Abstract
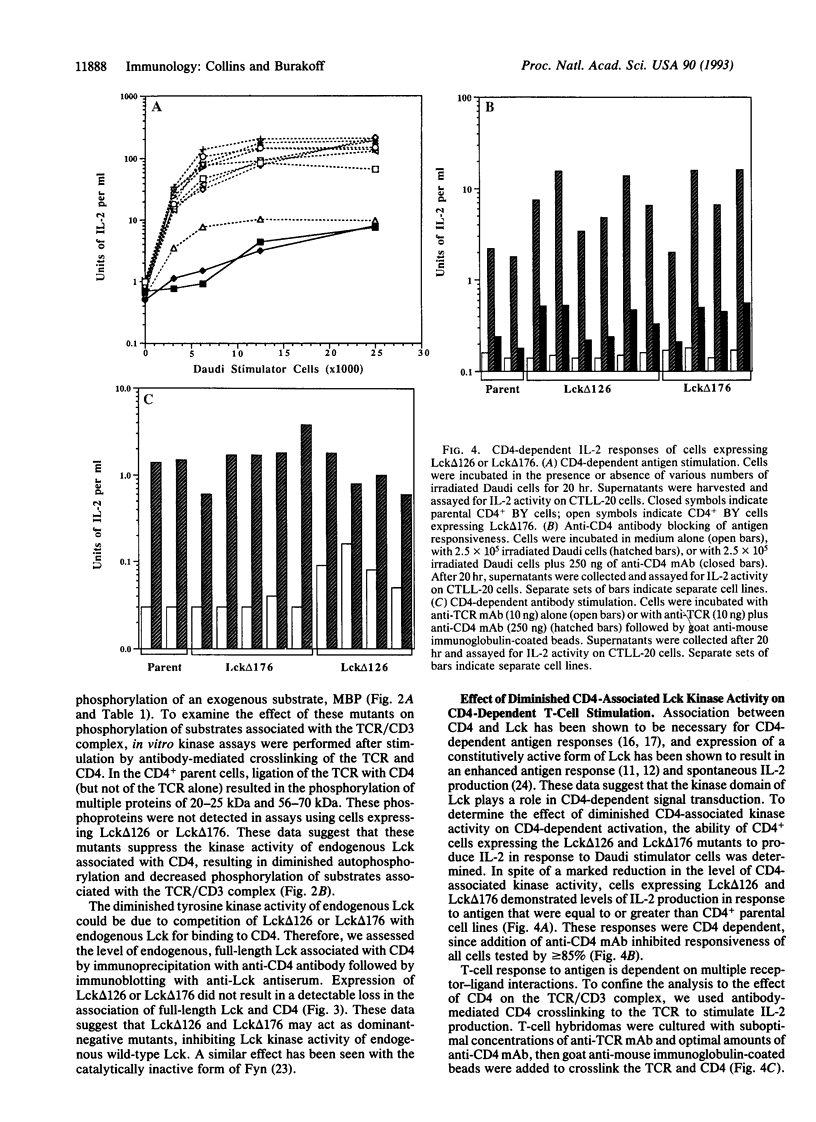
The lymphoid-specific tyrosine kinase p56lck (Lck) is critical for the development and activation of T lymphocytes, and Lck kinase activity has been implicated in both T-cell antigen receptor/CD3- and CD4-mediated signaling. CD4-dependent T-cell activation has been demonstrated to be dependent upon the association of CD4 with Lck. To examine the role of the kinase activity of Lck in CD4-dependent T-cell activation, we have generated several kinase-deficient mutants of Lck. When transfected into CD4+ murine T-cell hybridoma cells, these mutants cause approximately 90% diminution in CD4-associated Lck kinase activity. Specifically, upon CD4 crosslinking there is decreased Lck autophosphorylation and decreased phosphorylation of an exogenous substrate. When CD4 is crosslinked to the T-cell antigen receptor-CD3 complex, decreased phosphorylation of associated substrates is also observed. In spite of this striking inhibition of Lck kinase function, cells expressing the kinase-deficient mutants demonstrate normal or enhanced CD4-dependent antigen responsiveness. These data demonstrate that the level of Lck kinase activity does not correlate with its CD4-associated function and suggest that the kinase activity of Lck may not be required for CD4-mediated signaling.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Cahir McFarland E. D., Hurley T. R., Pingel J. T., Sefton B. M., Shaw A., Thomas M. L. Correlation between Src family member regulation by the protein-tyrosine-phosphatase CD45 and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron L., Abraham N., Pawson T., Veillette A. Structural requirements for enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine protein kinase p56lck. Mol Cell Biol. 1992 Jun;12(6):2720–2729. doi: 10.1128/mcb.12.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T. L., Uniyal S., Shin J., Strominger J. L., Mittler R. S., Burakoff S. J. p56lck association with CD4 is required for the interaction between CD4 and the TCR/CD3 complex and for optimal antigen stimulation. J Immunol. 1992 Apr 1;148(7):2159–2162. [PubMed] [Google Scholar]
- Eichmann K., Jönsson J. I., Falk I., Emmrich F. Effective activation of resting mouse T lymphocytes by cross-linking submitogenic concentrations of the T cell antigen receptor with either Lyt-2 or L3T4. Eur J Immunol. 1987 May;17(5):643–650. doi: 10.1002/eji.1830170510. [DOI] [PubMed] [Google Scholar]
- Emmrich F., Strittmatter U., Eichmann K. Synergism in the activation of human CD8 T cells by cross-linking the T-cell receptor complex with the CD8 differentiation antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8298–8302. doi: 10.1073/pnas.83.21.8298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaichenhaus N., Shastri N., Littman D. R., Turner J. M. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991 Feb 8;64(3):511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- Granja C., Lin L. L., Yunis E. J., Relias V., Dasgupta J. D. PLC gamma 1, a possible mediator of T cell receptor function. J Biol Chem. 1991 Sep 5;266(25):16277–16280. [PubMed] [Google Scholar]
- Hsi E. D., Siegel J. N., Minami Y., Luong E. T., Klausner R. D., Samelson L. E. T cell activation induces rapid tyrosine phosphorylation of a limited number of cellular substrates. J Biol Chem. 1989 Jun 25;264(18):10836–10842. [PubMed] [Google Scholar]
- Hurley T. R., Hyman R., Sefton B. M. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993 Mar;13(3):1651–1656. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin S. D., Anderson S. J., Forbush K. A., Perlmutter R. M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993 Apr;12(4):1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Batzer A., Daly R., Yajnik V., Skolnik E., Chardin P., Bar-Sagi D., Margolis B., Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993 May 6;363(6424):85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. S., Goldman S. J., Spitalny G. L., Burakoff S. J. T-cell receptor-CD4 physical association in a murine T-cell hybridoma: induction by antigen receptor ligation. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8531–8535. doi: 10.1073/pnas.86.21.8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992 Jan 25;267(3):1496–1501. [PubMed] [Google Scholar]
- Prasad K. V., Janssen O., Kapeller R., Raab M., Cantley L. C., Rudd C. E. Src-homology 3 domain of protein kinase p59fyn mediates binding to phosphatidylinositol 3-kinase in T cells. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7366–7370. doi: 10.1073/pnas.90.15.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Mayer B. J., Cicchetti P., Baltimore D. Identification of a ten-amino acid proline-rich SH3 binding site. Science. 1993 Feb 19;259(5098):1157–1161. doi: 10.1126/science.8438166. [DOI] [PubMed] [Google Scholar]
- Rojo J. M., Saizawa K., Janeway C. A., Jr Physical association of CD4 and the T-cell receptor can be induced by anti-T-cell receptor antibodies. Proc Natl Acad Sci U S A. 1989 May;86(9):3311–3315. doi: 10.1073/pnas.86.9.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., Fernley R., Wade J., Pawson T., Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993 May 6;363(6424):83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- Rudd C. E., Trevillyan J. M., Dasgupta J. D., Wong L. L., Schlossman S. F. The CD4 receptor is complexed in detergent lysates to a protein-tyrosine kinase (pp58) from human T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5190–5194. doi: 10.1073/pnas.85.14.5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleckman B. P., Peterson A., Jones W. K., Foran J. A., Greenstein J. L., Seed B., Burakoff S. J. Expression and function of CD4 in a murine T-cell hybridoma. Nature. 1987 Jul 23;328(6128):351–353. doi: 10.1038/328351a0. [DOI] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992 Aug 21;70(4):585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bolen J. B., Bookman M. A. Alterations in tyrosine protein phosphorylation induced by antibody-mediated cross-linking of the CD4 receptor of T lymphocytes. Mol Cell Biol. 1989 Oct;9(10):4441–4446. doi: 10.1128/mcb.9.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Bolen J. B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988 Oct 21;55(2):301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- Veillette A., Bookman M. A., Horak E. M., Samelson L. E., Bolen J. B. Signal transduction through the CD4 receptor involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature. 1989 Mar 16;338(6212):257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]