Abstract
Oxidative stress and reactive oxygen species (ROS) can elicit and modulate various physiological and pathological processes, including cell death. However, the mechanisms controlling ROS-induced cell death are largely unknown. Data from this study suggest that receptor-interacting protein (RIP) and tumor necrosis factor receptor (TNFR)-associated factor 2 (TRAF2), two key effector molecules of TNF signaling, are essential for ROS-induced cell death. We found that RIP−/− or TRAF2−/− mouse embryonic fibroblasts (MEF) are resistant to ROS-induced cell death when compared to wild-type cells, and reconstitution of RIP and TRAF2 gene expression in their respective deficient MEF cells restored their sensitivity to H2O2-induced cell death. We also found that RIP and TRAF2 form a complex upon H2O2 exposure, but without the participation of TNFR1. The colocalization of RIP with a membrane lipid raft marker revealed a possible role of lipid rafts in the transduction of cell death signal initiated by H2O2. Finally, our results demonstrate that activation of c-Jun NH2-terminal kinase 1 is a critical event downstream of RIP and TRAF2 in mediating ROS-induced cell death. Therefore, our study uncovers a novel signaling pathway regulating oxidative stress-induced cell death.
Oxidative stress refers to the imbalance with enhanced production of reactive oxygen species (ROS) and/or impaired function of the antioxidant system (50). ROS usually include superoxide anions, hydroxyl radicals, and hydrogen peroxide (H2O2) that are capable of reacting with and damaging various molecular targets including DNA, protein, and lipids. It is well known that ROS or oxidative stress plays an important role in various physiological and pathological processes such as aging, inflammation, carcinogenesis, neurodegenerative diseases, and cancer (15, 22). One important aspect of ROS biological effects is their regulatory roles on cell death: ROS can act either as direct activators of cell death or as second messengers in the cell death processes triggered by many other stimuli such as cancer chemotherapeutic agents, UV, ionizing radiation, and tumor necrosis factor (TNF) (6, 19, 45, 48). As direct stimuli, ROS could cause either apoptosis or necrosis, depending on the concentration used and the cell type tested (18, 53, 57). On the other hand, elevated levels of ROS have been detected in many apoptotic conditions, and mitochondria are believed to be the main source of intracellular ROS production (6, 7, 43). However, some important issues regarding the role of ROS and oxidative stress in cell death remain to be further studied. The molecular targets of ROS in cell death are largely elusive. Inconsistent reports often suggest contradictory results regarding the effects of ROS on some key effectors or regulatory molecules such as caspases, nuclear transcription factors NF-κB and activator protein 1, and some cell stress-activated kinases (6, 23, 43, 49). Apparently, the cell signaling pathways regulating ROS-induced cell death remain to be further investigated.
In recent years, extensive research on the TNF signaling pathway has greatly advanced our understanding of the cell death mechanisms. It is well known that receptor-interacting protein (RIP), TNF receptor (TNFR)-associated factor 2 (TRAF2), and Fas-associated death domain protein (FADD) are important effector molecules of TNFR1 signaling (4, 8, 38). In response to TNF, TNFR1 is trimerized and recruits TNFR-associated death domain protein (TRADD) as an adaptor molecule. The recruited TRADD interacts with FADD, which then interacts and activates caspase 8 to initiate the apoptotic cell death pathway. On the other hand, TRADD interacts with RIP and TRAF2 that is known to be important in TNF-induced activation of nuclear transcription factor NF-κB and mitogen-activated protein kinases (MAPK) (14).
Although it is well established that RIP and TRAF2 mainly act as cell survival factors to protect against TNF-induced apoptosis via NF-κB activation (4, 31, 37), little is known about their involvement in cell death elicited by other stimuli. An earlier study revealed that RIP is required for Fas-induced caspase-independent cell death in primary T cells (26), indicating diversified functions of RIP in the regulation of the cell death process. On the other hand, ROS and oxidative stress are known to be involved in TNF-induced cell death (12, 16, 46). However, currently there is no report concerning whether some of the key TNF signaling molecules such as RIP and TRAF2 serve as the molecular targets of ROS in cell death. In this study, we demonstrate that RIP, TRAF2, and FADD, three key TNF signaling molecules, are important regulators in H2O2-induced cell death. We found that mouse embryonic fibroblasts (MEF) deficient of RIP and TRAF2 are largely resistant, while FADD−/− MEF cells are highly sensitive to H2O2 cytotoxicity, when compared to wild-type (wt) cells. Reconstitution of these proteins significantly restores the responses of these knockout cells to H2O2. Moreover, RIP and TRAF2 form a complex upon H2O2 exposure, most probably through membrane lipid rafts but independently of TNFR1. Lastly, we demonstrate that activation of c-Jun NH2-terminal kinase 1 (JNK1) is the key downstream event responsible for H2O2-induced cell death. Thus, our study reveals a novel cell signaling mechanism that regulates oxidative stress-induced cell death.
MATERIALS AND METHODS
Reagents and plasmids.
The following antibodies were purchased from Santa Cruz: anti-TRAF2, anti-TNFR1, anti-JNK2, anti-MKK4/JNKK1, anti-MKK7/JNKK2, and anti-TRADD. Anti-RIP and anti-JNK1 were from BD Pharmingen. Anti-FADD (rabbit), anti-myc, and anti-GRP78/BiP were from Stressgen and Upstate. Cycloheximide (CHX), tert-butyl hydroperoxide, menadione, and fluorescein isothiocyanate (FITC)-cholera toxin B (CTxB) were obtained from Sigma. The specific JNK inhibitor SP600125 and various caspase inhibitors were from Calbiochem. mTNFα was purchased from R&D. Both wt RIP and RIP kinase dead RIPK45A constructs were reported previously (37).
Cell culture and transient transfection.
The wt and various knockout MEF cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The stable cell lines of the reconstituted cells were also cultured in the above complete medium with the presence of hygromycin (150 ng/ml). The wt Jurkat and RIP-deficient Jurkat cells were cultured in RPMI 1640 medium with the addition of 10% fetal bovine serum, glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml). The transient transfection was performed using Lipofectamine Plus reagent (Invitrogen) by following the manufacturer's instructions.
Detection of cell death.
Upon various designated treatments, cell death in MEF was quantified using a cytotoxicity detection kit (Roche) measuring the percentage of lactate dehydrogenase (LDH) leakage. In the transient transfection experiments, following H2O2 exposure, cells were fixed in 4% paraformaldehyde and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). H2O2-induced cell death was then quantified by calculating the percentage of the remaining blue-stained cells in each group in comparison to their respective controls (37).
Western blotting and coimmunoprecipitation assay.
For Western blotting, cells were first treated as described in the figure legends, and they were then collected and washed with phosphate-buffered saline (PBS) twice before they were lysed in M2 buffer (20 mM Tris at pH 7, 0.5% NP-40, 250 mM NaCl, 3 mM EDTA, 3 mM EGTA, 2 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 20 mM glycerol phosphate, 1 mM sodium vanadate, 1 μg of leupeptin/ml). The cell lysate was collected after centrifugation (12,879 × g, 5 min), and about 30 μg of protein was then fractionated on gradient sodium dodecyl (SDS)-4 to 20% polyacrylamide gel electrophoresis (PAGE) (Bio-Rad) and blotted onto polyvinylidene difluoride membrane (Millipore). After blocking in 5% milk in PBST (PBS with 0.05% Tween 20), the membrane was probed with various antibodies and developed with enhanced chemiluminescence by following the manufacturer's instructions (Amersham).
For the coimmunoprecipitation experiments, cell lysate was prepared as described above. The cell lysate was mixed and incubated with various antibodies and protein A-Sepharose beads (Roche) by incubation at 4°C overnight. After extensive washing with the lysis buffer, beads were boiled in SDS sample buffer, and the bound proteins were resolved on 4 to 20% gradient SDS-PAGE (Bio-Rad). Detection was accomplished by Western blot analysis.
JNK kinase assay.
After various treatments, cells were collected and lysed in M2 buffer. JNK1 was immunoprecipitated with anti-JNK1 antibody (BD Pharmingen) and harvested with protein A-Sepharose beads (Roche). After extensive washing, the kinase assay was performed in complete kinase assay buffer (20 mM HEPES [pH 7.5], 20 mM β-glycerol phosphate, 10 mM MgCl2, 1 mM dithiothreitol, 10 mM p-nithrophenyl phosphate, 50 μM sodium vanadate, 20 μM ATP) with the addition of p32-γ-ATP and glutathione S-transferase-c-Jun as substrate. After incubation at 30°C for 30 min, the reaction was stopped with the addition of SDS sample buffer. The samples were then separated on a 4 to 20% gradient SDS-PAGE and visualized by autoradiography.
Immunofluorescence staining and confocal microscopy.
The labeling of membrane lipid rafts with CTxB was conducted as previously reported (52). Cells were first stained with FITC-conjugated CTxB (10 μg/ml in PBS, 4°C for 30 min; Sigma), followed by H2O2 treatment (500 μM in DMEM, 37°C) for up to 30 min. Cells were then fixed in freshly prepared 3% paraformaldehyde, followed by permeabilization and blocking in 0.4% Triton X-100 with 20% goat serum. Immunofluorescence staining of RIP was performed using anti-RIP mouse monoclonal antibody (1:200; Transduction Laboratory) and a goat anti-mouse secondary antibody (1:1,000) (Alexa Fluor 594; Molecular Probes). All fluorescence images were obtained using a Zeiss confocal microscope (LSM 510 META).
RESULTS
RIP and TRAF2 are required for ROS-induced cell death.
ROS and oxidative stress are known to be important factors inducing cell death. However, the form of cell death induced by ROS and oxidative stress varies from apoptosis to necrosis, depending on the exact concentration applied and/or the cell type tested (18, 53, 57). For instance, low or moderate concentrations of H2O2 (100 to 500 μM) largely induced apoptotic cell death, while a high concentration of H2O2 (>2 mM) mainly caused necrosis in a number of cells, including human promonocytic cells (U-937) and murine L929 fibroblasts (18, 57). To confirm a recent finding that H2O2-induced cell death is caspase independent in MEF cells (62), we also investigated the role of caspases in H2O2-induced cell death in wt MEF cells by using a similar concentration (500 μM). It was found that general caspase inhibitors (zVAD and Boc-D), as well as specific inhibitors for caspase-3, caspase-8, and caspase-9, and the poxvirus protein Crm A, were all unable to block H2O2-induced cell death (data not shown). It is thus believed that H2O2 mainly induces caspase-independent cell death in wt MEF cells.
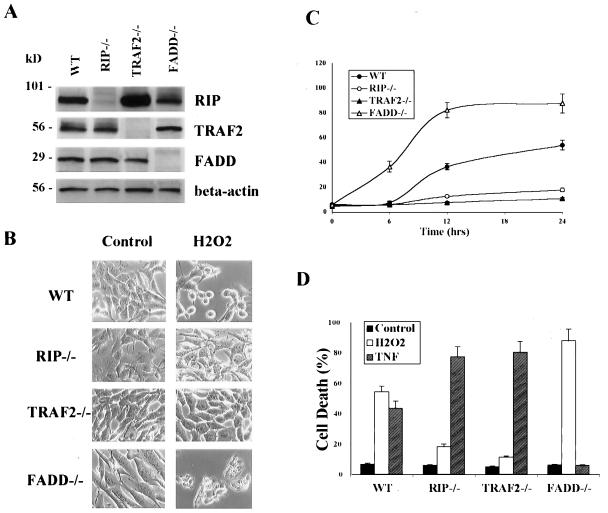
Despite the well known functions of RIP and TRAF2 in TNF signaling (4, 8, 38), it is not clear whether these cell signaling molecules are involved in other cell death signaling pathways. An early study suggested that RIP is involved in Fas- or TNF-induced caspase-independent cell death (26). Here, we examined whether RIP or TRAF2 is required for ROS-induced caspase-independent cell death. To do so, we compared H2O2-induced cell death in both wt and various knockout MEF cells in which the RIP, TRAF2, or FADD gene was disrupted (33, 61, 63) (Fig. 1A). When cells were treated with H2O2 (500 μM, 12 h) to undergo cell death, RIP−/− and TRAF2−/− cells were found to be H2O2 resistant when compared to wt MEF (Fig. 1B and C). In contrast, FADD−/− cells were more sensitive to H2O2-induced cell death than wt cells. Interestingly, such responses were found to be the opposite of TNF-induced apoptotic cell death (Fig. 1D): both RIP−/− and TRAF2−/− cells were hypersensitive to TNF-induced apoptosis, while FADD−/− cells were completely resistant. Therefore, RIP, TRAF2, and FADD play disparate roles in H2O2-induced cell death versus TNF-induced apoptosis, which depends on the activation of a caspase cascade initiated by caspase-8 activation (3, 4).
FIG. 1.
Different susceptibilities of RIP−/−, TRAF2−/−, and FADD−/− MEF to H2O2-induced cell death. (A) Western blotting confirmed the absence of RIP, TRAF2, and FADD proteins in their respective knockout cells. (B) H2O2-induced cell death examined by cell morphological changes after H2O2 treatment (500 μM, 12 h). Images were taken under a phase-contrast microscope (×200). (C) H2O2-induced cell death quantified by measuring the percentage of LDH leakage using a cytotoxicity test kit (Roche). Cells were treated with H2O2 (500 μM) for up to 24 h. (D) Opposite pattern of responses towards TNF-α-induced cell death from H2O2. RIP−/− and TRAF2−/− cells were highly sensitive and FADD−/− cells were completely resistant to TNF-induced cell death. Cells were treated with H2O2 (500 μM) or TNF-α (25 ng/ml plus CHX at 10 μg/ml) for 24 h. In both panels C and D, data are presented as means ± standard deviations from at least three independent experiments.
In order to strengthen our findings, we also tested the effects of other oxidants, such as tert-butyl hydroperoxide, an organic analog of H2O2 (21), and menadione, which is an intracellular superoxide generator (47). In the cell death induced by these two agents, the different gene disruptions had the same effect as in H2O2-induced cell death (Fig. 2A). To test whether RIP also plays a critical role in ROS-induced cell death in other types of cells, we used wt human leukemia Jurkat cells and RIP-deficient Jurkat cells (42) and found that RIP-null Jurkat cells were more resistant to H2O2-induced cell death than wt Jurkat cells, indicating that RIP plays a similar role in Jurkat cells as it did in MEF cells (Fig. 2B)
FIG. 2.
Other forms of oxidants induced patterns of cell death similar to that of H2O2 in RIP−/−, TRAF2−/−, and FADD−/− cells. (A) MEF cells were treated with H2O2 (500 μM), tert-butyl hydroperoxide (250 μM), or menadione (10 μM) for 12 h. (B) H2O2-induced cell death in Jurkat cells. Both wt and RIP-deficient Jurkat cells were treated with H2O2 (500 μM, 12 h) or TNF-α (25 ng/ml plus CHX at 10 μg/ml, 12 h). Cell death was quantified by the determination of LDH leakage. Data are presented as means ± standard deviations from at least three independent experiments.
To verify that the specific response of each cell type shown above is due to the absence of the gene product encoded by each disrupted gene, we examined the effect of protein reconstitution on the susceptibility of each cell type to H2O2-induced cell death. Stably transfected derivatives of RIP−/−, TRAF2−/−, and FADD−/− cells that express ectopic RIP, TRAF2, or FADD, respectively, were established (Fig. 3A). The reconstituted RIP−/− cells and the reconstituted TRAF2−/− cells showed restored sensitivity to H2O2-induced cell death (Fig. 3B). Similar results were also found when the cell death was examined using the TdT-mediated dUTP nick end labeling assay to determine the level of DNA fragmentation (data not shown). These results further support the notion that RIP and TRAF2 are important mediators of H2O2-induced cell death. In contrast, the reconstituted FADD−/− cells became less sensitive to H2O2-induced cell death than the parental cells (Fig. 3B), suggesting that FADD may attenuate H2O2-induced cell death. Currently, it is not clear how FADD inhibits H2O2-induced caspase-independent cell death. One possibility is that FADD may interfere in the function of RIP or TRAF2. For instance, RIP is the substrate of caspase-8, whose activation is mediated by FADD in TNF-induced apoptosis (36).
FIG. 3.
Protein expression reconstitution restored the responses of RIP−/−, TRAF2−/−, and FADD−/− cells to H2O2. (A) Restoration of RIP, TRAF2, and FADD protein expression in their respective reconstituted cells. (B) RIP and TRAF2 reconstitution restored the sensitivity of RIP−/− and TRAF2−/− cells to H2O2 treatment (500 μM, 12 h), while FADD reconstitution significantly reduced the sensitivity to H2O2-induced cell death. Cell death was quantified by the determination of LDH leakage, as described earlier. (C) Transient transfection in RIP−/− MEF cells with wt RIP and RIP kinase dead RIPK45A constructs restored the sensitivity to H2O2-induced cell death. RIP−/− cells were transiently transfected with control vector (pcDNA), wt RIP, or RIPK45A, together with Crm A and β-galactosidase expression vector. Following H2O2 treatment (500 μM, 18 h), cells were fixed and stained with X-Gal. The percentage of cell death was then calculated based on the number of the remaining positive-stained cells in each group.
It has been suggested that the kinase activity of RIP is required for Fas-mediated caspase-independent cell death (26). In this study, we thus examined the role of RIP kinase activity in H2O2-induced cell death. RIP−/− MEF cells were transiently transfected with wt RIP or RIPK45A, a RIP kinase dead construct, together with β-galactosidase expression vector. After H2O2 treatment, the remaining living cells with β-galactosidase staining in each group were then counted and compared. As shown in Fig. 3C, transient transfection of wt RIP or RIPK45A equally restored the sensitivity to H2O2-induced cell death, suggesting that the kinase activity of RIP is not required in this process.
H2O2-induced cell death is independent of TNFR1 and de novo protein synthesis.
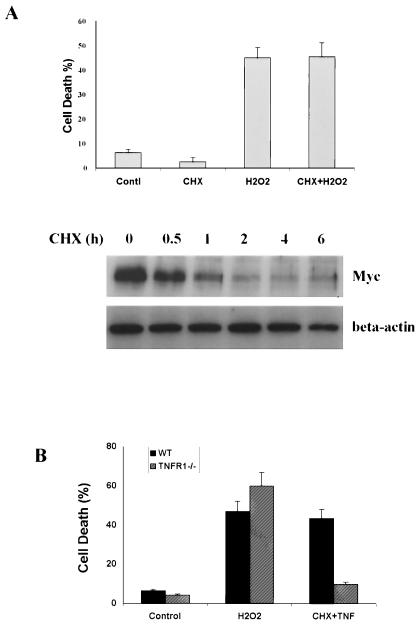
Two important nuclear transcription factors, NF-κB and activator protein 1, have been implicated in oxidative stress-induced cell death and proliferation (32). In this study, NF-κB was not activated by H2O2 treatment in wt MEF cells, based on the luciferase reporter assay (data not shown). Moreover, H2O2-induced cell death does not require de novo protein synthesis, as pretreatment with the protein synthesis inhibitor, CHX, failed to affect H2O2-induced cell death (Fig. 4A). The effectiveness of CHX treatment was confirmed by the time-dependent decrease of the c-Myc protein level. This result thus indicates that H2O2-induced cell death is not mediated through an autocrine pathway.
FIG. 4.
H2O2-induced cell death is independent of TNFR1 and de novo protein synthesis. (A) H2O2-induced cell death does not require de novo protein synthesis. The wt MEF cells were first pretreated with CHX (10 μg/ml, 30 min), followed by H2O2 exposure (500 μM, 12 h). The effectiveness of CHX treatment was confirmed by the decrease of the c-myc protein level in wt MEF cells detected by Western blotting (lower panel). (B) H2O2-induced cell death does not involve TNFR1. wt MEF cells and TNFR1−/− primary fibroblasts were treated with H2O2 (500 μM, 12 h) or TNF-α (25 ng/ml plus CHX at 10 μg/ml, 12 h). Cell death was quantified by the determination of LDH leakage. Data are presented as means ± standard deviations from at least three independent experiments.
Because RIP and TRAF2 are key molecules of TNFR1 signaling (4, 8, 38), we next examined whether H2O2 induces cell death through TNFR1. As shown in Fig. 4B, TNFR1−/− cells were found to be as susceptible to H2O2-induced cell death as wt cells while being resistant to TNF-induced apoptosis. These results indicated that TNFR1 does not play a critical role in H2O2-induced cell death.
RIP and TRAF2 form a complex in response to H2O2 exposure.
In order to understand the mechanism of RIP- and TRAF2-mediated ROS-induced cell death, we then investigated whether H2O2 can induce complex formation between RIP and TRAF2, as previously shown for TNF signaling (13, 28). While immunoprecipitation of TNFR1 failed to bring down any RIP and TRAF2 following H2O2 treatment (data not shown), a rapid and transient interaction between RIP and TRAF2 was found upon H2O2 exposure by immunoprecipitating TRAF2 (Fig. 5A). This interaction was detected as early as 5 min after H2O2 treatment and peaked at about 30 min. In contrast, TRADD, another important molecule in the TNF signaling pathway (29), was not detected in the complex (Fig. 5A). The TRAF2-RIP interaction is believed to be specific because other proteins, such as GRP78/BiP (20), were not part of the complex (Fig. 5A). The above finding was further confirmed by the reverse coimmunoprecipitation experiment using anti-RIP (Fig. 5B). Therefore, RIP and TRAF2 may mediate H2O2-induced cell death through forming a signal complex without TNFR1.
FIG. 5.
H2O2 exposure stimulates RIP and TRAF2 interaction. (A) H2O2 treatment stimulated an early RIP and TRAF2 interaction in vivo After cells were exposed to H2O2 (500 μM), coimmunoprecipitation (IP) was performed using TRAF2 antibody and immunoblotted with RIP, TRADD, GRP78, and TRAF2. Results are representatives of five independent experiments. (B) Reverse coimmunoprecipitation in wt MEF cells. After cells were treated with H2O2 (500 μM) for 15 and 30 min, coimmunoprecipitation was performed using RIP antibody and immunoblotted with TRAF2 and RIP antibodies. Results are representative of three independent experiments. (C) Immunofluorescence staining of membrane lipid rafts and RIP. Cells were first labeled with FITC-CTxB, followed by H2O2 treatment (500 μM in DMEM, 37°C) for up to 30 min and finally RIP immunofluorescence staining. Cells were examined using a Zeiss confocal microscopy. Bar, 20 μm.
Recently, the formation of lipid rafts has emerged as an important mechanism for regulating cell signaling, including TNF signaling (2, 35, 51). To shed light on how ROS induce the interaction of RIP and TRAF2, we investigated whether H2O2 induces the formation of membrane lipid rafts in MEF cells. To do so, wt MEF cells were first incubated with FITC-CTxB, which binds to the membrane lipid raft marker, GM1 (52). Cells were then treated with H2O2 treatment, followed by immunofluorescence staining of RIP. As shown in Fig. 5C, H2O2 rapidly induces the clustering of CTxB, followed by RIP colocalization. The colocalization of rafts and RIP is transient and starts to disappear by 30 min. Although the exact role of membrane lipid rafts in oxidative stress-induced cell death remains to be further elucidated, data from this study provide evidence that the formation of membrane lipid rafts and recruitment of some key signaling molecules, such as RIP, might be important upstream events to initiate the cell death pathway.
JNK1 mediates H2O2-induced cell death downstream of RIP and TRAF2.
The next important question was how RIP and TRAF2 mediate H2O2-induced cell death. JNK and p38 are members of the MAPK family that play pivotal roles in cellular responses to stress (30, 34). Although the functions of JNK and p38 in apoptosis have been extensively studied, their roles in oxidative stress-induced cell death have not been clearly defined (32, 39). Here, we first tested the involvement of p38 in H2O2-induced cell death by using MEF cells lacking p38 isozymes. MEF derived from p38α−/− mice, which are completely devoid of p38 activity (54), were as sensitive as wt MEF cells to H2O2-induced cell death (Fig. 6A). In contrast, MEF derived from JNK1−/− mice were resistant to H2O2, indicating that JNK1, but not p38, is required for H2O2-induced cell death.
FIG. 6.
Impaired JNK1 activation in RIP−/− and TRAF2−/− MEF cells is associated with their resistance to H2O2-induced cell death. (A) JNK1, but not JNK2 and p38, is involved in H2O2-induced cell death. wt MEF cells, JNK1−/−, JNK2−/−, and p38−/− primary fibroblasts were treated with H2O2 (500 μM) for 12 h. Cell death was quantified by the determination of LDH leakage. Data are presented as means ± standard deviations from at least three independent experiments. (B) H2O2-induced JNK activation in wt MEF cells. Cells were treated with H2O2 (500 μM) for up to 2 h. GST, glutathione S-transferase. (C) JNK activation in RIP−/−, TRAF2−/−, and FADD−/− cells upon H2O2 treatment (500 μM, 30 min). (D) JNK activation in TNFR1−/− cells upon H2O2 treatment (500 μM, 30 min). JNK kinase activity was determined using glutathione S-transferase-c-Jun as the substrate, as described in Materials and Methods. IgG, immunoglobulin G; IP, immunoprecipitation.
JNK proteins are coded by three different genes: the ubiquitously expressed jnk1 and jnk2 genes and the restrictedly expressed jnk3 gene (11). JNK1 and JNK2 have similar or differential effects on cell death depending on the cell type and the stimulus (9, 25). The differential roles of JNK1 and JNK2 in ischemia- and reoxygenation-mediated apoptosis have been suggested by using antisense oligonucleotides with high specificity for different JNK isoforms (17, 27). Here we found that oxidative stress-mediated cell death mainly involves JNK1 but not JNK2, as JNK2−/− MEF were as sensitive to H2O2 as p38−/− and wt cells (Fig. 6A). Thus, data from this study provide evidence that JNK1, but not JNK2, plays a key role in H2O2-induced cell death.
ROS are known to be potent activators of JNK, and JNK activation is also subject to redox regulation (55, 58). In this study, JNK activation by H2O2 could be detected as early as 5 min after treatment, with maximum levels reached by 30 to 60 min (Fig. 6B). The kinetics were found to be similar to those of H2O2-induced RIP and TRAF2 interaction as described earlier (Fig. 5A). More importantly, JNK1 activation by H2O2 was impaired in RIP−/− and TRAF2−/− cells (Fig. 6C). Thus, RIP and TRAF2 are needed for H2O2-induced JNK1 activation. In contrast, FADD is not involved in H2O2-mediated JNK1 activation, as FADD−/− cells exhibited levels of JNK activity after H2O2 treatment that were the same as for wt cells. Consistent with the insignificant role of TNFR1 in H2O2-induced cell death (Fig. 4C), H2O2-induced JNK activation is not impaired in TNFR1−/− cells (Fig. 6D), suggesting that H2O2 activates JNK independent of TNFR1. To shed light on the mechanism of H2O2-induced JNK1 activation, we examined whether RIP and TRAF2 mediate H2O2-induced JNK1 activation through the MAPK cascade (11). First, we tested if JNK1, MKK4/JNKK1, MKK7/JNKK2, MEKK1, or JNK-interacting protein (JIP) is present in the RIP and TRAF2 complex in response to H2O2. Surprisingly, only JNK1 was detected in the RIP-TRAF2 complex following H2O2 treatment (Fig. 6E and data not shown). This observation raised the possibility that RIP and TRAF2 mediate H2O2-induced JNK1 activation independently of the MAPK cascade. This possibility was further supported by the fact that dominant negative mutants of MKK4/JNKK1 and MKK7/JNKK2 had no effect on H2O2-induced JNK activation and cell death (data not shown).
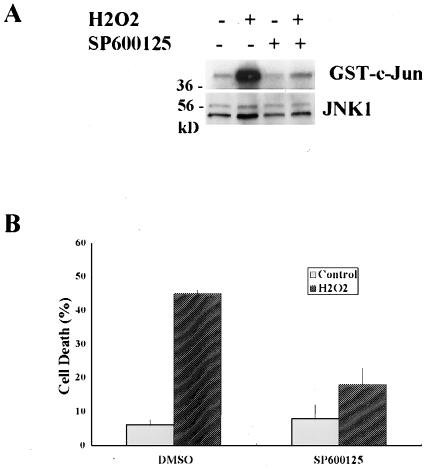
To further support the important role of JNK activation in H2O2-induced cell death, we utilized SP600125, a specific JNK inhibitor (24). As shown in Fig. 7, SP600125 prevented H2O2-induced JNK activation and efficiently blocked H2O2-induced cell death. Taken together, our data suggest that JNK1 activation, downstream of RIP and TRAF2, is essential for H2O2-induced cell death.
FIG. 7.
Inhibition of JNK protects H2O2-induced cell death. (A) Η2Ο2-induced JNK activation was completely inhibited by a specific JNK inhibitor (SP600125). The wt cells were pretreated with SP600125 (20 μM, 30 min) prior to H2O2 exposure (500 μM, 30 min). (B) The protective effects of SP600125 on H2O2-induced cell death in wt MEF cells. Cell death was quantified by the determination of LDH leakage after cells were pretreated with SP600125 (20 μM, 30 min), followed by H2O2 exposure (500 μM, 12 h). Data are presented as means ± standard deviations from at least three independent experiments. GST, glutathione S-transferase; DMSO, dimethyl sulfoxide.
DISCUSSION
Although tremendous effort has been made to study the mechanisms of ROS-induced cell death, the signaling pathway(s) that mediates this process remains elusive. An earlier study has demonstrated that RIP is required for TNF- or Fas-induced caspase-independent cell death, indicating that some TNF key signaling molecules may possess other signaling functions in cell death (26). Such a finding prompted us to investigate the involvement of RIP, together with TRAF2 and FADD, in H2O2-induced cell death, which is also found to be caspase independent. First, we provided evidence that RIP and TRAF2 are required for H2O2-induced cell death, while FADD acts to attenuate H2O2-induced cell death in MEF cells. The importance of these proteins in H2O2-induced cell death is further supported by the observation that reconstitution of these proteins significantly restores the responses of these knockout cells to H2O2. Moreover, RIP and TRAF2 form a complex upon H2O2 exposure, most probably through membrane lipid rafts but independently of TNFR1. Lastly, we demonstrate that activation of JNK1 is the key downstream event responsible for H2O2-induced cell death. Thus, our study reveals a novel cell signaling mechanism that regulates oxidative stress-induced cell death.
The involvement of RIP, TRAF2, and FADD in the TNFR1 signaling pathway has been well defined. Recruitment of FADD leads to the caspase cascade and apoptosis, while recruitments of the death domain kinase RIP and the adaptor protein TRAF2 mediate NF-κB and MAPK activation (4, 8, 38). However, data from this study suggest that these key TNF signaling molecules also play a role in H2O2-induced cell death: RIP and TRAF2 mediate H2O2-induced cell death through activating JNK1, while FADD has an inhibitory effect in this process. JNK is one of the major cell stress-responsive kinases (11). Although the involvement of JNK in TNF-induced apoptosis is still inconclusive, it has been suggested that activation of JNK is required for ROS-induced cell death (10, 11, 56). Consistent with previous studies, data from this study further supported the critical role of JNK in ROS-induced cell death. More importantly, our data identified JNK1 as the main isoform of the JNK family that is involved in ROS-induced cell death. Currently, a number of important points remain to be further addressed. First, how does RIP and TRAF2 interaction mediate JNK1 activation? TRAF2 is known to interact directly or indirectly with several MAPK kinase kinases, such as apoptosis signal regulation kinase 1, TAK1, and MEKK1 (5, 40, 41). However, we failed to detect any of these components in the RIP and TRAF2 complex (data not shown), suggesting an alternate signaling pathway in H2O2-treated cells. Our observation that JNK1, but not MKK4/JNKK1, MKK7/JNKK2, or JIP, is present in the RIP and TRAF2 complex in response to H2O2 further supported this possibility. It is quite likely that RIP/TRAF2-mediated H2O2-induced JNK1 activation is achieved, independently of the MAPK cascade, through a similar mechanism of TAB1-mediated p38 activation. Moreover, although RIP and TRAF2 interaction seems to be transient following H2O2 treatment, such a transient interaction of RIP and TRAF2 could be accountable for the prolonged JNK activation due to the absence of the activation of NF-κB in MEF (data not shown). Several recent reports have suggested that inhibition of NF-κB activation results in the prolonged JNK activation in response to TNF treatment, although the recruitment of RIP and TRAF2 to the TNFR1 complex is transient. Another critical issue remaining to be further investigated is the downstream events after JNK1 activation in H2O2-induced cell death. The proapoptotic function of JNK has been extensively studied (11); however, little is known about how JNK mediates the cell death process. A recent study suggested that poly(ADP-ribose) polymerase activation mediated caspase-independent cell death elicited by DNA-damaging agent and H2O2 through the function of apoptosis-inducing factor (62). It remains to be further determined whether JNK activation is involved in this process.
One of the interesting findings from this study is that H2O2-induced cell death is TNFR1 independent based on the following observation: (i) TNFR1−/− cells showed the same susceptibility as wt cells to H2O2-induced cell death (Fig. 4B), (ii) TNFR1 protein is not detected in the RIP-TRAF2 complex upon H2O2 exposure (Fig. 5A), and (iii) H2O2 activates JNK1 in TNFR1−/− cells as potently as in wt cells (Fig. 6D). TNFR1 is one of the death receptors that include proteins such as Fas and DR3 (4, 38). Since it has been reported that Fas mediated UV-induced cell death independently of FasL (1), we tested the involvement of Fas in H2O2-induced cell death. We found that the Fas-deficient mouse T-cell hybridoma VD-1 cells (60) did not differ significantly from wt 2B4 cells in the response to H2O2-induced cell death (data not shown). We also tested the possibility of the involvement of DR3 in this process by examining the presence of DR3 in the RIP-TRAF2 complex. No DR3 was associated with RIP or TRAF2 in response to H2O2 treatment (data not shown). Therefore, it is unlikely that either Fas or DR3 plays a major role in ROS-induced cell death. Nevertheless, the discovery that H2O2 rapidly induces the formation of membrane lipid rafts with RIP colocalization (Fig. 5C) implied that the cell death signal in response to H2O2 is initiated in the cell membrane, most likely, through a receptor. Since we found that JNK1 is associated with the RIP-TRAF2 complex following H2O2 treatment, we also examined the localization of JNK1 and we found that JNK1 also colocalized with lipid rafts in response to H2O2 (data not shown).
Under physiological or pathological conditions, high levels of ROS are produced either intracellularly from mitochondria or exogenously from some adjacent phagocytes in the case of acute inflammation (15, 44). Based on the fact that H2O2 is highly cell permeable and capable of diffusing through the cell membrane freely (59), it is possible that exogenously produced or applied ROS act in a similar pattern as those generated intracellularly. Mitochondria-derived ROS have been implicated in TNF-induced necrotic cell death discovered in certain cell types, such as mouse fibrosarcoma L929 cells (16). One of the mechanisms is found to be the persistent activation of MAPK by ROS, a process suppressed by NF-κB (46). Nevertheless, it remains to be seen whether those endogenously produced ROS in TNF-treated cells also require some of the upstream TNF signaling molecules such as RIP to convey the cell death signals.
Taken together, based on our study, it appears that RIP and TRAF2 have critical functions in a much broader spectrum of signal transduction pathways than was originally thought and act as the convergence point to relay different stimuli or stressors to different downstream signaling pathways that determine life and death for the cell.
Acknowledgments
We thank M. Kelliher for RIP−/− fibroblasts, W.-C. Yeh and T. W. Mak for TRAF2−/− fibroblasts, B. Seed for RIP-deficient Jurkat cells, S. Nedospasov for TNFR1−/− fibroblasts, and J. D. Ashwell for providing 2B4 and VD-1 cells.
H.-M.S. is on study leave from NUS and is supported in part by an Oncology Research Faculty Development Program from NIH.
REFERENCES
- 1.Aragane, Y., D. Kulms, D. Metze, G. Wilkes, B. Poppelmann, T. A. Luger, and T. Schwarz. 1998. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J. Cell Biol. 140:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arron, J. R., Y. Pewzner-Jung, M. C. Walsh, T. Kobayashi, and Y. Choi. 2002. Regulation of the subcellular localization of tumor necrosis factor receptor-associated factor (TRAF)2 by TRAF1 reveals mechanisms of TRAF2 signaling. J. Exp. Med. 196:923-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, S. J., and E. P. Reddy. 1998. Modulation of life and death by the TNF receptor superfamily. Oncogene 17:3261-3270. [DOI] [PubMed] [Google Scholar]
- 4.Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. [DOI] [PubMed] [Google Scholar]
- 5.Baud, V., Z. G. Liu, B. Bennett, N. Suzuki, Y. Xia, and M. Karin. 1999. Signaling by proinflammatory cytokines: oligomerization of TRAF2 and TRAF6 is sufficient for JNK and IKK activation and target gene induction via an amino-terminal effector domain. Genes Dev. 13:1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benhar, M., D. Engelberg, and A. Levitzki. 2002. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 3:420-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmody, R. J., and T. G. Cotter. 2001. Signalling apoptosis: a radical approach. Redox. Rep. 6:77-90. [DOI] [PubMed] [Google Scholar]
- 8.Chen, G., and D. V. Goeddel. 2002. TNF-R1 signaling: a beautiful pathway. Science 296:1634-1635. [DOI] [PubMed] [Google Scholar]
- 9.Chen, N., Q. B. She, A. M. Bode, and Z. Dong. 2002. Differential gene expression profiles of Jnk1- and Jnk2-deficient murine fibroblast cells. Cancer Res. 62:1300-1304. [PubMed] [Google Scholar]
- 10.Chen, Y. R., X. Wang, D. Templeton, R. J. Davis, and T. H. Tan. 1996. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 271:31929-31936. [DOI] [PubMed] [Google Scholar]
- 11.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 12.Denecker, G., D. Vercammen, W. Declercq, and P. Vandenabeele. 2001. Apoptotic and necrotic cell death induced by death domain receptors. Cell. Mol. Life Sci. 58:356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 14.Devin, A., Y. Lin, and Z. G. Liu. 2003. The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep. 4:623-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Droge, W. 2002. Free radicals in the physiological control of cell function. Physiol. Rev. 82:47-95. [DOI] [PubMed] [Google Scholar]
- 16.Fiers, W., R. Beyaert, W. Declercq, and P. Vandenabeele. 1999. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene 18:7719-7730. [DOI] [PubMed] [Google Scholar]
- 17.Garay, M., W. Gaarde, B. P. Monia, P. Nero, and C. L. Cioffi. 2000. Inhibition of hypoxia/reoxygenation-induced apoptosis by an antisense oligonucleotide targeted to JNK1 in human kidney cells. Biochem. Pharmacol. 59:1033-1043. [DOI] [PubMed] [Google Scholar]
- 18.Gardner, A. M., F. H. Xu, C. Fady, F. J. Jacoby, D. C. Duffey, Y. Tu, and A. Lichtenstein. 1997. Apoptotic vs. nonapoptotic cytotoxicity induced by hydrogen peroxide. Free Radic. Biol. Med. 22:73-83. [DOI] [PubMed] [Google Scholar]
- 19.Garg, A. K., and B. B. Aggarwal. 2002. Reactive oxygen intermediates in TNF signaling. Mol. Immunol. 39:509-517. [DOI] [PubMed] [Google Scholar]
- 20.Gething, M. J. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10:465-472. [DOI] [PubMed] [Google Scholar]
- 21.Haidara, K., I. Morel, V. Abalea, B. M. Gascon, and F. Denizeau. 2002. Mechanism of tert-butylhydroperoxide induced apoptosis in rat hepatocytes: involvement of mitochondria and endoplasmic reticulum. Biochim. Biophys. Acta 1542:173-185. [DOI] [PubMed] [Google Scholar]
- 22.Halliwell, B. 1994. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721-724. [DOI] [PubMed] [Google Scholar]
- 23.Hampton, M. B., and S. Orrenius. 1997. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 414:552-556. [DOI] [PubMed] [Google Scholar]
- 24.Han, Z., D. L. Boyle, L. Chang, B. Bennett, M. Karin, L. Yang, A. M. Manning, and G. S. Firestein. 2001. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Investig. 108:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hochedlinger, K., E. F. Wagner, and K. Sabapathy. 2002. Differential effects of JNK1 and JNK2 on signal specific induction of apoptosis. Oncogene 21:2441-2445. [DOI] [PubMed] [Google Scholar]
- 26.Holler, N., R. Zaru, O. Micheau, M. Thome, A. Attinger, S. Valitutti, J. L. Bodmer, P. Schneider, B. Seed, and J. Tschopp. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1:489-495. [DOI] [PubMed] [Google Scholar]
- 27.Hreniuk, D., M. Garay, W. Gaarde, B. P. Monia, R. A. McKay, and C. L. Cioffi. 2001. Inhibition of c-Jun N-terminal kinase 1, but not c-Jun N-terminal kinase 2, suppresses apoptosis induced by ischemia/reoxygenation in rat cardiac myocytes. Mol. Pharmacol. 59:867-874. [DOI] [PubMed] [Google Scholar]
- 28.Hsu, H., J. Huang, H. B. Shu, V. Baichwal, and D. V. Goeddel. 1996. TNF-dependent recruitment of the protein kinase RIP to the TNF receptor-1 signaling complex. Immunity 4:387-396. [DOI] [PubMed] [Google Scholar]
- 29.Hsu, H., H. B. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 30.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 31.Karin, M., and A. Lin. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221-227. [DOI] [PubMed] [Google Scholar]
- 32.Karin, M., T. Takahashi, P. Kapahi, M. Delhase, Y. Chen, C. Makris, D. Rothwarf, V. Baud, G. Natoli, F. Guido, and N. Li. 2001. Oxidative stress and gene expression: the AP-1 and NF-kappaB connections. Biofactors 15:87-89. [DOI] [PubMed] [Google Scholar]
- 33.Kelliher, M. A., S. Grimm, Y. Ishida, F. Kuo, B. Z. Stanger, and P. Leder. 1998. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8:297-303. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 35.Legler, D. F., O. Micheau, M. A. Doucey, J. Tschopp, and C. Bron. 2003. Recruitment of TNF receptor 1 to lipid rafts is essential for TNFalpha-mediated NF-kappaB activation. Immunity 18:655-664. [DOI] [PubMed] [Google Scholar]
- 36.Lin, Y., A. Devin, Y. Rodriguez, and Z. G. Liu. 1999. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 13:2514-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, Z. G., H. Hsu, D. V. Goeddel, and M. Karin. 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell 87:565-576. [DOI] [PubMed] [Google Scholar]
- 38.Mak, T. W., and W. C. Yeh. 2002. Signaling for survival and apoptosis in the immune system. Arthritis Res. 4(Suppl. 3):S243-S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martindale, J. L., and N. J. Holbrook. 2002. Cellular response to oxidative stress: signaling for suicide and survival. J. Cell. Physiol. 192:1-15. [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya-Tsuji, J., K. Kishimoto, A. Hiyama, J. Inoue, Z. Cao, and K. Matsumoto. 1999. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252-256. [DOI] [PubMed] [Google Scholar]
- 41.Nishitoh, H., M. Saitoh, Y. Mochida, K. Takeda, H. Nakano, M. Rothe, K. Miyazono, and H. Ichijo. 1998. ASK1 is essential for JNK/SAPK activation by TRAF2. Mol. Cell 2:389-395. [DOI] [PubMed] [Google Scholar]
- 42.Pimentel-Muinos, F. X., and B. Seed. 1999. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity 11:783-793. [DOI] [PubMed] [Google Scholar]
- 43.Raha, S., and B. H. Robinson. 2001. Mitochondria, oxygen free radicals, and apoptosis. Am. J. Med. Genet. 106:62-70. [DOI] [PubMed] [Google Scholar]
- 44.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 45.Riley, P. A. 1994. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 65:27-33. [DOI] [PubMed] [Google Scholar]
- 46.Sakon, S., X. Xue, M. Takekawa, T. Sasazuki, T. Okazaki, Y. Kojima, J. H. Piao, H. Yagita, K. Okumura, T. Doi, and H. Nakano. 2003. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22:3898-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sata, N., H. Klonowski-Stumpe, B. Han, D. Haussinger, and C. Niederau. 1997. Menadione induces both necrosis and apoptosis in rat pancreatic acinar AR4-2J cells. Free Radic. Biol. Med. 23:844-850. [DOI] [PubMed] [Google Scholar]
- 48.Scharffetter-Kochanek, K., M. Wlaschek, P. Brenneisen, M. Schauen, R. Blaudschun, and J. Wenk. 1997. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 378:1247-1257. [PubMed] [Google Scholar]
- 49.Schoonbroodt, S., and J. Piette. 2000. Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem. Pharmacol. 60:1075-1083. [DOI] [PubMed] [Google Scholar]
- 50.Sies, H. 1997. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 82:291-295. [DOI] [PubMed] [Google Scholar]
- 51.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 52.Sotgia, F., B. Razani, G. Bonuccelli, W. Schubert, M. Battista, H. Lee, F. Capozza, A. L. Schubert, C. Minetti, J. T. Buckley, and M. P. Lisanti. 2002. Intracellular retention of glycosylphosphatidyl inositol-linked proteins in caveolin-deficient cells. Mol. Cell. Biol. 22:3905-3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda, M., I. Shirato, M. Kobayashi, and H. Endou. 1999. Hydrogen peroxide induces necrosis, apoptosis, oncosis and apoptotic oncosis of mouse terminal proximal straight tubule cells. Nephron 81:234-238. [DOI] [PubMed] [Google Scholar]
- 54.Tamura, K., T. Sudo, U. Senftleben, A. M. Dadak, R. Johnson, and M. Karin. 2000. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 55.Torres, M. 2003. Mitogen-activated protein kinase pathways in redox signaling. Front. Biosci. 8:d369-d391. [DOI] [PubMed] [Google Scholar]
- 56.Tournier, C., P. Hess, D. D. Yang, J. Xu, T. K. Turner, A. Nimnual, D. Bar-Sagi, S. N. Jones, R. A. Flavell, and R. J. Davis. 2000. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 288:870-874. [DOI] [PubMed] [Google Scholar]
- 57.Troyano, A., P. Sancho, C. Fernandez, E. de Blas, P. Bernardi, and P. Aller. 2003. The selection between apoptosis and necrosis is differentially regulated in hydrogen peroxide-treated and glutathione-depleted human promonocytic cells. Cell Death Differ. 10:889-898. [DOI] [PubMed] [Google Scholar]
- 58.Wang, X., J. L. Martindale, Y. Liu, and N. J. Holbrook. 1998. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem. J. 333:291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Werner, E. 2003. Determination of cellular H2O2 production. Sci. STKE 2003:PL3. [DOI] [PubMed] [Google Scholar]
- 60.Yang, Y., M. Mercep, C. F. Ware, and J. D. Ashwell. 1995. Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J. Exp. Med. 181:1673-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeh, W. C., A. Shahinian, D. Speiser, J. Kraunus, F. Billia, A. Wakeham, J. L. de la Pompa, D. Ferrick, B. Hum, N. Iscove, P. Ohashi, M. Rothe, D. V. Goeddel, and T. W. Mak. 1997. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7:715-725. [DOI] [PubMed] [Google Scholar]
- 62.Yu, S. W., H. Wang, M. F. Poitras, C. Coombs, W. J. Bowers, H. J. Federoff, G. G. Poirier, T. M. Dawson, and V. L. Dawson. 2002. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297:259-263. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, J., D. Cado, A. Chen, N. H. Kabra, and A. Winoto. 1998. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature 392:296-300. [DOI] [PubMed] [Google Scholar]