Abstract
Background
Studies of cardiac disease among adult survivors of childhood cancer have generally relied upon self-reported or registry-based data.
Objective
Systematically assess cardiac outcomes among childhood cancer survivors
Design
Cross-sectional
Setting
St. Jude Children's Research Hospital
Patients
1,853 adult survivors of childhood cancer, ≥18 years old, and ≥10 years from treatment with cardiotoxic therapy for childhood cancer.
Measurements
History/physical examination, fasting metabolic and lipid panels, echocardiogram, electrocardiogram (ECG), 6-minute walk test (6MWT) all collected at baseline evaluation.
Results
Half (52.3%) of the survivors were male, median age 8.0 years (range: 0-24) at cancer diagnosis, 31.0 years (18-60) at evaluation. Cardiomyopathy was present in 7.4% (newly identified at the time of evaluation in 4.7%), coronary artery disease (CAD) in 3.8% (newly identified in 2.2%), valvular regurgitation/stenosis in 28.0% (newly identified in 24.8%), and conduction/rhythm abnormalities in 4.6% (newly identified in 1.4%). Nearly all (99.7%) were asymptomatic. The prevalences of cardiac conditions increased with age at evaluation, ranging from 3-24% among those 30-39 years to 10-37% among those ≥40 years. On multivariable analysis, anthracycline exposure ≥250 mg/m2 increased the odds of cardiomyopathy (odds ratio [OR] 2.7, 95% CI 1.1-6.9) compared to anthracycline unexposed survivors. Radiation to the heart increased the odds of cardiomyopathy (OR 1.9 95% CI 1.1-3.7) compared to radiation unexposed survivors. Radiation >1500 cGy with any anthracycline exposure conferred the greatest odds for valve findings.
Limitations
61% participation rate of survivors exposed to cardiotoxic therapies, which were limited to anthracyclines and cardiac-directed radiation. A comparison group and longitudinal assessments are not available.
Conclusions
Cardiovascular screening identified considerable subclinical disease among adult survivors of childhood cancer.
Funding
Cancer Center Support Grant (CA21765), U01 CA195547 1, American Lebanese Syrian Associated Charities
Introduction
Improvements in cancer therapies have led to an increasing number of survivors living many years following successful treatment. With overall 5-year survival rates in pediatric oncology exceeding 80%(1), the number of adult survivors of childhood or adolescent cancer, currently estimated to be 388,500 in the United States, is projected to surpass 500,000 by 2020.(2, 3) This success is tempered by recognition of adverse late effects of cancer therapy and elevated mortality years following treatment. An 8-fold increased risk of death has been reported among 5-year childhood cancer survivors compared to the age- and sex- matched general population.(4) Historically, the leading cause of death has been cancer recurrence. However, death from late-effects has become the leading cause of mortality 30 years from diagnosis, frequently attributed to premature cardiovascular disease.(5)
Given the rarity of childhood cancer and the challenges of following patients across the life spectrum, most studies have relied upon self-reported outcomes(6), registry(7) or death certificate data(8) to estimate the prevalence and incidence of adverse outcomes. Few have directly assessed survivors or performed detailed clinical evaluations.(9, 10) The aim of this study was to report clinically-evaluated cardiac outcomes among adults previously exposed to cardiotoxic therapies for the treatment of childhood cancer.
Methods
Participants
Cases for this analysis were participants who completed a baseline evaluation in the St. Jude Lifetime Cohort (SJLIFE), an ongoing study designed to facilitate longitudinal evaluation of health outcomes among adults previously treated for a pediatric malignancy. The study design and details have been previously published.(11) To be enrolled in the cohort participants must have been diagnosed and treated for a childhood cancer at St. Jude Children's Research Hospital, currently be ≥18 years old, and have survived ≥10 years from diagnosis. This cross-sectional analysis includes data collected during the baseline evaluation. Participants (recruited from 44 states and 28 countries) must have been treated with cardiotoxic therapy (anthracycline chemotherapy and/or cardiac-directed radiation therapy) and completed the initial on-campus SJLIFE comprehensive risk-based health evaluation as of April 30, 2013.
Survivors completed a detailed health questionnaire and underwent medical evaluation according to the Children's Oncology Group's Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers.(12) Assessments included: a history and physical examination, fasting laboratory battery (blood counts, metabolic panel, insulin level, glycosylated hemoglobin, and lipid panel), echocardiogram, and electrocardiogram (ECG). Two-dimensional (2D) Doppler ultrasound echocardiography was performed using a VIVID-7 machine (GE Medical Systems) (n=1834) or an iE33 (Phillips Healthcare) (n=19) using Harmonic Imaging per American Society of Echocardiography guidelines with 3D imaging for left ventricular volumes.(13) All images were centrally reviewed by the echocardiography laboratory in the Department of Cardiovascular Medicine at the Cleveland Clinic. A total of 1,586 (86%) participants had an evaluable echocardiogram sufficient for adequate determination of ejection fraction and 1,742 (94%) sufficient for evaluation of cardiac valves. Twelve-lead ECG (GE MAC 1200 machine) was obtained and centrally reviewed and coded per the Minnesota classification system by the Epidemiological Cardiology Research Center at Wake Forest School of Medicine, Winston-Salem, NC (1,798 participants completed an ECG).
Medical record abstraction was performed to capture cumulative anthracycline exposure in milligrams per body surface area in meters squared (mg/m2), mean cardiac radiation dose, and medical events during and after therapy. Anthracycline doses were converted to doxorubicin isotoxic equivalents by summing doxorubicin, daunorubicin (x0.83), epirubicin (x0.67), idarubicin (x5), and mitoxantrone (x4) doses.(14) Mean radiation dose to the heart in centigray (cGy) was estimated using previously established methods by the radiation physicists at MD Anderson Cancer Center, Houston, TX.(15) Radiation treatment details included energy source, tumor dose, and field locations. Scatter dose to the heart was estimated for each case, regardless of radiation site and target volume. The SJLIFE protocol and study documents were approved by the Institutional Review Board, and survivors provided informed consent prior to participation.
Outcomes
Outcomes analyzed included cardiomyopathy, coronary artery disease (CAD), valve function, and conduction/rhythm defects. Medical records were obtained to validate established cardiac outcomes reported prior to the SJLIFE assessment. Cardiomyopathy was defined as a systolic ejection fraction <50% on echocardiogram. Coronary artery disease was defined as a history of myocardial infarction, evidence of wall motion defect on echocardiogram, and/or ischemia on ECG. Valves were assessed for function (regurgitation or stenosis) and structure (thickening and/or calcification) identified by echocardiogram. Only functional valve findings (graded as mild, moderate, or severe) were used in the analysis.(16, 17) Conduction/rhythm disorders included major conduction abnormalities or arrhythmias (Appendix Table 1) as assessed by the Minnesota ECG Code.(18)
Appendix Table 1. Conduction/rhythm disorders per the Minnesota ECG Code*.
|
Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. Littleton, MA: John Wright-PSG; 1982. ECG = electrocardiogram, AV = atrio-ventricular, QT = Q wave T wave
Covariates
Physical activity was assessed by asking participants if they participated in “usual weekly vigorous activities for at least 10 minutes at a time such as: running, aerobics, wheelchair basketball, heavy yard work, or anything else that caused large increases in breathing or heart rate; or moderate activities for at least 10 minutes such as: brisk walking, bicycling, gardening, manual operation of a wheelchair, or anything else that caused small increases in breathing or heart rate” (http://www.cdc.gov/nchs/data/nhanes/nhanes_09_10/paq_f.pdf). The frequency of exercise sessions per week was multiplied by the duration of each session and weighted by the standardized classification of the energy expenditure in metabolic equivalents expressed as metabolic equivalent minutes/week (inactive ≤450). Physical fitness was assessed by the 6-minute walk test (6MWT)(19) and considered abnormal if the participant walked <490 meters.(20) Risky drinking was defined as alcohol consumption of ≥5 drinks on one occasion or ≥15 drinks per week for men, and ≥4 drinks on one occasion or ≥8 drinks per week for women (http://www.cdc.gov/alcohol/faqs.htm#heavyDrinking). Survivors who reported smoking within the last month were classified as current smokers, and those who smoked at least 100 cigarettes in their lifetime, but not within the last month, were considered past smokers. Dyslipidemia was defined as low-density lipoprotein cholesterol ≥160 mg/dl, high-density lipoprotein cholesterol <40 mg/dl (men) or <50 mg/dl (women), triglycerides >150 mg/dl, or on treatment for a lipid abnormality. Diabetes was defined as a fasting blood glucose ≥126 mg/dl, glycosylated hemoglobin ≥6.5%, or on an oral hypoglycemic agent or insulin for diabetes. Duplicate blood pressure readings were obtained in a seated position with both feet on the floor following a 5 minute rest with the lowest used for analysis. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or being on an anti-hypertensive agent.
Statistical analysis
Descriptive statistics were calculated for demographic, treatment, and cardiovascular risk factors (i.e. body mass index (BMI) at SJLIFE assessment, smoking, drinking, physical activity, hypertension, diabetes, and dyslipidemia). Chi-square tests were used to compare demographic and treatment characteristics between study participants and non-participants. Frequencies of cardiac conditions reported before or diagnosed during the on-campus evaluations were ascertained and prevalence by age at detection reported. Multivariable logistic models were used to evaluate associations between potential risk factors and cardiac conditions detected during the on-campus evaluations, excluding participants with cardiac outcomes detected prior to SJLIFE participation. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). Covariates were selected due to their known and previously reported associations with cardiac outcomes in the general population and among adult survivors of childhood cancer. Proportions of participants with poor physical fitness (6MWT <490 meters) were calculated and associations with cardiac conditions evaluated by multivariable logistic regression with age, sex, and height as covariates. To account for any missing cardiac outcomes, radiation dose (N=26), and 30 missing 6MWT (not due to a musculoskeletal cause), we used multiple imputation (SAS proc MI and proc MIANALYZE). Markov Chain Monte Carlo (MCMC) methods were used for partial imputation of non-monotone missing records.(21) Monotone missing data were imputed using logistic regression. Pooled estimates of 20 multiply imputed datasets of the multivariable analyses for each outcome are reported as OR with 95% CI. SAS version 9.3 (Cary, NC) was used for all analyses. All statistical inference was based on two-sided tests.
Role of the Funding Source
The funding sources had no role in the design, conduct, or analysis of the study or the decision to submit the manuscript for publication.
Results
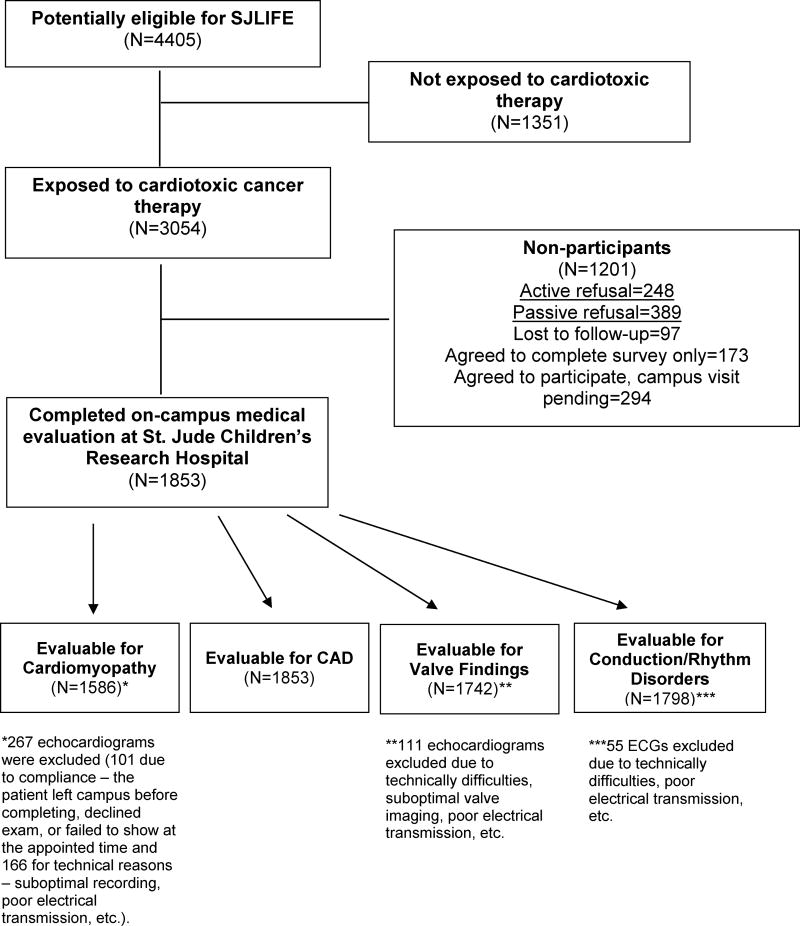
Among 3,054 participants exposed to cardiotoxic therapies, 1,853 (61%) returned to St. Jude Children's Research Hospital for a risk-based medical evaluation. Reasons for non-participation are shown in Appendix Figure 1. Compared to non-participants, participating survivors were more likely to be female, white, and have a history of leukemia. Additionally, participants were on average 8 months older and received on average less (9 mg/m2) total anthracycline exposure (Table 1). Just over half (52.3%) of participants were male with a median age at diagnosis of 8.0 years (range: 0-24) and 31.0 years (18-60) at time of study. Median time from diagnosis was 22.6 years (10-48); nearly two-thirds were more than 20 years from exposure. The majority of survivors had been treated for leukemia or lymphoma (67.2%). Other diagnostic categories included: sarcoma (14%), Wilms tumor (7.2%), neuroblastoma (4.5%), central nervous system (CNS) tumors (4.3%), and a variety of other tumors (2.8%).
Appendix Figure 1. Flow Diagram.
Table 1. Demographic, Diagnostic, Treatment, and Lifestyle Characteristics of Study Participants and Non-participants.
| Participant Survivors | Non-participants | ||||
|---|---|---|---|---|---|
| N=1853 | (%) | N=1201 | (%) | p-value | |
| Sex | |||||
| Female | 884 | (47.7) | 464 | (38.6) | <0.001 |
| Male | 969 | (52.3) | 737 | (61.4) | |
| Race | |||||
| White | 1589 | (85.8) | 977 | (81.3) | 0.005 |
| Black | 232 | (12.5) | 200 | (16.7) | |
| Other | 32 | (1.7) | 24 | (2.0) | |
| Diagnosis Group | |||||
| Leukemia | 763 | (41.2) | 473 | (39.4) | 0.012 |
| Hodgkin Lymphoma | 313 | (16.9) | 181 | (15.1) | |
| Non-Hodgkin Lymphoma | 169 | (9.1) | 154 | (12.8) | |
| Sarcomas | 260 | (14.0) | 148 | (12.3) | |
| Wilms Tumor | 133 | (7.2) | 94 | (7.8) | |
| Neuroblastoma | 84 | (4.5) | 57 | (4.8) | |
| Central Nervous System (CNS) | 79 | (4.3) | 52 | (4.3) | |
| Germ Cell Tumors | 11 | (0.6) | 11 | (0.9) | |
| Liver Malignancies | 7 | (0.4) | 3 | (0.3) | |
| Retinoblastoma | 6 | (0.3) | 13 | (1.1) | |
| Carcinoma | 19 | (1.0) | 9 | (0.8) | |
| Other | 9 | (0.5) | 6 | (0.5) | |
| Age at Diagnosis (years) | |||||
| 0 – 4 | 626 | (33.8) | 405 | (33.7) | 0.81 |
| 5 – 9 | 429 | (23.2) | 288 | (24.0) | |
| 10 – 14 | 461 | (24.9) | 282 | (23.5) | |
| ≥ 15 | 337 | (18.2) | 226 | (18.8) | |
| Age at SJLIFE Evaluation (years) | |||||
| 18 - 29 | 791 | (42.7) | 404 | (33.6) | 0.002 |
| 30 – 39 | 701 | (37.8) | 477 | (39.7) | |
| ≥ 40 | 361 | (19.5) | 320 | (26.6) | |
| Time Since Diagnosis (years) | |||||
| 10 – 20 | 671 | (36.2) | 350 | (29.1) | 0.118 |
| 20 – 30 | 753 | (40.6) | 495 | (41.2) | |
| > 30 | 429 | (23.2) | 356 | (29.6) | |
| Cardiac Radiation (cGy)* | |||||
| None | 1050 | (56.7) | 747 | (62.2) | 0.088 |
| ≤ 1500 | 366 | (19.8) | 217 | (18.1) | |
| > 1500 | 411 | (22.2) | 237 | (19.7) | |
| Unknown | 26 | (1.4) | 0 | (0.0) | |
| Anthracyclines (mg/m2) | |||||
| None | 332 | (17.9) | 203 | (16.9) | <0.001 |
| < 100 | 488 | (26.3) | 287 | (23.9) | |
| 100 – 249 | 647 | (34.9) | 513 | (42.7) | |
| ≥ 250 | 386 | (20.8) | 198 | (16.5) | |
| All variables below obtained at the time of the SJLIFE assessment | |||||
| BMI (kg/m2) | |||||
| Normal/underweight (< 25) | 717 | (38.7) | |||
| Overweight (25 – 29) | 525 | (28.3) | |||
| Obese (≥ 30) | 611 | (33.0) | |||
| Smoker | |||||
| Past | 217 | (11.7) | |||
| Current | 439 | (23.7) | |||
| Never | 1197 | (64.6) | |||
| Physical Activity | |||||
| Active (>450 metabolic equivalent minutes/week) | 934 | (50.4) | |||
| Inactive (≤450 metabolic equivalent minutes/week) | 919 | (49.6) | |||
| Risky drinking | |||||
| No | 1132 | (61.1) | |||
| Yes | 721 | (38.9) | |||
| Hypertension | |||||
| No | 1421 | (76.7) | |||
| Yes | 432 | (23.3) | |||
| Diabetes | |||||
| No | 1727 | (93.2) | |||
| Yes | 126 | (6.8) | |||
| Dyslipidemia | |||||
| No | 706 | (38.1) | |||
| Yes | 1147 | (61.9) | |||
| Six Minute Walk Test | |||||
| Normal (≥490 meters) | 1387 | (74.9) | |||
| Impaired (<490 meters) | 427 | (23.0) | |||
| Unknown | 39 | (2.1) | |||
Radiation dosimetry was not performed for non-participants and was, therefore, estimated from institutional records. SJLIFE = St. Jude Lifetime Cohort, cGy = centigray BMI = body mass index
Cardiotoxic exposures included anthracycline and radiation therapy. Over 82% were exposed to anthracyclines, with 20.8% receiving cumulative doses in excess of 250 mg/m2. Less than half received radiation to the heart, but 22.2% had a cardiac dose >1500 cGy. (Appendix Table 2) Cardiovascular risk factors present at the time of SJLIFE assessment included being overweight or obese (61.3%), smoking (23.7% current, 11.7% past), physical inactivity (49.6%), hypertension (23.3%), diabetes (6.8%), and dyslipidemia (61.9%).
Appendix Table 2. Distribution of cardiac radiation and anthracycline exposures.
| Anthracycline dose (mg/m2) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| None | < 250 mg/m2 | ≥ 250 mg/m2 | Total | |||||
|
|
||||||||
| Cardiac Radiation | N | (%) | N | (%) | N | (%) | N | (%) |
| None | 0 | (0) | 799 | (70.4) | 280 | (72.6) | 1079 | (58.2) |
| ≤ 1500 cGy | 127 | (38.3) | 183 | (16.1) | 53 | (13.7) | 363 | (19.6) |
| > 1500 cGy | 205 | (61.7) | 153 | (13.5) | 53 | (13.7) | 411 | (22.2) |
|
| ||||||||
| Total | 332 | (100) | 1135 | (100) | 386 | (100) | 1853 | (100) |
The prevalence of each outcome and the result of cardiovascular screening is shown in Table 2. Cardiomyopathy was identified in 7.4% of the population with more than half being detected during the SJLIFE assessment. Screening identified 4.7% with previously undiagnosed cardiomyopathy. Similarly, 3.8% and 4.6% of the population was found to have evidence of CAD or a conduction/rhythm disorder; 2.2% and 1.4%, respectively, newly identified by screening. Functional valve findings were frequent (28.0%) with most being identified during the SJLIFE assessment (24.8%). The prevalence and severity of regurgitation and stenosis in each valve is shown in Table 3. The majority were mild, with most being regurgitation of the tricuspid or pulmonary valves. Moderate or severe mitral regurgitation or stenosis was identified in 26 (1.5%) and 2 (0.1%) survivors, respectively. Sixty-four survivors (3.7%) had aortic regurgitation (16 were moderate, 3 severe) and 31 (1.8%) had aortic stenosis (4 moderate, 9 severe). Mitral valve thickening or annular calcifications were identified in 351 (20.2%) and 177 (10.2%), respectively, and 414 (23.8%) had aortic valve thickening and 107 (6.1%) aortic calcifications. One hundred sixty survivors (9.2%) had more than one valve involved.
Table 2. Prevalence of cardiac outcomes in the SJLIFE cohort and outcomes newly identified by cardiovascular screening.
| Cardiac outcome | Number evaluated (N) | Detected prior to SJLIFE Assessment | Detected at SJLIFE Assessment | Total Prevalence | Percent Identified by Cardiovascular Screening* | |||
|---|---|---|---|---|---|---|---|---|
| Cardiomyopathy | 1586ł | 46 | (2.9) | 72 | (4.5) | 118 | (7.4) | 4.7% |
| Coronary artery disease | 1853 | 29 | (1.6) | 40 | (2.2) | 69 | (3.8) | 2.2% |
| Valvular disease | 1742ł | 74 | (4.2) | 414 | (23.8) | 488 | (28.0) | 24.8% |
| Conduction/rhythm disorders | 1798ł | 53 | (3.0) | 25 | (1.4) | 78 | (4.6) | 1.4% |
Percent identified by cardiovascular screening = number of outcomes diagnosed during SJLIFE assessment ÷ the number evaluated minus outcomes diagnosed prior to SJLIFE assessment.
Only imaging that was successfully completed by the participants and of sufficient quality for interpretation was included, i.e. 1,586 completed the echocardiograms with images adequate for assessment of left ventricular function, 1,742 images were adequate for complete evaluation of valve function, and 1,798 participants completed the electrocardiogram. SJLIFE = St. Jude Lifetime Cohort
Table 3. Prevalence and severity of functional heart valve findings among 1742 survivors.
| Mitral Valve | Aortic Valve | Tricuspid Valve | Pulmonic Valve | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Regurgitation | Stenosis | Regurgitation | Stenosis | Regurgitation | Stenosis | Regurgitation | Stenosis | |||||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
|
|
||||||||||||||||
| Mild | 101 | (5.8) | 14 | (0.8) | 45 | (2.58) | 18 | (1.0) | 241 | (13.8) | 1 | (0.06) | 173 | (9.93) | 0 | 0 |
| Moderate | 19 | (1.1) | 1 | (0.06) | 16 | (0.92) | 4 | (0.23) | 40 | (2.3) | 0 | 0 | 12 | (0.69) | 1 | (0.69) |
| Severe | 7 | (0.4) | 1 | (0.06) | 3 | (0.17) | 9 | (0.52) | 2 | (0.11) | 0 | 0 | 0 | 0 | 0 | 0 |
Includes survivors with more than one functional valve finding.
The prevalence of each outcome increased with increasing age at evaluation and ranged from 10-37% among those more than 40 years old (Table 4). Nearly 5% of participants <30 years old had evidence of cardiomyopathy. However, most of the outcomes were identified in participants 30 years or older, with the exception of valvular regurgitation and/or stenosis, which was identified in over a quarter of survivors between 18-29 years.
Table 4. Prevalence of Cardiac Outcomes by Age at Detection.
| Cardiac outcome | Number evaluated | Age at detection (years) | Number evaluable | N | (%) |
|---|---|---|---|---|---|
| Cardiomyopathy | 1586ł | 18-29 | (n=706) | 33 | (4.7) |
| 30-39 | (n=610) | 56 | (9.2) | ||
| ≥ 40 | (n=270) | 29 | (10.7) | ||
| Coronary artery disease | 1853 | 18-29 | (n=791) | 7 | (0.9) |
| 30-39 | (n=701) | 24 | (3.4) | ||
| ≥ 40 | (n=361) | 38 | (10.5) | ||
| Valvular disease | 1742ł | 18-29 | (n=761) | 214 | (28.1) |
| 30-39 | (n=660) | 156 | (23.6) | ||
| ≥ 40 | (n=321) | 118 | (36.8) | ||
| Conduction/rhythm disorders | 1798ł | 18-29 | (n=770) | 21 | (2.7) |
| 30-39 | (n=677) | 21 | (3.1) | ||
| ≥ 40 | (n=350) | 36 | (10.3) |
Only imaging that was successfully completed by the participants and of sufficient quality for interpretation was included, i.e. 1,586 completed the echocardiograms with images adequate for assessment of left ventricular function, 1,742 images were adequate for complete evaluation of valve function, and 1,798 participants completed the electrocardiogram.
Table 5 shows the associations between sex, age at diagnosis, age at evaluation, treatment factors, and traditional cardiovascular risk factors for cardiomyopathy and valvular disease. The odds of cardiomyopathy were significantly associated with male sex, anthracycline doses ≥250 mg/m2, cardiac radiation exposure in excess of 1500cGy, and hypertension. Younger age at diagnosis and higher radiation doses were associated with increased odds of functional valve findings. Associations between radiation and valvular disease varied according to the anthracycline exposure (interaction p<0.001), with the highest odds being among those exposed to the highest doses of radiation and any anthracycline exposure. A reduction in the odds ratio for valve disease was noted among obese survivors and those with dyslipidemia. There were not enough CAD and conduction/rhythm abnormalities to support a fully adjusted multivariable model, yet it appeared these outcomes were more common with older age (≥40 years) and among those with cardiac radiation doses >1500 cGy. (Appendix Table 3).
Table 5. Cardiomyopathy and Valvular Disease by Cardiac Risk Factors* †.
| Cardiomyopathy | Valvular disease | |||
|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | |
|
|
||||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 1.9 | (1.1-3.3) | 0.8 | (0.6-1.0) |
| Age at Diagnosis (years) | ||||
| 0-4 | 0.5 | (0.3-1.1) | 1.5 | (1.1-2.2) |
| 5-9 | 0.6 | (0.3-1.2) | 1.3 | (0.9-1.9) |
| 10-14 | 0.9 | (0.5-1.7) | 1.0 | (0.7-1.5) |
| ≥ 15 | 1.0 | 1.0 | ||
| Age at SJLIFE Evaluation (years) | ||||
| 18-29 | 1.0 | 1.0 | ||
| 30-39 | 1.3 | (0.7-2.3) | 0.8 | (0.6-1.1) |
| ≥ 40 | 0.4 | (0.2-1.0) | 1.2 | (0.8-1.7) |
| BMI at SJLIFE assessment (kg/m2) | ||||
| Normal/underweight | 1.0 | 1.0 | ||
| Overweight (BMI=25.0 to <30) | 1.0 | (0.5-1.9) | 0.8 | (0.6-1.1) |
| Obese (BMI ≥ 30) | 1.2 | (0.6-2.3) | 0.4 | (0.3-0.6) |
| Anthracycline (mg/m2) | ||||
| None | 1.0 | - | -- | |
| < 250 | 1.2 | (0.5-2.8) | - | -- |
| ≥ 250 | 2.7 | (1.1-6.9) | - | -- |
| Average Cardiac Radiation dose (cGy) | ||||
| None | 1.0 | - | -- | |
| ≤ 1500 | 1.1 | (0.5-2.2) | - | -- |
| > 1500 | 1.9 | (1.1-3.7) | - | -- |
| Interaction Cardiac Radiation * Anthracycline | ||||
| ≤ 1500 cGy * No anthracycline | 1.0 | |||
| > 1500 cGy * ≥ 250 mg/m2 anthracycline | N/A | 4.5 | (1.9-10.7) | |
| ≤ 1500 cGy * ≥ 250 anthracycline | 0.9 | (0.3-2.3) | ||
| No radiation * ≥ 250 anthracycline | 1.1 | (0.6-2.2) | ||
| > 1500 cGy * ˂ 250 mg/m2 anthracycline | 3.1 | (1.5-6.2) | ||
| ≤ 1500 cGy * ˂ 250 mg/m2 anthracycline | 1.9 | (1.0-3.8) | ||
| No radiation * ˂ 250 mg/m2 anthracycline | 1.1 | (0.6-2.0) | ||
| > 1500 cGy * no anthracycline | 4.1 | (2.1-8.0) | ||
| Smoker (ever) | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.9 | (0.5-1.5) | 0.8 | (0.6-1.0) |
| Physical Activity | ||||
| Inactive | 1.0 | 1.0 | ||
| Active | 1.2 | (0.7-2.0) | 1.0 | (0.8-1.3) |
| Excessive alcohol | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.9 | (0.5-1.5) | 1.0 | (0.8-1.3) |
| Hypertension | ||||
| No | 1.0 | 1.0 | ||
| Yes | 3.0 | (1.7-5.2) | 1.2 | (0.9-1.7) |
| Diabetes | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.0 | (0.9-4.2) | 0.7 | (0.4-1.3) |
| Dyslipidemia | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.0 | (0.6-1.7) | 0.7 | (0.6-0.9) |
Estimates adjusted for all variables in the table.
Analysis limited to only those outcomes detected at baseline SJLIFE visit. SJLIFE = St. Jude Lifetime Cohort, BMI = body mass index, cGy = centigray
Appendix Table 3. Coronary Artery Disease and Conduction/rhythm disorders by Cardiac Risk Factors*.
| Coronary artery disease | Conduction/ rhythm disorders | |||
|---|---|---|---|---|
| OR* | 95% CI | OR* | 95% CI | |
|
|
||||
| Sex | ||||
| Female | 1.0 | 1.0 | ||
| Male | 1.7 | (0.9-3.2) | 1.1 | (0.5-2.4) |
| Age at Diagnosis (years) | ||||
| 0-4 | 0.5 | (0.2-1.3) | 1.1 | (0.3-3.9) |
| 5-9 | 0.8 | (0.3-1.9) | 1.8 | (0.6-5.4) |
| 10-14 | 0.4 | (0.2-1.1) | 1.0 | (0.3-3.2) |
| ≥ 15 | 1.0 | 1.0 | ||
| Age at SJLIFE Evaluation (years) | ||||
| 18-29 | 1.0 | 1.0 | ||
| 30-39 | 1.8 | (0.7-4.7) | 2.4 | (0.7-8.1) |
| ≥ 40 | 3.1 | (1.2-8.2) | 4.1 | (1.2-14.1) |
| Anthracycline (mg/m2) | ||||
| None | 1.0 | 1.0 | ||
| < 250 | 2.0 | (0.9-4.6) | 0.5 | (0.2-1.7) |
| ≥ 250 | 2.0 | (0.7-5.4) | 1.5 | (0.5-4.8) |
| Average Cardiac Radiation dose (cGy) | ||||
| None | 1.0 | 1.0 | ||
| ≤ 1500 | 2.2 | (0.7-7.1) | 1.3 | (0.3-4.9) |
| > 1500 | 10.5 | (4.2-26.3) | 3.5 | (1.1-10.7) |
Estimates adjusted for all variables in the table. SJLIFE = St. Jude Lifetime Cohort, cGy = centigray
Although most findings were asymptomatic (4 survivors reported intermittent chest pain; 1 palpitations) and only identified on screening, physical performance was significantly impaired. Survivors with cardiomyopathy and CAD had approximately twice the odds of having an abnormal 6MWT compared to those without these cardiac effects. (Table 6)
Table 6. Impaired Physical Function* and Cardiac Outcomes†.
| Odds Ratio | 95% CI | |
|---|---|---|
| Cardiomyopathy | 1.9 | (1.2-2.9) |
| Coronary Artery Disease | 2.2 | (1.3-3.8) |
| Valvular Disease | 0.7 | (0.6-1.0) |
| Conduction/rhythm Disorders | 1.4 | (0.8-2.4) |
Impaired physical function was defined as a 6MWT of <490 meters
Adjusted for current age, sex, height, and all cardiac conditions
Discussion
Cardiovascular disease and cancer, the leading causes of death among U.S. adults, have both been the target of therapeutic advances and survival gains over the last five decades. However, among adults previously treated for a pediatric malignancy, cardiovascular disease can present at an earlier age with substantial morbidity for which screening methods, modalities, and frequencies remain unclear.(6) Systematically screening the largest prospective cohort of adults previously treated for a childhood cancer, we identified subclinical cardiovascular disease among individuals generally too young for typical cardiovascular risk-stratification. While most cardiovascular disease was asymptomatic, significant associations with limited physical performance were identified.
Studies of cardiotoxicity following cancer therapy have largely focused on cardiomyopathy while only a few have included a broader range of outcomes, such as CAD, valvular disease, and conduction/ rhythm abnormalities.(6, 22-26) Frequencies of these findings have varied widely due to differing screening methods, diagnostic groups studied, outcome definitions, and study methodologies. In a systematic review, Kremer et al. reported subclinical cardiomyopathy ranging from 0-57%.(27) However, many of the studies focused on a particular diagnosis and a variety of definitions for cardiomyopathy. Among studies that evaluated a cross-section of childhood cancer diagnoses and more closely defined cardiomyopathy, the prevalence was narrower, between 5-20%.
Systematic screening identified a substantial number of survivors with asymptomatic cardiomyopathy at high risk for progression to symptomatic heart failure. At a median age of 31 years, we identified 7.5% of survivors with evidence of decreased systolic function; an estimate expected in a much older population. In a randomly selected healthy population, Redfield and colleagues found an EF ≤50% in 3% of those 45-54 years old and 4.8% and 7.1% among those 55-64 and 65-74 years, respectively.(28) The risk of progression to heart failure among persons with untreated systolic dysfunction is significant (29) and has been reported to be as high as 9% per year with a 3-year mortality of 16%.(30) In fact, according to the American College of Cardiology and American Heart Association classification system, these cancer survivors have already progressed from stage A (high risk for developing congestive heart failure) to stage B (asymptomatic) heart failure.(31)
The prevalence of CAD, valve findings, and conduction/rhythm defects has been less frequently described and commonly only within smaller disease-focused studies. Coronary artery disease, typically associated with cardiac directed radiation, has mostly been studied among survivors of Hodgkin lymphoma or breast cancer.(32, 33) However, the Childhood Cancer Survivor Study (CCSS) reported myocardial infarction in 0.7% of 14,358 childhood cancer survivors at a median age of 27 years.(6) In the SJLIFE cohort the prevalence of ischemic disease was higher (3.8%), likely due to the inclusion of subclinical findings, but also potentially influenced by the slightly older age of the cohort and longer follow-up time. Studies among Hodgkin lymphoma survivors have suggested a high fatality rate for radiation-induced CAD and a higher risk for patients treated under age 21 years.(34) CAD in this population is often proximal in the coronary tree, resulting in considerable myocardial loss following infarction. In fact, using CT angiography screening in a pilot study of Hodgkin lymphoma survivors from the SJLIFE cohort, we found obstructive and non-obstructive coronary disease, with 67% of the lesions located in the proximal coronary circulation.(35)
Valvular disease has also been mostly studied among survivors of Hodgkin lymphoma, due to the common use of mediastinal radiation therapy to treat this disease.(36) Based upon self-report, the prevalence of valve abnormalities in the CCSS cohort was 1.6% across a variety of diagnostic groups and was associated with radiation and anthracycline exposure (≥250 mg/m2).(6) The prevalence of valve findings was much higher as a result of systematic screening. In our initial report (26), we identified an abnormality in 29% of the SJLIFE cohort and 56.7% of those treated with radiation to the heart. In a recent study, van der Pal and colleagues(37) identified mild, moderate, and severe valve abnormalities in 169 of 545 (31%) childhood cancer survivors. In our analysis, 28% had a functional valve finding. Six survivors had a bicuspid aortic valve and none a known history of rheumatic fever. In a population-based study, Nkomo et al. estimated the prevalence of mild or greater left-sided valve disease in the general population to be 0.3% (0.2-0.3%) among 18-44 year olds and 11.7 % (11.0-12.5%) among those ≥75 years.(38) Notably, the risk of death among those with valve disease was 1.36 (95% CI 1.15-1.62) compared to those without. While most of the findings in our study were right-sided, 13.7% had a left-sided lesion (8.2% mitral, 5.5% aortic).
Like the CCSS, we also identified an association between valvular disease and anthracycline exposure. It is not entirely clear why anthracycline therapy might be associated with valvular disease but could result from anthracycline-induced cardiomyopathy with secondary annular dilatation leading to valvular incompetence or toxicity to the valvular endothelium. An inverse relationship was noted with BMI. However, this association has been described in the general population(39, 40) and may be due to reduced image quality in obese patients or hemodynamic changes related to body habitus. Follow-up and focused study of valvular disease in this population is warranted.
Conduction and rhythm abnormalities following cancer therapy can be of varied clinical significance and more difficult to characterize. Reports have frequently focused on particular diagnostic groups and been associated with radiation and/or anthracycline exposures.(7, 32, 41) Myocardial fibrosis can induce an arrhythmogenic focus or interrupt the normal electrical impulse. ECG abnormalities were identified in 4.6% of our cohort and significantly associated with radiation exposure. This prevalence is lower than our previous report that also included non-cardiotoxic therapies(26), suggesting that these disorders may occur more frequently than expected but may not be exclusively associated with particular cardiotoxic exposures. The estimated prevalence of a conduction/rhythm disorder beyond age 40 years was 10.3% in this study, higher than that reported in the CCSS.(42) The CCSS did not have the benefit of validated ECG screening of all survivors, thus could not report on subclinical abnormalities and may be an underestimate. CCSS also reported only Common Terminology Criteria for Adverse Events grade 3-5 abnormalities, thus, the lower prevalence might suggest that severe rhythm and conduction defects wane with time from diagnosis. A dedicated investigation and classification of ECG findings in cancer survivors following cardiotoxic and non-cardiotoxic therapies is needed.
Given the lack of randomized controlled trials to test screening practices, the current guidelines for the care of childhood cancer survivors were developed by incorporating best available evidence and expert clinical consensus. Only a few studies have investigated the effectiveness of adherence to these screening guidelines. In a clinic-based study of 370 childhood cancer survivors, median age at evaluation 23.9 years (5.3-57.2), Landier et al. classified screening practices, based upon the prevalence of late effects observed in the literature, as high (≥10%), intermediate (1 to <10%), and negligible (<1%) yield. Cardiomyopathy screening was determined to be of intermediate yield (6%) while yield of cardiac conduction screening was low (0.08%). Ischemic disease and cardiac valves were not evaluated. Two recent analyses assessed cost-effectiveness of echocardiographic screening of survivors for cardiomyopathy. Both concluded that the current guideline suggestions of imaging every 1-5 years is cost effective, though these benefits might be retained even if imaging was performed less frequently and/or with other modalities, such as cardiac magnetic resonance imaging.(43, 44) Our study provides important additional clinical parameters not available in these studies that relied upon self-reported cardiac outcomes. Future longitudinal studies, as can be conducted in the SJLIFE cohort, are needed to better clarify the progression of cardiomyopathy, the effects of treatment, and the implications for clinical practice guidelines related to cardiovascular health surveillance as this population ages. Furthermore, our study assessed additional cardiac outcomes and, to date, cost effectiveness studies have not been performed for those. The proportion of individuals with newly identified cardiac disease in our study was higher for all outcomes assessed than previously reported, albeit lowest for conduction/rhythm disorders.
A number of limitations should be considered when interpreting our results. Given the 61% participation, selection bias could potentially influence our findings. However, significant differences between participants and the SJLIFE source population have not been identified.(45) An on-campus evaluation was required, thus excluding survivors who may not have been able to travel due to commitments, illness, or death, potentially affecting our estimates of disease. Additionally, medical records were not routinely obtained for individuals who did not report a cardiac event, thus, potentially biasing our estimates. Comparisons were not made to a non-cancer control population and, while survivors will be invited to return for a repeat evaluation, longitudinal assessments are currently lacking. Only survivors with a history of cardiotoxic therapies were studied, limiting the ability to generalize these findings and potentially missing cardiac disease in survivors with other exposure histories. Finally, among those who did participate, 267 echocardiograms were missing (101 participants failed to complete scheduled cardiac evaluations and 166 for technical reasons – suboptimal recording, poor electrical transmission, etc.). All reasons were reviewed and treated as missing completely at random.
We identified considerable cardiovascular disease in this large cohort of adults cured of childhood cancer. The prevalence is inconsistent with the chronological age of the population and suggests a substantial future health care burden. Clinically, these data may guide risk factor stratification, screening practices, health counseling, and potential therapeutic measures aimed at changing the disease trajectory in this young adult population.
Acknowledgments
The authors with to thank Ms. Taryn Donley for technical support with manuscript preparation.
Grant Support: Supported by Cancer Center Support (CORE) Grant (CA21765) to St. Jude Children's Research Hospital, U01 CA195547 1 (PI: Dr. Melissa M. Hudson), and the American Lebanese Syrian Associate Charities (ALSAC), Memphis, TN.
Footnotes
Address for reprint requests: Daniel A. Mulrooney, MD, MS, St. Jude Children's Research Hospital, Department of Oncology, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Mailing addresses for authors:
Daniel A. Mulrooney, MD, MS, St. Jude Children's Research Hospital, Department of Oncology, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Gregory T. Armstrong, MD, MSCE, St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Sujuan Huang, MSPH, St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Kirsten K. Ness, PT, PhD, St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Matthew J. Ehrhardt, MD, MS, St. Jude Children's Research Hospital, Department of Oncology, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Vijaya M. Joshi, MD, University of Tennessee Health Science Center, Le Bonheur Research Center, 848 Adams Avenue, Suite L-400, Memphis, TN 38103
Juan Carlos Plana, MD, Baylor College of Medicine, Department of Medicine, 6620 Main Street, 12th Floor, Suite 1225, Houston, TX 77030
Elsayed Z. Soliman, MD MSc, MS, Wake Forest School of Medicine, Department of Epidemiology and Prevention & Department of Medicine-Cardiology, Medical Center Boulevard, Winston-Salem, NC 27157
Daniel M. Green, MD, St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Deokumar Srivastava, PhD St. Jude Children's Research Hospital, Department of Biostatistics, 262 Danny Thomas Place, MS768, Memphis, TN 38105
Aimee Santucci, PhD, St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control, 262 Danny Thomas Place, MS735, Memphis, TN 38105
Matthew J. Krasin, MD, St. Jude Children's Research Hospital, Department of Radiological Sciences, 262 Danny Thomas Place, MS210, Memphis, TN 38105
Leslie L. Robison, PhD St. Jude Children's Research Hospital, Department of Epidemiology & Cancer Control 262 Danny Thomas Place, MS735, Memphis, TN 38105
Melissa M. Hudson, MD St. Jude Children's Research Hospital, Department of Oncology, 262 Danny Thomas Place, MS735, Memphis, TN 38105
References
- 1.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2009. Bethesda, MD: National Cancer Institute; 2011. Vintage 2009 Populations. [Google Scholar]
- 2.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. doi: 10.1158/1055-9965.EPI-14-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–79. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2328–38. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Pal HJ, van Dalen EC, van Delden E, van Dijk IW, Kok WE, Geskus RB, et al. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30(13):1429–37. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 8.Tukenova M, Guibout C, Oberlin O, Doyon F, Mousannif A, Haddy N, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28(8):1308–15. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 9.Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30(10):1050–7. doi: 10.1200/JCO.2010.33.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, et al. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170(14):1247–55. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–36. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 13.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Le Deley MC, Leblanc T, Shamsaldin A, Raquin MA, Lacour B, Sommelet D, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol. 2003;21(6):1074–81. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 15.Stovall M, Weathers R, Kasper C, Smith SA, Travis L, Ron E, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166(1 Pt 2):141–57. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 16.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22(1):1–23. doi: 10.1016/j.echo.2008.11.029. quiz 101-2. [DOI] [PubMed] [Google Scholar]
- 18.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. Littleton, MA: John Wright-PSG; 1982. [Google Scholar]
- 19.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Pollentier B, Irons SL, Benedetto CM, Dibenedetto AM, Loton D, Seyler RD, et al. Examination of the six minute walk test to determine functional capacity in people with chronic heart failure: a systematic review. Cardiopulm Phys Ther J. 2010;21(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 21.Ratitch B, Lipkovich I, O'Kelly M. Combining Analysis Results from Multiply Imputed Categorical Data PharmaSUG. 2013 Paper SP03. [Google Scholar]
- 22.Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001;19(1):191–6. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 23.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. Eur J Cancer. 2006;42(18):3191–8. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Pein F, Sakiroglu O, Dahan M, Lebidois J, Merlet P, Shamsaldin A, et al. Cardiac abnormalities 15 years and more after adriamycin therapy in 229 childhood survivors of a solid tumour at the Institut Gustave Roussy. Br J Cancer. 2004;91(1):37–44. doi: 10.1038/sj.bjc.6601904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velensek V, Mazic U, Krzisnik C, Demsar D, Jazbec J, Jereb B. Cardiac damage after treatment of childhood cancer: a long-term follow-up. BMC Cancer. 2008;8:141. doi: 10.1186/1471-2407-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13(6):819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- 28.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108(8):977–82. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 30.Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigattors. N Engl J Med. 1992;327(10):685–91. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 31.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 32.Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin's disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–48. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 33.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 34.King V, Constine LS, Clark D, Schwartz RG, Muhs AG, Henzler M, et al. Symptomatic coronary artery disease after mantle irradiation for Hodgkin's disease. Int J Radiat Oncol Biol Phys. 1996;36(4):881–9. doi: 10.1016/s0360-3016(96)00295-7. [DOI] [PubMed] [Google Scholar]
- 35.Mulrooney DA, Nunnery SE, Armstrong GT, Ness KK, Srivastava D, Donovan FD, et al. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer. 2014;120(22):3536–44. doi: 10.1002/cncr.28925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heidenreich PA, Hancock SL, Lee BK, Mariscal CS, Schnittger I. Asymptomatic cardiac disease following mediastinal irradiation. J Am Coll Cardiol. 2003;42(4):743–9. doi: 10.1016/s0735-1097(03)00759-9. [DOI] [PubMed] [Google Scholar]
- 37.van der Pal HJ, van Dijk IW, Geskus RB, Kok WE, Koolen M, Sieswerda E, et al. Valvular Abnormalities Detected by Echocardiography in 5-Year Survivors of Childhood Cancer: A Long-Term Follow-Up Study. Int J Radiat Oncol Biol Phys. 2014 doi: 10.1016/j.ijrobp.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005–11. doi: 10.1016/S0140-6736(06)69208-8. [DOI] [PubMed] [Google Scholar]
- 39.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83(6):897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 40.Reid CL, Anton-Culver H, Yunis C, Gardin JM. Prevalence and clinical correlates of isolated mitral, isolated aortic regurgitation, and both in adults aged 21 to 35 years (from the CARDIA study) Am J Cardiol. 2007;99(6):830–4. doi: 10.1016/j.amjcard.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 41.Travis LB, Ng AK, Allan JM, Pui CH, Kennedy AR, Xu XG, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Natl Cancer Inst. 2012;104(5):357–70. doi: 10.1093/jnci/djr533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–80. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong FL, Bhatia S, Landier W, Francisco L, Leisenring W, Hudson MM, et al. Cost-effectiveness of the children's oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160(10):672–83. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160(10):661–71. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60(5):856–64. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]