Abstract
The eukaryotic origin recognition complex (ORC) selects the genomic sites where prereplication complexes are assembled and DNA replication begins. In proliferating mammalian cells, ORC activity appears to be regulated by reducing the affinity of the Orc1 subunit for chromatin during S phase and then preventing reformation of a stable ORC-chromatin complex until mitosis is completed and a nuclear membrane is assembled. Here we show that part of the mechanism by which this is accomplished is the selective association of Orc1 with Cdk1 (Cdc2)/cyclin A during the G2/M phase of cell division. This association accounted for the appearance in M-phase cells of hyperphosphorylated Orc1 that was subsequently dephosphorylated during the M-to-G1 transition. Moreover, inhibition of Cdk activity in metaphase cells resulted in rapid binding of Orc1 to chromatin. However, chromatin binding was not mediated through increased affinity of Orc1 for Orc2, suggesting that additional events are involved in the assembly of functional ORC-chromatin sites. These results reveal that the same cyclin-dependent protein kinase that initiates mitosis in mammalian cells also concomitantly inhibits assembly of functional ORC-chromatin sites.
Eukaryotic DNA replication is a highly conserved process that begins with binding of a six-subunit origin recognition complex (ORC) to DNA (reviewed in references 6 and 8). Proteins Cdc6 and Cdt1 (also called RLF-B) then load Mcm proteins 2 to 7 onto these ORC-chromatin sites to form prereplication complexes (pre-RCs). Mcm2 to Mcm7 hexamers constitute the helicases that unwind the DNA. Pre-RCs are activated upon binding of Mcm10 protein (62). Cdc6 is then released by the cyclin-dependent protein kinase Cdk2/cyclin A and replaced by Cdc45 with the help of the protein kinases Cdc7/Dbf4 and Cdk2/cyclin E. DNA polymerase-α-DNA primase, which is escorted to the complex by Cdc45, then initiates RNA-primed DNA synthesis (S phase).
One universal feature of eukaryotic DNA replication is that the genome is replicated once and only once each time a cell divides. This is accomplished in two ways. First, pre-RCs that are assembled during the M-to-G1 phase transition are inactivated during S phase, and second, new pre-RCs cannot be assembled until mitosis is complete and a nuclear membrane is present. Regulation of pre-RC assembly and activation occurs at multiple steps, such as the Cdc6, Cdt1, Mcm2 to Mcm7, and Cdk2 functions (reviewed in references 7, 16, 40, and 59). However, the premier step in determining both where and when DNA replication begins is the assembly of functional ORC-chromatin sites.
A variety of data suggest the existence of a eukaryotic ORC cycle in which ORC activity is regulated by modification of one or more ORC subunits (16). In both budding yeast and fission yeast, ORC remains intact and bound to DNA throughout the cell cycle. Nevertheless, ORC subunits in yeast undergo cell cycle-dependent phosphorylation that contributes to preventing reinitiation of DNA replication prior to mitosis. ORC is phosphorylated by Cdk1/cyclin B during the G1-to-S transition, hyperphosphorylated during the S-to-M phase transition, and then hypophosphorylated during early G1 phase when pre-RC assembly occurs (40, 57, 65). Saccharomyces cerevisiae ORC mutants that cannot be phosphorylated permit reinitiation of DNA replication under conditions in which Cdc6 and Mcm proteins fail to be exported from the nucleus (40). In Schizosaccharomyces pombe, Cdk1/cyclin B associates with replication origins during S phase and remains there during G2 and early M phases (65). This association is ORC dependent and prevents reinitiation of DNA replication.
Whether or not ORC activity is also regulated in the metazoa has been controversial. Not only do species-specific differences exist, but different studies of the same species have sometimes yielded conflicting results. Just as yeast ORC, Xenopus laevis ORC exists as a stable unit, at least in frog egg extracts, but in contrast to the affinity of ORC for DNA in yeast cells, the affinity of X. laevis ORC for DNA in egg extracts diminishes once pre-RCs are assembled. The extent of this change depends on whether or not the substrate is sperm chromatin, in which case X. laevis ORC becomes salt sensitive (49), or somatic-cell chromatin, in which case X. laevis ORC is released from chromatin under DNA replication conditions (51). Thus, the affinity of X. laevis ORC for DNA is influenced significantly by chromatin structure. Some data suggest that the affinity of X. laevis ORC for chromatin is further reduced during metaphase. X. laevis ORC is displaced from chromatin during mitosis in cultured cells (47), and X. laevis ORC in interphase eggs binds to chromatin while ORC in metaphase eggs does not (11, 20, 26, 49). Moreover, X. laevis ORC in metaphase eggs is hyperphosphorylated (9, 56), reminiscent of ORC in yeast cells. This hyperphosphorylation appears to result from a Cdk/cyclin A activity because addition of cyclin A to interphase egg extracts can release X. laevis ORC from chromatin (20, 26) and prevent binding of X. laevis ORC to chromatin (66) whereas equivalent amounts of either cyclin B or cyclin E do not have these effects. However, while it is clear that protein kinase inhibitors do not prevent X. laevis ORC from binding to chromatin, the question of whether or not Cdk activity prevents binding of ORC to chromatin in unactivated, metaphase egg extracts has not been addressed.
Some data suggest that Cdk activity regulates pre-RC assembly at ORC-chromatin sites rather than binding of ORC to chromatin. Metaphase egg extract has been reported to release X. laevis ORC from sperm chromatin in some cases (49) but not in others (20). Furthermore, ORC is associated with Cdk1/cyclin A in interphase egg extracts (48), where it would be expected to inhibit binding of ORC to DNA but clearly does not. One possibility is that Cdk1/cyclin A triggers release of X. laevis ORC following pre-RC assembly, concomitant with degradation of the Cdk-specific inhibitor Xic1 (67). However, inhibition of Cdk activity in interphase egg extracts has no effect on either binding of ORC to or release of ORC from somatic-cell chromatin (51, 66). A second possibility is that Cdk1/cyclin A inhibits loading of Mcm proteins onto ORC-chromatin sites, because inhibition of Cdk activity in geminin-depleted metaphase eggs strongly stimulates binding of Mcm to chromatin (52). Thus, while it is clear that the affinity of X. laevis ORC for chromatin is cell cycle dependent, the mechanisms involved are not clear and the role of Cdk1, if any, is ambiguous.
Some studies of mammalian cells have concluded that all six ORC subunits remain stably bound to chromatin throughout cell division (reference 42 and references therein), whereas other studies suggest that ORC activity is inactivated during S phase by reducing interaction between the largest ORC subunit, Orc1, and ORC-chromatin sites. Orc1 can be selectively eluted from chromatin during S and M phases but not during G1 phase (21, 33, 36, 37, 39). Restoration of stable binding of Orc1 to chromatin occurs during the M-to-G1 transition and follows the same time course as degradation of cyclin B (reference 37 and this report), suggesting that exiting mitosis triggers stable binding of Orc1 to chromatin. Furthermore, stable binding of Orc1 to chromatin precedes the appearance of functional pre-RCs at specific origins of bidirectional replication (35, 36, 39), suggesting that assembly of functional ORC-chromatin sites is the rate-limiting step in the assembly of pre-RCs at specific genomic sites.
These changes reflect changes in the binding of ORC to DNA. A DNA footprint at the human lamin B2 origin that encompasses the start sites for leading-strand DNA replication (2) changes from a large footprint in G1 phase to a smaller footprint in S and G2 phases and is absent during M phase (3). During G1 phase, Orc1, Orc2, Cdc6, and Mcm3 proteins can be cross-linked to this DNA sequence, but during S phase, only the Orc2 protein can be cross-linked, consistent with release of Orc1 and disassembly of a pre-RC (1). By M phase, neither Orc1 nor Orc2 can be cross-linked to DNA. This selective loss in the ability to cross-link Orc1 to DNA in S-phase cells has been reported for other mammalian replication origins as well (34). Since all six ORC subunits are required for ORC activity in yeast, release of Orc1 should prevent ORC function during the S-to-M transition, and in fact, mammalian metaphase chromatin lacks functional ORCs. Chromatin from metaphase hamster cells will not initiate DNA replication in a Xenopus egg extract that has been depleted of X. laevis ORC proteins (35, 39, 68).
What prevents Orc1 from binding tightly to chromatin during the S-to-M transition? One mechanism is ubiquitination. In human cells, Homo sapiens Orc1 is selectively polyubiquitinated and degraded by 26S proteasomes during S phase and then reappears during the M-to-G1 transition (21, 37, 46, 53). However, significant amounts of human Orc1 remain and nuclei from G2-phase HeLa cells can still replicate their DNA when incubated in an ORC-depleted Xenopus egg extract (47). Furthermore, Orc1 in hamster S-phase cells is selectively monoubiquitinated but it is not selectively degraded unless the cells are lysed (36). Monoubiquitination of Orc1 may be sufficient to inhibit assembly of functional ORCs.
Here we report a second mechanism. Hamster Orc1 was found to be selectively bound to a protein kinase activity predominantly, if not exclusively, during the G2/M phase of the cell division cycle. This Orc1-associated protein kinase was identified as Cdk1/cyclin A. It was able to hyperphosphorylate Orc1 in vivo as well as in vitro, and inhibition of its activity resulted in rapid and stable binding of Orc1 to chromatin. These results confirm those of previous studies in which the affinity of Orc1 for chromatin was observed to be markedly reduced in metaphase hamster cells (36, 39) and extends previous results by providing a specific regulatory mechanism that accounts for this phenomenon. The same cyclin-dependent protein kinase (Cdk1) that regulates the onset of mitosis also regulates ORC activity.
MATERIALS AND METHODS
Cell culture and synchronization.
Exponentially proliferating Chinese hamster ovary cells (CHO C400) were cultured in Dulbecco's modified Eagle's medium plus 5% fetal bovine serum plus nonessential amino acids in 5% CO2 at 37°C until 80% confluent. Synchronization of cells in metaphase was done by culturing them in 0.05 μg of nocodazole for 4 h and then recovering nonattached cells by shaking them free of the dish (23). The fraction of M-phase cells was monitored by staining with Giemsa and then either scoring the number with condensed chromatin or using fluorescence-activated cell sorter (FACS) analysis. FACS analysis was carried out with aliquots of suspended cells that were fixed by addition of cold 100% methanol to a final concentration of 70%, incubated on ice for 30 min, collected by sedimentation, washed once with phosphate-buffered saline (PBS), and resuspended in PBS (106 cells/ml). Samples were incubated with 50 μg of RNase A (Sigma)/ml at 37°C for 30 min, and the cells were then stained with propidium iodide (20 μg/ml). Alternatively, cells were synchronized at their G1/S-phase boundary by isoleucine and serum deprivation for 48 h followed by culture in complete medium supplemented with 10 mM aphidicolin for 12 h (31). The fraction of cells in S phase was monitored by incorporation of bromodeoxyuridine followed by immunostaining (23). Cells were released into the cell cycle by changing to complete medium without metabolic inhibitors and culturing as described above.
Antibodies.
Antibodies directed against either full-length Chinese hamster Orc1 or Orc2 recombinant proteins were prepared as described previously (36). Clone A17.1.1 mouse monoclonal antibody against Xenopus Cdk1 (Cdc2) protein (Oncogene Research Products) reacts specifically with p34cdc2 from a variety of species. Clone EG7.1 mouse monoclonal antibody against bovine p62 cyclin A protein (Oncogene Research Products) reacts specifically with cyclin A from a variety of species. Cyclin B1 and cyclin E polyclonal antibodies (Ab-3 and Ab-2, respectively; Oncogene Research Products) were specific for human, mouse, and rat proteins.
Protein analyses.
Whenever cells were trypsinized, they were washed first with complete medium. To assay total cellular proteins, cells were washed twice with PBS and resuspended in 20 volumes of Tris-glycine-sodium dodecyl sulfate (Tris-glycine-SDS) sample buffer (Invitrogen). To assay chromatin-bound and -unbound proteins, cells were washed twice with PBS and resuspended in 10 volumes of modified cytoskeleton (CSK) extraction buffer (10 mM HEPES [pH 7.5], 150 mM NaCl, 300 mM sucrose, 1 mM MgCl2, 1 mM Mg++-ATP, 1 mM EGTA, 1 mM dithiothreitol, protease inhibitor cocktail [Roche], 20 μM MG-132 [Calbiochem], and 0.1% Triton X-100) and then incubated on ice for 10 min before centrifugation in an Eppendorf microcentrifuge at 4,000 rpm and 4°C for 5 min (36). The pellet contained the chromatin-bound proteins, while the supernatant contained the chromatin-unbound proteins. All samples were heated immediately at 75°C for 5 min and then fractionated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (39).
SDS-PAGE was done on 4 to 20% gradient Tris-glycine gels (Invitrogen) under the reducing conditions suggested by the manufacturer. The bottom portion of the gel was stained with Simply Blue Safestain (Invitrogen) to visualize proteins. The top portion of the gel was subjected to immunoblotting as described by the antibody supplier. Prestained molecular weight standards were included (See-Blue+2; Invitrogen) in kinase assays and immunoblotting assays. Unstained molecular weight standards (Invitrogen) were included when gels were first stained to visualize proteins. Prior to autoradiography, gels were dried under a vacuum. About 105 cell equivalents was used per gel lane.
Immunoprecipitation.
Cells were washed first in complete medium and then in PBS, pelleted, resuspended in 10 pellet volumes of lysis buffer supplemented with protease inhibitor cocktail (Roche), and then incubated on ice for 10 min before centrifugation. Cells were lysed either in mammalian protein extraction reagent (M-PER; Pierce Biotechnology) according to the manufacturer's instructions or in CSK buffer containing 0.35 M NaCl as described previously (39). With both methods, ∼95% of the Orc1 and Orc2 was solubilized. Immunoprecipitation was carried out as previously described (36), except that the antibodies were immobilized onto Aminolink Plus coupling gel using an Aminolink Plus immobilization kit (Pierce) to prevent contamination of immunoprecipitates (IPs) with immunoglobulin G (IgG). Antibodies were added directly to M-PER lysates, but CSK lysates were first diluted twofold with CSK buffer without NaCl. Both methods gave the same results.
Protein kinase assay.
IPs from ∼2.5 × 105 cells were resuspended in a 20-μl assay mixture containing calf thymus histone H1 and 10 μCi of [γ-32P]ATP (13). Reaction mixtures were incubated at 35°C in a Thermomixer (Eppendorf) for 30 min at 800 rpm before fractionation of the proteins by SDS-PAGE.
RESULTS
The affinity of Orc1 for chromatin is reduced during mitosis.
Previous studies reported that the concentrations of Orc1, Orc2, and Orc4 in CHO C400 cells were constant throughout the cell cycle when assayed in SDS cell lysates but that when cells were lysed in a Triton X-100 buffer and then fractionated into chromatin-bound and -unbound proteins, all of the Orc2 and Orc4 remained bound to chromatin in both metaphase (M)- and G1-phase cells while the amount of bound Orc1 in M-phase cells was greatly reduced compared with that in G1-phase cells (36, 39, 42). However, in some cases, M-phase Orc1 appeared in the chromatin-unbound fraction (36, 39), whereas in other cases it did not (42), resulting in confusion as to whether or not the association of Orc1 with chromatin is cell cycle dependent. The problem is that mammalian Orc1 is sensitive to ubiquitination and degradation (36, 37); Orc1 in the chromatin-unbound fraction is degraded (even when samples are on ice), whereas Orc1 in the chromatin-bound fraction is not (36).
In the present study, this problem was avoided by including MG-132, a specific inhibitor of the 26S proteasome, in the cell lysis buffer along with a cocktail of other protease inhibitors. Asynchronous CHO C400 cells were incubated in nocodazole for 4 h for accumulation of M-phase cells, and one portion was then released into G1 phase. At the times indicated in the figures, aliquots of cells were either lysed with SDS to measure total Orc1 or lysed with CSK buffer and separated immediately into chromatin-bound and -unbound fractions that were fractionated by PAGE. As previously reported (36), chromatin-bound proteins were enriched with core histones while the unbound fraction was enriched with histone H1 (Fig. 1A). Under these conditions, the level of total cellular Orc1 remained constant but ∼94% of the Orc1 was not bound to chromatin in M-phase cells while ∼98% of the Orc1 was bound to chromatin in late-G1-phase cells (Fig. 1A). This phenomenon was not a nocodazole artifact, because >99% of Orc1 was not bound to chromatin in M-phase cells isolated in the absence of nocodazole (for examples, see Fig. 8A and C in reference 36 and Fig. 7A in reference 39). These results confirmed that the affinity of hamster Orc1 for chromatin was cell cycle dependent under the conditions used in the following experiments.
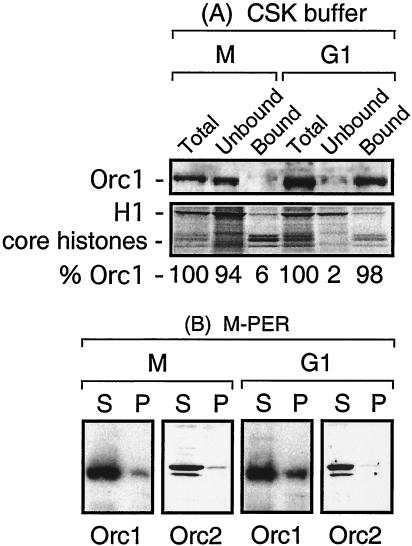
FIG. 1.
The affinity of Orc1 for chromatin changes during the M-to-G1 phase transition. (A) Orc1 could be selectively eluted from chromatin in M-phase cells but not from that in G1-phase cells. CHO C400 cells were synchronized at metaphase and then released into G1 phase for 4 h. Aliquots of cells were then lysed either with SDS to measure total Orc1 or with CSK buffer to measure chromatin-bound and -unbound Orc1. Fractions were then subjected to SDS-PAGE; the upper portion of the gel was assayed for Orc1 protein, and the lower portion was stained for total proteins. Proteins were identified by their antibody specificities and their migration patterns during SDS-PAGE. The fraction of Orc1 was calculated by defining the amounts of total Orc1 in M- and G1-phase cells each as 100%. The relative amount of Orc1 in each lane was normalized to the amount of either H1 or core histone present. (B) CHO C400 cells were lysed either in M-PER (shown here) or in CSK buffer containing 0.35 M NaCl (data not shown) in order to solubilize 90 to 95% of the Orc1 and Orc2 under conditions suitable for immunoprecipitation. S, soluble fraction; P, immunoprecipitate.
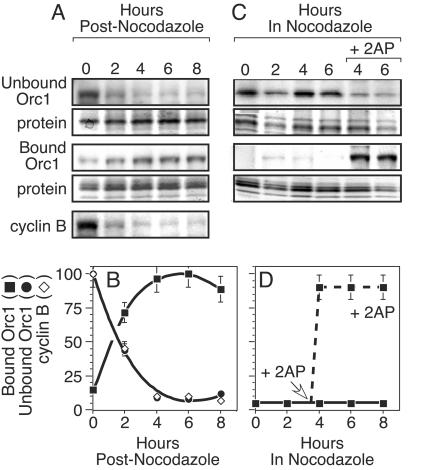
FIG. 8.
Inhibition of Cdk activity in M-phase-arrested cells causes rapid binding of Orc1 to chromatin. (A) M-phase-arrested cells (0) were released into G1 phase. At the times indicated, cells were lysed and proteins were separated into chromatin-bound and -unbound fractions. Following fractionation by SDS-PAGE, the top portion of the gel was assayed for Orc1 by immunoblotting while the bottom portion of the gel was stained with Simply Blue to detect proteins. Cyclin B was assayed in total cell extract by immunoblotting. (B) The relative amounts of chromatin-unbound Orc1, chromatin-bound Orc1, and cyclin B were quantified from results of three experiments by densitometry of data such as those in panel A. Results were normalized to the amount of protein in each lane. Amounts of Orc1 were calculated relative to the maximum amounts of Orc1 in the bound or unbound fraction. Amounts of cyclin B were calculated relative to the total amount of cyclin B in the extract. (C) M-phase-arrested cells were lysed at the indicated times after addition of nocodazole. Proteins were separated into chromatin-bound and -unbound fractions and treated as described for panel B. In one portion of cells, 10 mM 2AP was added to the culture medium 3.5 h after addition of nocodazole. (D) The fraction of chromatin-bound Orc1 (▪) before (solid line) and after (broken line) addition of 2AP was quantified from results of three experiments by densitometry of data such as those in panel C.
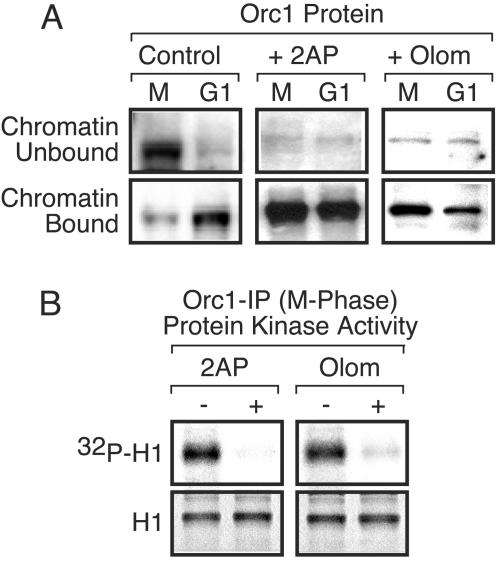
FIG. 7.
Cdk activity in M-phase-arrested cells prevents binding of Orc1 to chromatin. (A) The ratio of Orc1 in the chromatin-unbound and chromatin-bound fractions from M-phase-arrested cells and from cells 4 h after their release into G1 phase (G1) were assayed before (control) and after the cells had been incubated either with 10 mM 2AP (+ 2AP) or with 200 μM olomoucine (+ Olom) for 2 h prior to cell lysis. (B) Protein kinase activity was assayed in Orc1 IPs from M-phase-arrested cells that either had been treated with the indicated inhibitor (+) or had not been treated (−).
Orc1 is associated with a protein kinase during mitosis.
To determine whether or not cell cycle-dependent changes in the affinity of Orc1 for chromatin involve a protein kinase activity, cells were again synchronized in M phase and then released into G1 phase. At the times indicated, cells were lysed under conditions that released 90 to 95% of the Orc1 and Orc2 in either M-phase or G1-phase cells (Fig. 1B; data not shown). Orc1 and Orc2 were then individually immunoprecipitated from each cell extract and assayed for protein kinase activity by using histone H1 as the substrate. Throughout this and subsequent experiments, equivalent amounts of Orc1 and Orc2 were present in IPs from cells throughout the cell cycle, consistent with results of analyses of SDS cell lysates (Fig. 2). Furthermore, anti-Orc1 IPs contained Orc1 but not Orc2 and anti-Orc2 IPs contained Orc2 but not Orc1. Normal IgG IPs contained neither Orc1 nor Orc2. Examples from various experiments are shown in various figures. Proteins were identified by their antibody specificities and molecular weights.
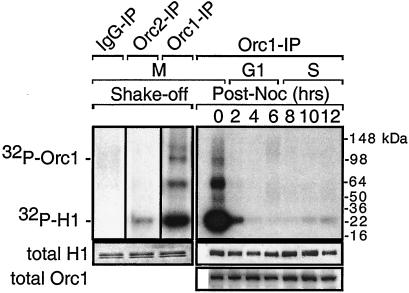
FIG. 2.
The only major Orc1-associated protein kinase activity appears during mitosis. Metaphase cells were collected either in the absence (shake-off) or in the presence of nocodazole and then released into G1 phase (post-noc) for the times indicated. Total ORC proteins were extracted and then immunoprecipitated with either anti-Orc1 (Orc1-IP), anti-Orc2 (Orc2-IP), or normal rabbit IgG (IgG-IP). IPs were assayed for protein kinase activity by labeling histone H1 with [γ-32P]ATP.
The results revealed that Orc1 was associated with a protein kinase activity during M phase but not during G1 or S phase (Fig. 2). To eliminate the possibility that this association was due to the nocodazole block, M-phase cells were isolated in the absence of any metabolic inhibitor by being shaken off the dish. Again Orc1 was associated with a strong protein kinase activity (Fig. 2). In comparison, little protein kinase activity was detected in Orc2 IPs and none in IgG IPs (Fig. 2; data not shown).
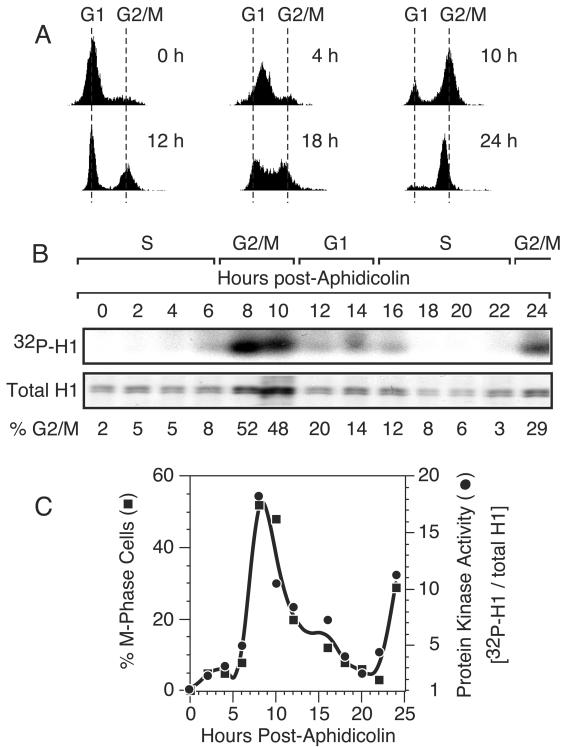
Cells were also synchronized at the beginning of S phase by inhibition of DNA synthesis with aphidicolin and then released into S phase by culturing of the cells in the absence of the drug. Cell cycle progress was monitored by FACS analysis (Fig. 3A), and the fraction of cells in G2/M phase was calculated from these data (Fig. 3B). Again, an Orc1-associated protein kinase activity was clearly present only during the G2/M period (Fig. 3B). Following the first mitosis, protein kinase activity was absent from the Orc1 IP until cells reentered mitosis. Quantifying these data confirmed that the level of Orc1-associated protein kinase activity correlated closely with the fraction of G2/M-phase cells (Fig. 3C). Protein kinase activity was not detected in Orc2 IPs from the same cell extracts (data not shown). The above-described results revealed that hamster Orc1 is selectively associated with a protein kinase primarily, if not exclusively, as cells transit G2/M phase.
FIG. 3.
The Orc1-associated protein kinase activity is specific to G2/M phase. (A) Cells were synchronized at the beginning of S phase with aphidicolin and then released. The fractions of cells in G1, S, and G2/M phases were determined by FACS analysis. Six examples are shown. (B) Protein kinase activity was assayed in the Orc1 IP from each fraction as described in the legend to Fig. 2. The fraction of cells in G2/M was calculated from FACS data by using CellQuest software. (C) The fraction of M-phase cells was compared with the ratio of protein kinase activity (intensity of the 32P-H1 protein band) normalized to histone H1 protein in each fraction. Gel bands were quantified with NIH Image software.
The Orc1-associated protein kinase is Cdk1/cyclin A.
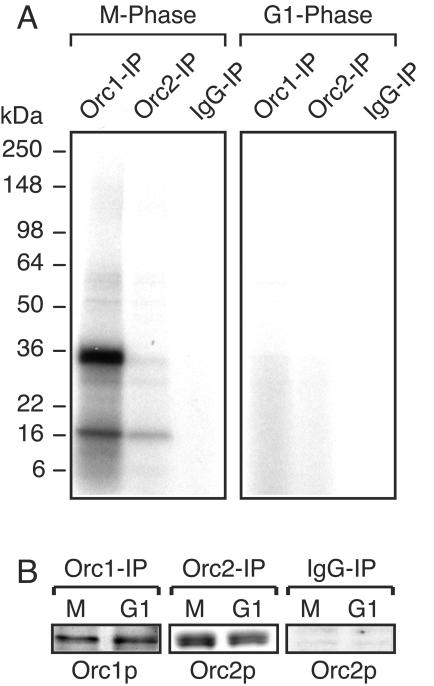
The Orc1-associated protein kinase activity was identified as Cdk1/cyclin A on the basis of its cell cycle specificity, ATP-binding affinity, molecular weight, antibody recognition profile, immunoprecipitation with either anti-Orc1 or anti-Cdk1 antibodies, and sensitivity to inhibitors. First, Orc1 and Orc2 IPs were prepared from either M-phase or late-G1-phase cell extracts and then incubated with [α-32P]ATP and exposed to UV radiation in order to cross-link ATP to ATP-binding proteins. The results revealed that Orc1 from mitotic cells was associated with a single, major ATP-binding protein of 34 kDa and a minor ATP-binding protein of 17 to 18 kDa whereas little, if any, of these ATP-binding proteins was detected in Orc1 IPs from G1-phase cells (Fig. 4). Under these conditions, an ATP-Orc1 complex was not detected, although this strategy can be adapted to identify ORC subunits that bind ATP (32). The major ATP-binding protein, and most of the minor ones, was absent from all Orc2 IPs and normal IgG IPs. Therefore, Orc1 is selectively associated with a 34-kDa ATP-binding protein only during the G2/M period of the cell cycle, suggesting that the Orc1-associated protein kinase was Cdk1, the cyclin-dependent protein kinase required for mitosis.
FIG. 4.
Orc1-associated protein kinase is a 34-kDa ATP-binding protein. Total ORC proteins were extracted from cells either after arrest in M phase with nocodazole or 4 h after release from nocodazole (G1 phase) and immunoprecipitated with either anti-Orc1 IgG, anti-Orc2 IgG, or normal IgG. (A) Each IP was resuspended in protein kinase assay cocktail without H1 protein, incubated on ice for 5 min in the presence of [α-32P]ATP, and then spotted onto a cell culture dish surface resting on ice and irradiated in a UV Stratelinker (0.2 J; Stratagene) to cross-link ATP to ATP-binding proteins. The reaction mixture was then fractionated by SDS-PAGE and subjected to autoradiography. (B) Aliquots from each IP were fractionated by SDS-PAGE, and the protein target was detected by immunoblotting. IgG IP was assayed for Orc2.
To test this hypothesis, Orc1 IPs were prepared from M- and late-G1-phase cells, fractionated by SDS-PAGE, and assayed for the presence of specific proteins by immunoblotting with specific antibodies. A monoclonal antibody specific for Cdk1 (24) detected only one protein in extracts of M- and G1-phase cells, and the molecular mass of this protein was 34 kDa, the same as that of Cdk1 (Fig. 5A). Moreover, this protein was present in Orc1 IPs from M-phase cells but little, if any, was present in Orc1 IPs from G1-phase cells, and the protein was absent from normal IgG IPs. Reciprocal IPs obtained using anti-Orc1 and anti-Cdk1 antibodies confirmed the association between Orc1 and Cdk1. Orc1 IPs from M-phase cells contained Cdk1, and Cdk1 IPs contained Orc1 (Fig. 5B). Moreover, both Cdk1 and Orc1 IPs from M-phase cells produced the same pattern of 32P-labeled proteins during in vitro protein kinase assays (Fig. 5B), confirming that the major protein kinase activity in Orc1 IPs was Cdk1.
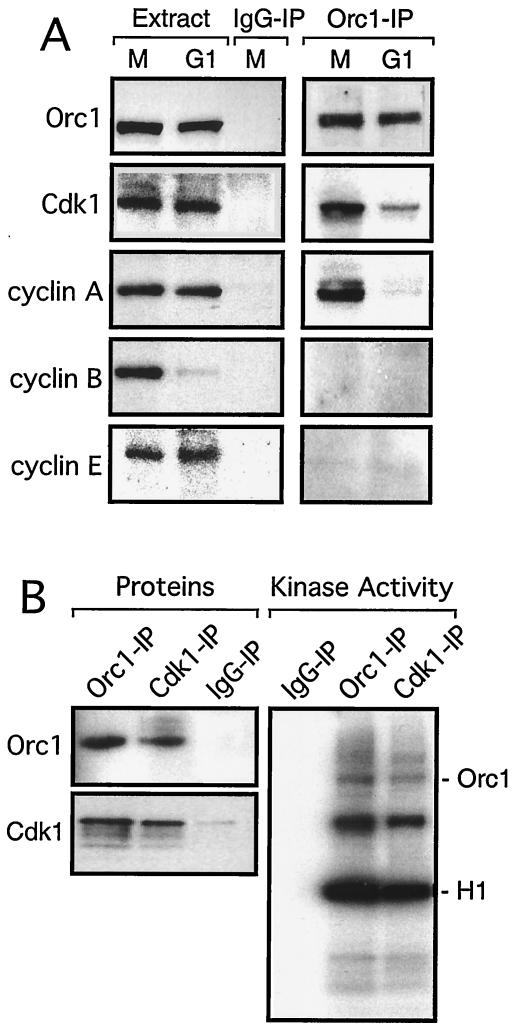
FIG. 5.
Cdk1/cyclin A is associated with Orc1 in M-phase cells. (A) Antibodies specific for Orc1 (96 kDa), Cdk1 (34 kDa), and cyclins A (62 kDa), B (62 kDa), and E (50 kDa) were used to detect these proteins in extracts of M-phase-arrested and G1-phase cells, prepared as described in the legend to Fig. 4, and in Orc1 IPs from these extracts that were first fractionated by SDS-PAGE and then immunoblotted with the indicated antibody. Normal IgG IPs provided a control. Proteins were identified by their antibody specificities and their migration patterns in SDS-PAGE. (B) Orc1, Cdk1, and normal IgG IPs were prepared from M-phase-arrested cells and assayed for either Orc1 or Cdk1 (proteins). Each IP was also assayed for protein kinase activity (kinase activity).
Since Cdk1 protein kinase activity requires a cyclin protein coactivator, assays for cyclins A, B, and E were carried out. A monoclonal antibody specific for cyclin A (4) detected a single protein in these cell extracts, and the molecular mass of this protein was 62 kDa, the same as that of cyclin A (Fig. 5A). Moreover, this antibody detected cyclin A in Orc1 IPs from M-phase cells but not in those from G1-phase cells (Fig. 5A), suggesting that Cdk1/cyclin A was the Orc1-associated protein kinase. This conclusion was strengthened by the fact that antibodies specific for cyclin B or cyclin E did not detect these proteins in Orc1 IPs from either M-phase or G1-phase cell extracts (Fig. 5A). This finding was not due to a lack of sensitivity, because cyclin B was easily detected in M-phase cell extracts but not in G1-phase extract and cyclin E was barely detectable in both M- and G1-phase extracts. These results were consistent with those of previous studies on the relative amounts of cyclins A, B, and E during proliferation of mammalian cells (5, 18, 41). None of these four proteins were detected in Orc2 IPs (data not shown) or normal IgG IPs from either M- or G1-phase cells (Fig. 5A).
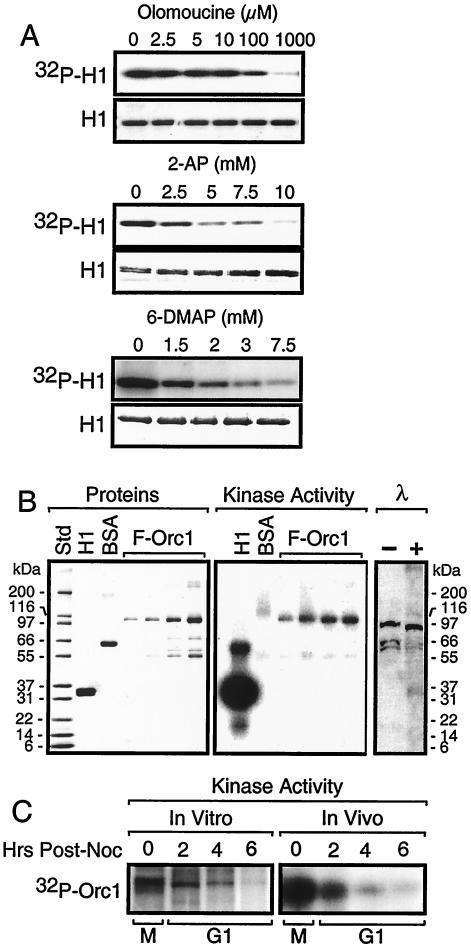
The Orc1-associated protein kinase activity was sensitive to inhibitors of cyclin-dependent protein kinases but not to inhibitors of protein kinase A or microtubule-associated protein (MAP) kinase. Olomoucine, a specific inhibitor of Cdk1, Cdk2, and Cdk5 (50% inhibitory concentration [IC50], 7 μM [30]), inhibited 50% of the protein kinase activity in Orc1 IPs from M-phase cell extracts at a concentration of ∼10 μM (Fig. 6A). 2-Aminopurine (2AP) and 6-dimethylaminopurine (6DMAP), general inhibitors of protein kinases that also inhibit Cdk activities, required 10-fold greater concentrations to inhibit this protein kinase to the same extent as olomoucine. Previous studies have shown that protein kinase A activation in late M phase is required for the subsequent S phase (12). Therefore, a specific inhibitor of protein kinase A (5-24 [CalBiochem]; Ki = 2.3 nM) was tested, but even 1 μM did not inhibit Orc1-associated protein kinase activity. Similarly, specific inhibitors of MAP kinase did not inhibit this activity (200 nM SB202190 [IC50 = 16 nM] and 100 nM SB203580 [IC50 = 34 nM]; CalBiochem). Taken together, these data are consistent with the conclusion that the Orc1-associated protein kinase is Cdk1/cyclin A.
FIG. 6.
Characterization of the Orc1-associated protein kinase activity from M-phase cells. (A) Inhibitor sensitivity. Orc1 IP from M-phase-arrested cells was assayed for protein kinase activity in the presence of the indicated concentrations of either olomoucine, 2AP, or 6DMAP. (B) Orc1 substrate sensitivity. Increasing amounts (0.1, 0.25, 0.5, and 1 μg) of FLAG-tagged Orc1 (F-Orc1) were added to an Orc1 IP from M-phase-arrested cells. One set of aliquots was fractionated by SDS-PAGE and stained with Simply Blue Safestain to detect proteins. Another set of aliquots was fractionated by SDS-PAGE and subjected to autoradiography to detect protein kinase activity. Lanes marked λ contained aliquots (10 μl) of M-phase extract that were incubated with (+) or without (−) 10 U of λ-phosphatase (Calbiochem) in 50 mM Tris-HCl (pH 7.8), 5 mM dithiothreitol, 2 mM MnCl2, and 100 μg of bovine serum albumin (BSA)/ml at 30°C for 1 h. The reaction mixtures were subjected to SDS-PAGE and assayed for Orc1 by immunoblotting. Std, molecular mass standard. (C) Orc1 IP protein kinase activity versus M-to-G1-phase protein kinase activity. Orc1 IPs were prepared from cells at the indicated times after addition of nocodazole (post-noc) and then assayed for protein kinase activity (in vitro). Alternatively, the same cells were cultured for 2 h with 10 μCi of 32Pi/ml (in vivo). Orc1 IPs were then prepared, fractionated by SDS-PAGE, and subjected to autoradiography.
Orc1 is hyperphosphorylated in M-phase cells.
The endogenous Orc1 in Orc1 IPs appeared to be phosphorylated by the Orc1-associated protein kinase, because a 32P-labeled protein of ∼96 kDa appeared in protein kinase assays (Fig. 2B). This conclusion was confirmed by adding purified FLAG-tagged hamster Orc1 to an Orc1 IP together with a known Cdk substrate, histone H1, and a nonsubstrate, bovine serum albumin, and then carrying out protein kinase assays. Both histone H1 and FLAG-Orc1 (∼97 kDa) were phosphorylated, but bovine serum albumin was not (Fig. 6B). FLAG-Orc2 also could be phosphorylated under the same conditions (data not shown).
To determine whether or not Orc1 was hyperphosphorylated in M-phase cells, an Orc1 IP from M-phase cell extract was treated with λ-phosphatase and Orc1 was assayed for a change in mobility during gel electrophoresis (Fig. 6B). Hamster Orc1 is 96 kDa (39). Orc1 from M-phase hamster cells was converted from a 100-kDa to a 96-kDa protein, consistent with a reduction in its phosphorylation. Similar results have been reported for Orc1 in M-phase human cells (54, 55).
Orc1 was phosphorylated in M-phase cells and dephosphorylated during the M-to-G1 transition, both in vitro and in vivo (Fig. 6C). In vitro, an Orc1 IP from M-phase cells phosphorylated the endogenous Orc1 in the IP whereas the extent of this phosphorylation decreased steadily when the Orc1 IP was prepared from G1-phase cell extracts taken at different times after cells were released from the nocodazole block. In vivo, incubation of 32Pi with M-phase cells radiolabeled Orc1 protein to a much greater extent than incubation of 32Pi with G1-phase cells. Thus, the Orc1-associated protein kinase activity appears to be responsible for the hyperphosphorylation of Orc1 during mitosis. The extent of endogenous Orc1 phosphorylation decreased at least 10-fold during the M-to-G1 transition.
Cdk activity prevents binding of Orc1 to chromatin in metaphase cells.
When hamster cells were arrested in M phase with nocodazole, at least 90% of Orc1 was in the chromatin-unbound fraction (see Fig. 1A; 7A, control lanes; and 8A, lane 0). When these cells were transferred to nocodazole-free culture medium, Orc1 rapidly bound to chromatin (see Fig. 1A; 7B; and 8A, lanes 2 to 8). The time course for the transition from chromatin-unbound to chromatin-bound Orc1 was the same as the time course for the disappearance of cyclin B during the M-to-G1 phase transition (see Fig. 8A and B).
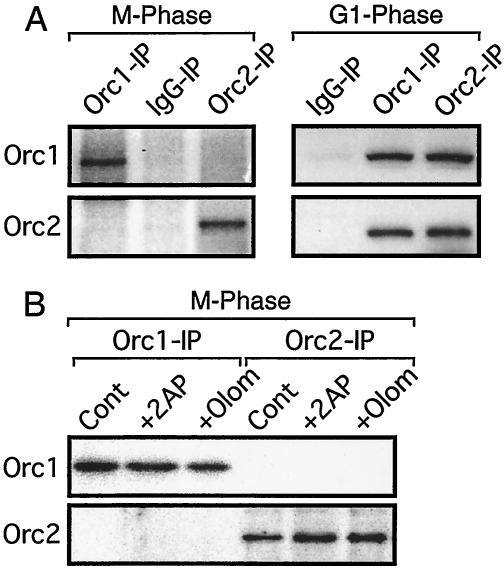
To determine whether or not a Cdk activity was required to prevent Orc1 from binding to metaphase chromatin, cells arrested in nocodazole and cells released into G1 phase for 4 h were cultured throughout this time in the presence or absence of either 2AP or olomoucine. In the absence of a protein kinase inhibitor, about 10% of the cells cultured in nocodazole were arrested in metaphase and 90% or more of the Orc1 in these cells was in the chromatin-unbound fraction (Fig. 7A, control lanes). In the presence of either 2AP or olomoucine, >99% of the Orc1-associated protein kinase activity was inhibited (Fig. 7B), about 3% of the cells were arrested in metaphase, and more than 95% of the Orc1 in the M-phase cells was in the chromatin-bound fraction (Fig. 7A). In contrast, neither protein kinase inhibitor altered the amount of Orc1 bound to chromatin in G1-phase cells (Fig. 7A).
To determine whether or not the effects of protein kinase inhibition were immediate, M-phase-arrested cells were treated either with 2AP (Fig. 8C) or with olomoucine (data not shown) after they had been exposed to nocodazole for a total of 3.5 h. Within 30 min, >90% of Orc1 was chromatin bound, whereas in the absence of either inhibitor, Orc1 remained in the chromatin-unbound fraction (Fig. 8C and D). Therefore, protein kinase activity was required both for entering mitosis and for preventing premature binding of Orc1 to chromatin.
Cdk activity does not prevent binding of Orc1 to Orc2 in metaphase cells.
Orc1 and Orc2 were not associated during mitosis, because Orc1 IPs from M-phase cell extracts did not contain Orc2 and Orc2 IPs did not contain Orc1 (Fig. 9A). This finding was consistent with the facts that Cdk1 protein and protein kinase activity were present in Orc1 IPs but not in Orc2 IPs (Fig. 2, 4, and 5) and that Orc1 (Fig. 5B) but not Orc2 (data not shown) was detected in Cdk1 IPs. However, when the same experiment was carried out with extracts from late-G1-phase cells, Orc1 and Orc2 were both precipitated using either anti-Orc1 or anti-Orc2 IgG (Fig. 9A). Therefore, as cells transit from M to G1 phase, Orc1 and Orc2 form a stable complex that presumably includes other ORC subunits as well. Since Orc2 and the other ORC subunits are stably bound to chromatin in M-phase cells, this change in the affinity of Orc1 for other ORC subunits would account for binding of Orc1 to chromatin at those sites where ORC subunits 2 to 6 are located.
FIG. 9.
Cdk activity does not prevent association of Orc1 with Orc2 during mitosis. (A) Orc1 IPs, Orc2 IPs, and normal IgG IPs were prepared from M-phase and G1-phase cells, as described in the legend to Fig. 4, and the presence of either Orc1 or Orc2 was assayed, as described in the legend to Fig. 5. (B) The experiment described for panel A was repeated except that 1 aliquot of M-phase-arrested cells was treated either with 10 mM 2AP (+ 2AP) or with 30 μM olomoucine (+ Olom) for 2 h before preparation of cell extracts. Cont, control.
To determine whether or not a protein kinase activity in M phase was responsible for preventing Orc1 association with Orc2 during mitosis, M-phase-arrested cells were treated either with 2AP or with olomoucine, as described above. However, in contrast to the change in Orc1 association with chromatin, no change was detected in the association between Orc1 and Orc2. Neither inhibitor induced formation of a detectable Orc1/Orc2 complex in metaphase-arrested cells (Fig. 9B). Therefore, the G2/M-phase-specific Orc1-associated protein kinase activity was not solely responsible for preventing association between Orc1 and Orc2 during mitosis.
DISCUSSION
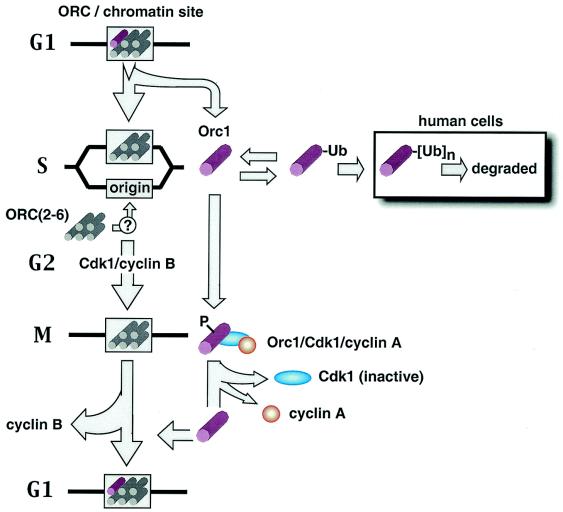
Eukaryotic cells appear to regulate the assembly of functional ORC-chromatin sites through cell cycle-dependent modifications of one or more ORC subunits (see the introduction). In mammalian cells, one essential feature of this ORC cycle is that Orc1 is selectively prevented from rebinding tightly to ORC-chromatin sites until mitosis is completed and a nuclear membrane is assembled (outlined in Fig. 10). In both human (37) and hamster (Fig. 8B) cells, rebinding of Orc1 to chromatin is inversely related to degradation of cyclin B. Since cyclin B is degraded during metaphase (10), stable binding of Orc1 to chromatin is triggered during the transition from M to G1 phase. In hamster cells, the total amount of Orc1 in M-phase cells was essentially the same as that in G1-phase cells, and most if not all of it was not ubiquitinated. Nevertheless, most of the Orc1 in metaphase cells was recovered in the chromatin-unbound fraction whereas most of the Orc1 in G1-phase cells was recovered in the chromatin-bound fraction, revealing that the affinity of Orc1 for chromatin is cell cycle dependent (Fig. 1A) (36, 39). In fact, when metaphase cells were not arrested in nocodazole, the amount of chromatin-bound Orc1 was <1% of the amount bound to G1-phase chromatin (Fig. 8C) (36, 39). When cells were arrested in nocodazole, from 5 to 20% of the Orc1 became stably bound to chromatin with time (Fig. 8A and C) (36).
FIG. 10.
Mammalian ORC cycle. DNA synthesis in mammalian cells triggers a change in the affinity of Orc1 protein for chromatin that allows Orc1 to be selectively eluted while the remaining ORC subunits remain chromatin bound. In S-phase hamster cells, Orc1 becomes monoubiquitinated and remains in the nucleus. In human cells, most but not all of the Orc1 becomes polyubiquitinated and degraded. It seems likely, although it is not known, that ORC core complexes bind to newly created origins during S phase. During the S-to-M transition in hamster cells, the monoubiquitinated form of Orc1 disappears and a hyperphosphorylated form of Orc1 appears that is associated with Cdk1/cyclin A. As cells exit mitosis and enter G1 phase, cyclin B is degraded, Cdk1 and cyclin A presumably dissociate, Orc1-associated protein kinase activity disappears, and Orc1 is hypophosphorylated and bound to chromatin, presumably at ORC-chromatin sites.
Regulation of ORC activity during mitosis in mammalian cells.
What prevents Orc1 from binding to chromatin during mitosis in mammalian cells? Metaphase hamster chromatin is not inherently resistant to binding ORC, because it rapidly binds Xenopus ORC proteins and initiates DNA replication when incubated in Xenopus egg extract (51). Furthermore, binding of Orc1 to chromatin during the M-to-G1 transition does not require protein synthesis (42). Therefore, some posttranslational modification must occur, either on preexisting Orc1 and/or on chromatin, that determines the affinity of Orc1 for chromatin. The results presented here show that Orc1 associates with Cdk1/cyclin A protein kinase during mitosis and that this activity prevents Orc1 from binding to chromatin.
Virtually all of the Orc1-associated protein kinase activity was detected specifically in G2/M-phase cells, and this association was independent of the method by which M-phase cells were collected or synchronized (Fig. 2 and 3B). The predominant Cdk activity in G2/M-phase cells is Cdk1. In fact, the Orc IP from M-phase cells contained a single major ATP-binding protein and this protein had the same molecular mass as Cdk1 (34 kDa) (Fig. 4). Moreover, this protein was not detected in Orc IPs from G1-phase cell extracts. The Orc1 IP from M-phase cells also contained a single protein that reacted with an antibody specific for Cdk1, and this protein was 34 kDa (Fig. 5A). The association of Cdk1 with Orc1 was specific, because Cdk1 IPs contained Orc1 but not Orc2 (Fig. 5B); Orc2 IPs contained only a small fraction of the protein kinase activity detected in Orc1 IPs (Fig. 2). The same Orc1 IP also contained cyclin A but not cyclin B or E. In contrast, Orc1 IPs from G1-phase cells contained a little Cdk1 but no cyclin A, B, or E (Fig. 5A). This would account for the absence of Orc1-associated protein kinase activity in G1-phase cells (Fig. 2 and 3). Orc1-associated protein kinase activity was also inhibited strongly by olomoucine, a specific inhibitor of Cdk1, Cdk2, and Cdk5 (30), as well as by general protein kinase inhibitors such as 2AP and 6DMAP, but not by specific inhibitors of protein kinase A or MAP kinase (Fig. 6A; data not shown). Finally, Cdk1 IPs phosphorylated the same proteins that were phosphorylated in Orc1 IPs (Fig. 5B), consistent with the conclusion that Cdk1 is the major protein kinase in both preparations.
Based on the information summarized above and the specificity of olomoucine, we conclude that the Cdk1/cyclin A activity during mitosis is responsible for preventing stable binding of Orc1 to chromatin. Addition of either olomoucine or 2AP to metaphase-arrested cells resulted in rapid inhibition of the Orc1-associated protein kinase activity with concomitant binding of Orc1 to chromatin (Fig. 7 and 8). Moreover, endogenous Orc1 in M-phase cells was hyperphosphorylated (Fig. 6B and C), as previously reported for human cells (54, 55), and the ability of the G2/M-phase-specific Orc1-associated protein kinase to phosphorylate Orc1 in vitro paralleled the ability of M-phase and G1-phase hamster cells to phosphorylate Orc1 in vivo (Fig. 6B and C). Therefore, it is likely that this Orc1-associated protein kinase is responsible for hyperphosphorylating Orc1 during G2/M phase and that hyperphosphorylation of Orc1 inhibits its association with chromatin.
This conclusion is consistent with findings of earlier studies that manipulated the ratio of Orc1 to Cdk1. Expression of human Orc1 in Schizosaccharomyces pombe led to continuous DNA synthesis in the absence of mitosis, and this phenomenon required the Cdk-targeted portion of human Orc1 (63). Conversely, reduction of Cdk1 activity in human cells not only prevented mitosis but also induced rereplication of the genome (27). This study reveals a role for Cdk1 in regulating DNA replication as well as mitosis. The results presented here suggest that this role is to prevent assembly of functional ORC-chromatin sites. It should be noted that reduction of Cdk1 activity does not always induce reinitiation of DNA replication, because inactivation of a temperature-sensitive Cdk1 mutant in a mouse cell line arrests cells in G2 phase without affecting DNA replication (25), consistent with the fact that initiation of DNA replication is regulated through multiple coherent pathways. Rereplication prior to mitosis may require overriding more than one control mechanism (40, 59).
The expression patterns of Cdk1 and cyclins are consistent with the role proposed for Cdk1/cyclin A in regulating DNA replication. Cdk1 is present at approximately constant levels throughout the mammalian cell cycle (60), although active Cdk1/cyclin B protein kinase, the primary trigger for mitosis, first appears on the centrosomes during prophase (28). All three cyclins can be detected throughout the cell cycle, but the amount and location of each one is cell cycle dependent (18, 41). Cyclin A accumulation begins with the onset of DNA synthesis and continues throughout S phase (19), and cyclin A is then degraded during prometaphase (14, 22). Cyclin A is located in the nucleus during interphase, where Cdk2/cyclin A is required for DNA replication (43) and for inactivation of Cdc6 (29, 44). Cyclin A also binds to Cdk1 in G2 phase, where it is required for entrance into mitosis (43). Cyclin A is associated with chromosomes during prophase but not during metaphase (45), consistent with assembly of an Orc1/Cdk1/cyclin A complex. In contrast, cyclin B is located in the cytoplasm during interphase, translocated to the nucleus during the G2-to-M phase transition, and then degraded during metaphase (10). This accounts for its relative absence in G1-phase cells (Fig. 5A and 8A). Cyclin E accumulates in the nucleus during late G1 phase, where, in cooperation with Cdc6, it stimulates pre-RC assembly (13). Cyclin E is degraded during S phase, leaving barely detectable amounts from late S phase to the subsequent early G1 phase.
Association between ORC proteins and chromatin.
Does Orc1 dissociate from chromatin during the S-to-M phases of the cell cycle, or does it remain associated with chromatin but with an altered affinity that inhibits its ability to form a functional ORC-chromatin site? The available data support the second conclusion. During the S-to-M-phase period of cell division, Orc1 can be selectively released from chromatin under conditions in which the other ORC subunits remain bound (see the introduction). The same conditions that selectively elute Orc1 from chromatin also selectively elute histone H1 (Fig. 1A) (36). In fact, Orc1, like H1, remains with the chromatin fraction when metaphase cells are permeabilized with digitonin under low-salt conditions that support initiation of DNA replication (39), and ectopically expressed Orc1 remains associated with chromatin even in metaphase cells (42). However, the fact that metaphase chromatin cannot replicate in an ORC-depleted Xenopus egg extract reveals that although mammalian ORC proteins are present, they are not functional (35, 39, 68). When metaphase chromatin is incubated in complete egg extract, X. laevis ORC proteins rapidly bind to the chromatin, initiate DNA replication, and are then released spontaneously (51). Thus, both the intact frog ORC and the mammalian Orc1 subunit exhibit a cell cycle-dependent change in their affinities for mammalian chromatin.
This change in affinity of Orc1 for chromatin is reflected in change in the binding of Orc1 to DNA. During G1 phase, Orc1 can be cross-linked to DNA either with formaldehyde (34) or with UV irradiation (1), but not during the S-to-M transition. An earlier report that human Orc1 remained stably bound to chromatin throughout the cell cycle and could be UV cross-linked to DNA in metaphase cells (54) was later called into question, because the antibody (3A2A) used to detect human Orc1 in those experiments was found to cross-react with the 100-kDa polypyrimidine tract binding protein-associated splicing factor (53). A new antibody prepared against full-length human Orc1 revealed that human Orc1 was selectively degraded by the 26S proteasome during S phase (53).
What causes ORC to alter its affinity for chromatin? In frog egg extracts, reduced affinity between X. laevis ORC and chromatin is triggered solely by completion of pre-RC assembly; inhibitors of either Cdks or DNA replication have no effect (49, 51). However, chromatin structure does have an effect. X. laevis ORC becomes salt sensitive after pre-RC assembly on sperm chromatin (49), but it is spontaneously released after pre-RC assembly on hamster chromatin (51). Interestingly, the affinity of Orc1 for chromatin in mammalian cells is not determined simply by its affinity for other ORC subunits, because Cdk activity in metaphase cells was solely responsible for preventing stable binding of Orc1 to chromatin but it was not responsible for preventing binding of Orc1 to Orc2 (Fig. 9B). Since the affinity between Orc1 and Orc2 did increase during the M-to-G1 phase transition (Fig. 9A), a second event must occur during the M-to-G1 phase transition that assembles a functional ORC on the chromatin. This event may correspond to the origin decision point (64), that time during G1 phase when functional pre-RCs appear at specific sites on mammalian genomes. If the Orc2 to Orc5 core complex (17, 58) remains bound to specific genome sites throughout the cell cycle, then Orc1 may determine which of these sites is selected for pre-RC assembly based on the interaction of Orc1 with chromatin-bound proteins other than ORC subunits (35, 36, 39). Moreover, Orc1 may chaperon Cdc6 to specific ORC-chromatin sites. Cdc6 can bind to Orc1 (50), and Cdc6 can facilitate binding of Xenopus ORC to somatic cell chromatin (51) and Saccharomyces cerevisiae ORC to origin-containing DNA (38). Thus, the ORC cycle provides a mechanistic basis for the Jesuit Model (15) of initiation site selection in mammalian cells.
Relationship between Orc1 ubiquitination and phosphorylation.
In hamster cells, Orc1 is selectively monoubiquitinated, but it is not selectively degraded (Fig. 10) (36, 42). In human cells, Orc1 is selectively polyubiquitinated and degraded, although as much as 30% remains during mitosis (Fig. 10) (37, 46, 53). Polyubiquitination and degradation of hamster Orc1 has been observed, but only in cell lysates (36). Similarly, degradation of human Orc1 by the 26S proteasome in some human cells appears to occur only after cell lysis, because degradation is prevented by addition of MG-132 to the lysis buffer (46). Whether ubiquitination is responsible for releasing chromatin-bound Orc1 or is simply a mechanism for preventing Orc1 from rebinding to chromatin during S phase remains to be determined. What is clear is that by the time cells have entered mitosis, the Orc1 that is present is not ubiquitinated. This can occur in three ways: (i) degradation of polyubiquitinated Orc1 by the 26S proteasome and resynthesis of Orc1; (ii) removal of mono- or diubiquitinated residues by ubiquitin hydrolases (61); and (iii) degradation and resynthesis of Orc1 by other pathways. The half-life for both hamster Orc1 and Orc2 in vivo is ∼3 h (36, 42), which allows sufficient time for at least 75% of the ubiquitin-Orc1 pool to be replaced by Orc1 without the help of 26S proteasomes.
In addition to the role of Cdk1/cyclin A described in this paper, Cdk2/cyclin A is involved in preventing premature reassembly of pre-RCs during S phase (13). Cdk2/cyclin A can also associate with human Orc1 and phosphorylate it in vitro (37). However, since these studies were done with exponentially proliferating 293 cells, only about 1% of which would be in mitosis, the G2/M-phase-specific association between Orc1 and Cdk1/cyclin A would not have been detected. In fact, the data presented here (Fig. 1 and 2) reveal that the amount of protein kinase activity associated with Orc1 during G1 and S phases is <1% of the amount associated with Orc1 during G2/M phase. The relationship between Cdk2 activity and ORC-chromatin interaction, if any, remains to be demonstrated.
Role of Cdk1 in other eukaryotic ORC cycles.
Similarities between the results presented here and those from yeast cells suggest that Cdk1 activity regulates not only mitosis throughout the eucarya but ORC activity as well. In yeast, Cdk1/cyclin B phosphorylates several ORC subunits during the S-to-M transition, and this modification inhibits ORC activity during mitosis and perhaps during S phase as well by preventing the same ORC from being used a second time. In mammals, Cdk1/cyclin A phosphorylates a single ORC subunit, Orc1, in a cell cycle-dependent manner. Although Cdk1 can phosphorylate other ORC subunits, such as Orc2, in vitro, there is no evidence that this occurs in vivo. Moreover, the fact that inhibition of protein kinase activity during mitosis does not induce association of Orc1 with Orc2 suggests that Cdk1 does not mediate assembly of ORC subunits. Results from Xenopus egg extracts (see the introduction) are more difficult to reconcile with the model in Fig. 10. We suggest that the compressed cell cycle in rapidly cleaving frog eggs (30 min) and the lack of a G1 phase preclude dissociation of X. laevis ORC from Cdk1/cyclin A and that the X. laevis ORC remains intact, nonubiquitinated, and associated with Cdk1/cyclin A throughout these rapid S-to-M phases. We suggest that ORC-associated Cdk1 activity is inhibited by Xic1, a Cdk-specific peptide inhibitor, that is degraded only after assembly of pre-RCs on chromatin (67).
Acknowledgments
We thank Jonathon Pines (Cancer Research Institute, Cambridge, United Kingdom) for information about cyclin-dependent protein kinases.
REFERENCES
- 1.Abdurashidova, G., M. B. Danailov, A. Ochem, G. Triolo, V. Djeliova, S. Radulescu, A. Vindigni, S. Riva, and A. Falaschi. 2003. Localization of proteins bound to a replication origin of human DNA along the cell cycle. EMBO J. 22:4294-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdurashidova, G., M. Deganuto, R. Klima, S. Riva, G. Biamonti, M. Giacca, and A. Falaschi. 2000. Start sites of bidirectional DNA synthesis at the human lamin B2 origin. Science 287:2023-2026. [DOI] [PubMed] [Google Scholar]
- 3.Abdurashidova, G., S. Riva, G. Biamonti, M. Giacca, and A. Falaschi. 1998. Cell cycle modulation of protein-DNA interactions at a human replication origin. EMBO J. 17:2961-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adamczewski, J. P., J. V. Gannon, and T. Hunt. 1993. Simian virus 40 large T antigen associates with cyclin A and p33cdk2. J. Virol. 67:6551-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arooz, T., C. H. Yam, W. Y. Siu, A. Lau, K. K. Li, and R. Y. Poon. 2000. On the concentrations of cyclins and cyclin-dependent kinases in extracts of cultured human cells. Biochemistry 39:9494-9501. [DOI] [PubMed] [Google Scholar]
- 6.Bell, S. P. 2002. The origin recognition complex: from simple origins to complex functions. Genes Dev. 16:659-672. [DOI] [PubMed] [Google Scholar]
- 7.Bell, S. P., and A. Dutta. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71:333-374. [DOI] [PubMed] [Google Scholar]
- 8.Bogan, J. A., D. A. Natale, and M. L. DePamphilis. 2000. Initiation of eukaryotic DNA replication: conservative or liberal? J. Cell. Physiol. 184:139-150. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter, P. B., and W. G. Dunphy. 1998. Identification of a novel 81-kDa component of the Xenopus origin recognition complex. J. Biol. Chem. 273:24891-24897. [DOI] [PubMed] [Google Scholar]
- 10.Clute, P., and J. Pines. 1999. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat. Cell Biol. 1:82-87. [DOI] [PubMed] [Google Scholar]
- 11.Coleman, T. R., P. B. Carpenter, and W. G. Dunphy. 1996. The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell 87:53-63. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo, V., E. V. Avvedimento, M. E. Gottesman, J. Gautier, and D. Grieco. 1999. Protein kinase A is required for chromosomal DNA replication. Curr. Biol. 9:903-906. [DOI] [PubMed] [Google Scholar]
- 13.Coverley, D., H. Laman, and R. A. Laskey. 2002. Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat. Cell Biol. 4:523-528. [DOI] [PubMed] [Google Scholar]
- 14.den Elzen, N., and J. Pines. 2001. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J. Cell Biol. 153:121-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePamphilis, M. L. 1993. Eukaryotic DNA replication: anatomy of an origin. Annu. Rev. Biochem. 62:29-63. [DOI] [PubMed] [Google Scholar]
- 16.DePamphilis, M. L. 2003. The “ORC cycle”: a novel pathway for regulating eukaryotic DNA replication. Gene 310:1-15. [DOI] [PubMed] [Google Scholar]
- 17.Dhar, S. K., L. Delmolino, and A. Dutta. 2001. Architecture of the human origin recognition complex. J. Biol. Chem. 276:29067-29071. [DOI] [PubMed] [Google Scholar]
- 18.Dulic, V., E. Lees, and S. I. Reed. 1992. Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257:1958-1961. [DOI] [PubMed] [Google Scholar]
- 19.Erlandsson, F., C. Linnman, S. Ekholm, E. Bengtsson, and A. Zetterberg. 2000. A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp. Cell Res. 259:86-95. [DOI] [PubMed] [Google Scholar]
- 20.Findeisen, M., M. El-Denary, T. Kapitza, R. Graf, and U. Strausfeld. 1999. Cyclin A-dependent kinase activity affects chromatin binding of ORC, Cdc6, and MCM in egg extracts of Xenopus laevis. Eur. J. Biochem. 264:415-426. [DOI] [PubMed] [Google Scholar]
- 21.Fujita, M., Y. Ishimi, H. Nakamura, T. Kiyono, and T. Tsurumi. 2002. Nuclear organization of DNA replication initiation proteins in mammalian cells. J. Biol. Chem. 277:10354-10361. [DOI] [PubMed] [Google Scholar]
- 22.Geley, S., E. Kramer, C. Gieffers, J. Gannon, J. M. Peters, and T. Hunt. 2001. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153:137-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert, D. M., H. Miyazawa, and M. L. DePamphilis. 1995. Site-specific initiation of DNA replication in Xenopus egg extract requires nuclear structure. Mol. Cell. Biol. 15:2942-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodger, N. M., J. Gannon, T. Hunt, and P. R. Morgan. 1996. The localization of p34cdc2 in the cells of normal, hyperplastic, and malignant epithelial and lymphoid tissues of the oral cavity. J. Pathol. 178:422-428. [DOI] [PubMed] [Google Scholar]
- 25.Hamaguchi, J. R., R. A. Tobey, J. Pines, H. A. Crissman, T. Hunter, and E. M. Bradbury. 1992. Requirement for p34cdc2 kinase is restricted to mitosis in the mammalian cdc2 mutant FT210. J. Cell Biol. 117:1041-1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hua, X. H., and J. Newport. 1998. Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2. J. Cell Biol. 140:271-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itzhaki, J. E., C. S. Gilbert, and A. C. Porter. 1997. Construction by gene targeting in human cells of a “conditional” CDC2 mutant that rereplicates its DNA. Nat. Genet. 15:258-265. [DOI] [PubMed] [Google Scholar]
- 28.Jackman, M., C. Lindon, E. A. Nigg, and J. Pines. 2003. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat. Cell Biol. 5:143-148. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, W., N. J. Wells, and T. Hunter. 1999. Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc. Natl. Acad. Sci. USA 96:6193-6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knockaert, M., P. Greengard, and L. Meijer. 2002. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 23:417-425. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi, T., T. Rein, and M. L. DePamphilis. 1998. Identification of primary initiation sites for DNA replication in the hamster dihydrofolate reductase gene initiation zone. Mol. Cell. Biol. 18:3266-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong, D., T. R. Coleman, and M. L. DePamphilis. 2003. Xenopus origin recognition complex (ORC) initiates DNA replication preferentially at sequences targeted by Schizosaccharomyces pombe ORC. EMBO J. 22:3441-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreitz, S., M. Ritzi, M. Baack, and R. Knippers. 2001. The human origin recognition complex protein 1 dissociates from chromatin during S phase in HeLa cells. J. Biol. Chem. 276:6337-6342. [DOI] [PubMed] [Google Scholar]
- 34.Ladenburger, E. M., C. Keller, and R. Knippers. 2002. Identification of a binding region for human origin recognition complex proteins 1 and 2 that coincides with an origin of DNA replication. Mol. Cell. Biol. 22:1036-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, C. J., J. A. Bogan, D. A. Natale, and M. L. DePamphilis. 2000. Selective activation of pre-replication complexes in vitro at specific sites in mammalian nuclei. J. Cell Sci. 113:887-898. [DOI] [PubMed] [Google Scholar]
- 36.Li, C. J., and M. L. DePamphilis. 2002. Mammalian Orc1 protein is selectively released from chromatin and ubiquitinated during the S-to-M transition in the cell division cycle. Mol. Cell. Biol. 22:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendez, J., X. H. Zou-Yang, S. Y. Kim, M. Hidaka, W. P. Tansey, and B. Stillman. 2002. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell 9:481-491. [DOI] [PubMed] [Google Scholar]
- 38.Mizushima, T., N. Takahashi, and B. Stillman. 2000. Cdc6p modulates the structure and DNA binding activity of the origin recognition complex in vitro. Genes Dev. 14:1631-1641. [PMC free article] [PubMed] [Google Scholar]
- 39.Natale, D. A., C. J. Li, W. H. Sun, and M. L. DePamphilis. 2000. Selective instability of Orc1 protein accounts for the absence of functional origin recognition complexes during the M-G(1) transition in mammals. EMBO J. 19:2728-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen, V. Q., C. Co, and J. J. Li. 2001. Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411:1068-1073. [DOI] [PubMed] [Google Scholar]
- 41.Ohtsubo, M., A. M. Theodoras, J. Schumacher, J. M. Roberts, and M. Pagano. 1995. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol. Cell. Biol. 15:2612-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuno, Y., A. J. McNairn, N. den Elzen, J. Pines, and D. M. Gilbert. 2001. Stability, chromatin association and functional activity of mammalian pre-replication complex proteins during the cell cycle. EMBO J. 20:4263-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen, B. O., J. Lukas, C. S. Sorensen, J. Bartek, and K. Helin. 1999. Phosphorylation of mammalian CDC6 by cyclin A/CDK2 regulates its subcellular localization. EMBO J. 18:396-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pines, J., and T. Hunter. 1991. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol. 115:1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritzi, M., K. Tillack, J. Gerhardt, E. Ott, S. Humme, E. Kremmer, W. Hammerschmidt, and A. Schepers. 2003. Complex protein-DNA dynamics at the latent origin of DNA replication of Epstein-Barr virus. J. Cell Sci. 116:3971-3984. [DOI] [PubMed] [Google Scholar]
- 47.Romanowski, P., M. A. Madine, A. Rowles, J. J. Blow, and R. A. Laskey. 1996. The Xenopus origin recognition complex is essential for DNA replication and MCM binding to chromatin. Curr. Biol. 6:1416-1425. [DOI] [PubMed] [Google Scholar]
- 48.Romanowski, P., J. Marr, M. A. Madine, A. Rowles, J. J. Blow, J. Gautier, and R. A. Laskey. 2000. Interaction of Xenopus Cdc2 cyclin A1 with the origin recognition complex. J. Biol. Chem. 275:4239-4243. [DOI] [PubMed] [Google Scholar]
- 49.Rowles, A., S. Tada, and J. J. Blow. 1999. Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins. J. Cell Sci. 112:2011-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saha, P., J. Chen, K. C. Thome, S. J. Lawlis, Z. H. Hou, M. Hendricks, J. D. Parvin, and A. Dutta. 1998. Human CDC6/Cdc18 associates with Orc1 and cyclin-cdk and is selectively eliminated from the nucleus at the onset of S phase. Mol. Cell. Biol. 18:2758-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun, W. H., T. R. Coleman, and M. L. DePamphilis. 2002. Cell cycle-dependent regulation of the association between origin recognition proteins and somatic cell chromatin. EMBO J. 21:1437-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tada, S., A. Li, D. Maiorano, M. Mechali, and J. J. Blow. 2001. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat. Cell Biol. 3:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatsumi, Y., S. Ohta, H. Kimura, T. Tsurimoto, and C. Obuse. 2003. The ORC1 cycle in human cells. I. Cell cycle-regulated oscillation of human ORC1. J. Biol. Chem. 278:41528-41534. [DOI] [PubMed] [Google Scholar]
- 54.Tatsumi, Y., T. Tsurimoto, K. Shirahige, H. Yoshikawa, and C. Obuse. 2000. Association of human origin recognition complex 1 with chromatin DNA and nuclease-resistant nuclear structures. J. Biol. Chem. 275:5904-5910. [DOI] [PubMed] [Google Scholar]
- 55.Thome, K. C., S. K. Dhar, D. G. Quintana, L. Delmolino, A. Shahsafaei, and A. Dutta. 2000. Subsets of human origin recognition complex (ORC) subunits are expressed in non-proliferating cells and associate with non-ORC proteins. J. Biol. Chem. 275:35233-35241. [DOI] [PubMed] [Google Scholar]
- 56.Tugal, T., X. H. Zou-Yang, K. Gavin, D. Pappin, B. Canas, R. Kobayashi, T. Hunt, and B. Stillman. 1998. The Orc4p and Orc5p subunits of the Xenopus and human origin recognition complex are related to Orc1p and Cdc6p. J. Biol. Chem. 273:32421-32429. [DOI] [PubMed] [Google Scholar]
- 57.Vas, A., W. Mok, and J. Leatherwood. 2001. Control of DNA rereplication via Cdc2 phosphorylation sites in the origin recognition complex. Mol. Cell. Biol. 21:5767-5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vashee, S., P. Simancek, M. D. Challberg, and T. J. Kelly. 2001. Assembly of the human origin recognition complex. J. Biol. Chem. 276:26666-26673. [DOI] [PubMed] [Google Scholar]
- 59.Vaziri, C., S. Saxena, Y. Jeon, C. Lee, K. Murata, Y. Machida, N. Wagle, D. S. Hwang, and A. Dutta. 2003. A p53-dependent checkpoint pathway prevents rereplication. Mol. Cell 11:997-1008. [DOI] [PubMed] [Google Scholar]
- 60.Welch, P. J., and J. Y. Wang. 1992. Coordinated synthesis and degradation of cdc2 in the mammalian cell cycle. Proc. Natl. Acad. Sci. USA 89:3093-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wilkinson, K. D. 2000. Ubiquitination and deubiquitination: targeting of proteins for degradation by the proteasome. Semin. Cell Dev. Biol. 11:141-148. [DOI] [PubMed] [Google Scholar]
- 62.Wohlschlegel, J. A., S. K. Dhar, T. A. Prokhorova, A. Dutta, and J. C. Walter. 2002. Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Mol. Cell 9:233-240. [DOI] [PubMed] [Google Scholar]
- 63.Wolf, D. A., D. Wu, and F. McKeon. 1996. Disruption of re-replication control by overexpression of human ORC1 in fission yeast. J. Biol. Chem. 271:32503-32506. [DOI] [PubMed] [Google Scholar]
- 64.Wu, J. R., and D. M. Gilbert. 1996. A distinct G1 step required to specify the Chinese hamster DHFR replication origin. Science 271:1270-1272. [DOI] [PubMed] [Google Scholar]
- 65.Wuarin, J., V. Buck, P. Nurse, and J. B. Millar. 2002. Stable association of mitotic cyclin B/Cdc2 to replication origins prevents endoreduplication. Cell 111:419-431. [DOI] [PubMed] [Google Scholar]
- 66.Xu, H., Z. H. Lu, and G. H. Leno. 2002. The binding of ORC2 to chromatin from terminally differentiated cells. Exp. Cell Res. 274:334-341. [DOI] [PubMed] [Google Scholar]
- 67.You, Z., K. Harvey, L. Kong, and J. Newport. 2002. Xic1 degradation in Xenopus egg extracts is coupled to initiation of DNA replication. Genes Dev. 16:1182-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu, G., J. R. Wu, and D. M. Gilbert. 1998. Analysis of mammalian origin specification in ORC-depleted Xenopus egg extracts. Genes Cells 3:709-720. [DOI] [PubMed] [Google Scholar]