Abstract
BRCA1 mutations and estrogen use are risk factors for the development of breast cancer. Recent work has identified estrogen receptors localized at the plasma membrane that signal to cell biology. We examined the impact of BRCA1 on membrane estrogen and growth factor receptor signaling to breast cancer cell proliferation. MCF-7 and ZR-75-1 cells showed a rapid and sustained activation of extracellular signal-related kinase (ERK) in response to estradiol (E2) that was substantially prevented by wild-type (wt) but not mutant BRCA1. The proliferation of MCF-7 cells induced by E2 was significantly inhibited by PD98059, a specific ERK inhibitor, or by dominant negative ERK2 expression and by expression of wt BRCA1 (but not mutant BRCA1). E2 induced the synthesis of cyclins D1 and B1, the activity of cyclin-dependent kinases Cdk4 and CDK1, and G1/S and G2/M cell cycle progression. The intact tumor suppressor inhibited all of these. wt BRCA1 also inhibited epidermal growth factor and insulin-like growth factor I-induced ERK and cell proliferation. The inhibition of ERK and cell proliferation by BRCA1 was prevented by phosphatase inhibitors and by interfering RNA knockdown of the ERK phosphatase, mitogen-activated kinase phosphatase 1. Our findings support a novel tumor suppressor function of BRCA1 that is relevant to breast cancer and identify a potential interactive risk factor for women with BRCA1 mutations.
Mutations of the BRCA1 and BRCA2 genes strongly increase the risk of developing breast and ovarian cancer in women and prostate cancer in men (2, 21, 46). The intact BRCA1 protein has several functions that prevent cancer development, including DNA repair, activation of cell cycle checkpoints, and induction of chromosome stability (10, 17, 22, 40, 46, 49). BRCA1 may induce p53-independent cell cycle arrest in breast cancer cells when highly overexpressed (11, 38) but more often collaborates with p53 for this function. The partnering of BRCA1 with the BARD protein activates ubiquitin E3 ligase activity and leads to proteasomal degradation of relevant proteins (1). These or other undefined functions that are compromised by BRCA1 mutation contribute substantially to carcinogenesis.
Cells that transform in a background of BRCA1 mutation are initially hormone responsive. In one such malignancy, breast cancer, estradiol (E2) use after menopause is a risk factor (2, 35). Although many BRCA1 mutant-related breast tumors lack estrogen receptors (ER) at the time of diagnosis (16), some studies suggest that an interaction between estrogen and BRCA1 may contribute to early tumor pathogenesis. Bilateral prophylactic ovariectomy is associated with a significantly reduced incidence of breast cancer in women carrying mutant BRCA1 (33), while men with single allelic BRCA1 mutations have a much lower incidence of breast malignancy than women carrying the same mutation (42). Although E2 upregulates BRCA1 expression, this may indirectly reflect the proliferative actions of the sex steroid (19). It is unclear whether BRCA1 upregulation has biological consequences. More important, however, may be the functional interactions between the sex steroid and the wild-type (wt) tumor suppressor protein. BRCA1 inhibits both ligand-independent (54) and -dependent (6) transcription induced by nuclear ER. This inhibition results in part from the ability of the N terminus of BRCA1 (amino acids 1 to 300) to physically interact with the AF-2 domain of nuclear ERα (7). However, the impact of the interactions between BRCA1 and nuclear ER on breast cancer biology is unclear.
E2 traditionally has been described to induce transcription through nuclear ER (48). However, E2 also rapidly activates both transcription and the modification of protein function through kinase activation (24, 29). Plasma membrane-associated ER usually mediates this signaling, activating G proteins and the extracellular signal-regulated protein kinase (ERK) cascade (32); this signal significantly influences the survival of the breast cancer cell (29). The majority of studies also suggest the possibility of a role for ERK in E2-induced cell proliferation (18, 30, 36). Breast cancer growth factors such as epidermal growth factor (EGF) or insulin-like growth factor I (IGF-I) utilize similar signaling pathways to stimulate cell proliferation, which results from the activation of their membrane tyrosine kinase receptors (44, 52). Furthermore, membrane ER signaling to ERK in breast cancer results from cross talk to EGF receptor (EGFR) transactivation (8). Thus, it is potentially important to understand whether BRCA1 influences this mechanism of both membrane ER/E2 and growth factor action as a novel tumor suppressor function.
MATERIALS AND METHODS
Cell lines and materials.
MCF-7, ZR-75-1, and HCC-1937 breast cancer cells were obtained from the American Type Culture Collection. Estradiol was obtained from Sigma, and ICI182780 was obtained from AstraZeneca (kindly provided by Alan Wakeling). EGF, IGF-I, tyrphostin AG1287, and PD98059 were purchased from Calbiochem. Antibodies were from Santa Cruz Biotechnology, Inc. Duplexed RNA oligomers for mitogen-activated kinase phosphatase 1 (MKP-1), green fluorescent protein (GFP), and BRCA1 were synthesized by QIAGEN.
Kinase activity studies.
For ERK activity assays, the cells were synchronized for 24 h in serum, phenol red, and growth factor-free medium. The cells were then exposed to 10 nM E2 for 9 min with or without additional substances, and the cells were then lysed and immunoprecipitated for ERK2 as previously described (25). Immunoprecipitated ERK2 samples were resuspended in 40 μl of kinase buffer containing [32P]ATP and myelin basic protein (Sigma) as the substrate for the in vitro assay (25). Equal aliquots of immunoprecipitated ERK from each condition were also immunoblotted to show equal gel loading. All experiments were repeated two to three times.
For phosphatidylinositol 3-kinase (PI3K), phosphorylation of AKT at serine 473 was determined after 15 min of exposure to E2 as an indication of activation (25). Cultured cell lysates were pelleted and dissolved in sodium dodecyl sulfate sample buffer, boiled, separated, and then transferred to nitrocellulose. Phosphorylated AKT was detected by using phosphospecific monoclonal antibodies (Santa Cruz) and an ECL Western blot kit (Amersham). For Cdk4 activity, studies methodologically similar to that described for ERK were carried out at 16 h using the retinoblastoma protein as a substrate (26). For CDK1 activity, samples were obtained from cells after 36 h of incubation under various conditions, including HCC-1937 cells expressing wt BRCA1. CDK1 was immunoprecipitated using monoclonal antibody (Santa Cruz), and activity was determined by an in vitro assay with histone H1 as a substrate.
Transient transfection and constructs.
Fusion plasmids encoding wt BRCA1 (pcBRCA1-385) and mutant BRCA1 proteins (the 185delAG, 5677insA, and T300G mutants; kindly provided by Michael Erdos) utilize the expression vector pcDNA3. A constitutively active MEK-1 plasmid was obtained from Upstate Biotechnology. HCC-1569 or MCF-7 cells were grown to 40 to 50% confluence and then transiently transfected with 0.5 to 10 μg of fusion plasmids, depending on the plate size and the amount of cells, with Lipofectamine reagent (GIBCO-BRL, Grand Island, N.Y.); cells were incubated with liposome-DNA complexes at 37°C for 5 h, followed by overnight recovery in 10% fetal bovine serum. Then, prior to experimental treatment, the cells were synchronized in serum-free Dulbecco's minimal essential-F-12 medium for 24 h and then treated with 17-β-E2 and/or related compounds. Cotransfections with a GFP expression vector indicated 50 to 63% efficiency of transfection. Additional plasmids included mouse ERα in pcDNA3, containing nucleotides 17 to 2001 of the steroid receptor, or the pcDNA3 backbone vector and were kindly provided by Ken Korach (5). ERK2(Y185F), a potent dominant negative construct for the mitogen-activated protein (MAP) kinase, was a kind gift from Melanie Cobb (34), and in additional studies, a small interfering RNA (siRNA) for BRCA1 was expressed. The DNA sequence against which double-stranded RNA for BRCA1 was created is 5′-TGCCAAAGUAGCTGATGTA-3′. Double-stranded RNA was transfected into MCF-7 cells, by using Oligofectamine, as 0.3 μg of siRNA/well of a six-well plate. In some studies, MCF-7 cells were transfected to express wt BRCA1, recovered, and then incubated with actinomycin D 6 h prior to the incubation with E2. ERK activity was then determined over time.
Proliferation studies.
HCC-1937 cells were transfected to express ERα with or without wt BRCA1 alone or with dominant negative ERK2(Y185F). The cells were recovered overnight in serum and synchronized without serum for 12 to 24 h. The cells were then incubated in 0.2% serum (to prevent apoptosis of control cells) with or without 10 nM E2 and other substances added daily in fresh medium for 72 h, trypsinized, and counted with a Coulter counter or hemocytometer. Viability was determined by trypan blue exclusion analysis, and the counts were adjusted. Some experiments used MCF-7 or ZR-75-1 cells transfected with mutant or wt BRCA1. In additional studies, the E domain of ERα was transiently expressed and targeted in HCC-1937 cells to either the nucleus (E-Nuc-ECFP) or the plasma membrane (E-Mem-ECFP) (13) as previously described (31, 32). Proliferation was also detected in MCF-7 cells by bromodeoxyuridine (BrdU) labeling. After 24 h of treatment with 10 nM E2, the cells were incubated for 1 h with BrdU (dilution, 1:100) according to the manufacturer's protocol (Zymed, South San Francisco, Calif.). The cells were then fixed with 70% ethanol, and the incorporated BrdU was detected by an indirect immunoperoxidase method (Amersham, Arlington Heights, Ill.). Briefly, the cultured cells were incubated for 1 h with biotin-linked, mouse anti-BrdU antibody. After being washed in 20 mM Tris-500 mM NaCl-0.05% Tween 20 solution (pH 7.5), the cells were further incubated with biotinylated goat anti-mouse immunoglobulin for 10 min. The cells were then washed and incubated with peroxidase conjugates for 10 min at room temperature, and immunoreactivity was revealed by the addition of chromogen as a substrate. The cells were counterstained with hematoxylin, and the BrdU-labeled cells were counted. The study was repeated twice.
Cell cycle and immunofluorescence studies.
The cell cycle distribution of the cells was determined after exposing MCF-7 cells to 10 nM E2 for 16 h (G1/S) and 36 h (G2/M). The cells were stained with propidium iodide, and the distribution was determined by fluorescence-activated cell sorting (FACS). For the localization of cyclin B1 during the G2/M transition, MCF-7 cells were fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. Indirect immunofluorescent confocal microscopy was carried out with a monoclonal antibody to cyclin B1 and a fluorescein isothiocyanate-conjugated second antibody.
Western blot analysis.
Immunoblot analyses of cell lysates were carried out for AKT, cyclins D1 and B1, or MKP-1 with monoclonal antibodies after the cells were exposed to E2 for 15 min (AKT), 16 h (cyclin D1), or 36 h (cyclin B1 and MKP-1) as described previously (31, 32). Proteins were detected with an ECL Western blot kit (Amersham).
MKP-1 studies.
HCC-1937 cells were transfected to express ERα plus pcDNA3 or wt BRCA1, recovered, and synchronized. The cells were then exposed (or not exposed) to 10 nM E2 for 9 min with or without 1 μM sodium vanadate (tyrosine phosphatase inhibitor) or 0.1 μM okadaic acid (serine/threonine phosphatase inhibitor). The phosphatase inhibitors were added 20 min prior to the addition of E2, and ERK activity was determined. In additional studies, double-stranded RNA for MKP-1 or GFP (control) was transfected into MCF-7 cells. Immunoblotting for MKP-1 was done daily with lysed cells over a 5-day period (temporal profile). Based upon the significant knockdown of MKP-1 at 72 h, we expressed in MCF-7 cells the siRNA for GFP (control) or MKP-1, recovered the cells over 24 h, and then expressed wt BRCA1. The cells were again recovered and synchronized over 48 h, and then ERK activity was determined in response to 10 nM E2. The DNA sequence against which double-stranded RNA for MKP-1 (QIAGEN) was created is 5′-GGACATGCTGGATGCCTTG-3′.
Phosphatase activity assay.
ERK-directed phosphatase activity was determined by modifying a phosphatase activity protocol from New England Biolabs. MCF-7 cells were grown to 80% confluence and then labeled with inorganic 32P (specific activity, 100 μCi/ml). The cells were lysed, and the lysate was subjected to immunoprecipitation with agarose bead-conjugated polyclonal antibody against ERK2 (Santa Cruz). This complex was extensively washed to remove unincorporated 32P. After protein determination, equal amounts of labeled ERK were used as a substrate for determining phosphatase activity under various treatment conditions. Two days earlier, a second set of MCF-7 cells had been transfected with pcDNA3 (control) or wt BRCA1 and then recovered and synchronized. The cells were then incubated with or without 10 nM E2 for 9 min. The cells were washed twice with Dulbecco's minimal essential medium, scraped, and then sonicated in phosphatase activity buffer (New England Biolabs). After centrifugation, the supernatants (100 μl) from the cells subjected to each treatment were added in separate tubes to equal aliquots of 32P-labeled ERK. The mixtures were then incubated with 100 μl of phosphatase activity buffer at 37°C for 30 min. After microcentrifugation, 50 μl of each supernatant was counted in a beta-counter for released 32P, reflecting the ERK-directed phosphatase activity.
Apoptosis.
The influence of wt BRCA expression in MCF-7 cells on cell death was determined as previously described (29). MCF-7 cells were grown on 18-mm-diameter coverslips in 12-well culture dishes in Dulbecco's minimal essential-F-12 medium without phenol red but with 0.2% charcoal-stripped serum added. The cells were transfected with pcDNA3 or wt BRCA1 expression plasmids, recovered, and then incubated in the presence or absence of 10 nM E2 for 72 h. At the end of the incubation, the cells were washed with phosphate-buffered saline and fixed with 1% freshly prepared paraformaldehyde in phosphate-buffered saline, pH 7.4, at 40°C overnight. Apoptosis was then determined by the terminal deoxynucleotidyltransferase-stimulated incorporation of nucleotides into the 3-OH end of damaged DNA in the cell, detected by fluorescent antibodies to the nucleotides (TUNEL) with a kit from Intergen, Purchase, N.Y. For each experimental condition, 400 cells were visually scored for apoptosis and viewed by fluorescence microscopy with standard fluorescein excitation and emission filters. The study was repeated. Apoptosis was also determined by FACS detection of Annexin V binding by use of a kit (Becton-Dickinson).
RESULTS
Estrogen activation of ERK is inhibited by BRCA1.
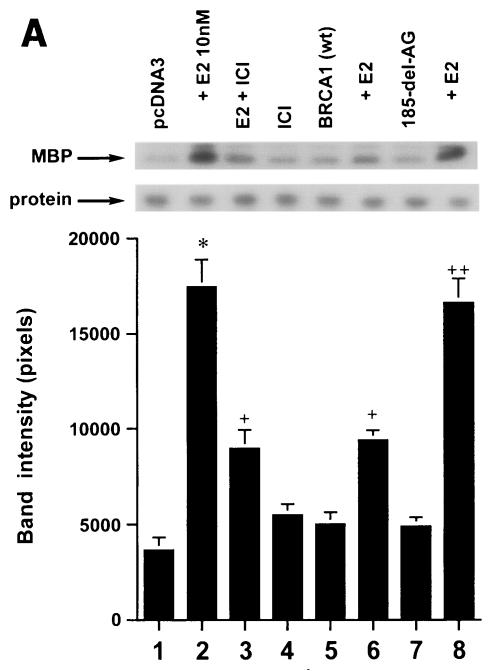
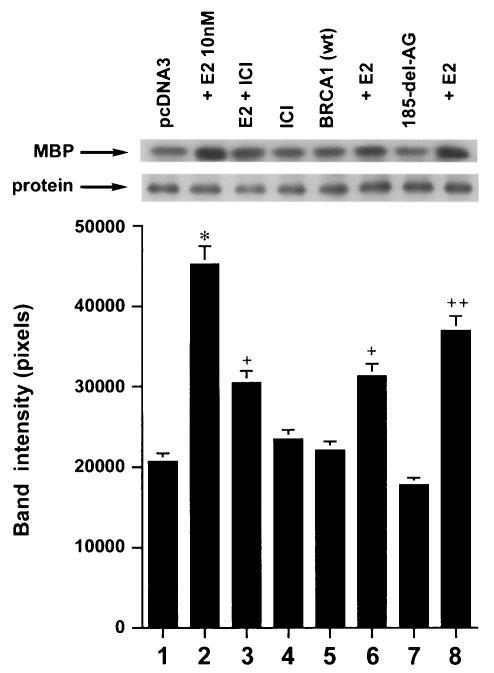
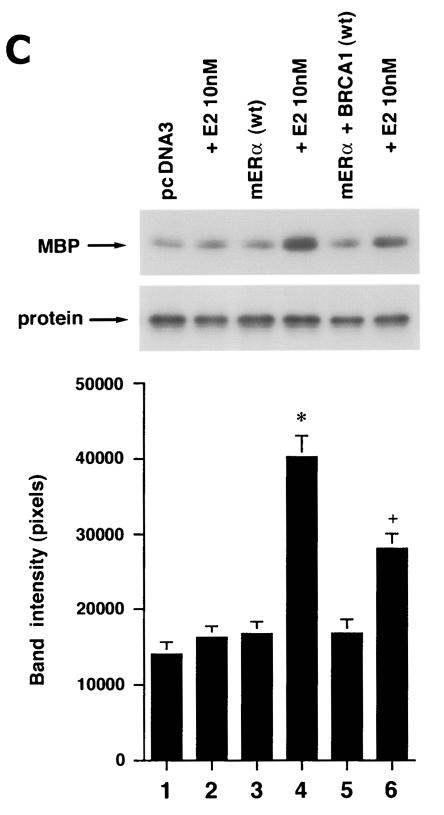
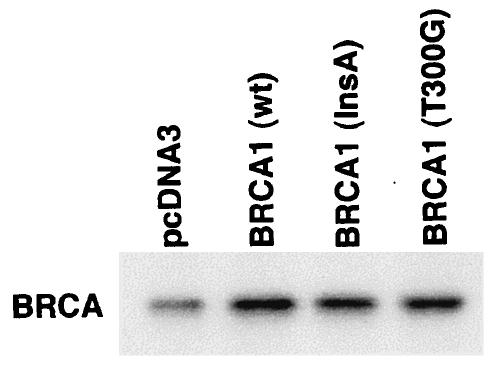
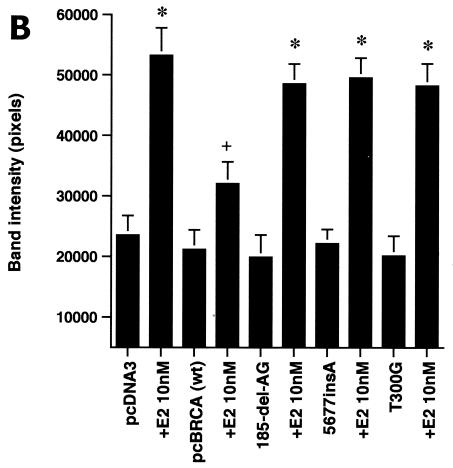
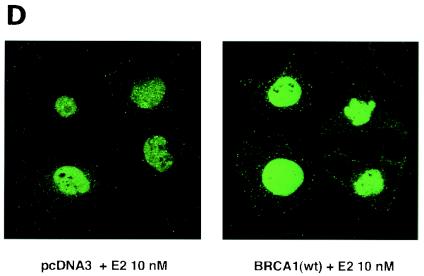
We first determined that in MCF-7 and ZR-75-1 cells E2 rapidly stimulates ERK activation (Fig. 1A), which is substantially prevented by the ER antagonist, ICI182780. It was previously shown that ERK activation results from membrane ER ligation by E2 (31). Expression of wt BRCA1 also inhibited ERK activation. In contrast, transfection of DNA for 185delAG BRCA1, a null mutant protein found in women with breast cancer, did not affect E2-induced ERK. Expression of two other mutant BRCA1 proteins from women with familial breast cancer, 5677insA BRCA1 and T300G BRCA1, also failed to significantly alter E2-induced ERK (Fig. 1B). This failure occurred despite the fact that wt and mutant BRCA1 proteins were comparably expressed after transfection in MCF-7 cells (Fig. 1C, right). We also examined HCC-1937 cells that have an endogenous 5382insC mutation in BRCA1 (45) and lack ER. We expressed ERα with and without wt BRCA1 in the cells to levels of protein(s) comparable to those of wt MCF-7 cells (data not shown). The ability of E2 to stimulate ERK in the ER-transfected cells was significantly inhibited when wt BRCA1 was coexpressed (Fig. 1C, left). Finally, we determined that endogenous BRCA1 produced in MCF-7 cells (Fig. 1D, left) localized to the nucleus, as did expressed wt BRCA1 (Fig. 1D, right).
FIG. 1.
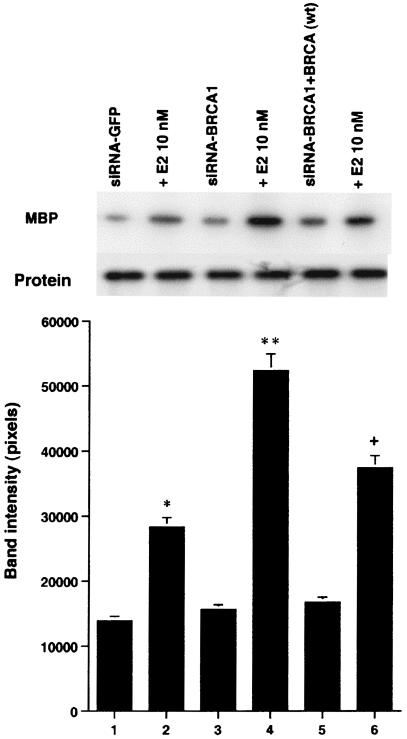
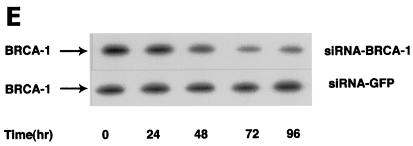
wt BRCA1 but not mutant BRCA1 inhibits E2-induced ERK activation. (A) MCF-7 (left) and ZR-75-1 (right) cells, both ER positive, were transfected to express wt BRCA1 or 185delAG mutant BRCA1 or were transfected with pcDNA3 (control), recovered in serum, synchronized overnight without serum, and then incubated with 10 nM E2 for 9 min. ERK activity against myelin basic protein was then determined. ERK immunoblots are shown below activity to normalize activity for total ERK protein. The bar graph shows the combined results of three experiments. Values are means ± standard errors of the means, determined by analysis of variance plus Scheffe's test (P values of <0.05 are considered significant). Significance of results: *, P of <0.05 for pcDNA3 versus same plus E2; +, P of <0.05 for pcDNA3 plus E2 versus BRCA1 (wt) plus E2 or versus E2 plus ICI182780; ++, P of <0.05 for the 185delAG BRCA1 mutant versus same plus E2. (B) MCF-7 cells were transfected to express pcDNA3 (control), wt BRCA1, or the 185delAG, 5677insA, or T300G BRCA1 mutant, and E2-induced ERK activity was determined. Significance of results: *, P of <0.05 for pcDNA3 or mutant alone versus same plus E2; +, P of <0.05 for pcDNA3 plus E2 versus BRCA1 (wt) plus E2. (C) (Left) HCC-1937 cells were transfected with pcDNA3 or ERα, with or without a wt BRCA1 expression plasmid, and E2-induced ERK activity was determined. Significance of results: *, P of <0.05 for mouse ERα (mERa)-expressing cells versus same incubated with E2; +, P of <0.05 for ER-expressing cells plus E2 versus ER plus wt BRCA1-expressing cells plus E2. The data are from three experiments. (Right) Expression levels of wt BRCA1 and mutant BRCA1 proteins in MCF-7 cells are comparable. MCF-7 cells were transfected with pcDNA3 (control) or expression plasmids for wt BRCA1 or two mutant BRCA1 proteins, 5677insA BRCA1 (InsA) and T300G BRCA1. The cells were recovered, and 24 h later, Western blotting of the cell lysate was carried out with an N-terminal-directed antibody to BRCA1. (D) Endogenous or expressed BRCA1 localizes to the nucleus of MCF-7 cells. MCF-7 cells on coverslips were transfected with pcDNA3 or wt BRCA1, incubated with 10 nM E2 for 36 h, and then fixed for 10 min, as described in Materials and Methods. BRCA1 expression was determined by immunofluorescent confocal microscopy using a first antibody directed against the C terminus and a second antibody conjugated to fluorescein isothiocyanate. The study was repeated twice. (E) Endogenous BRCA1 restrains E2/ER signaling to ERK. (Top) Time course of BRCA1 protein knockdown by siRNA. MCF-7 cells were transfected to express an interfering RNA for BRCA1 or GFP (control), and BRCA1 protein knockdown was determined by Western blotting. (Bottom) Expression of siRNA for BRCA1 augments E2-induced ERK. MCF-7 cells transfected to express siRNA for BRCA1 or GFP were incubated 72 h later with 5 nM E2, and ERK activity was determined. wt BRCA1 was also cotransfected under one condition. A bar graph reflecting the combined results of three experiments is shown. Significance of results: *, P of <0.05 for siRNA-GFP versus same plus E2; **, P of <0.05 for siRNABRCA1 versus same plus E2; +, P of <0.05 for siRNA-BRCA1 plus E2 versus same plus wt BRCA1.
We then asked whether endogenous wt BRCA1 restrains E2 signaling to ERK. First, MCF-7 cells were transfected with double-stranded RNA oligomers to BRCA1 or GFP (control), and BRCA1 protein knockdown was determined over 96 h. The siRNA for BRCA1 (and not the siRNA for GFP) produced significant inhibition of endogenous BRCA1 expression (Fig. 1E, top). We then examined the effects of this siRNA on E2-induced ERK. E2 (10 nM) stimulated ERK activity in MCF-7 cells transfected with siRNA to GFP (Fig. 1E, bottom). However, this effect was enhanced in cells transfected with the siRNA to BRCA1. When wt BRCA1 was transfected, it reversed the effects of the siRNA and restored E2-induced ERK. This suggests a novel role for endogenous, intact BRCA1, to restrain ERK activation by E2. Overall, wt but not mutant BRCA1 proteins inhibit this signal from the membrane ER.
Estrogen and BRCA1 modulate ERK-induced cell proliferation.
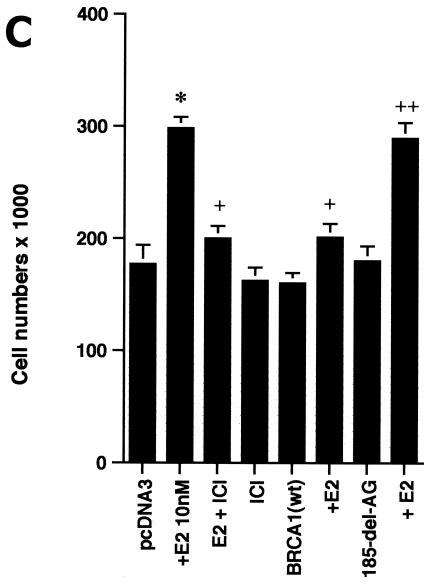
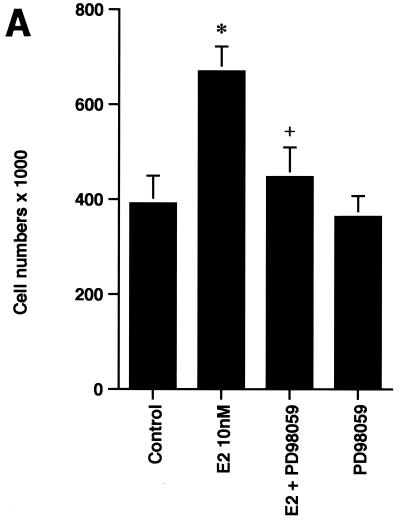
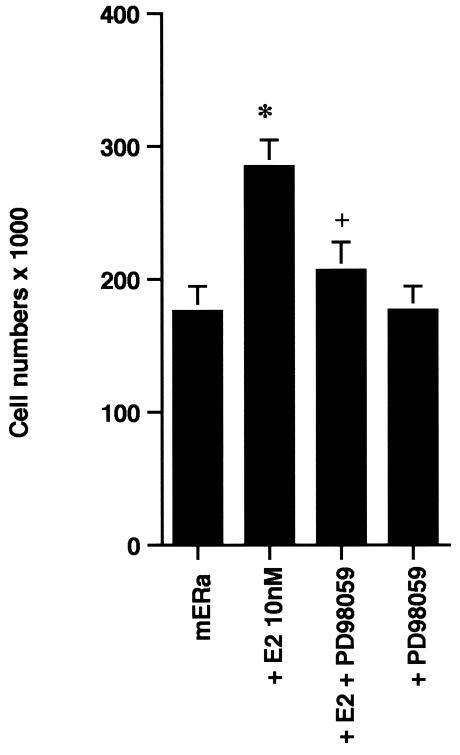
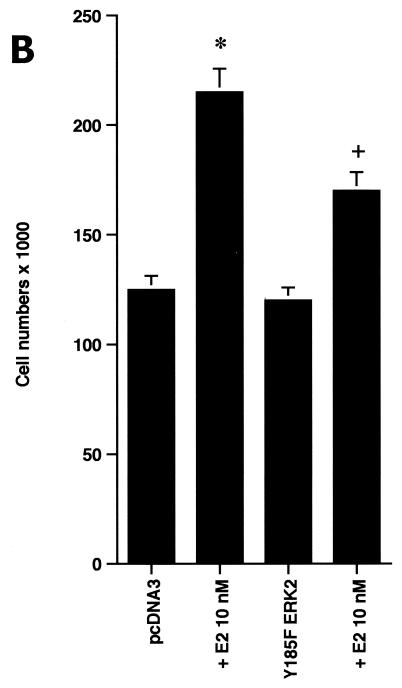
In order to understand the potential importance of the ability of wt BRCA1 to inhibit E2-induced signaling through ERK, we cultured MCF-7 cells with 10 nM E2 with and without PD98059, a specific ERK kinase (MEK) inhibitor (25). The cells were cultured for 3 days in the absence of other exogenous growth factors but in the presence of 0.2% serum to prevent apoptosis of the control cells. After 3 days, E2 caused a 70% increase in cell number, and this increase was 75% reversed by the MEK inhibitor (Fig. 2A, left). Similar effects occurred in ERα-expressing HCC-1937 cells (Fig. 2A, right). To corroborate the role of ERK, we expressed a dominant negative ERK2 construct (34) in the MCF-7 cells and compared steroid-induced proliferation in this setting to that in MCF-7 cells expressing the empty vector. We found that E2 was significantly less able to stimulate cell proliferation, despite a 53% efficiency of transfection of the mutant ERK2 (Fig. 2B). Thus, signaling from the membrane to ERK significantly contributes to E2-induced proliferation.
FIG. 2.
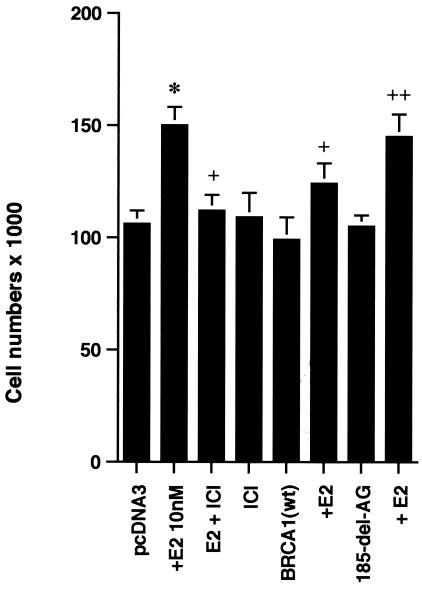
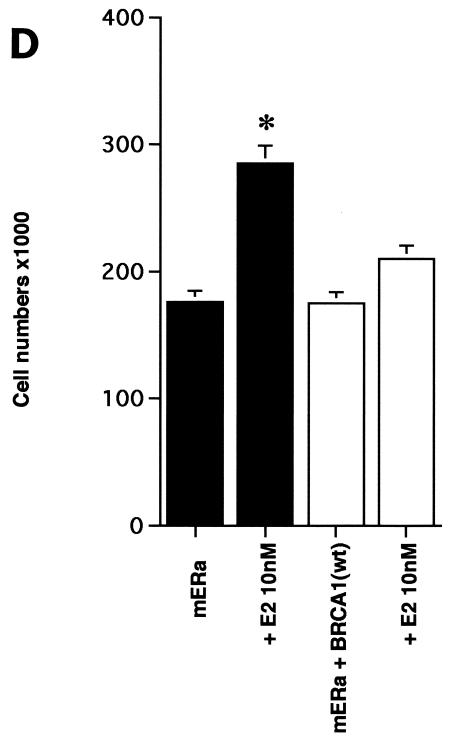
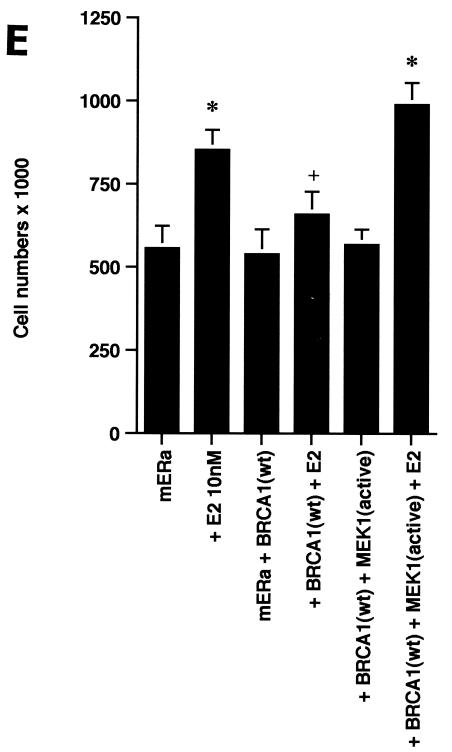
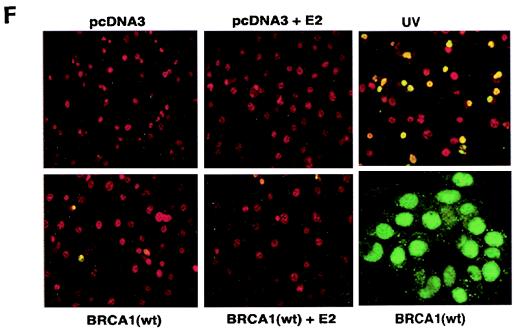
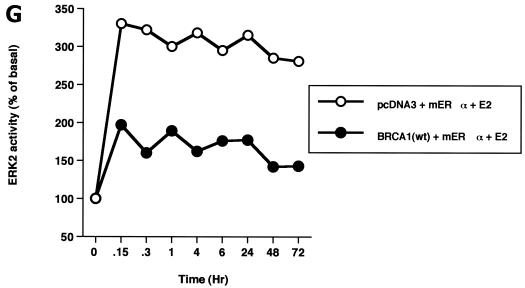
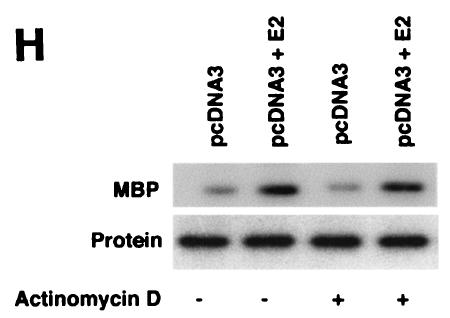
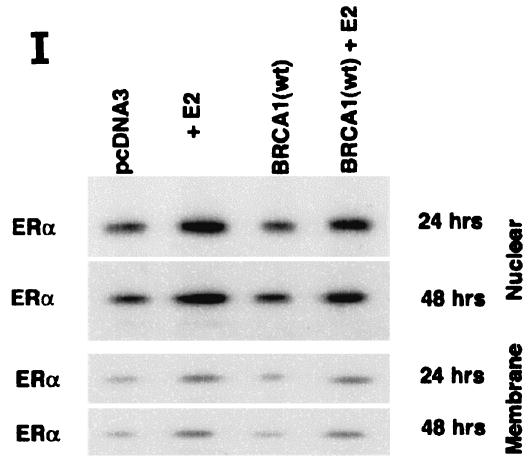
ERK activation by E2 is important for cell proliferation. (A) MCF-7 (left) or HCC-1937 (right) cells, the latter transfected to express ERα, were incubated with 0.2% serum (control) with or without 10 nM E2 and with or without the MEK-1 inhibitor PD98059. After 3 days, the cells were trypsinized and counted. The bar graph represents the combined results from triplicate wells for each of two experiments. Significance of results: *, P of <0.05 for control versus E2; +, P of <0.05 for E2 versus E2 plus PD98059. (B) Dominant negative ERK2(Y185F) prevents E2-induced cell proliferation. Cells were transfected with dominant negative ERK2(Y185F) or pcDNA3 (control), recovered, and then incubated with 10 nM E2 for 3 days. The results are from three experiments (n = 3). Significance of results: *, P of <0.05 for control versus E2, +, P of <0.05 for E2 versus ERK2(Y185F) plus E2. (C) Cell proliferation induced by E2 is prevented by wt BRCA1. MCF-7 (left) or ZR-75-1 (right) cells were transfected with pcDNA3, wt BRCA1, or 185delAG mutant BRCA1, recovered, and incubated with 10 nM E2 for 3 days. To some wells, 1 μM ICI182780 (ER antagonist) was added (n = 3). Significance of results: *, P of <0.05 for control versus E2; +, P of <0.05 for E2 versus same plus ICI182780 or E2 plus wt BRCA1. ++, P of <0.05 for BRCA1 (wt) plus E2 versus 185delAG mutant BRCA1 plus E2. (D) wt BRCA1 expression inhibits E2-induced proliferation in HCC-1937 cells. These cells were transfected with mERa plus pcDNA3 or wt BRCA1 and incubated with 10 nM E2 for 3 days. (E) Active MEK-1 reverses BRCA1 inhibition of E2-induced proliferation. HCC-1937 cells were transfected with mERa plus pcDNA3, or mERa plus wt BRCA1, with and without active MEK-1 (n = 3). Significance of results: *, P of <0.05 for control versus E2, or ERα plus BRCA1 (wt) plus MEK-1 versus same in the presence of E2 (last two bars); +, P of <0.05 for E2 versus same plus BRCA1 in the presence of ERα for both. (F) wt BRCA1 does not induce apoptosis of E2-treated MCF-7 cells. MCF-7 cells were transfected to express pcDNA3 or wt BRCA1 and then recovered and incubated with and without 10 nM E2 for 72 h. Other cells were exposed to UV irradiation (50 J over 2 min). BRCA1 immunostaining revealed a 60% transfection efficiency. Apoptosis was determined by TUNEL staining. (G) Temporal kinetics of E2-induced ERK activity. HCC-1937 cells were transfected with mERa without (open circles) or with (closed circles) wt BRCA1. E2 was added daily in fresh medium, and ERK activity, normalized for ERK protein, was determined over 72 h. (H) ERK activation by E2 is unaffected by transcription inhibition. MCF-7 cells were transfected with a wt BRCA1 expression plasmid, recovered, and then incubated with the transcription inhibitor actinomycin D, at 4 μM, for 6 h prior to the addition of 10 nM E2. The cells were subsequently processed for ERK activity at 9 min after E2 addition. Total ERK2 protein is shown in this representative study of two experiments. (I) ERα protein is not modulated by wt BRCA1 expression. MCF-7 cells were transfected to express pcDNA3 (control) or wt BRCA1, recovered, and then incubated with 10 nM E2 for 24 or 48 h. Membrane and nuclear fractions were isolated by sucrose gradient centrifugation, and Western blotting was carried out using the H-222 antibody (directed against the ligand binding domain).
The inhibition by wt (but not mutant) BRCA1 of membrane ER signaling to ERK possibly impairs cell proliferation. To test this possibility, we carried out additional proliferation assays. E2 incubation for 3 days induced a significant increase in numbers of MCF-7, ZR-75-1, and ERα-transfected HCC-1937 cells, blocked by the ER antagonist, ICI182780 (Fig. 2C). When wt BRCA1 was transfected, MCF-7 (Fig. 2C, left) and ZR-75-1 (Fig. 2C, right) cells underwent a significant reduction in E2-related proliferation. In contrast, transfection of the 185delAG BRCA1 mutant did not significantly alter E2-induced proliferation. Similarly, E2-treated HCC-1937 cells transfected with ERα alone were greater in number than cells expressing both ERα and wt BRCA1 (Fig. 2D). To support the idea that wt BRCA1 acts through the suppression of ERK, we expressed a constitutively active MEK-1 construct that had been validated previously (30). MEK-1 directly stimulates ERK activity. Active MEK-1 reversed the ability of BRCA1 to inhibit E2-induced cell proliferation in transfected HCC-1937 cells (Fig. 2E). At this level of expression, active MEK did not by itself induce cell proliferation; it did so only in the setting of E2.
It is conceivable that wt BRCA1 expression induced cell death in the setting of E2 exposure. To test this possibility, MCF-7 cells on coverslips were transfected to express pcDNA3 or wt BRCA1, recovered, and then incubated with 10 nM E2 for 72 h. As shown in Fig. 2F, wt BRCA1 expression alone caused less than 5% cell death compared to that caused by cells incubated with 0.2% serum (pcDNA3, control) or with E2. Most importantly, we could detect only an occasional cell undergoing cell death when wt BRCA1-expressing cells were incubated with E2. As a positive control, UV exposure induced significant cell death, shown by TUNEL staining. Similar results were determined by FACS analysis of Annexin V staining (data not shown). Thus, wt BRCA1 inhibits the growth but does not induce the death of E2-treated MCF-7 cells.
We then determined the kinetics of E2-induced ERK and its regulation by BRCA1 over 72 h. E2-induced ERK was rapidly upregulated by 9 min (first point assessed) and slightly declined over the next 50 min but remained significantly increased during the 3-day study. wt BRCA1 always suppressed E2-induced ERK activity by as much as 70% (Fig. 2G). These results are consistent with a role of ERK in the interactions of E2 and BRCA1, to modulate cell proliferation over the same time period.
We also determined whether the inhibition of transcription modulated E2-induced ERK (Fig. 2H). We found that signaling by the steroid at 9 min was unaffected by 6 h of pretreatment with 4 μM actinomycin D. These results are consistent with our previous reports: targeting the E domain or full-length ERα to the cell membranes of previously ER-negative breast cancer or CHO cells results in E2-induced ERK activity. In contrast, targeting of these constructs to the nucleus does not support this signaling (30, 32). Finally, we asked whether BRCA1 might downregulate ER expression, leading to the inhibition of E2 signaling. Endogenous ERα expression was determined by Western blot analysis of both nuclear and membrane compartments of MCF-7 cells after isolation of the fractions by sucrose gradient centrifugation (30, 31). E2 caused the moderate stimulation of ERα protein expression in both compartments, but this stimulation was unaffected by BRCA1 expression at both 24 and 48 h during E2 incubation (Fig. 2I).
E domain of membrane and nuclear ERα contributes to cell proliferation.
Targeting the ligand binding domain (E domain) of ERα to the plasma membrane (but not the nucleus) of ER-negative breast cancer cells allows E2-induced ERK activation (31, 32) and the rescue of osteoblasts and HeLa cells from apoptosis (13). Rapid signaling to ERK by E2 also results in neuronal cell survival (39). We speculate here that the E domain is sufficient for E2-induced signaling from the membrane to cell proliferation. However, this does not preclude a contribution to cell proliferation by a separate action of the nuclear ER. The effects of discrete pools of ER on the stimulation of breast cancer cell proliferation have not been previously compared, and the ability of BRCA1 to prevent the two pools of ER from inducing cell proliferation is unknown.
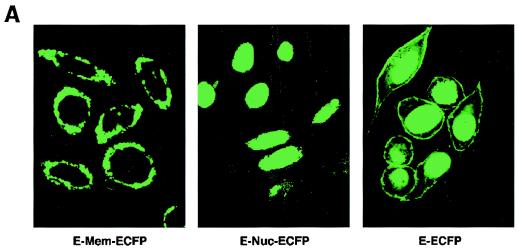
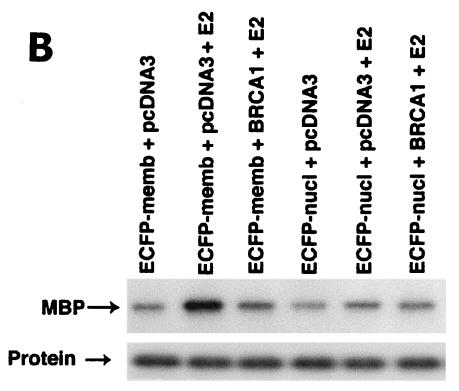
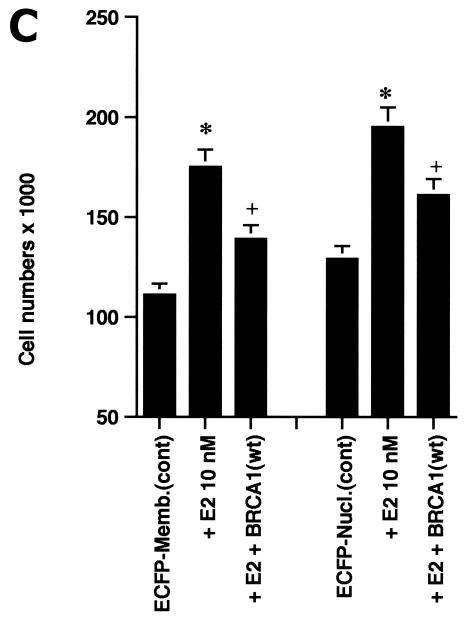
We therefore modeled this hypothesis by targeting the E domain of ERα to the plasma membrane or nucleus of HCC-1937 cells. The nonoverlapping cell localization of the two constructs is shown in Fig. 3A. The membrane-targeted E domain reflects this portion of ERα localized to the cytoplasmic face of the membrane, with a small amount of expression perhaps taking place in ribosomes. Ligand binding studies using sucrose gradient-isolated cell compartments confirm that there is a paucity of ER in the cytoplasmic fractions and none in the nucleus. In contrast, the nucleus-targeted E domain is found exclusively in the nucleus. In our first studies, we found that only the membrane-targeted E domain of ERα supported E2-induced ERK (Fig. 3B). This effect was substantially inhibited by coexpression of wt BRCA1. We then carried out proliferation studies. E2 induced a significant increase in the number of cells expressing either the membrane- or nucleus-targeted E domain (Fig. 3C). Significantly, wt BRCA1 expression prevented E2-induced proliferation of cells expressing either membrane- or nucleus-targeted E domain. Expression of BRCA1 in the absence of E2 had little effect on the number of cells (data not shown).
FIG. 3.
(A) Distribution in HCC-1937 cells of the transfected E domain of ERα, targeted to the membrane (E-Mem-ECFP) or nucleus (E-Nuc-ECFP), or nontargeted (E-ECFP), as revealed by immunofluorescent confocal microscopy. Note that the membrane-targeted E domain appears only at the cell surface, while the nucleus-targeted E domain is localized only to the nucleus. (B) The membrane-targeted but not the nucleus-targeted E domain of ERα supports E2-induced ERK. HCC-1937 cells were transfected with one of two targeted E-domain vectors, pcDNA3 or wt BRCA1, recovered overnight, and then treated with 10 nM E2 for 9 min. ERK activity was then determined. The results of a representative study, repeated twice, are shown. (C) Membrane- and nucleus-targeted E domain contributes to E2-induced cell proliferation. Transfected HCC-1937 cells were recovered and then synchronized in the absence of serum or E2 for 16 h. The cells were then exposed to 0.2% serum plus 10 nM E2 for 3 days. Some cells were transfected to also express wt BRCA1. The bar graph represents the combined results of three experiments. Significance of results: *, P of <0.05 for control versus E2; +, P of <0.005 for E2 versus same plus BRCA1.
BRCA1 selectively affects signaling to cell proliferation.
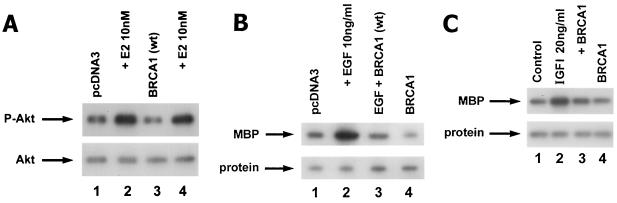
Several molecules, including the PI3K/AKT pathway (9), often mediate cell proliferation that results from signaling initiated at the membrane (44). As a function of PI3K, E2 stimulates the activating phosphorylation of AKT on threonine 473 (Fig. 4A), consistent with our previous findings (24). This pathway contributes to E2-induced cell cycle events (see below). However, BRCA1 expression does not affect PI3K/AKT activity, indicating a relatively specific function of BRCA1, to block ERK activity-related proliferation.
FIG. 4.
BRCA1 does not block E2-induced AKT activity. (A) MCF-7 cells were transfected to express pcDNA3 or wt BRCA1 and recovered, and E2-induced AKT activation and phosphorylation on serine 473 were determined by immunoblotting after 15 min of incubation. Protein loading of total AKT is shown below the activity immunoblot; the study was repeated twice. (B) EGF-induced ERK is inhibited by wt BRCA1 expression. MCF-7 cells were transfected to express pcDNA3 (control) or wt BRCA1 and then treated with 10 ng of EGF/ml for 9 min, and ERK activity was determined. (C) IGF-I-induced ERK in MCF-7 cells is inhibited by BRCA1. MCF-7 cells were transfected to express pcDNA3 or wt BRCA1. After recovery, the cells were incubated with IGF-I at 20 ng/ml for 9 min, and ERK was determined. The results from a representative study, repeated twice, are shown.
BRCA1 inhibits growth factor signaling to proliferation.
Signaling to ERK by other important breast cancer growth factors, such as EGF and IGF-I, rapidly occurs after these proteins bind specific tyrosine kinase growth factor receptors in the plasma membrane (52). Activation of ERK by these growth factor receptors is also important for cell proliferation (12, 44). We therefore asked whether BRCA1 could also inhibit this mechanism of EGF or IGF-I to stimulate breast cancer proliferation.
We first determined that BRCA1 prevents EGF-induced ERK activation (Fig. 4B). This action coincided with the ability of BRCA1 to suppress EGF-induced proliferation of HCC-1937 cells (Table 1). The native cells express EGFR but not ER and respond to EGF (but not E2) with significantly increased proliferation (Table 1). EGF action was substantially reversed by tyrphostin AG1478, a specific inhibitor of EGFR tyrosine kinase activity. In cells transfected to express ERα, E2 induced a strong proliferative response that was modestly enhanced by coincubation with EGF (Table 1). The lack of enhanced proliferation in response to the combination of E2 and EGF may reflect the fact that E2 strongly induces EGFR transactivation (8, 32) so that the addition of EGF is not significant. Notably, the proliferative responses here to only EGF or E2 were nearly identical. We also found that proliferation in response to E2 was inhibited by tyrphostin AG1478 (Table 1). In contrast, addition of tyrphostin to the platelet-derived growth factor receptor kinase activity had no influence on E2-induced proliferation (data not shown). Thus, membrane ER cross talk to the EGFR is essential for ERK activation (32) and breast cancer cell proliferation, as shown in the present study. We also found that wt BRCA1 expression inhibits E2-, EGF-, or E2-plus-EGF-induced proliferation, consistent with the ability of the tumor suppressor to downregulate ERK signaling. Finally, we investigated the interactions of BRCA1 and IGF-I. IGF-I stimulated ERK activity (Fig. 4C) and cell proliferation (data not shown), and these actions were prevented by wt BRCA1 expression. Thus, wt BRCA1 broadly suppresses signaling by established mitogens in breast cancer cells, a novel action.
TABLE 1.
Interactions of estradiol and EGF with BRCA1 in regulating HCC-1937 cell proliferationa
| Expression vector | Condition | No. of cells (103) |
|---|---|---|
| pcDNA3 | Control | 148 ± 15 |
| E2 (10 nM) | 158 ± 17 | |
| EGF (10 ng/ml) | 227 ± 20b | |
| EGF + tyrphostin AG1478 | 154 ± 17c | |
| EGF + wtBRCA1 | 171 ± 19c | |
| Tyrphostin AG1478 | 152 ± 17 | |
| ERα | Control | 134 ± 14 |
| E2 (10 nM) | 211 ± 21b | |
| E2 + EGF | 227 ± 18b | |
| E2 + tyrphostin AG1478 | 160 ± 16c | |
| EGF | 212 ± 19b | |
| EGF + tyrphostin AG1478 | 127 ± 15c | |
| E2 + EGF + wtBRCA1 | 174 ± 17c | |
| EGF + wtBRCA1 | 170 ± 15c | |
| wtBRCA1 | 124 ± 15 |
Cells were transfected to express pcDNA3 or ERα with and without wtBRCA1 and incubated with E2 or EGF and tyrphostin AG1478 (EGFR tyrosine kinase inhibitor) for 3 days. Data are means ± standard errors of the results of three experiments combined, with triplicate determinations for each condition in each experiment.
P of <0.05 for control versus treated cells as determined by analysis of variance plus Scheffe's test.
P of <0.05 for cells treated with E2 or EGF versus the same plus tyrphostin or BRCA1.
E2 induces G1/S and G2/M cell cycle progression that is prevented by BRCA1.
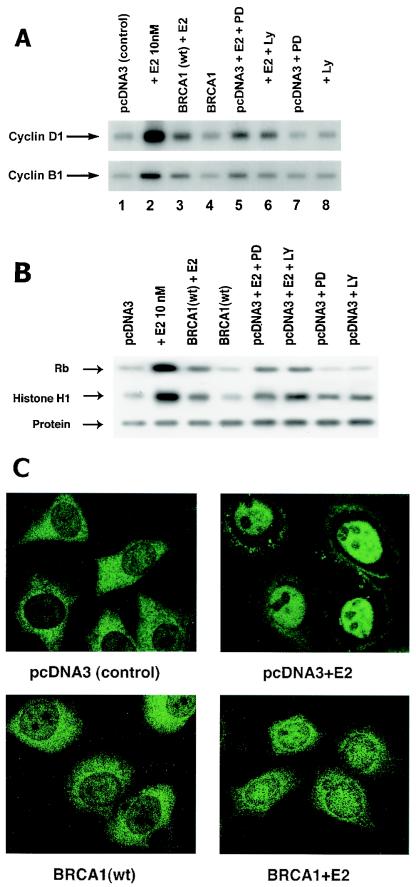
What proliferation-related actions of E2 are dependent on membrane ER signaling through ERK and are opposed by wt BRCA1? The ability of E2 to induce cyclin D1 production is significantly dependent upon ERK activation in breast cancer (30). Cyclin D1 upregulates Cdk4 activity that contributes to the inactivating phosphorylation of the retinoblastoma protein, leading to G1/S-phase cell cycle progression. This is considered to be important for the proliferation of breast cancer (51). Here, we found that E2 induces cyclin D1 protein in MCF-7 cells and that expression of wt BRCA1 significantly prevents this (Fig. 5A). E2 stimulates cyclin D1 through ERK and PI3K-related actions since the effects of E2 were partially reversed by PD98059 (MEK inhibitor) and LY294002 (PI3K inhibitor). Cdk4 activity was also induced by E2 via ERK and PI3K actions, and this was substantially reduced by BRCA1 expression (Fig. 5B). These results were corroborated by FACS analysis showing that the number of cells in S phase doubled after 16 h of E2 incubation. The passage of cells into S phase was reduced by 60% by BRCA1 transfection or 70% by incubation with PD98059.
FIG. 5.
BRCA1 inhibits key cell cycle actions induced by E2. (A) MCF-7 cells were transfected with wt BRCA1 or pcDNA3 (control) and then incubated with or without 10 nM E2 for 16 h (cyclin D1) or 36 h (cyclin B1). For some cells, 1 μM PD98059, a MEK inhibitor, or 10 μM LY294002, a PI3K inhibitor, was added. Cyclins were detected by Western blotting. (B) MCF-7 cells were incubated with E2 after recovery from transfection. Cdk4 activity was determined after 16 h of incubation by immunoprecipitating the kinase from cell lysates and adding a portion to an in vitro activity assay utilizing retinoblastoma protein as the substrate. CDK1 activity was determined after 36 h of incubation by an assay with histone H1 as the substrate. CDK1 total protein blots are shown below each sample. The results of three experiments are represented. (C) Transfected MCF-7 cells were incubated with or without E2 for 36 h. Cyclin B1 cell distribution was determined by immunofluorescent microscopy during G2/M.
Regarding the G2/M checkpoint, the cyclin B1 protein controls the activity of CDK1 kinase, which is necessary for passage into M phase (27). We found that E2 induces increased cyclin B1 protein levels via both ERK and PI3K and that wt BRCA1 expression partially prevents this (Fig. 5A). E2 also induced CDK1 activity (Fig. 5B), which is significantly prevented by wt BRCA1 expression. BRCA1 has previously been reported to downregulate cyclin B1 production through unknown mechanisms that impact gene regulation. To further understand the interactions between E2/ER and BRCA1 during the critical G2/M transition, we determined the subcellular localization of cyclin B1. Cyclin B1 is diffusely distributed throughout the cytoplasm in pcDNA3-transfected MCF-7 cells (Fig. 5C). Expression of wt BRCA1 in the absence of E2 similarly results in typical cytoplasmic localization of cyclin B1. Following 36 h of incubation with E2, cyclin B1 translocates substantially to the nucleus (Fig. 5C, upper right). However, when wt BRCA1 is expressed and these cells are incubated with E2, cyclin B1 remains predominantly cytoplasmic. Thus, wt BRCA1 inhibits E2-induced cell cycle events that are essential to G1/S and G2/M progression.
BRCA1 induces a specific ERK phosphatase.
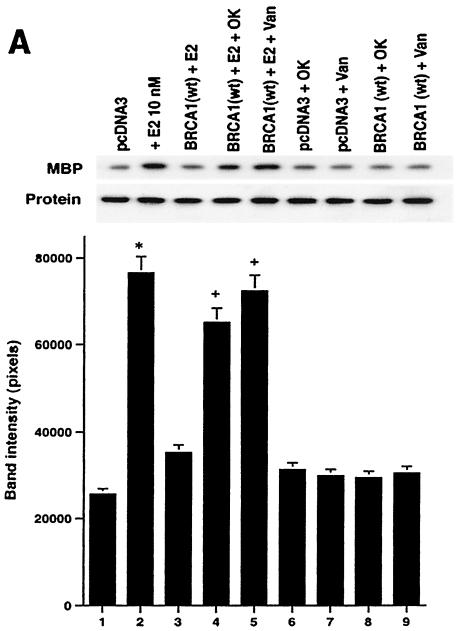
We have established that BRCA1 inhibits E2 signaling through ERK and that this is important for the growth regulatory actions of the tumor suppressor. wt BRCA1 is predominantly a nuclear protein and sometimes transactivates genes or stabilizes proteins that contribute to its role as a tumor suppressor (37). Therefore, we postulated that BRCA1 induces a phosphatase with strong activity against nuclear ERK. To test this idea, we determined whether the ability of expressed wt BRCA1 to inhibit ERK in ER-transfected HCC-1937 cells was dependent on phosphatase activity. BRCA1 inhibited E2-induced ERK, but this inhibition was partially reversed by either tyrosine or threonine/serine phosphatase inhibitors (Fig. 6A). Phosphatase inhibitors or BRCA1 alone had little effect when ERK activity was normalized for protein. We also found similar results for the reversal of BRCA1 inhibition of EGF- or IGF-I-induced ERK activity (data not shown).
FIG. 6.
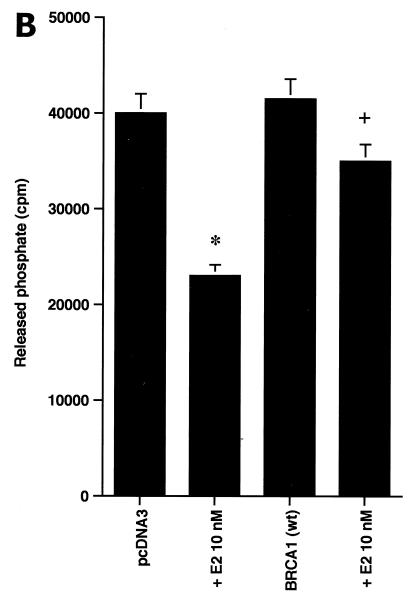
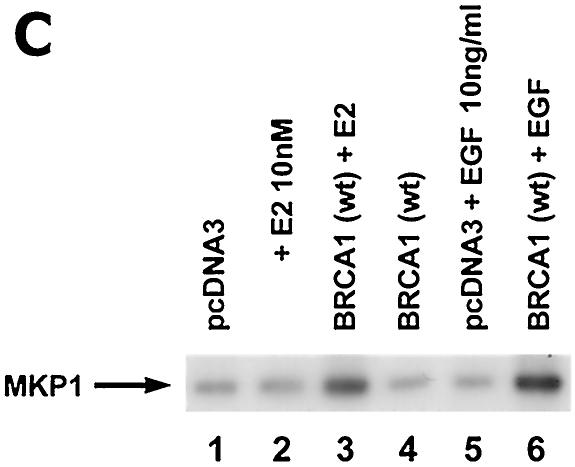
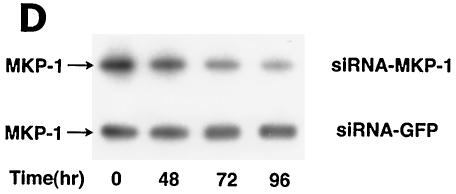
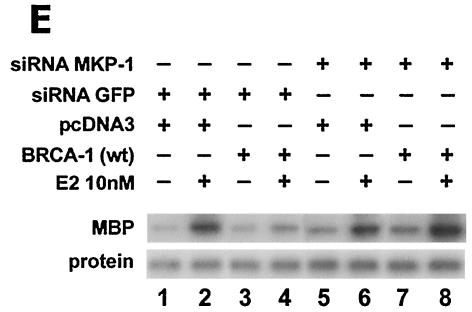
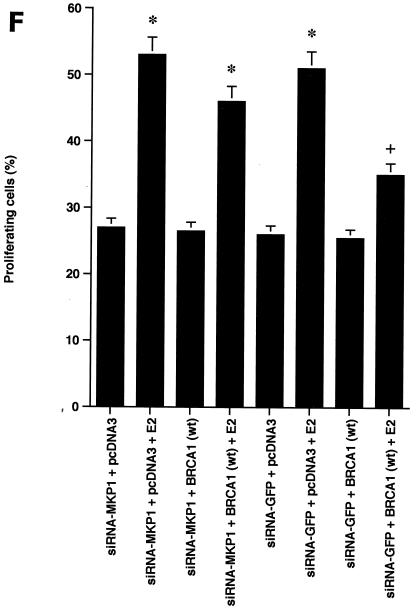
BRCA1 activates phosphatase activity to downregulate ERK activity. (A) Phosphatase inhibitors reverse BRCA1 inhibition of E2-stimulated ERK. HCC-1937 cells were transfected to express ER plus pcDNA3 or wt BRCA1, recovered and synchronized for 48 h, and then exposed (or not exposed [pcDNA3, lane 1]) to 10 nM E2 for 9 min. Under some conditions, the cells were also incubated with 1 μM of sodium vanadate (Van) or 0.1 μM okadaic acid (OK) 20 min prior to E2 addition. Phosphatase inhibitors or BRCA1 alone had little effect when ERK activity was normalized for protein. The bar graph reflects the combined results of three experiments. Significance of results: *, P of <0.05 for pcDNA3 versus same plus E2; +, P of <0.05 for BRCA1 (wt) plus E2 versus same plus okadaic acid or same plus Van. (B) BRCA1 prevents E2-induced downregulation of ERK phosphatase activity. 32P-labeled ERK2 was prepared as described in Materials and Methods and used as a substrate for an in vitro phosphatase assay. MCF-7 cells transfected with pcDNA3 (control) or wt BRCA1 were incubated with or without 10 nM E2 for 9 min, and the cells were then lysed. Equal protein aliquots of cell lysate were then added to 32P-labeled ERK2 protein aliquots, and phosphatase activity was determined. The data are from triplicate determinations for each condition and are representative of two separate studies. (C) BRCA1 upregulates MKP-1 protein. MCF-7 cells were transfected with pcDNA3 (control) or wt BRCA1, recovered, synchronized in the absence of steroid or serum, and then incubated in medium with or without 10 nM E2 for 24 h. Western blot analyses from cell lysates were carried out for immunoprecipitated MKP-1. The representative study was repeated twice. (D) siRNA for MKP-1 downregulates MKP-1 protein. MCF-7 cells were transfected with annealed, double-stranded RNA for MKP-1 or GFP by using Oligofectamine, as described in Materials and Methods. Western blot analyses for MKP-1 were accomplished in cells lysed at 48, 72, and 96 h posttransfection. (E) siRNA for MKP-1 reverses BRCA1 inhibition of E2-induced ERK. MCF-7 cells were sequentially transfected with 0.3 μg of double-stranded RNA oligomers (to GFP or MKP-1) on day 1, recovered, and then transfected 24 h later with pcDNA3 or wt BRCA1. After recovery and synchronization over 48 h in 0.2% serum, E2 was added for 9 min. ERK activity was determined 72 h after siRNA transfection. The experiment was repeated twice. (F) siRNA for MKP-1 prevents wt BRCA1 inhibition of E2-induced proliferation. MCF-7 cells were transfected with pcDNA3 or wt BRCA1, recovered, and then transfected with siRNA for GFP or MKP-1. The cells were recovered and then incubated with 10 nM E2 for 23 h, followed by BrdU pulsing for 1 h. The cells were fixed, and BrdU labeling was detected as described in Materials and Methods. The data are the means ± standard errors of the means for results with 1,000 cells counted per condition in each of three separate experiments and then combined.
We hypothesized that BRCA1 might induce a dual-specificity phosphatase with activity against ERK, such as MKP-1 (4). We speculated that at 9 min, BRCA1 upregulates ERK-directed phosphatase activity and modulates the longer-term inhibition of kinase through the induction of MKP-1 protein. We first determined phosphatase activity directed against 32P-labeled ERK. E2 (10 nM) alone significantly reduced ERK-directed phosphatase activity in the MCF-7 cell lysate after 9 min of incubation, probably contributing to the activation of this kinase by the steroid (Fig. 6B). In contrast, BRCA1 substantially reversed this action of E2, correlating with the acute inhibition of E2-induced ERK.
To assess the longer-term effect of BRCA1, MCF-7 cells transfected to express BRCA1 or pcDNA3 (control) were incubated or not incubated with E2 for 24 h. MKP-1 protein levels were then determined. BRCA1 significantly induced MKP-1 protein only in the setting of cotreatment with E2 (Fig. 6C). Thus, it is in the state of ERK activation (stimulated by E2) that BRCA1 upregulates MKP-1 expression. This could reflect a transcriptional upregulation of MKP-1 in the setting of both E2 and BRCA1. Comparable to the interaction with E2, BRCA1 strongly induced MKP-1 when the cells were exposed to EGF.
To specifically implicate MKP-1, we utilized an siRNA approach. We first transfected MCF-7 cells with double-stranded RNA for GFP (control) or MKP-1 to determine protein knockdown over 5 days. At 72 h, specifically the siRNA for MKP-1 lowered the expression of this protein (Fig. 6D). We then carried out ERK activation studies. Expression of the siRNA for GFP, sequentially followed by transfection of pcDNA3 (control), did not affect the strong activation of ERK by E2 (Fig. 6E). When wt BRCA1 was expressed with the siRNA for GFP, the activation of ERK by E2 was barely evident. Upon expression of the siRNA for MKP-1, followed by transfection of pcDNA3, E2 strongly activated ERK. In contrast, when the siRNA for MKP-1 and the plasmid containing wt BRCA1 were sequentially expressed, E2 activation of ERK was no longer inhibited.
We then determined whether MKP-1 knockdown also affected cell proliferation. BrdU incorporation into the MCF-7 cells was stimulated by E2 and inhibited by wt BRCA1 expression. However, in the presence of the siRNA for MKP-1 (but not the siRNA for GFP), BRCA1 inhibition of proliferation was substantially prevented (Fig. 6F). Therefore, the ability of BRCA1 to upregulate ERK phosphatase activity via the MKP-1 protein is functionally important in blocking E2-induced ERK and cell proliferation.
DISCUSSION
It is estimated that up to 80% of women expressing mutant BRCA1 ultimately develop breast cancer (21), but it is unknown why such mutations only predispose to cancer of hormonally responsive tissues. This predisposition perhaps results from a tumor-promoting interaction of mutant BRCA1 with estrogen. Here, we report the novel finding that intact BRCA1 inhibits E2 signaling to ERK and breast cancer cell proliferation. In contrast, three mutations of BRCA1 that are commonly found in women with breast cancer fail to suppress E2 signaling to proliferation. Both effects of intact BRCA1 result substantially from the ability of this tumor suppressor to induce MAP kinase phosphatase production and activity. As an additional antiproliferative mechanism, the ability of BRCA1 to block nuclear ER-induced transcription (6, 7) might also contribute. We definitively implicated MKP-1 by use of an interfering RNA approach. This finding is consistent with BRCA1 being localized mostly to the nucleus (3), where it inhibits the final step (effector) in membrane ER signaling to ERK activation and cell cycle progression. Furthermore, BRCA1-induced MKP-1 protein can accomplish the longer-term inhibition of E2-induced ERK, demonstrated in the present study over 3 days. This is relevant to breast cancer since it is the sustained ERK signaling induced by growth factors in this disease that stabilizes oncogenic proteins, such as c-fos (23).
The rapid activation of ERK stems from steroid ligation of the plasma membrane pool of ER. Membrane targeting of either (i) ERα that are deficient in the nuclear localization sequence (53) or (ii) the E domain of ERα in ER null cells (31) is sufficient for the rapid activation of this kinase by E2. In contrast, targeting of only the E domain (31, 32) or full-length receptor to the nucleus (unpublished observations) does not result in E2-induced ERK. We report here that the activation of ERK by E2 is not prevented by actinomycin D. This result suggests that an important effect of the steroid is to modulate acutely the activity of the kinase in part through inhibition of phosphatase activity as shown in the present study. It is also possible that E2 prolongs the survival of ERK protein, contributing to the long-term signaling by the sex steroid. We demonstrate the importance of E2 signaling to ERK activation in that E2-induced breast cancer cell proliferation is substantially prevented by (i) a soluble inhibitor of ERK kinase or (ii) expression of a dominant negative ERK2 protein. E2 signaling through ERK to cyclin D1 production and Cdk4 activity underlies the passage of breast cancer cells through G1 to S phase of the cell cycle (30; present study). We also report the novel finding that E2-induced signaling through ERK (and PI3K) leads to cyclin B1 and CDK1 activity upregulation and passage into M phase. These cell cycle effects of E2 are prevented by BRCA1 and affirm in this setting the ability of this tumor suppressor to induce G1/S and G2/M checkpoint activities (40, 49). Supporting a causal relationship between BRCA1 and ERK, constitutively active MEK protein reverses BRCA1 inhibition of E2-induced proliferation.
Breast cancer cells such as MCF-7 cells typically express both membrane and nuclear ER. In vivo, the administration of antibodies to ERα blocks the growth of human breast cancer xenografts in nude mice, presumably through the prevention of membrane ER signaling to ERK and PI3K previously demonstrated in vitro (20). In addition, ERK and PI3K signaling stimulates gene transcription that is relevant to breast cancer (18, 47). The traditional function of the nuclear ER of transactivating relevant genes in this disease is important. However, in breast cancer, an overexpressed and truncated MTA1 protein sequesters ER away from the nucleus and strongly reduces E2-activated transcription yet promotes increased ERK signaling and aggressive tumor behavior (14). These findings suggest the additional importance of nontranscriptional actions of ERK to the promotion of malignancy, and it was previously reported that E2 signaling from the membrane increases cell survival through posttranslational effects (29).
We show here the first comparison of the contributions of distinct ER pools to breast cancer proliferation. Upon targeting the E domain of ERα to the plasma membranes of HCC-1937 cells, E2 significantly stimulates cell division. This occurs despite the fact that the nuclear ER is absent. Thus, some events critical for E2-induced breast cancer cell proliferation may require only the membrane E-domain function. It was recently shown that targeting the E domain to the cell membrane of ER-negative breast cancer cells caused a series of G-protein-coupled signaling events that led to the transactivation of the EGF receptor and subsequent activation of ERK (32). Targeting the E domain to the nucleus did not support this signaling. However, targeting the E domain to the nucleus, as reported here, results in E2-stimulated growth of the tumor cells. This result supports the idea that both ER populations contribute to breast cancer proliferation and impact common key targets, such as cyclin D1 production. It is well recognized that membrane growth factor signaling can augment nuclear ER function (reviewed in reference 15), which occurs through several mechanisms, including the activating phosphorylation of nuclear ER and the recruitment of coactivator proteins. Since membrane ER transactivates EGFR and ErbB2 (8, 32, 41), we propose an integrated model wherein membrane ER signaling though the tyrosine kinase growth factor receptors augments nuclear ER function in MCF-7 cells, thereby promoting proliferation. This proliferation occurs in addition to the direct effects of kinase activation that modify the functions of existing proteins and transactivate genes (24). As determined here, BRCA1 inhibits proliferation arising from either E-domain model.
An important finding is that intact BRCA1 also prevents EGF and IGF-I signaling through ERK to cell proliferation. EGF and IGF-1 are strongly implicated in the biology of human breast cancer, where they signal through this member of the MAP kinase family to cell growth (27, 52). EGF serves as a ligand for important heterodimers of the EGFR family, including the EGFR/ErbB2 heterodimer that is implicated in breast cancer pathogenesis or aggressiveness. It was recently demonstrated that E2 activates ERK in breast cancer via transactivation of the EGF receptor (8, 32). We find here that a specific EGFR tyrosine kinase inhibitor strongly prevents E2-induced cell proliferation. Thus, the ability of BRCA1 to inhibit both membrane ER and EGFR-induced ERK activation and subsequent proliferation is consistent with a functional role of this cross talk between steroid and growth factor receptors (28). There is also abundant evidence that ER/IGF-I receptor cross talk participates in the biology of this malignancy (reviewed in reference 15). Thus, in ER-positive cells, the ability of intact BRCA1 to oppose individual and collective signaling from the membrane is likely to be important as a tumor suppressor mechanism.
Yan et al. recently reported that BRCA1 overexpression in MCF-7 cells results in c-Jun N-terminal kinase and ERK activation (50). The latter signaling contributed to cell survival of BRCA1, only in MCF-7 cells. However, those investigators did not determine the interactions between E2 and BRCA1 nor the effects of mutant BRCA1. Different subsets of MCF-7 cells have been identified, and a minority actually respond to estrogen with cell death. We find that BRCA1 expression stimulates c-Jun N-terminal kinase and significantly induces apoptosis in MCF-7 cells only during an additional stress (UV radiation or paclitaxel); this stimulation is inhibited by E2 (unpublished observations). Here we show that BRCA1 does not stimulate apoptosis, particularly when E2 is present. BRCA1 inhibits ERK as induced by E2 and growth factors for as long as 72 h. This finding is consistent with a tumor-suppressive action of BRCA1. We further report that BRCA1 inhibits E2-induced ERK in three different breast cancer cell lines, including HCC-1937 cells transfected to express intact BRCA1, which represents a nonoverexpression model for BRCA1 action.
Estrogen use is a moderate risk factor for the development of breast cancer in women (35). This is attributed to the ability of the sex steroid to promote cell proliferation and survival and underlies the rationale for using tamoxifen, a drug that prevents the in vivo development or recurrence of ER-positive breast cancer (43). BRCA1 may serve as an endogenous restraint on both steroid and growth factor signaling to proliferation in women, the majority of whom have intact BRCA1. We propose that the loss of signaling restraint due to BRCA1 mutation might be a determining stimulus that promotes the development of breast cancer. This possibility could be relevant to both ER-positive and ER-negative tumor development, as the latter is dependent upon growth factor receptor signaling.
Acknowledgments
This work was supported by grants from the Research Service of the Department of Veterans Affairs, Avon Products Breast Cancer Research Foundation, Department of Defense Breast Cancer Research Program (grant no. BC990915), and the NIH (HL-59890) (to E.R.L.), and by NCI grants CA82599 and CA800000, a Department of Defense Breast Cancer Research Program grant (BC999254), and Susan G. Komen Breast Cancer Foundation grant 99-003255S (to E.M.R.).
REFERENCES
- 1.Baer, R., and T. Ludwig. 2002. The BRCA1/BARD1 heterodimer, a tumor suppressor complex with ubiquitin E3 ligase activity. Curr. Opin. Genet. Dev. 12:86-91. [DOI] [PubMed] [Google Scholar]
- 2.Casey, G. 1995. The BRCA1 and BRCA2 breast cancer genes. Curr. Opin. Oncol. 9:88-93. [DOI] [PubMed] [Google Scholar]
- 3.Chen, Y., A. A. Farmer, C.-F. Chen, D. C. Jones, P.-L. Chen, and W.-H. Lee. 1996. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer Res. 56:3168-3172. [PubMed] [Google Scholar]
- 4.Chu, Y., P. A. Solski, R. Khosravi-Far, C. J. Der, and K. Kelly. 1996. The mitogen-activated protein kinase phosphatases PAC1, MKP-1, and MKP-2 have unique substrate specificities and reduced activity in vivo toward the ERK2 sevenmaker mutation. J. Biol. Chem. 271:6497-6501. [DOI] [PubMed] [Google Scholar]
- 5.Couse, J. F., S. W. Curtis, T. F. Washburn, J. Lindzey, T. S. Golding, D. B. Lubahn, O. Smithies, and K. S. Korach. 1995. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol. Endocrinol. 9:1441-1454. [DOI] [PubMed] [Google Scholar]
- 6.Fan, S., J.-A. Wang, R. Yuan, Y. Ma, Q. Meng, M. R. Erdos, R. G. Pestell, F. Yuan, K. J. Auborn, I. D. Goldberg, and E. M. Rosen. 1999. BRCA1 inhibition of estrogen receptor signaling in transfected cells. Science 284:1354-1356. [DOI] [PubMed] [Google Scholar]
- 7.Fan, S., Y. X. Ma, C. Wang, R. Q. Yuan, Q. Meng, J. A. Wang, M. Erdos, I. D. Goldberg, P. Webb, P. J. Kushner, R. G. Pestell, and E. M. Rosen. 2001. Role of direct interaction in BRCA1 inhibition of estrogen receptor activity. Oncogene 20:77-87. [DOI] [PubMed] [Google Scholar]
- 8.Filardo, E. J., J. A. Quinn, K. I. Bland, and A. R. Frackelton. 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, gpr30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 14:1649-1660. [DOI] [PubMed] [Google Scholar]
- 9.Fruman, D. A., R. E. Meyers, and L. C. Cantley. 1998. Phosphoinositide kinases. Annu. Rev. Biochem. 67:481-507. [DOI] [PubMed] [Google Scholar]
- 10.Gowen, L. C., A. V. Avrutskaya, A. M. Latour, B. H. Koller, and S. A. Leadon. 1998. BRCA1 required for transcription-coupled repair of oxidative DNA damage. Science 281:1009-1012. [DOI] [PubMed] [Google Scholar]
- 11.Harkin, D. P., J. M. Bean, D. Miklos, Y.-H. Song, V. B. Truong, C. Englert, F. C. Christians, L. W. Ellisen, S. Maheswaran, J. D. Oliner, and D. A. Haber. 1999. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell 97:575-586. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann, K., and G. Thiel. 2002. Epidermal growth factor and thrombin induced proliferation of immortalized human keratinocytes is coupled to the synthesis of Egr-1, a zinc finger transcriptional regulator. J. Cell. Biochem. 85:381-391. [DOI] [PubMed] [Google Scholar]
- 13.Kousteni, S., T. Bellido, L. I. Plotkin, C. A. O'Brien, D. L. Bodenner, L. Han, K. Han, G. B. DiGregorio, J. A. Katzenellenbogen, B. S. Katzenellenbogen, P. K. Roberson, R. S. Weinstein, R. L. Jilka, and S. C. Manolagas. 2001. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719-730. [PubMed] [Google Scholar]
- 14.Kumar, R., E.-A. Wang, A. Mazumdar, A. H. Talukder, M. Mandal, Z. Yang, R. Bagheri-Yarmand, A. Sahin, G. Hortobagyi, L. Adam, C. J. Barnes, and R. K. Vadlamudi. 2002. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature 418:654-657. [DOI] [PubMed] [Google Scholar]
- 15.Levin, E. R. 2003. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol. 17:309-317. [DOI] [PubMed] [Google Scholar]
- 16.Loman, N., O. Johannsson, P. O. Bendahl, A. Borg, M. Ferno, and H. Olsson. 1998. Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer 83:310-319. [PubMed] [Google Scholar]
- 17.MacLachlan, T. K., R. Takimoto, and W. S. El-Deiry. 2002. BRCA1 directs a selective p53-dependent transcriptional response towards growth arrest and DNA repair targets. Mol. Cell. Biol. 22:4280-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marino, M., F. Acconcia, F. Bresciani, A. Weisz, and A. Trentalance. 2002. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol. Biol. Cell. 13:3720-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marks, J. R., G. Huper, J. P. Vaughn, P. L. Davis, J. Norris, D. P. McDonnell, R. W. Wiseman, P. A. Futreal, and J. D. Iglehart. 1997. BRCA1 expression is not directly responsive to estrogen. Oncogene 14:115-121. [DOI] [PubMed] [Google Scholar]
- 20.Marquez, D. C., and R. J. Pietras. 2001. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene 20:5420-5430. [DOI] [PubMed] [Google Scholar]
- 21.Miki, Y., J. Swensen, D. Shattuck-Eidens, P. A. Futreal, K. Harshman, S. Tavtigian, Q. Liu, C. Cochran, L. M. Bennett, W. Ding, et al. 1994. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science 266:66-72. [DOI] [PubMed] [Google Scholar]
- 22.Moynahan, M. E., J. W. Chiu, B. H. Koller, and M. Jasin. 1999. Brca1 controls homology-directed DNA repair. Mol. Cell 4:511-518. [DOI] [PubMed] [Google Scholar]
- 23.Murphy, L. O., S. Smith, R. H. Chen, D. C. Fingar, and J. Blenis. 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell. Biol. 4:556-564. [DOI] [PubMed] [Google Scholar]
- 24.Pedram, A., M. Razandi, M. Aitkenhead, C. C. W. Hughes, and E. R. Levin. 2002. Integration of the non-genomic and genomic actions of estrogen: membrane initiated signaling by steroid (MISS) to transcription and cell biology. J. Biol. Chem. 277:50768-50775. [DOI] [PubMed] [Google Scholar]
- 25.Pedram, A., M. Razandi, and E. R. Levin. 2002. Deciphering VEGF signaling to vascular permeability: inhibition by atrial natriuretic peptide. J. Biol. Chem. 277:44385-44398. [DOI] [PubMed] [Google Scholar]
- 26.Pedram, A., M. Razandi, R.-M. Hu, and E. R. Levin. 1998. Astrocyte progression through G1-S phase of the cell cycle depends upon multiple protein interactions. J. Biol. Chem. 273:13966-13972. [DOI] [PubMed] [Google Scholar]
- 27.Pines, J., and T. Hunter. 1992. Cyclins A and B1 in the human cell cycle. Ciba Found. Symp. 170:187-196. [PubMed] [Google Scholar]
- 28.Prenzel, N., E. Zwick, M. Leserer, and A. Ullrich. 2000. Tyrosine kinase signalling in breast cancer. Epidermal growth factor receptor: convergence point for signal integration and diversification. Breast Cancer Res. 2:184-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razandi, M., A. Pedram, and E. R. Levin. 2000. Estrogen signals to preservation of endothelial cell form and function. J. Biol. Chem. 275:38540-38546. [DOI] [PubMed] [Google Scholar]
- 30.Razandi, M., G. Alton, A. Pedram, S. Ghonshani, P. Webb, and E. R. Levin. 2003. Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol. Cell. Biol. 23:1633-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Razandi, M., P. Oh, A. Pedram, J. Schnitzer, and E. R. Levin. 2002. Estrogen receptors associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol. Endocrinol. 16:100-115. [DOI] [PubMed] [Google Scholar]
- 32.Razandi, M., A. Pedram, S. Parks, and E. R. Levin. 2003. Proximal events in ER signaling from the plasma membrane. J. Biol. Chem. 278:2701-2712. [DOI] [PubMed] [Google Scholar]
- 33.Rebbeck, T. R., A. M. Levin, A. Eisen, C. Snyder, P. Watson, L. Cannon-Albright, C. Isaacs, O. Olopade, J. E. Garber, A. K. Godwin, M. B. Dal, S. A. Narod, S. L. Neuhausen, H. T. Lynch, and B. L. Weber. 1999. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J. Natl. Cancer Inst. 91:1475-1479. [DOI] [PubMed] [Google Scholar]
- 34.Robbins, D. J., E. Zhen, H. Owaki, C. A. Vanderbilt, D. Ebert, T. D. Geppert, and M. H. Cobb. 1993. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J. Biol. Chem. 268:5097-5106. [PubMed] [Google Scholar]
- 35.Santen, R. J., and G. R. Petroni. 1999. Relative versus attributable risk of breast cancer from estrogen replacement. J. Clin. Endocrinol. Metabol. 84:1875-1881. [DOI] [PubMed] [Google Scholar]
- 36.Santen, R. J., R. X. Song, R. McPherson, R. Kumar, L. Adam, M. H. Jeng, and W. Yue. 2002. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 80:239-256. [DOI] [PubMed] [Google Scholar]
- 37.Scully, R., and D. M. Livingston. 2000. In search of the tumour-suppressor functions of BRCA1 and BRCA2. Nature 408:429-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao, N., Y. L. Chai, E. Shyam, P. Reddy, and V. N. Rao. 1996. Induction of apoptosis by the tumor suppressor protein BRCA1. Oncogene 13:1-7. [PubMed] [Google Scholar]
- 39.Singer, C. A., X. A. Figueroa-Masot, R. H. Batchelor, and D. M. Dorsa. 1999. The mitogen activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. J. Neurosci. 19:2455-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somasundaram, K., H. Zhang, Y.-X. Zeng, Y. Houvras, Y. Peng, H. Zhang, G. S. Wu, J. D. Licht, B. L. Weber, and W. S. El-Deiry. 1997. Arrest of the cell cycle by the tumor-suppressor BRCA1 requires the CDK-inhibitor p21waf1/CiP1. Nature 280:187-189. [DOI] [PubMed] [Google Scholar]
- 41.Stoica, G. E., T. F. Franke, A. Wellstein, F. Czubayko, H. J. List, R. Reiter, E. Morgan, M. B. Martin, and A. Stoica. 2003. Estradiol rapidly activates Akt via the ErbB2 signaling pathway. Mol. Endocrinol. 17:818-830. [DOI] [PubMed] [Google Scholar]
- 42.Stratton, M. R., D. Ford, S. Neuhasen, S. Seal, R. Wooster, L. S. Friedman, M. C. King, V. Egilsson, P. Devilee, R. McManus, et al. 1994. Familial male breast cancer is not linked to the BRCA1 locus on chromosome 17q. Nat. Genet. 7:103-107. [DOI] [PubMed] [Google Scholar]
- 43.Stuart, N. S., J. Warwick, G. R. Blackledge, D. Spooner, C. Keen, A. R. Taylor, C. Tyrell, D. J. Webster, and H. Earl. 1996. A randomized phase III cross-over study of tamoxifen versus megestrol acetate in advanced and recurrent breast cancer. Eur. J. Cancer 32:1888-1892. [DOI] [PubMed] [Google Scholar]
- 44.Svegliati-Baroni, G., F. Ridolfi, A. Di Sario, A. Casini, L. Marucci, G. Gaggiotti, P. Orlandoni, G. Macarri, L. Perego, A. Benedetti, and F. Folli. 1999. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology 29:1743-1751. [DOI] [PubMed] [Google Scholar]
- 45.Tomlinson, G. E., T. T.-L. Chen, V. A. Stastny, A. K. Kirmani, M. A. Spillman, V. Tonk, J. L. Blum, N. R. Schneider, I. I. Wistuba, J. W. Shay, J. D. Minna, and A. F. Gazdar. 1998. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 58:3237-3242. [PubMed] [Google Scholar]
- 46.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 47.Watters, J. J., T. Y. Chun, Y. N. Kim, P. J. Bertics, and J. Gorski. 2000. Estrogen modulation of prolactin gene expression requires an intact mitogen-activated protein kinase signal transduction pathway in cultured rat pituitary cells. Mol. Endocrinol. 14:1872-1881. [DOI] [PubMed] [Google Scholar]
- 48.White, R., and M. G. Parker. 1998. Molecular mechanisms of steroid hormone action. Endocr.-Rel. Cancer 5:1-14. [Google Scholar]
- 49.Xu, X., Z. Weaver, S. P. Linke, C. Li, J. Gotay, X.-W. Wang, C. C. Harris, T. Ried, and C.-X. Deng. 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3:389-395. [DOI] [PubMed] [Google Scholar]
- 50.Yan, Y., J. P. Haas, M. Kim, M. K. Sgagias, and K. H. Cowan. 2002. BRCA1-induced apoptosis involves inactivation of ERK1/2 activities. J. Biol. Chem. 277:33422-33430. [DOI] [PubMed] [Google Scholar]
- 51.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411:1017-1021. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, X., and D. Yee. 2000. Tyrosine kinase signaling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res. 2:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Z., B. Maier, R. J. Santen, and R. X. Song. 2002. Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation. Biochem. Biophys. Res. Commun. 294:926-933. [DOI] [PubMed] [Google Scholar]
- 54.Zheng, L., L. A. Annab, C. A. Afshari, W.-H. Lee, and T. G. Boyer. 2001. BRCA1 mediates ligand-independent transcriptional repression of the estrogen receptor. Proc. Natl. Acad. Sci. USA 98:9587-9592. [DOI] [PMC free article] [PubMed] [Google Scholar]