to the EGFP antisense riboprobe; section N was hybridized to the EGFP sense riboprobe. Sections C, G, and M are higher magnifications of sections B, F, and L, respectively.
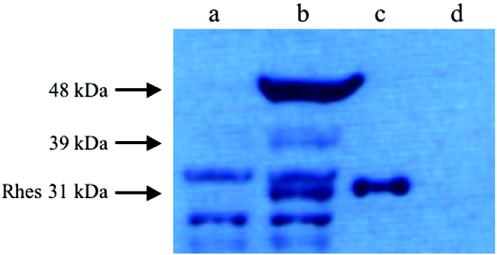
To explore the presence of the Rhes protein, we prepared a rabbit anti-Rhes polyclonal antibody. We proved the efficacy and the specificity of the antibody by Western blotting on HeLa cell extracts transfected with either a Rhes expression vector or an empty vector. Rhes protein was only detected in the cells transfected with the Rhes expression vector (Fig. 6, lane c) while no cross-reacting proteins were seen in the control extracts (Fig. 6, lane d). However, Western blotting carried out on striatum protein extracts with the same antibody revealed the presence of protein bands that were present both in wild-type (Fig. 6, lane b) and knockout (Fig. 6, lane a) animals that we interpreted as nonspecific. Three bands were present only in the wild-type mice and were completely absent in knockout extracts. The lower band displayed a mobility very similar to that of Rhes expressed in HeLa cells. The slight difference could be due to different posttranslational modifications happening in the in vitro and in vivo models. The upper bands identified proteins of higher molecular mass. Interestingly, the Rhes locus shows several transcripts, one of which (accession number BC036988) encodes a protein containing Rhes at the C terminus and extending 48 additional amino acids at the N terminus without an initiator methionine, suggesting that the actual protein could be longer, like the one(s) that we detected in the Western blot.
FIG. 6.
Expression of Rhes protein in wild-type and Rhes−/− striatum. Rhes−/− (a) and wild-type (b) striatum protein extracts (100 μg of total protein/lane) were analyzed by Western blotting with anti-Rhes polyclonal antibody; 0.4 μg of total protein from HeLa cells transfected with a Rhes construct (c) was used as the positive control; and 0.4 μg of total protein from HeLa cells transfected with the empty vector (d) was used as the negative control. Arrows indicate proteins present in wild-type and absent in knockout extracts.
Taking together the RNA and protein data on Rhes expression, we conclude that the targeted Rhes allele presented in this paper does not produce any detectable Rhes protein. We have also presented evidence of a novel Rhes-related protein whose presence was also abolished in the knockout mouse that we generated.
Rhes affects body weight.
We observed that knockout mice weighed less than wild-type mice, as clearly shown by a significant main effect of genotype [F(1, 16) = 9.247 and P = 0.0078; see Fig. 8]. Furthermore, a genotype-age interaction [F(2, 32) = 3.487 and P = 0.0427] but not a genotype-sex interaction was found. At each age analyzed (postnatal days 70, 100, and 130), a marked weight difference between wild-type and knockout mice in both males and females was revealed by post hoc analysis (P < 0.01; Fig. 7). Since Rhes is expressed in the thyroid gland and in pancreatic β-cells (14bis), we tested the thyroid-stimulating hormone, glucose, and amylase levels in age-matched homozygous knockout and wild-type mice. No statistically significant differences were found (data not shown).
FIG. 8.
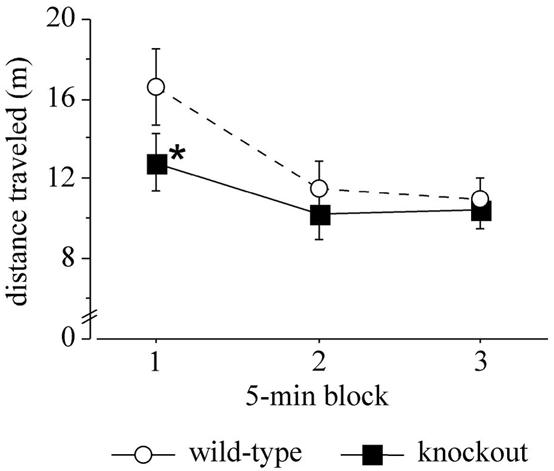
Locomotor activity in the open-field test. The distance traveled by wild-type and knockout mice is shown. Data are means ± standard error of the mean. Data for males and females were pooled (n = 10). The asterisk indicates a significant difference between wild-type and knockout mice (P < 0.05).
FIG. 7.
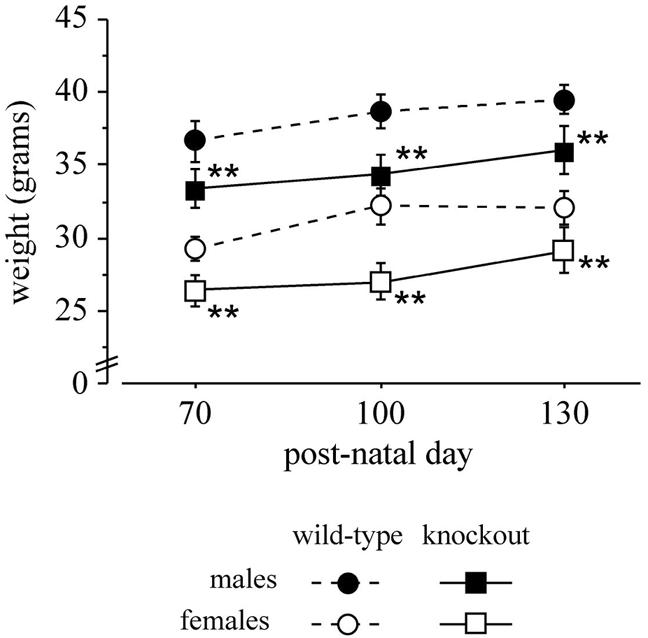
Body weights of wild-type and knockout mice measured on postnatal days 70, 100, and 130. Data are means ± standard error of the mean, n = 5. The double asterisk indicates a significant difference between wild-type and knockout mice (P < 0.01).
Behavioral analysis of Rhes−/− mice. (i) Passive avoidance and Morris water maze tests.
In both the passive avoidance and Morris water maze tests, no significant difference between wild-type and knockout mice was found (data not shown).
(ii) Open-field test.
In the first 15 min of the open-field test test, no main effect of genotype was found in distance moved, and the genotype × 5-min block interaction missed statistical significance [F(2, 36) = 2.704 and P = 0.0805; Fig. 8]. However, post hoc comparison revealed that knockout mice had an altered locomotor profile, moving less than wild-type mice during the first 5 min (post hoc, P < 0.05; Fig. 8).
(iii) Elevated plus-maze test.
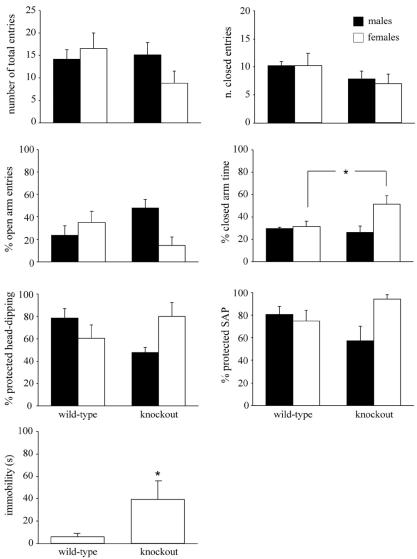
In agreement with previous studies (26, 51), wild-type males displayed higher anxiety levels than wild-type females in the elevated plus-maze test. This profile was found inverted in knockout mice, females being more anxious than males, as illustrated by a significant genotype × sex interaction observed in the following measures (Fig. 9): percent of open arm entries [F(1, 16) = 7.011 and P = 0.0175], number of open arm entries [F(1, 16) = 4.438 and P = 0.0500], percent of time spent in the open arms [F(1, 16) = 5.059 and P = 0.0389], and percent of time spent in the closed arms [F(1, 16) = 4.878 and P = 0.0421]. In the last measure, a clear difference between wild-type and knockout females was evident (post hoc, P < 0.05), indicating abnormally high anxiety levels in knockout females compared to wild-type females. Other measures confirmed increased anxiety levels in knockout mice, especially in knockout females (Fig. 9). A significant genotype-sex interaction was observed in percent of head-dipping [F(1, 16) = 6.347 and P = 0.0228] and percent of stretch attend postures [F(1, 16) = 5.998 and P = 0.0262], both performed in the protected area. Moreover, knockout mice showed less locomotion than wild-type mice, spending a long time in immobility [F(1, 16) = 4.336 and P = 0.0500]. It is worth noting that four knockout mice (two males and two females) out of 10 fell from the plus-maze during the test, while none of the wild-type mice did (P < 0.05; Fisher test).
FIG. 9.
Behavioral endpoints in the plus-maze test. The number of total and closed arm entries, the percentage of open arm entries, the percentage of closed arm time, the percentage of protected head dipping and stretch attend postures, and immobility shown by wild-type and knockout mice are indicated. Data are means ± standard error of the mean (n = 5). For immobility, data for males and females were pooled (n = 10). The asterisk indicates a significant difference between wild-type and knockout mice (P < 0.05).
(iv) Rota-rod test.
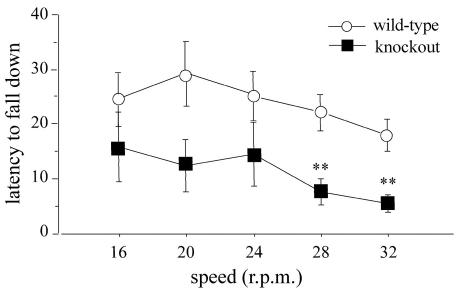
In the rota-rod test, though both genotypes displayed a similar over-trial profile, decreasing their tendencies to fall from the mast with the increase in speed (Friedman χ2 in the overall group = 17.73, P = 0.0014), knockout mice always had worse performances than wild-type mice (main effect of genotype; Mann-Whitney U = 81.5, P = 0.0172) (Fig. 10). Specifically, knockout mice fell within significantly shorter times at the two fastest speeds (post hoc, P < 0.01).
FIG. 10.
Balance and motor coordination in the rota-rod test. The time before falling at each speed level shown by wild-type and knockout mice is indicated. Data are means ± standard error of the mean. Data for males and females were pooled (n = 10). The double asterisk indicates a significant difference between wild-type and knockout mice (P < 0.01).
DISCUSSION
The mechanism responsible for impairment of brain function in thyroid hormone deficiencies is not well understood. It is known that several genes in the brain are under thyroid hormone control, but it is not clear which gene(s), when deregulated, is responsible for the phenotype observed in thyroid hormone deficiencies or how it does so. In this study we focused on the role of Rhes, a gene under thyroid hormone control that is expressed in the striatum. Rhes is a member of a new subfamily of Ras-related small GTP-binding proteins recently identified. To this end, we generated mice homozygous for the Rhes null mutation. We checked the absence of Rhes protein in the knockout mouse striatum by Western blot analysis. This analysis also showed the absence of another protein which probably originates from the Rhes locus by alternative transcription initiation and splicing.
The knockout mice were viable, and their general condition did not reveal gross abnormalities with the exception of a reduced body weight. The macroscopic analysis of adult tissues which express Rhes did not show any gross abnormalities between knockout and wild-type mice. The knockout animals mated and were fertile, and the litter sizes were indistinguishable from those of wild-type matings. No alteration of thyroid and pancreatic gland functions was observed, even though Rhes is expressed in the wild-type glands. Given that the absence of thyroid hormone determines severe damage to the developing striatum in neurological cretinism (8, 9, 39), we analyzed in detail the behavioral features of Rhes knockout mice. These mice showed a significant decrease in locomotor activity compared to wild-type mice. Interestingly, Rhes deletion influenced the anxiety response in the plus-maze test in a gender-specific manner. The impact of gender on the anxiety test has been widely studied in mice, and in the plus-maze test, females generally show lower anxiety levels than males (35, 48). In the present study, wild-type mice behaved as expected, while an opposite trend has been found in Rhes−/− mice, females showing higher anxiety levels than males in most of the endpoints considered.
The main behavioral effect of Rhes deletion was a marked impairment in motor coordination. In particular, knockout mice showed a clear impairment in the rota-rod test. This task has been proven to be very sensitive to striatum integrity (11, 25, 56) and has also been used to detect the progressive decline of striatal function in R6/2 Huntington gene transgenic mice (27, 31). Thus, the motor coordination impairment shown by Rhes knockout mice in the rota-rod test, confirmed by the number of falls in the plus-maze, is strongly concordant with the main striatal localization of the Rhes protein (24, 59).
The striatum is reportedly involved in cognitive abilities, from motor planning to reward seeking and procedural learning (4, 32). Consistent with the role played by the striatum in motor activity and learning processes, these abilities are dramatically impaired in advanced Parkinson's disease (47). Rhes knockout mice showed no learning or memory impairment in the water maze and passive avoidance tests, suggesting that Rhes protein may be involved only in selected striatal processes not influencing learning and memory. This finding indicates that mental retardation linked to hypothyroidism may be independent of alteration in Rhes levels or function.
In conclusion, the modest behavioral deficits of Rhes knockout mice indicate that Rhes is involved in selected striatal competencies, mainly locomotor activity and motor coordination, suggesting that its downregulation in hypothyroidism could be responsible only for a subset of symptoms, such as the striatopallidal syndrome (8, 9, 39).
Acknowledgments
We thank Mario De Felice for advice in generation of knockout mice and Giovanni Maraviglia for technical assistance. We also thank Tommaso Russo for glucose and amylase determination and Andreas Nagy for providing the R1 ES cell line.
This work was supported in part by Telethon grant GP0208Y01, by a grant from the Associazione Italiana per la Ricerca sul Cancro (to R.D.L.), by Ministero dell'Università e della Ricerca Scientifica e Tecnologica grant “I geni dell'uomo” cluster 01, and by Italian Ministry of Health project ALZ1 (to E.A.). A.R., M.T.P., P.M., and A.A. were supported by Biogem s.c.a.r.l., Italy.
REFERENCES
- 1.Alvarez-Dolado, M., J. M. Gonzalez-Sancho, J. Bernal, and A. Munoz. 1998. Developmental expression of the tenascin-C is altered by hypothyroidism in the rat brain. Neuroscience 84:309-322. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Dolado, M., T. Iglesias, A. Rodriguez-Pena, J. Bernal, and A. Munoz. 1994. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res. Mol. Brain Res. 27:249-257. [DOI] [PubMed] [Google Scholar]
- 3.Baldini, G., G. Wang, M. Weber, M. Zweyer, R. Bareggi, J. W. Witkin, and A. M. Martelli. 1998. Expression of Rab3D N135I inhibits regulated secretion of ACTH in AtT-20 cells. J. Cell Biol. 140:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berke, J. D., and S. E. Hyman. 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25:515-532. [DOI] [PubMed] [Google Scholar]
- 5.Bernal, J., J. Numez. 1995. Thyroid hormones and brain development. Eur. J. Endocrinol. 4:390-398. [DOI] [PubMed] [Google Scholar]
- 6.Betz, U. A., C. A. Vosshenrich, K. Rajewsky, and W. Muller. 1996. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr. Biol. 6:1307-1316. [DOI] [PubMed] [Google Scholar]
- 7.Birnberg, N. C., P. J. Stork, and L. M. Hemmick. 1992. Expression of the c-Harvey ras oncogene alters peptide synthesis in the neurosecretory cell line AtT20. J. Biol. Chem. 267:15464-15468. [PubMed] [Google Scholar]
- 8.Boyages, S. C. 1993. Clinical review 49: Iodine deficiency disorders. J. Clin. Endocrinol. Metab. 77:587-591. [DOI] [PubMed] [Google Scholar]
- 9.Boyages, S. C., and J. P. Halpern. 1993. Endemic cretinism: toward a unifying hypothesis. Thyroid 3:59-69. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla, R., N. Gnesutta, L. Minichiello, G. White, A. J. Roylance, C. E. Herron, M. Ramsey, D. P. Wolfer, V. Cestari, C. Rossi-Arnaud, S. G. Grant, P. F. Chapman, H. P. Lipp, E. Sturani, and R. Klein. 1997. A role for the Ras signaling pathway in synaptic transmission and long-term memory. Nature 390:281-286. [DOI] [PubMed] [Google Scholar]
- 11.Brandon, E. P., S. F. Logue, M. R. Adams, M. Qi, S. P. Sullivan, A. M. Matsumoto, D. M. Dorsa, J. M. Wehner, G. S. McKnight, and R. L. Idzerda. 1998. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J. Neurosci. 18:3639-3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calamandrei, G., A. Venerosi, I. Branchi, and E. Alleva. 1999. Effects of prenatal zidovudine treatment on learning and memory capacities of preweanling and young adult mice. Neurotoxicology 20:17-25. [PubMed] [Google Scholar]
- 13.Calamandrei, G., A. Venerosi, I. Branchi, F. Chiarotti, A. Verdina, F. Bucci, and E. Alleva. 1999. Effects of prenatal AZT on mouse neurobehavioral development and passive avoidance learning. Neurotoxicol. Teratol. 21:29-40. [DOI] [PubMed] [Google Scholar]
- 14.Calamandrei, G., A. Venerosi, I. Branchi, A. Valanzano, and E. Alleva. 2000. Prenatal exposure to anti-HIV drugs. long-term neurobehavioral effects of lamivudine (3TC) in CD-1 mice. Neurotoxicol. Teratol. 22:369-379. [DOI] [PubMed] [Google Scholar]
- 15.Chandler, L. A., C. P. Ehretsmann, and S. Bourgeois. 1994. A novel mechanism of Ha-ras oncogene action: regulation of fibronectin mRNA levels by a nuclear posttranscriptional event. Mol. Cell. Biol. 14:3085-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 17.Costa, L. G., and S. D. Murphy. 1982. Passive avoidance retention in mice tolerant to the organophosphorus insecticide disulfoton. Toxicol. Appl. Pharmacol. 65:451-458. [DOI] [PubMed] [Google Scholar]
- 18.Cuadrado, A., J. Bernal, and A. Munoz. 1999. Identification of the mammalian homolog of the splicing regulator Suppressor-of-white-apricot as a thyroid hormone regulated gene. Brain Res. Mol. Brain Res. 71:332-340. [DOI] [PubMed] [Google Scholar]
- 19.Dathan, N., R. Parlato, A. Rosica, M. De Felice, and R. Di Lauro. 2002. Distribution of the titf2/foxe1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev. Dyn. 224:450-456. [DOI] [PubMed] [Google Scholar]
- 20.Davis, R. J. 1995. Transcriptional regulation by MAP kinases. Mol. Reprod. Dev. 42:459-467. [DOI] [PubMed] [Google Scholar]
- 21.De Felice, M., C. Ovitt, E. Biffali, A. Rodriguez-Mallon, C. Arra, K. Anastassiadis, P. E. Macchia, M. G. Mattei, A. Mariano, H. Scholer, V. Macchia, and R. Di Lauro. 1998. A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat. Genet. 19:395-398. [DOI] [PubMed] [Google Scholar]
- 22.Dugbartey, A. T. 1998. Neurocognitive aspects of hypothyroidism. Arch. Intern. Med. 158:1413-1418. [DOI] [PubMed] [Google Scholar]
- 23.Ellis, S., and H. Mellor. 2000. Regulation of endocytic traffic by rho family GTPases. Trends Cell Biol. 10:85-88. [DOI] [PubMed] [Google Scholar]
- 24.Falk, J. D., P. Vargiu, P. E. Foye, H. Usui, J. Perez, P. E. Danielson, D. L. Lerner, J. Bernal, and J. G. Sutcliffe. 1999. Rhes: A striatal-specific Ras homolog related to Dexras1. J. Neurosci. Res. 57:782-788. [PubMed] [Google Scholar]
- 25.Fernagut, P. O., S. Chalon, E. Diguet, D. Guilloteau, F. Tison, and M. Jaber. 2003. Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscience 116:1123-1130. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes, C., M. I. Gonzalez, C. A. Wilson, and S. E. File. 1999. Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol. Biochem. Behav. 64:731-738. [DOI] [PubMed] [Google Scholar]
- 27.Ferrante, R. J., O. A. Andreassen, B. G. Jenkins, A. Dedeoglu, S. Kuemmerle, J. K. Kubilus, R. Kaddurah-Daouk, S. M. Hersch, and M. F. Beal. 2000. Neuroprotective effects of creatine in a transgenic mouse model of Huntington's disease. J. Neurosci. 20:4389-4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher, D. A. 1991. Clinical review 19: management of congenital hypothyroidism. J. Clin. Endocrinol. Metab. 72:523-529. [DOI] [PubMed] [Google Scholar]
- 29.Guilbault, G. G., and E. B. Rietz. 1976. Enzymatic, fluorometric assay of alpha-amylase in serum. Clin. Chem. 22:1702-1704. [PubMed] [Google Scholar]
- 30.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Hockly, E., P. M. Cordery, B. Woodman, A. Mahal, A. van Dellen, C. Blakemore, C. M. Lewis, A. J. Hannan, and G. P. Bates. 2002. Environmental enrichment slows disease progression in R6/2 Huntington's disease mice. Ann. Neurol. 51:235-242. [DOI] [PubMed] [Google Scholar]
- 32.Hyman, S. E., and R. C. Malenka. 2001. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2:695-703. [DOI] [PubMed] [Google Scholar]
- 33.Johnson, D. I. 1999. Cdc42: an essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63:54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson, L., D. Greenbaum, K. Cichowski, K. Mercer, E. Murphy, E. Schmitt, R. T. Bronson, H. Umanoff, W. Edelmann, R. Kucherlapati, and T. Jacks. 1997. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 11:2468-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston, A. L., and S. E. File. 1991. Sex differences in animal tests of anxiety. Physiol. Behav. 49:245-250. [DOI] [PubMed] [Google Scholar]
- 36.Joyner, A. L. 2000. Gene targeting: a practical approach, 2nd ed.
- 37.Kemppainen, R. J., and E. N. Behrend. 1998. Dexamethasone rapidly induces a novel ras superfamily member-related gene in AtT-20 cells. J. Biol. Chem. 273:3129-3131. [DOI] [PubMed] [Google Scholar]
- 38.Kjoller, L., and A. Hall. 1999. Signaling to Rho GTPases. Exp. Cell Res. 253:166-179. [DOI] [PubMed] [Google Scholar]
- 39.Ma, T., Z. C. Lian, S. P. Qi, E. R. Heinz, and G. R. DeLong. 1993. Magnetic resonance imaging of brain and the neuromotor disorder in endemic cretinism. Ann. Neurol. 34:91-94. [DOI] [PubMed] [Google Scholar]
- 40.Maruta, H., H. He, A. Tikoo, T. Vuong, and E. K. M. Nur. 1999. G proteins, phosphoinositides, and actin-cytoskeleton in the control of cancer growth. Microsc. Res. Tech. 47:61-66. [DOI] [PubMed] [Google Scholar]
- 41.McCormick, F. 1995. Ras-related proteins in signal transduction and growth control. Mol. Reprod. Dev. 42:500-506. [DOI] [PubMed] [Google Scholar]
- 42.Morris, R. 1984. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11:47-60. [DOI] [PubMed] [Google Scholar]
- 43.Munoz, A., A. Rodriguez-Pena, A. Perez-Castillo, B. Ferreiro, J. G. Sutcliffe, and J. Bernal. 1991. Effects of neonatal hypothyroidism on rat brain gene expression. Mol. Endocrinol. 5:273-280. [DOI] [PubMed] [Google Scholar]
- 44.Nagy, A., J. Rossant, R. Nagy, W. Abramow-Newerly, and J. C. Roder. 1993. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl. Acad. Sci. USA 90:8424-8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngsee, J. K., A. M. Fleming, and R. H. Scheller. 1993. A rab protein regulates the localization of secretory granules in AtT-20 cells. Mol. Biol. Cell 4:747-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Novick, P., and M. Zerial. 1997. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9:496-504. [DOI] [PubMed] [Google Scholar]
- 47.Olanow, C. W., and W. G. Tatton. 1999. Etiology and pathogenesis of Parkinson's disease. Annu. Rev. Neurosci. 22:123-144. [DOI] [PubMed] [Google Scholar]
- 48.Palanza, P. 2001. Animal models of anxiety and depression: how are females different? Neurosci. Biobehav. Rev. 25:219-233. [DOI] [PubMed] [Google Scholar]
- 49.Palomba, S., T. Russo, F. Orio, Jr., A. Sammartino, F. M. Sbano, C. Nappi, A. Colao, P. Mastrantonio, G. Lombardi, and F. Zullo. 2004. Lipid, glucose and homocysteine metabolism in women treated with a GnRH agonist with or without raloxifene. Hum. Reprod. 19:415-421. [DOI] [PubMed] [Google Scholar]
- 50.Pfeffer, S. R. 1994. Rab GTPases: master regulators of membrane trafficking. Curr. Opin. Cell Biol. 6:522-526. [DOI] [PubMed] [Google Scholar]
- 51.Pryce, C. R., J. Lehmann, and J. Feldon. 1999. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacol. Biochem. Behav. 64:753-759. [DOI] [PubMed] [Google Scholar]
- 52.Riis, B., S. I. Rattan, B. F. Clark, and W. C. Merrick. 1990. Eukaryotic protein elongation factors. Trends Biochem. Sci. 15:420-424. [DOI] [PubMed] [Google Scholar]
- 53.Rodgers, R. J., and N. J. Johnson. 1995. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol. Biochem. Behav. 52:297-303. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez-Pena, A., N. Ibarrola, M. A. Iniguez, A. Munoz, and J. Bernal. 1993. Neonatal hypothyroidism affects the timely expression of myelin-associated glycoprotein in the rat brain. J. Clin. Investig. 91:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosman, N. P. 1972. The neuropathology of congenital hypothyroidism. Adv. Exp. Med. Biol. 30:337-366. [PubMed] [Google Scholar]
- 56.Sedelis, M., R. K. Schwarting, and J. P. Huston. 2001. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav. Brain Res. 125:109-125. [DOI] [PubMed] [Google Scholar]
- 57.Silva, J. E., and P. Rudas. 1990. Effects of congenital hypothyroidism on microtubule-associated protein-2 expression in the cerebellum of the rat. Endocrinology 126:1276-1282. [DOI] [PubMed] [Google Scholar]
- 58.St Croix, B., C. Rago, V. Velculescu, G. Traverso, K. E. Romans, E. Montgomery, A. Lal, G. J. Riggins, C. Lengauer, B. Vogelstein, and K. W. Kinzler. 2000. Genes expressed in human tumor endothelium. Science 289:1197-1202. [DOI] [PubMed] [Google Scholar]
- 59.Vargiu, P., B. Morte, J. Manzano, J. Perez, R. de Abajo, J. Gregor Sutcliffe, and J. Bernal. 2001. Thyroid hormone regulation of rhes, a novel Ras homolog gene expressed in the striatum. Brain Res. Mol. Brain Res. 94:1-8. [DOI] [PubMed] [Google Scholar]
- 60.Walker, P., M. E. Weichsel, Jr., D. Eveleth, and D. A. Fisher. 1982. Ontogenesis of nerve growth factor and epidermal growth factor in submaxillary glands and nerve growth factor in brains of immature male mice: correlation with ontogenesis of serum levels of thyroid hormones. Pediatr. Res. 16:520-524. [DOI] [PubMed] [Google Scholar]
- 61.Wasylyk, B., J. Hagman, and A. Gutierrez-Hartmann. 1998. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem. Sci. 23:213-216. [DOI] [PubMed] [Google Scholar]