Abstract
RalA and RalB constitute a family of highly similar (85% identity) Ras-related GTPases. Recently, active forms of both RalA and RalB have been shown to bind to the exocyst complex, implicating them in the regulation of cellular secretion. However, we show here that only active RalA enhances the rate of delivery of E-cadherin and other proteins to their site in the basolateral membrane of MDCK cells, consistent with RalA being a regulator of exocyst function. One reason for this difference is that RalA binds more effectively to the exocyst complex than active RalB does both in vivo and in vitro. Another reason is that active RalA localizes to perinuclear recycling endosomes, where regulation of vesicle sorting is thought to take place, while active RalB does not. Strikingly, analysis of chimeras made between RalA and RalB reveals that high-affinity exocyst binding by RalA is due to unique amino acid sequences in RalA that are distal to the common effector-binding domains shared by RalA and RalB. Moreover, these chimeras show that the perinuclear localization of active RalA is due in part to its unique variable domain near the C terminus. This distinct localization appears to be important for RalA effects on secretion because all RalA mutants tested that failed to localize to the perinuclear region also failed to promote basolateral delivery of E-cadherin. Interestingly, one of these inactive mutants maintained binding to the exocyst complex, suggesting that RalA binding to the exocyst is necessary but not sufficient for RalA to promote basolateral delivery of membrane proteins.
RalA and RalB constitute a family of proteins within the Ras branch of small GTPases (5). They are highly similar, with over 85% amino acid sequence identity. Like all members of the GTPase family, Ral proteins cycle between the active GTP and inactive GDP-bound states (for a review, see reference 40). What distinguishes Ral proteins from other GTPases is that they are activated by a unique set of guanine nucleotide exchange factors, which promote GDP-to-GTP exchange in response to specific upstream signals. Ral proteins also bind to and alter the activity of a distinct set of downstream target proteins when in the active GTP-bound state. Finally, Ral proteins are inactivated by a unique GTPase-activating protein (GAP) (for a review, see reference 9).
Ral proteins are present in the plasma membrane, but a major fraction of the proteins reside on intracellular vesicles (2, 21, 26). These include components of both the endocytic and exocytic compartments, with both RalA and RalB present at particularly high levels in synaptic vesicles and in platelet granules. It is likely, however, that RalA and RalB have different subcellular distributions because, although both proteins are posttranslationally modified by geranylgeranylation (20), a major difference in their amino acid sequences is in the “variable domain” near their C termini. In other Ras GTPases, this region participates in targeting the proteins to specific membrane compartments.
A family of Ral-specific guanine nucleotide exchange factors that couple Ral activation to a wide variety of upstream signals exist (33). The best-studied example is a set of Ral-specific guanine nucleotide exchange factors that are activated by binding activated Ras. Since Ras is activated by many types of stimuli, Ral proteins also have the potential to participate in mediating the action of a wide variety of extracellular signals. Ral proteins can also be activated by Ras-independent mechanisms, which are not well understood (1, 13, 34). RalA and RalB are identical in regions thought to respond to guanine nucleotide exchange factors, and to date no differences in the responsiveness of RalA and RalB to upstream signals have been reported.
Active RalA has a wide range of effects on cells. It can activate transcription factors, promote cell proliferation, and even contribute to oncogenic transformation (for a review, see reference 9). The mechanism by which Ral influences these processes remains unclear, because the specific downstream target proteins responsible for these effects are not known. However, at least three downstream targets of both Ral proteins that potentially allow Ral proteins to influence vesicle sorting and the actin cytoskeleton have been identified. The first Ral target identified was RalBP1/RLIP (4, 16), which connects Ral proteins with components of the cellular machinery involved in receptor-mediated endocytosis. For example, RalBP1 forms a complex with proteins such as adaptin (17), POB1/Reps1 (18, 44), and epsin and eps15 (22). Although active RalB suppresses receptor-mediated endocytosis (17, 25), how it influences endocytosis through these proteins is not yet clear. Active RalA has since been shown to interact with the actin-binding protein filamin and as such to promote filapodium-like changes in cell morphology (28).
Recently, the exocyst complex has been shown to be another Ral effector (3, 23, 31, 39). The exocyst is a multiprotein complex that contains at least eight different subunits, and active RalA and active RalB have been shown to bind to both the sec5 and exo84 subunits in a GTP-dependent manner (23, 24, 39). The exocyst was first identified in Saccharomyces cerevisiae, where it directs vectorial targeting of secretory vesicles to specific sites on the plasma membrane (for a review, see reference 27). A similar complex also exists in mammalian cells, where it also participates in delivering vesicles to the plasma membrane (14, 19). In polarized epithelial cells, the exocyst participates in the delivery of membrane components destined for the basolateral but not the apical side of the cells (12). In neurons, the exocyst appears to participate in delivering synaptic vesicles (42) and calcium channels, like the N-methyl-d-aspartate glutamate receptor (36) to newly generated synapses, although its abundance in the synaptic endings of adult animals (43) suggests that it may also contribute to the maintenance of synaptic function (31). The exocyst also targets Glut4-containing vesicles to the plasma membrane in response to insulin (15).
How Ral influences secretion through exocyst binding is just beginning to be revealed. Depletion of RalA by interfering RNA expression leads to dissociation of the exocyst complex and a breakdown in polarized membrane delivery (23), suggesting that RalA proteins promote exocyst complex formation. However, for reasons that are not yet clear, expression of a constitutively activated RalB protein caused the same inhibitory phenotype as RalA depletion (23). Interactions of RalA with the exocyst have also been implicated in the formation of filopodia (39). Interestingly, this effect was not due to the expected role of the exocyst in membrane delivery, suggesting that this complex may also influence the actin cytoskeleton associated with filopodium formation.
In this paper we show that active Ral can enhance basolateral delivery of membrane components through the exocyst. However, this is true only for RalA, not for RalB, because the latter has a significantly lower affinity for the exocyst due to a segment of the Ral protein not previously thought to be involved in effector protein binding. Another reason for this difference is that active RalA and RalB localize differently in cells. Finally, the data show that RalA binding to components of the exocyst may not be sufficient to promote secretion, implying that another RalA target protein involved in this process remains to be identified.
MATERIALS AND METHODS
Antibodies and reagents.
Anti-RalA monoclonal antibody and anti-RalB polyclonal antibody were from Transduction Laboratories; anti-Sec6 and Sec8 monoclonal antibodies were from Stressgen; anti-Sec5 polyclonal antibodies were a kind gift from Y. Ohta (Harvard University); anti-E-cadherin rr1 monoclonal antibody was from the University of Iowa Developmental Study Hybridoma Bank; anti-cadherin polyclonal antibodies were a kind gift from W. J. Nelson (Stanford University); anti-gp135 monoclonal antibody was a kind gift from G. K. Ojakian (State University of New York, Brooklyn); adenovirus expressing human transferrin receptor was kindly provided by I. Mellman (Yale University); and anti-TGN-38 polyclonal antibodies were a kind gift from G. Banting (University of Bristol). Donkey anti-mouse immunoglobulin G Fab fragments labeled with cyanine 2 (Cy2) or indocarbocyanine (Cy3) or unlabeled were from Jackson Immunoresearch. All other reagents were either from Sigma or Fisher Scientific unless noted otherwise.
Cell culture.
MDCK cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) with 5% fetal calf serum (HyClone) supplemented with the necessary antibiotics. To establish inducible cell lines, T23 MDCK cells (a kind gift from J. E. Casanova, University. of Virginia) were cotransfected with a hygromycin resistance plasmid and the pBIG vector (Clontech) with a bidirectional tet-off promoter and lacZ on one side and Ral mutant cDNA inserted on the other side. Cell lines were selected in medium supplemented with 0.25 mg of hygromycin and 0.3 μg of doxycycline per ml. The resulting cell lines were first tested for β-galactosidase induction, and only cell lines where all cells stained for β-galactosidase were selected. After that, the cell lines were tested for induction of Ral mutant expression. Cells were maintained in medium supplemented with 0.3 μg of doxycycline per ml.
Synthesis and surface delivery assays.
Cells from confluent 100-mm culture dishes were trypsinized, and 25% of the cells were put into 24-mm Transwell filter inserts (0.45-μm pore size; Costar). Cells were grown for 3 days, with the medium being changed daily. Formation of tight junctions was checked by measuring diffusion of fluorescein isothicyanate (FITC)-dextran across the cell monolayer. The cells were starved in DMEM without methionine and cysteine for 1 h and then pulse-labeled by placing 100-μl drop of starvation medium with 2 mCi of 35S-Tran label (ICN) on the sheet of Parafilm in the 37°C water bath and placing filters with cells onto these drops for 15 min; 500 μl of starvation medium with 20 mM HEPES (pH 7.5) was added to upper chambers of filter inserts. Then medium from the upper chambers was removed, and inserts were put into the six-well clusters with 2 ml of regular DMEM/well, and 1 ml of DMEM was added to the upper chambers of the inserts. Cells were then incubated at 37°C for various periods of time.
For polarized delivery assays, filter inserts were washed with ice-cold Dulbecco's phosphate-buffered saline (PBS), and apical or basal surfaces were biotinylated on ice for 30 min with 1 mg of Sulfo-SS-NHS-biotin (Pierce) per ml in Dulbecco's PBS. Biotinylation was stopped by washing filters twice with ice-cold Tris-buffered Dulbecco's PBS, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer, and E-cadherin or gp135 was immunoprecipitated. Beads with antibody-antigen complex were then boiled with 20 μl of 3% sodium dodecyl sulfate (SDS), diluted with 1 ml of reprecipitation buffer (20 mM Tris [pH 7.5], 150 mM NaCl, 1 mmM EDTA, 1% Triton X-100), supernatant was reprecipitated with avidin beads, and precipitated proteins were then run in SDS gels and analyzed by phosphoimager analysis.
For assays of E-cadherin processing, after chase filter inserts were washed with ice-cold Dulbecco's PBS, cells were lysed in RIPA buffer and cadherin was immunoprecipitated with anticadherin polyclonal antibody. Immunoprecipitates were run on SDS-protein gels, dried, and subjected to phosphoimager analysis.
Immunofluorescence.
Cells were induced or uninduced for expression of Ral mutants and seeded onto polylysine-treated 10-mm coverslips. Cells were washed with cold PBS, fixed with 4% paraformaldehyde in PBS, permeabilized with 0.075% Saponin in PBS for 15 min, and blocked with 1% bovine serum albumin in PBS with 0.075% saponin. Cells were then incubated with primary antibody in PBS with 1% bovine serum albumin and 0.075% saponin for 2 h at room temperature, washed with PBS, and incubated with secondary antibody in PBS with 1% bovine serum albumin and 0.075% saponin for 1 h at room temperature, washed three times with PBS, and mounted in PBS with 50% glycerol and 0.2% n-propylgallate.
For colocalization of Sec6 and RalA, cells were fixed with either paraformaldehyde or methanol, permeabilized as described above, and incubated sequentially with intermittent washes with the following antibodies, all in 1% bovine serum albumin-0.075% saponin in PBS: mouse anti-Sec6 antibody, donkey anti-mouse Fab-Cy2, excess unlabeled donkey anti-mouse Fab, mouse anti-RalA antibody, and donkey anti-mouse Fab-Cy3. Cells were then washed and mounted as described above. Images were acquired with an Axioplan (Carl Zeiss) microscope and 40× objective equipped with a digital camera and processed with Adobe Photoshop 6.0.1 for Macintosh.
Loading of recycling endosomes with transferrin.
RalA72L T23 MDCK cells were induced for RalA72L expression, infected with adenovirus expressing human transferrin receptor, and sparsely plated onto polylysine-treated 10-mm coverslips. Coverslips with cells were incubated in serum-free DMEM for 40 min, inverted onto 100-μl drops of DMEM with 0.1 mg of Texas Red-labeled human transferrin (Molecular Probes) per ml on the Parafilm sheets on ice, and incubated on ice for 20 min to allow transferrin binding. Coverslips were then transferred to 37°C DMEM with 5% fetal calf serum and chased for 20 min. Cells were fixed with 4% paraformaldehyde, permeabilized with 0.075% saponin, and processed for RalA staining as described above.
Images were acquired with a Leica TCS SP2 confocal microscope and 63× objective and processed with Adobe Photoshop 6.0.1 for Macintosh.
Immunoprecipitation.
For coimmunoprecipitation experiments, 293T cells were seeded at 50% confluency on a 100-mm dish and transfected with N-terminally Myc-tagged cDNAs of RalA or RalB mutants with Lipofectamine 2000 (Invitrogen), and 48 h later the cells were washed twice with ice-cold PBS and lysed in 1 ml of lysis buffer (20 mM Tris [pH 7.5], 50 mM NaCl, 1 mM MgCl2, 0.5% Triton X-100, freshly supplemented with protease inhibitors). RalA and RalB mutants were immunoprecipitated from 900 ul of lysate with 2 μg of anti-Myc 9E10 monoclonal antibody (Tufts University/New England Medical Center facility) prebound to 15 μl of protein A-Sepharose beads. The beads were washed three times with lysis buffer, boiled with SDS sample buffer, run in SDS gels, transferred to nitrocellulose membranes, and probed with the appropriate antibodies.
For high-salt wash beads with immunoprecipitated Ral mutants were washed twice with 1 ml of high-salt buffer (lysis buffer supplemented with 900 mM NaCl) and then twice with lysis buffer and either boiled with SDS sample buffer or incubated with lysate from untransfected 293T cells for 2 h at 4°C and then washed three times with lysis buffer, boiled with SDS sample buffer, run in SDS gels, and processed for Western blotting with the appropriate antibodies.
RESULTS
Expression of constitutively activated RalA enhances cellular activities associated with the exocyst complex.
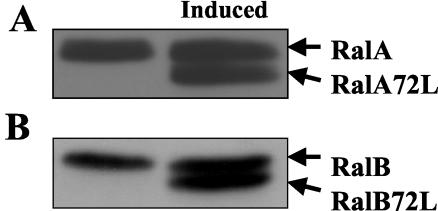
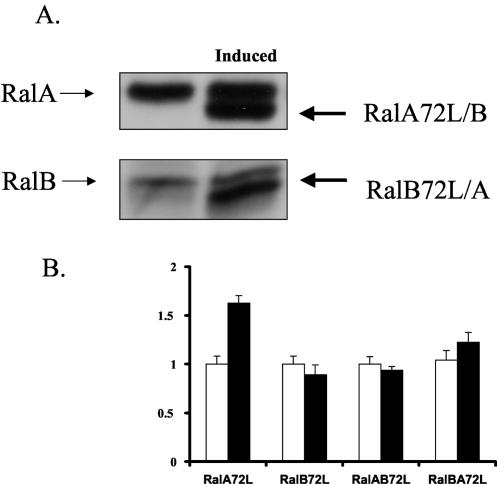
We previously identified the exocyst complex as a putative effector protein for RalA by demonstrating that it can be immunoprecipitated from cells with the constitutively active but not constitutively inactive form of the GTPase (31). To test whether active RalA affects cells in a manner consistent with a regulator of exocyst function, an MDCK epithelial cell line that expresses a constitutively active mutant of RalA (RalA72L) under the control of the tetracycline repressor was established. Since this mutant RalA has a slightly faster mobility in SDS-protein gels than wild-type RalA, its expression can easily be detected by immunoblotting. Figure 1A shows that active RalA was expressed at a level similar to that of its endogenous counterpart. This cell line was then used to measure the effect of transient expression of active RalA on the delivery of membrane components specifically to the basolateral surface of cells, a function known to be mediated by the exocyst complex.
FIG. 1.
Inducible expression of RalA72L and of RalB72L in T23 MDCK cell lines. (A) T23 MDCK cells expressing RalA72L under the control of a bidirectional tet-off promoter were either grown in the presence of doxycycline (left) or induced to make RalA72L by removal of doxycycline for 24 h (right). Both endogenous (RalA) and activated RalA72L were detected by immunoblotting with anti-RalA antibodies. (B) T23 MDCK cells expressing RalB72L by the same system as in A were either grown in the presence of doxycycline (left) or induced to synthesize active RalB72L by removing doxycycline for 24 h (right). Both endogenous RalB and activated RalB72L were detected by immunoblotting with anti-RalB antibodies.
To detect polarized delivery of membrane components in MDCK cells, E-cadherin and gp135 were studied because they are known to be delivered specifically to the basolateral and apical surfaces of cells, respectively (6, 29). In particular, MDCK cells were grown on Transwell filter inserts and allowed to form a polarized epithelium. Newly synthesized proteins were labeled with [35S]methionine/cysteine and chased with unlabeled medium for various times. The basolateral and apical surfaces of the epithelium were then labeled by biotinylation. Marker proteins were immunoprecipitated, dissociated from the immune complex, and isolated again by avidin affinity precipitation. The amount of labeled protein at each surface was compared in uninduced and induced cells.
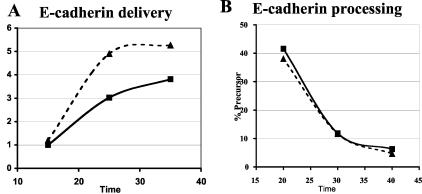
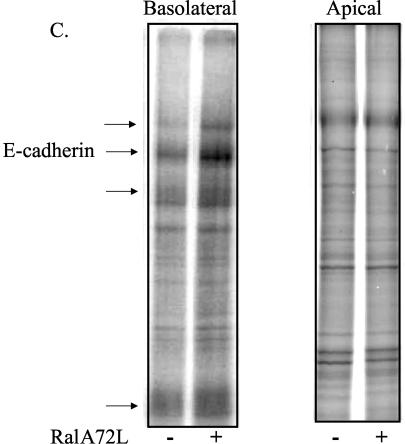
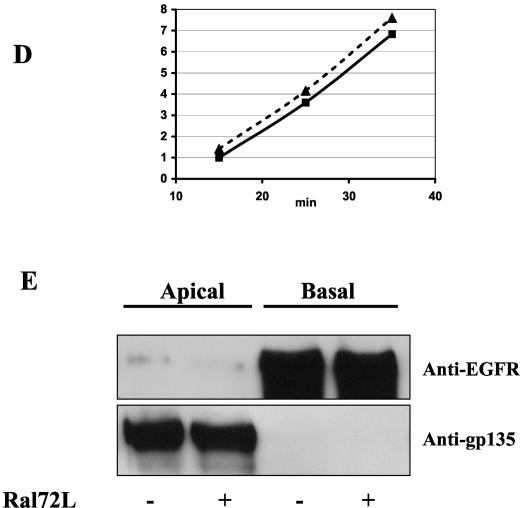
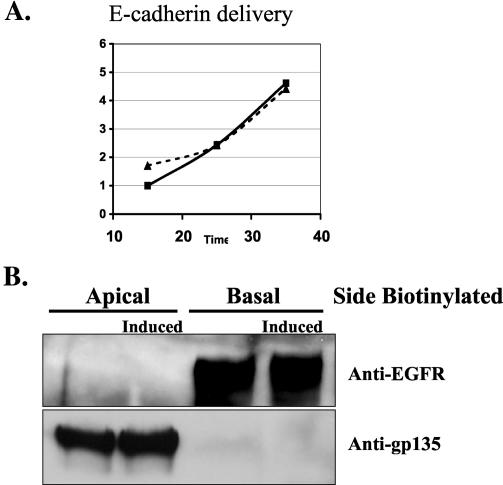
Transient induction of active RalA 72L expression in MDCK cells resulted in an enhanced rate (≈2-fold) of delivery of E-cadherin to the basolateral membrane compared to its rate of delivery in uninduced control cells (Fig. 2A). The rate of synthesis of E-cadherin was not changed significantly during this time period in induced cells (data not shown). E-cadherin is initially synthesized as a 130-kDa precursor that is cleaved in the trans-Golgi compartment to form a mature 120-kDa protein (38). The kinetics of this processing were unchanged upon expression of RalA 72L (Fig. 2B), indicating that RalA enhances delivery of vesicles containing E-cadherin between the trans-Golgi and the basolateral plasma membrane. The effect of RalA was not limited to E-cadherin, since investigation of the entire population of newly synthesized basolateral proteins labeled with biotin and then isolated by avidin binding revealed enhanced delivery of many proteins (Fig. 2C). Importantly, the rate of delivery of gp135 (Fig. 2D) and other newly synthesized biotinylated proteins to the apical surface (Fig. 2C) was not significantly different after induction of RalA72L expression.
FIG. 2.
RalA enhances delivery of membrane proteins to the basolateral but not apical membrane surface of MDCK cells. (A) Basolateral delivery of E-cadherin. T23 RalA72L cells were plated on 24-mm Trans-well inserts, and half of the filters were induced for expression (dashed line) and half were left uninduced (solid line). After 3 days, cells were pulse labeled with [35S]cysteine/methionine and chased for different periods of time, and the basolateral surface was biotinylated. E-cadherin was immunoprecipitated, dissociated from beads, and reprecipitated with streptavidin beads, and precipitates were run on SDS gels and exposed to a phosphorimager. Signals were quantified with ImageQuant software and normalized to relative total protein incorporation of 35S label. The resulting data were normalized as increase over the first data point of uninduced (−) cells and plotted as a function of time. The experiment was repeated three times. The data are from a representative experiment. (B) E-cadherin processing. The T23 RalA72L cell line was plated and processed as in panel A except that the biotinylation step was omitted and E-cadherin was immunoprecipitated, and precipitates were run on SDS gels and exposed to phosphorimager analysis. The amount of E-cadherin precursor was expressed as a percentage of total E-cadherin and plotted as a function of time. The data are representative of two independent experiments. (C) Basolateral and apical protein delivery of total membrane proteins. T23 RalA72L cells were plated, induced, and pulse labeled as in panel A. After 30 min of chase, either the basolateral or apical surface was biotinylated. Total apical and basolateral membrane proteins were then isolated on streptavidin beads, and the samples were run on SDS gels and exposed to a phosphorimager. Proteins whose appearance on the basolateral surface increased significantly in response to RalA72L expression, including the position in the gel where E-cadherin migrates, are marked with arrows. (D) Apical delivery of gp135. The RalA72L T23 cell line was plated and processed as in panel A except that apical side was biotinylated, gp135 was immunoprecipitated and processed as in panel A. Delivery of gp135 to the apical membrane was plotted as the increase over the first data point of uninduced cells (−) as a function of time. (E) Cell polarity. T23 RalA72L cells were plated and grown as in A, apical or basal surfaces were biotinylated, and biotinylated proteins were precipitated with streptavidin beads, run on SDS gels, transferred to the membrane, and probed with anti-epidermal growth factor receptor (EGFR) antibody (basolateral marker); the membrane was then stripped and reprobed with anti-gp135 antibody (apical marker).
In addition, cells expressing active RalA maintained their ability to partition apical (epidermal growth factor receptors) and basolateral (gp135) proteins faithfully (Fig. 2E). Finally, and as expected, a control CAAX box mutant RalA72 that cannot be targeted to cell membranes failed to activate basolateral delivery in these assays (data not shown). Taken together, these findings support the idea that RalA functions as a positive mediator of exocyst-related functions.
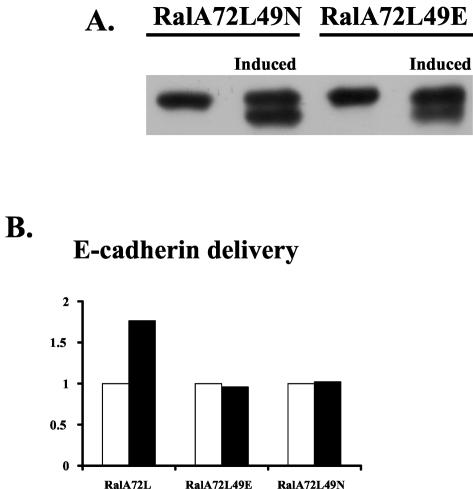
To support the idea that RalA interaction with the exocyst is involved in its ability to influence cellular secretion, effector domain mutants of Ral, previously identified on the basis of their loss of binding affinity for specific Ral effector proteins, were investigated. In particular, inducible cell lines expressing RalA72L49E, which fails to bind to the Sec5 and Exo84 subunits of the exocyst (23, 24), or RalA72L49N, which retains the ability to bind to both exocyst components (23, 24, 31), were generated and tested in secretion assays as described above (Fig. 3A). Expression of RalA72L49E failed to promote enhanced delivery of E-cadherin to the basolateral surface of MDCK cells (Fig. 3B). Thus, as expected, exocyst binding to active RalA is needed for the GTPase to enhance secretion. Surprisingly, expression of RalA72L49N also failed to enhance basolateral membrane delivery (Fig. 3B), despite the fact that this mutant can still bind to the exocyst. These findings suggest that exocyst binding is necessary but not sufficient for RalA to enhance secretion.
FIG. 3.
Effector domain mutations block RalA72L effects on delivery of basolateral membrane proteins. (A) Inducible expression of effector binding mutants. T23 MDCK cells expressing RalA72L49E or RalA72L49N under the control of a bidirectional tet-off promoter were induced to make Ral proteins for 24 h, and cell lysates from uninduced and induced cells were run on SDS gels and probed with anti-RalA antibody. (B) Basolateral delivery of E-cadherin. The T23 MDCK RalA72L, RalA72L49N, and RalA72L49E cell lines were plated and processed as in Fig. 2A except that the chase time was fixed at 30 min. The E-cadherin delivery assay was performed, and the data are presented as in Fig. 2A. Open bars, uninduced cells; solid bars, induced cells. The experiment was repeated three times, and data are from a representative experiment.
Active RalB does not bind efficiently to the exocyst due to amino acid sequences just distal to the effector-binding domain.
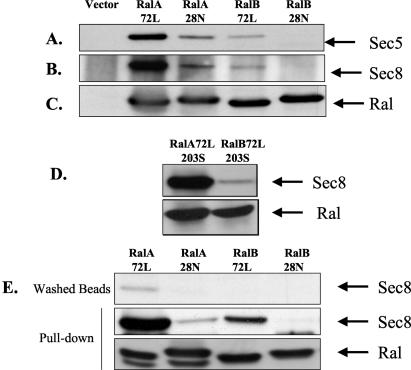
RalB is 88% identical to RalA in its first 162 amino acids, and within this region the two proteins have identical effector-binding domains, which are involved in Sec5 binding. Thus, one might have assumed that active RalA and active RalB would interact equally with the exocyst. To test this hypothesis, active RalB was tested for the ability to bind to the exocyst in cells as we had done for active RalA (Fig. 4). Myc-tagged versions of active (72L) and inactive (28N) RalA and RalB were transfected into 293T cells, and cell lysates were immunoprecipitated with anti-Myc antibody and immunoblotted with antibodies to exocyst components (Fig. 4A to C). As shown previously, active RalA72L formed a complex with Sec5 (panel A), Sec8 (panel B), and Sec6 (data not shown) much more efficiently than did inactive RalA28N. Surprisingly, the exocyst components examined bound much less efficiently to active RalB72L than to active RalA72L (≈5-fold). In fact, by analyzing silver-stained gels of immunoprecipitates, all of the exocyst components that we detected previously bound to active RalA (31), including Exo84, were present at much lower levels on immunoprecipitates of active RalB than active RalA (data not shown). Although active RalB bound less well to the exocyst complex, it still displayed activity-dependent binding (compare RalB72L to RalB28N). Figure 4C shows that all Ral proteins were expressed at similar levels. Moreover, both RalA72L and RalB72 were present almost exclusively in the GTP-bound state in cells (data not shown).
FIG. 4.
RalB72L coimmunoprecipitates poorly with exocyst components. (A to D) Myc-RalA and Myc-RalB mutants were transfected into 293T cells and immunoprecipitated with an anti-Myc monoclonal antibody. The presence of exocyst components in the immunoprecipitates was detected by immunoblotting. (E) Immunoprecipitated Ral proteins were washed with high-salt buffer to remove bound proteins (top row) and then incubated with fresh 293T cell lysates. The presence of exocyst components was then detected as in panels A to D (middle row). Total Ral proteins in cell lysates are shown in the bottom row.
To determine whether this difference in binding in cells was due to intrinsic differences in exocyst binding ability between RalA and RalB or to differences in the cellular localizations of the proteins, the binding properties of both proteins were compared when they were removed from their membrane localizations to the cytosol by cysteine-to-serine substitutions at position 203 in their C-terminal CAAX boxes. Even when RalA and RalB were located similarly in the cytoplasm, active RalA still bound to Sec5 and associated components of the exocyst more effectively than RalB (Fig. 4D). To perform experiments in vitro, the immunoprecipitated Ral proteins prepared as in Fig. 4A and B were freed from bound exocyst components by exposure to high salt (panel E, top row) and used to precipitate exocyst components from fresh cell lysates. The same result was obtained (Fig. 4E, bottom two rows), confirming that cell localization is not responsible for binding differences. Instead, despite having identical effector-binding domains, active RalA intrinsically binds better to the exocyst complex than active RalB.
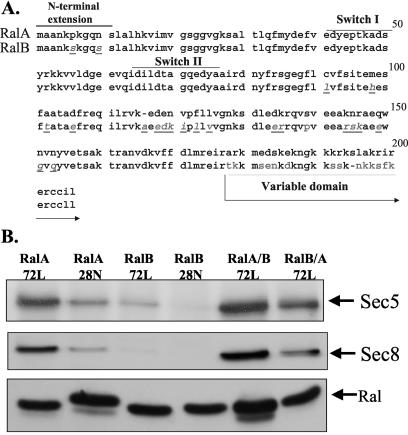
By aligning the amino acid sequences of RalA and RalB, it becomes clear that the major distinctions between the two proteins are in the segments just distal to the switch 2 effector-binding domain (amino acids 91 to 153) and the variable domain near the C terminus, outside the core GTPase domain (Fig. 5A). The two amino acid differences in the N-terminal extension are not involved in binding differences between RalA and RalB, because their deletion had no effect on exocyst binding (data not shown). To determine which of the other regions are involved, the variable domains of RalA and RalB were switched by making chimeric Ral/RalB proteins at amino acid 176. The chimeric proteins were tested in binding assays as described above (Fig. 5B). The RalA/RalB(variable domain) chimera retained high-affinity binding to Sec5 and Sec8 in cells. The RalB/RalA(variable domain) chimera retained lower binding ability than RalA, although its binding activity did rise. These results indicate that the region between the switch 2 region of the “effector domain” and the variable domain (amino acids 91 to 153) plays a major role in the differences in the exocyst binding potential of RalA and RalB. In addition, the variable domain may also contribute, possibly by altering the localization of the proteins (see below).
FIG. 5.
Binding activity of chimeric Ral proteins. (A) Amino acid differences and key regions of RalA and RalB are shown. The sites of the variable domain and common C terminus that was switched in the chimeras are also shown. (B) Chimeras were compared to normal active RalA and RalB in binding activity to exocyst proteins in cells as described for Fig. 4.
Active RalB does not promote basolateral membrane delivery of E-cadherin.
To determine whether the weakness in exocyst binding in cells was reflected in a reduced ability of active RalB to promote exocyst-related functions in cells, an MDCK cell line inducibly expressing activated RalB72L to levels comparable to endogenous RalB was generated (Fig. 1B). Uninduced control cells and cells induced to express active RalB were then compared in the polarized secretion assay described previously for cells expressing active RalA. Consistent with poor binding to the exocyst, active RalB did not enhance the delivery of E-cadherin to the basolateral side of MDCK cells (Fig. 6A). Expression of active RalB also did not disrupt polarized delivery of basolateral or apical membrane proteins (Fig. 6B). Thus, despite their similarity, RalA but not RalB is a regulator of exocyst-related functions.
FIG. 6.
RalB72L has no effect on basolateral membrane proteins delivery in MDCK cells. (A) Basolateral delivery of E-cadherin. T23 MDCK RalB72L cells were plated and processed as in Fig. 2A. The E-cadherin delivery assay was performed, and the results are plotted as described for Fig. 2A. The experiment was repeated three times, and the results are from a representative experiment. (B) Membrane polarity. Biotinylation of apical and basolateral surfaces of cells and the polarity assay were performed as described for Fig. 2C.
The regions of RalA and RalB responsible for this biological difference were then tested by making MDCK cell lines with inducible expression of the chimeras (Fig. 7A) to use in the membrane delivery assays described above. The RalA/RalB(variable domain) chimera failed to promote basolateral delivery of E-cadherin (Fig. 7B), despite the fact that it retained high-affinity binding for the exocyst (see Fig. 5B). Thus, high-affinity binding to the exocyst is not sufficient for RalA to enhance basolateral secretion; the variable domain also plays a role. The RalB/RalA chimera actually displayed a small increase in activity above baseline activity seen with RalB, suggesting that the variable domain of RalA can complement to some degree the lower binding ability of RalB.
FIG. 7.
Biological activity of RalA/RalB chimeras. (A) The expression levels of chimeric RalA/RalB(variable domain) and RalB/RalA(variable domain) proteins under tet-off promoter control were assayed by immunoblotting with appropriate Ral antibodies under uninduced and induced conditions. Endogenous RalA and RalB are also shown. (B) Delivery of E-cadherin to the basolateral membrane was assayed, and the data were plotted as in Fig. 3B. Each data point was performed in duplicate, and the experiment was repeated twice. The error bars represent the range of experimental points.
RalA and RalB are localized differently in MDCK cells.
Because the C-terminal variable domain is known to influence the cellular localization of GTPases, we investigated whether RalA and RalB are localized differently in cells. Therefore, MDCK cells were fixed with paraformaldehyde, permeabilized with saponin, and stained with either RalA (Fig. 8A to C and E) or RalB (Fig. 8F and G) antibodies. RalA antibodies generated a weak plasma membrane staining of endogenous RalA at the sites of cell-cell contacts, with diffuse punctate staining throughout the cytoplasm (Fig. 8A). RalB antibody produced denser punctate staining of endogenous RalB throughout the cytoplasm, but little if any membrane staining was observed (Fig. 8F). Since the exocyst complex is known to accumulate between MDCK cells (Fig. 8D), these subtle differences hinted that distinct cellular localizations of the two Ral proteins could be significant.
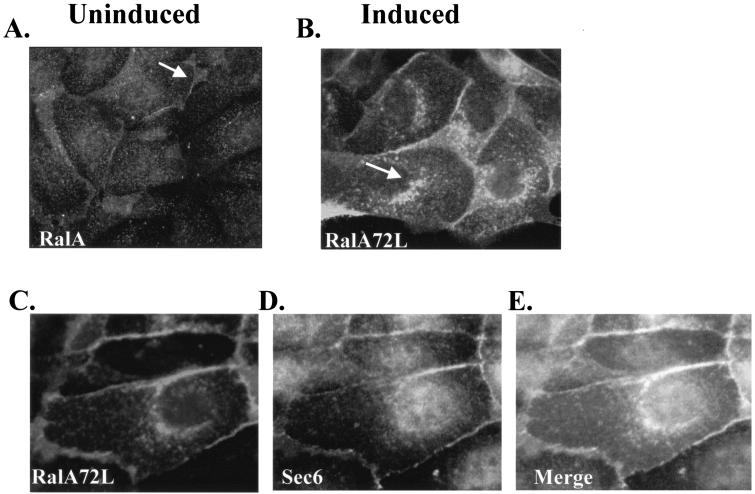
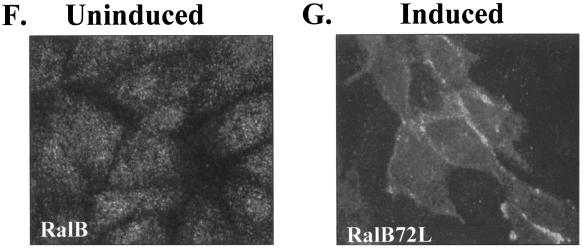
FIG. 8.
Active RalA and active RalB are localized differently in MDCK cells. MDCK T23 RalA72L and RalB72L cells were either left uninduced or induced for expression. The next day cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% saponin, and stained with anti-RalA, anti-RalB, or anti-Sec6 antibody. Cells were viewed by fluorescence microscopy. (A) RalA staining in uninduced cells. (B) RalA staining in induced RalA72L cells. (C to E) T23 RalA72L induced cells were fixed and permeabilized as in panels A and B and then sequentially incubated with anti-Sec6 monoclonal antibody, Cy2-labeled donkey anti-mouse Fab, an excess of unlabeled donkey anti-mouse Fab, anti-RalA monoclonal antibody, and Cy3-labeled donkey anti-mouse Fab. Cells were then viewed and photographed. Control cells with Sec6 antibody omitted showed no specific staining. Exposure for Sec6 (Cy2) staining was 5 s (C) and for RalA (Cy3) staining was 0.5 s (D). (E) A merged image of panels C and D. (F) RalB staining of uninduced T23 RalB72L cells. (G) RalB staining of induced T23 RalB72L cells.
To investigate this possibility further, the cellular distributions of the constitutively active forms of RalA and RalB were compared, since the differences in biological activities of the two Ral isoforms were observed when the expression of these proteins was induced in cells. RalA antibody staining of RalA72L-induced MDCK cells yielded more intense staining of the plasma membrane between cells where the exocyst also exists than in uninduced cells (Fig. 8B). Interestingly, a newly appearing intense perinuclear staining was observed for RalA72L (Fig. 8B). Costaining of the same cells with antibodies to both RalA and the exocyst component Sec6 showed extensive colocalization at the plasma membrane between cells and some costaining in perinuclear regions (Fig. 8C, D, and E).
RalB antibody staining of RalB72L-expressing cells also yielded intense staining of the plasma membrane between cells (Fig. 8G). Importantly, however, no accumulation of active RalB was observed in perinuclear regions (Fig. 8G).
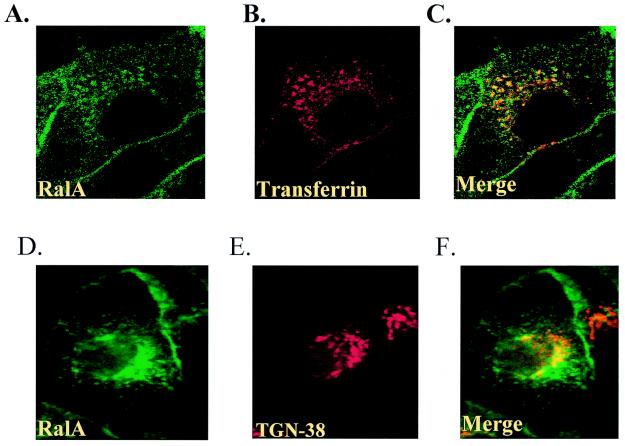
Since this perinuclear staining was reminiscent of recycling endosomes, this vesicle population was stained by exposing MDCK cells (infected with human transferrin receptor-encoding virus) with Texas Red-conjugated transferrin and allowing uptake for 20 min (37). A cross-section of cells viewed by confocal microscopy displayed a very similar staining pattern for transferrin and activated RalA (Fig. 9A to C). In contrast, significantly less colocalization of active RalA and the trans-Golgi marker TGN-38 was observed (Fig. 9D to F), suggesting that active RalA but not active RalB accumulates predominantly in recycling endosomes.
FIG. 9.
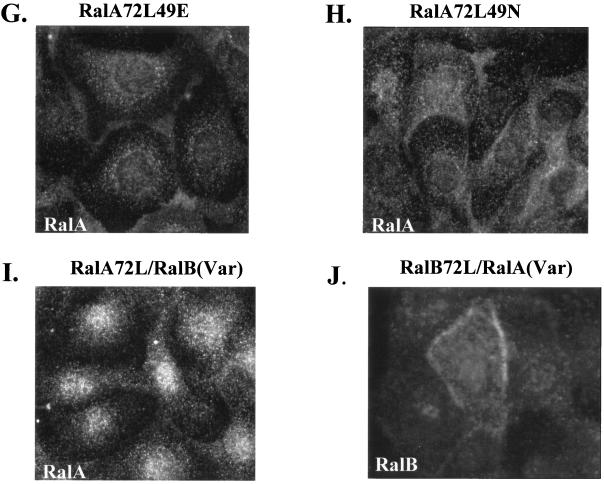
Costaining of active RalA72L but not functionally inactive mutants with transferrin. (A to C) T23 RalA72L induced cells were infected with human transferrin receptor-encoding adenovirus, incubated on ice with Texas Red-labeled transferrin for 30 min, washed, shifted to 37°C for 20 min, fixed and permeabilized as in Fig. 8, and incubated with anti-RalA antibodies followed by Cy2-labeled secondary antibody. A cross section of cells was viewed by confocal microscopy. (A) RalA staining. (B) Direct visualization of Texas Red-labeled transferrin. (C) Merged images. (D to F) RalA72L-expressing cells were incubated with anti-RalA monoclonal antibody (D) and anti-TGN38 rabbit polyclonal antibodies (E), followed with Cy2-labeled anti-mouse and Cy3-labeled anti-rabbit immunoglobulin secondary antibodies. (F) Merged images. (G) RalA72L49E. (H) RalA72L49N. (I) RalA72L/RalB(variable domain). (J) RalB72L/RalA(variable domain)-expressing T23 MDCK cells were fixed and permeabilized as in Fig. 8 and stained with anti-RalA monoclonal antibody and Cy2-labeled anti-mouse immunoglobulin secondary antibodies (G, H, and I) or with anti-RalB and Cy3-labeled anti-rabbit immunoglobulin secondary antibodies (J) and viewed by standard fluorescence microscopy as in Fig. 8.
Since this localization distinguishes active RalA from active RalB, its role in mediating RalA effects on secretion was assessed by visualizing the localization of RalA mutants that have lost their ability to activate polarized secretion. In particular, MDCK cells expressing either RalA72L49E or RalA72L49N, both of which no longer promote secretion, were stained with anti-RalA antibodies. Figure 9G and H show that both mutants failed to accumulate in the perinuclear region (compare to Fig. 8B), arguing that this cellular site is important for Ral function. In addition, since the RalA72L49E mutant maintained its ability to bind to Sec5 and Exo84, a second putative Ral effector protein may be required for proper targeting of active RalA to this cellular compartment.
Analysis of the chimeric RalA/RalB proteins was also revealing (Fig. 9I and J). Both chimeras also failed to accumulate in recycling endosomes. Since these mutants were defective in enhancing secretion, these results reinforce the significance of the recycling endosome localization of active RalA for its effects on basolateral delivery of membrane proteins. The failure of the RalA72L/RalB(variable domain) chimera to localize correctly also demonstrates that, as expected, the variable region also participates in localizing active RalA to a site in the cell where it can promote basolateral membrane delivery.
DISCUSSION
Ras family GTPases function as molecular switches in cells by becoming activated in response to specific extracellular signals and then by propagating these signals through interactions with specific cytoplasmic proteins. One level of signaling specificity derives from the fact that different Ras family members perform different functions because they contain distinct effector-binding domains that allow them to influence a specific subset of cellular proteins. However, most individual GTPases have the ability to bind to more than one “effector” protein through this domain, and so another level of signaling specificity derives from mechanisms that allow differential activation of individual effector proteins (35). However, isoforms of Ras family members exist that have identical effector binding domains, such as RalA and RalB. How these similar proteins carry out specific functions has remained less clear. Here, we document that active RalA enhances functions associated with the exocyst while active RalB does not, due both to differences in the ability of the active proteins to interact with one of their potential cellular targets, the exocyst, and to their different subcellular localizations.
In particular, active RalA binds to the exocyst complex much more efficiently than active RalB does. Consistent with this difference, only active RalA, not active RalB, promotes vesicle trafficking expected of a regulator of exocyst function, i.e., delivery of membrane proteins to the basolateral surface of cells. The finding of a difference in intrinsic target protein binding potential between active RalA and active RalB is surprising because they have identical effector domains that in other GTPases determine binding to target proteins. Recent structural analysis shows that a major binding interaction between RalA and Sec5 of the exocyst occurs at residues 38 to 47 of the switch 1 region of the GTPase (11). Other GTPases also interact with targets through their switch 2 regions, although this could not be assessed because that region of RalA is not resolved well in the crystal structure. However, in the present work we find that the binding difference between RalA and RalB was encoded in RalA-specific amino acids 91 to 153, whereas no direct interactions between Sec5 and RalA are present in the structural study.
Interestingly, some of the amino acids that distinguish RalA and RalB in this region are found in helix 3. Our previous study on the generation of the specificity of interactions between exchange factors and the closely related H-Ras and R-Ras GTPases suggested that the switch 2 regions in the effector domains of those proteins are influenced by differences in amino acids in their helix 3 regions (41). Thus, it is possible that differences in the intramolecular interactions between helix 3 and the effector domains of RalA and RalB cause the observed differences in their ability to bind to exocyst components.
Alternatively, amino acids between 91 to 153 may bind to an additional site on Sec5 or to another exocyst component such as Exo84 to generate high-affinity binding, since the structure experiments used only a small part of the Sec5 protein, while we studied binding to the exocyst complex in cells, not to individual components. Since the structure of amino acids 91 to 153 of RalA is not affected by nucleotide binding, this region is not likely involved in nucleotide-dependent binding of the exocyst, but rather in promoting high-affinity binding required for biological activity in cells. This model is consistent with the fact that although RalB binds with relatively low affinity to the exocyst, it still does so in a GTP-dependent manner. Support for this model comes from structural studies on the interaction of Rab3A with its effector rabphilin (30). In this report, additional binding sites for rabphilin outside the effector domain that are proposed to contribute to Rab family member binding specificity. Interestingly, one of these sites is in a region analogous to the one that we have implicated in RalA-exocyst binding.
Immunofluorescence experiments in this study show that active RalA72L and active RalB72L distribute differently in cells. Both active RalA and active RalB are enriched in the plasma membrane between MDCK cells, along with the components of the exocyst complex. However, active RalA also accumulates in a perinuclear compartment that appears to be the recycling endosome, based on its complete colocalization with internalized transferrin and less consistent colocalization with the trans-Golgi marker TGN 38. In contrast, active RalB does not. Although early studies implied that exocyst proteins function at the plasma membrane (12, 45), new studies implicated it in recycling endosomes (10, 32), and we find significant overlap of active RalA and exocyst component Sec6 in this perinuclear region.
The importance of this localization to RalA function is supported by the fact that all Ral proteins studied that do not promote basolateral secretion also do not accumulate in recycling endosomes. These inactive Ral proteins include active RalB72L, effector-domain mutants of RalA72L, and chimeras of RalA72L and RalB72L. The fact that active RalA, which enhances the membrane delivery of newly synthesized proteins, localizes to recycling endosomes, a compartment also known to be involved in endocytosis, highlights the idea that RalA and its targets may function at the junction of exocytosis and endocytosis regulation. Adding support to this notion is our recent finding that another recycling endosome protein, Rab11-FIP2, is part of a signaling complex associated with RalBP1, a Ral effector implicated in endocytosis (8).
Furthermore, we find that while the interaction of Ral with the exocyst is necessary for enhanced secretion, it may not be sufficient because an effector domain mutant of RalA (RalA72L49N) that still interacts with the Sec5 and Exo84 in cells does not enhance the rate of basolateral membrane delivery of vesicle components. This mutant also lacks the ability to accumulate in recycling endosomes. Since this effector mutant does not bind to RalBP1, RalBP1 or a novel Ral effector with similar Ral-binding properties may function with the exocyst to promote secretion.
The fact that the effector-binding domain contributes to the subcellular localization of a GTPase is consistent with that GTPase's functioning in vesicle sorting, since an effector protein may be used to target a GTPase-containing vesicle to a specific site in the cell. However, for Ral proteins, the variable domain near the C terminus of the Ral proteins also plays a key role in the specific localization of active RalA, since this region, not the effector domain, is different between RalA and RalB. In fact, we find that the chimeric protein containing RalA with the variable region of RalB does not colocalize to the recycling endosome even though it maintains high-affinity binding potential for the exocyst. Thus, the variable region and the effector domain likely work in concert to place the active form of the protein in the correct location for active RalA to promote basolateral membrane delivery.
The data from this study also suggests that an additional Ral effector protein, besides the two exocyst components already identified, is needed for correct localization of active RalA and for secretion regulation. Since all of these effectors require interaction with the same effector-binding domain on Ral, it is likely that they cannot bind simultaneously. Thus, proper localization of active RalA may require sequential binding of multiple effectors, possibly one early in the secretory process in recycling endosomes and one at the plasma membrane.
The levels of constitutively activated Ral proteins used in these experiments are comparable to those of their endogenous GTPase counterparts. Thus, they represent the most active Ral protein that could ever exist in an MDCK cell. Nevertheless, this level of active RalB does not enhance basolateral membrane delivery of E-cadherin or other biotinylatable membrane proteins, indicating that RalB is not a regulator of exocyst function, at least for basolateral membrane protein delivery in MDCK cells. A previous study showed that when active RalB is expressed at levels many times higher than endogenous RalB in MDCK cells it still fails to enhance basolateral delivery of membrane proteins (23). In fact, not only do these high levels of active RalB fail to enhance polarized secretion, they actually disrupt polarized secretion, since basolateral membrane proteins such as epidermal growth factor receptor are found on the apical side of the membrane. This disruption, which we do not observe when activated RalB is expressed at levels comparable to endogenous RalB (Fig. 6B), is also observed when endogenous Ral activity is blocked in cells (23), showing that high levels of active RalB actually inhibit exocyst-related function.
Our studies suggest that high levels of activated RalB expression overcomes the relatively weak binding of RalB to the exocyst in cells. As such, it targets a significant enough fraction of the exocyst to a nonfunctional site in the cell to disrupt exocyst function. This explanation is consistent with our finding that placing the RalB variable domain on active RalA blocks its activity in cells despite that fact that the protein maintains high affinity for the exocyst.
This model can also explain why Moskalenko et al. (23) found that a D49N mutation, which blocks RalBP1 but not exocyst binding, has no effect on the ability of active RalB to disrupt polarized secretion, while we find that the same mutation suppresses the ability of active RalA to enhance polarized secretion. This difference would be expected if active RalB does, in fact, prevent polarized secretion by removing the exocyst from its active site in cells, since such a “dominant negative” effect would be expected to require the removal of only one critical effector from its natural location. In contrast, the present study suggests that a positive effect of RalA on secretion may require interaction of RalA with more than one effector protein.
The fact that active RalA enhances polarized secretion of many basolateral proteins together with the finding in a previous study that suppression of RalA protein expression inhibits polarized secretion of multiple basolateral proteins (23) firmly implicates RalA as a positive regulator of a key cellular process that contributes to the maintenance of polarity in epithelial cells. Additional studies, however, will be needed to explain how RalA enhances basolateral membrane delivery and secretion.
Finally, if RalB does not regulate secretion, what is its function in cells? A body of evidence has implicated Ral proteins and their target RalBP1 in an early step in receptor-mediated endocytosis (17, 22, 25). Interestingly, all of theses experiments used activated RalB, not RalA, to show an effect on receptor uptake. Thus, it is interesting to speculate that although RalA and RalB have identical effector-binding domains, differences in their ability to bind specific effector proteins and differences in their subcellular localization allow them to carry out unique functions. A recent study has shown, with short interfering RNA-induced depletion of individual Ral isoforms, that RalA is required for the growth of tumor cells in suspension, while RalB is required for tumor cell survival (7). Whether the differences in the biochemical and biological activities of RalA and RalB identified here are related to these intriguing differences in how the two isoforms affect tumor cell growth remains to be determined.
Acknowledgments
The research was supported by grants to L.A.F. from the NCI and the GRASP Digestive Disease Center (P30-DK34928 GRASP).
We thank, J. Casanova for tetracycline-inducible MDCK cells, G. Banting, Y. Ohta, W. J. Nelson, and G. K. Ojakian for kindly providing antibodies, and I. Mellman for providing adenovirus expressing the human transferrin receptor.
REFERENCES
- 1.Bhattacharya, M., P. H. Anborgh, A. V. Babwah, L. B. Dale, T. Dobransky, J. L. Benovic, R. D. Feldman, J. M. Verdi, R. J. Rylett, and S. S. Ferguson. 2002. Beta-arrestins regulate a Ral-GDS Ral effector pathway that mediates cytoskeletal reorganization. Nat. Cell. Biol. 4:547-555. [DOI] [PubMed] [Google Scholar]
- 2.Bielinski, D. F., N. Y. Pyun, K. Linko-Stentz, I. Macara, and R. E. Fine. 1993. Ral and Rab3a are major GTP binding proteins of axonal rapid transport vesicles of synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exoytosis. Biochim. Biophys. Acta 1151:246-256. [DOI] [PubMed] [Google Scholar]
- 3.Brymora, A., V. A. Valova, M. R. Larsen, B. D. Roufogalis, and P. J. Robinson. 2001. The brain exocyst complex interacts with RalA in a GTP-dependent manner: identification of a novel mammalian Sec3 gene and a second Sec15 gene. J. Biol. Chem. 276:29792-29797. [DOI] [PubMed] [Google Scholar]
- 4.Cantor, S., T. Urano, and L. A. Feig. 1995. Identification and characterization of RalBP1, a potential downstream target of Ral GTPases. Mol. Cell. Biol. 15:4578-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chardin, P., and A. Tavitian. 1986. The ral gene: a new ras-related gene isolated by the use of a synthetic probe. EMBO J. 5:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y. T., D. B. Stewart, and W. J. Nelson. 1999. Coupling assembly of the E-cadherin/beta-catenin complex to efficient endoplasmic reticulum exit and basal-lateral membrane targeting of E-cadherin in polarized MDCK cells. J. Cell Biol. 144:687-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien, Y., and M. A. White. 2003. RAL GTPases are linchpin modulators of human tumour-cell proliferation and survival. EMBO Rep. 4:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullis, D. N., B. Philip, J. D. Baleja, and L. A. Feig. 2002. Rab11-FIP2, an adaptor protein connecting cellular components involved in internalization and recycling of epidermal growth factor receptors. J. Biol. Chem. 277:49158-49166. [DOI] [PubMed] [Google Scholar]
- 9.Feig, L. A. 2003. Ral GTPases: Approaching their 15 minutes of fame. Trends Cell Biol. 13:419-425. [DOI] [PubMed] [Google Scholar]
- 10.Folsch, H., M. Pypaert, S. Maday, L. Pelletier, and I. Mellman. 2003. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J. Cell Biol. 163:351-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukai, S., H. T. Matern, J. R. Jagath, R. H. Scheller, and A. T. Brunger. 2003. Structural basis of the interaction between RalA and Sec5, a subunit of the sec6/8 complex. EMBO J. 22:3267-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grindstaff, K. K., C. Yeaman, N. Anandasabapathy, S. C. Hsu, E. Rodriguez-Boulan, R. H. Scheller, and W. J. Nelson. 1998. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell 93:731-740. [DOI] [PubMed] [Google Scholar]
- 13.Hofer, F., R. Berdeaux, and G. S. Martin. 1998. Ras-independent activation of Ral by a Ca2+-dependent pathway. Curr. Biol. 14:839-842. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, S. C., C. D. Hazuka, D. L. Foletti, and R. H. Scheller. 1999. Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol. 9:150-153. [DOI] [PubMed] [Google Scholar]
- 15.Inoue, M., L. Chang, J. Hwang, S. H. Chiang, and A. R. Saltiel. 2003. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422:629-633. [DOI] [PubMed] [Google Scholar]
- 16.Jullien-Flores, V., O. Dorseuil, F. Romero, F. Letourneur, S. Saragosti, R. Berger, A. Tavitian, G. Gacon, and J. H. Camonis. 1995. Bridging Ral GTPase to Rho pathways. J. Biol. Chem. 270:22473-22477. [DOI] [PubMed] [Google Scholar]
- 17.Jullien-Flores, V., Y. Mahe, G. Mirey, C. Leprince, B. Meunier-Bisceuil, A. Sorkin, and J. H. Camonis. 2000. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J. Cell Sci. 113:2837-2844. [DOI] [PubMed] [Google Scholar]
- 18.Kariya, K., S. Koyama, S. Nakashima, T. Oshiro, K. Morinaka, and A. Kikuchi. 2000. Regulation of complex formation of POB1/epsin/adaptor protein complex 2 by mitotic phosphorylation. J. Biol. Chem. 275:18399-18406. [DOI] [PubMed] [Google Scholar]
- 19.Kee, Y., J. S. Yoo, C. D. Hazuka, K. E. Peterson, S. C. Hsu, and R. H. Scheller. 1997. Subunit structure of the mammalian exocyst complex. Proc. Natl. Acad. Sci. USA 94:14438-14443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinsella, B. T., and W. A. Maltese. 1991. Carboxyl-terminal isoprenylation of ras-related GTP binding proteins rac1, rac2 and ralA. J. Biol. Chem. 266:9786-9794. [PubMed] [Google Scholar]
- 21.Mark, B. L., O. Jilkina, and R. P. Bhullar. 1996. Association of Ral GTP-binding protein with human platelet dense granules. Biochem. Biophys. Res. Commun. 225:40-46. [DOI] [PubMed] [Google Scholar]
- 22.Morinaka, K., S. Koyama, S. Nakashima, T. Hinoi, K. Okawa, A. Iwamatsu, and A. Kikuchi. 1999. Epsin binds to the EH domain of POB1 and regulates receptor-mediated endocytosis. Oncogene 18:5915-5922. [DOI] [PubMed] [Google Scholar]
- 23.Moskalenko, S., D. O. Henry, C. Rosse, G. Mirey, J. H. Camonis, and M. A. White. 2002. The exocyst is a Ral effector complex. Nat. Cell. Biol. 4:66-72. [DOI] [PubMed] [Google Scholar]
- 24.Moskalenko, S., C. Tong, C. Rosse, J. Camonis, and M. A. White. 2003. Ral GTPases regulate exocyst assembly through dual subunit Interactions. Electronic publication ahead of print. J. Biol. Chem. 278:51743-51748. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima, S., K. Morinaka, S. Koyama, M. Ikeda, M. Kishida, K. Okawa, A. Iwamatsu, S. Kishida, and A. Kikuchi. 1999. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 18:3629-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ngsee, J. K., L. A. Elferink, and R. H. Scheller. 1991. A family of ras-like GTP-binding proteins expressed in electromotor neurons. J. Biol. Chem. 266:2675-2680. [PubMed] [Google Scholar]
- 27.Novick, P., and W. Guo. 2002. Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 12:247-249. [DOI] [PubMed] [Google Scholar]
- 28.Ohta, Y., N. Suzuki, S. Nakamura, J. H. Hartwig, and T. P. Stossel. 1999. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. USA 96:2122-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ojakian, G. K., R. Schwimmer, and R. E. Herz. 1990. Polarized insertion of an intracellular glycoprotein pool into the apical membrane of MDCK cells. Am. J. Physiol. 258:C390-C398. [DOI] [PubMed] [Google Scholar]
- 30.Ostermeier, C., and A. T. Brunger. 1999. Structural basis of Rab effector specificity: crystal structure of the small G protein Rab3A complexed with the effector domain of rabphilin-3A. Cell 96:363-374. [DOI] [PubMed] [Google Scholar]
- 31.Polzin, A., M. Shipitsin, T. Goi, L. A. Feig, and T. J. Turner. 2002. Ral-GTPase influences the regulation of the readily releasable pool of synaptic vesicles. Mol. Cell. Biol. 22:1714-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prigent, M., T. Dubois, G. Raposo, V. Derrien, D. Tenza, C. Rosse, J. Camonis, and P. Chavrier. 2003. ARF6 controls post-endocytic recycling through its downstream exocyst complex effector. J. Cell Biol. 163:1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quilliam, L. A., J. F. Rebhun, and A. F. Castro. 2002. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog. Nucleic Acid Res. Mol. Biol. 71:391-444. [DOI] [PubMed] [Google Scholar]
- 34.Rebhun, J. F., H. Chen, and L. A. Quilliam. 2000. Identification and characterization of a new family of guanine nucleotide exchange factors for the Ras-related GTPase Ral. J. Biol. Chem. 275:13406-13410. [DOI] [PubMed] [Google Scholar]
- 35.Rusanescu, G., T. Gotoh, X. Tian, and L. A. Feig. 2001. Regulation of ras signaling specificity by protein kinase C. Mol. Cell. Biol. 21:2650-2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sans, N., K. Prybylowski, R. S. Petralia, K. Chang, Y. X. Wang, C. Racca, S. Vicini, and R. J. Wenthold. 2003. NMDA receptor trafficking through an interaction between PDZ proteins and the exocyst complex. Nat. Cell. Biol. 5:520-530. [DOI] [PubMed] [Google Scholar]
- 37.Sheff, D. R., E. A. Daro, M. Hull, and I. Mellman. 1999. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J. Cell Biol. 145:123-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shore, E. M., and W. J. Nelson. 1991. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney epithelial cells. J. Biol. Chem. 266:19672-19680. [PubMed] [Google Scholar]
- 39.Sugihara, K., S. Asano, K. Tanaka, A. Iwamatsu, K. Okawa, and Y. Ohta. 2002. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat. Cell. Biol. 4:73-78. [DOI] [PubMed] [Google Scholar]
- 40.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-208. [DOI] [PubMed] [Google Scholar]
- 41.Tian, X., and L. A. Feig. 2001. Basis for signaling specificity difference between Sos and Ras-GRF guanine nucleotide exchange factors. J. Biol. Chem. 276:47248-47256. [DOI] [PubMed] [Google Scholar]
- 42.Vega, I. E., and S. C. Hsu. 2001. The exocyst complex associates with microtubules to mediate vesicle targeting and neurite outgrowth. J. Neurosci. 21:3839-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vik-Mo, E. O., L. Oltedal, E. A. Hoivik, H. Kleivdal, J. Eidet, and S. Davanger. 2003. Sec6 is localized to the plasma membrane of mature synaptic terminals and is transported with secretogranin II-containing vesicles. Neuroscience 119:73-85. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi, A., T. Urano, T. Goi, and L. A. Feig. 1997. An Eps homology (EH) domain protein that binds to the Ral-GTPase target, RalBP1. J. Biol. Chem. 272:31230-31234. [DOI] [PubMed] [Google Scholar]
- 45.Yeaman, C., K. K. Grindstaff, J. R. Wright, and W. J. Nelson. 2001. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J. Cell Biol. 155:593-604. [DOI] [PMC free article] [PubMed] [Google Scholar]