Abstract
In slaughterhouses, the biological risk is present not only from the direct or indirect contact with animal matter, but also from the exposure to bioaerosols. Fungal contamination was already reported from the floors and walls of slaughterhouses. This study intends to assess fungal contamination by cultural and molecular methods in poultry, swine/bovine and large animal slaughterhouses. Air samples were collected through an impaction method, while surface samples were collected by the swabbing method and subjected to further macro- and micro-scopic observations. In addition, we collected air samples using the impinger method in order to perform real-time quantitative PCR (qPCR) amplification of genes from specific fungal species, namely A. flavus, A. fumigatus and A. ochraceus complexes. Poultry and swine/bovine slaughterhouses presented each two sampling sites that surpass the guideline of 150 CFU/m3. Scopulariopsis candida was the most frequently isolated (59.5%) in poultry slaughterhouse air; Cladosporium sp. (45.7%) in the swine/bovine slaughterhouse; and Penicillium sp. (80.8%) in the large animal slaughterhouse. Molecular tools successfully amplified DNA from the A. fumigatus complex in six sampling sites where the presence of this fungal species was not identified by conventional methods. This study besides suggesting the indicators that are representative of harmful fungal contamination, also indicates a strategy as a protocol to ensure a proper characterization of fungal occupational exposure.
Keywords: fungal burden, assessment strategy, slaughterhouses
1. Introduction
Airborne and settled particulate material of biological origin is referred to as organic dust in the field of occupational health [1]. This organic dust is composed of non-viable particles, generated from different sources, such as feces, litter, feed and feather formation (which produces a high quantity of allergen dandruff), but also by viable particulate matter (also called bioaerosols). Bioaerosols are comprised of airborne bacteria, fungi, viruses and their by-products, endotoxins and mycotoxins [1]. Fungal spores are complex agents that may contain multiple hazardous components. Health hazards may differ across species and strains because fungi may produce different allergens and also mycotoxins, and some species can infect humans [2].
In the period of 1950–1980, several fungal species were identified as causes of hypersensitivity pneumonitis (also called allergic alveolitis) in a number of professional occupations, including farmers, malt workers and wood workers [2].
In slaughterhouses, the biological risk is present not only from the direct or indirect contact with animal matter (feces, innards, feathers), but also from the exposure to bioaerosols [3]. Moreover, in some studies, Aspergillus, Penicillium, Cladosporium and Mucor genera were isolated from the floors and walls of slaughterhouses [4,5]. In addition, ventilation systems in slaughtering and processing facilities have been identified as an additional reservoir for the aerosolization and distribution of airborne microorganisms [6]. Poultry slaughterhouses are the ones that have been most assessed regarding their bioaerosols exposure [3,6,7,8,9,10], but others have been assessed too, namely cattle, sheep and reindeer slaughterhouses [8,11].
This study intends to assess fungal contamination by cultural and molecular methods in poultry, swine/bovine and large animal (bovine and horses) slaughterhouses, more precisely in the different processing areas from each unit. Fungal burden characterization will be helpful to know the background level of fungal contamination and to identify suitable indicator parameters for these settings regarding occupational exposures.
2. Materials and Methods
2.1. Assessed Settings
Three slaughterhouses were assessed between January and June from 2015 during a normal working day. One poultry slaughterhouse, one of both a swine and a bovine slaughterhouse and one large animal slaughterhouse were selected.
The poultry slaughterhouse (PS) is located in Coimbra district. It has 400 workers distributed by several production phases. The main activities are slaughtering (8500 chickens·h−1), evisceration (6000 chickens·h−1) and meat preparation for storage and selling. The swine/bovine slaughterhouse is located in Setubal district and it has 189 workers. The main activity is slaughtering (150 tons/day). The large animal slaughterhouse (LAS) is located in Lisbon district, and it has 31 workers. The average of animals killed per week is 280. All of the three units have Portuguese and International quality certification regarding food safety.
The sampling sites selected for each of these settings were chosen based on the high amounts of time spent by the workers in those places during their occupational activity (Table 1).
Table 1.
Sampling sites selected from each slaughterhouse.
| Poultry Slaughterhouse (PS) | Swine/Bovine Slaughterhouse (SBS) | Large Animal Slaughterhouse (LAS) |
|---|---|---|
| Birds hanging | Gut room swine | Bovine line |
| Reception | Gut room bovine | Paws room |
| Stacker | Bleeding swine | Heads room |
| Bleeding | Cutting swine | Gut room |
| Evisceration | Bleeding bovine | Expedition |
| Cutting | Cutting bovine | Barn |
In addition to conventional methods, molecular methods were also applied to detect fungal DNA (Table 2). This approach was performed to overcome some limitations of the culture-based methods and whenever specific species/strains needed to be detected. Besides the working clothes worn in all units for hygienic purposes, only the workers from bird hanging at the poultry slaughterhouse use protective masks and protection glasses as protection devices.
Table 2.
Number of samples collected and the fungal species targeted.
| Slaughterhouses | Conventional Methods | Molecular Biology | Fungal Species Assessed by Molecular Biology | |
|---|---|---|---|---|
| Air Samples | Surface Samples | Air Samples | ||
| Poultry | 6 | 6 (floor) | 6 |
A. flavus complex (toxigenic strains) A. fumigatus complex A. ochraceus complex |
| Swine | 6 | 6 (walls) | 6 | |
| Large animal | 6 | 6 (floor) | 5 | |
| Total of samples | 18 | 18 | 17 | |
2.2. Sample Collection
2.2.1. Conventional Methodologies
Air samples were collected by the use of conventional methods (Table 2). The amount of collected air ranged from 100 L (from poultry and swine/bovine slaughterhouses) to 250 L (large animal slaughterhouse). Air samples were collected through the impaction method with a flow rate of 140 L/min onto malt extract agar (MEA) supplemented with chloramphenicol (0.05%), using the Millipore air Tester (Millipore). Samplers were placed at a height of 0.6–1.5 m above the floor, approximately at the breathing zone level, and as close as possible to the worker during a normal working day. An outdoor sample was also collected to be used as a reference. Surface samples were collected by swabbing the surfaces of the same indoor sites, using a 10 by 10 cm square stencil disinfected with 70% alcohol solution between samples according to the International Standard ISO 18593 (2004). The obtained swabs were then streaked onto MEA.
2.2.2. Molecular Methodologies
Air samples of 300 L were collected using the impinger Coriolis μ air sampler (Bertin Technologies), at 300 L/min airflow rate. Samples were collected onto 10 mL of sterile phosphate-buffered saline with 0.05% Triton X-100, and the collection liquid was subsequently used for DNA extraction.
2.3. Sample Preparation and Analysis
2.3.1. Conventional Methodologies
All of the collected samples were incubated at 27 °C for 5–7 days. After laboratory processing and incubation of the collected samples, quantitative (colony-forming units: CFU/m3 and CFU/m2) and qualitative results were obtained, with the identification of the isolated fungal species. For species identification, microscopic mounts were performed using tease mount or Scotch tape mount and lactophenol cotton blue mount procedures. Morphological identification was achieved through macro- and micro-scopic characteristics, as noted by De Hoog et al. [12]
2.3.2. Molecular Methodologies
Five milliliters of the collection liquid were centrifuged at 2500× g for 10 min; the supernatant was removed, and DNA was then extracted using the ZR Fungal/Bacterial DNA MiniPrep Kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s recommendations.
Molecular identification of the different species/strains (Table 2) was achieved by real-time PCR (RT-PCR) using the Rotor-Gene 6000 qPCR Detection System (Corbett). Reactions included 1 × iQ Supermix (Bio-Rad), 0.5 μM of each primer (Table 3) and 0.375 μM of TaqMan probe in a total volume of 20 μL. Amplification followed a three-step PCR: 40 cycles with denaturation at 95 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 30 s. A non-template control was used in every PCR reaction. As positive controls for the species, DNA samples were obtained from reference strains from the Mycology Laboratory from the National Institute of Health Doutor Ricardo Jorge.
Table 3.
Sequence of primers and TaqMan probes used for real-time PCR.
| Fungal Species Targeted | Sequences |
|---|---|
| A. flavus complex (Toxigenic Strains) | |
| Primer Forward | 5‘-GTCCAAGCAACAGGCCAAGT-3‘ |
| Primer Reverse | 5‘-TCGTGCATGTTGGTGATGGT-3‘ |
| Probe | 5‘-TGTCTTGATCGGCGCCCG-3‘ |
| A. fumigatus complex | |
| Primer Forward | 5‘-CGCGTCCGGTCCTCG-3‘ |
| Primer Reverse | 5‘-CGTGAGGCGGGAGCA-3‘ |
| Probe | 5‘-CCAACCTCCCACCCGTG-3‘ |
| A. ochraceus complex | |
| Primer Forward | 5‘-CGGGTCTAATGCAGCTCCAA-3‘ |
| Primer Reverse | 5‘-CGGGCACCAATCCTTTCA-3‘ |
| Probe | 5‘-CGTCAATAAGCGCTTTT-3‘ |
2.4. Data Analysis
The data analysis was performed and used descriptive statistics using frequency, median and graphical representations appropriate for the nature of the data. To compare CFU/m3 in air and CFU/m2 on surfaces between slaughterhouses (PS, SBS and LAS), the Kruskal–Wallis test was used. For the statistical analysis, we used the statistical software SPSS V21. The results are considered significant at a 5% significance level.
3. Results
3.1. Fungal Load
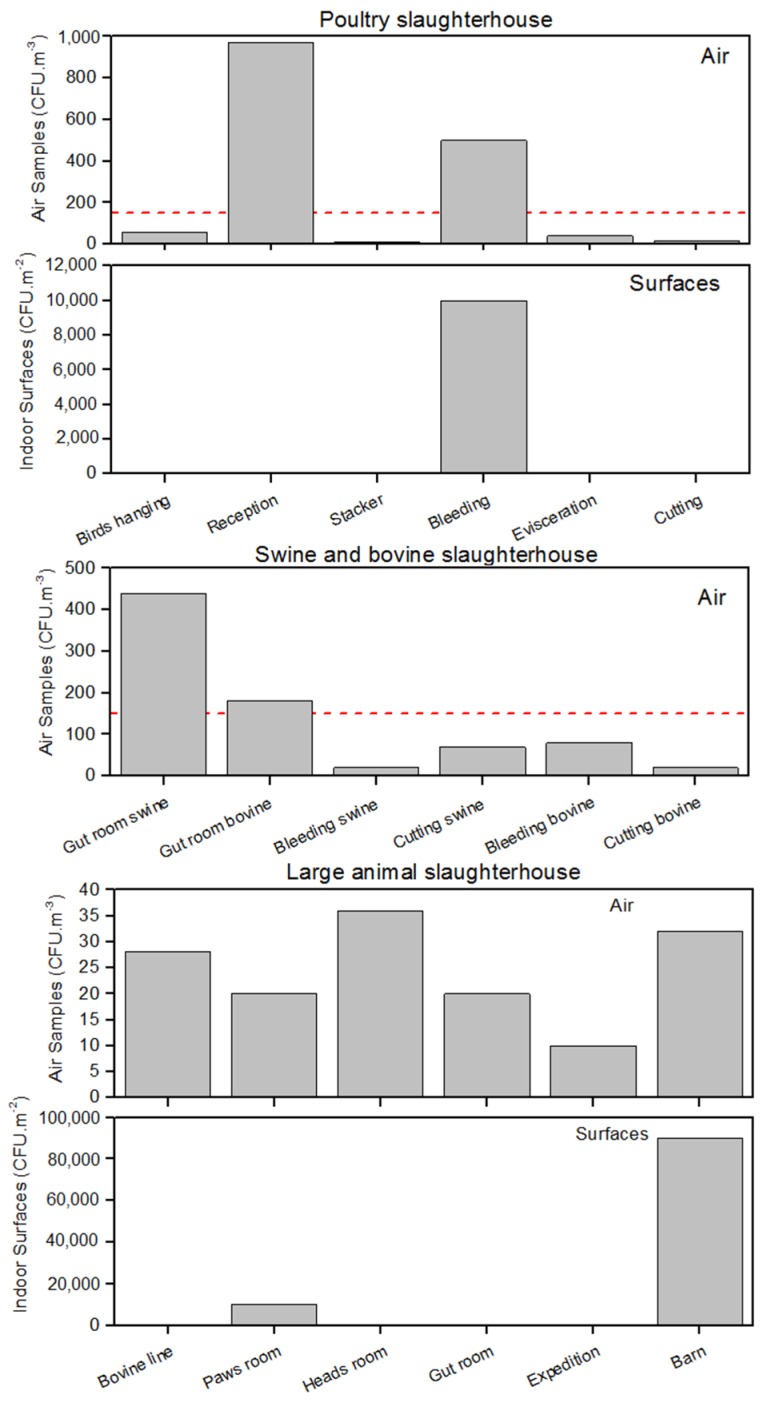
Air fungal load ranged from 16 CFU/m3 to 970 CFU/m3 in the poultry slaughterhouse, 20 CFU/m3 to 440 CFU/m3 in the swine/bovine slaughterhouse and 10 CFU/m3 to 36 CFU/m3 in the large animal slaughterhouse (Figure 1). The surfaces present results that ranged from 0 CFU/m2 to 10,000 CFU/m2 in the poultry slaughterhouse and 0 CFU/m2 to 90,000 CFU/m2 in the large animal slaughterhouse. No fungal isolates were found in swine/bovine slaughterhouse surfaces (Figure 1). Poultry and swine and bovine slaughterhouses presented each two sampling sites that surpass the guideline proposed by World Health Organization (WHO) (maximum value of 150 CFU/m3) [13].
Figure 1.
Fungal load distribution in the three assessed slaughterhouses. The dashed line represents the reference limits suggested by the World Health Organization (WHO).
In the three units assessed, 11 out of the 18 samples collected presented a higher indoor fungal load when compared to the outdoor sampling, the poultry slaughterhouse being the one with the highest number of samples with increased load indoors (six out of the six samples collected).
Comparing the CFU/m3 in air and the CFU/m2 on surfaces among the three units, no statistically-significant differences were detected.
3.2. Fungal Identification
3.2.1. Poultry Slaughterhouse
Eight different fungal species were detected in indoor air in a total of 1596 isolates. Scopulariopsis candida was the most frequently-isolated species (59.5%) followed by Penicillium sp. (32.8%). Other fungi were also identified in this unit, namely: isolates belonging to the Aspergillus fumigatus complex; Aspergillus niger complex; Mucor sp.; Geotrichum sp.; Paecilomyces sp.; and Trichoderma sp. Only Mucor genera were isolated on surfaces (Table 4).
Table 4.
Most common fungi isolated in the three slaughterhouses.
| Fungal Identification | Fungal Quantification |
|---|---|
| Indoor air samples (PS) | (CFU/m3; %) |
| Scopulariopsis sp. | 950; 59.52 |
| Penicillium sp. | 524; 32.83 |
| Aspergillus fumigatus complex | 50; 3.13 |
| Others | 72; 4.5 |
| Indoor surfaces samples (PS) | (CFU/m2; %) |
| Mucor sp. | 10,000; 100 |
| Indoor air samples (SBS) | (CFU/m3; %) |
| Cladosporium sp. | 370; 45.7 |
| Penicillium sp. | 270; 33.3 |
| Aureobasidium sp. | 90; 11.1 |
| Others | 80; 9.9 |
| Indoor air samples (LAS) | (CFU/m3; %) |
| Penicillium sp. | 118; 80.8 |
| Aspergillus ochraceus complex | 8; 5.5 |
| Cladosporium sp. | 8; 5.5 |
| Others | 12; 8.2 |
| Indoor surfaces samples (LAS) | (CFU/m2; %) |
| Scopulariopsis brumptii | 40,000; 40 |
| A. terreus complex | 30,000; 30 |
| Others | 30,000; 30 |
3.2.2. Swine and Bovine Slaughterhouse
Seven different fungal species were detected in indoor air in a total of 810 isolates. Cladosporium sp. was the most frequently-isolated genus (45.7%) followed by Penicillium sp. (33.3%). Other fungi were also identified in this unit, namely: isolates belonging to the Aspergillus fumigatus complex; Aureobasidium sp.; Chrysonilia sp.; Fusarium poae; and Alternaria sp. Regarding surfaces, no filamentous fungi were isolated (Table 4).
3.2.3. Large Animal Slaughterhouse
Six different fungal species were detected in indoor air in a total of 146 isolates. Penicillium sp. was the most frequently isolated (80.8%). Other fungi were also identified in this unit, namely: isolates from the Aspergillus ochraceus complex; Aureobasidium sp.; Cladosporium sp.; Chrysonilia sp.; and Chrysosporium inops. Five different fungal species were detected on surfaces in a total of 10 × 104 isolates, Scopulariopsis brumptii being the most found (40.0%) followed by isolates belonging to the Aspergillus terreus complex (30.0%). Other fungi also isolated were: Eurotium herbariorum (teleomorph form of Aspergillus glaucus complex); Acremonium sp.; and Chrysosporium sp. (Table 4).
In both slaughterhouses where fungal isolates were obtained on surfaces, different species (from the ones found in air samples) were detected. In poultry slaughterhouse, Mucor sp. was found only on surfaces; in large animal slaughterhouse, five species were found only on surfaces (Scopulariopsis brumptii; Aspergillus terreus complex; Eurotium herbariorum; Acremonium sp.; and Chrysosporium sp.).
3.3. Fungal Detection
DNA from toxigenic strains of the A. flavus complex and the A. ochraceus complex was not amplified by qPCR. However, qPCR analysis successfully amplified DNA from the A. fumigatus complex in six sampling sites where the presence of this fungal species was not identified by conventional methods (Table 5). Of note, samples with lower cycle threshold (CT) values very likely exhibit higher levels of the A. fumigatus complex.
Table 5.
Conventional quantification of isolates and molecular detection from A. fumigatus complex in the three slaughterhouses.
| Sampling Sites | Fungal Quantification | Fungal Detection | |
|---|---|---|---|
| Poultry slaughterhouse (PS) | Air (CFU/m3) | Surfaces (CFU/m2) | Real-time PCR (Ct, cycle threshold) |
| Birds hanging | - | - | n. d. |
| Reception | - | - | n. d. |
| Stacker | 10 | - | 35.7 |
| Bleeding | 30 | - | 35.92 |
| Evisceration | 10 | - | 36.53 |
| Cutting | - | - | n.d. |
| Swine and bovine slaughterhouse (SBS) | Air (CFU/m3) | Surfaces (CFU/m2) | Real-time PCR (Ct, cycle threshold) |
| Gut room swine | - | - | 33.91 |
| Gut room bovine | 10 | - | n.d. |
| Bleeding swine | - | - | 33.53 |
| Cutting swine | - | - | 33.61 |
| Bleeding bovine | - | - | n.d. |
| Cutting bovine | 35.89 | ||
| Large animal slaughterhouse (LAS) | Air (CFU/m3) | Surfaces (CFU/m2) | Real-time PCR (Ct, cycle threshold) |
| Bovine line | - | - | n.d. |
| Paws room | - | - | 35.85 |
| Heads room | - | - | n. d. |
| Gut room | - | - | n.d. |
| Expedition | - | - | 34.47 |
n.d.: not detected.
4. Discussion
This is the first Portuguese study to comprehensively assess fungal contamination by cultural methods and molecular tools in several animal slaughterhouses and in different processing areas of those slaughterhouses.
Health data under the context of bioaerosol exposure were previously analyzed [14,15,16]; on the date of these studies, the available data were not sufficient to derive exposure limits for bioaerosols [14,15,16]. To overcome this limitation, the worst case approach was used, allowing a low cost exposure assessment. This assessment strategy will prioritize interventions in the assessed setting aiming at the implementation of safety measures [17]. It is noteworthy that only short-term air sampling was performed, and variations in fungal contamination are expected. Nevertheless, the most critical scenario was selected to perform sampling, ensuring a more demanding results analyses.
Since there are no exposure limits, the guideline proposed by the World Health Organization (WHO) (maximum value of 150 CFU/m3) [13] was compared to the obtained fungal load background, since it is the most strict for occupational assessment purposes. Overall, the poultry slaughterhouse presented the most critical fungal load, not only indicating two sampling sites that surpass the selected guideline, but also because all air samples presented a higher indoor fungal load when compared to the outdoor sample, which could mean the existence of indoor fungal contamination sources [18]. However, we must point out that viable bioaerosol particles constitute a small percentage of the total concentration of microorganisms [19], and therefore, we should consider a bias regarding fungal burden in all units assessed.
One of the most important stages of risk control is to verify the existence of indicator species/strains that are representative of harmful fungal contamination in the analyzed setting. For that, it is important to identify what are the best indicators for slaughterhouses’ occupational environment [20]. Furthermore, a protocol with standardized methods that ensure a proper identification and detection of the selected indicators will be very helpful [20]. In this kind of occupational environment, expected to be highly contaminated, verification should be carried out applying conventional and molecular methods [21].
Several studies that intend to assess occupational exposure to fungi already applied these methodologies [22,23,24,25,26]. Regarding the results obtained through conventional (cultural) methods, Penicillium sp. was one of the most frequent in all of the assessed units (the most prevalent in LAS and the second in PS and in SBS), and among this genus, we should consider targeting some specific species/strains, such as Penicillium polonicum (belonging to Penicillium aurantiogriseum complex) with toxigenic potential [27], related to high contamination levels in feed and food [28] and already isolated in a similar occupational environment [3]. Although not being the most prevalent genus in any of the slaughterhouses analyzed, Aspergillus sp. was present in all units with isolates belonging to different species/complexes. The Fumigati section was present in PS and in SBS; the Nigri section only in PS; Circumdati, Terrei and Aspergilli sections were isolated only in LAS. Besides the presence of the A. fumigatus complex requiring implementation of corrective measures according to the American Industrial Hygiene Association (AIHA 1996) in the Field Guide for the Determination of Biological Contaminants in Environmental Samples, we should highlight that most of the species/strains isolated from Aspergillus sections have toxigenic potential [27].
No fungal isolates were recovered from the surfaces of SBS. Instead of the floor, walls were the surfaces sampled, since the floor was covered with animal’s blood. In the other two units, all of the fungal species/complexes isolated on surfaces were not found in air, meaning that it is crucial to collect samples from both, air and surfaces, to ensure a complete fungal contamination assessment [29]. Among the species identified exclusively on surfaces, we must point out the Aspergillus terreus complex that can cause invasive infections in humans [30,31].
The target species-complexes selected for this study should be considered as good indicators complexes/species/strains of harmful fungal contamination in this specific occupational environment. The A. flavus complex (toxigenic strains) produces a mycotoxin (Aflatoxin B1 (AFB1)) that is genotoxic and a potent hepatocarcinogen [32,33]. High risks of occupational exposure to this mycotoxin through inhalation were already reported [33,34,35,36,37,38]. Besides, in previous studies developed in Portuguese poultries, toxigenic strains from the Flavi section were detected [25], and occupational exposure of poultry slaughterhouse workers [39] and poultry workers [40] to AFB1 was already reported. The Circumdati section, mainly A. ochraceus, is known to be one of the predominant producers of ochratoxin A (OTA) detected worldwide in various food and feed sources [27]. Exposure to OTA has been related to several diseases in both animals and humans, predominantly affecting the kidney. However, besides nephrotoxicity, other toxic effects have been associated with OTA exposure, such as neurotoxicity, immunotoxicity, myelotoxicity, reproductive toxicity and teratogenicity [41,42,43]. Additionally, and since 1993, OTA has been classified as a possible carcinogen to humans in Group 2B [32], based on its known tumorigenicity in rodents. Occupational exposure to OTA has already been reported in other occupational settings, such as waste management [44], coffee production [45] and observed a high presence in the settled dust of grain elevators [46].
Aspergillus fumigatus is the species with higher clinical relevance [47,48] and the most common cause of invasive aspergillosis [49]. Moreover, one of the most abundantly-produced metabolites by this fungus is gliotoxin, which exhibits a diverse array of biologic effects on the immune system [50]. Besides, at least in two different Portuguese settings, it was proven that molecular tools increase the detection of this species-complex, namely poultries [25] and in the waste industry [51].
The use of the two types of analytical methods in this study, culture analysis and PCR-based detection, has given a more accurate scenario regarding fungal contamination in this occupational environment. This strategy allows the quantification and identification of fungal species that pose higher occupational risk due to inhalation and also the comparison of their levels with a selected guideline. In addition, with molecular tools, we can target selected species (indicators) that can be inhibited with other fungal species with fast growth rates [25,26,52]. However, the Circumdati section was identified in low counts in LAS, but not detected by the molecular tools applied. The same happens in one sampling site for the Fumigati section in SBS. False negatives are often a problem in PCR assays for microbial detection. These might be caused by several factors, including the ineffective release of microbial DNA content from the cells, poor DNA recovery after extraction and purification steps and inappropriate removal of PCR inhibitors in the analyzed samples [53]. In our study, in particular, one of the inhibition sources could potentially be the presence of particles in the air, as already observed in previous studies [54].
Prevention of fungal dissemination in slaughterhouses is of great importance in order to avoid mycotoxin production, since this can result in mycotoxin exposure for workers and, even in some cases, for consumers [55].
In order to reduce the potential exposure to fungal burden, protective measures should be taken. Preventive measures should be implemented, such as disinfection and also wearing personal protection devices, such as filtration half masks and gloves.
5. Conclusions
Besides the given information regarding fungal contamination background in this specific occupational environment, this study suggests the indicators that are representative of harmful fungal contamination. The applied strategy, using cultural-based methods and molecular tools in parallel, as a protocol to ensure the proper characterization of fungal contamination occupational exposure is also an added value from this assessment.
Moreover, this specific occupational environment is a good example of the reality of occupational exposures: co-exposure to several risks factors by different exposure routes. This implies the need for caution when comparing exposure results with occupational exposure limits and also for considering the cumulative risk assessment as the best option when performing risk assessment.
Acknowledgments
The authors are grateful to the Occupational Health Services from the analyzed slaughterhouses. This study was supported by the Environment & Health Research Group from Lisbon School of Health Technology and Associação Para o Desenvolvimento de Conhecimento e Inovação (POLITEC & ID).
Author Contributions
Carla Viegas and Susana Viegas conceived of and designed the experiments. Anita Quintal Gomes, Carla Viegas, Tiago Faria and Mateus dos Santos performed the experiments. Anita Quintal Gomes, Carla Viegas, Elisabete Carolino, Raquel Sabino and Susana Viegas analyzed the data. Tiago Faria, Mateus dos Santos and Elisabete Carolino contributed reagents, materials and analysis tools. Carla Viegas wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Oppliger A., Charrie N., Droz P.O., Rinsoz T. Exposure to Bioaerosols in Poultry Houses at Different Stages of Fattening; Use of Real-time PCR for Airborne Bacterial Quantification. Ann. Occup. Hyg. 2008;52:405–412. doi: 10.1093/annhyg/men021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eduard W. Fungal spores: A critical review of the toxicological and epidemiological evidence as a basis for occupational exposure limit setting. Crit. Rev. Toxicol. 2009;39:799–864. doi: 10.3109/10408440903307333. [DOI] [PubMed] [Google Scholar]
- 3.Paba E., Chiominto A., Marcelloni A.M., Proiettol A.R., Sisto R. Exposure to Airborne Culturable Microorganisms and Endotoxin in Two Italian Poultry Slaughterhouses. J. Occup. Environ. Hyg. 2014;11:469–478. doi: 10.1080/15459624.2013.877141. [DOI] [PubMed] [Google Scholar]
- 4.Refai M., Nada M.A., El Naggar A., Abdel-Aziz A. Fungal flora in modern Egyptian abattoirs. Fleischwirtsch. 1993;73:172–174. [Google Scholar]
- 5.Mansour N.K.M. Thesis in Veterinary Medicine; Munchen, Germany: 1986. Zum Vorkommen von Schimmelpilzen der Gattung Cladosporium Link ex Fries auf Schaffleisch. [Google Scholar]
- 6.White P., Collins L.D., McGill K., Monahan C., O’Mahoni H. Distribution and prevalence of airborne microorganisms in three commercial poultry processing plants. J. Food Prot. 2001;64:388–391. doi: 10.4315/0362-028x-64.3.388. [DOI] [PubMed] [Google Scholar]
- 7.Haas D., Posch J., Schmidt S., Wust G., Sixl W., Feierl G., Marth E., Reinthaler F.F. A case study of airborne culturable microorganisms in a poultry slaughterhouse in Styria, Austria. Aerobiologia. 2005;21:193–201. doi: 10.1007/s10453-005-9003-x. [DOI] [Google Scholar]
- 8.Laitinen S., Kangas J., Husman K., Susitaival P. Evaluation of exposure to airborne bacterial endotoxins and Peptidoglycans in selected work environments. Ann. Agric. Environ. Med. 2001;8:213–219. [PubMed] [Google Scholar]
- 9.Hagmar L., Schlitzl A., Hallberg T., Sjoholm A. Health effects of exposure to endotoxins and organic dust in poultry slaughter-house workers. Int. Arch. Occup. Environ. Health. 1990;62:159–164. doi: 10.1007/BF00383592. [DOI] [PubMed] [Google Scholar]
- 10.Lues J.F., Theron M.M., Venter P., Rasephei M.H. Microbial Composition in Bioaerosols of a High-Throughput Chicken-Slaughtering Facility. Poult. Sci. 2007;86:142–149. doi: 10.1093/ps/86.1.142. [DOI] [PubMed] [Google Scholar]
- 11.Hall R.J., Leblanc-Maridor M., Wang J., Ren X., Moore N.E., Brooks C.R., Peacey M., Douwes J., McLean D.J. Metagenomic Detection of Viruses in Aerosol Samples from Workers in Animal Slaughterhouses. PLoS ONE. 2013;8:297. doi: 10.1371/journal.pone.0072226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Hoog G.S., Guarro J., Gebé J., Figueras M.J. Atlas of Clinical Fungi. 2nd ed. Centraalbureau voor Schimmelcultures; Utrecht, The Netherlands: 2000. [Google Scholar]
- 13.WHO Regional Office for Europe . WHO Regional Office for Europe; Geneva, Swiss: 2009. WHO Guidelines for Indoor Air Quality: Dampness and Mould. [PubMed] [Google Scholar]
- 14.Swan J.R.M., Kelsey A., Crook B., Gilbert E.J. Occupational and Environmental Exposure to Bioaerosols from Composting and Potential Health Effects—A critical Review of Published Data. [(accessed on 28 January 2016)]; Available online: http://www.hse.gov.uk/research/rrpdf/rr130.pdf.
- 15.O’Connor A.M., Auvermann B., Bickett-Weddle D., Kirkhorn S., Sargeant J.M., Ramirez A., Essen S.G.V. The Association between Proximity to Animal Feeding Operations and Community Health: A Systematic Review. PLoS ONE. 2010;5:297. doi: 10.1371/journal.pone.0009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson C., Littlewood E., Douglas P., Robertson S., Gant T.W., Hansell A.L. Exposures and health outcomes in relation to bioaerosol emissions from composting facilities: A systematic review of occupational and community studies. J. Toxicol. Environ. Health B Crit. Rev. 2015;18:43–69. doi: 10.1080/10937404.2015.1009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.European Committee for Standardization . European Standard EN 14042:2003: Workplace Atmospheres—Guide for the Application and Use of Procedures for the Assessment of Exposure to Chemical and Biological Agents. European Committee for Standardization; Brussels, Belgium: 2003. [Google Scholar]
- 18.Wouters I., Spaan S., Douwes J., Doekes G., Heederik D. Overview of personal occupational exposure levels to inhalable dust, endotoxin, beta(1→3)—Glucan and fungal extracellular polysaccharides in the waste management chain. Ann. Occup. Hyg. 2006;50:39–53. doi: 10.1093/annhyg/mei047. [DOI] [PubMed] [Google Scholar]
- 19.Górny R.L., Harkawy A.S., Ławniczek-Wałczyk A., Karbowska-Berent J., Wlazło A., Niesler A., Gołofit-Szymczak M., Cyprowski M. Exposure to Culturable and Total Microbiota in Cultural Heritage Conservation. Int. J. Occup. Med. Environ. Health. 2016;29:255–275. doi: 10.13075/ijomeh.1896.00630. [DOI] [PubMed] [Google Scholar]
- 20.Walser S.M., Gerstner D.G., Brenner B., Bünger J., Eikmann T., Janssen B., Kolb S., Kolk A., Nowak D., Raulf M., et al. Evaluation of exposure–response relationships for health effects of microbial bioaerosols—A systematic review. Int. J. Hyg. Environ. Health. 2015;218:577–589. doi: 10.1016/j.ijheh.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Viegas C., Pinheiro C., Sabino R., Viegas S., Brandão J., Veríssimo C. Environmental Mycology in Public Health: Fungi and Mycotoxins Risk Assessment and Management. Academic Press; Cambridge, UK: 2015. [Google Scholar]
- 22.Coenen G.J., Dahl S., Ebbehøj N., Ivens U., Stenbæk E., Würtz H. Immunglobulins and peak expiratiory flow measurements in waste collectorsin relation to bioaerosol exposure. Ann. Agric. Environ. 1997;4:75–80. [Google Scholar]
- 23.Rimac D., Macan J., Varnai V.M., Vucemilo M., Matkovic K., Prester L., Orct T., Trosic I., Pavicic I. Exposure to poultry dust and health effects in poultry workers: Impact of mould and mite allergens. Int. Arch. Occup. Environ. Health. 2010;83:9–19. doi: 10.1007/s00420-009-0487-5. [DOI] [PubMed] [Google Scholar]
- 24.Sabino R., Faisca V.M., Carolino E., Verissimo C., Viegas C. Occupational exposure to Aspergillus by swine and poultry farm workers in Portugal. J. Toxicol. Environ. Health A. 2012;75:1381–1391. doi: 10.1080/15287394.2012.721170. [DOI] [PubMed] [Google Scholar]
- 25.Viegas C., Malta-Vacas J., Sabino R., Viegas S., Veríssimo C. Accessing indoor fungal contamination using conventional and molecular methods in Portuguese poultries. Environ. Monit. Assess. 2014;3:1951–1959. doi: 10.1007/s10661-013-3509-4. [DOI] [PubMed] [Google Scholar]
- 26.Viegas C., Sabino R., Botelho D., dos Santos M., Quintal Gomes A. Assessment of exposure to the Penicillium glabrum complex in cork industry using complementing methods. Arh. Hig. Rada. Toksikol. 2015;66:203–207. doi: 10.1515/aiht-2015-66-2614. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen K.F. Mycotoxin production by indoor molds. Fungal Genet. Biol. 2003;39:103–117. doi: 10.1016/S1087-1845(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 28.Stoev S.D., Dutton M.F., Njobeh P.B., Mosonik J.S., Steenkamp P.A. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Endemic Nephropathy. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010;27:72–88. doi: 10.1080/02652030903207227. [DOI] [PubMed] [Google Scholar]
- 29.Viegas C., Faria T., Meneses M., Carolino E., Viegas S., Quintal G.A., Sabino R. Analysis of surfaces for the characterization of fungal burden—Does it matter? Int. J. Occup. Med. Environ. Health. 2016 doi: 10.13075/ijomeh.1896.00562. in press. [DOI] [PubMed] [Google Scholar]
- 30.Balajee S.A. Aspergillus terreus complex. Med. Mycol. 2009;47:42–46. doi: 10.1080/13693780802562092. [DOI] [PubMed] [Google Scholar]
- 31.Lackner M., Coassin S., Haun M., Binder U., Kronenberg F., Haas H., Jank M., Maurer E., Meis J.F., Hagen F., et al. Geographically predominant genotypes of Aspergillus terreus species complex in Austria: S microsatellite typing study. Clin. Microbiol. Infect. 2015;22:270–276. doi: 10.1016/j.cmi.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 32.IARC . Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Press; Lyon, France: 1993. Ochratoxin A; pp. 489–521. [Google Scholar]
- 33.Dash B., Afriyie-Gyawu E., Huebneret H.J., Porter W., Wang J.S., Jolly P.E., Phillips T.D. Determinants of the variability of aflatoxin-albumin adduct in levels in Ghanaians. Toxicol. Environ. Health A. 2007;70:58–67. doi: 10.1080/15287390600748880. [DOI] [PubMed] [Google Scholar]
- 34.Dvorackova I. Aflatoxin inhalation and alveolar cell carcinoma. Br. Med. J. 1976;20:691. doi: 10.1136/bmj.1.6011.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dvorackova I., Pichova V. Pulmonary interstitial fibrosis with evidence of aflatoxin B1 in lung tissue. J. Toxicol. Environ. Health. 1986;18:153–157. doi: 10.1080/15287398609530856. [DOI] [PubMed] [Google Scholar]
- 36.Baxter C.S., Wey H.E., Burg W.R. A prospective analysis of the potential risk associated with inhalation of aflatoxin contaminated grain dusts. Food Cosmet. Toxicol. 1981;19:765–769. doi: 10.1016/0015-6264(81)90535-6. [DOI] [PubMed] [Google Scholar]
- 37.Hayes R.B., van Nieuwenhuize J.P., Raatgever J.W., ten Kate F.J. Aflatoxin exposures in the industrial setting: an epidemiological study of mortality. Food Chem. Toxicol. 1984;22:39–43. doi: 10.1016/0278-6915(84)90050-4. [DOI] [PubMed] [Google Scholar]
- 38.Popendorf W., Donham K.J., Easton D.N., Silk J. A synopsis of agricultural respiratory hazards. Am. Ind. Hyg. Assoc. J. 1985;46:154–161. doi: 10.1080/15298668591394572. [DOI] [PubMed] [Google Scholar]
- 39.Viegas S., Veiga L., Almeida A., dos Santos M., Carolino E., Viegas C. Occupational Exposure to Aflatoxin B1 in a Portuguese Poultry Slaughterhouse. Ann. Occup. Hyg. 2015;60:176–183. doi: 10.1093/annhyg/mev077. [DOI] [PubMed] [Google Scholar]
- 40.Viegas S., Veiga L., Malta-Vacas J., Sabino R., Figueiredo P., Almeida A., Viegas C., Carolino E. Occupational exposure to aflatoxin (afb1) in poultry production. J. Toxicol. Environ. Health A. 2012;75:1330–1340. doi: 10.1080/15287394.2012.721164. [DOI] [PubMed] [Google Scholar]
- 41.WHO . Evaluation of Certain Mycotoxins in Food: Fifty-sixth Report of the Joint FAO/WHO Expert Committee on Food Additives. WHO; Geneva, Swiss: 2002. pp. 1–62. [PubMed] [Google Scholar]
- 42.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 43.Costa J.G., Saraiva N., Guerreiro P.S., Louro H., Silva M.J., Miranda J.P., Castro M., Batinic-Haberle I., Fernandes A.S., Oliveira N.G. Ochratoxin A-induced cytotoxicity, genotoxicity and reactive oxygen species in kidney cells: An integrative approach of complementary endpoints. Food Chem. Toxicol. 2016;87:65–76. doi: 10.1016/j.fct.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Degen G.H., Blaskewicz M., Lektarau Y., GrLiner C. Ochratoxin A Analyses of blood samples from workers at waste. Mycotoxin Res. 2003;19:3–7. doi: 10.1007/BF02940082. [DOI] [PubMed] [Google Scholar]
- 45.Brera C., Caputi R., Miraglia M., Iavicolib I., Salernoc A., Carellib G. Exposure assessment to mycotoxins in workplaces: Aflatoxins and ochratoxin A occurrence in airborne dusts and human sera. Microchem. J. 2002;73:167–173. doi: 10.1016/S0026-265X(02)00061-9. [DOI] [Google Scholar]
- 46.Mayer S., Curtui V., Usleber E., Gareis M. Airborne mycotoxins in dust from grain elevators. Mycotoxin Res. 2007;23:94–100. doi: 10.1007/BF02946033. [DOI] [PubMed] [Google Scholar]
- 47.Dagenais T., Keller N. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin. Microbiol. Rev. 2009;22:447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCormick A., Loeffler L., Ebel F. Aspergillus fumigatus: contours of an opportunistic human pathogenic. Cell. Microbiol. 2010;12:1535–1543. doi: 10.1111/j.1462-5822.2010.01517.x. [DOI] [PubMed] [Google Scholar]
- 49.Stanzani M., Orciuolo E., Lewis R., Kontoyiannis D., Martins S., St. John L., Komanduri K. Aspergillus fumigatus suppresses the human cellular immune response via gliotoxin-mediated apoptosis of monocytes. Blood. 2005;15:2258–2265. doi: 10.1182/blood-2004-09-3421. [DOI] [PubMed] [Google Scholar]
- 50.Lewis R., Wiederhold N., Lionakis M., Prince R., Kontoyiannis D. Frequency and species distribution of gliotoxin-producing Aspergillus isolates recovered from patients at a tertiary-care cancer center. J. Clin. Microbiol. 2005;43:6120–6122. doi: 10.1128/JCM.43.12.6120-6122.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viegas C., Gomes A.Q., Faria T., Sabino R. Prevalence of Aspergillus fumigatus complex in waste sorting and incineration plants: An occupational threat. Int. J. Environ. Waste Manag. 2016 doi: 10.1504/IJEWM.2015.074939. in press. [DOI] [Google Scholar]
- 52.Bellanger A.P., Reboux G., Murat J.B., Bex V., Millon L. Detection of Aspergillus fumigatus by quantitative polymerase chain reaction in air samples impacted on low-melt agar. Am. J. Infect. Control. 2010;38:195–198. doi: 10.1016/j.ajic.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Yang S., Rothman R.E. PCR-based diagnostics for infectious diseases: Uses, limitations, and future applications in acute-care settings. Lancet Infect. Dis. 2004;4:337–348. doi: 10.1016/S1473-3099(04)01044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDevitt J.J., Lees P.S.J., Merz W.G., Schwab K.J. Inhibition of quantitative PCR analysis of fungal conidia associated with indoor air particulate matter. Aerobiologia. 2007;23:35–45. doi: 10.1007/s10453-006-9047-6. [DOI] [Google Scholar]
- 55.Veskoviã-Moraåanin S.M., Borovié B.R., Velebit B.M., Rašeta M.P., Milicevic D.R. Identification of Mycobiota in Serbian Slaughterhouses. Proc. Nat. Sci. Matica. Srpska. Novi Sad. 2009;117:45–49. doi: 10.2298/ZMSPN0917045V. [DOI] [Google Scholar]