Abstract
Hypoxia occurs during the development of the placenta in the first trimester and correlates with both trophoblast differentiation and the induction of telomerase activity through hTERT expression. We sought to determine the mechanism of regulation of hTERT expression during hypoxia. We show that hypoxia-inducible factor 1α (HIF-1α) and hTERT expression in the human placenta decrease with gestational age and that these are overexpressed in preeclamptic placenta, a major complication of pregnancy. Hypoxia not only transactivates the hTERT promoter activity but also enhances endogenous hTERT expression. The hTERT promoter region between −165 and +51 contains two HIF-1 consensus motifs, and in vitro reporter assays show that these are essential for hTERT transactivation by HIF-1. Introduction of an antisense oligonucleotide for HIF-1 diminishes hTERT expression during hypoxia, indicating that upregulation of hTERT by hypoxia is directly mediated through HIF-1. Our results provide persuasive evidence that the regulation of hTERT promoter activity by HIF-1 represents a mechanism for trophoblast growth during hypoxia and suggests that this may be a generalized response to hypoxia in various human disorders including resistance to cancer therapeutics by upregulating telomerase.
Trophoblasts are unique cells that derived from the outer cell layer of the blastocyst and exist as undifferentiated cytotrophoblasts in the placenta (17). During early pregnancy the proliferation of trophoblasts is very active, similar to malignant cells, as they invade the endometrium and maternal blood vessels in the stroma. However, unlike tumor invasion, trophoblastic invasion into the endometrium is under strict control (2). This control limits invasion, so that it primarily remains confined to the endometrial aspect of the myometrium and continues only until midgestation (39). Trophoblasts during early gestation grow more rapidly than in the late gestational period, particularly during the first 10 weeks of placental development when the placenta resides in a relatively hypoxic environment (34). Low oxygen tension-triggered trophoblast proliferation can result in early onset preeclampsia and is the major cause of maternal morbidity and mortality (4). The role of O2 tension in modulating proliferation and/or differentiation within the human placenta prompted us to investigate the importance of hypoxia inducible factor 1 (HIF-1) function in controlling this process.
HIF-1 is a heterodimeric transcription factor composed of the basic helix-loop-helix-PAS proteins HIF-1α and the arylhydrocarbon receptor nuclear translocator (ARNT). HIF-1 mediates the transcriptional response to O2 deprivation by binding to hypoxia response elements (HREs) within promoters or enhancers of genes involved in glycolysis, glucose transport, erythropoiesis, and angiogenesis (3, 42). HIF-1 activity is critical for normal development; mouse embryos lacking functional HIF-1 complexes die on or before E10.5. Hif-1−/− embryos show developmental arrest by E9.0, with significant mesenchymal cell death and impaired vascular development (15, 33). Arnt−/− animals die by E10.5, displaying deficiencies in the yolk sac and/or in placental vascularization (18, 21). Furthermore, Arnt−/− yolk sacs show decreased numbers of multilineage hematopoietic progenitors (1). HIF-1 activity is therefore essential for the proliferation, survival, and/or differentiation of multiple embryonic tissues.
It was previously reported that trophoblasts from trophoblastic diseases and normal chorionic villi in early pregnancy expressed relatively high levels of telomerase activity and that the levels of telomerase activity in normal human villi tended to decrease depending on gestational age (19, 29). The expression of the human telomerase catalytic subunit (hTERT) correlates with telomerase activity (24, 26, 41). While telomerase activity is an important factor in cell proliferation, hypoxia exposure has been shown to increase hTERT gene expression, suggesting that telomerase activation may also be a mechanism that protects against genetic stress induced by hypoxia (25, 35). However, the molecular mechanisms by which hypoxia activates telomerase have not been examined in any detail. Interestingly, computer-assisted homology searches have revealed potential binding sites for HIF-1 in the hTERT proximal promoter.
Based on these observations, we propose that early in the first trimester (<10 weeks) the low oxygen tension environment maintains trophoblasts in a relatively immature, proliferative state, mediated by hTERT through HIF-1. The present study was designed to test if HIF-1 is involved in hypoxia-induced activation of the hTERT promoter in the placental JAR and JEG-3 cells. We provide direct evidence that induction of hTERT promoter activity by hypoxia is mediated by HIF-1 through two HIF-1 consensus binding sites (HREs) located at −165 to −158 and + 44 to + 51 in the hTERT proximal promoter. This study adds hTERT to the list of hypoxia-inducible genes regulated by this transcription factor.
MATERIALS AND METHODS
Samples.
The samples were obtained with informed consent from patients undergoing surgery such as cesarean section for normal placentas and uterine evacuation for normal villi. The tissues were finely minced into small pieces with scissors, washed in 0.9% sterile saline to avoid contamination with red blood cells that may interfere with PCR, snap frozen, and stored at −80°C.
Cell culture.
The human choriocarcinoma cell lines JEG-3 and JAR were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, Calif.) supplemented with 10% fetal bovine serum. For hypoxic exposure, cells were subjected to 1% oxygen in a water-jacketed CO2 incubator (NAPCO, Winchester, Va.) at 37°C in a humidified atmosphere with 5% CO2 for the indicated times. In experiments with transfected cells, each transfection was performed prior to treatment with exposure to hypoxia.
DNA plasmids.
The HIF-1α and ARNT expression vectors were described previously (13). pGL3-181-luc is an hTERT promoter-luciferase reporter plasmid in which a 181-bp sequence upstream of the transcription start site of hTERT (designated nucleotide [nt] +1) and a 77-bp sequence (5′ untranslated region) downstream of the transcription start site are cloned into pGL3-Basic (Promega) at MluI and BglII sites (see Fig. 3) (41). Reporter plasmids pGL3-181mt1-luc, pGL3-181mt2-luc, and pGL3-181mt-luc (20) contain one or two mutations in the two putative binding sites of HIF-1 at nt −165 and + 44, which were introduced by PCR-based site-specific mutagenesis (see Fig. 3).
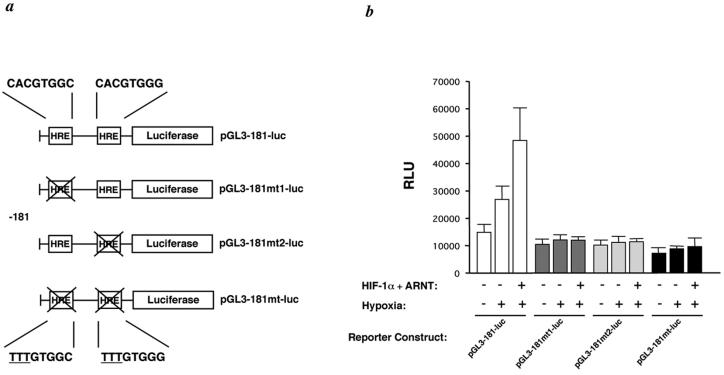
FIG. 3.
Transactivation of hTERT promoter activity by hypoxia exposure and HIF-1 overexpression. (a) Schematic representations of the HIF-1 response elements and the pGL181-luc, pGL181mt1-luc, pGL181mt2-luc, and pGL181mt-luc reporters are shown. (b) JEG-3 cells were cotransfected with the pGL181-luc, pGL181mt1-luc, pGL181mt2-luc, and pGL181mt-luc (0.3 μg each) constructs and 1.0 μg (each) of the HIF-1 expression constructs (HIF-1α and ARNT) or the empty vector pcDNA3 under hypoxic conditions (1% O2); activity is reported as relative luminescence units (RLU). Error bars indicate standard deviations in triplicate assays.
Transfections and luciferase assays.
Each cell line was seeded at 5 × 105 cells per 35-mm dish and incubated overnight at 37°C in a 5% CO2 incubator. For each transfection, 1.0 μg of empty vector and/or expression vectors along with 0.3 μg of promoter-luciferase DNA were mixed in 0.2 ml of Opti-MEM (Invitrogen), and a precipitate was formed by using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommendations. Cells were washed with Opti-MEM, and complexes were applied to the cells. Cells were then exposed to hypoxia conditions (1% oxygen). At 24 h after transfection, cells were harvested and extracts were prepared with luciferase cell lysis buffer (Pharmingen, San Diego, Calif.). Luciferase activity was measured in extracts from triplicate samples by using a luciferase assay kit (Pharmingen). Plasmid pCMV-GAL (Invitrogen) was used as a reporter for transfection efficiency. We used a Gal assay kit (Invitrogen) to determine Gal activity. hTERT reporter gene activity is given as a ratio of luciferase activity to Gal activity.
Antisense oligonucleotide and transfections.
To inhibit the expression of endogenous HIF-1α, we prepared a high-performance liquid chromatography-purified antisense (AS) phosphorothioate oligonucleotide and, as a control, a sense (SE) oligonucleotide based on the sequence of the HIF-1α gene (5). The sequences of the AS and SE HIF-1α oligonucleotides were 5′-GCCGGCGCCCTCCAT-3′ and 5′-ATGGAGGGCGCCGGC-3′, respectively. All nucleotides were phosphorothioated. The AS oligonucleotide or SE oligonucleotide (0.6 μM) was complexed with Lipofectamine 2000 reagent (Invitrogen) and applied to cells. Cells were then exposed to hypoxia conditions (1% oxygen). At 24 h after transfection, cells were harvested, and lysates were prepared for Western blot analysis.
Western blot analysis.
Cells were harvested and lysed on ice for 30 min in lysis buffer (10 mM Tris [pH 8.0], 1 mM EDTA, 400 mM NaCl, 10% glycerol, 0.5% NP-40, 5 mM sodium fluoride, 0.1 mM phenylmethylsulfonyl fluoride, 1 mM dithiothreitol) containing complete protease inhibitor cocktail (Roche Applied Science, Indianapolis, Ind.). Equal amounts of protein (40 μg) were loaded onto a sodium dodecyl sulfate-polyacrylamide gel (4 to 12% polyacrylamide) and subjected to electrophoresis at 200 V for 50 min. The protein was transferred onto a polyvinylidene difluoride membrane and probed with anti-hTERT antibodies (H-231; Santa Cruz Biotechnology, Santa Cruz, Calif.), anti-HIF-1α antibody (OZ15; Lab Vision Corporation, Fremont, Calif.), and anti-actin antibody (C4; Roche Applied Science). The same blot was probed after the membrane was stripped with the different antibodies. Each protein was detected by horseradish peroxidase-conjugated secondary antibody coupled with enhanced chemiluminescence Western blotting detection reagents (Amersham Biosciences, Piscataway, N.J.).
TRAP assay.
Protein extraction for telomerase assays was performed according to protocols published previously, with minor modifications (16, 14). Briefly, cells were harvested in CHAPS (3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate) lysis buffer (Intergen, Gaithersburg, Md.) and incubated for 30 min on ice. After centrifugation at 15,000 × g for 20 min at 4°C, the supernatants were used for telomerase activity assays. Telomerase activity was measured by the PCR-based telomerase repeat amplification protocol (TRAP) assay using a TRAP-eze telomerase detection kit (Intergen). The bands were visualized by silver staining.
Real-time reverse transcription (RT)-PCR analysis.
Cells were seeded after a 20-h exposure to hypoxia. Transfected cells were harvested, and total cellular RNA was isolated by using Isogen reagent (Nippon Gene, Tokyo, Japan). Total RNAs (5 μg) were reverse transcribed into cDNAs by using a First-strand cDNA Synthesis kit (Roche Applied Science). Real-time PCR was performed for quantitative estimation of hTERT mRNA and glyceraldehyde-3-phosphate dehydrogenase by a Light Cycler (Roche Applied Science).
In vitro transcription and translation.
HIF-1 protein was synthesized in vitro in the presence of unlabeled amino acids by using the pcDNA3-HIF-1α and pcDNA3-ARNT expression constructs with the coupled transcription and translation system from Promega (Madison, Wis.). Translated products were analyzed by Western blotting by using anti-HIF-1α antibody (OZ15; Lab Vision Corporation).
Electrophoretic mobility shift assays (EMSA).
Oligonucleotides containing the HIF-1 consensus DNA binding site were purchased as single-stranded DNAs from Genosys Biotechnologies (Woodlands, Tex.). Double-stranded oligonucleotides were prepared by annealing complementary oligonucleotides in a buffer containing 10 mM Tris (pH 8.0), 500 mM NaCl, and 1 mM EDTA. The sequences of the complementary pairs are as follow: TERT, 5′-GCGCTCCCCACGTGGCGGAGGG-3′, and HRE1, 5′-CCCTCCGCCACGTGGGGAGCGC-3′; TERT, 5′-CTGCTGCGCACGTGGGAAGCCC-3′, and HRE2, 5′-GGGCTTCCCACGTGCGCAGCAG-3′; HRE, 5′-TCTGTACGTGACCACACTCACCTC-3′ and 5′-GAGGTGAGTGTGGTCACGTACAGA-3′; TERT, 5′-GCGCTCCCTTTGTGGCGGAGGG-3′, and HREmt1, 5′-CCCTCCGCCACAAAGGGAGCGC-3′; and TERT, 5′-CTGCTGCGTTTGTGGGAAGCCC-3′, and HREmt2, 5′-GGGCTTCCCACAAACGCAGCAG-3′.
The double-stranded oligonucleotides were end labeled with 32P by using T4 polynucleotide kinase and [γ-32P]ATP. For the EMSA, end-labeled double-stranded oligonucleotides (5,000 cpm) were incubated with 2 μl of HIF-1 protein prepared by in vitro transcription and translation at room temperature (22°C) for 30 min in the presence of a binding buffer containing 25% glycerol, 250 mM NaCl, 50 mM Tris (pH 8.0), 2.5 mM dithiothreitol, 5 mM EDTA (pH 7.5), and 150 ng of poly(dI-dC). When competition assays were performed, an unlabeled HRE, TERT/HRE1, or TERT/HRE2 oligonucleotide was incubated with protein and buffer for 5 min prior to the addition of each labeled oligonucleotide. When supershift assays were performed, 0.5 μg of anti-HIF-1α antibody (OZ15; Lab Vision Corporation) was incubated with protein and buffer for 5 min prior to the addition of each labeled oligonucleotide. Samples (20 μl) were loaded onto a 5% nondenaturing polyacrylamide gel and subjected to electrophoresis. After electrophoresis, gels were exposed to Kodak XAR film with intensifying screens at −80°C.
RESULTS
HIF-1α and hTERT expression in human placentas.
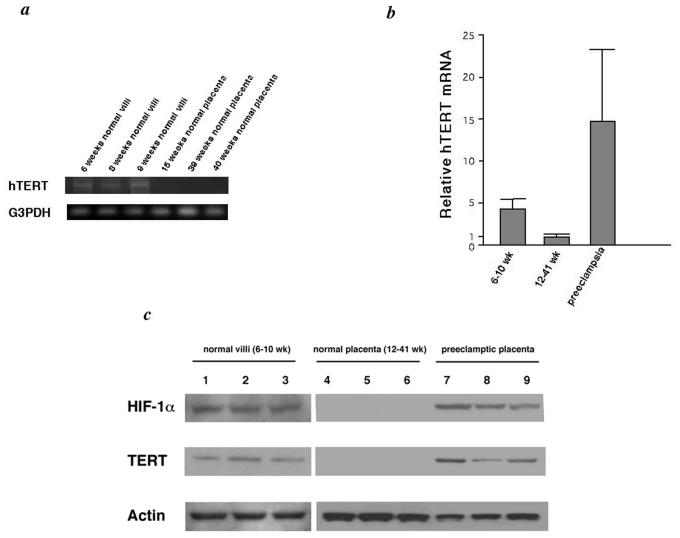
We examined hTERT mRNA expression in 22 normal chorionic villi obtained from 6 to 10 weeks of gestation and 15 placentas from 12 to 41 weeks by RT-PCR by using previously reported methods (7, 28, 29). Although 20 of the normal chorionic villi from the 6- to 10-week gestational period had hTERT expression, placenta tissues from the 12- to 41-week period were negative except for four cases (Fig. 1a). Real-time RT-PCR results confirmed that normal chorionic villi from early pregnancy (before 10 weeks of gestation) expressed high levels of hTERT mRNA, whereas the level of expression from placenta tissues after 10 weeks of gestation was dramatically lower (Fig. 1b). Similar results were obtained in hTERT protein expression by Western blot analysis (Fig. 1c). We next examined HIF-1α protein expression by Western blot analysis and found that 19 of the 22 normal chorionic villi obtained before 10 weeks of gestation expressed HIF-1α (Fig. 1c). In addition, there was no observable HIF-1α protein expression in placenta samples after 10 weeks of gestation, and HIF-1α protein expression strongly correlated with hTERT expression.
FIG. 1.
(a) Representative hTERT mRNA expression in placenta. Normal villi and placenta were analyzed by RT-PCR for hTERT and glyceraldehyde-3-phosphate dehydrogenase expression. (b) Relative mRNA level of hTERT by real-time RT-PCR. (c) Representative hTERT protein expression in placentas analyzed by Western blotting for HIF-1α, hTERT, and actin as follows: 6-, 8-, and 9-week normal villi (lanes 1 to 3, respectively); 15-, 39-, and 40-week normal placentas (lanes 4 to 6, respectively); and 32-, 35-, and 38-week preeclamptic placentas (lanes 7 to 9, respectively).
We next examined both hTERT and HIF-1α expression in preeclampsia placentas (number of placentas, 8). Based on real-time RT-PCR results, hTERT mRNA expression in preeclampsia placentas was substantially higher than that in normal placenta tissues from mid to late gestation (Fig. 1b). By Western blot analysis, all preeclampsia placentas expressed the HIF-1α protein expression (Fig. 1c).
Hypoxia induces HIF-1α and hTERT expression.
Hypoxia has been reported to upregulate both HIF-1α expression and telomerase activity (25, 35, 36). We first examined the effect of hypoxia on telomerase activity in JEG-3 choriocarcinoma cancer cells. Cells were cultured in hypoxia (1% O2) or normoxia (20% O2) for 24 h, and cell pellets were analyzed by TRAP assays. Significant upregulation of telomerase activity was induced by hypoxic exposure (Fig. 2a). Because transcriptional regulation of hTERT is the major mechanism of telomerase activation, we next examined the effect of hypoxia on hTERT mRNA and protein expression. To analyze whether there is a correlation between the two, we examined the expression of the two genes in choriocarcinoma cells in response to 1% O2. Total RNA was isolated from the controls, and cells were exposed to 1% O2 for 1, 3, 6, 12, and 24 h. Real-time RT-PCR revealed that hTERT mRNA expression was increased significantly by hypoxic exposure in JEG-3 (Fig. 2b) and JAR (data not shown) cells. A marked increase in hTERT mRNA expression was observed at 6 h, with peak induction at 12 and 24 h (Fig. 2b). Whole-cell extracts prepared from controls and cells exposed to 1% O2 for 24 h were subjected to Western blot analysis. Both HIF-1α and hTERT expression were increased significantly when cells were exposed to 1% O2 (Fig. 2c).
FIG. 2.
Hypoxia induces telomerase and hTERT expression. (a) JEG-3 cells were cultured at 20% O2 or 1% O2 for 24 h. Whole-cell extracts were prepared and subjected to TRAP assays. (b) JEG-3 cells were cultured at 20% O2 or 1% O2 for 1, 3, 6, 12, and 24 h. Total RNA was prepared and subjected to real-time RT-PCR analysis. (c) JEG-3 and JAR cells were cultured at 20% O2 or 1% O2 for 24 h. Whole-cell extracts (40 μg) were prepared and subjected to Western blot analysis.
To examine whether induction of hTERT expression by hypoxia is mediated via a transcriptional mechanism, JEG-3 cells were transfected with pGL3-181-luc (Fig. 3a) and exposed to 1% O2 for 24 h. Whole-cell extracts were prepared, and promoter activity was examined by the measurement of luciferase activity. Activation of the hTERT promoter was increased by about twofold by hypoxia (Fig. 3b). We next performed cotransfection assays by using the reporter pGL3-181-luc with HIF-1α and ARNT. HIF-1 overexpression transactivated the hTERT promoter by three- to fourfold over the hTERT promoter activity in normoxia (Fig. 3b). We prepared reporter constructs, pGL3-181mt1-luc, pGL3-181mt2-luc, and pGL3-181mt-luc, where the one or two putative HREs were mutated by replacement of CAC with TTT (Fig. 3a). Hypoxia and HIF-1 overexpression did not increase the activity of these reporter constructs in cotransfection assays (Fig. 3b). Furthermore, these mutations slightly decrease the promoter activity (Fig. 3b). Similar results were obtained with JAR cells (data not shown).
Disruption of hTERT expression by an AS oligonucleotide of HIF-1α.
An AS oligonucleotide has been previously used to inhibit HIF-1α expression (5). Thus, we next used the HIF-1α AS oligonucleotide to examine whether hypoxia-induced hTERT expression is mediated by upregulation of HIF-1α. When JEG-3 and JAR cells were transfected with the AS oligonucleotide and exposed to hypoxia, the AS oligonucleotide downregulated hTERT expression by more than 50%, compared with the level of expression in SE oligonucleotide-transfected cells (Fig. 4a). As a control, the level of actin was monitored and found to be unaltered by the HIF-1α AS oligonucleotide (Fig. 4a). We also examined the effect of the HIF-1α AS oligonucleotide on telomerase activity and found that significant downregulation of telomerase activity was induced by transfection of the AS oligonucleotide (Fig. 4b). These results indicate that HIF-1α induces telomerase activity through direct interaction with hTERT regulatory regions.
FIG. 4.
Disruption of hTERT expression by an AS oligonucleotide of HIF-1α. (a) JEG-3 and JAR cells were transfected with an AS or SE oligonucleotide relative to the HIF-1α cDNA sequence by using Lipofectamine 2000. Cells were then exposed to hypoxic conditions (1% O2). A cell lysate was prepared after 24 h and analyzed by Western blotting for protein level by using antibodies to HIF-1α, hTERT, and actin. (b) JEG-3 cells were transfected with the HIF-1α AS or SE oligonucleotide by using Lipofectamine 2000. Cells were then exposed to hypoxic conditions (1% O2). Whole-cell extracts were prepared and subjected to a TRAP assay.
HIF-1 interacts with putative HREs in the hTERT promoter.
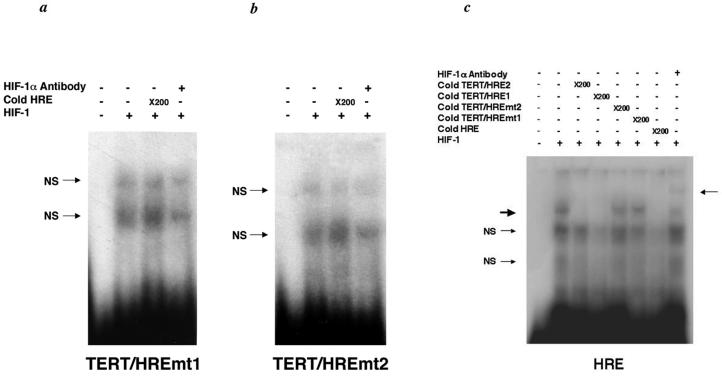
To determine whether HIF-1 has direct interaction with the putative HREs derived from the hTERT promoter, EMSA were performed. Two oligonucleotides corresponding to nt −173 to −158 (TERT/HRE1) and + 36 to + 57 (TERT/HRE2) of the hTERT promoter were incubated with HIF-1α and ARNT prepared by in vitro transcription and translation and subjected to electrophoresis. A DNA-protein complex was formed when either TERT/HRE1 or TERT/HRE2 was incubated with HIF-1-programmed rabbit reticulocyte lysate (Fig. 5a and b) but not with unprogrammed lysate (data not shown). The addition of a 50-fold molar excess of cold HIF-1 consensus oligonucleotide (Santa Cruz Biotechnology) markedly reduced binding (Fig. 5a and b). To further substantiate HIF-1 binding to these oligonucleotides, we performed competition assays using an end-labeled HIF-1 consensus oligonucleotide and cold TERT/HRE1 or TERT/HRE2 as competitors. While both unlabeled TERT/HRE1 and TERT/HRE2 competed the HIF-1 binding to the wild-type probe, when present in the reaction at a 200-fold molar excess, the cold HIF-1 consensus oligonucleotide competed more efficiently at a 50-fold molar excess (Fig. 5c). These results confirm that HIF-1 binds to both TERT/HRE1 and TERT/HRE2 and indicate that the affinity of HIF-1 binding to the TERT/HRE1 or TERT/HRE2 is lower than to the consensus HIF-1 sequence. This finding indicates that hypoxia-induced hTERT expression is due to the enhanced activity of HIF-1α on the hTERT promoter.
FIG. 5.
Analysis of HIF-1 interaction with the putative HREs in the hTERT promoter. (a and b) 32P end-labeled TERT/HRE1 or 32P end-labeled TERT/HRE2 oligonucleotide was used as a probe. For the competition assays, a 50-fold or 200-fold molar excess of the HRE was used. For the supershift assay, anti-HIF-1α antibody (0.5 μg) was added to the binding reaction. The thick arrow indicates HIF-1/DNA complexes, and the thin arrows indicate supershifted bands. (c) The HIF-1 consensus oligonucleotide was end-labeled with 32P. For the competition assays, a 50-fold or 200-fold molar excess of the TERT/HRE1 and of the TERT/HRE2 oligonucleotides were added to the binding reaction, respectively. For the supershift assay, anti-HIF-1α antibody (0.5 μg) was added to the binding reaction. The thick arrow indicates HIF-1/DNA complexes, and the thin arrows indicate supershifted bands. NS, nonspecific.
We performed additional EMSA using TERT/HREmt1 or TERT/HREmt2 as probes. These oligonucleotides did not form a DNA-protein complex with HIF-1 (Fig. 6a and b). We next performed competition assays with an end-labeled HIF-1 consensus oligonucleotide and cold TERT/HREmt1 or TERT/HREmt2 as competitors. While the cold TERT/HRE1 and TERT/HRE2 competed the HIF-1 binding to the wild-type probe, the cold TERT/HREmt1 and TERT/HREmt2 did not compete (Fig. 6c). Taken together, the results indicate that the putative HIF-1 binding sites in the hTERT promoter (between −165 and −158 and between +44 and + 51) are essential for the regulation of hTERT by HIF-1.
FIG. 6.
Analysis of HIF-1 interaction with the mutated HREs in the hTERT promoter. (a and b) 32P end-labeled TERT/HREmt1 or 32P end-labeled TERT/HREmt2 oligonucleotide was used as a probe. For the competition assays, a 200-fold molar excess of the HRE was used. For the supershift assay, anti-HIF-1α antibody (0.5 μg) was added to the binding reaction. (c) The HIF-1 consensus oligonucleotide was end labeled with 32P. For the competition assays, 200-fold molar excesses of TERT/HRE1, TERT/HRE2, TERT/HREmt1, TERT/HREmt2, and HRE were added to the binding reactions. For the supershift assay, anti-HIF-1α antibody (0.5 μg) was added to the binding reaction. The thick arrow indicates HIF-1/DNA complexes, and thin arrows indicate supershifted bands. NS, nonspecific.
DISCUSSION
Placental hypoxia is one of the most critical situations during pregnancy. Continuous hypoxia exposure results in an increased risk for stillbirths and pregnancy-related diseases, such as preeclampsia (4). There has been much recent information about new vasoactive and proproliferative factors being secreted by the placenta in response to hypoxia (4, 31). Some of these reports show that the abundance of HIF-1α protein in the human placenta is significantly decreased with gestational age, and we confirm this in the present study (4, 6). The finding of increased HIF-1α protein expression in the first trimester is consistent with the low intervillous blood flow and physiological hypoxia that have been reported during the early stages of placental development (32). In the present study we also show that the HIF-1α expression correlates with hTERT expression (and telomerase activity), which decreases with increased gestational age (19, 29). For the first time we show that the transcription factor HIF-1 induces hTERT expression.
Telomerase correlates with hTERT expression and plays an important role in cell growth, cell immortalization, and cancer progression (12, 27, 38). Hypoxia also induces cell proliferation (10, 30). It has been previously reported that the exposure of human cells to hypoxic conditions results in the induction of telomerase activity through hTERT expression (25, 35). However, until our present report it has been unclear what mechanisms are involved in hypoxia-induced hTERT expression. There are at least two possibilities to consider about the relationship between hypoxia and induction of telomerase activity. First, hypoxic conditions could induce a DNA damage response by causing telomere damage. In response to this damage HIF-1α may induce telomerase in order to heal the damaged chromosome ends. Alternatively, the hypoxia induction of telomerase could trigger an antiapoptotic response as has been reported previously (40, 45). While the present experiments did not distinguish between these possibilities, we did determine the molecular mechanism for the activation of the hTERT gene during hypoxia. We examined the induction of the hTERT promoter activity in choriocarcinoma cells induced by hypoxia and HIF-1 in a transient expression system and found that hypoxia markedly increased the expression of HIF-1α. In addition, the level of hTERT increased under hypoxic conditions as determined by real-time RT-PCR analysis. In choriocarcinoma cell lines, cotransfection with both HIF-1α and ARNT expression vectors and the hTERT promoter-luciferase reporter plasmid revealed that the hTERT promoter was activated by HIF-1. When AS oligonucleotides were used to reduce HIF-1α expression, hTERT expression was also significantly diminished during hypoxia. Using EMSA, we identified the active HIF-1 responsive elements in the hTERT core promoter. To test the generality of these results, we confirmed by ChIP assays in ME180 cervical cancer cells that these elements were crucial for HIF-1 binding (data not shown).
The HREs (5′-RCGTG-3′) overlap the E box (CACGTG), which is known to bind several nuclear factors, such as c-Myc, Max, and Mad (20, 37, 43, 44). In the present study, the hTERT promoter activity was decreased by mutations of the HREs in normoxic conditions (Fig. 3b). HIF-1 may compete with these factors for binding to these sites. Recent findings show that hypoxia downregulates c-Myc expression (23). Under hypoxic conditions, HIF-1α may play a predominant role in regulating the promoter activity of hTERT. In support of this, we found that in ME180 cancer cells, HIF-1 upregulates the hTERT promoter and endogenous hTERT levels (data not shown). Taken together, these data strongly indicate that hypoxia-induced hTERT expression is due to the enhanced activity of HIF-1 on the hTERT promoter. Furthermore, the HREs in the hTERT promoter are essential for the upregulation of hTERT during hypoxia since the hTERT promoter was not transactivated by hypoxia when the HREs were mutated by using hTERT promoter luciferase constructs.
While there have been several descriptive reports of various markers associated with preeclampsia, including tumor necrosis factor α, vascular endothelial growth factor, and insulin-like growth factors (8, 9, 11, 22), the precise role, if any, of these proteins in preeclampsia remains unclear. The data presented here demonstrate not only that abnormalities in HIF-1α expression are associated with preeclampsia but also that hTERT expression may restore the proliferating capability of preeclamptic trophoblasts. In placenta predisposed to preeclampsia, HIF-1α expression remains abnormally elevated under hypoxic conditions, and trophoblasts remain in a relatively immature state of differentiation. As a direct consequence, trophoblast invasion into the uterus is limited and uteroplacental perfusion is reduced (5). This conclusion is consistent with the clinical manifestations of preeclampsia, including shallow trophoblast invasion into the uterus and abnormally high trophoblast proliferation. Nevertheless, the aberrant expression of a large number of factors in preeclamptic pregnancies suggests that the pathogenesis of this disease is complex and likely involves many regulatory systems. The results of the present experiments suggest that monitoring HIF-1α and hTERT expression may be a useful diagnostic marker of preeclampsia. Equally important, these data suggest a novel target for therapeutic intervention to ameliorate or prevent this life-threatening condition.
In conclusion, we demonstrate that hTERT expression is upregulated following the induction of HIF-1 during exposure of hypoxia and that HIF-1 directly induces the hTERT transcription. Given that low oxygen levels and hTERT activation are crucial for placental development, this function of HIF-1 may represent an important mechanism of regulating placental growth mediated by hTERT during hypoxia.
Acknowledgments
We thank C. Higuma for excellent technical assistance. We thank Eric Huang for providing the HIF-1 expression vectors.
This study was supported in part by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, and by the Public Trust Haraguchi Memorial Cancer Research Fund.
REFERENCES
- 1.Adelman, D. M., E. Maltepe, and M. C. Simon. 1999. Multilineage embryonic hematopoiesis requires hypoxic ARNT activity. Genes Dev. 13:2478-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkotz, R. S., and D. P. Goldstein. 1996. Chorionic tumors. N. Engl. J. Med. 335:1740-1748. [DOI] [PubMed] [Google Scholar]
- 3.Bunn, H. F., and R. O. Poyton. 1996. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 76:839-885. [DOI] [PubMed] [Google Scholar]
- 4.Caniggia, I., S. Grisaru-Gravnosky, M. Kuliszewsky, M. Post, and S. J. Lye. 1999. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J. Clin. Investig. 103:1641-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caniggia, I., H. Mostachfi, J. Winter, M. Gassmann, S. J. Lye, M. Kuliszewski, and M. Post. 2000. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGF beta(3) J. Clin. Investig. 105:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter, A. M. 1997. When is the maternal placental circulation established in man? Placenta 18:83-87. [DOI] [PubMed] [Google Scholar]
- 7.Chen, R. J., C. T. Chu, S. C. Huang, S. N. Chow, and C. Y. Hsieh. 2002. Telomerase activity in gestational trophoblastic disease and placental tissue from early and late human pregnancies. Hum. Reprod. 17:463-468. [DOI] [PubMed] [Google Scholar]
- 8.Conrad, K. P., and D. F. Benyo. 1997. Placental cytokines and the pathogenesis of preeclampsia. Am. J. Reprod. Immunol. 37:240-249. [DOI] [PubMed] [Google Scholar]
- 9.Crossey, P. A., C. C. Pillai, and J. P. Miell. 2002. Altered placental development and intrauterine growth restriction in IGF binding protein-1 transgenic mice. J. Clin. Investig. 110:411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsyth, N. R., A. P. Evans, J. W. Shay, and W. E. Wright. 2003. Developmental differences in the immortalization of lung fibroblasts by telomerase. Aging Cell 2:235-243. [DOI] [PubMed] [Google Scholar]
- 11.Gratton, R. J., H. Asano, and V. K. Han. 2002. The regional expression of insulin-like growth factor II (IGF-II) and insulin-like growth factor binding protein-1 (IGFBP-1) in the placentae of women with pre-eclampsia. Placenta 23:303-310. [DOI] [PubMed] [Google Scholar]
- 12.Holt, S. E., J. W. Shay, and W. E. Wright. 1996. Refining the telomere-telomerase hypothesis of aging and cancer. Nat. Biotechnol. 14:836-839. [DOI] [PubMed] [Google Scholar]
- 13.Huang, L. E., J. Gu, M. Schau, and H. F. Bunn. 1998. Regulation of hypoxia-inducible factor 1 alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 95:7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isaka, K., H. Nishi, Y. Sagawa, T. Nakada, Y. Osakabe, H. Serizawa, Y. Ebihara, and M. Takayama. 2003. Establishment of a new human cell line (EN) with TP53 mutation derived from endometrial carcinoma. Cancer Genet. Cytogenet. 141:20-25. [DOI] [PubMed] [Google Scholar]
- 15.Iyer, N. V., L. E. Kotch, F. Agani, S. W. Leung, E. Laughner, R. H. Wenger, M. Gassmann, J. D. Gearhart, A. M. Lawler, A. Y. Yu, and G. L. Semenza. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12:149-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 17.Kliman, H. J., R. F. Feinberg, and J. E. Haimowitz. 1990. Human trophoblast-endometrial interactions in an in vitro suspension culture system. Placenta 11:349-367. [DOI] [PubMed] [Google Scholar]
- 18.Kozak, K. R., B. Abbott, and O. Hankinson. 1997. ARNT-deficient mice and placental differentiation. Dev. Biol. 191:297-305. [DOI] [PubMed] [Google Scholar]
- 19.Kyo, S., M. Takakura, M. Tanaka, T. Kanaya, T. Sagawa, T. Kohama, H. Ishikawa, T. Nakano, K. Shimoya, and M. Inoue. 1997. Expression of telomerase activity in human chorion. Biochem. Biophys. Res. Commun. 241:498-503. [DOI] [PubMed] [Google Scholar]
- 20.Kyo, S., M. Takakura, T. Taira, T. Kanaya, H. Itoh, M. Yutsudo, H. Ariga, and M. Inoue. 2000. Sp1 cooperates with c-Myc to activate transcription of the human telomerase reverse transcriptase gene (hTERT). Nucleic Acids Res. 28:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maltepe, E., J. V. Schmidt, D. Baunoch, C. A. Bradfield, and M. C. Simon. 1997. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 386:403-407. [DOI] [PubMed] [Google Scholar]
- 22.Maynard, S. E., J. Y. Min, J. Merchan, K. H. Lim, J. Li, S. Mondal, T. A. Libermann, J. P. Morgan, F. W. Sellke, I. E. Stillman, F. H. Epstein, V. P. Sukhatme, and S. A. Karumanchi. 2003. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 111:649-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazure, N. M., C. Chauvet, B. Bois-Joyeux, M. A. Bernard, H. Nacer-Cherif, and J. L. Danan. 2002. Repression of alpha-fetoprotein gene expression under hypoxic conditions in human hepatoma cells: characterization of a negative hypoxia response element that mediates opposite effects of hypoxia inducible factor-1 and c-Myc. Cancer Res. 62:1158-1165. [PubMed] [Google Scholar]
- 24.Meyerson, M., C. M. Counter, E. N. Eaton, L. W. Ellisen, P. Steiner, S. D. Caddle, L. Ziaugra, R. L. Beijersbergen, M. J. Davidoff, Q. Liu, S. Bacchetti, D. A. Haber, and R. A. Weinberg. 1997. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell 90:785-795. [DOI] [PubMed] [Google Scholar]
- 25.Minamino, T., S. A. Mitsialis, and S. Kourembanas. 2001. Hypoxia extends the life span of vascular smooth muscle cells through telomerase activation. Mol. Cell. Biol. 21:3336-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama, J., H. Tahara, E. Tahara, M. Saito, K. Ito, H. Nakamura, T. Nakanishi, E. Tahara, T. Ide, and F. Ishikawa. 1998. Telomerase activation by hTRT in human normal fibroblasts and hepatocellular carcinomas. Nat. Genet. 18:65-68. [DOI] [PubMed] [Google Scholar]
- 27.Nelson, N. J. 1996. Researchers debate clinical role of telomerase. J. Natl. Cancer Inst. 88:1021-1023. [DOI] [PubMed] [Google Scholar]
- 28.Nishi, H., K. Ohyashiki, A. Fujito, N. Yahata, J. H. Ohyashiki, K. Isaka, and M. Takayama. 1999. Expression of telomerase subunits and localization of telomerase activation in hydatidiform mole. Placenta 20:317-323. [DOI] [PubMed] [Google Scholar]
- 29.Nishi, H., N. Yahata, K. Ohyashiki, K. Isaka, K. Shiraishi, J. H. Ohyashiki, K. Toyama, and M. Takayama. 1998. Comparison of telomerase activity in normal chorionic villi to trophoblastic diseases. Int. J. Oncol. 12:81-85. [DOI] [PubMed] [Google Scholar]
- 30.Parrinello, S., E. Samper, A. Krtolica, J. Goldstein, S. Melov, and J. Campisi. 2003. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 5:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajakumar, A., and K. P. Conrad. 2000. Expression, ontogeny, and regulation of hypoxia-inducible transcription factors in the human placenta. Biol. Reprod. 63:559-569. [DOI] [PubMed] [Google Scholar]
- 32.Rodesch, F., P. Simon, C. Donner, and E. Jauniaux. 1992. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 80:283-285. [PubMed] [Google Scholar]
- 33.Ryan, H. E., J. Lo, and R. S. Johnson. 1998. HIF-1alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 17:3005-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schammel, D. P., and T. Bocklage. 1996. p53 PCNA, and Ki-67 in hydropic molar and nonmolar placentas: an immunohistochemical study. Int. J. Gynecol. Pathol. 15:158-166. [DOI] [PubMed] [Google Scholar]
- 35.Seimiya, H., M. Tanji, T. Oh-hara, A. Tomida, I. Naasani, and T. Tsuruo. 1999. Hypoxia up-regulates telomerase activity via mitogen-activated protein kinase signaling in human solid tumor cells. Biochem. Biophys. Res. Commun. 260:365-370. [DOI] [PubMed] [Google Scholar]
- 36.Semenza, G. L. 2000. Surviving ischemia: adaptive responses mediated by hypoxia-inducible factor 1. J. Clin. Investig. 106:809-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semenza, G. L. 2000. HIF-1 and human disease: one highly involved factor. Genes Dev. 14:1983-1991. [PubMed] [Google Scholar]
- 38.Shay, J. W., and W. E. Wright. 1996. Telomerase activity in human cancer. Curr. Opin. Oncol. 8:66-71. [DOI] [PubMed] [Google Scholar]
- 39.Shimonovitz, S., A. Hurwitz, M. Dushnik, E. Anteby, T. Geva-Eldar, and S. Yagel. 1994. Developmental regulation of the expression of 72 and 92 kd type 4 collagenases in human trophoblasts: a possible mechanism for control of trophoblast invasion. Am. J. Obstet. Gynecol. 171:832-838. [DOI] [PubMed] [Google Scholar]
- 40.Stewart, S. A., and R. A. Weinberg. 2000. Telomerase and human tumorigenesis. Semin. Cancer Biol. 10:399-406. [DOI] [PubMed] [Google Scholar]
- 41.Takakura, M., S. Kyo, T. Kanaya, M. Tanaka, and M. Inoue. 1998. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 58:1558-1561. [PubMed] [Google Scholar]
- 42.Wenger, R. H. M. 1997. Oxygen(es) and the hypoxia-inducible factor-1. Biol. Chem. 378:609-616. [PubMed] [Google Scholar]
- 43.Wu, D., N. Popov, M. Hou, Q. Wang, M. Bjorkholm, A. Gruber, A. R. Menkel, and M. Henriksson. 2001. Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. Proc. Natl. Acad. Sci. USA 98:3826-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, K. J., C. Grandori, M. Amacker, N. Simon-Vermot, A. Polack, J. Lingner, and R. Dalla-Favera. 1999. Direct activation of TERT transcription by c-MYC. Nat. Genet. 21:220-224. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, P., S. L. Chan, W. Fu, M. Mendoza, and M. P. Mattson. 2003. TERT suppresses apoptosis at a premitochondrial step by a mechanism requiring reverse transcriptase activity and 14-3-3 protein-binding ability. FASEB J. 17:767-769. [DOI] [PubMed] [Google Scholar]